Abstract
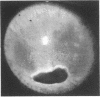
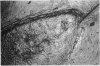
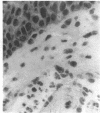
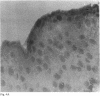
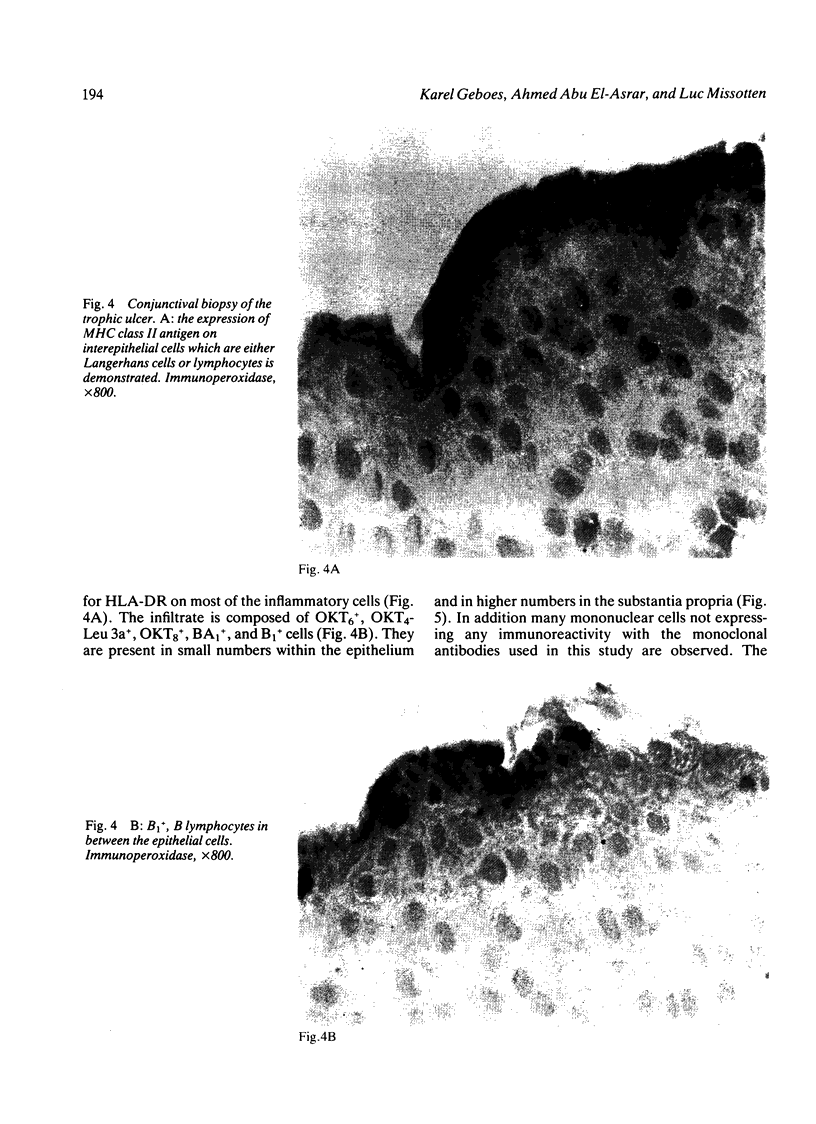
The corneal surface was examined by means of replica histology, and the excised limbic conjunctiva was examined by routine histological and immunohistochemical methods with monoclonal antibodies directed against major histocompatibility class II antigens, lymphocyte subsets, Langerhans cells (HLA-DR, OKT4-Leu3a, OKT8, BA1, B1, and OKT6) and immunoglobulins A, G, M, and D. The findings were compared with those found in normal conjunctiva. No inflammatory cells were present in the replica of the corneal surface. An inflammatory infiltrate composed of B lymphocytes and null cells, in addition to T lymphocytes, Langerhans cells, and polymorphs, was present in the epithelium as well as in the stroma of the limbic conjunctiva. The composition of the infiltrate points towards the involvement of cell mediated immunity as well as humoral immunity. No immunoglobulins were bound to the conjunctival epithelium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson C. S., Kersey J. H., LeBien T. W. A monoclonal antibody (BA-1) reactive with cells of human B lymphocyte lineage. J Immunol. 1981 Jan;126(1):83–88. [PubMed] [Google Scholar]
- Berman M. B., Kerza-Kwiatecki A. P., Davison P. F. Characterization of human corneal collagenase. Exp Eye Res. 1973 Mar;15(3):367–373. doi: 10.1016/0014-4835(73)90152-8. [DOI] [PubMed] [Google Scholar]
- Binder P. S. Herpes simplex keratitis. Surv Ophthalmol. 1977 Jan-Feb;21(4):313–331. doi: 10.1016/0039-6257(77)90113-8. [DOI] [PubMed] [Google Scholar]
- Brown S. I. Mooren's ulcer. Histopathology and proteolytic enzymes of adjacent conjunctiva. Br J Ophthalmol. 1975 Nov;59(11):670–674. doi: 10.1136/bjo.59.11.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. I. What is Mooren's ulcer? Trans Ophthalmol Soc U K. 1978 Sep;98(3):390–392. [PubMed] [Google Scholar]
- Dawson C. R., Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol. 1976 Sep-Oct;21(2):121–135. doi: 10.1016/0039-6257(76)90090-4. [DOI] [PubMed] [Google Scholar]
- Fithian E., Kung P., Goldstein G., Rubenfeld M., Fenoglio C., Edelson R. Reactivity of Langerhans cells with hybridoma antibody. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2541–2544. doi: 10.1073/pnas.78.4.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster C. S., Kenyon K. R., Greiner J., Greineder D. K., Friedland B., Allansmith M. R. The immunopathology of Mooren's ulcer. Am J Ophthalmol. 1979 Aug;88(2):149–159. doi: 10.1016/0002-9394(79)90459-8. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Habu S., Akamatsu K., Tamaoki N., Okumura K. In vivo significance of NK cell on resistance against virus (HSV-1) infections in mice. J Immunol. 1984 Nov;133(5):2743–2747. [PubMed] [Google Scholar]
