Abstract
Vasculogenic cell therapies have emerged as a powerful tool to increase vascularization and promote tissue repair/regeneration. Current approaches to cell therapies, however, rely mostly on progenitor cells, which pose significant risks (e.g., uncontrolled differentiation, tumorigenesis, and genetic/epigenetic abnormalities). Moreover, reprogramming methodologies used to generate induced endothelial cells (iECs) from induced pluripotent stem cells rely heavily on viral vectors, which pose additional translational limitations. This work describes the development of engineered human extracellular vesicles (EVs) capable of driving reprogramming-based vasculogenic therapies without the need for progenitor cells and/or viral vectors. The EVs were derived from primary human dermal fibroblasts (HDFs), and were engineered to pack transcription factor genes/transcripts of ETV2, FLI1, and FOXC2 (EFF). Our results indicate that in addition of EFF, the engineered EVs were also loaded with transcripts of angiogenic factors (e.g., VEGF-A, VEGF-KDR, FGF2). In vitro and in vivo studies indicate that such EVs effectively transfected HDFs and drove direct conversions towards iECs within 7–14 days. Finally, wound healing studies in mice indicate that engineered EVs lead to improved wound closure and vascularity. Altogether, our results show the potential of engineered human vasculogenic EVs to drive direct reprogramming processes of somatic cells towards iECs, and facilitate tissue repair/regeneration.
Keywords: engineered EVs, induced endothelium, ischemic disorders, vasculogenic therapies
Graphical Abstract
Engineered human EVs loaded with ETV2, FLI1, and FOXC2 (EFF), can effectively drive vasculogenic reprogramming in human dermal fibroblasts and aid in in vivo tissue repair and regeneration. Such EVs have the potential to serve as a powerful nanocarrier platform for vasculogenic factors to treat a myriad of ischemic disorders.
INTRODUCTION
Extracellular vesicles (EVs) are spherical lipid-bilayer nanoparticles secreted by all healthy and diseased cells in the body. EVs originate from the endosome or plasma membrane and are classified into three sub-populations: exosomes, microvesicles, and apoptotic bodies, according to their release mechanism and size1–3. EVs are capable of packing and transporting payloads of miRNAs, mRNAs, DNA, and proteins among other molecules4. The molecular cargo is protected from extracellular degradation by their lipid bilayer membrane. EVs have shown to be implicated in physiological processes like paracrine, autocrine, and immune system signaling5. They also serve an important role in surface molecular trafficking and horizontal transfer of DNA, mRNA, and proteins among different cell types6. Cells can incorporate the molecular cargo derived from EVs through several mechanisms, including membrane receptor-ligand interactions1, or direct fusion of the EVs with the membrane of recipient cells via endocytosis. The increase in knowledge on EV composition, biogenesis, and their role in intercellular communication, has given rise to a research field that aims at understanding how EVs could be implicated in diseases onset and propagation7–10. Additionally, our lab and others have shown their significant potential to serve as efficient nanocarriers for an extensive array of therapeutic applications, including regenerative medicine and cancer7, 11–20.
Ischemic disorders and tissue injury often result in deficient vascularization, which can lead to infections, inflammation, and impaired tissue regeneration (e.g., non-healing ulcers, partial necrosis)21, 22. Cell therapies, involving the use of vascular progenitor cells or induced endothelial cells (iECs), have emerged as a powerful approach to increase neovascularization and promote tissue repair and regeneration under multiple conditions, including myocardial ischemia, limb ischemia, and stroke, among others19, 23–26. iECs provide growth factors, such as vascular endothelial growth factor (VEGF), and iEC-driven neovasculature serves as a scaffolding structure to support and guide cell migration and proliferation, promoting tissue repair27, 28. Current approaches to vasculogenic cell therapies, however, rely on transplantation of progenitor/stem-like cells29, 30, which pose significant risks in terms of uncontrolled/undesired differentiation, tumorigenesis, genetic/epigenetic abnormalities, immunogenicity, and ethical considerations31, 21, 28, 32, 33. Moreover, reprogramming methodologies used to generate induced pluripotent stem cells (iPSCs)-derived endothelial cells (ECs) are heavily dependent on viral vectors, which pose additional biosafety and translational concerns.
Recent studies by us and others have shown that transcription factor genes/transcripts Ets variant 2 (ETV2), Friend leukemia virus integration 1 (FLI1), and Forkhead box C2 (FOXC2) (EFF) can be used to directly reprogram somatic cells into iECs18, 19, 28, 34–37. ETV2 (Ets variant 2) has recently been reported as a pioneering transcription factor, implicated in the embryonic development of endothelial and hematopoietic lineages38 39, 40. ETV2 plays an important role inducing relaxation of the chromatin by the recruitment of factors such as Brahma-related gene-1 (BRG1) and ETS Transcription Factor (ELK3), this recruitment opens the chromatin and increases H3K27ac deposition, resulting in the activation of gene expression38. Recent reports suggest that ETV2 can induce Rhoj expression, and that the ETV2/Rhoj network plays a key role in regulating the migration of early endothelial progenitor cells during embryonic development41. An essential function of ETV2 is its interaction with forkhead transcription factors, like a FOXC2, via the FOX:ETS motif, which is a composite cis-element. This ETV2/FOXC2 complex can regulate at least 30% of endothelial gene expression42. A complete knockdown of ETV2 affects the expression of genes like FLI1, and can lead to embryonic lethality43. FLI1 is a key regulator of hematopoietic progenitor cell differentiation and maintenance, and vascularization during embryonic development43. FLI1 and Ets-related gene (ERG), are implicated in hematopoietic and endothelium development, while FOXC2 is an important regulator of essential angiogenesis processes like cell migration, remodeling, and maturation42, 44–46. On the other hand, growth factors such as FGF2 and VEGF-A synergistically promote angiogenesis, and proliferation of endothelial cells 40,47, 48. Additionally, recent studies suggest that FGF2 is involved in maintenance of vascular integrity in adult vasculature by regulating VE-cadherin/p120-catenin coupling49. These VE-cadherins/p120-catenin interactions are essential for maintaining endothelial barrier function and the prevention of endocytosis50.
Recently we reported that in vitro cell nanotransfection or in vivo tissue nanotransfection (TNT) with EFF can drive the development of iECs and improve tissue vascularization and repair/regeneration under different models of tissue ischemia18, 19, 25, 26. While in both cases we document the release of EFF-loaded EVs from EFF-nanotransfected cells and tissues, currently no study has looked into the production and characterization of human EFF-loaded EVs for therapeutic applications. Here we show that primary human dermal fibroblasts (HDFs) can be driven to release EFF-loaded EVs via non-viral transfection of expression plasmids for EFF, and that such EVs can induce direct conversion of somatic cells into iECs in vitro and in vivo. Finally, we showed that EFF-loaded human EVs can lead to improved wound closure in mice. Our results highlight the potential use of engineered EVs as a novel cell-free therapy to help overcome many of the limitations of current progenitor/stem cell-based therapies.
