Abstract
Background
Anastomotic leak is one of the most feared complications of colorectal surgery, and probably linked to poor blood supply to the anastomotic site. Several technologies have been described for intraoperative assessment of bowel perfusion. This systematic review and meta-analysis aimed to evaluate the most frequently used bowel perfusion assessment modalities in elective colorectal procedures, and to assess their associated risk of anastomotic leak. Technologies included indocyanine green fluorescence angiography, diffuse reflectance spectroscopy, laser speckle contrast imaging, and hyperspectral imaging.
Methods
The review was preregistered with PROSPERO (CRD42021297299). A comprehensive literature search was performed using Embase, MEDLINE, Cochrane Library, Scopus, and Web of Science. The final search was undertaken on 29 July 2022. Data were extracted by two reviewers and the MINORS criteria were applied to assess the risk of bias.
Results
Some 66 eligible studies involving 11 560 participants were included. Indocyanine green fluorescence angiography was most used with 10 789 participants, followed by diffuse reflectance spectroscopy with 321, hyperspectral imaging with 265, and laser speckle contrast imaging with 185. In the meta-analysis, the total pooled effect of an intervention on anastomotic leak was 0.05 (95 per cent c.i. 0.04 to 0.07) in comparison with 0.10 (0.08 to 0.12) without. Use of indocyanine green fluorescence angiography, hyperspectral imaging, or laser speckle contrast imaging was associated with a significant reduction in anastomotic leak.
Conclusion
Bowel perfusion assessment reduced the incidence of anastomotic leak, with intraoperative indocyanine green fluorescence angiography, hyperspectral imaging, and laser speckle contrast imaging all demonstrating comparable results.
Anastomotic leak (AL) is one of the most feared complications of colorectal surgery and strongly linked to poor blood supply to the anastomotic site. Several technologies have been developed for intraoperative assessment of the bowel’s blood supply. This study found that using an intervention to measure bowel perfusion reduced the rate of AL. Indocyanine green fluorescence angiography, hyperspectral imaging, and laser speckle contrast imaging demonstrated comparable results, and an ability to reduce the risk of AL when used during surgery. The field would benefit from a large-scale RCT comparing perfusion assessment modalities.
Background
Colorectal cancer is a common cancer with an increasing incidence1,2. For most patients, potentially curative treatment requires major surgical resection of the affected bowel. In England and Wales in 2018, this equated to around 19 000 of the 31 000 cancers diagnosed3. After resection, when feasible, it is preferable to anastomose the remaining bowel4. It is generally accepted that a good blood supply is required to allow anastomotic healing. With inadequate blood supply, the anastomosis is likely to leak5,6.
Anastomotic leakage is a serious complication of colorectal resection associated with a significant increase in mortality and morbidity at 30 days after operation and beyond7. Leak rates in colorectal surgery are estimated at around 1–19 per cent and have a mortality rate of up to 35 per cent, depending on both patient and operative factors7. The relationship between hypoperfusion and an increased incidence of anastomotic leakage has been well documented, and is probably the most important risk factor for leakage5.
Several intraoperative techniques to measure tissue perfusion at the site of anastomosis have been described. One of the most researched methods is indocyanine green fluorescence angiography (ICG-FA). This procedure requires the injection of a dye and subjective intraoperative assessment of perfusion by the surgeon. Laser speckle contrast imaging (LSCI) is another technique described, whereby the movement of blood within the tissue changes the observed laser speckle pattern projected by the device, producing a contrast agent-free measurement of perfusion5. Another method gaining prominence is hyperspectral imaging (HSI). HSI does not require the use of any specific contrast product, but can offer a real-time analysis of perfusion using visible light, as well as near-infrared light in one commercial system This system enables each pixel to be analysed to provide an estimation of local tissue oxygenation8,9. A final perfusion method is diffuse reflectance spectroscopy (DRS), which analyses diffuse reflected light at discrete pinpoint locations in contact with the serosa of the bowel to identify colonic oxygen saturations10.
The aim of this systematic review and meta-analysis was to assess the various types of intraoperative perfusion measurement and evaluate their impact on anastomotic leak rates after surgery.
Methods
Study design
This was a systematic review of all available data on the use of intraoperative bowel perfusion imaging during colorectal surgery. The study was registered with PROSPERO before initiation of searches (CRD42021297299, 9 December 2021). Expert surgeons, librarians, and statisticians were consulted on the study design, search methodology, and statistical analysis.
Inclusion and exclusion criteria
RCTs and comparative studies that used intraoperative perfusion assessment methods to identify bowel perfusion, and documented the rates of anastomotic leakage after surgery, were included. Procedures performed must have been elective and were not limited to minimally invasive surgery. All studies must have been clinical and related to humans, with both demographic and anastomotic leakage data available. A time limit of 21 years (2001–2022) was used to ensure that only relevant studies of intraoperative perfusion imaging were included in what is a relatively new intervention, first described in 199311. Participants in the studies must have been adults with a colorectal pathology requiring intervention. Only English-language studies were included. Studies were excluded if full articles were not accessible or they were systematic reviews or case reports. Where studies used multiple methods of perfusion assessment, only the modality that was used first was included as this was most likely to influence the decision regarding anastomotic site.
Outcomes
The primary outcome was the incidence of anastomotic leak within 30 days of major colorectal surgery involving intraoperative use of bowel perfusion imaging. Secondary outcomes included anastomotic site location changes, tissue oxygenation measurements, threshold of perfusion cut-offs, and sensitivities.
Search strategy
Sources searched included Embase, MEDLINE, Cochrane Library, Scopus, and Web of Science. The first literature search was undertaken on 28 December 2021, with subsequent searches carried out on 1 March 2021 and 29 July 2022. Searches were supplemented by reviews of studies included in relevant systematic reviews to that ensure all available studies were included. The following Boolean search terms were applied: (Ascending Colon OR Colon OR Sigmoid OR Colorectal Surgery OR Bowel) AND (Surgical Anastomosis OR Postoperative Complications OR Anastomotic Leak OR Postoperative Complications) AND (Perfusion OR Perfusion Index OR Perfusion Imaging OR Ischemia OR Imagery).
Data extraction
A data extraction template was completed by two researchers to collect study-level information for each study meeting the inclusion criteria. Data collected included: study data (study design, year, country), demographic data (number of patients included, age, sex, BMI), outcome data (operative focus, number of anastomotic leaks, perfusion modality used, site relocation data, definition of anastomotic leak used), and device data (specific device used, sensitivity, perfusion threshold). Any disagreements between reviewers were resolved through discussion. Where a case–control methodology had been used, the total number of participants was included as well as the case and control numbers.
Assessment of risk of bias in included studies
Methodological quality was assessed using the Methodological Index for Non-Randomized Studies (MINORS) scoring system12. The MINORS criteria were chosen to enable the assessment of both randomized and non-randomized studies. The MINORS criteria were modified to fit the characteristics of included studies; scoring criteria can be seen in the supplementary material. Publication bias was assessed using a funnel plot (supplementary material).
