Abstract
The antibody responses to human and bovine retinal S antigen in the sera of patients with uveitis from various causes were compared with those of a group of healthy volunteers who were fully screened for signs of eye disease. Antiretinal antibodies were found with equal frequency and through the same range of titres in patients and controls, irrespective of the nature or activity of the uveitis. These findings were confirmed by spectrotypic analysis of sera from patients and controls where the predominant serum antibody response was polyclonal. In a small group of patients with retinal vasculitis there was an additional monoclonal response, indicating clonal expansion of a single lymphocyte subset. The prevalence of serum antibodies to retinal antigens in the normal population may indicate a protective role for 'natural' autoantibodies, as has recently been suggested for autoimmune diseases generally.
Full text
PDF

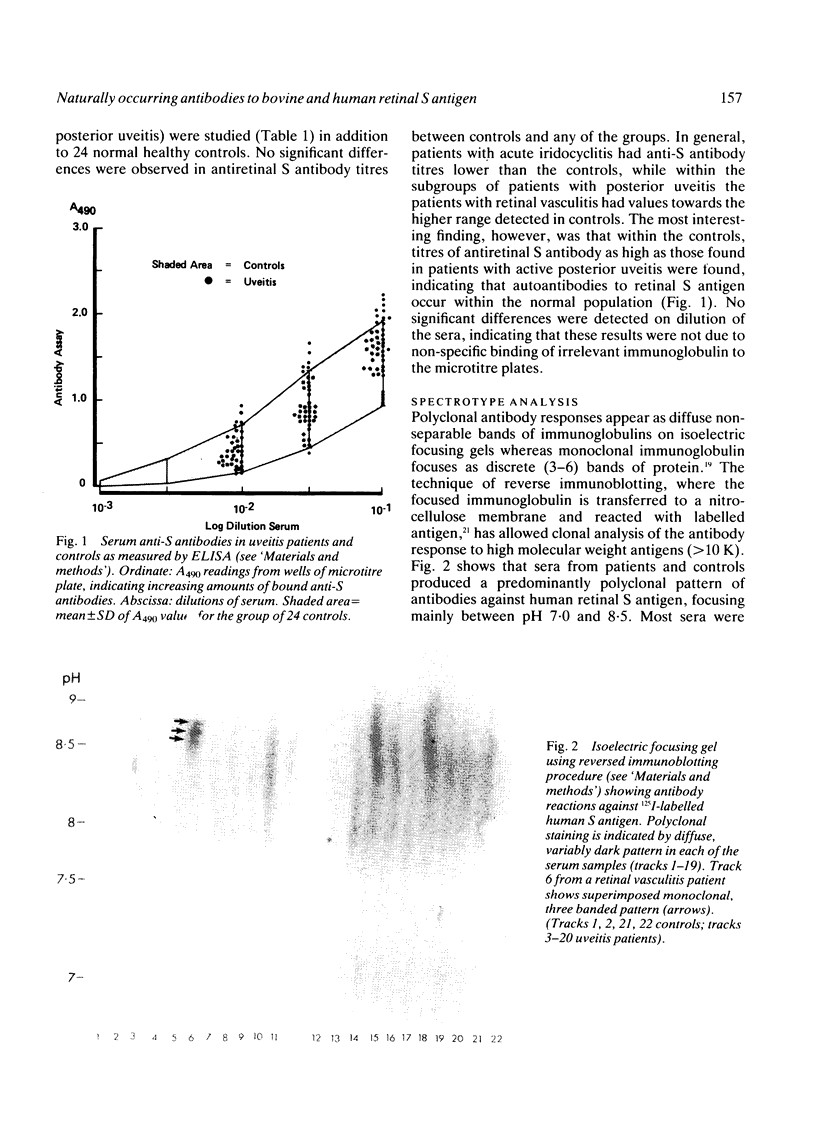
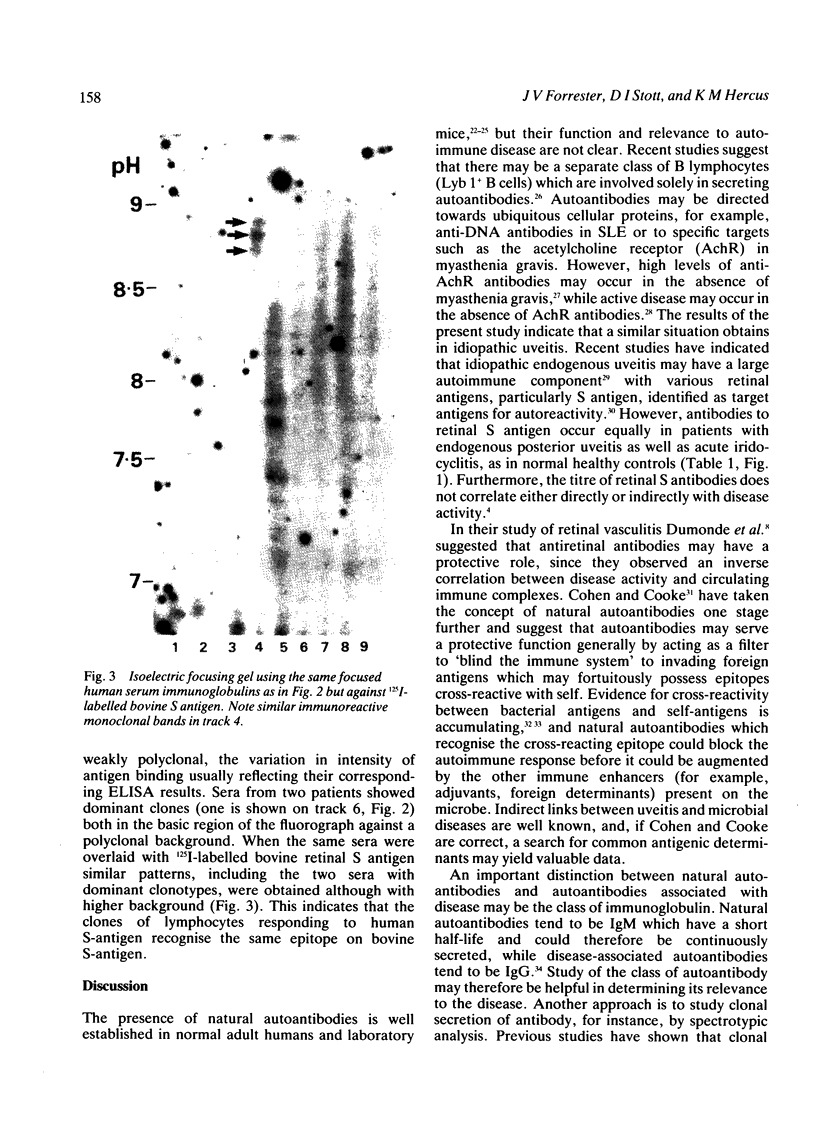

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams I. W., Gregerson D. S. Longitudinal study of serum antibody responses to retinal antigens in acute ocular toxoplasmosis. Am J Ophthalmol. 1982 Feb;93(2):224–231. doi: 10.1016/0002-9394(82)90418-4. [DOI] [PubMed] [Google Scholar]
- Al-Mahdawi S., Forrester J. V., Lee W. R. A simplified method for the isolation of highly purified bovine retinal S antigen. J Neuroimmunol. 1987 Feb;14(1):99–108. doi: 10.1016/0165-5728(87)90104-4. [DOI] [PubMed] [Google Scholar]
- Bionda A., De Baets M. H., Tzartos S. J., Lindstrom J. M., Weigle W. O., Theophilopoulos A. N. Spectrotypic analysis of antibodies to acetylcholine receptors in experimental autoimmune myasthenia gravis. Clin Exp Immunol. 1984 Jul;57(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clough J. D., Valenzuela R. Relationship of renal histopathology in SLE nephritis to immunoglobulin class of anti-DNA. Am J Med. 1980 Jan;68(1):80–85. doi: 10.1016/0002-9343(80)90169-2. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982 Jun;128(6):2788–2792. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Drachman D. B. Myasthenia gravis (first of two parts). N Engl J Med. 1978 Jan 19;298(3):136–142. doi: 10.1056/NEJM197801192980305. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Kasp-Grochowska E., Graham E., Sanders M. D., Faure J. P., de Kozak Y., van Tuyen V. Anti-retinal autoimmunity and circulating immune complexes in patients with retinal vasculitis. Lancet. 1982 Oct 9;2(8302):787–792. doi: 10.1016/s0140-6736(82)92679-4. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Borthwick G. M. Clinical relevance of S-antigen induced experimental uveoretinitis. Trans Ophthalmol Soc U K. 1983;103(Pt 5):497–502. [PubMed] [Google Scholar]
- Forrester J. V., Borthwick G. M., McMenamin P. G. Ultrastructural pathology of S-antigen uveoretinitis. Invest Ophthalmol Vis Sci. 1985 Sep;26(9):1281–1292. [PubMed] [Google Scholar]
- Forrester J. V. Chronic intraocular inflammation. Trans Ophthalmol Soc U K. 1985;104(Pt 3):250–255. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Abrahams I. W. Immunologic and biochemical properties of several retinal proteins bound by antibodies in sera from animals with experimental autoimmune uveitis and uveitis patients. J Immunol. 1983 Jul;131(1):259–264. [PubMed] [Google Scholar]
- Gregerson D. S., Abrahams I. W., Thirkill C. E. Serum antibody levels of uveitis patients to bovine retinal antigens. Invest Ophthalmol Vis Sci. 1981 Nov;21(5):669–680. [PubMed] [Google Scholar]
