Abstract
Objective.
To assess changes in walking function and walking-related prefrontal cortical activity following two post-stroke rehabilitation interventions: an accurate adaptability (ACC) walking intervention and a steady state (SS) walking intervention.
Design.
Randomized, single blind, parallel group clinical trial.
Setting.
Hospital research setting.
Subjects.
Adults with chronic post-stroke hemiparesis and walking deficits.
Interventions.
ACC emphasized stepping accuracy and walking adaptability, while SS emphasized steady state, symmetrical stepping. Both included 36 sessions led by a licensed physical therapist. ACC walking tasks recruit cortical regions that increase corticospinal tract activation, while SS walking activates the corticospinal tract less intensely.
Main Measures.
The primary functional outcome measure was preferred steady state walking speed. Prefrontal brain activity during walking was measured with functional near infrared spectroscopy to assess executive control demands. Assessments were conducted at baseline, post-intervention (3 months), and follow-up (6 months).
Results.
Thirty eight participants were randomized to the study interventions (mean age 59.6±9.1 years; mean months post-stroke 18.0±10.5). Preferred walking speed increased from baseline to post-intervention by 0.13±0.11 m/s in the ACC group and by 0.14±0.13 m/s in the SS group. The Time × Group interaction was not statistically significant (p=0.86). Prefrontal fNIRS during walking decreased from baseline to post-intervention, with a marginally larger effect in the ACC group (p=0.05).
Conclusions
The ACC and SS interventions produced similar changes in walking function. fNIRS suggested a potential benefit of ACC training for reducing demand on prefrontal (executive) resources during walking.
Keywords: Walking, Rehabilitation, Stroke, Brain, cognition
Introduction
Rehabilitation strategies for people post-stroke should target specific neural impairments important to control of walking. One such impairment is damage to the corticospinal tract, which has an important role for both steady state walking and for tasks requiring accuracy or adaptability of walking1–3. The latter refers to gait modifications needed to meet behavioral task goals (e.g., obstacle negotiation) and demands of the environment (e.g., safe placement of the foot on uneven terrain).4 Walking adaptability tasks generally require substantial demands on attention, visual information, somatosensory feedback, and precision. These demands elicit recruitment of cortical association areas (prefrontal, premotor, sensory) that in turn increase neural drive to the corticospinal tract.2,3,5–9 In contrast, steady state walking is thought to activate the corticospinal tract less intensely.1
The objective of this study is to test the hypothesis that an intervention focused on accurate walking adaptability tasks (obstacle crossing/avoidance, accurate foot placement, etc.) will be superior to an intervention that focuses on steady state walking for recovery of walking function after stroke. By targeting a central mechanism of impairment (corticospinal control of walking), better recovery is expected to generalize across various measures of walking function, including preferred steady state walking speed.
A secondary focus is to investigate how each intervention affects the demand on executive control of walking. Executive control refers to conscious attention and planning dedicated to the movement.10 Following stroke, heightened executive demand is considered to be a compensatory strategy to help overcome sensorimotor control deficits including the aforementioned corticospinal tract impairment.8,10 We hypothesize that accurate adaptability training will be more effective at reducing the executive demand of walking, as measured by functional near infrared spectroscopy (fNIRS) of prefrontal cortex.
Methods
This is a randomized, single blind, parallel group clinical trial that is registered with ClinicalTrials.gov (NCT02132650; www.clinicaltrials.gov). The study procedures were approved by the University of Florida Institutional Review Board and VA Human Research Protections Program (approval number IRB201500910). Funding was provided by the US Department of Veterans Affairs Rehabilitation Research and Development Service. The study began in June 2014 and concluded in May 2019.
Research participants were recruited using local clinical and research databases of people post-stroke, posting of flyers at rehabilitation clinics, newspaper advertisements, and community health fairs. All participants provided written informed consent at the time of enrollment. Study procedures were conducted in a hospital research setting.
Inclusion criteria included: age > 21 years, time since stroke between six and 48 months, medical stability, ability to follow three-step commands, hemiparesis with asymmetrical gait pattern, ability to walk without support from another person, 10-meter walking speed < 0.9 m/s, and Fugl-Meyer Assessment lower extremity (FMA-LE) score < 30.11,12
Exclusion criteria, consisting of major health conditions that would interfere with safety or compliance, included: uncontrolled hypertension, pain, severe obesity (BMI > 40), major cardiovascular, pulmonary or renal conditions, significant visual and/or vestibular impairment, lower motor neuron injury, bone fracture or joint replacement in the prior six months, or diagnosis of a terminal illness.
A parallel groups design with 1:1 allocation ratio was used. Group assignment was conducted after baseline assessment. The Principal Investigator was responsible for contacting the study statistician when a new participant was ready to be randomized. The statistician used a computer algorithm to determine group assignment, which include stratifying participants by sex and by stroke severity, then randomly assigning to either the accurate adaptability walking intervention or steady state walking intervention. Stroke severity was evaluated by calculating the synergy sub-score of the FMA-LE (parts two, three, and four)13, with scores ≤14 rated as more severe and >14 as less severe (highest possible score is 22). The statistician reported the group assignment directly to the Principal Investigator, who then notified the therapy team.
