Abstract
The STP oncoproteins of the herpesvirus saimiri (HVS) subgroup A strain 11 and subgroup C strain 488 are now found to be stably associated with tumor necrosis factor receptor-associated factor (TRAF) 1, 2, or 3. Mutational analyses identified residues of PXQXT/S in STP-A11 as critical for TRAF association. In addition, a somewhat divergent region of STP-C488 is critical for TRAF association. Mutational analysis also revealed that STP-C488 induced NF-κB activation that was correlated with its ability to associate with TRAFs. The HVS STP-C488 P10→R mutant was deficient in human T-lymphocyte transformation to interleukin-2-independent growth but showed wild-type phenotype for marmoset T-lymphocyte transformation in vitro and in vivo. The STP-C488 P10→R mutant was also defective in Rat-1 fibroblast transformation, and fibroblast cell transformation was blocked by a TRAF2 dominant-negative mutant. These data implicate TRAFs in STP-C488-mediated transformation of human lymphocytes and rodent fibroblasts. Other factors are implicated in immortalization of common marmoset T lymphocytes and may also be critical in the transformation of human lymphocytes and rodent fibroblasts.
Members of the tumor necrosis factor (TNF) receptor (TNFR) superfamily are important for lymphoid organ development, lymphocyte activation, acute-phase responses, cell growth, and apoptosis (3, 22, 49). The TNFR superfamily includes TNFR1, TNFR2, CD27, CD30, CD40, Fas (CD95), 4-1BB, and OX40 (45). The cytoplasmic regions of TNFR1, TNFR2, CD30, or CD40 are required for receptor-mediated signaling and interact with TNF receptor-associated factors (TRAFs) (8, 23, 43, 48). TRAF2 and TRAF3 have an amino terminal RING finger structure, and all TRAFs have more than one zinc finger. TRAFs also have a predicted extended alpha-helical coiled-coiled hydrophobic heptad repeat domain (8). A carboxyl-terminal TRAF domain of approximately 200 amino acids can be further divided into the TRAF-N and TRAF-C subdomains. The highly conserved carboxyl-terminal TRAF-C domain mediates interaction with TNFRs (48). The cytoplasmic region of LMP1, which is a key effector of Epstein-Barr virus (EBV)-mediated transformation, also binds to TRAFs, and this interaction is essential for B lymphocyte growth transformation (24, 38). Human and simian LMP1, CD40, and CD30 and a cytoplasmic TRAF-interacting protein, TANK, share a PXQXT/S core sequence through which they interact with TRAFs (8, 20, 21). TRAF2 is a mediator of NF-κB and Jun kinase activation from the TNFRs and LMP1 (13, 35, 46, 54).
Herpesvirus saimiri (HVS), a gamma-2 herpesvirus or rhadinovirus, infects most squirrel monkeys without causing apparent disease (11, 17). In other nonhuman primates, however, HVS induces rapidly fatal T-cell lymphoproliferative diseases (19, 27). Sequence divergence among HVS isolates is most extensive at the left end of the viral genomic DNA and is the basis for the classification of HVS into subgroups A, B, and C (9, 36). Sequence variation in the STP gene in this region correlates with different capacities for immortalizing T lymphocytes in vitro and for inducing lymphoma in nonhuman primates (5, 9, 12, 32). Subgroup A and subgroup C viruses can immortalize common marmoset T lymphocytes to interleukin-2 (IL-2)-independent proliferation (12, 47). Highly oncogenic subgroup C strains are also able to immortalize human, rabbit, and rhesus monkey lymphocytes and induce fulminant lymphoma in rhesus monkeys (2, 4, 6, 37).
The STPs of subgroup A or C strains (STP-A or STP-C) can transform rodent fibroblast cells in vitro. STP-C is considerably more potent (28, 30). STP-C488 associates with cellular Ras in transformed cells (25). Mutations that disrupt Ras association disrupt the transforming ability of the STP-C488 oncogene (25). In contrast, STP-A binds to the SH2 domain of Src kinase and is phosphorylated by the associated Src kinase (34). Transgenic mice expressing STP-C488 developed invasive epithelial cell tumors (39), while STP-A11 transgenic mice developed peripheral pleomorphic T-cell lymphomas (33). Deletion of STP from the group C strain 488 or from the group A strain 11 yields viruses that are no longer capable of immortalizing lymphocytes in vitro or of inducing fatal lymphomas in common marmosets (9, 10, 12, 14, 32, 40). Since HVS lacking STP can be repeatedly isolated from the peripheral blood of common marmosets for months or years, STP is not required for viral replication or persistence in vivo, but it is essential for transformation in cell culture and for lymphoma induction in common marmosets (9, 14).
STP-A11 and STP-C488 are similar in genome location and orientation and have limited sequence similarity (5, 28). Both open reading frames encode a highly acidic amino terminus. STP-C488 has 18 direct repeats of a collagen-like motif (Gly-Pro-Pro or Gly-Pro-Gln) that comprises more than 50% of the protein and is predicted to have an alpha-helical triple structure (5). A mutation which disrupts the collagen repeats has been shown to disrupt the transforming activity of STP-C488 (26). STP-A11 is also glycine and proline rich. STP-A11 and STP-C488 proteins have highly hydrophobic carboxyl termini which are sufficient for membrane interaction (26). Since STP-C likely oligomerizes through its collagen-like motif and associates with cellular membranes, STP-C may mimic a ligand-independent constitutively active receptor, like the EBV LMP1 protein. STP-A could have similar properties.
In this report, we investigate this hypothesis and find that STP-A and STP-C associate with TRAF 1, 2, or 3. Furthermore, the STP-C488 TRAF binding site is required for NF-κB activation and cell growth transformation.
MATERIALS AND METHODS
Cell culture and virus propagation.