RESULTS
EFF transfected human dermal fibroblasts (HDFs) release vasculogenic EVs with tailored cargo
EFF loaded EVs were derived from HDFs after electroporation with plasmids encoding for ETV2, FLI1, and FOXC2 (EFF)18 (Figure 1A). HDFs transfected with a sham plasmid (i.e., empty vector with the same backbone) were used to derive control EVs. HDF transfection efficiencies ranged between 30 and 40% for the sham (control) and EFF groups, respectively (Supplemental Figure 1). Dynamics of engineered EV release revealed a peak 48 hours after transfection of the donor HDFs, with particle concentrations ranging around 7×109 EVs/mL for EFF-loaded EVs, and 5×109 EVs/mL for control EVs, respectively (Figure 1B). EV size remained consistent over time, around 200 nm for both EFF-loaded and control EVs, as confirmed via nanoparticle tracking analysis by NanoSight, and cryo-transmission electron microscopy (cryo-TEM) (Figure 1C–D and Supplemental Figure 2). Western blot characterization confirmed the presence of the EV marker, CD63, in both EFF and control EV preparations (Figure 1E). Quantitative and absolute reverse transcription polymerase chain reaction (qRT-PCR and aRT-PCR) analysis confirmed robust and sustained upregulation of EFF expression in donor HDFs for 12–48 hours after transfection, with a significant increase at 48 hours for ETV2 and FLI1, and slightly higher expression for FOXC2 at 24 hours (Figure 1F). qRT-PCR characterization of the EVs, on the other hand, shows a significant and sustained increase in EFF loading between 12–48 hours post-transfection, with 48 hours consistently showing one of the highest levels of each factor, thus suggesting that 48 hours post-HDF transfection may represent the best timepoint for EV collection for downstream use (Figure 1G). To determine whether the EVs were loaded with plasmid DNA, intact EVs were treated with DNase to remove potential coprecipitates (Supplemental Figure 3A). Subsequently, the presence of plasmid DNA within the EVs was confirmed by amplification of the poly-linker region of each plasmid via conventional PCR (Supplemental Figure 3B). Moreover, absolute PCR characterization showed significant packing of both plasmid DNA and mRNA content in EFF EVs compared to control EVs (Supplemental Figure 3C–3E). Further characterization of the transcript content packed in the EFF-loaded EVs suggested the presence of additional regulators of vasculogenesis and angiogenesis, specifically VEGF-A and Vascular endothelial growth factor receptor 2 (VEGF-R2/KDR), and FGF-2 (Figure 1H).
Figure 1. Vasculogenic EVs fabrication, isolation, and characterization.

A) Schematic describing how engineered vasculogenic EVs are derived from primary human dermal fibroblasts after electroporation with plasmids encoding for the vasculogenic transcription factors genes ETV2, FLI1, and FOXC2 (EFF). Nanoparticle tracking analysis showing B) particle concentration and C) particle size distribution for EFF and control EVs isolated at 12, 24, and 48 hours after electroporation of the donor cells. D) CryoEM images showing single EFF and control EVs isolated 48 hours post-electroporation of the donor cells, showing their intact morphology with a defined lipid bilayer. E) Western blot characterization of EFF and control EVs showing positive expression of the EV marker, CD63 for both EV preparations. F) ETV2, FLI1, and FOXC2 (EFF) upregulation in donor cells at 12, 24, and 48 hours after electroporation compared to sham electroporated cells. G) Dynamics of transcript packing in EFF and control EVs isolated at 12, 24, and 48 hours after electroporation of donor cells. H) Transcript levels of additional vasculogenic factors (VEGF-A, VEGF-KDR, and FGF-2) packed in EFF and control EVs isolated 48 hours after electroporation of donor cells. Data represented as standard deviation Mean+/−SD. # Indicates significant difference with respect to control EVs. * # P < 0.05 (n = 3 – 6, One way ANOVA with Fisher LSD multiple comparison test).
Engineered vasculogenic EVs are capable of transfecting HDFs and inducing direct reprogramming towards iECs capable of forming mature vasculature in vivo
EFF EVs collected 48 hours after electroporation of donor cells, were effectively captured and incorporated by recipient cells as early as 6 hours after treatment, with uptake efficiencies of 90.6% and 70.9% for EFF and control EVs, respectively (Figure 2A–B). qRT-PCR analyses confirmed that recipient cells, in this case HDFs, were successfully transfected with the vasculogenic cargo and showed sustained overexpression of EFF for up to 12 hours after treatment with EFF EVs compared to cells treated with control EVs (Figure 2C). Recipient cells showed sustained EFF expression at weeks 1 and 2 post-EV treatment (Figure 2D). We also observed that HDFs treated with EFF EVs were capable of further releasing EFF-loaded EVs for up to 2-weeks after treatment (Figure 2E). Further characterization confirmed the presence of both EFF plasmid DNA and mRNA in these EVs (Supplemental Figure 4A–B). Finding the plasmid DNA in the EVs 1-week after treatment of the HDFs with EFF EVs, suggests that there is a mechanism of EV uptake and subsequent release.
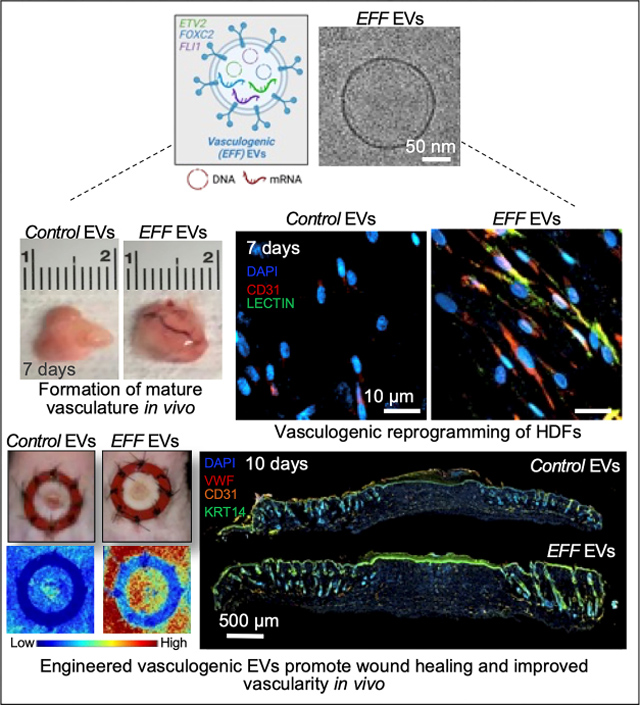
Figure 2. EV uptake by recipient cells and positive transfection of primary HDFs after EFF EV treatment.
A) Representative immunofluorescence micrographs of HDFs treated with fluorescently labeled (red) EFF or control EVs for 6 and 12 hours (cell nuclei were counterstained with DAPI), showing positive uptake of the EVs into subcellular compartments; and B) respective quantification of EV uptake as percentage of positive cells. C) Upregulation of ETV2, FLI1, and FOXC2 (EFF) in recipient cells (HDFs) at 6 and 12 hours after EV treatment. D) Sustained upregulation of ETV2, FLI1, and FOXC2 (EFF) in recipient cells (HDFs) at 1 and 2-weeks after EV treatment. E) Further release of EFF EVs by recipient cells at 1 and 2-weeks after EV treatment. Data represented as Mean+/−SD. * P < 0.05 (n = 3, B-D - Holm-Sidak method and two tailed t-test, E - One way ANOVA with Fisher LSD multiple comparison test).
Additional experiments where the skin of mice was transfected via EFF EVs or via standard bulk electroporation-based transfection were conducted to compare transfection efficiency in vivo. These results demonstrated that in vivo, EFF EVs are more effective in gene transfer compared to standard bulk electroporation-based transfection (Supplemental Figure 5A–B).
Long-term culture experiments also demonstrated the ability of EFF EVs to successfully induce direct reprograming of HDFs into iECs, whereby HDFs treated with EFF EVs showed positive expression of the vascular marker Lectin and the cluster of differentiation 31 (CD31) as early as 1-week after treatment compared to cells treated with control EVs (Figure 3A–B). Positive expression of markers of more mature endothelium such as, von-Willebrand-Factor (VWF), ERG and FGF-2 was observed at 2- and 3-weeks after EV treatment (Figure 3C–G).