Demographic data collected included age, sex, race, time since stroke, paretic side, and body mass index (BMI). Additional assessments conducted to characterize the participants included the Mini-Mental State Exam (MMSE) for global cognition14, Charlson Index for comorbidities 15, and Patient Health Questionnaire for depressive symptoms (PHQ-9).16
Participants who were enrolled underwent study assessments at baseline (within two weeks prior to starting the intervention), post-intervention (within one week after completing the 3-month intervention), and follow-up (approximately three months after completing the intervention; 6 months total from baseline). All clinical assessments were conducted by a licensed physical therapist. For a given participant, the same physical therapist assessed outcomes at each time point. The assessment therapists were blinded to group assignment. Assessments that were conducted at each time point to gauge intervention efficacy included 10-meter preferred walking speed (primary outcome), 10-meter obstacle walking speed (foam obstacles with three inch height and depth placed at two meters, five meters, and eight meters), Dynamic Gait Index (DGI)17, Activities-Specific Balance Confidence Scale (ABC Scale)18, Fugl-Meyer Lower Extremity Motor Assessment (FMA-LE)11, and satisfaction with mobility function and recovery.19 Some participants used an ankle/foot orthosis and/or a cane if needed to safely complete the walking assessments (see Table 1). For consistency across time points, these participants used the same assistive device for assessments at post-intervention and follow up.
Table 1.
Demographic and Health Data
| Steady State Walking | Accurate Walking | |||
|---|---|---|---|---|
| Intervention (SS, n=18) | Intervention (ACC, n=20) | |||
| Mean ± SD | Range | Mean ± SD | Range | |
| Age (years) | 58.6 ± 8.4 | 41 – 71 | 60.5 ± 9.9 | 43 – 80 |
| Time Since Stroke (months) | 20.9 ± 10.5 | 6 – 47 | 15.4 ± 10.0 | 5 – 36 |
| Body Mass Index (kg/m2) | 29.5 ± 5.3 | 19.5 – 40.1 | 27.6 ± 3.2 | 22.9 – 33.1 |
| Mini Mental State Exam (points out of 30) | 25.7 ± 3.8 | 17 – 30 | 27.7 ± 2.0 | 23 – 30 |
| Patient Health Questionnaire (points out of 27) | 3.7 ± 3.9 | 0 – 14 | 3.1 ± 2.6 | 0 – 8 |
| Charlson Index (points) | 3.4 ± 1.5 | 2 – 7 | 3.2 ± 1.2 | 2 – 6 |
| Count (percent) | Count (percent) | |||
| Sex (female) | 7 (38.9%) | 8 (40.0%) | ||
| Paretic Side (left) | 8 (44.4%) | 12 (60.0%) | ||
| Assistive Device (Any) | 9 (50.0%) | 7 (35.0%) | ||
| Ankle Foot Orthosis (AFO) | 5 (27.8%) | 3 (15.0%) | ||
| Cane | 1 (5.6%) | 3 (15.0%) | ||
| AFO and Cane | 3 (16.7%) | 1 (5.0%) | ||
Groups did not differ significantly for any measure (p>0.05).
At baseline and post-intervention time points only, functional near infrared spectroscopy (fNIRS) was used to assess prefrontal cortical activity during walking adaptability tasks. The walking task order was randomized and included typical walking at preferred speed (Typical task), obstacle walking (Obstacles task), and dual-task walking while performing serial-7 subtraction (Dual-Task). Participants also performed serial-7 subtraction while seated (Serial7 task) as a control task to evaluate single-task cognitive performance. For each walking task, participants walked at their preferred speed for up to three consecutive laps on an 18-meter oval-shaped walking path (slower walkers took fewer laps). For both Serial7 and Dual-Task, participants were asked to continuously subtract by seven beginning from a randomly assigned number between 91 and 99. If the participant reached zero prior to the end of the task (one minute for Serial7; walking task duration for Dual-Task) a new number was immediately assigned. Participants were not given any specific instructions pertaining to prioritization of task performance during Dual-Task. After each walking task participants sat down to rest for at least three minutes. A small number of participants had expressive aphasia with consequent difficulty verbalizing their responses. These individuals were instructed to perform the serial-7 subtraction task silently to minimize confounding effects.
During Typical, Obstacles, Dual-Task, and Serial-7, activity in anterior prefrontal cortex during each task was quantified with a commercially available fNIRS monitor (Niro 200NX, Hamamatsu Photonics, Japan) based on changes in oxygenated hemoglobin concentration (O2Hb). We have previously published the fNIRS acquisition procedures for this study.8,20 Briefly, optodes were placed on each side of the forehead over anterior prefrontal cortex (Brodmann Area 10), which is involved with executive functions. Infrared light at continuous wavelengths of 735 nm and 810 nm were used to estimate changes in O2Hb using the modified Beer-Lambert Law. Inter-optode distance was three centimeters, and movement artifact from sensors or wires were minimized by using adhesive tape and elastic straps. Just prior to beginning each walking task, participants stood quietly for approximately one minute to provide a reference level of prefrontal activity.
The steady state walking and accurate adaptability walking interventions were designed to be similar in frequency, duration, and intensity. The steady state walking intervention emphasized steady state, repetitive, symmetrical stepping. In contrast, the accurate adaptability walking intervention emphasized stepping accuracy (foot trajectory/placement) and walking adaptability (modifying the walking pattern).4,21 Additional details of each intervention are provided below. Both interventions were delivered three days/week for 12 weeks (i.e., 36 sessions), with each session consisting of 30 minutes of walking practice. The first 15 minutes of each session consisted of treadmill walking and the second 15 minutes of each session consisted of overground walking. Rest was provided as needed but was not counted toward the 30-minute training time. A licensed physical therapist with experience in neurologic physical therapy led all sessions, and the same therapists conducted both the accurate adaptability walking intervention and steady state walking intervention. The treadmill practice involved partial body weight support, but only to the extent necessary for the participant to meet the walking duration goal and/or to avoid use of poor quality movement strategies such as circumduction of the paretic leg or markedly asymmetric gait.
For the steady state walking intervention, a rehabilitation team of two to three people (always led by a licensed physical therapist) provided hands-on cueing and/or manual assistance at the pelvis, knee, and/or ankle, particularly during treadmill walking. The rationale was to facilitate proper timing and quality of the gait pattern. The therapists emphasized independent stepping with maximal weight bearing on the paretic leg and encouragement of appropriate movement strategies. Verbal feedback of gait quality was also provided if necessary to reduce the use of compensatory gait modifications (e.g., “avoiding swinging your leg to the side”).
