Abstract
Simple Summary
The cells of the immune system can exert a dual effect on cancer development and growth. On the one hand, the immune system can be activated by tumor antigens and can elicit an antitumor response. On the other, the inflammatory milieu in the tumor microenvironment can trigger immune effector mechanisms that promote tumor growth. In the oral cavity, the balance between protumor and antitumor immunity can influence the progression from premalignancy to carcinoma. In this article, we review the cells and mechanisms that are thought to be the most important immune determinants of oral cancer development and progression.
Abstract
A still unresolved issue surrounding tumor formation concerns the role that the immune system plays in preventing the formation and progression of neoplasia, including oral squamous cell carcinoma (OSCC). Antitumor immunity has historically been seen as a critical barrier for cancer cells to develop, grow and spread, and this can be modulated using immunotherapies to achieve antitumor clinical responses. However, it has recently become clear that tumor-associated immunity, particularly the inflammatory microenvironment, has the paradoxical effect of enhancing tumorigenesis and progression. In this review, we discuss the multifaceted function of infiltrating immune cells in suppressing or promoting premalignancy and cancer. In particular, we report on the evidence supporting a role for T lymphocytes, dendritic cells, macrophages, and neutrophils in the development and progression of oral potentially malignant disorders (OPMD) and OSCC. We also draw attention to the clinical relevance of immune cell phenotypes and associated molecules for use as biomarkers and to the translatability of current research findings to improve classification systems and precision medicine in patients with OSCC.
Keywords: lymphocytes, macrophages, oral cancer, oral potentially malignant disorders, tumor microenvironment, tumor immunoediting
1. Introduction
Head and neck cancer (HNC) is one of the most common malignancies worldwide, with the majority of HNCs arising from the stratified epithelium of the oral cavity. Worryingly, over 300,000 new cases of oral squamous cell carcinoma (OSCC) are diagnosed every year, particularly in the Indian subcontinent and Southeast Asia, where OSCC is overall the third most common type of cancer [1]. Current evidence supports the existence of a multi-step process of oral carcinogenesis, which clinically translates into the onset of oral potentially malignant diseases (OPMDs) in the early stages of progression to malignancy. OPMDs eventually progress to oral cancer following the acquisition of a number of additional mutations [2]. Thanks to the relatively easy inspection of the oral cavity, it is possible to detect early changes in the oral mucosa before these progress to overt malignancy, and thus reduce mortality [3]. However, while oral carcinogenesis consists in an accumulation of genetic and epigenetic alternations, growing evidence calls into question the role of the surrounding microenvironment in the development of malignancy [4].
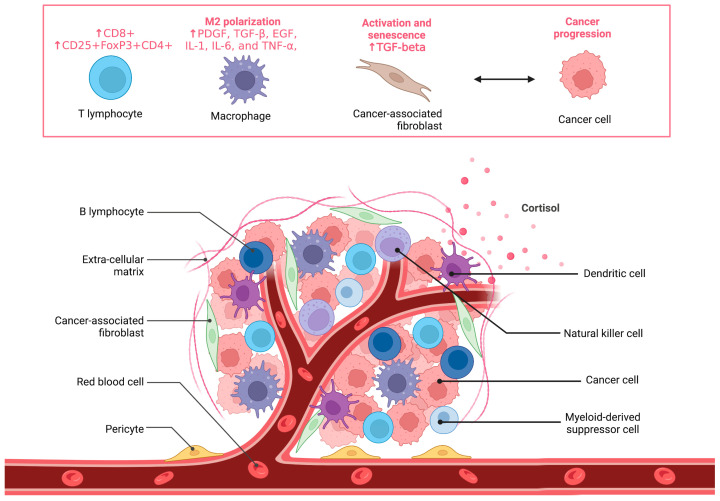
The microenvironment is a complex milieu of extracellular matrix components, blood vessels and non-malignant cells, such as immune cells and fibroblasts, that surround the tumor [5] or its pre-malignant lesion (Figure 1). The continuous exchange of different signals among these components leads to changes in the environment, which keep evolving during the progression from normal to cancerous tissue [6]. For example, we have shown that stromal fibroblasts found in cancer tissues (cancer-associated fibroblasts, CAFs) promote epithelial cancer progression via paracrine mechanisms that involve oxidative stress and cellular senescence [7,8].
Figure 1.
Graphical representation of cancer tissue. In addition to malignant cells, cancer tissue includes extra-cellular matrix components, blood vessels and non-malignant cells. The cell types depicted on top of the panel (T lymphocytes, macrophages and activated fibroblasts) play a key role in cancer progression.
As knowledge of cancer mechanisms progresses, it has become apparent that the normal cell progressively evolves to a neoplastic state by evading growth suppression, enabling replicative immortality and resisting apoptosis (cell-intrinsic mechanisms) as well as via cell-extrinsic mechanisms, such as escape of immune surveillance [9]. In this scenario, inflammation has acquired a paradoxical role, since the immune cells involved in the elimination of altered cells may be educated to promote carcinogenesis by different mechanisms [10]. In agreement with this view, higher incidence of OSCC is observed in OPMDs characterized by chronic inflammation [11], and concomitant production of anti-inflammatory, immunosuppressive molecules is observed in cancer tissues, including OSCC [12]. Strikingly, cancer cells can themselves produce immunomodulatory molecules, such as cortisol [13], hence driving the immune response to facilitate or repress cancer growth and immune surveillance.
In this review, we will examine the immune cells involved in OSCC-associated inflammation, their role in oral carcinogenesis, as well as their clinical significance from a prognostic standpoint.
2. Immune Cells of the Tumor Microenvironment in Oral Cancer Development and Progression
The idea that the immune system can recognize and destroy nascent-transformed cells finds its roots in the nineteenth century with Virchow’s work and was later conceptualized in the cancer immunosurveillance hypothesis of Burnet and Thomas [14]. In the last 30 years, this hypothesis has been substantiated by a growing body of experimental evidence. Interestingly, this work has shown that the immune system can also function to promote or select tumor variants with reduced immunogenicity, thereby providing cancer cells with a mechanism to escape immunologic detection and elimination. These findings have led to the development of the cancer immunoediting hypothesis, which acknowledges both the antitumor and tumor-promoting actions of the immune system in tumor development. Here, we discuss the role that immune cells play in the multistep process of oral carcinogenesis.