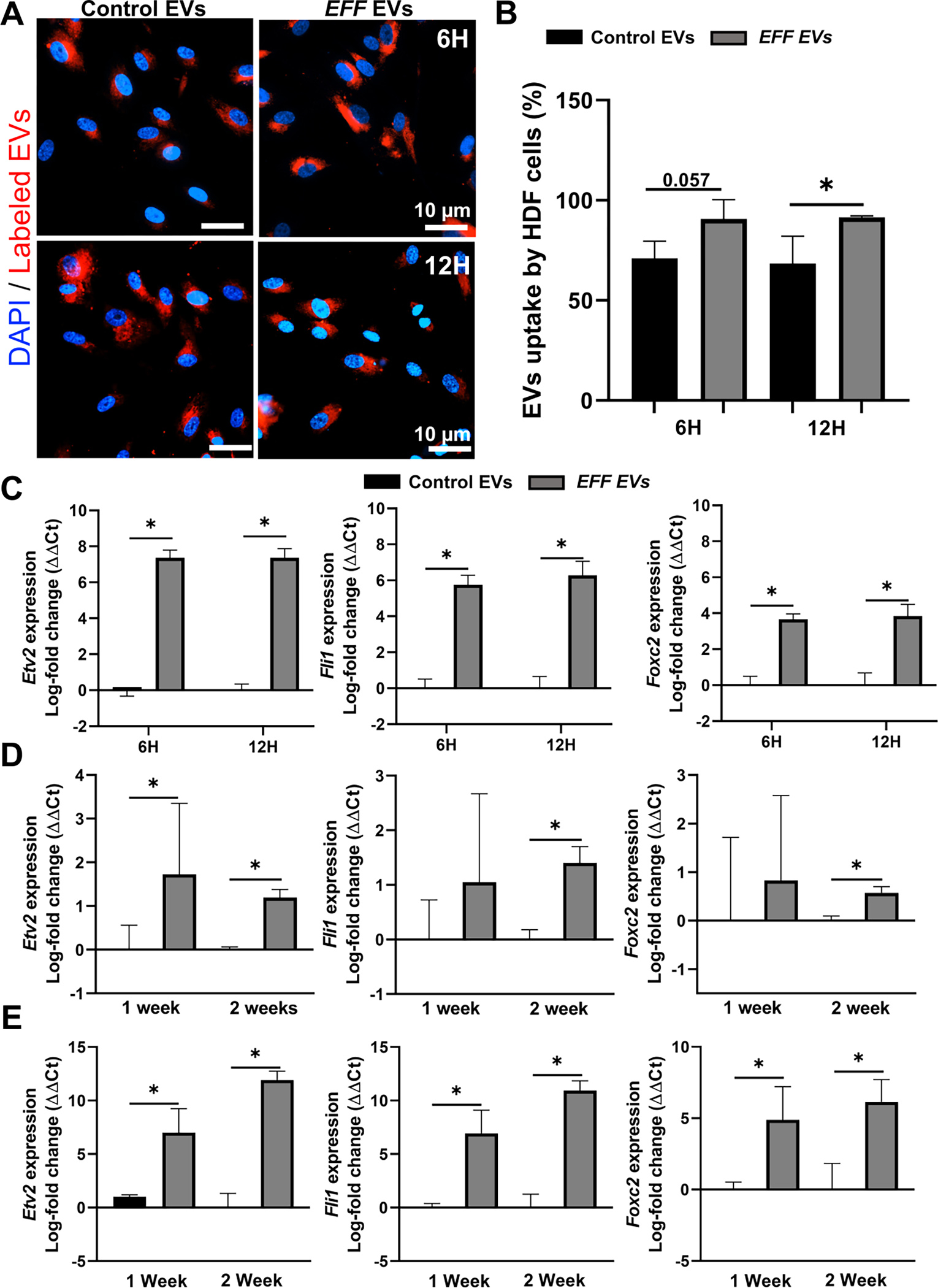
Figure 3. EFF EVs induce direct endothelial reprograming of primary HDFs.
A) Successful reprogramming of HDFs towards iECs after treatment with EFF EVs, with positive expression of the endothelia markers CD31 (red) and lectin (green) at 1-week after EV treatment (cell nuclei were counterstained with DAPI), and B) respective quantification of the mean fluorescence intensity for CD31 and Lectin positive cells. C) Immunofluorescence micrographs of iECs showing expression of the more mature endothelial markers VWF (red) at 2 and 3 weeks after EV treatment (cell nuclei were counterstained with DAPI), and D) respective quantification of the mean fluorescence intensity for VWF positive cells. Upregulation of the mature endothelial makers ERG and FGF2 at E) 1-week, F) 2-weeks, and G) 3 weeks post-EV treatment. Data represented as Mean+/− SD. * P < 0.05 (n = 3 – 4, One way ANOVA with Fisher LSD multiple comparison test and Tukey test).
To stablish the ability of EFF EV treated cells to promote new blood vessel formation and increased angiogenesis, 14 days after EFF or control EV treatment, HDFs were embedded in a Matrigel plug and injected in the flank of immunodeficient nude mice (NU/J). (Figure 4A). At day 7 after implantation the animals were euthanized and the Matrigel plugs and surrounding tissues (i.e., skin and muscle) were harvested and cryopreserved for immunohistochemistry (IHC) analysis. Animals implanted with EFF EV-treated cells showed increased angiogenesis with significantly higher vessel area compared to animals implanted with cells treated with control EVs (Figure 4B–C). IHC analysis revealed significantly higher expression of the endothelial markers, VWF and Lectin, in the harvested plugs and the surrounding skin and muscle tissue, for animals implanted with the EFF EV treated cells compared to controls (Figure 4D–E). This data shows the potential of EFF engineered EVs to promote angiogenesis locally, and in the surrounding tissues.
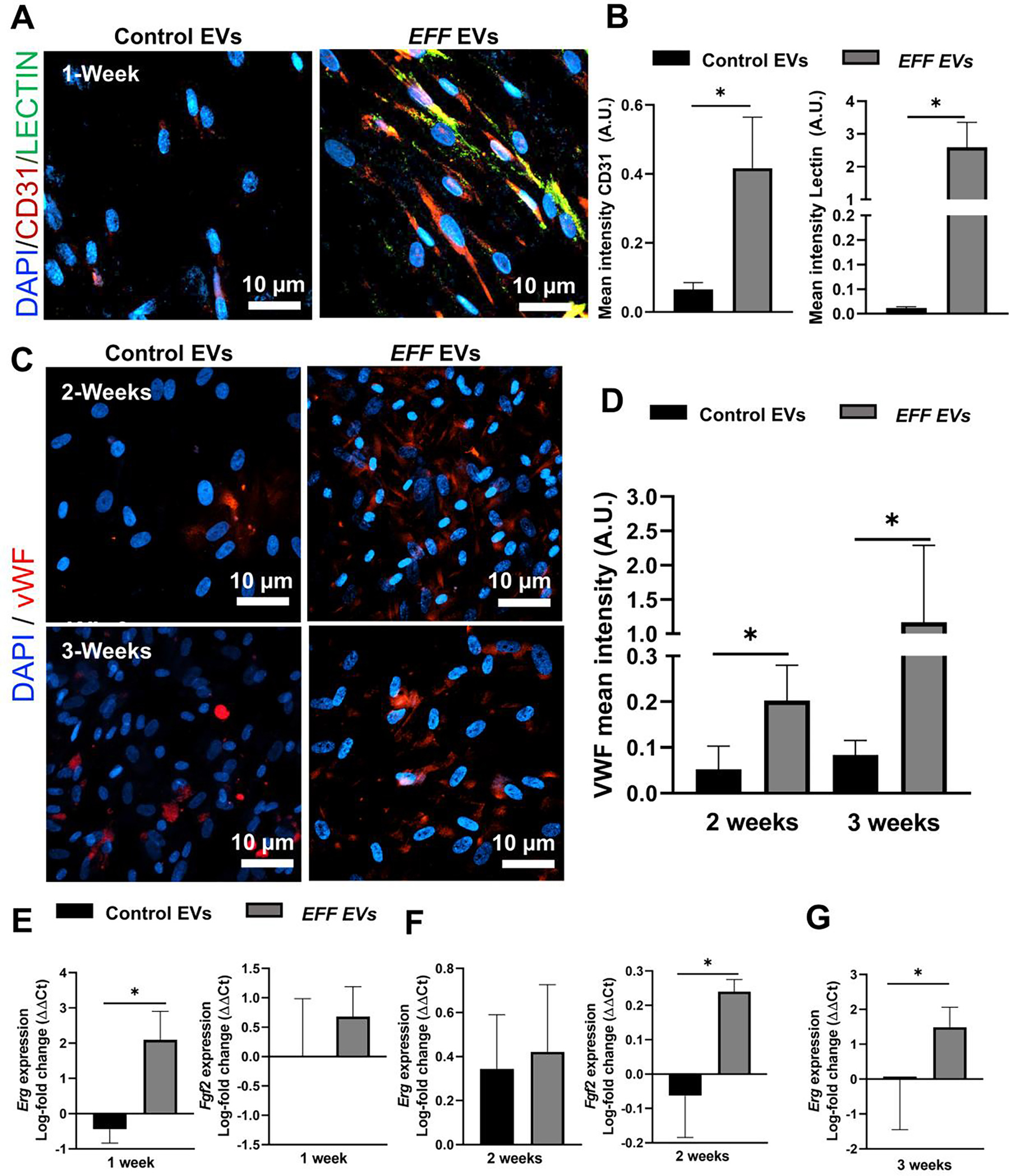
Figure 4. iECs derived from EFF EV treated primary HDFs had the potential to induce formation of mature vasculature in vivo.