The accurate adaptability walking intervention emphasized stepping accuracy and walking adaptability tasks. The accurate walking tasks were stepping on targets, stepping over obstacles, stepping through a gait ladder (overground only) and navigating around obstacles (overground only). Each task was practiced for a similar amount of time during each session. Therapist involvement was similar to what is described above for the steady state intervention. However, compared to the steady state intervention, there was less emphasis on symmetrical stepping and less hands-on cueing.
Here we briefly describe each task used in the accurate adaptability intervention, along with methods for progressing the accuracy and/or adaptability demands:
For targeted stepping during treadmill walking, one or more laser pointers were used to project static targets onto the treadmill surface. For targeted stepping during overground walking, small squares (about 1.5 inches) of rubber sheet material were placed on the floor to form a path of targets. Progression of task complexity involved use of unilateral versus bilateral targets, alternating between multiple targets based on verbal instructions from the therapist, and stepping onto a target with either forefoot, midfoot, or heel, based on verbal instructions.
For stepping over obstacles on the treadmill and overground, foam blocks were placed on the walking path. The participant was instructed to step over each block while minimizing foot clearance. Progression of task complexity involved varying the size, spacing, and bilateral placement of the obstacles.
The ladder training used a commercially available unit with adjustable rungs. The ladder laid flat on the floor and participants would place their feet between the rungs. Progression of task complexity started with unilateral evenly spaced rungs and advanced to bilateral irregularly spaced rungs.
For navigating around obstacles, participants walking around a series of mini traffic cones and/or foam blocks. Progression of task complexity started with obstacles that were evenly spaced in the forward and side-to-side directions, and became gradually more irregular in spacing. This required more precise foot placement to negotiate tighter turns.
During the first three weeks of the intervention, the accurate adaptability training tasks were presented in a “blocked practice” format such that each task was practiced for a few minutes before moving on to the next task.22 Weeks four through six used a “random practice” format in which participants would switch between and repeat the tasks frequently to add variety and challenge.22 The final six weeks of training used a “combined practice” format in which multiple tasks were performed simultaneously such as stepping onto targets placed within the gait ladder.
Progression of the intensity of training was accomplished by minimizing body weight support on the treadmill, minimizing therapist assistance, encouraging faster walker speed, and/or encouraging larger steps (including using larger obstacles for the accurate adaptability walking intervention). Throughout both interventions we monitored training intensity and progression. Participants’ rating of perceived exertion (RPE) was monitored multiple times during the treadmill and overground walking periods for each visit, with the goal of maintaining RPE at approximately 5 (out of a maximum of 10) on the Borg Category Ratio 10 Scale. This corresponds to “hard” exertion. Walking speed was adjusted as needed to keep participants near the target rating of perceived exertion. Cadence was also measured intermittently throughout the session for both treadmill and overground walking by manually counting the number of steps taken in 30 seconds (which was later multiplied by two to calculate cadence as steps per minute). Preferred 10-meter walking speed was measured at the beginning of every intervention session.
We have previously published the fNIRS data analysis procedures for this study 8,20. Briefly, mean bilateral prefrontal change in oxygenated hemoglobin concentration (ΔO2Hb) was calculated between a reference period of quiet standing and the active walking task. For the active period, ΔO2Hb was measured over a 30-second period that began seven seconds after task onset to allow for cerebral blood flow changes to stabilize.23
Intention to treat analysis was applied to all study data. For participants who withdrew during the intervention period we carried baseline data forward to the post-intervention and follow-up time points. For participants who withdrew after post-intervention assessment but before follow-up, we carried the post-intervention assessment forward to the follow-up time point.
Statistical analyses were conducted in JMP version 14.0.0. Demographic data were compared between groups using two-sample t-tests (continuous variables) or chi square tests (nominal variables). Behavioral performance measures were evaluated with repeated measures ANOVA with main effects of Group, Time point, and Group × Time point interaction. Separate models were used to compare Baseline versus Post-intervention time points and Baseline versus Follow-Up time points for each variable. Criteria for performing ANOVA were tested using Mauchly’s Test of Sphericity and Shapiro-Wilk’s test. The False Discovery Rate procedure was used to correct for multiple comparisons.24 fNIRS data were evaluated with repeated measures ANOVA accounting for Group, Task, Time point, and interaction effects. Post hoc analysis of main effects were conducted as warranted using separate repeated measures ANOVA models.
Results
A flow chart of participant randomization and retention is shown in Figure 1. Fifty adults with post-stroke hemiparesis attended the onsite screening visit, and thirty-eight were subsequently randomized to the accurate adaptability intervention group (n=20) or the steady state walking group (n=18). There was a total of five adverse events reported in the accurate adaptability intervention group and six adverse events reported in the steady state intervention group. None of these were considered to be related to the study. Adverse events or other factors that affected study participation are shown in Figure 1. For those who completed the study, all planned interventions and procedures were conducted in accordance with the original research plan. Enrollment stopped when the study funding expired. The study was conducted between June 2014 and May 2019.
Figure 1. Study Flow Diagram.