2.1. T Lymphocytes
A key immune-modulatory element in oral precursor lesions is the tumor-infiltrating lymphocytes (TILs), particularly CD8+ T lymphocytes, which are abundant in OPMDs [15]. Several studies have revealed an association between TILs and higher grading in dysplastic lesions [16,17,18]. In one example, the ratio between CD8+ T lymphocytes and CD4+ cells increased following dysplastic change, which may represent an attempt of the immune system to eliminate altered cells [18,19]. Similarly, Gannot et al. found an increase of CD4+ CD8+ T lymphocytes and B cells in moderate and severe dysplasia and OSCC compared to hyperkeratotic lesions [20], suggesting that antitumor immune response mounts during the development of OSCC. Conversely, IgA and IgG-secreting B cells were found in leukoplakia with dysplasia and were decreased during the progression to malignancy [21], which might signal a reduction in humoral antitumor immune surveillance.
Crucially, Strauss et al. demonstrated a switch towards an infiltrating CD25+FoxP3+ CD4+ phenotype in patients with HNSCC. This unique subpopulation of T cells secreting interleukin-10 (IL-10) and transforming growth factor (TGF)-β1 mediates immune system suppression in the tumor microenvironment and hence favours pro-tumour immunity [22]. Consistently, other studies have found that CD25+ and FoxP3+ lymphocytes were associated to OPMD progressing to OSCC [15,23,24]. Gan et al. recently performed an immunohistochemical and transcriptomic profiling of diverse severity OPMDs and early OSCCs. They found that infiltrating lymphocytes were present in 80% of high-risk OPMDs and OSCCs, compared to 9% of benign lesions. In high-risk OPMDs, transcriptomic profiling revealed the existence of T-cell inflamed and non-immune reactive subtypes. T-cell inflamed subtype was characterized by T lymphocytes, interferon and PD/PD-L1 pathway signatures, suggesting the presence of an impaired immune surveillance [25]. This study supported the results from Yagyuu et al., who found increased immunohistochemical expression of PD-L1, CD163+ macrophages and CD8+ lymphocytes in high-grade dysplasia [12].
The results of these studies have informed the recent development of an immunoreactivity score based on the expression of PD1, PD-L1, FoxP3, IL-6, IL-10 and TGF-β1. A higher number of T-reg cells and the expression of such markers correlated to higher grades of dysplasia in OPMDs and the strongest correlation was found between PD1 and PD-L1. Collectively, these results would suggest that immune suppression and PD1/PD-L1 axis are instrumental in the progression of OPMDs to OSCC [17].
2.2. Macrophages
Tumor-associated macrophages (TAMs) are the most abundant immune cells in the tumor microenvironment and act in concert with TILs. In particular, complex interactions between lymphocytes and macrophages can either promote or contrast the formation of a pro-inflammatory and immunosuppressive environment [26].
Oral dysplastic lesions can recruit macrophages by expression of human beta-defensin 3 [27], and an increase in macrophages and myeloid-derived suppressive cells (MDSCs) correlates with malignant progression, in particular by M2 macrophage polarization [12,24,28,29,30,31]. In several studies, M2 were characterized by CD163+ and CD204+ and correlated with the presence of FoxP3 and CD25+ lymphocytes; specifically, both cell types increased with the worsening of dysplasia [15,30]. Despite the strong evidence of a pro-tumor activity of M2 macrophages [32], conflicting results have also been reported. In contrast to their own results in a smaller cohort of patients, Yagyuu et al. could not confirm a prognostic role of M2 CD163+ in malignant transformation although some differences in study design and inclusion might have led to this inconsistency [12,33]. In a mouse study, M2 signatures were predictive of longer oral cancer-free survival [34]. Nevertheless, canonical markers of M2 might be functionally associated to an M1 phenotype. For example, Weber et al. found CD163+ macrophages co-expressing CD11c, which is also known to be a marker for M1 polarization [29]. Mori et al. observed an increase of CD163+ macrophages in higher grade dysplasia, but this occurred in an immunosuppressive environment regulated by CD4+ lymphocytes and associated with the expression of CXCR3, CCR5, CXCL9, STAT1 and interferon-induced gene products [35].
The role of external etiopathogenetic factors in the modulation of the cancer immune response has also been considered. For example, smoking was shown to determine an immunosuppressive environment characterized by M2 infiltration, arginase-1 and IL-10 and lower TNFα and iNOS [36]. Stasikowska-Kanicka et al. found an increase in CD68+, CD163+ (M2), iNOS+ (M1), CD4+, CCR4+ (Th2) and CCR5+ (Th1) in the progression to metastatic OSCC, whereas the presence of CD8+ cells negatively correlated to both CD163+ and iNOS+ macrophages regardless of the presence of metastasis [37]. Ye et al. showed that signal regulatory protein α (SIRPα) correlated with the number of CD68+ macrophages while advancing from normal to OPMD to OSCC, while CD163+ negatively correlated with SIRPα expression. In an in vitro model of co-culture of macrophages with oral cancer cells, the blockade of SIRPα led to M2 polarization with inhibition of phagocytosis, IL-6 and TNF-α, and secretion of IL-10 and TGF-β [38]. Hence, M1-M2 phenotype switch might be overlapping during the whole process of oral carcinogenesis [39]. Moreover, another important detail to be considered is that all these different studies evaluated and characterized different makers of expression and different tissue localization of macrophages, such as stroma, periphery, sub- and intraepithelial which may contribute to these contrasting results and differential M1-M2 phenotype characterization [12,18,28,29,30,33,35,37,39,40,41].
2.3. Dendritic Cells
While macrophages and lymphocytes may be considered the main characters of the immune microenvironment, many other cells types contribute to immunosurveillance, and their reciprocal interaction may promote or suppress OPMD/OSCC development [42]. In the context of mucosal microenvironment, dendritic cells (DCs) are involved in major histocompatibility complex call I pathway by presenting antigens to T cells [43,44]. The most abundant type of DC in the oral cavity is dendritic Langerhans cells (LCs) [45] and current evidence demonstrates contrasting roles in malignant progression. Specifically, several studies found an increase of CD1a+ LCs while progressing from normal to mild and severe dysplasia [18,45,46,47,48,49] while others found a decrease of this cell population [50,51]. Of interest, in the study of Wang et al., nine patients going towards malignant transformation reported significantly lower LCs. It is plausible, therefore, that changes in the number of infiltrating LCs affects the immunosurveillance ability during early carcinogenesis [46,51].