- Gregerson D. S., Fling S. P., Wohlhueter R. M. Characterization of immunologically active cyanogen bromide peptide fragments of bovine and human retinal S-antigen. Exp Eye Res. 1986 Nov;43(5):803–818. doi: 10.1016/s0014-4835(86)80011-2. [DOI] [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Holmberg D., Forsgren S., Ivars F., Coutinho A. Reactions among IgM antibodies derived from normal, neonatal mice. Eur J Immunol. 1984 May;14(5):435–441. doi: 10.1002/eji.1830140510. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Murray P. Serum autoantibodies and uveitis. Br J Ophthalmol. 1986 Apr;70(4):266–268. doi: 10.1136/bjo.70.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persselin J. E., Louie J. S., Stevens R. H. Clonally restricted anti-IgG antibodies in rheumatoid arthritis. Arthritis Rheum. 1984 Dec;27(12):1378–1386. doi: 10.1002/art.1780271208. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Steinberg A. D. Autoimmunity--a perspective. Annu Rev Immunol. 1983;1:175–210. doi: 10.1146/annurev.iy.01.040183.001135. [DOI] [PubMed] [Google Scholar]
- Stefansson K., Dieperink M. E., Richman D. P., Gomez C. M., Marton L. S. Sharing of antigenic determinants between the nicotinic acetylcholine receptor and proteins in Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. Possible role in the pathogenesis of myasthenia gravis. N Engl J Med. 1985 Jan 24;312(4):221–225. doi: 10.1056/NEJM198501243120407. [DOI] [PubMed] [Google Scholar]
- Stott D. I., McLearie J. Isoelectric focusing and reverse immunoblotting of autoantibodies against high molecular weight antigens. Immunol Invest. 1986 Apr;15(2):113–122. doi: 10.3109/08820138609094137. [DOI] [PubMed] [Google Scholar]
- Stott D. I., McLearie J., Neilson L. Analysis of the clonal origins of autoantibodies against thyroglobulin and DNA in autoimmune thyroiditis and systemic lupus erythematosus. Clin Exp Immunol. 1986 Sep;65(3):520–533. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker W. B., Donoso L. A., Kalsow C. M., Yankeelov J. A., Jr, Organisciak D. T. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977 Dec;119(6):1949–1958. [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]