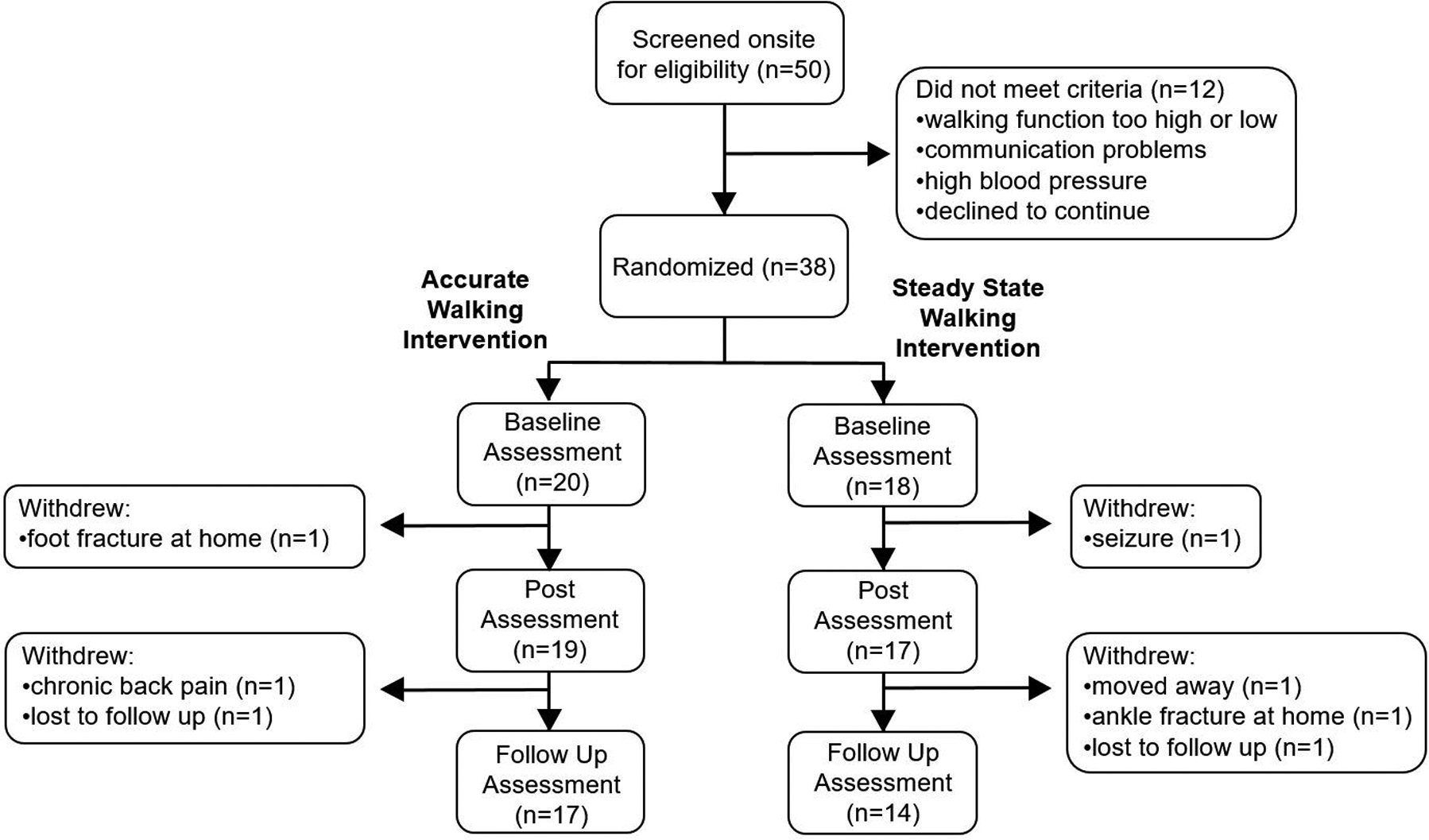
The flow of participants through the study is displayed, along with reasons for exclusion or withdrawal. Post-intervention assessment was conducted within one week after the final intervention session. Follow-up assessment was conducted approximately 12 weeks after the final intervention assessment.
Demographic data for each group are presented in Table 1. The accurate adaptability intervention group and steady state intervention group did not differ significantly (p>0.05) for age, sex, time since stroke, body mass index, sensorimotor impairment (Fugl Meyer Assessment for the lower extremity), global cognition (Mini Mental State Examination), depression (Patient Health Questionnaire-9), or comorbidities (Charlson Index). The racial distribution of participants who were randomized included 24 white, 12 black, one Asian, and one American Indian.
Rehabilitation intensity and progression data across sessions are presented in Figure 2. Walking outcome measures are presented in Table 2. For the primary outcome of preferred walking speed, Mauchly’s Test of Sphericity indicated that the assumption of sphericity had not been violated (χ2 = .43, p = .80). Furthermore, the distribution of model residuals was found to be normal based on Shapiro Wilk’s test (p>0.05). The Time × Group interaction was not statistically significant for any functional outcome measure (p>0.05).
Figure 2. Intervention Intensity and Progression Data.