Rat-1, COS-1, BOSC23, and owl monkey kidney (OMK 1637) cells cultivated in Dulbecco’s modified Eagle’s medium or minimal essential medium supplemented with penicillin, streptomycin, l-glutamine, and 10% (vol/vol) heat-inactivated fetal bovine serum (GIBCO BRL, Grand Island, N.Y.) were used for the propagation of the HVS strain C488. Low-passage-number OMK cells (<30 passages) were used for the transfections. Primary human and common marmoset (Callithrix jacchus) peripheral blood mononuclear cells (PBMCs) were purified by using lymphocyte separation medium (Organon Teknika Corp., Malvern, Pa.). Cultures of human and common marmoset PBMCs in immortalization assays with HVS recombinants were performed in RPMI 1640 medium supplemented with penicillin, streptomycin, Fungizone, l-glutamine, 20% (vol/vol) heat-inactivated fetal bovine serum, and 5 mg of β-mercaptoethanol per liter. A DEAE or calcium phosphate transfection was used for transient expression in COS-1 or BOSC23 cells, respectively. Rat-1 cells were transfected with plasmid pcDNA3-TRAF2 Δ6–86 by the calcium phosphate protocol, followed by the selection with 500 μg of G418 per ml. Subsequently, these cells were transfected with pBabe-puro or pBabe-STP-C488 and selected with 5 μg of puromycin/ml.
Plasmid constructions.
All STP-C488 mutants have been described previously (26). Mutations in STP-A11 were generated by PCR by using oligonucleotide-directed mutagenesis (16). Oligonucleotide mutant primers from complementary strands of STP-A11 were synthesized with specific restriction enzyme sites within 5′ and 3′ primers to facilitate cloning into pBluescript KS(+) (Stratagene, San Diego, Calif.). PCR was carried out with a DNA thermal cycler (Perkin-Elmer Cetus Instruments, Norwalk, Conn.) under the following conditions: 30 cycles of 1 min at 55°C for annealing, 4 min at 72°C for polymerization, and 1 min at 94°C for denaturation. The amplified DNA fragments containing mutations in STP-A11 were purified and cloned into the pBluescript KS(+) vector. Each STP-A11 mutant was completely sequenced to verify the presence of the mutation and the absence of any other changes. After confirmation of the DNA sequence, DNA containing the desired STP-A11 mutation was recloned into EcoRI and BglII cloning sites of the pFJ vector for gene expression. TRAF2 Δ6–86 and flag-tagged TRAF1, TRAF2 and TRAF3 have been described previously (31). Flag-tagged TRAF3-C and TRAF3 ΔC were constructed by PCR by using oligonucleotide-directed mutagenesis and completely sequenced to verify the presence of the mutation and the absence of any other changes.
Virion DNA isolation.
HVS virion preparations were obtained from the virus-containing medium by removal of OMK cell debris by low-speed centrifugation, followed by pelleting of the virus at 18,000 rpm for 2 h in an SS-34 rotor. To purify intact virion DNA, the virus was disrupted at 60°C for 2 h in lysis buffer containing 10 mM Tris (pH 8.5), 1 mM EDTA, 1% (vol/vol) Sarkosyl, and 0.1 mg of proteinase K/ml. Extraction of the aqueous solution, first with an equal volume of phenol and then twice with chloroform, was sufficient to purify the virion DNA for use in transfections. Sterile cut pipette tips were used for manipulating virion DNA without shearing.
Construction of recombinant HVS.
Generation of the P10→R mutation in STP-C488 by oligonucleotide-directed mutagenesis has been described previously (26). A 3.6-kb clone, pNEB-C488-PX, containing the tyrosine kinase-interacting protein tip, STP-C488, and herpesvirus saimiri U RNAs (HSURs) (5, 15), was used to provide a subcloning vector with flanking sequence adequate to facilitate recombination during cotransfection. Digestion of this vector with EcoRV and SpeI permitted insertion of the corresponding STP fragment containing the P10→R mutation.
Linearized plasmid DNA containing the 3.6-kb viral DNA with the STP-C488/P10→R mutation was cotransfected into OMK cells with HVSΔSTP/SV40-SEAP virion DNA by the calcium phosphate protocol. A pure form of recombinant virus with the secreted engineered alkaline phosphatase (SEAP) reporter replaced with STP-C488/P10→R was isolated by limiting dilution and repeated selection of SEAP-negative virus onto OMK cell monolayers in 48-well tissue culture plates performed as described previously (15). SEAP production was detected by a liquid scintillation counter; the chemiluminescence produced in cell culture medium was assayed by using Phospha-Light reagents (Tropix Inc., Bedford, Mass.) according to the manufacturer’s recommendations.
In vitro immortalization of human and common marmoset lymphocytes.
Assays of lymphocyte immortalization in vitro have been described previously (14). PBMCs were isolated from heparinized blood specimens from human donors and common marmosets (C. jacchus) by centrifugation through lymphocyte separation medium (Organon Teknika Corp.) followed by washing in RPMI culture medium. PBMCs from each donor were individually washed, resuspended in RPMI, and then distributed in 1-ml volumes containing approximately 106 cells into 12-well tissue culture plates. A single well containing PBMCs from each donor was then infected at a multiplicity of infection ranging from 1 to 5 with 1 ml of fresh, purified HVS viral stocks. Cells were maintained with RPMI 1640 growth medium which was changed every 3 to 4 days. Immortalization or cell death was assessed microscopically.
Experimental infection of common marmosets.
The in vivo oncogenicity of the HVS-C488 recombinants was assessed by experimental infection of common marmosets. Marmosets were injected intramuscularly with 105 50% tissue culture infective doses of virus in a volume of 1 ml. Sera and blood cell pellets were collected and frozen at −70°C weekly during the first 4 weeks and every 2 weeks thereafter. Viral loads in PBMC specimens were assessed periodically by duplicate plating of 106 PBMCs and serial threefold dilutions of PBMCs on OMK cells in 24-well tissue culture plates (14). Animals that became moribund were euthanized and received complete necropsies. Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Immunoprecipitation, immunoblotting, and antibodies.