2.4. Mast Cells
Another supporting component in this multicellular microenvironment are mast cells (MCs), which have been emerging as possible players in the malignant progression from OPMD to OSCC. Telagi et al. found an increase of MCs in patients with dysplasia and oral submucous fibrosis, which was particularly prominent in the presence of inflammation [52]. Mast cells were also increased in leukoplakia, lichen planus and actinic cheilitis [47,53,54,55,56,57,58]. Mechanisms by which MCs might contribute to malignant progression are still not well understood [52,59]. Piecemeal degranulation is an unconventional secretory pathway characterized by vesicular transport of small packets of selected materials from the cytoplasmic secretory granules to the cell surface and might represent one of the main mechanisms by which MCs contribute to cancer progression. These granules may be enriched of cytokines, proteases, arachidonic acid derivates and growth factors, representing a heterogeneous phenotypic expression of MCs [59,60,61,62]. A systematic review on this topic showed that the highest number of MCs was found in OPMD while their presence declined in OSCC. Indeed, it appears that MCs are abundant in an inflammatory tumor microenvironment although it is not clear whether these cells exert an anti- or pro-tumor activity [59]. Several studies found an increase of MCs’ density while moving from normal mucosa to dysplasia to OSCC, which positively correlated to microvessel density (CD34+/CD31+). This would suggest MC activity parallels neo-angiogenesis [63,64,65,66,67,68,69,70,71]. Oliveira-Neto found a decrease of MCs in OSCC and transforming OPMDs, as a consequence in microenvironment changes, and reported an inhibition of MC migration that could reflect impaired control during tumor initiation [72]. Similar results were documented by Singh et al. [73]. This dichotomous role may reflect an attempt of the immune system to fight altered cells by promoting cytotoxic effect in the early phases; however, once the tumor is established, MCs become educated to produce pro-angiogenic and pro-tumoral factors [59].
2.5. Myeloid-Derived Suppressive Cells (MDSCs)
MDSCs have recently gained a more prominent role in the pro-tumorigenic tumor microenvironment, although current studies are limited to patients with a diagnosis of OSCC or in vivo animal models of OPMD [28,74]. MDSCs’ accumulation and malignant progression was associated to porphyromonas gingivalis infection by increase in CXCL2, CCL2, IL-6 and IL-8, which drew malignant progression [75]. The presence of MDSCs also correlated with CD4+FoxP3+ lymphocytes and IL-1β secretion in a dectin-1 depending signal in a model of fungal infection [76].
2.6. Neutrophils and Eosinophils
Scarce evidence exists with regards to neutrophils and eosinophils as very few studies have explored their role in the malignant progression of OPMDs [77]. Neutrophils were mostly investigated by their ability to form extracellular traps (NETs) which was observed to be a prominent ability in patients with oral lichen planus, thus suggesting a possible role in the progression to OSCC [78]. Eosinophils were also found increased in patients with leukoplakia and the mean number correlated to the advancement of dysplasia [77] although this finding was not confirmed in other studies [65,79,80].
2.7. The Immune Function of Cancer-Associated Fibroblasts
Although not properly considered part of the immune system, fibroblasts represent the main cellular component of extracellular matrix and are known to produce several cytokines with emerging roles in innate immune response [81,82,83]. Fibroblasts/CAFs are prominent cell types in the tumor microenvironment and hence could interfere in the immunosurveillance mechanisms in cancer involving a cross-talk with primary immune cells [84,85,86,87]. While these α-smooth muscle actin positive (α-SMA) (myo)fibroblasts are virtually absent in the normal oral mucosa [88,89], it has been reported that myofibroblasts significantly increase in number while progressing to OPMD and to OSCC [88,89,90,91,92,93]. Other authors could not find evidence of myofibroblasts in normal mucosa or in patients with oral leukoplakia or dysplasia but only in OSCC [94,95,96]. Current evidence shows a wide heterogeneity in the role of myofibroblasts in the progression to malignancy [97]. In this regard, a recent systematic review suggested that myofibroblasts may be involved in the progression of oral submucous fibrosis but not in patients with leuko-erythroplakia [97]. Indeed, it has been reported that increased inflammatory response in the stroma is inversely associated to myofibroblasts [98,99], and OSCC—fibroblasts contact is necessary for induction of myofibroblast phenotype [100,101]. Conversely, findings from our group show that a paracrine cross-talk between malignant keratinocytes and fibroblasts is sufficient to drive tumor migration and invasion in a TGF-β-dependent manner [7,8]. These data are in line with the results obtained in an in vitro model where OSCC cell line was able to transdifferentiate fibroblasts to myofibroblasts via secretion of TGF-β1 [99].
3. Immunopathogenic Mechanisms in OSCC and Precursor Lesions
Although a functioning immune system is instrumental for the elimination of neoplastic cells, when the immune response becomes regulated by the tumor microenvironment, this produces an opportunity for the development of malignant cells that are capable of escaping the destructive effects of the immune system. The immune microenvironment contributes to tumorigenesis by impairing normal immune cell activity via immune suppression and tolerance, as well as by enhancing angiogenesis and ROS production [102,103,104].
The mechanisms of this dual pro- and anti-cancer role of the immune cells will be discussed here.
3.1. Acquisition of Tolerance during the Progression to Malignancy
While forming cancer cells express antigens that are recognized and targeted by the immune system, it is more difficult to explain why a similar immune dysregulation is witnessed in OPMD. One fascinating hypothesis on how transforming cells evade the immune surveillance is that precursor lesions express tumor antigens, which facilitate the acquisition of tolerance over time. Consistently, a pattern of different tumoral antigens has been shown in OPMDs, such as MAGE cancer testis antigens, NY-ESO-1, MUC1 and neo-antigens [105,106,107]. It is possible that the emergence of CD25+ and FoxP3+ lymphocytes observed in OPMD progressing to OSCC [15,23,24] might represent the immune system adaptation and education provided by the transformation of premalignant cells [28]. One of these adaptive changes includes CD8+ T-lymphocytes’ ability to recognize antigens and deliver their cytotoxic effect [108]. The functional activity exerted by cytotoxic CD8+ T-lymphocytes in this context has not been definitively proven [109]. More likely, inflammation may promote genomic instability in the early stages; while later, once genomic changes have occurred, new mechanisms might exert an immunosuppressive conditioning, which facilitates the progression from OPMD to over carcinoma [28,110].
3.2. Expression of Immune Checkpoint Markers
Immune checkpoints are stimulatory and inhibitory pathways that modulate the immune response while maintaining self-tolerance. However, the expression of some of these immune-checkpoint proteins by malignant epithelial cells (rather than immune cells) dysregulates the antitumor immunity and favors the growth and expansion of cancer cells. In the context of oral carcinogenesis, it has been shown that the elevated number of T-reg cells and the expression of immune checkpoint markers correlated to higher grades of dysplasia in OPMDs, with the strongest correlation found between PD1 and PD-L1 [19]. This suggests that the PD1/PD-L1 axis may be responsible for the progression of OPMDs to OSCC, possibly leading to T-cell exhaustion and immunosuppressive environment [17]. Strategies that target these regulatory pathways to enhance immunological activity against tumor cells are being developed, with mixed results [111,112]. The most successful immune checkpoint blockade strategy is anti-PD-1/PD-L1 therapy that has been approved to treat a wide variety of cancer types, such as blood, skin, lung, liver, bladder and kidney cancers. While the more durable response of checkpoint blockade compared to chemo- or targeted therapies may be related to mechanisms of immunological memory, a relatively low response rate has been observed in most cancers, including HNSCC [113]. Therefore, further immune checkpoint inhibitors besides those targeting the PD-L1/PD-1 pathway need to be explored for therapeutic use.