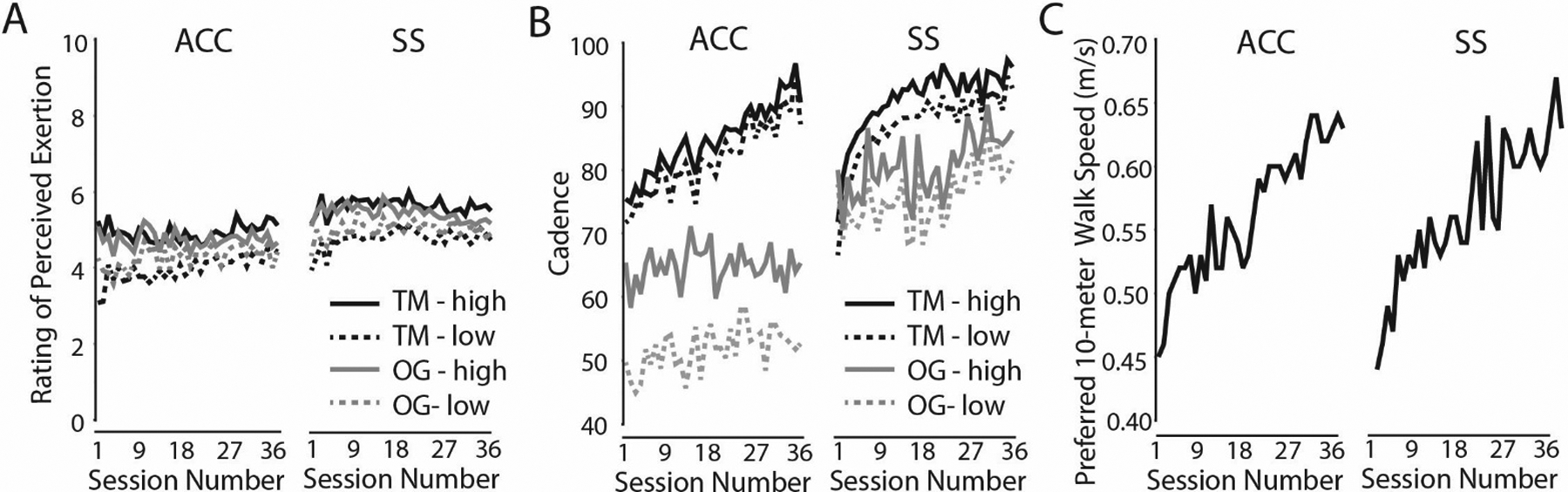
For each group, average walking cadence (Panel A) and rating of perceived exertion (Panel B) are shown at each session for training on the treadmill (TM; black lines) and training overground (OG; gray lines). Within each session there were multiple measurement timepoints for each variable. Only the highest values (solid lines: TM-high and OG-high) and lowest values (dashed lines: TM-low and OG-low) per session for each participant were used to calculate group means. Preferred 10-meter walking speed is shown in Panel C. This was recorded at each session prior to rehabilitation in order to provide information about the trajectory of walking function improvements.
Table 2.
Functional Outcome Measures
| Post vs. Baseline | Follow-Up vs. Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Baseline | Post | Follow Up | Change | Time | Time × Group | Change | Time | Time × Group | |
| Typical Walking Speed | ACC | 0.63 ± 0.27 | 0.76 ± 0.35 | 0.73 ± 0.30 | 0.13 ± 0.11 | F(1,36)=48.2, p<0.0001 | F(1,36)=0.03, p=0.86 | 0.10± 0.12 | F(1,36)=20.5, p<0.0001 | F(1,36)=0.74, p=0.39 |
| (meters/second) | SS | 0.58 ± 0.24 | 0.72 ± 0.30 | 0.65 ± 0.24 | 0.14 ± 0.13 | 0.07 ± 0.11 | ||||
| Obstacles Walking Speed | ACC | 0.53 ± 0.26 | 0.61 ± 0.30 | 0.63 ± 0.28 | 0.09 ± 0.11 | F(1,36)=24.5, p<0.0001 | F(1,36)=0.05, p=0.83 | 0.12 ± 0.12 | F(1,36)=21.3, p<0.0001 | F(1,36)=0.99, p=0.32 |
| (meters/second) | SS | 0.46 ± 0.25 | 0.55 ± 0.28 | 0.53 ± 0.23 | 0.12 ± 0.12 | 0.10 ± 0.12 | ||||
| Dual-Task Walking Speed | ACC | 0.49 ± 0.22 | 0.56 ± 0.26 | - | 0.08 ± 0.12 | F(1,35)=18.5, p<0.0001 | F(1,34)=0.06, p=0.81 | - | - | - |
| (meters/second) | SS | 0.45 ± 0.20 | 0.52 ± 0.22 | - | 0.07 ± 0.08 | - | ||||
| Dynamic Gait Index | ACC | 13.7 ± 4.9 | 16.2 ± 3.6 | 15.9 ± 3.6 | 2.5 ± 3.5 | F(1,36)=23.0, p<0.0001 | F(1,36)=0.44, p=0.51 | 2.2 ± 3.6 | F(1,36)=22.3, p<0.0001 | F(1,36)=0.001, p=0.97 |
| (points out of 24) | SS | 12.7 ± 2.9 | 14.6 ± 3.3 | 14.9 ± 2.9 | 1.9 ± 1.9 | 2.2 ± 1.7 | ||||
| ABC Scale | ACC | 57.5 ± 21.2 | 66.1 ± 15.4 | 71.0 ± 15.4 | 8.6 ± 15.1 | F(1,36)=11.3, p=0.002 | F(1,36)=0.004, p=0.95 | 13.5 ± 17.7 | F(1,36)=12.7, p<0.0001 | F(1,36)=1.32, p=0.26 |
| (% confidence) | SS | 58.1 ± 21.0 | 66.4 ± 18.3 | 65.0 ± 24.4 | 8.3 ± 14.2 | 6.9 ± 17.4 | ||||
| Fugl-Meyer LE Score | ACC | 24.2 ± 5.8 | 25.5 ± 6.0 | 25.6 ± 5.8 | 1.3 ± 1.6 | F(1,36)=3.4, p=0.07 | F(1,36)=5.7, p=0.02 | 1.4 ± 2.9 | F(1,36)=8.0, p=0.008 | F(1,36)=0.18, p=0.68 |
| (points out of 34) | SS | 25.6 ± 5.1 | 25.4 ± 5.7 | 26.6 ± 5.5 | −0.2 ± 2.2 | 1.0 ± 2.0 | ||||
| Satisfaction with Recovery | ACC | 26.2 ± 6.8 | 30.8 ± 6.1 | 29.4 ± 5.3 | 4.6 ± 5.6 | F(1,36)=19.3, p<0.0001 | F(1,36)=0.000, p=0.99 | 3.2 ± 5.5 | F(1,36)=14.1, p=0.006 | F(1,36)=0.55, p=0.46 |
| (points out of 44) | SS | 24.6 ± 6.9 | 29.1 ± 6.8 | 29.3 ± 8.1 | 4.6 ± 7.1 | 4.8 ± 7.6 | ||||
| Serial7 cognitive performance | ACC | 0.15 ± 0.10 | 0.16 ± 0.13 | - | 0.01 ± 0.06 | F(1,33)=0.07, p=0.79 | F(1,33)=1.04, p=0.32 | - | - | - |
| (correct responses/second) | SS | 0.15 ± 0.18 | 0.13 ± 0.15 | - | −0.01 ± 0.06 | - | ||||
| Dual-Task cognitive | ACC | 0.13 ± 0.10 | 0.13 ± 0.12 | - | 0.01 ± 0.05 | F(1,33)=1.87, p=0.18 | F(1,33)=0.31, p=0.58 | - | - | - |
| performance | SS | 0.11 ± 0.11 | 0.12 ± 0.15 | - | 0.02 ± 0.05 | - | ||||
| (correct responses/second) | ||||||||||
ACC - Accurate adaptability walking intervention; SS - steady state walking intervention; LE - lower extremity
Post-intervention assessment was conducted within one week after the final intervention session. Follow-up assessment was conducted approximately 12 weeks after the final intervention assessment.