Cells were harvested and lysed with lysis buffer (0.15 M NaCl, 0.5% Nonidet P-40, and 50 mM HEPES buffer [pH 8.0]) containing 1 mM Na2VO3, 1 mM NaF, and protease inhibitors (leupeptin, aprotinin, phenylmethylsulfonyl fluoride, pepstatin, and bestatin). Precleared lysates were used for immunoprecipitation or immunoblot analysis. The rabbit polyclonal antibody 109 and mouse monoclonal antibody A1.4 directed against STP-C488 used in these experiments have been described previously (28). Flag and AU-1 antibodies were purchased from KODAK IBI (New Haven, Conn.) and BABCO Biotech (Berkeley, Calif.), respectively.
Isolation of genomic DNA and PCR analysis.
Genomic DNA was isolated with a Qiagene genomic isolation kit according to the manufacturer’s protocol. Five micrograms of purified genomic DNA was used for PCR amplification performed by using a 5′ primer which corresponds to the upstream sequence of the STP-C488 gene and a 3′ primer which corresponds to the downstream sequence of STP-C488. Amplified DNA was cloned into the TA cloning vector (Invitrogen, San Diego, Calif.). Both strands from each of five independent clones were subsequently sequenced using an ABI PRISM 377 automatic DNA sequencer.
Reporter assays.
All transfections included pGKβgal, which expresses β-galactosidase from a phosphoglucokinase promoter, together with 3X-κB-luc, which has three copies of the NF-κB binding site from the murine major histocompatibility complex class I promoter upstream of a minimal fos promoter and a luciferase gene. At 48 h posttransfection, cells were washed once in phosphate-buffered saline and lysed in 200 μl of reporter lysis buffer (Promega, Madison, Wis.). Assays for luciferase were performed with a Luminometer by using a luciferase assay (Promega). Values were normalized to β-galactosidase activity.
RESULTS
HVS subgroup A and subgroup C STPs interact with TRAFs.
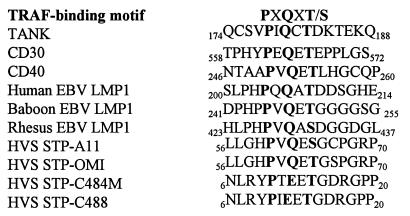
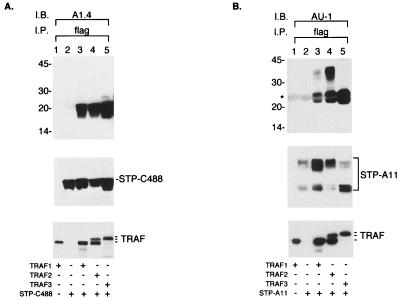
HVS STP-A has a PXQXT sequence similar to that in LMP1, CD30, CD40 and TANK (7, 20, 21), while STP-C488 and STP-C484M have more divergent sequences with glutamic acid in place of glutamine (Fig. 1). The potential interaction of STP with TRAFs was therefore investigated by transfecting BOSC23 cells with expression vectors for STP-A11 or STP-C488 and for TRAF1, TRAF2, or TRAF3. The amino termini of the TRAFs and STP-A11 were tagged with Flag and AU-1 epitopes, respectively. After transfection, TRAF complexes were precipitated with an anti-Flag antibody and STP-A11 or STP-C488 was detected by immunoblotting. STP-C488 was readily evident at 20 kDa in the TRAF 1, 2, or 3 precipitates (Fig. 2A). STP-A11 was expressed as 26- and 35-kDa proteins (34). STP-A11 and STP-C488 were not detected in precipitates from negative control cell lysates (Fig. 2A and B, lanes 2). These tests demonstrate that STP-A11 and STP-C488 interacted specifically with TRAFs in BOSC23 cells.
FIG. 1.
Sequence comparison of the amino-terminal regions of STPs with TRAF binding motifs. The sequences of the amino-terminal regions of STPs from HVS subgroup A and C strains were aligned with TRAF-binding motifs of LMP1, CD30, CD40, and TANK. STP-A11 and STP-OMI belong to subgroup A, and STP-C484M and STP-C488 belong to subgroup C. Bold letters indicate the conserved amino acid sequence.
FIG. 2.
Interaction of STP with TRAF. BOSC23 cells were transfected with STP-A11 or STP-C488 expression vector together with TRAF1, TRAF2, or TRAF3 expression vector as shown at the bottom of the figure. After 48 h, cell extracts were used for immunoprecipitation (I.P.) with Flag antibody (flag). The upper panels show Flag immune complexes that were subjected to immunoblot assay (I.B.) with A1.4 antibody to detect STP-C488 (A) or AU-1 antibody to detect STP-A11 (B). The expression levels of TRAFs (bottom panel) and STP-A11 or STP-C488 (middle panel) in BOSC23 cells were evaluated by immunoblotting with the specific antibodies, and the results are shown in the bottom two panels of the figure. The asterisk indicates the light chains of immunoglobulin.
Different regions of TRAF are required for binding to STP-A and STP-C.