3.3. Role of Cancer Stem Cells in Immune Evasion
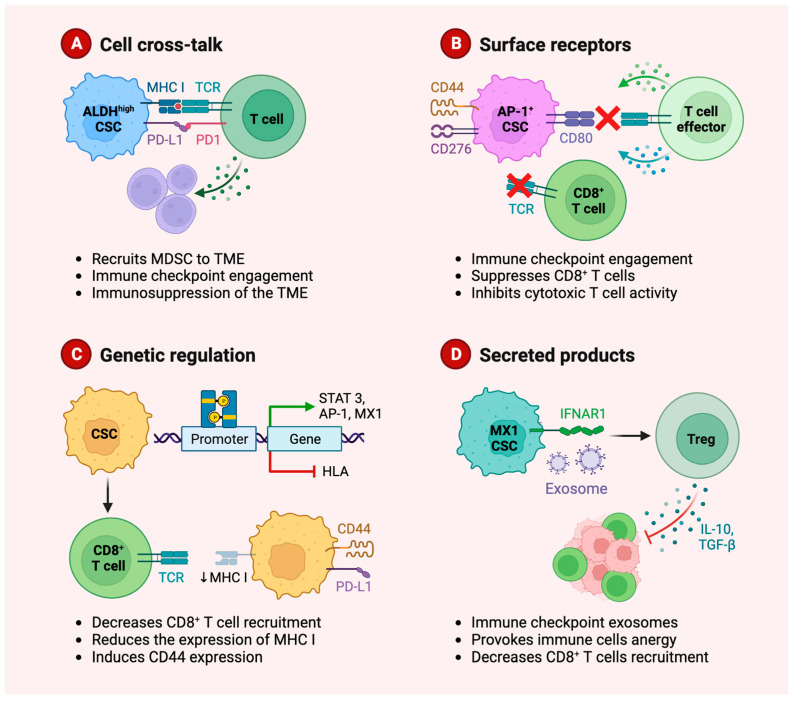
Cancer stem cells (CSCs) play a critical role in carcinogenesis, progression to metastasis, and resistance to antineoplastic treatment of head and neck tumors [114]. Since CSCs act as tumor-initiating cells, these cell populations may develop intrinsic mechanisms to evade immune surveillance both via direct contact and paracrine regulation of immune cells involving secreted molecules and exosomes (Figure 2).
Figure 2.
Graphical representation of the mechanisms of immune evasion by cancer stem cells (CSCs). CSCs promote an immunosuppressive microenvironment via direct interaction with T cells (A,B), genetic regulation leading to reduced immunogenicity (C) and paracrine mechanisms provoking immune cells anergy and suppression of the immune response (D). MDSC, Myeloid-derived suppressor cells; TME, tumor microenvironment.
Studies have shown that CSCs express low levels of molecules involved in processing and presenting tumor antigens to T cell receptors (TCRs), a crucial stimulatory signal to T-cell response, and escape from recognition by anti-tumor immunity [115]. In addition, enriched PD-L1 expression in CSCs has been suggested to facilitate CSC immune evasion in head and neck cancers by suppressing T-cell-mediated immunity [116]. Subsequent studies characterized these TIL subsets and found that T cell infiltration was enriched in an effector memory phenotype (CD45RA−/CCR7−). Naïve T cells (CD45RA+/CCR7+) were decreased in the microenvironment compared to PBMC of patients while regulatory T cells (CD4+/CD25+/CD127 low and CD4+/CD39+) were elevated [117]. Notably, the immunomodulatory molecule cortisol induces the formation of stem cell-like populations in epithelial cancers [118] and glucocorticoid receptor inhibition by mifepristone mediates anti-proliferative effect on ovarian mesenchymal stem cells [119]. It is possible, therefore, that cancer-derived cortisol promotes immune evasion both directly by immune suppression and indirectly by enriching CSC subpopulations.
3.4. Role of Immune Modulatory Cytokines
The role of cytokines in cancer, including OSCC, has been studied extensively and the main effector molecules regulating the pro-tumor immune activity are CSF-1, IL-6, VEGF, PGE-2, TGF-β and IL-10 [120]. The immune modulatory role of these cytokines in the tumor microenvironments of head and neck squamous cell carcinomas has been reviewed in detail recently [121]. Notably, the clinical prognostic value of tumor-infiltrating cell types depends on their secretory cytokine profile. For example, the negative correlation between patient outcome and level of tumor-associated macrophages (TAM) is reliant upon TAM expression of PDGF, TGF-β, EGF, IL-1, IL-6 and TNF-α, which generates a favorable environment for tumor growth [122]. In one example, TGF-β produces an immunosuppressive and tumor-promoting microenvironment in OSCC tissues by stimulating the production of Treg cells and CAFs, which then results in the inhibition of cytotoxic T lymphocytes (CTLs) and natural killer cells. We have shown that CAFs, in turn, produce high levels of TGF-β1 and TGF-β2 and promote oral carcinogenesis and local invasion via TGF-β-dependent mechanisms [8,123,124]. Therefore, the tumor-limiting vs. tumor-promoting action of cytokines is not necessarily related to their production by the immune infiltrate; rather, it will result from the interplay between different cell types in the context of tumor microenvironment.
4. Clinical Significance of Immune Biomarkers in OSCC
While considerable effort has been directed to the study of the tumor-related host response to OSCC, the principles, mechanisms and molecules governing this process have not been translated to clinical practice to date. In one example, the 8th Edition of the American Joint Committee on Cancer (AJCC) has provided some important novelties in prognostic stratification, including the addition of depth of invasion (DOI) and extranodal extension (ENE) for the evaluation of T (Tumor) and N (Nodal) parameters [125]; however, no immune-related features have been included. Furthermore, prognostication is still based on staging at the time of diagnosis, which only represents a “snapshot” of dimensionality and site involvements of the disease. In other words, the current approach still lacks a “global” assessment of tumor aggressiveness, which makes it difficult to inform precision treatments. For example, tumors presenting at the same stage tend to be treated with the same therapeutic means, yet they show very different biological behaviour and clinical responses. This exemplifies the clinical importance of diving deeper into the discovery and validation of new biomarkers that can reliably predict tumor behaviour and prognosis.