fNIRS data are presented in Figure 3 and Table 3. fNIRS analysis indicated significant effects of Group (p = 0.048), Timepoint (p = 0.017), Group × Timepoint (p = 0.058), and Group × Timepoint × Task (p = 0.052). Post hoc analysis revealed that the Group × Timepoint effect was primarily driven by Dual-Task, with the accurate adaptability intervention group exhibiting a larger reduction in prefrontal activity from baseline to post-intervention. Between-group effect sizes for change score (Cohen’s d; baseline – post) were 0.36, 0.22, and 0.88 for Typical, Obstacles, and Dual-Task, respectively.
Figure 3. Prefrontal activity measured by fNIRS.
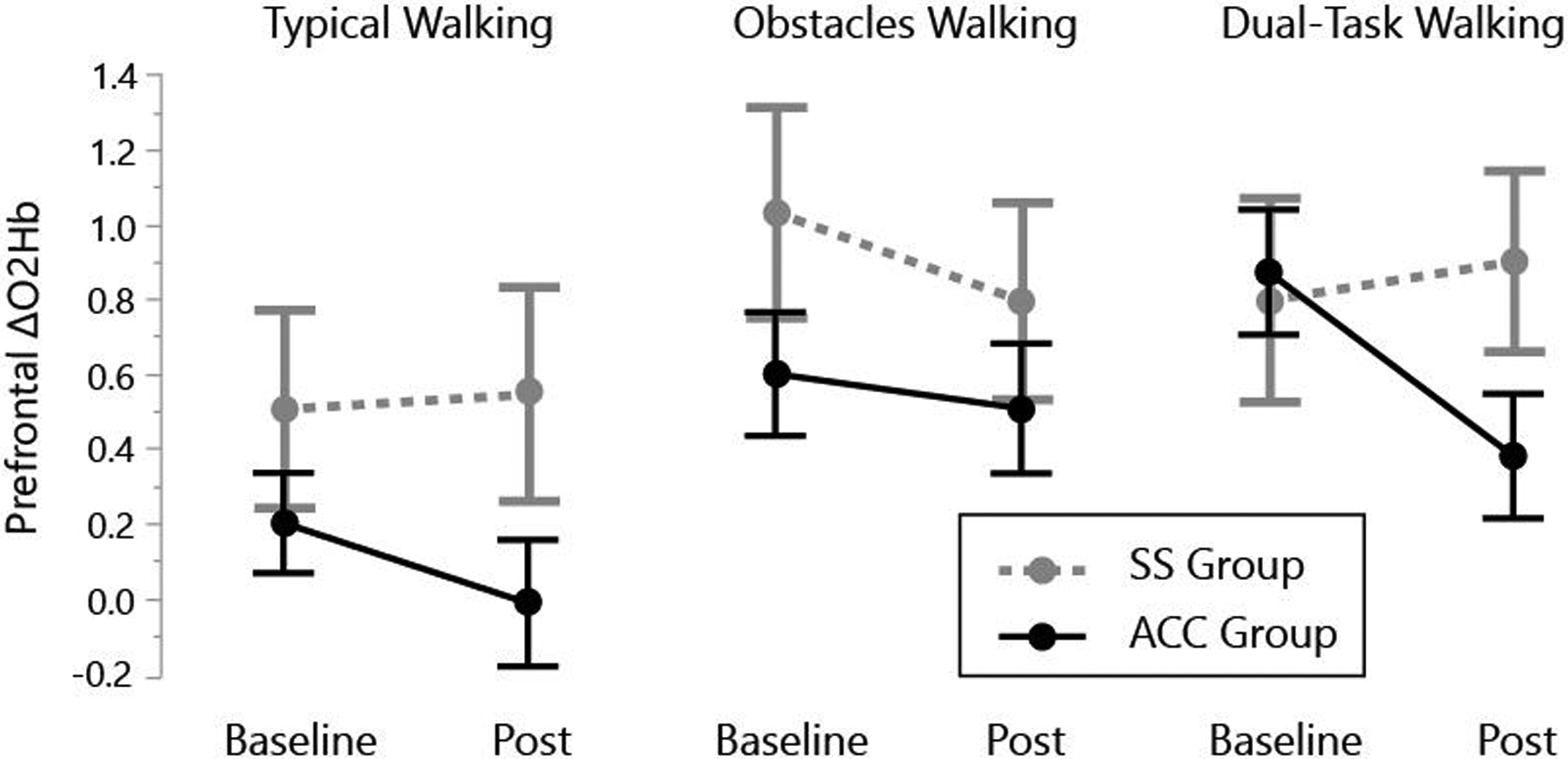
The change in prefrontal oxygenated hemoglobin concentration (ΔO2Hb; active minus rest periods) is shown at Baseline and Post-Intervention for Typical Walking, Obstacles Walking, and Dual-Task Walking (i.e., walking while performing serial-7 subtraction). The accurate adaptability intervention (ACC) group had a significant reduction in prefrontal activity between Baseline and Post-intervention. Error bars are standard error of the mean.