A) Schematic diagram of the Matrigel plug assay, where HDFs were embedded in a Matrigel plug at 2-weeks after EV treatment and injected into the flank of immunodeficient nude mice (NU/J) for 1-week. B) Matrigel plugs recovered at 1-week after implantation, showing the potential of iECs to induce formation of mature vasculature in vivo, and C) respective quantification of blood formation in terms of vessel area. D) Representative immunofluorescence micrographs of the Matrigel plugs and surrounding skin and muscle at 1-week post-implantation, showing increased vascularity with upregulation of the endothelial markers VWF (red) and lectin (green) (cell nuclei were counterstained with DAPI), and E) respective quantification of the mean fluorescence intensity for VWF and lectin positive cells. Data represented as Mean+/−SD. * P < 0.05 (n = 5, One way ANOVA with Fisher LSD multiple comparison test).
Engineered vasculogenic EVs promote wound healing
An excisional wound assay was conducted to verify the effect of EFF EVs on wound healing (Figure 5A). EFF or control EVs were topically administered to all animals at day-0 and day-3 post-wounding. Reduction in the wound area was measured at 0, 2, 4, 6, 8, and 10 days after wounding. A significant reduction in wound area was observed as early as 4 days after wounding for animals treated with EFF EVs compared to those treated with control EVs (Figure 5B–C). Animal treated with EFF EVs showed a substantial increase in perfusion by day-10 compared to animals treated with control EVs (Figure 5D–E). Further analysis of skin biopsies at day-10, revealed that animals treated with EEF EVs had improved vascularity with marked expression of the endothelial markers VWF and CD31 (Figure 5F–H). Both EFF EV and control EV treated animals showed positive expression of keratin 5 (KRT5) and keratin 14 (KRT14), which are constitutively expressed in the basal layer of the skin and are involved in proliferation and differentiation of its stratified epithelia51 (Supplemental Figure 6). Additional experiments were conducted to verify that EVs were successfully internalized by both epidermal and dermal cells (Supplemental Figure 7). However, from previous studies conducted by our group we know that the cells more likely to respond to EFF mediated reprogramming are fibroblasts18.
Figure 5. EFF EVs promote wound healing in vivo.
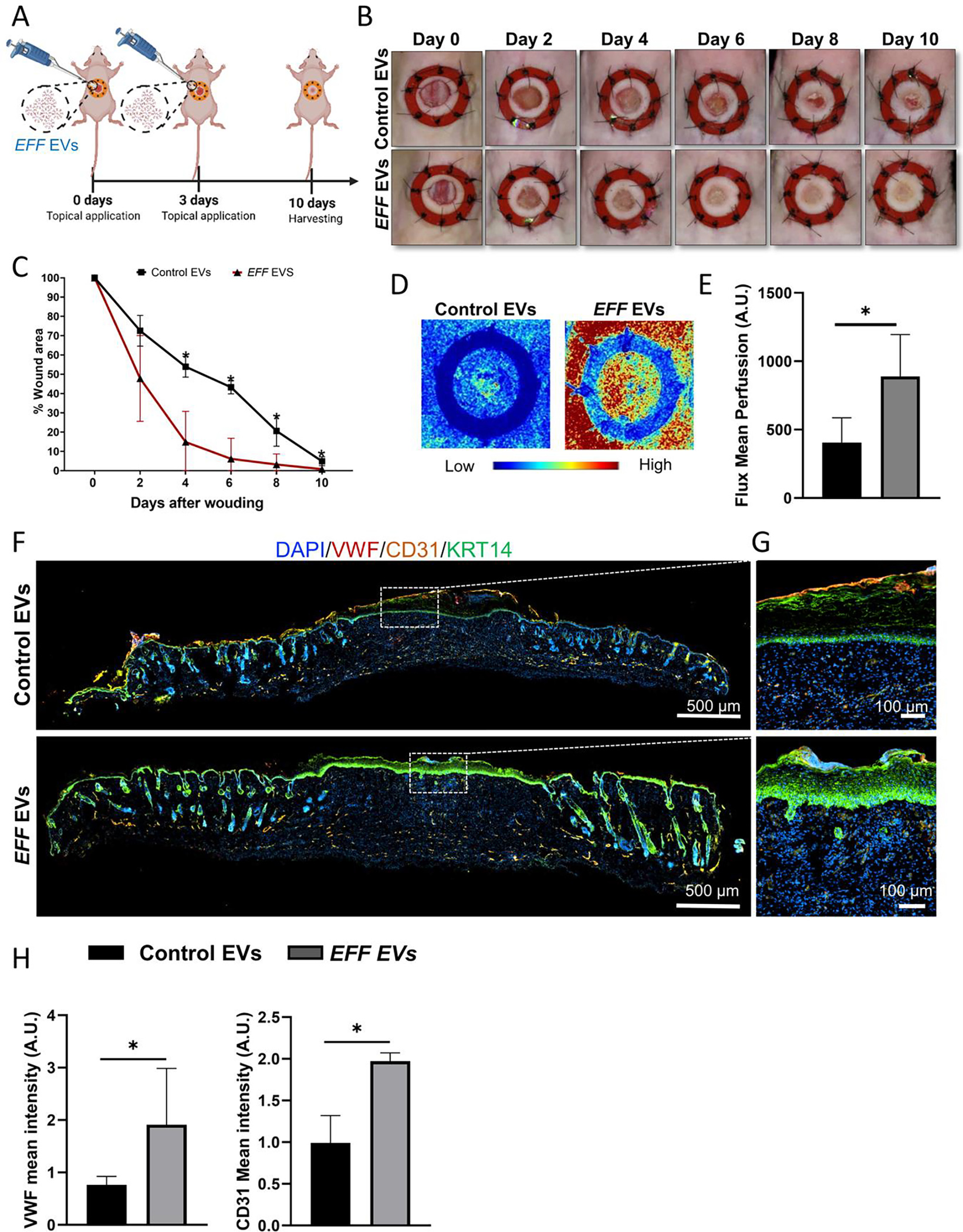
A) Schematic illustration of the excisional wound healing assay in nude mice and treatment with EFF or control EVs administrated topical at 0, and 3-days post-wounding. B) Representative images showing accelerated wound closure over time for animals treated with EFF EVs compared to animals treated with control EVs, and C) respective quantification of the percentage of wound closure at 0-, 2-, 4-, 6-, 8-, and 10-days after wounding, showing a significant increase in wound closure by day-4 after wounding for animals treated with EFF EVs. D) Skin perfusion at 10-days post-wounding for animals treated with EFF or control EVs, and E) respective perfusion assessment. F) Representative immunofluorescence micrographs of skin biopsies collected at day-10 post wounding for animals treated with EFF or control EVs, showing positive expression of the endothelial markers vWF (red) and CD31 (yellow), as well as presence of the epithelia marker KRT14 (green), with G) respective zoom-in regions, showing how EFF EV treated animals showed presence of invaginations of the epidermis, resembling hair follicles. H) respective quantification of the mean fluorescence intensity for VWF and CD31 in the wound bed area. Data represented as Mean+/−SD. * P < 0.05 (n = 3, One tailed t-test and One way ANOVA with Fisher LSD multiple comparison test).