Prognostic biomarkers may be defined as specific biological characteristics that can be quantified at baseline and that could help predict clinical outcomes (e.g., death, recurrence, progression) occurring in the future. A preliminary assessment of future disease behavior may be useful in tailoring clinical decision making and in modulating treatment approaches. The study of the tumor-related immune response seems to be one of the most promising sources of information for prognostic stratification, as it provides information that is not captured by the current TNM classification system, such as the ability of the host’s immune system to fight against cancer cells.
Several studies have proven that tumors at the same stage differ in their immune response capability, which has led to the definition of three different cancer-immune phenotypes: the immune-desert phenotype, the immune–excluded phenotype, and the inflamed phenotype [126]. Each of these profiles seems to be associated with specific pathological mechanisms that may hamper the host immune response’s ability to kill cancer cells [127]. Such profiles can be easily assessed through a comprehensive analysis of spatial immune infiltration patterns (“topography”) and across various immune cell types [128]. A study from our group revealed that this approach is easily applied in routine histological analysis and can detect a subgroup of immune-desert tongue squamous cell carcinoma characterized by a very poor prognosis [129]. However, a simple histologic evaluation has its own limitations and may not be useful for more accurate stratification among different immune cell types.
Recent guidelines of the International Immuno-oncology Working Group have been extensively utilized for the analysis of tumor-infiltrating lymphocytes (TILs) and should be used as a milestone for implementation in clinical practice [130]. More broadly, the identification of specific immune subpopulations of immune cells through the use of molecular biomarkers seems to be a promising approach. An ever-increasing number of studies has been published on this topic, thus raising the need of literature synthesis through data meta-analysis, and good quality evidence is present for some of these immune cell subpopulations. In particular, prognostic studies of OSCC tumors with stromal abundance of CD163+ tumor-associated macrophages (TAMs) evaluated by immunohistochemistry showed a significantly worse prognosis [131].
Another important aspect to take into consideration is the possibility of making a systemic assessment of the tumor immune response by evaluating the population of cells present in the blood. Among these, pre-treatment quantification of the neutrophil-to-lymphocyte ratio significantly impacts the prognosis of head and neck SCC patients [132]. Studies on such a type of prognostic biomarkers are abundant and dispersive, and they represent just an intermediate step toward biomarker implementation in clinical practice [133]. The next step should be to combine multiple predictors in a prognostic model whereby risk of a specific endpoint can be calculated for individual patients [134]. The routine application of such tools requires multiple validations from diverse patient cohorts in different countries and the implementation of their graphical representation for an easier use by physicians [135].
Ultimately, these efforts should be aimed at developing a staging system that includes immune-related features, so that a TNM-Immune Staging System can be implemented [136].
5. Conclusions
In this article, we presented evidence supporting the role of immune cells in oral carcinogenesis. Lymphocytes and macrophages are key actors in this process and have gained an important role in OPMDs and in immunoediting. The switch from CD8+ to CD4+ and cytokine profile have been associated with malignant progression. Another mechanism may involve the communication between FoxP3+ CD4+ T lymphocytes, which increase in the progression from OPMD to OSCC, together with the abundanceof CD68+ macrophages in an IL-10-enriched environment. Fibroblasts and secreted molecules including cortisol may exert immunoregulatory functions that are crucial for promoting immune escape and cancer development. So far, the advances in the understanding of the immune function of the TME have led to relatively little improvements in treatment modalities for OSCC and have not been translated into more accurate diagnostic/staging systems. Filling this gap will be instrumental for the development of precision oncology.
Acknowledgments
The authors would like to thank STEMM Research (UK) and The University of Melbourne for making the necessary resources available.
Author Contributions
Conceptualization, N.C., G.T. and V.C.A.C.; investigation, V.C.A.C., K.Z., G.T. and N.C.; resources, L.L.M., N.C.; writing—original draft preparation, V.C.A.C., K.Z., G.T. and N.C.; writing—review and editing, V.C.A.C., K.Z., G.T., L.L.M. and N.C.; supervision, L.L.M. and N.C.; project administration, L.L.M. and N.C.; funding acquisition, L.L.M. and N.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the European Union—NextGenerationEU through the Italian Ministry of University and Research under PNRR—M4C2-I1.3 Project PE_00000019 “HEAL ITALIA HEALTH EXTENDED ALLIANCE FOR INNOVATIVE THERAPIES, ADVANCED LAB-RESEARCH, AND INTEGRATED APPROACHES OF PRECISION MEDICINE”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pahwa V., Nair S., Shetty R.S., Kamath A. Prevalence of Oral Premalignant Lesions and Its Risk Factors among the Adult Population in Udupi Taluk of Coastal Karnataka, India. Asian Pac. J. Cancer Prev. 2018;19:2165–2170. doi: 10.22034/APJCP.2018.19.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Sayans M., Somoza-Martin J.M., Barros-Angueira F., Reboiras-Lopez M.D., Gandara Rey J.M., Garcia-Garcia A. Genetic and molecular alterations associated with oral squamous cell cancer (Review) Oncol. Rep. 2009;22:1277–1282. doi: 10.3892/or_00000565. [DOI] [PubMed] [Google Scholar]
- 3.Sankaranarayanan R., Ramadas K., Thomas G., Muwonge R., Thara S., Mathew B., Rajan B. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet. 2005;365:1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 4.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman P., Mielgo A. Cancer-Associated Fibroblast Mediated Inhibition of CD8+ Cytotoxic T Cell Accumulation in Tumours: Mechanisms and Therapeutic Opportunities. Cancers. 2020;12:2687. doi: 10.3390/cancers12092687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beacham D.A., Cukierman E. Stromagenesis: The changing face of fibroblastic microenvironments during tumor progression. Semin. Cancer Biol. 2005;15:329–341. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Lim K.P., Cirillo N., Hassona Y., Wei W., Thurlow J.K., Cheong S.C., Pitiyage G., Parkinson E.K., Prime S.S. Fibroblast gene expression profile reflects the stage of tumour progression in oral squamous cell carcinoma. J. Pathol. 2011;223:459–469. doi: 10.1002/path.2841. [DOI] [PubMed] [Google Scholar]