Table 3.
Prefrontal activity measured with fNIRS as the change in oxygenated hemoglobin concentration (active walking period minus standing rest period).
| Group | Baseline | Post | |
|---|---|---|---|
| Typical Walking | ACC | 0.20 ± 0.60 | −0.01 ± 0.76 |
| SS | 0.50 ± 1.13 | 0.54 ± 1.22 | |
| Obstacles Walking | ACC | 0.60 ± 0.75 | 0.51 ± 0.77 |
| SS | 1.03 ± 1.20 | 0.79 ± 1.12 | |
| Dual-Task Walking | ACC | 0.87 ± 0.75 | 0.38 ± 0.75 |
| SS | 0.80 ± 1.16 | 0.90 ± 1.03 |
ACC - accurate adaptability walking intervention;
SS - steady state walking intervention;
LE - lower extremity
Post-intervention assessment was conducted within one week after the final intervention session.
Discussion
The primary finding of this study is that gains in walking function did not differ for the accuracy adaptability intervention and the steady state walking intervention. The lack of difference between groups might be due to the comparable level of training frequency, duration, and intensity. For instance, the intervention design specified a target for rating of perceived exertion of approximately 5 (“high”) on the Borg Category Ratio10 scale. As shown in Figure 2A, both groups on average maintained the prescribed RPE throughout the intervention. Accumulating evidence suggests that training intensity and amount of practice are key factors in determining walking performance outcomes, regardless of the specific mode of rehabilitation training.25,26 Our study findings seem consistent with that assertion. The results of this study are comparable to two prior investigations of post-stroke rehabilitation that compared variable walking task training to steady state walking training, and found similar beneficial outcomes for both walking interventions.27,28
The accurate adaptability intervention group exhibited a larger reduction in prefrontal activity during walking, particularly for the Dual-Task condition. This finding suggests that the accurate adaptability intervention may have contributed to reduced demand for executive control of walking (improved “automaticity”10) and/or more efficient cognitive processing.20 This is generally consistent with prior studies in older adults that have also reported a reduction in prefrontal activity during walking following coordination/walking interventions.29,30 A possible explanation for the change we detected is that reduced prefrontal activity in the accurate adaptability intervention group is a training response indicating a more automatic walking control strategy. It might also indicate better efficiency of brain activity for cognitive processing.20 In either case, the finding might be indicative of an advantage for the accurate adaptability intervention group, such that reliance on prefrontal/executive control is reduced or more efficient due to beneficial neuroplasticity associated with engaging this brain region during accurate adaptability training.
It should be acknowledged that the cognitive domains used for accurate walking differ from those used for a serial subtraction task. Therefore, the mechanisms that underlie a training response of reduced prefrontal activity during our Dual-Task condition is not fully clear. Furthermore, the group differences in prefrontal activity did not yield a dual-task performance benefit for the accurate adaptability intervention group. Specifically, both groups exhibited similar intervention effects on dual-task performance with similarly increased walking speed but absence of improved subtraction task performance.
A weakness of this study is the relatively small sample size. To examine this further we have conducted a post-hoc power analysis for our primary measure of change in preferred walking speed. For the whole study sample, the standard deviation of the change in walking speed (post minus baseline time points) is 0.12. Using this standard deviation value and based on our actual sample size, a two-sample t-test with power=0.80 and alpha=0.05 would allow us to detect a group difference as small as 0.11 m/s. This statistical power is comparable to what is needed to detect “clinically meaningful” changes in walking speed for the stroke population. Perera et al. report that “substantial changes are near 0.10 for gait speed”, based on both distribution-based and anchor-based analysis approaches.31 Tilson et al. report that a change in walking speed of 0.16 m/s is the minimal clinically important difference for walking speed in individuals post-stroke (although in a more acute population).32 Therefore it is reasonable to expect that our sample size would be sufficient to detect a clinically meaningful difference between groups, had such a difference existed. Although both of our experimental groups demonstrated highly significant gains in walking speed, there was essentially no difference between groups. Assuming that we tested a representative sample of stroke participants (we have no reason to suspect otherwise), a larger sample size would not be expected to change the outcome.
More research is needed to understand the potential benefit of accurate adaptability walking training after stroke, such as the potential significance of changes in prefrontal activity. Although we found no obvious link between changes in prefrontal activity and walking function from our study, it may be possible that traditional performance-based metrics like walking speed do not sufficiently capture the relative contributions of neural automaticity and executive control strategies. For example, a real world walking situation involving unexpected obstacles or maneuvering in crowded places might benefit in a way that is not evidence from controlled lab-based walking measures. Future research should therefore assess outcome measures that may be more sensitive to neural control.
Other interesting outcome measures for future research are self-efficacy and falls. The present data show that the accurate adaptability intervention group had a nearly two-fold larger improvement than the steady state intervention group in balance confidence at three-month follow up (gains of 13.5 and 6.9 percentage points, respectively). Although this study was underpowered for detecting a group difference in this outcome, one could hypothesize that a more automatic control strategy might instill balance confidence during walking.