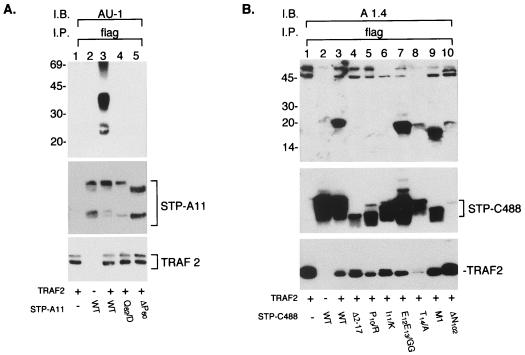
To confirm that TRAFs bind through their TRAF-C domain to the STP-A11 or STP-C488, we evaluated the association of STP-A11 or STP-C488 with TRAF3-C, which contains the TRAF-C domain only, or with TRAF3 ΔC, from which the TRAF-C domain has been deleted. Flag-tagged TRAF3, TRAF3 ΔC, or TRAF3-C was expressed in COS-1 cells along with STP-A11 or STP-C488. Anti-Flag antibody was used to precipitate TRAF complexes from cell lysates. TRAF3-C bound to STP-A11 as efficiently as wild-type (wt) TRAF3, whereas the TRAF3 ΔC did not bind to STP-A11 (Fig. 3A). In contrast, both TRAF3-C and TRAF3 ΔC failed to bind to STP-C488, while wt TRAF3 bound efficiently to STP-C488. Under these conditions, similar amounts of TRAF3, TRAF3-C, and TRAF3 ΔC and of STP-A11 and STP-C488 were expressed in COS-1 cells (Fig. 3). These results demonstrate that the TRAF-C domain is necessary and sufficient for its binding to STP-A11, as it is for its binding to CD40 or LMP1, while a domain(s) in addition to the TRAF-C domain is necessary for binding to STP-C488.
FIG. 3.
Different regions of TRAF are required for binding to STP-A11 and STP-C488. COS-1 cells were transfected with TRAF3 (lanes 1, 2, and 3), TRAF3 ΔC (lanes 4, 5, and 6), or TRAF3-C (lanes 7, 8, and 9) together with STP-A11 (lanes 2, 5, and 8) or STP-C488 (lanes 3, 6, and 9) expression vector. After 48 h, cell lysates were used for immunoprecipitation with Flag antibody (flag) (A and B). The immune complex was subjected to immunoblot assay (I.B.) with AU-1 antibody to detect STP-A11 (A) or A1.4 antibody to detect STP-C488 (B). The expression levels of TRAF (C), STP-A11 (D), and STP-C488 (E) were demonstrated by immunoblotting of cell lysates with specific antibodies. Asterisks indicate the heavy and light chains of immunoglobulin, and arrows indicate each protein as labelled.
Mutational analysis of the putative STP-A11 and STP-C488 TRAF binding motifs.
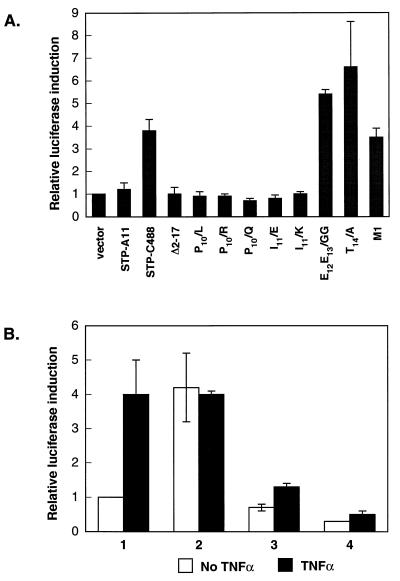
To investigate whether the putative STP TRAF binding motif is required for TRAF association, two point mutations in the putative TRAF binding motif in STP-A11 were generated by site-directed mutagenesis; codon 60 was deleted from one mutant (STP-A11 ΔP60) and glutamine encoded by codon 62 was replaced by alanine in a second mutant (STP-A11 Q62/A). Seven STP-C488 mutants (P10/R, I11/K, E12E13/GG, T14/A, M1, ΔN102, and Δ2–17) described previously (25, 26) were also evaluated for the TRAF binding activity. P10/R, I11/K, E12E13/GG, and T14/A are point mutations in the putative TRAF binding motif. Δ2–17 is deleted for amino-terminal residues 2 to 17, M1 has a three-amino-acid insertion in the collagen repeats, and ΔN102 is deleted for the carboxyl-terminal amino acid (25, 26). Δ2–17 and M1 mutants have been shown to be defective in Rat-1 transformation, whereas ΔN102 mutant shows wt Rat-1 transformation (25, 26).
After cotransfection of the STP genes and the TRAF2 genes into BOSC23 cells, an anti-Flag antibody was used to precipitate TRAF complexes. The ΔP60 and Q62/A mutations in the STP-A11 PXQXT motif abolished complex formation with TRAF2 (Fig. 4A). Under these conditions, similar amounts of STP-A11 and its mutants were expressed. The STP-C488 P10/R, I11/K, and Δ2–17 mutations also abrogated TRAF2 binding activity, whereas the E12E13/GG, T14/A, M1, and ΔN102 mutations did not (Fig. 4B). Despite weak expression, the ΔN102 mutant was readily detected in TRAF2 complexes (Fig. 4B). The STP-C488 mutants migrated somewhat anomalously (Fig. 4B) as described previously (26). These results indicate that the STP-A11 putative TRAF binding motif is important for TRAF association. Furthermore, the STP-C488 putative TRAF binding motif appears to be part of the TRAF binding domain. However, only the amino-terminal P10 and I11 residues of the core site appear to be critical for TRAF.
FIG. 4.
Mutational analysis of STP-A11 and STP-C488 for binding to TRAF. BOSC23 cells were transfected with STP-A11 mutants (A) or STP-C488 mutants (B) together with TRAF2 expression vector as indicated at the bottom of the figure. Cell lysates were used for immunoprecipitation (I.P.) with Flag antibody (flag), followed by an immunoblot assay (I.B.) with AU-1 antibody to detect STP-A11 (top panel of A) or A1.4 antibody to detect STP-C488 (top panel of B). The levels of STPs and TRAF2 expression were demonstrated by immunoblotting with their specific antibodies as indicated at the bottom of the figure.
STP-C488 activates NF-κB activity.