Statistical analysis
Results were subjected to a meta-analysis in which the main findings from the studies were combined and synthesized13,14. The main outcomes analysed were the number of patients with anastomotic leaks expressed as proportions of the total number of patients observed. A meta-analysis was performed using both a common-effect and random-effects models13. The estimation of effects and their confidence intervals was conducted on proportions transformed to logit units15; once estimations had been obtained, for reporting purposes, the results were converted back to the original units (proportions) to ease interpretation. Forest plots were produced to illustrate the results. Interstudy variance was estimated using the DerSimonian and Laird method16 as recommended by Wang15. Summary effect sizes were estimated as weighted means of the observed effects of individual studies. Once the results had been obtained, sensitivity analysis was performed. The magnitude of heterogeneity of study effects was quantified using the level of between-study variance represented by τ2. Q statistics were used, which form part of the formal test of the null hypothesis stating that τ2 = 0, with P values also reported in forest plots. P < 0.050 was considered to indicate a significant level of heterogeneity between studies. Heterogeneity was also measured in terms of the I2 index, which indicates the percentage of the total variability accounted for by between-study variance13. Significant levels of study heterogeneity were defined by values of I2 exceeding 50 per cent17. All the above mentioned indices and P values (Q statistics) were reviewed together as summary information from which the conclusions about the existence of an important level of heterogeneity among study effects were derived. All analyses were undertaken in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study characteristics
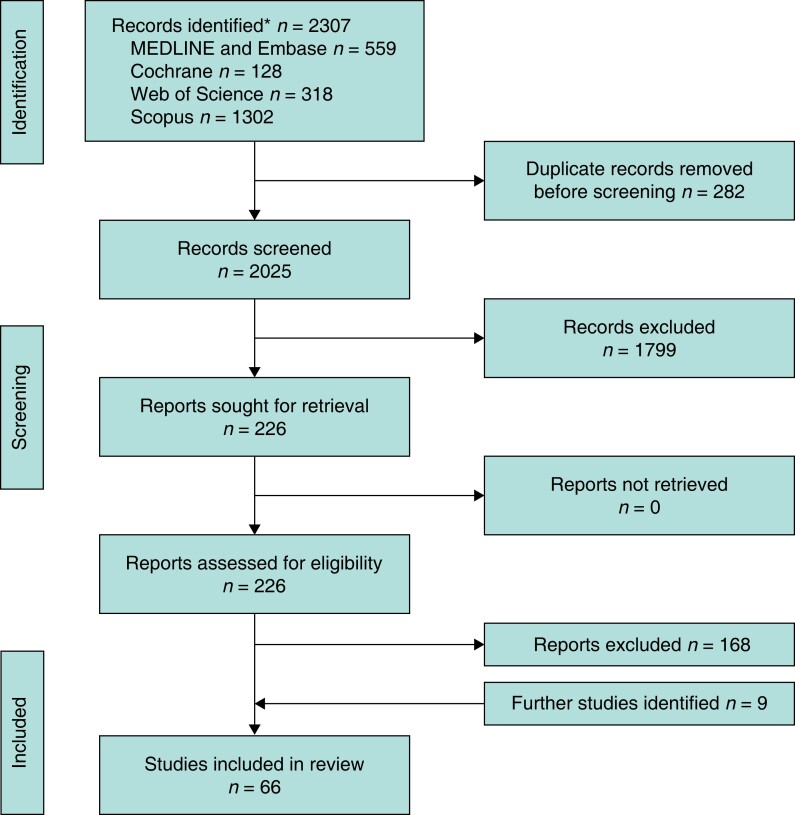
The initial search yielded 2307 studies. After removal of 282 duplicates, the titles and abstracts of the remaining 2025 records were screened for appropriateness and a further 1799 were excluded. A total of 226 studies were retrieved for full-text review by two independent researchers and 168 studies were excluded. After repeated searching, eight more studies were added and a total of 66 studies were included in this systematic review (Fig. 1)5–81,82. Related recent systematic reviews83–90 on intraoperative perfusion were also reviewed to ensure that no studies had been missed.
Fig. 1.
PRISMA flow diagram showing selection of articles for review
†Reasons for exclusion listed in supplementary material.
The 66 included studies included data from 11 560 patients. A summary of each study, including patient characteristics, can be found in the supplementary material. Fifty-six studies were from a single centre and 10 were multicentre in design. Included studies were from a total of 17 nations and 1 study spanned multiple nations. Seventeen studies took place in Japan, 10 in the USA, 10 in Italy, 8 in Germany, and 3 in China; 2 or fewer were conducted by other nations.
Intraoperative perfusion measuring modalities in current use
The searches identified four methods of assessing intraoperative colonic perfusion that are currently in use. The most assessed intervention was ICG-FA with 10 789 patients enrolled in 52 studies, followed by DRS with 321 patients across 6 studies, HSI with 265 patients across 5 studies, and LSCI with 185 patients across 3 studies. ICG-FA uses near-infrared technology to fluoresce ICG. DRS uses diffuse light reflectance technology in contact with the bowel at discrete locations to give a serosal tissue oxygenation (Sto2) value. LSCI uses speckle patterns and flow to assess perfusion at a distance using a camera system. HSI uses reflection of a light source to assess colonic perfusion (also Sto2) at a distance. ICG-FA, HSI, and LSCI rely on blood flow to generate perfusion data, whereas DRS measures oxygenated and deoxygenated blood directly.
An overview of the devices used, along with the benefits and drawbacks of each technology, taken from the manufacturer’s information where available, is shown in Table 1.
Table 1.
Benefits and drawbacks of technologies included in this review
| Modality | Devices used | Benefits | Drawbacks |
|---|---|---|---|
| ICG-FA | Firefly™ robotic surgical system, Intuitive Surgical (L) Photodynamic Eye© PC6100 C9830–10, Hamamatsu (O) Novadaq SPY, Stryker© (O) SPY Elite System, Stryker© (O) PINPOINT Endoscopic Fluorescence Imaging System, Stryker© (L) 1588 Advanced Imaging Modalities, Stryker© (L) D-light P system, Karl Storz© (L) IC-View, Pulsion Medical Systems© (O) VISERA ELITE2 system, Olympus© (L) Opto-cam 2100, Optomedic (L) HyperEye Medical System, Mizuho Medical Company (O) The Quest Artemis, Quest Medical Imaging© (O) VisionSense™ VS Iridium, Medtronic (L) |
Visual markers of well perfused areas Many studies evaluating its use Many devices available |
Lack of objective marker of blood supply Requires dark operating environment if not laparoscopic Requires injection of dye No standardized protocol, concentration, uptake time |
| DRS | O2C, LEA-Medizintechnik© (O) T-Stat, Spectros Corporation© (L) IntraOx device, ViOptix© (L) INVOS, Medtronic© (L) |
Gives quantitative value for oxygen levels No need for medications |
Can only monitor a very localized area of bowel at one time Requires tissue contact Repeat measurements required across bowel for clinical decision-making for wide-field analysis Not all included devices have been cleared for commercial use Few validated studies |
| HSI | TIVITA® Tissue system, Diaspective Vision© (O) | No medications required Can provide objective measurement of Sto2 visually and numerically |
Current systems not real time Open surgery system requires background light to be turned off Few validated studies Included system not laparoscopic |
| LSCI | LSFG device, Softcare Co© (L) MoorFLPI-2, Moor Instruments© (L) |
No medication required Demonstrates non-quantitative blood flow |
System susceptible to tissue/imaging device motion Included systems not real time and images must be superimposed on an existing image Few validated studies |
O, device used only for open surgery; L, device can be used laparoscopically. ICG-FA, indocyanine green fluorescence angiography; DRS, diffuse reflectance spectroscopy; HSI, hyperspectral imaging; Sto2, serosal tissue oxygenation; LSCI, laser speckle contrast imaging. Further details of devices can be found in supplementary material.
Intuitive Surgical (Sunnyvale, California (CA), United States), Hamamatsu (Shizuoka, Japan), Stryker (Kalamazoo, Michigan (Mich), United States), Karl Storz (Tuttlingen, Germany), Pulsion Medical Systems (Midlothian, UK), Olympus (Tokyo, Japan), Optomedic (Guangdong, China), HyperEye Medical System, Mizuho Medical Co. (Tokyo, Japan), Quest Medical Imaging (Wieringerwerf, The Netherlands), LEA-Medizintechnik (Tuttlingen, Germany), Spectros Corp. (Texas, TA, USA), ViOptix Inc (Newark, CA, United States), Medtronic (Dublin, Ireland), Diaspective Vision GmbH (Salzhaff, Germany), Softcare Co. (Japan), Moor Instruments (Devon, UK).