DISCUSSION
Our study demonstrates that engineered vasculogenic EVs can be obtained after electroporation of primary HDFs with a cocktail containing expression plasmids encoding for the vasculogenic transcription factor genes, ETV2, FLI1, and FOXC2 (EFF) (Figure 1A–E). We confirmed that these engineered EVs are loaded with plasmid DNA and mRNA encoding for EFF (Supplemental Figure 3). The presence of plasmid DNA content in the EVs has the potential to enhance the number of transcripts produced and packed by donor cells. Moreover, the potential risk of genomic integration is mitigated by the fact that circular plasmids, which are less susceptible to degradation by exonucleases, were used to engineer the donor cells. However, further studies will need to be conducted to evaluate the incidence of plasmid integration, if any52, 53. Overall, this data highlights the ability of engineered EVs to transfer exogenous DNA to target cells.
Additional vasculogenic and angiogenic factors endogenously expressed by the transfected donor cells, such as vascular VEGF-A and -KDR, were successfully packed in the engineered EVs (Figure 1F–H). To the best of our knowledge this is the first report of engineered vasculogenic EVs derived from HDFs. Our results demonstrate that EFF EVs are effectively captured and internalized by recipient cells, and effectively transfect them. A single dose treatment with EFF EVs can promote epigenetic changes in the recipient cells, which induced reprograming of HDFs toward an endothelial phenotype (Figure 3A–D). Moreover, EFF EV treated cells were able to further release vasculogenic EVs for up to 2-weeks after treatment (Figure 2E).
EFF EVs can be incorporated by recipient cells through direct membrane fusion or via the endocytic pathway1. Endocytosis is divided into two principal sub-types, pinocytosis, and phagocytosis. Recent reports have shown that the pinocytosis pathway is the major route for EVs internalization 54, 55. Although the mechanisms of EVs trafficking within the cell after EV incorporation remains unknown, EVs can effectively escape the endosomal and lysosomal degradative pathways56, 57. Thus, after EV uptake their molecular cargo like plasmid DNA and mRNA can be released into the cytosol without degradation by the lysosomal pathway55, 58. EVs mRNA and plasmid DNA content would be translated in the recipient cells to produce target proteins.
Engineered EVs have the potential to enable novel therapeutic cell-free, non-viral, strategies that can overcome the limitations exhibited by stem cell and progenitor cell-based therapies. Recently, EVs isolated from umbilical mesenchymal stem cells (MSCs) have shown to induce cutaneous wound healing by promoting tissue repair, and angiogenesis59 60. Moreover, EVs derived from MSCs have shown to improve functional recovery, induce neurovascular remodeling, and increase axonal density and synaptophysin-positive area in the ischemic boundary zone using a rat model for middle cerebral artery occlusion (MCAO)61. However, the EVs isolated from stem cells conserved molecular cues from their donor cells, which may pose additional risks in terms of tumorigenesis. The implementation of human dermal fibroblasts as donor cells circumvents this issue.
Our results indicate that EFF EVs can be used to effectively induce direct reprogramming of adult HDFs to iECs, with positive expression of the endothelial marker CD31 as early as 1-week after EV treatment and sustained expression of VWF for up to 3 weeks after EV treatment (Figure 3B–D). CD31 is a transmembrane protein, which is expressed in early endothelial development62, while VWF has heterogeneous levels of expression in vascular tissues (e.g., veins, arteries, arterioles, and capillarity beds), for this reason it is used as an angiogenesis marker63. These epigenetic changes are facilitated by the additional vasculogenic factors packed in the EFF-loaded EVs, such as VEGF-A and -KDR, and FGF2 (Figure 1G–H); as well as those further expressed by the treated cells such as ERG and FGF2 (Figure 3E–G).
Engineered vasculogenic EVs offer a benign method to effectively transfect primary HDFs. Using engineered EVs as nanocarriers to deliver genetic material to target cells can help circumvent limitations imposed by standard electroporation techniques, where the whole cell membrane is exposed to an electric field to achieve transient poration and transfer of the genetic material via electrophoresis. Although effective, this technique can alter the cell membrane composition and integrity, which in turn can affect cell viability, biological structure, and uptake of molecular cargo by target cell64. Our results highlight how engineered EV-based nanocarriers offer specific advantages over synthetic and/or viral delivery methods, where EFF EVs are captured with uptake efficiencies of 90.6% and 91.4% at 6- and 12-hours post-EV treatment (Figure 2A–B), they can transfect primary HDFs and induce reprogramming under simple culture conditions (i.e., without the need for differentiation factors). Our findings indicate that human iECs obtained after treatment with EFF EVs can form mature vasculature once transplanted into immunodeficient nude mice (NU/J). Moreover, we observed a robust effect of EFF EVs on wound closure, perfusion, and vascularization at day-10 post-EV treatment. (Figure 4–5 and Supplemental Figure 3).
CONCLUSIONS
EVs have increasingly gained attention due to their capacity to inherently mediate cell-to-cell communication, which makes them a promising candidate for drug and gene delivery applications. Ours study indicates that EFF-loaded EVs can successfully induce direct reprograming of primary HDFs into endothelial cells (iECs). The iECs generated after a single dose treatment with EFF EVs express early endothelial markers such as CD31 and lectin as early as 1-week after treatment, and more mature markers such as VWF as early as 2-weeks post-EV treatment. iECs reprogramed via EFF EV treatment can form mature vasculature in Matrigel plugs transplanted in mice at 1week after EV treatment. In vivo wound healing experiments showed that EFF EVs induced more rapid wound closure compared to animals treated with control EVs. This response can be partially mediated by the sustained release of vasculogenic EVs from recipient cells for up 2-weeks post-EV treatment. These results demonstrate the feasibility of using engineered vasculogenic EVs derived from adult skin fibroblasts, as effective nanocarriers to deliver gene therapies for ischemic disorders.
MATERIALS & METHODS
Cell culture
Primary human dermal fibroblasts (HDF) were isolated from surgical discard skin from healthy female and male patients under IRB approval (2019H028) as previously described65. HDF were cultured in DME (Thermo Fisher Scientific) supplemented with 1% penicillin streptomycin, 4% fetal bovine serum (FBS; Gemini BioProducts, West Sacramento, CA), 5 μg/mL bovine insulin (Sigma), 0.1 mM l ascorbic acid-2-phosphate (Sigma), 0.5 μg/mL hydrocortisone (Sigma), and 10 ng/mL epidermal growth factor at 5% CO2 in a humidified incubator.
DNA plasmid preparation and cell transfection
ETV2, FLI1 and FOXC2 plasmids were purchased from OriGene. All plasmids were expanded via bacterial inoculation. The plasmid purification was made using a DNA ZymoPure II kit (Zymo research, USA. Cat. No. D4201) following the procedure described by the manufacturer. DNA concentrations and quality were assessed using a Nanodrop 2000c Spectrophotemeter (Thermo Fisher Scientific). A cocktail containing plasmids encoding for ETV2, FLI1 and FOXC2 (EFF) were transfected into the donor cells at a 1:1:1 ratio (with 0.05 μg/uL per plasmid) using a Neon™ Transfection System (Thermo Fisher Scientific). At 1425V, with 1 pulse, for 30ms After transfection, cells were maintained in DME media with 4% of exosome depleted fetal bovine serum (GIBCO cat: A27208–01). The plasmids used in this study included: human-ETV2 (cat: RG213907, OriGene), human-FOX2 (cat: RG223412, OriGene), human-FLI1- (cat: RG200695, OriGene) and the sham vector pCMV6 (cat: PS100010 OriGene) used to produced control EVs.