- 8.Hassona Y., Cirillo N., Lim K.P., Herman A., Mellone M., Thomas G.J., Pitiyage G.N., Parkinson E.K., Prime S.S. Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-β. Carcinogenesis. 2013;34:1286–1295. doi: 10.1093/carcin/bgt035. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 10.DeNardo D.G., Andreu P., Coussens L.M. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chadwick J.W., Macdonald R., Ali A.A., Glogauer M., Magalhaes M.A. TNFalpha Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021;11:741013. doi: 10.3389/fonc.2021.741013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagyuu T., Hatakeyama K., Imada M., Kurihara M., Matsusue Y., Yamamoto K., Obayashi C., Kirita T. Programmed death ligand 1 (PD-L1) expression and tumor microenvironment: Implications for patients with oral precancerous lesions. Oral. Oncol. 2017;68:36–43. doi: 10.1016/j.oraloncology.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Cirillo N., Morgan D.J., Pedicillo M.C., Celentano A., Lo Muzio L., McCullough M.J., Prime S.S. Characterisation of the cancer-associated glucocorticoid system: Key role of 11β-hydroxysteroid dehydrogenase type 2. Br. J. Cancer. 2017;117:984–993. doi: 10.1038/bjc.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnet M. Cancer: A biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouketsu A., Sato I., Oikawa M., Shimizu Y., Saito H., Tashiro K., Yamashita Y., Takahashi T., Kumamoto H. Regulatory T cells and M2-polarized tumour-associated macrophages are associated with the oncogenesis and progression of oral squamous cell carcinoma. Int. J. Oral. Maxillofac. Surg. 2019;48:1279–1288. doi: 10.1016/j.ijom.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Migliorati C.A., Migliorati E.K., Silverman S., Jr Greenspan D., Greenspan J.S. Phenotypic identification of mononuclear cells in oral premalignant lesions and cancer by monoclonal antibodies. J. Oral. Pathol. 1986;15:352–358. doi: 10.1111/j.1600-0714.1986.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 17.Kujan O., Agag M., Smaga M., Vaishnaw Y., Idrees M., Shearston K., Farah C.S. PD-1/PD-L1, Treg-related proteins, and tumour-infiltrating lymphocytes are associated with the development of oral squamous cell carcinoma. Pathology. 2021;54:409–416. doi: 10.1016/j.pathol.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Bondad-Palmario G.G. Histological and immunochemical studies of oral leukoplakia: Phenotype and distribution of immunocompetent cells. J. Philipp. Dent. Assoc. 1995;47:3–18. doi: 10.2330/joralbiosci1965.36.87. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Hidalgo A., Murrah V., Fedoriw Y., Padilla R.J. Relationship of infiltrating intraepithelial T lymphocytes in the diagnosis of oral lichen planus versus oral epithelial dysplasia: A pilot study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2019;127:e123–e135. doi: 10.1016/j.oooo.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Gannot G., Gannot I., Vered H., Buchner A., Keisari Y. Increase in immune cell infiltration with progression of oral epithelium from hyperkeratosis to dysplasia and carcinoma. Br. J. Cancer. 2002;86:1444–1448. doi: 10.1038/sj.bjc.6600282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loning T., Burkhardt A. Plasma cells and immunoglobulin-synthesis in oral precancer and cancer. Correlation with dysplasia, cancer differentiation, radio- and chemotherapy. Virchows Arch. A Pathol. Anat. Histol. 1979;384:109–120. doi: 10.1007/BF00427156. [DOI] [PubMed] [Google Scholar]
- 22.Strauss L., Bergmann C., Szczepanski M., Gooding W., Johnson J.T., Whiteside T.L. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Pt 1Clin. Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J., Wang Z., Han J., Qiu X., Pan J., Chen J. Increased frequency of CD4+ CD25+ FOXP3+ cells correlates with the progression of 4-nitroquinoline1-oxide-induced rat tongue carcinogenesis. Clin. Oral. Investig. 2014;18:1725–1730. doi: 10.1007/s00784-013-1146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y., Liu N., Guan X., Wu H., Sun Z., Zeng H. Immunosuppression Induced by Chronic Inflammation and the Progression to Oral Squamous Cell Carcinoma. Mediators Inflamm. 2016;2016:5715719. doi: 10.1155/2016/5715719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gan C., Lee B.K.B., Lau S.H., Kallarakkal T.G., Zaini Z.M., Zain R.B., Sathasivam H.P., Yeong J.P.S., Savelyeva N., Thomas G., et al. 911 Immune profiling reveals enrichment of distinct immune signatures in high-risk oral potentially malignant disorders. J. ImmunoTherapy Cancer. 2021;9:A957. doi: 10.1136/jitc-2021-SITC2021.911. [DOI] [Google Scholar]
- 26.Chang D.T., Jones J.A., Meyerson H., Colton E., Kwon I.K., Matsuda T., Anderson J.M. Lymphocyte/macrophage interactions: Biomaterial surface-dependent cytokine, chemokine, and matrix protein production. J. Biomed. Mater. Res. A. 2008;87:676–687. doi: 10.1002/jbm.a.31630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawsar H.I., Weinberg A., Hirsch S.A., Venizelos A., Howell S., Jiang B., Jin G. Overexpression of human beta-defensin-3 in oral dysplasia: Potential role in macrophage trafficking. Oral. Oncol. 2009;45:696–702. doi: 10.1016/j.oraloncology.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Rangel R., Pickering C.R., Sikora A.G., Spiotto M.T. Genetic Changes Driving Immunosuppressive Microenvironments in Oral Premalignancy. Front. Immunol. 2022;13:840923. doi: 10.3389/fimmu.2022.840923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber M., Wehrhan F., Baran C., Agaimy A., Büttner-Herold M., Öztürk H., Neubauer K., Wickenhauser C., Kesting M., Ries J. Malignant transformation of oral leukoplakia is associated with macrophage polarization. J. Transl. Med. 2020;18:11. doi: 10.1186/s12967-019-02191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigeoka M., Koma Y.I., Nishio M., Komori T., Yokozaki H. CD163(+) macrophages infiltration correlates with the immunosuppressive cytokine interleukin 10 expression in tongue leukoplakia. Clin. Exp. Dent. Res. 2019;5:627–637. doi: 10.1002/cre2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shigeoka M., Koma Y.I., Kanzawa M., Akashi M., Yokozaki H. Intraepithelial Macrophage Expressing CD163 Is a Histopathological Clue to Evaluate the Malignant Potency of Oral Lichenoid Condition: A Case Report and Immunohistochemical Investigation. Diagnostics. 