Outcome assessment
The primary outcome was the variation in anastomotic leak rates across the different perfusion methods. The overall pooled incidence of anastomotic leak was 7.4 per cent across the four included groups when perfusion measurements were used, compared with 12.4 per cent in the control groups (Table 2).
Table 2.
Anastomotic leak rates across four perfusion measurement modalities
| Modality | No. of articles | No. of participants | No. of cases | No. of controls | No. with AL* | Site relocation cases* | |
|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||
| ICG-FA | 52 | 10 789 | 5739 | 5050 | 238 (4.1) | 454 (9) | 533 (10.7) |
| DRS | 6 | 321 | 321 | 0 | 43 (13.4) | – | 3 (9.4) |
| HSI | 5 | 265 | 265 | 0 | 21 (7.9) | – | 30 (60) |
| LSCI | 3 | 185 | 71 | 114 | 3 (4.3) | 18 (15.8) | 0 (0) |
| Total | 66 | 11 560 | 6396 | 5164 | 305 (7.4)† | 472 (12.4)† | 566 (20)† |
Values are n (%). †Average as opposed to total. Site relocation data consider only studies that documented the parameter. AL, anastomotic leak; ICG-FA, indocyanine green fluorescence angiography; DRS, diffuse reflectance spectroscopy; HSI, hyperspectral imaging; LSCI, laser speckle contrast imaging. Percentages may not total 100% due to rounding.
Secondary outcomes included the rates of reoperation to resite the anastomosis, tissue oxygenation measurements, and threshold of perfusion cut-offs. The rate of resiting of the anastomotic transection margin and anastomosis ranged between 0 and 100 per cent across all groups within studies. DRS and ICG-FA were associated with similar relocation rates of 9.38 and 10.69 per cent respectively for studies that included these data. The impact of intraoperative assessment of tissue perfusion on duration of operation was reported infrequently. Of 21 ICG-FA studies, the mean increase in operating time was 5.4 (range –22 to 38.1) min. Only one LSCI study50 reported operating times, and documented an average increase of 56 min with intraoperative use of the technology. Reoperation rates were also variable across the groups. The reoperation rate was 14 per cent across 2 DRS studies, 0 per cent across 2 LSCI studies, and 4 per cent across 28 studies in the ICG-FA group. Reoperation rates were not reported for the HSI studies.
Meta-analysis
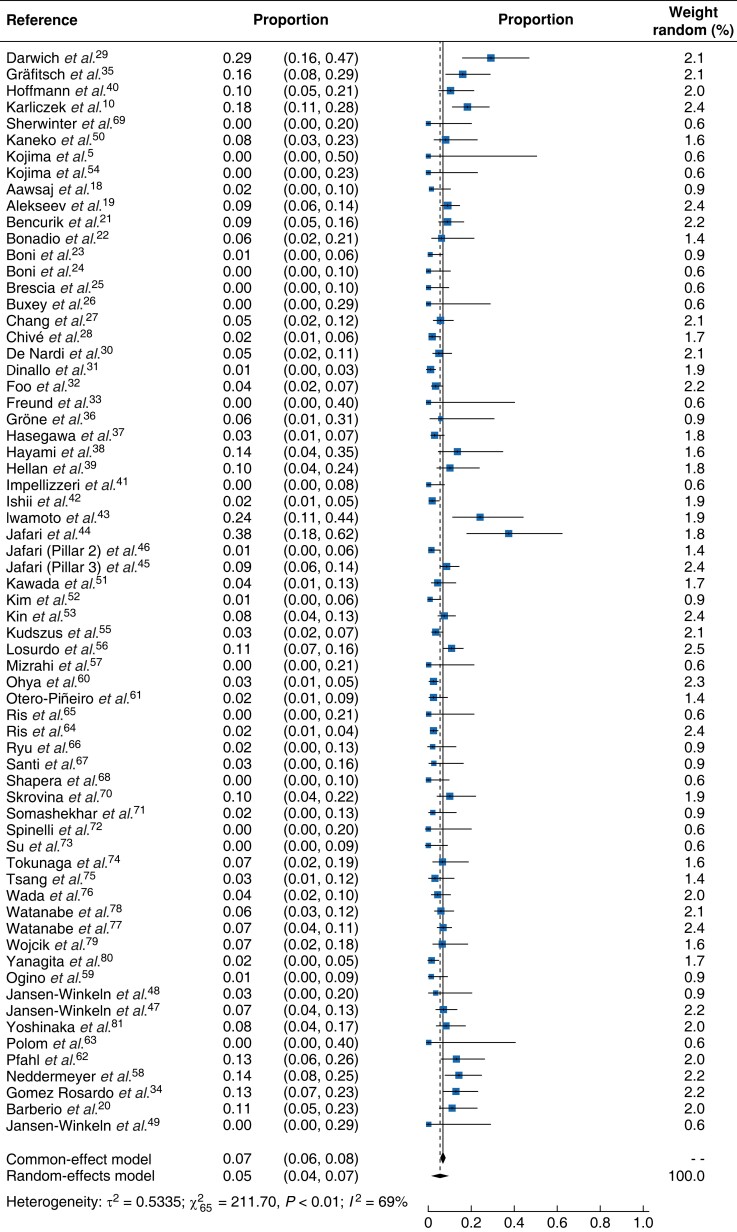
Detailed results of the meta-analysis are presented in a form of forest plot (Fig. 2). The pooled effects of anastomotic leak per case derived from the random-effects model was 0.05 (95 per cent c.i. 0.04 to 0.07). The effect derived from the common-effect model was very similar at 0.07 (0.06, 0.08). The estimated effects ranged from 0 to 0.3844. There was heterogeneity in the data (I2 = 69 per cent, τ2 = 0.5335, χ2 = 211.70, 65 d.f., P < 0.01). Figure 3 illustrates the effect each perfusion assessment modality on anastomotic leak rates. The effects of different modalities were estimated using a random-effects model with a modality factor included as categorical predictor. Different modalities in this equation were represented by dummy variables, with ICG-FA fixed as a reference category. The results of this analysis indicated that 19.8 per cent of the overall heterogeneity of studies related to the different perfusion assessment modalities. The test of residual heterogeneity indicated that, after accounting for different modalities, there remained significant heterogeneity in the effects caused by other factors (QE (62 d.f.) = 172.8238, P < 0.001). All effects were significantly different from zero, indicating that the modalities tested reduced the risk of an adverse outcome (Table 3) which in this instance was anastomotic leak. The detailed subgroup effects, along with their 95 per cent confidence intervals and heterogeneity estimate, are presented in Table 3. The omnibus test for the equality of effects for different modalities indicated that they also differed significantly between each other (QM (4 d.f.) = 646.1490, P < 0.001). Further tests revealed that significant differences in results existed between ICG-FA and DRS, as well as between pairs HSI and DRS, and LSCI and DRS. The estimated effect for the pooled cohort of all control cases, where no perfusion assessment was used, was 0.10 (0.08, 0.12).
Fig. 2.
Forest plot with all studies included (cases only) demonstrating risk of anastomotic leak within 30 days
Proportions are shown with 95 per cent confidence intervals.