Since TRAF2 can mediate NF-κB activation by EBV LMP1 or TNF receptors (24, 38, 43), we have investigated the effect of STP-C488 expression on NF-κB activation in 293 cells by using an NF-κB-driven luciferase reporter plasmid, 3X-κB-L, and a control β-galactosidase expression plasmid, pGKβgal. Relative luciferase values were normalized to β-galactosidase activity for transfection efficiency. Three independent assays showed that wt STP-C488 increased NF-κB activity approximately 3.5- to 6-fold. The STP-C488 E12E13/GG, T14/A, and M1 mutants were similar to wt STP-C488, whereas no activation was induced by the STP-C488 P10/R, P10/Q, P10/L, I11/E, I11/K, or Δ2–17 mutants (Fig. 5A). Luciferase activity required a wt NF-κB element (data not shown). These results indicate that STP-C488 can activate NF-κB activity and that this activity is dependent upon the STP-C488 amino terminus, including P10 and I11, which are critical for TRAF association. In striking contrast, STP-A11 did not induce NF-κB activation under the same conditions (Fig. 5A).
FIG. 5.
Activation of NF-κB activity by STP. (A) Activation of NF-κB activity by STP. 293 cells were transfected with STP-A11, STP-C488, or mutant STP-C488. (B) Suppression of NF-κB activation by the dominant-negative TRAF2 Δ6–86 mutant. Columns 1, Rat-babe; columns 2, Rat-STP-C488; columns 3, Rat-TRAF2 Δ6–86; and columns 4, Rat-STP-C488/TRAF2 Δ6–86. Cells were transfected with an NF-κB-driven luciferase reporter 3XκB plasmid together with the pGKβgal plasmid, which controlled for transfection efficiency. Forty-eight hours after transfection, cell lysates were used for luciferase and β-galactosidase assays. Where indicated, Rat-1 cells were treated with 10 ng of mouse TNF-α for 16 h. Luciferase activity was determined and normalized on the basis of β-galactosidase activities. The fold activation expresses the normalized luciferase activity relative to that of the cells transfected with vector alone (panel A) or to that of Rat-babe cells (panel B). Values are the averages of three independent experiments.
Generation of HVS STP-C488 P10→R recombinant.
To investigate the importance of the TRAF-binding activity of STP in virus-induced transformation, we constructed a recombinant HVS C488 with the STP-C488 P10→R mutation, which disrupts TRAF association and NF-κB activation. The parental virion DNA from which the mutant was constructed had a SEAP reporter expression cassette in place of the STP-C488 gene and is nontransforming in culture and nononcogenic in common marmosets (14). After cotransfection with the parental virion DNA and a linearized plasmid containing the P10→R mutation in STP-C488 in the context of a flanking sequence homologous to the parental DNA, SEAP-negative virus was isolated by limiting dilution. PCR and sequence analysis confirmed the presence of STP-C488 P10→R in the putative recombinant virus; the rest of the STP-C488 open reading frame was confirmed to be of the wt.
Transforming activity of recombinant HVS STP-C488 P10→R in common marmosets.
Primary common marmoset T lymphocytes were infected with recombinant HVS STP-C488 P10→R, wt HVS, or the parental HVS ΔSTP-SV40-SEAP. HVS, HVS ΔSTP-SV40-SEAP, and HVS STP-C488 P10→R at equivalent titers were separately added to individually purified, unstimulated, common marmoset PBMCs. HVS STP-C488 P10→R immortalized marmoset primary T lymphocytes to IL-2-independent growth within about 1 month postinfection and was similar to wt HVS in immortalizing efficiency, whereas HVS ΔSTP-SV40-SEAP was unable to immortalize the marmoset T cells (Table 1).
TABLE 1.
In vitro and in vivo oncogenicity of HVS STP-C488 P10→R
| Virus | No. of positive samples or animals/no. tested
|
Survival (no. of days) | |||
|---|---|---|---|---|---|
| Immortalization of:
|
Virus recoverya | In vivo lymphoma | |||
| Primary common marmoset T lymphocytes | Primary human T lymphocytes | ||||
| None | 0/9 | ||||
| HVS C488 | 9/9 | 3/5 | 2/2 | 2/2 | 19, 20 |
| HVS ΔSTP-SV40-SEAP | 0/9 | 0/5 | 2/2 | 0/2 | >370 |
| HVS STP-C488 P10→R | 9/9 | 0/5 | 2/2 | 2/2 | 20 |
Virus was recovered from PBMCs of two infected marmosets as described previously (14).
HVS STP-C488 P10→R was also compared with wt HVS in experimental common marmoset infection. HVS STP-C488 P10→R and wt HVS caused fatal lymphoproliferative disease with a roughly equivalent time course. Animals infected with wt HVS became moribund and were sacrificed on days 19 and 20; animals infected with HVS STP-C488 P10→R became moribund and were sacrificed on day 20 postinfection (Table 1). Necropsies of these animals revealed multicentric lymphoma that is consistent with previously described HVS-induced pathology (9). HVS STP-C488 P10→R-induced lymphoma was indistinguishable from that induced by wt HVS.
To confirm that HVS STP-C488 P10→R was present in the transformed common marmoset lymphocytes from tumor-bearing animals and in in vitro-immortalized cells, the lymphocytes were cocultivated with OMK cells. Virus preparations were made from supernatant media and tested by PCR for STP-C488 DNA. The DNA sequencing of five independent clones of amplified DNA confirmed the presence of the P10→R mutation. These results indicated that recombinant HVS STP-C488 P10→R was fully capable of immortalizing common marmoset lymphocytes to continuous growth and of inducing lymphoma in common marmosets. Thus, the TRAF binding activity of STP-C488 is not essential for virus-induced transformation of primary marmoset lymphocytes in vitro and in vivo.
Transformation of human lymphocytes by recombinant HVS STP-C488 P10→R.