Extracellular Vesicle isolation
EVs were isolated from culture media at 12-, 24- and 48-hours after transfection of the donor cells. Supernatants were centrifuged at 2000 g for 30 minutes and 4°C to remove dead cells and debris. After centrifugation, total exosome isolation reagent (Thermo Fisher Scientific) was added to the cell-free supernatant and the samples were incubated overnight at 4°C. All samples were subsequently centrifuged at 10,000 g and 4°C for 1 hour and the resulting pelleted EVs were stored at −80°C for subsequent use. EV concentration and size distribution were quantified via nanoparticle tracking analysis using a Nanosight system (Malvern NS300). If needed the samples were diluted to ~107-109 particles/mL to properly fit the detection range for this system66. Effective cell transfection of donor and recipient cells were evaluated via qRT-PCR using specific Taqman primers (see Table 1).
Table 1.
Human primers (Taq-Man,ThermoFisher) used to evaluate gene expression analysis.
| Gene symbol | Gene name | Gene aliases | Catalog number |
|---|---|---|---|
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | G3PD, GAPD, HEL-S-162eP | Hs02786624_g1 |
| ETV2 | Human ets variant 2 | ER71, ETSRP71 | Hs00403699_m1 |
| FLI1 | Human Friend leukemia virus integration 1 | EWSR2, SIC-1 | Hs00956709_m1 |
| FOXC2 | Human forkhead box C2 | FKHL14, LD, MFH-1, MFH1 | Hs00270951_s1 |
| ERG | ETS transcription factor | Erg-3, p55 | Hs01554629_m1 |
| VEGF-A | vascular endothelial growth factor A | MVCD1, VEGF, VPF | Hs00900055_m1 |
| VEGF-D | c-fos induced growth factor | VEGFD, FIGF | Hs01128657_m1 |
| FGF2 | fibroblast growth factor 2 | BFGF, FGF-2, FGFB, HBGF-2 | Hs00266645_m1 |
| KDR | kinase insert domain receptor | CD309, FLK1, VEGFR, VEGFR2 | Hs00911700_m1 |
Western blot Analysis
Total protein isolation from HDF donor cells, control and EFF EVs was conducted using a lysis buffer containing radioimmunoprecipitation assay buffer (reference no. 89900, Thermo Fisher Scientific), complete protease inhibitor cocktail (reference no. 4693116001, Roche), phosphatase inhibitor cocktail 2 (reference no. P5726–1, Sigma-Aldrich), and 1 mM phenylmethylsulfonyl fluoride (PMSF). All samples were incubated in lysis buffer for 15 min at 4°C and overnight at −20°C; the samples were subsequently vortexed for 15 min, and centrifuged at 10,000 RPM, 4°C for 30 min. The protein concentration for all samples was determined via a standard Bradford protein assay (reference no. 500006, Bio-Rad). 13 ug of total protein was mixed with NuPAGE™ LDS Sample Buffer (4X) (Thermo Fisher Scientific, cat: NP007) and NuPAGE™ Sample Reducing Agent (10X) (Thermo Fisher Scientific, cat: NP009). Then, the samples were heated to 85°C for 2 minutes. The protein samples were separated using a 10–20% Tris-glycine gel (Invitrogen, cat: XP10202BOX) and then transferred onto a nitrocellulose membrane (Thermo Fisher Scientific, cat: LC2001). The membrane was subsequently blocked with 5% BSA at room temperature for 1 hour. The antibodies were diluted in TBS-T (1X) with 2.5% BSA and incubated overnight at 4°C. The following antibodies and dilutions were used for this study: β-Actin 1:500 (Cell signaling technology, cat: 8h10d10) and CD63 1:200 (Abcam, cat: ab216130). Lastly, membrane visualization was done using a C-DiGitLI-CORblot scanner.
Gene expression analysis
Total RNA from HDF donor cells, EFF EVs, and control EVs were extracted using Trizol reagent (Thermoscientific cat: 15596018). DNase treatment was performed to remove genomic and plasmid DNA (Thermoscientific cat: 18068015). RNA concentration was measured using a Nanodrop 2000c Spectrophotometer (Thermo Fisher Scientific). Reverse transcription reactions were performed using superscript VILO cDNA synthesis kit (Termo Fisher) with 1000–2500 ng of RNA in 20 μL. ETV2, FLI1, and FOXC2 TaqMan primers were used to evaluate the level of expression via quantitative and absolute real time PCR. Real-time PCR reactions were performed with TaqMan fast advance chemistry (Thermo Scientifc) under the following conditions: 95 °C for 10 min, 40 cycles of 95 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min using a QuantStudio 3 Real-Time PCR System (Termo Fisher). Human GAPDH (TaqMan, Thermo Fisher. Cat: Hs02786624_g1) was used as a normalizing gene in all qRT-PCR reactions. For the absolute PCRs 10-fold serial dilution of each factor (ETV2, FLI1, and FOXC2) were used as standard curve. Table 1 lists all the primers used in this study.
The plasmid DNA content in the EVs was evaluated via conventional PCR. DNase treatment was performed before the DNA and RNA isolation. Specific primers that target the poly-linker of the engineered plasmid were used VP1.5-Forward (5`-GGACTTTCCAAAATGTCG-3`) and XL39-reverse (3ÀTTAGGACAAGGCTGGTGGG-5`) from OriGene. The following conditions were used to perform the PCRs 1 cycle at 95°C for 1 min, 30 cycles at 95°C for 30 sec, 55°C for 30 sec, and 72°C for 1 min, and 1 cycle at 72°C for 5 min. Products were analyzed by 0.8% agarose gel electrophoresis.
Analysis of EV uptake
Approximately 1.5×105 HDF cells were seeded and allowed to adhere on glass cover slips coated with gelatin (Fisherbrand™ cat: 1254580). EVs were stained with PKH26 Red Fluorescent Cell Linker (Sigma-Aldrich, cat: PKH26GL-1KT) following the procedure described by the manufacturer. Pellets of EFF or control EVs were resuspended in DME media with 4% of exosome depleted fetal bovine serum (GIBCO cat: A27208–01), and recipient cells were treated with 3.34×102EVs/cell for 6 and 12 hours.
In vitro reprogramming assays
EFF EVs were used to reprogram HDFs into iEC. For this end, approximately 1.5×105 HDF cells were seeded and allowed to adhere on glass cover slips coated with gelatin (Fisherbrand™ cat: 1254580). Pellets of EFF or control EVs were resuspended in DME media with 4% of exosome depleted fetal bovine serum (GIBCO cat: A27208–01). Recipient cells were treated with 1.04 ×104 EVs/cell for 48 hours. At 48 hours after EV treatment, the cells were maintained in culture using endothelial cell growth basal medium-2 (EBM; Lonza cat: CC-3156) for 1, 2, and 3 weeks.