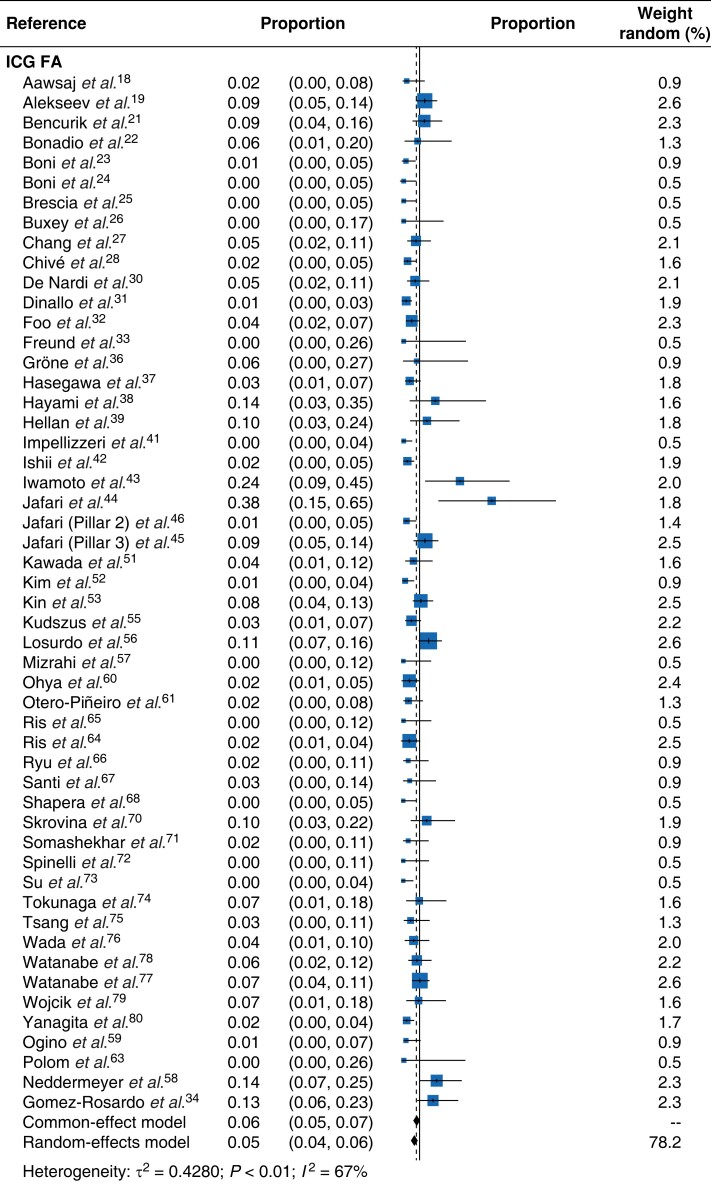
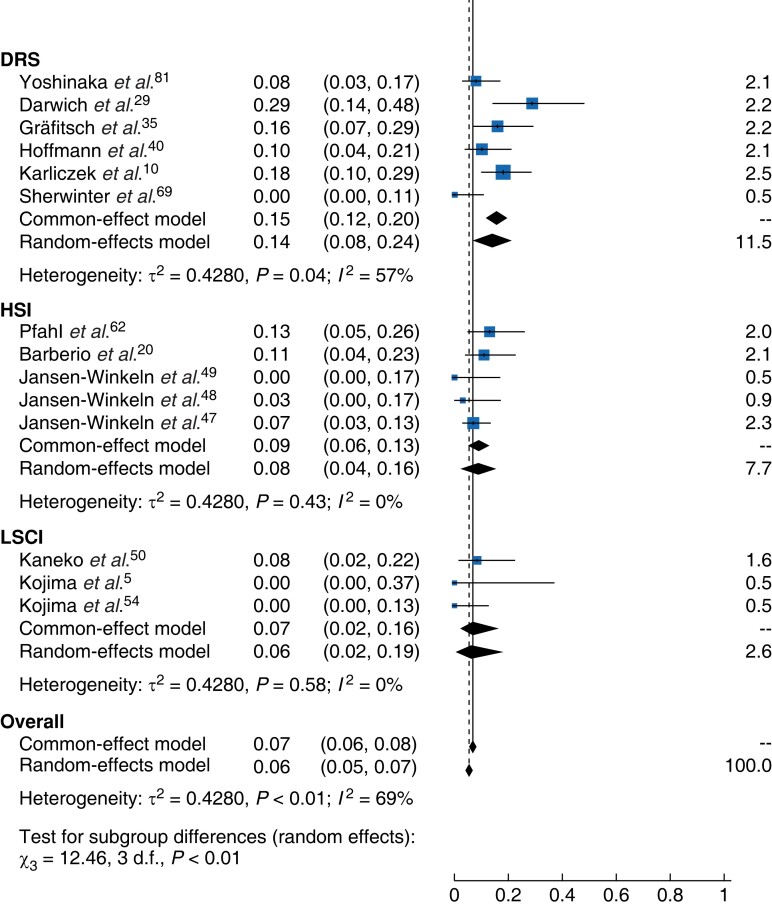
Fig. 3.
Forest plot for each perfusion assessment method and risk of anastomotic leak
Proportions are shown with 95 per cent confidence intervals. ICG-FA, indocyanine green fluorescence angiography; DRS, diffuse reflectance spectroscopy; HSI, hyperspectral imaging; LSCI, laser speckle contrast imaging.
Table 3.
Results of meta-analysis investigating risk of anastomotic leak by perfusion assessment modality and overall
| Modality | AL estimates taken from random-effects model* | Heterogeneity | Weight (%) | |||
|---|---|---|---|---|---|---|
| I 2 (%) | τ2 | χ2 | P | |||
| All modalities | 0.06 (0.05, 0.07) | 69 | 0.4280 | = 211.70 | <0.01 | 100 |
| ICG-FA | 0.05 (0.04, 0.06) | 67 | 0.4280 | = 156.37 | <0.01 | 78.2 |
| DRS | 0.14 (0.08, 0.24) | 57 | 0.4280 | = 11.55 | 0.04 | 11.5 |
| HSI | 0.08 (0.04, 0.16) | 0 | 0.4280 | = 3.8 | 0.43 | 7.7 |
| LSCI | 0.06 (0.02, 0.19) | 0 | 0.4280 | = 1.09 | 0.58 | 2.6 |
| Control | 0.10 (0.08, 0.12) | 80 | 0.3464 | = 162.52 | <0.01 | 100 |
Values in parentheses are 95% confidence intervals. Amount of total heterogeneity accounted for by modalities (R2) = 19.8%. AL, anastomotic leak; ICG-FA, indocyanine green fluorescence angiography; DRS, diffuse reflectance spectroscopy; HSI, hyperspectral imaging; LSCI, laser speckle contrast imaging.
Because the data revealed a substantial level of heterogeneity, further procedures were employed, to search for possible explanations for the observed differences in effect sizes between studies. A subgroup analysis was used for this purpose. The main focus of the subgroup analysis was performance modality. The effects of other potential moderators were not a subject of interest here as a number of other meta-analyses are available in the literature87–91. The summary effect sizes for each modality (ICG-FA, DRS, HSI, LSCI) were calculated and reported in a separate forest plot (Fig. 3). The final part of the statistical analysis was to investigate possible publication bias; the funnel plot was symmetrical (supplementary material)15.