To further examine the role of STP-TRAF interaction in transformation, HVS STP-C488 P10→R was compared with wt HVS and HVS ΔSTP-SV40-SEAP in primary human T-lymphocyte immortalization assays. Equivalent titers of HVS STP-C488 P10→R, wt HVS, and HVS ΔSTP-SV40-SEAP were used to infect unstimulated, human PBMCs from five donors. HVS transformed the primary human lymphocytes from three of the five donors to IL-2-independent growth within about 2 months postinfection, while HVS STP-C488/P10→R and parental HVSΔSTP-SV40-SEAP failed to immortalize primary human T lymphocytes to IL-2-independent growth (Table 1). These results indicate that STP-TRAF interaction is required for primary human lymphocyte transformation.
STP-C488 TRAF binding is also implicated in rodent fibroblast transformation.
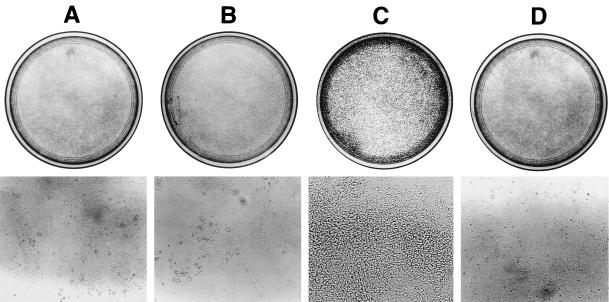
STP-C488 P10→R mutant, which fails to bind to TRAFs, has been shown to be defective in Rat-1 cell transformation (26). To further investigate the specific role of TRAF-mediated signaling in STP transformation, we used a dominant-negative mutant of TRAF2, TRAF2 Δ6–86, which is deleted for the ring finger domain. The TRAF2 ring finger domain is not required for binding to TNFR2, CD40, or LMP1, and this mutant can block NF-κB activation induced by LMP1 (31). Rat-1 cells stably transfected with pcDNA3-TRAF2 Δ6–86 or pcDNA3 vector were derived by selection in the presence of 500 μg of neomycin/ml. Rat-1 and Rat-TRAF Δ6–86 cells were then transfected with a pBabe-puro vector or a pBabe-STP-C488 vector and selected with 5 μg of puromycin/ml. STP-C488 and TRAF2 Δ6–86 expression was monitored by the immunoblot assay with anti-STP-C488 or anti-Flag antibody (data not shown). The growth properties of Rat-babe, Rat-STP-C488, Rat-TRAF2 Δ6–86, and Rat-STP-C488/TRAF2 Δ6–86 cells were examined to investigate whether TRAF Δ6–86 could block STP-C488-mediated transformation. As was shown previously (26, 30), STP-C488 transformed Rat-1 cells, resulting in focus formation (Fig. 6C). Foci were recognizable even before cells reached confluence. The expression of TRAF2 Δ6–86 drastically suppressed the transformation of Rat-1 cells by STP-C488 (Fig. 6D). The number of foci observed for Rat-STP-C488 cells was over 1,000 per 106 cells, whereas Rat-STP-C488/TRAF Δ6–86 cells grew in flat monolayers and formed less than 40 foci per 106 cells. These results demonstrate that the expression of the dominant-negative TRAF2 Δ6–86 mutant blocks Rat-1 cell transformation by STP-C488.
FIG. 6.
Inhibition of transformation of STP-C488 by the dominant-negative TRAF2 mutant. Rat-1 fibroblast cells were plated at 106 cells per 100-mm-diameter tissue culture dishes. After 14 days’ incubation, cells were photographed to show foci of transformed cells at magnification of ×100 (bottom) and after staining with methylene blue (top). A, Rat-babe; B, Rat-TRAF2 Δ6–86; C, Rat-STP-C488; and D, Rat-STP-C488/TRAF2 Δ6–86.
Rat-babe, Rat-STP-C488, Rat-TRAF2 Δ6–86, and Rat-STP-C488/TRAF2 Δ6–86 cells were also used to measure the level of NF-κB activity. Cells were treated with TNF-α as controls. TNF-α treatment or STP-C488 expression induced NF-κB activity in Rat-1 cells approximately fourfold more than that in control cells (Fig. 5B). However, TNF-α treatment did not further induce NF-κB activity in Rat-STP-C488 cells (Fig. 5B). In contrast, the expression of the dominant-negative mutant TRAF2 Δ6–86 abolished NF-κB activation by STP-C488 or TNF-α treatment (Fig. 5B). These results indicate that TRAF-mediated signaling is important for STP-C488-induced transformation and NF-κB activation in Rat-1 cells.
DISCUSSION
In this report we demonstrate that STP of HVS subgroups A and C can associate with TRAFs. Furthermore, STP-C488 activates NF-κB and this activation is dependent on the same STP-C488 residues that are required for TRAF association. Moreover, an HVS STP-C488 P10→R mutation that codes for an STP-C488 mutant, which does not interact with TRAF, cannot transform primary human T lymphocytes or Rat-1 cells in vitro. Thus, TRAF interaction is implicated in HVS STP-C488 transforming activity. However, HVS STP-C488 P10→R is able to transform marmoset T lymphocytes and induce malignant lymphoma in marmosets. TRAF binding is therefore only one component of STP-C488 transforming activity.
The observation that STP interaction with TRAF is required for human T-lymphocyte and Rat-1-cell transformation but not for marmoset T-lymphocyte transformation indicates that TRAF interaction is important for transformation in restricted genetic or developmental backgrounds. This is not surprising, since HVS has a number of genes which may contribute to cell growth transformation, including those encoding tip, v-cyclin, vIL-17, orf14 superantigen homolog, vCD59, vbcl-2, vFLIP, and vIL-8 receptor, as well as the rest of the STP open reading frame (1, 29, 42, 44, 50, 52, 53). Since many of these genes are likely expressed in transformed marmoset T lymphocytes (18), STP-TRAF-mediated signaling may be less important in this cell type. In contrast, HVS is tightly latent in transformed human T lymphocytes, where the single bicistronic STP and tip transcript has been detected (18). Rat-1-cell transformation requires only STP-C488. Thus, HVS may be considerably less dependent on STP for marmoset lymphocyte transformation because of other viral genes expressed in these cells. In addition, STP-C488 binding to cellular Ras is also important for Rat-1 cell transformation (25), and the role of Ras interaction in marmoset and human lymphocyte transformation remains to be investigated.