Cryo-Electron Microscopy (CryoEM)
The size and morphology of individual EVs was evaluated via Cryo-Electron Microscopy (CryEM). CryoEM grids were frozen using Vitrobot Mark IV system (Thermo Fisher Scientific, Hillsboro). To make the carbon surface hydrophilic, the grids were pretreated using a Pelco EasiGlow system for 30s at 20mA. A small aliquot (3μl) of sample was then applied to the carbon side of the glow-discharged Lacey carbon grid. After blotting away excess liquid, the grid was immediately plunged into liquid ethane to rapidly form a thin film of amorphous ice. The Vitrobot was operated at 4°C and 100% humidity. The blotting force was set at +1. The blotting time was 4s. The frozen grids were clipped into AutoGrids and stored in liquid nitrogen. CryoEM images were captured using a Falcon 3EC direct electron detector under linear mode on a Thermo Scientific™ Glacios™ CryoTEM. The microscope was operated at an acceleration voltage of 200kV. The images were collected using EPU software at 57,000x or 6,700x nominal magnification.
Immunocytochemistry
Cells were fixed using 10% formalin for 15 minutes at room temperature, blocked with 5% normal goat serum and incubated with primary antibodies against the endothelial antibody markers VWF (rabbit, ab6994, 1/1000) and CD31 (mouse, ab9498, 1/200), followed by incubation with the secondary antibodies: Alexa 568-tagged α-rabbit (1:200), or Alexa 647-tagged α-mouse (1:200), respectively. Cell nuclei were counterstained with DAPI. Images were captured using a NikonTi2e microscope. Cells incubated without primary antibodies were used as a negative control for staining specificity. The fluorescence intensity was quantified using ImageJ/Fiji and normalized by the area of interest.
Matrigel plug assay
For this assay iECs were used at 14 days after EV treatment. A total of 1×106 iECs were resuspended in 500 uL of Matrigel (ThermoFisher, cat: #3632) and Endothelial Cell Basal Medium-2 (Lonza) without FBS. The mix was injected subcutaneously into the rear flank of 10-week-old male immunodeficient nude mice (NU/J) (Jackson Laboratory, Strain #002019). At 7 days post-implantation the Matrigel plugs, and surrounding tissues (including the skin above and muscle underneath the plugs) were collected, preserved in optimal cutting temperature (OCT) compound (Fisher Healthcare, cat: 4585), and stored at −80°C for subsequent analysis. The Matrigel plugs were sectioned to a thickness of 20 μm using a cryostat. All animal studies performed were approved by the Institutional Animal Care and Use Committee at The Ohio State University (protocol # 016A00000074-R2).
Characterization of transfection efficiency in vivo
C57BL/6 mice (10- to 16-weeks-old, female and male) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were depilated 24 hours before the procedure. The mice were anesthetized by continuous 1–3% isoflurane administration throughout the procedure. For in vivo bulk electroporation (BEP), 0.01 ug/uL of the EFF plasmid cocktail was injected intradermally using a U-100 insulin syringe (BD 329424). Then, the injected skin was gently clamped between two electrodes, and a pulse electric field was applied to facilitate electroporation and transfection of the plasmids (200V, 10 ms pulses, 10 pulses). For the EFF EVs, a saline solution containing 1 ×108 EVs/mL was injected intradermally using a U-100 insulin syringe. After 24 hours, a 10 mm skin biopsy was collected. Transfection efficiency was evaluated via qRT-PCRs. pCMV6 plasmid and control EVs were used for comparison purposes for BEP and EFF EVs, respectively. Additionally, uptake of the engineered EVs by skin cells in vivo was confirmed via fluorescence imaging at 24 hours after intradermal injection of fluorescently (red) labeled EFF or control EVs (1 ×108 EVs/mL) using a U-100 insulin syringe. All animal procedures performed in this study were approved by the Institutional Animal Care and Use Committee at The Ohio State University (protocol # 2016A00000074-R2).
Excisional wound healing assay
8-weeks-old male immunodeficient nude mice (NU/J) (Jackson Laboratory, Strain #002019) were used of this assay. Briefly, the dorsal skin of the mice was depilated 24 hours before the procedure. The mice were anesthetized by continuous 1–3% isoflurane administration throughout the duration of the procedure. The back skin was cleaned and sterilized with betaine and 70% ethanol. A sterile 6 mm biopsy punch was used to create a wound on the back skin of each animal. A 0.5 mm thick silicon sheet was placed around the wound, and it was secured with eight interrupted sutures of non-absorbable 6–0 Nylon (Henry Schein, cat: 1016053). After surgery, the mice were treated with EFF or control EVs at a final concentration of 1.8 ×108 EVs/mL in saline solution, and control mouse were treated with saline solution. Finally, a single dose of buprenorphine was administered subcutaneously to control pain. The animals were housed individually after surgery. Skin perfusion was assessed at 10 days after EV treatment, immediately before euthanasia using a high-resolution laser doppler imager (MOORLDI2-HIR, Moor Instruments). All animal procedures performed in this study were approved by the Institutional Animal Care and Use Committee at The Ohio State University (protocol # 2016A00000074-R2).
Immunohistochemistry
Skin biopsies were collected at 10 days post-EV treatment. For this end, 12 mm of skin biopsies were collected, preserved in optimal cutting temperature (OCT) compound (Fisher Healthcare, cat: 4585), and then stored at ~80 °C for subsequent analysis. Preserved skin samples were sectioned and processed for immunohistochemistry staining. Briefly, the samples were fixed with 10% formalin for 15 minutes at room temperature, blocked with 5% normal goat serum and incubated with primary antibodies against the endothelial markers VWF (rabbit, ab6994, 1/1000) and CD31 (mouse, ab9498, 1/200), Keratin 14 (Chicken, 1/200, Biolegend), and Cytokeratin 5 (rabbit, ab52635, 1/200) Skin biopsies collected 24 hours after EFF or control EV injection, were stained using the primary antibody for vimentin (rabbit, ab 92547,1/200) and Keratin 14 (Chicken, 1/200, Biolegend). followed by an incubation with the pertinent secondary antibodies: Alexa 568-tagged α-rabbit (1/200), Alexa 647-tagged α-mouse (1/200), or Alexa 488-tagged α-Chicken (1/200). Cell nuclei were counterstained with DAPI. Skin sections incubated without primary antibodies were used as negative control for staining specificity. All images were captured using a NikonTi2e microscope. For wound the wound healing assay, the fluorescence intensity was quantified in the wound bed using ImageJ/Fiji and all measurements were normalized by the area.
Statistical analysis
Statistical analyses were performed using SigmaPlot 12. Analysis of normality for all data sets were evaluated using a Shapiro-Wilk test. Normal data was evaluated using one tailed t-test, two tailed t-test, and one-way analysis of variance (ANOVA), while non-normally distributed data was analyzed via post hoc Dunńs non-parametric comparison. All data are reported as mean ± standard deviation (SD). The graphics were made using GraphPad Prism7.
Supplementary Material
ACKNOWLEDGEMENTS
All illustrations were created using BioRender. This work was partially funded by NIH award R01AR079485 to NHC, and NIH awards DP1DK126199 and DP2EB028110 to DGP.
Footnotes
DECLARATION OF INTEREST
The authors declare no conflict of interests.