Quality assessment
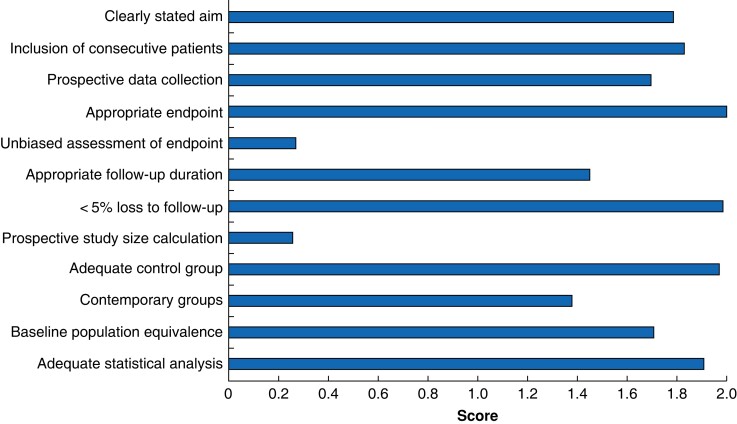
The average MINORS score for controlled trials was 18 (range 13–24) of 24. For non-controlled trials it was 11 (8–14) of 16. Most studies lost points for lack of blinding, or for not calculating study size or powers, and so were deemed to have a high risk of bias in these domains. The breakdown for individual categories is shown in Fig. 4. The greater the score for each domain, the less susceptible it is to bias. Full MINORS scores for each paper are documented in the supplementary material.
Fig. 4.
Average (mean) MINORS scores for all studies
The last four categories were only applicable to controlled trials. Maximum scores per category were 2 and minimum scores available were 0. MINORS, Methodological Index for Non-Randomized Studies.
Discussion
This meta-analysis found that assessment of bowel perfusion before the formation of a colorectal anastomosis reduced the incidence of anastomotic leak. ICG-FA, DRS, LSCI, and HSI all reduced the risk of anastomotic leak occurring for the included populations.
This comprehensive review compared four methods of bowel perfusion assessment. The major limitation of the study pertained to the limited number of studies and case populations in all groups other than ICG-FA, meaning that studies describing other imaging modalities were potentially underpowered. With ICG-FA accounting for 78.2 per cent of all cases, the total anastomotic leak rate is likely to be skewed more towards the mean for ICG-FA, rather than the true mean across all studies. Additionally, MINORS scores for the included studies were low for blinding and study sizes. This is likely to have influenced the results in favour of the interventions investigated. Owing to the heterogeneity of the results, it was decided to rely on results obtained from random-effects model methodology as it incorporates more realistic assumptions about heterogeneity of effects across studies. The data sets from the DRS and ICG-FA groups demonstrated the most heterogeneity. Factors increasing study heterogeneity included different study methodologies, definitions of anastomotic leak, surgical techniques, and device use. Studies investigating HSI and LSCI had lower heterogeneity as they were trials of similar origin.
The current literature is conflicting regarding the efficacy of ICG-FA. Similar to other work, based on the present review, the authors propose that ICG-FA has a role in reducing the risk of anastomotic leak when deployed during operation for lower gastrointestinal cancers61,86,88,92. However, some of the larger case–control studies evaluating ICG-FA drew contrasting conclusions; some42,45 suggested a trend towards reduced anastomotic leak rates with the use of ICG-FA, whereas others19,32,77 demonstrated that ICG-FA could significantly reduce leakage rates. Of note, there are also a number of ongoing studies assessing the benefits of ICG-FA in reducing anastomotic leak rates in colorectal cancers, including EssentiAL93, IntAct, and AVOID.
The total random-effects results demonstrated that DRS was not as effective as HSI, LSCI, and ICG-FA in reducing the risk of anastomotic leak. This is also supported by the differences between DRS and the other imaging modalities in omnibus testing. A potential reason for this is that DRS looks at a very small area of tissue oxygenation and so lacks the overall picture that ICG-FA, HSI or LSCI may provide; further research to test this hypothesis is recommended.
At present, there is no consensus regarding the definition of reduced perfusion across all modalities. For ICG-FA, a visual marker of perfusion is generated and studies31,53,56,60,68 have used various uptake time cut-offs from 25 to 60 s, and differing volumes of ICG, to define optimal perfusion. However, this relies on an effective systemic vascular supply. There is also variation in the literature concerning the range of healthy tissue saturation levels. Mean colonic Sto2 measurements from the DRS group indicated that a reduced risk of anastomotic leak was associated with a value of between 58 and 79.4 per cent, and that the lowest Sto2 measurement for a viable anastomosis was 51 per cent10,35,40. Additionally, in the DRS group, it was proposed that an Sto2 rise of 2 per cent after anastomosis formation had to occur to avoid leakage10,38. The wide range of proposed healthy tissue saturation may be explained by the specific equipment used, with certain systems having lower cut-offs.
The lack of objective measurement with ICG-FA is an active area of research. One of the included studies considered the development of quantitative fluorescence measurement within ICG-FA, with the aim of measuring the fluorescence of ICG objectively and relaying it back to the surgeon for a strengthened anastomotic line. They proposed an arbitrary unit cut-off but were limited by hypertension and location of the anastomosis, as well as a lack of real-time evaluation as data were processed after operation34. The development of a quantitative cut-off for adequate perfusion and its validation during surgery would likely enhance its use and uptake.
There also remain drawbacks in implementing the other imaging methods investigated. DRS uses a probe-based measurement of serosal oxygenation, where only a small amount of tissue is measured; LSCI requires a separate camera system to view data that can be used for surgical visualization; and, at present, HSI has a near-to but not real-time laparoscopic system8. In the fields of HSI and LSCI, studies are being set up to assess whether wide-field imaging for perfusion measurements and concurrent tissue differentiation can reliably be performed in real time94,95.
Finally, none of the included perfusion assessment methods currently have validated protocols documented in the reviewed literature and no standard perfusion assessment modality exists for colorectal resection. LSCI, HSI, and DRS are emerging technologies, as evidenced by the smaller number of included studies and limited numbers of patients. Future work across all modalities will require the development of standardized protocols for ease of adoption and use. The use of adjunctive bowel perfusion measurement technologies is unlikely to negate the importance of surgical skill and experience, but rather should promote safer surgery during bowel resections, and surgical centres wishing to adopt new technology should take these factors into consideration.
Supplementary Material
Acknowledgements
For the purpose of open access, the authors have applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission.
Contributor Information
Maxwell S Renna, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK; Department of General Surgery, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
Mariusz T Grzeda, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK.
James Bailey, Department of General Surgery, University of Nottingham, Nottingham, UK.
Alison Hainsworth, Department of General Surgery, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
Sebastien Ourselin, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK; Hypervision Surgical Ltd, London, UK.
Michael Ebner, Hypervision Surgical Ltd, London, UK.
Tom Vercauteren, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK; Hypervision Surgical Ltd, London, UK.
Alexis Schizas, Department of General Surgery, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
Jonathan Shapey, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK; Hypervision Surgical Ltd, London, UK; Department of Neurosurgery, King’s College Hospital, London, UK.
Funding
This work was supported by core funding from the Wellcome (WT203148/Z/16/Z), EPSRC Engineering and Physical Sciences Research Council (EPSRC, NS/A000049/1), and received funding from the National Institute for Health and Care Research (NIHR) (NIHR202114). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. T.V. is supported by a Medtronic and the Royal Academy of Engineering (RCSRF1819/7/34).
Author contributions
Maxwell Renna (Conceptualization, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing), Mariusz Grzeda (Methodology, Software, Validation, Writing—review & editing), James Bailey (Data curation, Formal analysis, Investigation, Resources, Validation, Writing—original draft, Writing—review & editing), Alison Hainsworth (Writing—review & editing), Sebastien Ourselin (Conceptualization, Writing—review & editing), Michael Ebner (Methodology, Resources, Visualization, Writing—review & editing), Tom Vercauteren (Methodology, Project administration, Resources, Validation, Visualization, Writing—review & editing), Alexis Schizas (Conceptualization, Writing—review & editing), and Jonathan Shapey (Conceptualization, Formal analysis, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing).
Disclosure
S.O. is co-founder and shareholder of Hypervision Surgical, and declares research funding from Medtronic and Siemens Healthineers. M.E., T.V., and J.S. are co-founders and shareholders of Hypervision Surgical.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The full data set is available to view in the supplementary material. Should further information or data be required, the corresponding author can be contacted.