HVS strains are classified into three subgroups (subgroups A, B, and C) based on sequence divergence in the STP and tip genes (5, 36). Subgroups A and C are highly oncogenic and are able to immortalize common marmoset T lymphocytes, inducing transformation to growth in an IL-2-independent manner in vitro, while subgroup B does not have these properties (13, 37). Subgroup C strains are in addition capable of immortalizing human, rabbit, and rhesus monkey lymphocytes into continuously proliferating T-cell lines (1, 3). All STP-A and STP-C isolates which have been sequenced so far appear to be similar around the TRAF-binding sites that have been revealed by the biochemical and genetic analyses described here (18, 34). These experiments show that oncogenes from the transforming gamma herpesviruses EBV and HVS employ TRAF as a common cellular factor for their activities. Previous experiments with EBV LMP1 point up the importance of both aggregation and TRAF binding in signaling (24, 38). STP-C488 can also oligomerize through its collagen-like motif (unpublished results). Thus, the HVS STP-C488 and EBV LMP1 may mimic a ligand-independent constitutively active receptor.
While STPs of HVS subgroups A and C bind to TRAF 1, 2, and 3, only STP-C488 interaction with TRAF appears to activate NF-κB activity. This may explain why STP-C488 is considerably more potent in Rat-1-cell transformation than STP-A11 (30). On the other hand, STP-A11 may use TRAF interaction for the activation of downstream effectors other than NF-κB, e.g., activation of stress-activated protein kinase or inhibitors of apoptosis (41, 51, 54). Further studies are needed to elucidate the detailed mechanisms of HVS STP-TRAF-mediated signal transduction.
ACKNOWLEDGMENTS
We thank R. Desrosiers, K. Williams, L. Alexander, and R. Means for critical reading of the manuscript and G. Mosialos for providing reagents. We also thank Kristen Toohey for photography support.
This work was supported by U.S. Public Health Service grants CA31363 and RR00168.
REFERENCES
- 1.Ahuja S K, Murphy P M. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem. 1996;268:20691–20694. [PubMed] [Google Scholar]
- 2.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 Nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B, Cerami A. Tumor necrosis, cachexia, shock and inflammation: a common mediator. Annu Rev Biochem. 1988;57:505–518. doi: 10.1146/annurev.bi.57.070188.002445. [DOI] [PubMed] [Google Scholar]
- 4.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesinger B, Trimble J J, Desrosiers R C, Fleckenstein B. The divergence between two oncogenic herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990;176:505–514. doi: 10.1016/0042-6822(90)90020-r. [DOI] [PubMed] [Google Scholar]
- 6.Bröker B M, Tsygankov A Y, Müller-Fleckenstein I, Guse A H, Chitaev N A, Biesinger B, Fleckenstein B, Emmrich F. Immortalization of human T cell clones by Herpesvirus saimiri. J Immunol. 1993;151:1184–1192. [PubMed] [Google Scholar]
- 7.Cheng G, Baltimore D. TANK, a co-inducer with TRAF2 of TNF- and CD40L-mediated NF-κB activation. Genes Dev. 1996;10:963–973. doi: 10.1101/gad.10.8.963. [DOI] [PubMed] [Google Scholar]
- 8.Cheng G, Cleary A M, Ye Z-S, Hong D I, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1497. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 9.Desrosiers R C, Bakker A, Kamine J, Falk L A, Hunt R D, King N W. A region of the herpesvirus saimiri genome required for oncogenicity. Science. 1985;228:184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- 10.Desrosiers R C, Burghoff R L, Bakker A, Kamine J. Construction of replication-competent herpesvirus saimiri deletion mutants. J Virol. 1984;49:343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desrosiers R C, Falk L A. Herpesvirus saimiri strain variability. J Virol. 1982;43:352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desrosiers R C, Silva D, Waldron L M, Letvin N L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986;57:701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devergne O, McFarland E C, Mosialos G, Izumi K M, Ware C F, Kieff E. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duboise S M, Guo J, Czajak S, Desrosiers R C, Jung J U. STP and tip are essential for herpesvirus saimiri oncogenicity. J Virol. 1998;72:1308–1313. doi: 10.1128/jvi.72.2.1308-1313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duboise S M, Guo J, Desrosiers R C, Jung J U. Use of virion DNA as a cloning vector for the construction of mutant and recombinant herpesviruses. Proc Natl Acad Sci USA. 1996;93:11389–11394. doi: 10.1073/pnas.93.21.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du Z, Regier D A, Desrosiers R C. Improved recombinant PCR mutagenesis procedure that uses alkaline-denatured plasmid template. BioTechniques. 1995;18:376–378. [PubMed] [Google Scholar]
- 17.Falk L, Wolfe L, Deinhardt F. Isolation of herpesvirus saimiri from blood of squirrel monkeys (Saimiri sciureus) J Natl Cancer Inst. 1972;48:1499–1505. [PubMed] [Google Scholar]
- 18.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleckenstein B, Desrosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. New York, N.Y: Plenum Publishing Corporation; 1982. pp. 253–332. [Google Scholar]
- 20.Franken M, Devergne O, Rosenzweig M, Annis B, Kieff E, Wang F. Comparative analysis identifies conserved tumor necrosis factor receptor-associated factor 3 binding sites in the human and simian Epstein-Barr virus oncogene LMP1. J Virol. 1996;70:7819–7826. doi: 10.1128/jvi.70.11.7819-7826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gedrich R W, Gilfillan M C, Duckett C S, Van Dongen J L, Thompson C B. CD30 contains two binding sites with different specificities for members of the tumor necrosis factor receptor-associated factor family of signal transducing proteins. J Biol Chem. 1996;271:12852–12858. doi: 10.1074/jbc.271.22.12852. [DOI] [PubMed] [Google Scholar]