2020;10:624. doi: 10.3390/diagnostics10090624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Geng X., Hou J., Wu G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021;21:389. doi: 10.1186/s12935-021-02089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagyuu T., Funayama N., Imada M., Kirita T. Effect of smoking status and programmed death-ligand 1 expression on the microenvironment and malignant transformation of oral leukoplakia: A retrospective cohort study. PLoS ONE. 2021;16:e0250359. doi: 10.1371/journal.pone.0250359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouaoud J., Foy J.-P., Tortereau A., Michon L., Lavergne V., Gadot N., Boyault S., Valantin J., De Souza G., Zrounba P., et al. Early changes in the immune microenvironment of oral potentially malignant disorders reveal an unexpected association of M2 macrophages with oral cancer free survival. Oncoimmunology. 2021;10:1944554. doi: 10.1080/2162402X.2021.1944554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori K., Haraguchi S., Hiori M., Shimada J., Ohmori Y. Tumor-associated macrophages in oral premalignant lesions coexpress CD163 and STAT1 in a Th1-dominated microenvironment. BMC Cancer. 2015;15:573. doi: 10.1186/s12885-015-1587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y., Zhang S., Sun J., Wang T., Liu Q., Wu G., Qian Y., Yang W., Wang Y., Wang W. Cigarette smoke promotes oral leukoplakia via regulating glutamine metabolism and M2 polarization of macrophage. Int. J. Oral. Sci. 2021;13:25. doi: 10.1038/s41368-021-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stasikowska-Kanicka O., Wagrowska-Danilewicz M., Danilewicz M. T cells are involved in the induction of macrophage phenotypes in oral leukoplakia and squamous cell carcinoma-a preliminary report. J. Oral. Pathol. Med. 2018;47:136–143. doi: 10.1111/jop.12657. [DOI] [PubMed] [Google Scholar]
- 38.Ye X., Zhang J., Lu R., Zhou G. Signal regulatory protein alpha associated with the progression of oral leukoplakia and oral squamous cell carcinoma regulates phenotype switch of macrophages. Oncotarget. 2016;7:81305–81321. doi: 10.18632/oncotarget.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shigeoka M., Koma Y.I., Nishio M., Akashi M., Yokozaki H. Alteration of Macrophage Infiltrating Compartment: A Novel View on Oral Carcinogenesis. Pathobiology. 2021;88:327–337. doi: 10.1159/000515922. [DOI] [PubMed] [Google Scholar]
- 40.Mori K., Hiroi M., Shimada J., Ohmori Y. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers. 2011;3:3726–3739. doi: 10.3390/cancers3043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shigeoka M., Koma Y.I., Kodama T., Nishio M., Akashi M., Yokozaki H. Intraepithelial CD163(+) macrophages in tongue leukoplakia biopsy: A promising tool for cancer screening. Oral. Dis. 2020;26:527–536. doi: 10.1111/odi.13269. [DOI] [PubMed] [Google Scholar]
- 42.Dadi S., Chhangawala S., Whitlock B.M., Franklin R.A., Luo C.T., Oh S.A., Toure A., Pritykin Y., Huse M., Leslie C.S., et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell. 2016;164:365–377. doi: 10.1016/j.cell.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 44.Zitvogel L. Dendritic and natural killer cells cooperate in the control/switch of innate immunity. J. Exp. Med. 2002;195:F9–F14. doi: 10.1084/jem.20012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.-J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y.P., Chen I.C., Wu Y.H., Wu Y.C., Chen H.M., Yu-Fong Chang J. Langerhans cell counts in oral epithelial dysplasia and their correlation to clinicopathological parameters. J. Formos. Med. Assoc. 2017;116:457–463. doi: 10.1016/j.jfma.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Araújo C.P., Gurgel C.A.S., Ramos E.A.G., Freitas V.S., Júnior A.d.A.B., Ramalho L.M.P., dos Santos J.N. Accumulation of CD1a-positive Langerhans cells and mast cells in actinic cheilitis. J. Mol. Histol. 2010;41:357–365. doi: 10.1007/s10735-010-9297-z. [DOI] [PubMed] [Google Scholar]
- 48.Kindt N., Descamps G., Seminerio I., Bellier J., Lechien J.R., Pottier C., Larsimont D., Journé F., Delvenne P., Saussez S. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral. Oncol. 2016;62:1–10. doi: 10.1016/j.oraloncology.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Rani S.V., Aravindha B., Leena S., Balachander N., Malathi L.K., Masthan M.K. Role of abnormal Langerhans cells in oral epithelial dysplasia and oral squamous cell carcinoma: A pilot study. J. Nat. Sci. Biol. Med. 2015;6((Suppl. 1)):S128–S133. doi: 10.4103/0976-9668.166120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Upadhyay J., Rao N.N., Upadhyay R.B. A comparative analysis of langerhans cell in oral epithelial dysplasia and oral squamous cell carcinoma using antibody CD-1a. J. Cancer Res. Ther. 2012;8:591–597. doi: 10.4103/0973-1482.106565. [DOI] [PubMed] [Google Scholar]
- 51.Da Silva L.C., Fonseca F.P., de Almeida O.P., de Almeida Mariz B.A.L., Lopes M.A., Radhakrishnan R., Sharma M., Kowalski L.P., Vargas P.A. CD1a+ and CD207+ cells are reduced in oral submucous fibrosis and oral squamous cell carcinoma. Med. Oral. Patol. Oral. Cir. Bucal. 2020;25:e49–e55. doi: 10.4317/medoral.23177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Telagi N., Ahmed Mujib B.R., Kulkarni P.G., Naik R. The master switch: Comparative study of mast cell in oral epithelial dysplasia, oral submucous fibrosis and oral squamous cells carcinoma and their association with inflammation and angiogenesis. J. Oral. Maxillofac. Pathol. 2015;19:25–29. doi: 10.4103/0973-029X.157196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madhuri RAnkle A.D.K., Nayak R. Mast cells are increased in leukoplakia, oral submucous fibrosis, oral lichen planus and oral squamous cell carcinoma. J. Oral. Maxillofac. Pathol. 2007;11:18–22. [Google Scholar]
- 54.Bhatt A., Dholakia H. Mast cell density in oral submucous fibrosis. J. Indian. Dent. Assoc. 1977;49:187–191. [Google Scholar]
- 55.Zhao Z.Z., Savage N.W., Pujic Z., Walsh L.J. Immunohistochemical localization of mast cells and mast cell-nerve interactions in oral lichen planus. Oral. Dis. 1997;3:71–76. doi: 10.1111/j.1601-0825.1997.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhao Z.Z., Sugerman P.B., Zhou X.J., Walsh L.J., Savage N.W. Mast cell degranulation and the role of T cell RANTES in oral lichen planus. Oral. Dis. 2001;7:246–251. doi: 10.1034/j.1601-0825.2001.70408.x. [DOI] [PubMed] [Google Scholar]
- 57.Jontell M., Hansson H.A., Nygren H. Mast cells in oral lichen planus. J. Oral. Pathol. 1986;15:273–275. doi: 10.1111/j.1600-0714.1986.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Z.Z., Savage N.W., Sugerman P.B., Walsh L.J. Mast cell/T cell interactions in oral lichen planus. J. Oral. Pathol. Med. 2002;31:189–195. doi: 10.1034/j.1600-0714.2002.310401.x. [DOI] [PubMed] [Google Scholar]