References
- 1. Office for National Statistics . Cancer Registration Statistics, England.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland (accessed 7 November 2022)
- 2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–691 [DOI] [PubMed] [Google Scholar]
- 3. Healthcare Quality Improvement Partnership (HQIP) . National Bowel Cancer Audit Annual Report, England and Wales (2014–2019).https://www.hqip.org.uk/resource/national-bowel-cancer-audit-annual-report-2019/ (accessed 7 November 2022)
- 4. National Institute for Health and Care Excellence . Colorectal Cancer. NICE Guideline (NG151).https://www.nice.org.uk/guidance/ng151 (accessed 7 November 2022) [PubMed]
- 5. Kojima S, Sakamoto T, Nagai Y, Matsui Y, Nambu K, Masamune K. Laser speckle contrast imaging for intraoperative quantitative assessment of intestinal blood perfusion during colorectal surgery: a prospective pilot study. Surg Innov 2019;26:293–301 [DOI] [PubMed] [Google Scholar]
- 6. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 2009;208:269–278 [DOI] [PubMed] [Google Scholar]
- 7. Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal Aet al. . Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 2015;220:195–206 [DOI] [PubMed] [Google Scholar]
- 8. Shapey J, Xie Y, Nabavi E, Bradford R, Saeed SR, Ourselin Set al. . Intraoperative multispectral and hyperspectral label-free imaging: a systematic review of in vivo clinical studies. J Biophotonics 2019;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmer A, Marotz J, Wahl P, Dau M, Kämmerer PW. Hyperspectral imaging in perfusion and wound diagnostics—methods and algorithms for the determination of tissue parameters. Biomed Tech 2018;63:587–594 [DOI] [PubMed] [Google Scholar]
- 10. Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, Van Dam GM. Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis 2010;12:1018–1025 [DOI] [PubMed] [Google Scholar]
- 11. Hammond D, Lane F, Mackeigan J, Passinault W. Endoscopic tattooing of the colon: clinical experience. Am Surg 1993;59:205–210 [PubMed] [Google Scholar]
- 12. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg 2003;73:712–716 [DOI] [PubMed] [Google Scholar]
- 13. Borenstein M, Hedges L V, Higgins J, Rothstein HR. Introduction to Meta-Analysis. West Sussex: John Wiley & Sons, 2009. [Google Scholar]
- 14. Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. London: Springer, 2015. [Google Scholar]
- 15. Wang N. How to Conduct a Meta-Analysis of Proportions in R: a Comprehensive Tutorial.https://www.researchgate.net/publication/325486099_How_to_Conduct_a_Meta-Analysis_of_Proportions_in_R_A_Comprehensive_Tutorial?channel=doi&linkId=5b1107bc4585150a0a5e427f&showFulltext=true (accessed 7 November 2022)
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 17. Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 18. Aawsaj Y, Mustafa A, Winstanley J, O’loughlin P. The impact of indocyanine green fluorescence angiography on intraoperative decision making in right hemicolectomy. Surg Laparosc Endosc Percutan Tech 2021;32:209–212 [DOI] [PubMed] [Google Scholar]
- 19. Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Colorectal Dis 2020;22:1147–1153 [DOI] [PubMed] [Google Scholar]
- 20. Barberio M, Lapergola A, Benedicenti S, Mita M, Barbieri V, Rubichi Fet al. . Intraoperative bowel perfusion quantification with hyperspectral imaging: a guidance tool for precision colorectal surgery. Surg Endosc 2022;36:8520–8532 [DOI] [PubMed] [Google Scholar]
- 21. Bencurik V, Skrovina M, Bartos J, Klos K, Andel P, Holaskova Eet al. . Evaluation of intestinal perfusion by indocyanine green (ICG) fluorescence imaging during procedures on the rectum and colon sigmoideum. Surg Endosc Other Interv Tech 2017;31:S24 [Google Scholar]
- 22. Bonadio L, Iacuzzo C, Cosola D, Cipolat Mis T, Giudici F, Casagranda Bet al. . Indocyanine green-enhanced fluorangiography (ICGf) in laparoscopic extraperitoneal rectal cancer resection. Updates Surg 2020;72:477–482 [DOI] [PubMed] [Google Scholar]
- 23. Boni L, David G, Dionigi G, Rausei S, Cassinotti E, Fingerhut A. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc 2016;30:2736–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc 2017;31:1836–1840 [DOI] [PubMed] [Google Scholar]
- 25. Brescia A, Pezzatini M, Romeo G, Cinquepalmi M, Pindozzi F, Dall’Oglio Aet al. . Indocyanine green fluorescence angiography: a new ERAS item. Updates Surg 2018;70:427–432 [DOI] [PubMed] [Google Scholar]
- 26. Buxey K, Lam F, Muhlmann M, Wong S. Does indocyanine green improve the evaluation of perfusion during laparoscopic colorectal surgery with extracorporeal anastomosis? ANZ J Surg 2019;89:E487–E491 [DOI] [PubMed] [Google Scholar]
- 27. Chang YK, Foo CC, Yip J, Wei R, Ng KK, Lo Oet al. . The impact of indocyanine-green fluorescence angiogram on colorectal resection. Surgeon 2019;17:270–276 [DOI] [PubMed] [Google Scholar]
- 28. Chivé E, Sabbagh C, Guérin O, Pellegrin A, Dembinski J, Regimbeau JM. Is intraoperative fluorescence imaging with indocyanine green associated with a lower incidence of anastomotic leakage after colorectal surgery? A propensity score matching study. Surg Open Dig Adv 2021;2:100014 [Google Scholar]
- 29. Darwich I, Rustanto D, Friedberg R, Willeke F. Spectrophotometric assessment of bowel perfusion during low anterior resection: a prospective study. Updates Surg 2019;71:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti Eet al. . Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc 2020;34:53–60 [DOI] [PubMed] [Google Scholar]
- 31. Dinallo AM, Kolarsick P, Boyan WP, Protyniak B, James A, Dressner RMet al. . Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks? A retrospective cohort analysis. Am J Surg 2019;218:136–139 [DOI] [PubMed] [Google Scholar]
- 32. Foo CC, Ng KK, Tsang J, Wei R, Chow F, Chan TYet al. . Colonic perfusion assessment with indocyanine-green fluorescence imaging in anterior resections: a propensity score-matched analysis. Tech Coloproctol 2020;24:935–942 [DOI] [PubMed] [Google Scholar]
- 33. Freund MR, Kent I, Agarwal S, Wexner SD. Use of indocyanine green fluorescence guidance in redo ileocolic resection for Crohn’s disease. Colorectal Dis 2021;23:3190–3195 [DOI] [PubMed] [Google Scholar]
- 34. Gomez-Rosado JC, Valdes-Hernandez J, Cintas-Catena J, Cano-Matias A, Perez-Sanchez A, del Rio-Lafuente FJet al. . Feasibility of quantitative analysis of colonic perfusion using indocyanine green to prevent anastomotic leak in colorectal surgery. Surg Endosc 2022;36:1688–1695 [DOI] [PubMed] [Google Scholar]
- 35. Gräfitsch A, Kirchhoff P, Soysal SD, Däster S, Hoffmann H. Dynamic serosal perfusion assessment during colorectal resection using visible light spectroscopy. Eur Surg Res 2021;62:25–31 [DOI] [PubMed] [Google Scholar]
- 36. Gröne J, Koch D, Kreis ME. Impact of intraoperative microperfusion assessment with pinpoint perfusion imaging on surgical management of laparoscopic low rectal and anorectal anastomoses. Colorectal Dis 2015;17(Suppl ):22–28 [DOI] [PubMed] [Google Scholar]
- 37. Hasegawa H, Tsukada Y, Wakabayashi M, Nomura S, Sasaki T, Nishizawa Yet al. . Impact of intraoperative indocyanine green fluorescence angiography on anastomotic leakage after laparoscopic sphincter-sparing surgery for malignant rectal tumors. Int J Colorectal Dis 2020;35:471–480 [DOI] [PubMed] [Google Scholar]