REFERENCES
- 1.De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV & Verhaar MC Extracellular vesicles: potential roles in regenerative medicine. Front Immunol 5, 608 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abels ER & Breakefield XO Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol 36, 301–312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakarla R, Hur J, Kim YJ, Kim J & Chwae YJ Apoptotic cell-derived exosomes: messages from dying cells. Exp Mol Med 52, 1–6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C et al. Active cargo loading into extracellular vesicles: Highlights the heterogeneous encapsulation behaviour. J Extracell Vesicles 10, e12163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asare-Werehene M et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 39, 1600–1616 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer S et al. Indication of Horizontal DNA Gene Transfer by Extracellular Vesicles. PLoS One 11, e0163665 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apodaca LA et al. Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimers Res Ther 13, 57 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Monserrate Z et al. Delayed Processing of Secretin-Induced Pancreas Fluid Influences the Quality and Integrity of Proteins and Nucleic Acids. Pancreas 50, 17–28 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmanouilidou E et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30, 6838–6851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding H, Li LX, Harris PC, Yang J & Li X Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nat Commun 12, 4548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otero-Ortega L et al. Low dose of extracellular vesicles identified that promote recovery after ischemic stroke. Stem Cell Res Ther 11, 70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortega-Pineda L et al. Designer Extracellular Vesicles Modulate Pro-Neuronal Cell Responses and Improve Intracranial Retention. Adv Healthc Mater, e2100805 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte-Sanmiguel S, Higuita-Castro N & Gallego-Perez D Nanoelectroporation and Collection of Genetically Modified Exosomes in Primary Cultures of Dendritic Cells. Methods in molecular biology (Clifton, N.J.) 2050, 79–84 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Duarte-Sanmiguel S et al. In Situ Deployment of Engineered Extracellular Vesicles into the Tumor Niche via Myeloid-Derived Suppressor Cells. Adv Healthc Mater, e2101619 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang S et al. Non-viral reprogramming of human nucleus pulposus cells with FOXF1 via extracellular vesicle delivery: an in vitro and in vivo study. Eur Cell Mater 41, 90–107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang TT, Lv LL, Lan HY & Liu BC Extracellular Vesicles: Opportunities and Challenges for the Treatment of Renal Diseases. Front Physiol 10, 226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J Control Release 327, 688–702 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Gallego-Perez D et al. Topical tissue nano-transfection mediates non-viral stroma reprogramming and rescue. Nat Nanotechnol 12, 974–979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemmerman LR et al. Nanotransfection-based vasculogenic cell reprogramming drives functional recovery in a mouse model of ischemic stroke. Science Advances 7, eabd4735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou Y, Li J, Guan S & Witte F The therapeutic potential of MSC-EVs as a bioactive material for wound healing. Engineered Regeneration 2, 182–194 (2021). [Google Scholar]
- 21.Frueh FS, Menger MD, Lindenblatt N, Giovanoli P & Laschke MW Current and emerging vascularization strategies in skin tissue engineering. Crit Rev Biotechnol 37, 613–625 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Atashgah RB et al. Restoring Endogenous Repair Mechanisms to Heal Chronic Wounds with a Multifunctional Wound Dressing. Mol Pharm 18, 3171–3180 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Weber A et al. Magnetic resonance mapping of transplanted endothelial progenitor cells for therapeutic neovascularization in ischemic heart disease. Eur J Cardiothorac Surg 26, 137–143 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto K et al. Molecular evaluation of endothelial progenitor cells in patients with ischemic limbs: therapeutic effect by stem cell transplantation. Arterioscler Thromb Vasc Biol 24, e192–196 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Starokozheva L et al. Early Intervention in Ischemic Tissue with Oxygen Nanocarriers Enables Successful Implementation of Restorative Cell Therapies. Cell Mol Bioeng 13, 435–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore JT et al. Nanochannel-Based Poration Drives Benign and Effective Nonviral Gene Delivery to Peripheral Nerve Tissue. Adv Biosyst 4, e2000157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dikici S, Claeyssens F & MacNeil S Pre-Seeding of Simple Electrospun Scaffolds with a Combination of Endothelial Cells and Fibroblasts Strongly Promotes Angiogenesis. Tissue Eng Regen Med 17, 445–458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita R et al. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U S A 112, 160–165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olgasi C et al. Patient-Specific iPSC-Derived Endothelial Cells Provide Long-Term Phenotypic Correction of Hemophilia A. Stem cell reports 11, 1391–1406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh W et al. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem cells 23, 1571–1578 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki H et al. A novel strategy to engineer pre-vascularized 3-dimensional skin substitutes to achieve efficient, functional engraftment. Sci Rep 9, 7797 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholas MN, Jeschke MG & Amini-Nik S Methodologies in creating skin substitutes. Cell Mol Life Sci 73, 3453–3472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomatto M et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int J Mol Sci 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han JK et al. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation 130, 1168–1178 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Kim JE, Johnson BA, Andukuri A & Yoon YS Direct reprogramming into endothelial cells: a new source for vascular regeneration. Regen Med 12, 317–320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan G et al. Engineering vascularized skeletal muscle tissue with transcriptional factor ETV2-induced autologous endothelial cells. Protein Cell 10, 217–222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SY et al. Etv2- and Fli1-Induced Vascular Progenitor Cells Enhance Functional Recovery in Ischemic Vascular Disease Model-Brief Report. Arterioscler Thromb Vasc Biol 40, e105–e113 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Gong W et al. ETV2 functions as a pioneer factor to regulate and reprogram the endothelial lineage. Nat Cell Biol 24, 672–684 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Lammerts van Bueren K & Black BL Regulation of endothelial and hematopoietic development by the ETS transcription factor Etv2. Curr Opin Hematol 19, 199–205 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Liu F et al. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep 16, 654–669 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh BN et al. ETV2 (Ets Variant Transcription Factor 2)-Rhoj Cascade Regulates Endothelial Progenitor Cell Migration During Embryogenesis. Arterioscler Thromb Vasc Biol 40, 2875–2890 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Koyano-Nakagawa N et al. Feedback Mechanisms Regulate Ets Variant 2 (Etv2) Gene Expression and Hematoendothelial Lineages. J Biol Chem 290, 28107–28119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abedin MJ et al. Fli1 acts downstream of Etv2 to govern cell survival and vascular homeostasis via positive autoregulation. Circ Res 114, 1690–1699 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kataoka H et al. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRalpha+ primitive mesoderm. Blood 118, 6975–6986 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Sumanas S & Choi K ETS Transcription Factor ETV2/ER71/Etsrp in Hematopoietic and Vascular Development. Curr Top Dev Biol 118, 77–111 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Kume T Foxc2 transcription factor: a newly described regulator of angiogenesis. Trends Cardiovasc Med 18, 224–228 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kano MR et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci 118, 3759–3768 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Seghezzi G et al. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol 141, 1659–1673 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murakami M et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest 118, 3355–3366 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrett JP, Lowery AM, Adam AP, Kowalczyk AP & Vincent PA Regulation of endothelial barrier function by p120-cateninVE-cadherin interaction. Mol Biol Cell 28, 85–97 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam H, Sehgal L, Kundu ST, Dalal SN & Vaidya MM Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol Biol Cell 22, 4068–4078 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nafissi N et al. DNA ministrings: highly safe and effective gene delivery vectors. Mol Ther Nucleic Acids 3, e165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orefice NS Development of New Strategies Using Extracellular Vesicles Loaded with Exogenous Nucleic Acid. Pharmaceutics 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costa Verdera H, Gitz-Francois JJ, Schiffelers RM & Vader P Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release 266, 100–108 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Nakase I, Kobayashi NB, Takatani-Nakase T & Yoshida T Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep 5, 10300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy DE et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med 51, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonsergent E et al. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat Commun 12, 1864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrmann IK, Wood MJA & Fuhrmann G Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol 16, 748–759 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Zhang B et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta-catenin pathway. Stem Cells Transl Med 4, 513–522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He C et al. Comparison of two cell-free therapeutics derived from adipose tissue: small extracellular vesicles versus conditioned medium. Stem Cell Res Ther 13, 86 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xin H et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33, 1711–1715 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sweeney M & Foldes G It Takes Two: Endothelial-Perivascular Cell Cross-Talk in Vascular Development and Disease. Front Cardiovasc Med 5, 154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Randi AM, Smith KE & Castaman G von Willebrand factor regulation of blood vessel formation. Blood 132, 132–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batista Napotnik T, Polajzer T & Miklavcic D Cell death due to electroporation - A review. Bioelectrochemistry 141, 107871 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Blackstone BN et al. Fractional CO2 laser micropatterning of cell-seeded electrospun collagen scaffolds enables rete ridge formation in 3D engineered skin. Acta Biomater 102, 287–297 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Koritzinsky EH, Street JM, Star RA & Yuen PS Quantification of Exosomes. J Cell Physiol 232, 1587–1590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