- KAUFMAN H. E. EPITHELIAL EROSION SYNDROME: METAHERPETIC KERATITIS. Am J Ophthalmol. 1964 Jun;57:983–987. doi: 10.1016/0002-9394(64)91045-1. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missotten L., Maudgal P. C. The replica technique used to study superficial corneal epithelium in vivo. Am J Ophthalmol. 1977 Jul;84(1):104–111. doi: 10.1016/0002-9394(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Mondino B. J., Brown S. I., Rabin B. S. Autoimmune phenomena of the external eye. Ophthalmology. 1978 Aug;85(8):801–817. doi: 10.1016/s0161-6420(78)35618-9. [DOI] [PubMed] [Google Scholar]
- Murray P. I., Rahi A. H. Pathogenesis of Mooren's ulcer: some new concepts. Br J Ophthalmol. 1984 Mar;68(3):182–187. doi: 10.1136/bjo.68.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler L. M., Ritz J., Hardy R., Pesando J. M., Schlossman S. F., Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981 Jan;67(1):134–140. doi: 10.1172/JCI110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Further characterization of the human inducer T cell subset defined by monoclonal antibody. J Immunol. 1979 Dec;123(6):2894–2896. [PubMed] [Google Scholar]
- Saksela E., Timonen T., Ranki A., Häyry P. Morphological and functional characterization of isolated effector cells responsible for human natural killer activity to fetal fibroblasts and to cultured cell line targets. Immunol Rev. 1979;44:71–123. doi: 10.1111/j.1600-065x.1979.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Shore B., Leopold I. H., Henley W. L. Cellular immunity in chronic ophthalmic disorders. 2. Leukocyte migration inhibition in diseases of the cornea. Am J Ophthalmol. 1972 Jan;73(1):62–67. doi: 10.1016/0002-9394(72)90306-6. [DOI] [PubMed] [Google Scholar]
- Thorsby E., Berle E., Nousiainen H. HLA-D region molecules restrict proliferative T cell responses to antigen. Immunol Rev. 1982;66:39–56. doi: 10.1111/j.1600-065x.1982.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Wildy P., Gell P. G. The host response to herpes simplex virus. Br Med Bull. 1985 Jan;41(1):86–91. doi: 10.1093/oxfordjournals.bmb.a072032. [DOI] [PubMed] [Google Scholar]
- van den Oord J. J., de Wolf-Peeters C., Vanstapel M. J., Desmet V. J. Improved immunohistochemical visualization of helper/inducer T-cells by the simultaneous application of two noncrossblocking monoclonal antibodies. Stain Technol. 1985 Jan;60(1):45–49. doi: 10.3109/10520298509113890. [DOI] [PubMed] [Google Scholar]