- 22.Goeddel D V, Aggarwal B B, Gray P W, Leung D W, Nedwin G E, Palladino G E, Patton J S, Pennica D, Shepard H M, Sugarman B J, Wong G H W. Tumor necrosis factors: gene structure and biological activities. Cold Spring Harbor Symp Quant Biol. 1986;51:597–609. doi: 10.1101/sqb.1986.051.01.072. [DOI] [PubMed] [Google Scholar]
- 23.Hu H M, O’Rourke K, Boguski M S, Dixit V M. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J Biol Chem. 1994;269:30069–30076. [PubMed] [Google Scholar]
- 24.Izumi K M, Kaye K M, Kieff E D. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung J U, Desrosiers R C. Association of the viral oncoprotein STP-C488 with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung J U, Desrosiers R C. Distinct functional domains of STP-C488 of herpesvirus saimiri. Virology. 1994;204:751–758. doi: 10.1006/viro.1994.1590. [DOI] [PubMed] [Google Scholar]
- 27.Jung J U, Desrosiers R C. Herpesvirus saimiri and ateles. In: Webster R, Granoff A, editors. Encyclopedia of virology. Philadelphia, Pa: Saunders Scientific Publications, Inc.; 1994. pp. 614–622. [Google Scholar]
- 28.Jung J U, Desrosiers R C. Identification and characterization of the herpesvirus saimiri oncoprotein STP-C488. J Virol. 1991;65:6953–6960. doi: 10.1128/jvi.65.12.6953-6960.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung J U, Stäger M, Desrosiers R C. Virus-encoded cyclin. Mol Cell Biol. 1994;14:7235–7244. doi: 10.1128/mcb.14.11.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung J U, Trimble J J, King N W, Biesinger B, Fleckenstein B W, Desrosiers R C. Identification of transforming genes of subgroup A and C strains of herpesvirus saimiri. Proc Natl Acad Sci USA. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye K M, Devergne O, Harada J N, Izumi K M, Yalamanchili R, Kieff E, Mosialos G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koomey J M, Mulder C, Burghoff R L, Fleckenstein B, Desrosiers R C. Deletion of DNA sequences in a nononcogenic variant of herpesvirus saimiri. J Virol. 1984;50:662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretschmer C, Murphy C, Biesinger B, Beckers J, Fickenscher H, Kirchner T, Fleckenstein B, Rüther U. A Herpes saimiri oncogene causing peripheral T-cell lymphoma in transgenic mice. Oncogene. 1996;12:1609–1616. [PubMed] [Google Scholar]
- 34.Lee H, Trimble J J, Yoon D-W, Regier D, Desrosiers R C, Jung J U. Genetic variation of herpesvirus saimiri subgroup A transforming protein and its association with cellular src. J Virol. 1997;71:3817–3825. doi: 10.1128/jvi.71.5.3817-3825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 36.Medveczky P, Szomolayi E, Desrosiers R C, Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984;52:938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittrücker H-W, Müller-Fleckenstein I, Fleckenstein B, Fleishcher B. CD2-mediated autocrine growth of herpes virus saimiri-transformed human T lymphocytes. J Exp Med. 1995;176:900–913. doi: 10.1084/jem.176.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 39.Murphy C, Kretschmer C, Biesinger B, Beckers J, Jung J, Desrosiers R C, Müller-Hermelink H K, Fleckenstein B W, Rüther U. Epithelial tumors induced by a herpesvirus oncogene in transgenic mice. Oncogene. 1994;9:221–226. [PubMed] [Google Scholar]
- 40.Murthy S C S, Trimble J J, Desrosiers R C. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J Virol. 1989;63:3307–3314. doi: 10.1128/jvi.63.8.3307-3314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;10:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 42.Nava V E, Cheng E H-Y, Veliuona M, Zou S, Clem R J, Mayer M L, Hardwick J M. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothe M, Wong S C, Henzel W J, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 44.Rother R P, Rollins S A, Fodor W L, Albrecht J-C, Setter E, Fleckenstein B, Squinto S P. Inhibition of complement-mediated cytolysis by the terminal complement inhibitor of herpesvirus saimiri. J Virol. 1994;68:730–737. doi: 10.1128/jvi.68.2.730-737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 46.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szomolanyi E, Medveczky P, Mulder C. In vitro immortalization of marmoset cells with three subgroups of herpesvirus saimiri. J Virol. 1987;61:3485–3490. doi: 10.1128/jvi.61.11.3485-3490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi M, Rothe M, Goeddel D V. Anatomy of TRAF2. J Biol Chem. 1996;271:19935–19942. doi: 10.1074/jbc.271.33.19935. [DOI] [PubMed] [Google Scholar]
- 49.Tartaglia L A, Goeddel D V. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 50.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schröter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 51.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S J. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 52.Yao Z, Fanslow W C, Seldin M F, Rousseau A M, Painter S L, Comeau M R, Cohen J I, Spriggs M K. Herpesvirus saimiri encodes a new cytokine IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 53.Yao Z, Maraskovsky E, Spriggs M K, Cohen J I, Armitage R J, Alderson M R. Herpesvirus saimiri open reading frame 14, a protein encoded by a T lymphotropic herpesvirus, binds to MHC class II molecules and stimulates T cell proliferation. J Immunol. 1996;156:3260–3266. [PubMed] [Google Scholar]
- 54.Yuasa T, Ohno S, Kehrl J H, Kyriakis J M. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J Biol Chem. 1998;273:22681. doi: 10.1074/jbc.273.35.22681. [DOI] [PubMed] [Google Scholar]