- 38. Hayami S, Matsuda K, Iwamoto H, Ueno M, Kawai M, Hirono Set al. . Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech Coloproctol 2019;23:973–980 [DOI] [PubMed] [Google Scholar]
- 39. Hellan M, Spinoglio G, Pigazzi A, Lagares-Garcia JA. The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc 2014;28:1695–1702 [DOI] [PubMed] [Google Scholar]
- 40. Hoffmann H, Delko T, Kirchhoff P, Rosenthal R, Schäfer J, Kraljević Met al. . Colon perfusion patterns during colorectal resection using visible light spectroscopy. World J Surg 2017;41:2923–2932 [DOI] [PubMed] [Google Scholar]
- 41. Impellizzeri HG, Pulvirenti A, Inama M, Bacchion M, Marrano E, Creciun Met al. . Near-infrared fluorescence angiography for colorectal surgery is associated with a reduction of anastomotic leak rate. Updates Surg 2020;72:991–998 [DOI] [PubMed] [Google Scholar]
- 42. Ishii M, Hamabe A, Okita K, Nishidate T, Okuya K, Usui Aet al. . Efficacy of indocyanine green fluorescence angiography in preventing anastomotic leakage after laparoscopic colorectal cancer surgery. Int J Colorectal Dis 2020;35:269–275 [DOI] [PubMed] [Google Scholar]
- 43. Iwamoto H, Matsuda K, Hayami S, Tamura K, Mitani Y, Mizumoto Yet al. . Quantitative indocyanine green fluorescence imaging used to predict anastomotic leakage focused on rectal stump during laparoscopic anterior resection. J Laparoendosc Adv Surg Tech 2020;30:542–546 [DOI] [PubMed] [Google Scholar]
- 44. Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJet al. . The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 2013;27:3003–3008 [DOI] [PubMed] [Google Scholar]
- 45. Jafari MD, Pigazzi A, McLemore EC, Mutch MG, Haas E, Rasheid SHet al. . Perfusion assessment in left-sided/low anterior resection (PILLAR III): a randomized, controlled, parallel, multicenter study assessing perfusion outcomes with PINPOINT near-infrared fluorescence imaging in low anterior resection. Dis Colon Rectum 2021;64:995–1002 [DOI] [PubMed] [Google Scholar]
- 46. Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DAet al. . Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 2015;220:82–92.e1 [DOI] [PubMed] [Google Scholar]
- 47. Jansen-Winkeln B, Dvorak M, Köhler H, Maktabi M, Mehdorn M, Chalopin Cet al. . Border line definition using hyperspectral imaging in colorectal resections. Cancers (Basel) 2022;14:1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jansen-Winkeln B, Germann I, Köhler H, Mehdorn M, Maktabi M, Sucher Ret al. . Comparison of hyperspectral imaging and fluorescence angiography for the determination of the transection margin in colorectal resections—a comparative study. Int J Colorectal Dis 2021;36:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jansen-Winkeln B, Holfert N, Köhler H, Moulla Y, Takoh JP, Rabe SMet al. . Determination of the transection margin during colorectal resection with hyperspectral imaging (HSI). Int J Colorectal Dis 2019;34:731–739 [DOI] [PubMed] [Google Scholar]
- 50. Kaneko T, Funahashi K, Ushigome M, Kagami S, Yoshida K, Koda Tet al. . Noninvasive assessment of bowel blood perfusion using intraoperative laser speckle flowgraphy. Langenbecks Arch Surg 2020;405:817–826 [DOI] [PubMed] [Google Scholar]
- 51. Kawada K, Hasegawa S, Wada T, Takahashi R, Hisamori S, Hida Ket al. . Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc 2017;31:1061–1069 [DOI] [PubMed] [Google Scholar]
- 52. Kim JC, Lee JL, Yoon YS, Alotaibi AM, Kim J. Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot Comput Assist Surg 2016;12:710–717 [DOI] [PubMed] [Google Scholar]
- 53. Kin C, Vo H, Welton L, Welton M. Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum 2015;58:582–587 [DOI] [PubMed] [Google Scholar]
- 54. Kojima S, Sakamoto T, Matsui Y, Nambu K, Masamune K. Clinical efficacy of bowel perfusion assessment during laparoscopic colorectal resection using laser speckle contrast imaging: a matched case–control study. Asian J Endosc Surg 2020;13:329–335 [DOI] [PubMed] [Google Scholar]
- 55. Kudszus S, Roesel C, Schachtrupp A, Höer JJ. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 2010;395:1025–1030 [DOI] [PubMed] [Google Scholar]
- 56. Losurdo P, Mis TC, Cosola D, Bonadio L, Giudici F, Casagranda Bet al. . Anastomosis leak: is there still a place for indocyanine green fluorescence imaging in colon-rectal surgery? A retrospective, propensity score-matched cohort study. Surg Innov 2022;29:511–518 [DOI] [PubMed] [Google Scholar]
- 57. Mizrahi I, Abu-Gazala M, Rickles AS, Fernandez LM, Petrucci A, Wolf Jet al. . Indocyanine green fluorescence angiography during low anterior resection for low rectal cancer: results of a comparative cohort study. Tech Coloproctol 2018;22:535–540 [DOI] [PubMed] [Google Scholar]
- 58. Neddermeyer M, Kanngießer V, Maurer E, Bartsch DK. Indocyanine green near-infrared fluoroangiography is a useful tool in reducing the risk of anastomotic leakage following left colectomy. Front Surg 2022;9:850256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ogino T, Okuyama M, Hata T, Kawada J, Okano M, Kim Yet al. . Evaluation of blood flow on the remnant distal bowel during left-sided colectomy. World J Surg Oncol 2018;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ohya H, Watanabe J, Suwa H, Suwa Y, Ishibe A, Masui Het al. . Incidence and risk factors for fluorescence abnormalities on near-infrared imaging using indocyanine green in stapled functional end-to-end anastomosis in laparoscopic colectomy. Int J Colorectal Dis 2020;35:2011–2018 [DOI] [PubMed] [Google Scholar]
- 61. Otero-Piñeiro AM, de Lacy FB, Van Laarhoven JJ, Martín-Perez B, Valverde S, Bravo Ret al. . The impact of fluorescence angiography on anastomotic leak rate following transanal total mesorectal excision for rectal cancer: a comparative study. Surg Endosc 2021;35:754–762 [DOI] [PubMed] [Google Scholar]
- 62. Pfahl A, Radmacher GK, Köhler H, Maktabi M, Neumuth T, Melzer Aet al. . Combined indocyanine green and quantitative perfusion assessment with hyperspectral imaging during colorectal resections. Biomed Opt Express 2022;13:3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Polom W, Migaczewski M, Skokowski J, Swierblewski M, Cwalinski T, Kalinowski Let al. . Multispectral imaging using fluorescent properties of indocyanine green and methylene blue in colorectal surgery—initial experience. J Clin Med 2022;11:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ris F, Liot E, Buchs NC, Kraus R, Ismael G, Belfontali Vet al. . Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg 2018;105:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ris F, Hompes R, Cunningham C, Lindsey I, Guy R, Jones Oet al. . Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc 2014;28:2221–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ryu S, Suwa K, Kitagawa T, Aizawa M, Ushigome T, Okamoto Tet al. . Evaluation of anastomosis with ICG fluorescence method using VISERA ELITE2 during laparoscopic colorectal cancer surgery. Anticancer Res 2020;40:373–377 [DOI] [PubMed] [Google Scholar]