Another avenue of future research could be to assess longer term training and/or retention of gains. Both of our experimental groups showed steady gains in walking function over the three-month intervention period, which did not plateau (Figure 2C). This suggests that a longer intervention could have yielded further gains for one or both groups. A longer term follow-up, such as one year, might also have been an interesting addition to assess durability of gains. There is a continued need to study accuracy and adaptability tasks in rehabilitation research. There is no evidence of a disadvantage to this approach, as long as training intensity, duration, and amount of practice are maintained at a high level.
Clinical Messages.
In adults with chronic post-stroke hemiparesis, gains in walking function did not differ for the accurate adaptability intervention and steady state walking intervention.
Preliminary evidence of a larger reduction of prefrontal cortical activity during walking from the accurate adaptability intervention suggests a possible beneficial reduction in executive demand of walking.
Funding
This work was supported by the US Department of Veterans Affairs, Rehabilitation Research and Development (RR&D) Service [B1149R, B9252C]; National Institutes of Health T32 Neuromuscular Plasticity Training Pre-Doctoral Fellowship [T32HD043730]; and the Foundation for Physical Therapy (doctoral student scholarships). Resources were provided by the North Florida/South Georgia Veterans Health System and the Veterans Affairs Brain Rehabilitation Research Center. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
- 1.Nielsen JB. How we walk: Central control of muscle activity during human walking. Neuroscientist 2003; 9: 195–204. [DOI] [PubMed] [Google Scholar]
- 2.Lodha N, Chen YT, McGuirk TE, et al. Emg synchrony to assess impaired corticomotor control of locomotion after stroke. J Electromyogr Kinesiol 2017; 37: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark DJ, Kautz SA, Bauer AR, et al. Synchronous emg activity in the piper frequency band reveals the corticospinal demand of walking tasks. Annals of biomedical engineering 2013; 41: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian CK, Clark DJ, Fox EJ. Walking adaptability after a stroke and its assessment in clinical settings. Stroke research and treatment 2014: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew T Motor cortical cell discharge during voluntary gait modification. Brain Res 1988; 457: 181–187. [DOI] [PubMed] [Google Scholar]
- 6.Drew T, Jiang W, Kably B, et al. Role of the motor cortex in the control of visually triggered gait modifications. Canadian Journal of Physiology and Pharmacology 1996; 74: 426–442. [PubMed] [Google Scholar]
- 7.Drew T, Jiang W, Widajewicz W Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Brain Res Rev 2002; 40: 178–191. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins KA, Fox EJ, Daly JJ, et al. Prefrontal over-activation during walking in people with mobility deficits: Interpretation and functional implications. Hum Mov Sci 2018; 59: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark DJ, Rose DK, Ring SA, et al. Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults. Front Aging Neurosci 2014; 6(217): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark DJ. Automaticity of walking: Functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci 2015; 9(246): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 12.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair 2002; 16: 232–240. [DOI] [PubMed] [Google Scholar]
- 13.Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: Relationship of the fugl-meyer assessment to hemiparetic locomotion. Neurorehabilitation and Neural Repair 2010; 24: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of chronic diseases 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of prime-md: The phq primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. Jama 1999; 282: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 17.Jonsdottir J, Cattaneo D Reliability and validity of the dynamic gait index in persons with chronic stroke. Arch Phys Med Rehabil 2007; 88: 1410–1415. [DOI] [PubMed] [Google Scholar]
- 18.Powell LE, Myers AM. The activities-specific balance confidence (abc) scale. J Gerontol A Biol Sci Med Sci 1995; 50A: M28–34. [DOI] [PubMed] [Google Scholar]
- 19.Robinson CA, Shumway-Cook A, Ciol MA, et al. Participation in community walking following stroke: Subjective versus objective measures and the impact of personal factors. Phys Ther 2011; 91: 1865–1876. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee SA, Fox EJ, Daly JJ, et al. Interpreting prefrontal recruitment during walking after stroke: Influence of individual differences in mobility and cognitive function. Front Hum Neurosci 2019; 13(194): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark DJ, Neptune RR, Behrman AL, et al. A locomotor adaptability task promotes intense and task-appropriate output from the paretic leg during walking. Archives of Physical Medicine and Rehabilitation 2016; 97: 493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merbah S, Meulemans T Learning a motor skill: Effects of blocked versus random practice. Psychologica Belgica 2011; 51: 15–48. [Google Scholar]
- 23.Vitorio R, Stuart S, Rochester L, et al. Fnirs response during walking - artefact or cortical activity? A systematic review. Neuroscience and biobehavioral reviews 2017; 83: 160–172. [DOI] [PubMed] [Google Scholar]
- 24.Curran-Everett D Multiple comparisons: Philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1–8. [DOI] [PubMed] [Google Scholar]
- 25.Hornby TG, Straube DS, Kinnaird CR, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil 2011; 18: 293–307. [DOI] [PubMed] [Google Scholar]
- 26.Hornby TG, Reisman DS, Ward IG, et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. Journal of neurologic physical therapy : JNPT 2020; 44: 49–100. [DOI] [PubMed] [Google Scholar]
- 27.DePaul VG, Wishart LR, Richardson J, et al. Varied overground walking-task practice versus body-weight-supported treadmill training in ambulatory adults within one year of stroke: A randomized controlled trial protocol. BMC neurology 2011; 11: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornby TG, Henderson CE, Plawecki A, et al. Contributions of stepping intensity and variability to mobility in individuals poststroke. Stroke 2019; 50: 2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eggenberger P, Wolf M, Schumann M, et al. Exergame and balance training modulate prefrontal brain activity during walking and enhance executive function in older adults. Front Aging Neurosci 2016; 8(66): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godde B, Voelcker-Rehage C Cognitive resources necessary for motor control in older adults are reduced by walking and coordination training. Frontiers in human neuroscience 2017; 11(156): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society 2006; 54: 743–749. [DOI] [PubMed] [Google Scholar]
- 32.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys Ther 2010; 90: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]