- 67. Santi CS, Violi VV, Casali LC, Franzini CF, Rollo AR. Applications of indocyanine green enhanced fluorescence in laparoscopic colorectal resections. Surg Endosc 2018;32(Suppl):S535. [DOI] [PubMed] [Google Scholar]
- 68. Shapera E, Hsiung RW. Assessment of anastomotic perfusion in left-sided robotic assisted colorectal resection by indocyanine green fluorescence angiography. Minim Invasive Surg 2019;2019:3267217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sherwinter D, Chandler P, Martz J. The use of tissue oxygen measurements compared to indocyanine green imaging for the assessment of intraoperative tissue viability of human bowel. Surg Endosc 2022;36:2192–2196 [DOI] [PubMed] [Google Scholar]
- 70. Skrovina M, Bencurik V, Martinek L, Machackova M, Bartos J, Andel Pet al. . The significance of intraoperative fluorescence angiography in miniinvasive low rectal resections. Wideochir Inne Tech Maloinwazyjne2020;15:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Somashekhar SP, Reddy RG, Rohit Kumar C, Ashwin KR. Prospective study comparing clinical vs indocyanine green fluorescence-based assessment of line of transection in robotic rectal cancer surgery—Indian study. Indian J Surg Oncol 2020;11:642–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Spinelli A, Carvello M, Kotze PG, Maroli A, Montroni I, Montorsi Met al. . Ileal pouch–anal anastomosis with fluorescence angiography: a case-matched study. Colorectal Dis 2019;21:827–832 [DOI] [PubMed] [Google Scholar]
- 73. Su H, Wu H, Bao M, Luo S, Wang X, Zhao Cet al. . Indocyanine green fluorescence imaging to assess bowel perfusion during totally laparoscopic surgery for colon cancer. BMC Surg 2020;20:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tokunaga T, Shimada M, Higashijima J, Yoshikawa K, Nishi M, Kashihara Het al. . Intraoperative thermal imaging for evaluating blood perfusion during laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech 2020;31:281–284 [DOI] [PubMed] [Google Scholar]
- 75. Tsang Y, Leung LHA, Lau C, Tang C. Indocyanine green fluorescence angiography to evaluate anastomotic perfusion in colorectal surgery. Int J Colorectal Dis 2020;35:1133–1139 [DOI] [PubMed] [Google Scholar]
- 76. Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa Set al. . ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 2017;31:4184–4193 [DOI] [PubMed] [Google Scholar]
- 77. Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota M, Kunisaki Cet al. . Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: a propensity score-matched cohort study. Surg Endosc 2020;34:202–208 [DOI] [PubMed] [Google Scholar]
- 78. Watanabe J, Ota M, Suwa Y, Suzuki S, Suwa H, Momiyama Met al. . Evaluation of the intestinal blood flow near the rectosigmoid junction using the indocyanine green fluorescence method in a colorectal cancer surgery. Int J Colorectal Dis 2015;30:329–335 [DOI] [PubMed] [Google Scholar]
- 79. Wojcik M, Doussot A, Manfredelli S, Duclos C, Paquette B, Turco Cet al. . Intra-operative fluorescence angiography is reproducible and reduces the rate of anastomotic leak after colorectal resection for cancer: a prospective case-matched study. Colorectal Dis 2020;22:1263–1270 [DOI] [PubMed] [Google Scholar]
- 80. Yanagita T, Hara M, Osaga S, Nakai N, Maeda Y, Shiga Ket al. . Efficacy of intraoperative ICG fluorescence imaging evaluation for preventing anastomotic leakage after left-sided colon or rectal cancer surgery: a propensity score-matched analysis. Surg Endosc 2021;35:2373–2385 [DOI] [PubMed] [Google Scholar]
- 81. Yoshinaka H, Takakura Y, Egi H, Shimizu W, Sumi Y, Mukai Set al. . Prediction of anastomotic leakage after left-sided colorectal cancer surgery: a pilot study utilizing quantitative near-infrared spectroscopy. Surg Today 2022;52:971–977 [DOI] [PubMed] [Google Scholar]
- 82. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Clifford RE, Fowler H, Manu N, Sutton P, Vimalachandran D. Intra-operative assessment of left-sided colorectal anastomotic integrity: a systematic review of available techniques. Colorectal Dis 2021;23:582–591 [DOI] [PubMed] [Google Scholar]
- 84. Degett TH, Andersen HS, Gögenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 2016;401:767–775 [DOI] [PubMed] [Google Scholar]
- 85. James DRC, Ris F, Yeung TM, Kraus R, Buchs NC, Mortensen NJet al. . Fluorescence angiography in laparoscopic low rectal and anorectal anastomoses with pinpoint perfusion imaging—a critical appraisal with specific focus on leak risk reduction. Colorectal Dis 2015;17:16–21 [DOI] [PubMed] [Google Scholar]
- 86. Kryzauskas M, Bausys A, Jakubauskas M, Valciukiene J, Makunaite G, Jasiunas Eet al. . Intraoperative testing of colorectal anastomosis and the incidence of anastomotic leak. Medicine (Baltimore) 2020;99:e23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lin J, Zheng B, Lin S, Chen Z, Chen S. The efficacy of intraoperative ICG fluorescence angiography on anastomotic leak after resection for colorectal cancer: a meta-analysis. Int J Colorectal Dis 2021;36:27–39 [DOI] [PubMed] [Google Scholar]
- 88. Pang HY, Chen XL, Song XH, Galiullin D, Zhao LY, Liu Ket al. . Indocyanine green fluorescence angiography prevents anastomotic leakage in rectal cancer surgery: a systematic review and meta-analysis. Langenbecks Arch Surg 2021;406:261–271 [DOI] [PubMed] [Google Scholar]
- 89. Shen R, Zhang Y, Wang T. Indocyanine green fluorescence angiography and the incidence of anastomotic leak after colorectal resection for colorectal cancer: a meta-analysis. Dis Colon Rectum 2018;61:1228–1234 [DOI] [PubMed] [Google Scholar]
- 90. Song M, Liu J, Xia D, Yao H, Tian G, Chen Xet al. . Assessment of intraoperative use of indocyanine green fluorescence imaging on the incidence of anastomotic leakage after rectal cancer surgery: a PRISMA-compliant systematic review and meta-analysis. Tech Coloproctol 2021;25:49–58 [DOI] [PubMed] [Google Scholar]
- 91. Blanco-Colino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 2018;22:15–23 [DOI] [PubMed] [Google Scholar]
- 92. Cassinotti E, Boni L, Della Porta M, Baldari L. The role of indocyanine green performing a minimally invasive right colectomy. Ann Laparosc Endosc Surg 2021;6:30 [Google Scholar]
- 93. Ichiro Takemasa J, Watanabe M, Kotake S, Noura M, Ikeda H, Suwa Met al. . Randomized phase III trial evaluating the efficacy of ICG fluorescence imaging on anastomotic leakage in laparoscopic surgery for rectal cancer (EssentiAL study). In: 30th International Congress of the European Association for Endoscopic Surgery (EAES), 2022. Abstract O123. Kraków, Poland, 5-8 July 2022
- 94. Lu G, Fei B. Medical hyperspectral imaging: a review. J Biomed Opt 2014;19:010901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu Y, Shah S, Sanders C, Nwaiwu C, Dechert A, Mehrotra Set al. . Utility and usability of laser speckle contrast imaging (LSCI) for displaying real-time tissue perfusion/blood flow in robot-assisted surgery (RAS): comparison to indocyanine green (ICG) and use in laparoscopic surgery. Surg Endosc 2022:1–9 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full data set is available to view in the supplementary material. Should further information or data be required, the corresponding author can be contacted.