- 59.Ashish Shrestha S.K., Raut T. Evaluation of Mast Cells in Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Int. J. Dent. 2021;2021:5. doi: 10.1155/2021/5609563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribatti D., Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Dvorak A.M. Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem. Immunol. Allergy. 2005;85:135–184. doi: 10.1159/000086516. [DOI] [PubMed] [Google Scholar]
- 62.Coussens L.M., Raymond W.W., Bergers G., Laig-Webster M., Behrendtsen O., Werb Z., Caughey G.H., Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes. Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michailidou E.Z., Markopoulos A.K., Antoniades D.Z. Mast cells and angiogenesis in oral malignant and premalignant lesions. Open Dent. J. 2008;2:126–132. doi: 10.2174/1874210600802010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iamaroon A., Pongsiriwet S., Jittidecharaks S., Pattanaporn K., Prapayasatok S., Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2003;32:195–199. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 65.Saxena S., Singh A., Singh P., Sundaragiri K.S., Sankhla B., Bhargava A. Evaluating the Role of Immunological Cells (Tissue Eosinophils and Mast Cells) in Progression of Oral Squamous Cell Carcinoma. Mymensingh Med. J. 2018;27:382–388. [PubMed] [Google Scholar]
- 66.Laishram D., Rao K., Devi H.S.U., Priya N.S., Smitha T., Sheethal H.S. Mast cells and angiogenesis in malignant and premalignant oral lesions: An immunohistochemical study. J. Oral. Maxillofac. Pathol. 2017;21:229–238. doi: 10.4103/jomfp.JOMFP_111_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jyothsna M., Rammanohar M., Kumar K. Histomorphometric Analysis of Angiogenesis using CD31 Immunomarker and Mast Cell Density in Oral Premalignant and Malignant Lesions: A Pilot Study. J. Clin. Diagn. Res. 2017;11:ZC37–ZC40. doi: 10.7860/JCDR/2017/23870.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramsridhar S., Narasimhan M. Immunohistochemical Evaluation of Mast Cells in Leukoplakia and Oral Squamous Cell Carcinoma. J. Clin. Diagn. Res. 2016;10:ZC100–ZC103. doi: 10.7860/JCDR/2016/19297.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sathyakumar M., Sriram G., Saraswathi T., Sivapathasundharam B. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral precancerous lesion-leukoplakia. J. Oral. Maxillofac. Pathol. 2012;16:343–348. doi: 10.4103/0973-029X.102481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinra M., Ramalingam K., Sarkar A., Rehman F., Girish K. Comparison of mast cell count and mast cell density in normal mucosa, oral leukoplakia, oral lichen planus, oral submucous fibrosis and oral squamous cell carcinoma–a study on 50cases. J. Pharm. Sci. Inn. 2012;1:4–11. [Google Scholar]
- 71.Sabarinath B., Sriram G., Saraswathi T.R., Sivapathasundharam B. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral submucous fibrosis. Indian. J. Dent. Res. 2011;22:116–121. doi: 10.4103/0970-9290.80009. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira-Neto H.H., Leite A.F., Costa N.L., Alencar R.C., Lara V.S., Silva T.A., Leles C.R., Mendonça F.E., Batista A.C. Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cells. Oral. Oncol. 2007;43:484–490. doi: 10.1016/j.oraloncology.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Singh S., Gupta V., Vij R., Aggarwal R., Sharma B., Nagpal M. Evaluation of mast cells in oral premalignant and malignant lesions: A histochemical study. Natl. J. Maxillofac. Surg. 2018;9:184–190. doi: 10.4103/njms.NJMS_49_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pang X., Fan H.-Y., Tang Y.-L., Wang S.-S., Cao M.-X., Wang H.-F., Dai L.-L., Wang K., Yu X.-H., Wu J.-B., et al. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE. 2020;15:e0229089. doi: 10.1371/journal.pone.0229089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen L., Mu W., Lu H., Wang X., Fang J., Jia Y., Li Q., Wang D., Wen S., Guo J., et al. Porphyromonas gingivalis Promotes Oral Squamous Cell Carcinoma Progression in an Immune Microenvironment. J. Dent. Res. 2020;99:666–675. doi: 10.1177/0022034520909312. [DOI] [PubMed] [Google Scholar]
- 76.Bhaskaran N., Jayaraman S., Quigley C., Mamileti P., Ghannoum M., Weinberg A., Thuener J., Pan Q., Pandiyan P. The Role of Dectin-1 Signaling in Altering Tumor Immune Microenvironment in the Context of Aging. Front. Oncol. 2021;11:669066. doi: 10.3389/fonc.2021.669066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madhura M.G., Gajalakshmi S., Kumar B.V., Suma S., Sarita Y., Shweta R.D. Role of tissue eosinophils in oral Leukoplakia: A pilot study. J. Oral. Maxillofac. Pathol. 2015;19:286–290. doi: 10.4103/0973-029X.174647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jablonska E., Garley M., Surazynski A., Grubczak K., Iwaniuk A., Borys J., Moniuszko M., Ratajczak-Wrona W. Neutrophil extracellular traps (NETs) formation induced by TGF-beta in oral lichen planus—Possible implications for the development of oral cancer. Immunobiology. 2020;225:151901. doi: 10.1016/j.imbio.2019.151901. [DOI] [PubMed] [Google Scholar]
- 79.Jain M., Kasetty S., Sudheendra U.S., Tijare M., Khan S., Desai A. Assessment of tissue eosinophilia as a prognosticator in oral epithelial dysplasia and oral squamous cell carcinoma-an image analysis study. Patholog Res. Int. 2014;2014:507512. doi: 10.1155/2014/507512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deepthi G., Kulkarni P.G. SRKN Eosinophils: An imperative histopathological prognostic indicator for oral squamous cell carcinoma. J. Oral. Maxillofac. Pathol. 2019;23:307. doi: 10.4103/jomfp.JOMFP_111_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Correa-Gallegos D., Jiang D., Rinkevich Y. Fibroblasts as confederates of the immune system. Immunol. Rev. 2021;302:147–162. doi: 10.1111/imr.12972. [DOI] [PubMed] [Google Scholar]
- 82.Bautista-Hernandez L.A., Gomez-Olivares J.L., Buentello-Volante B., Bautista-de Lucio V.M. Fibroblasts: The Unknown Sentinels Eliciting Immune Responses Against Microorganisms. Eur. J. Microbiol. Immunol. 2017;7:151–157. doi: 10.1556/1886.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chansard A., Dubrulle N., De Molliens M.P., Falanga P.B., Stephen T., Hasan M., Van Zandbergen G., Aulner N., Shorte S.L., David-Watine B. Unveiling Interindividual Variability of Human Fibroblast Innate Immune Response Using Robust Cell-Based Protocols. Front. Immunol. 2020;11:569331. doi: 10.3389/fimmu.2020.569331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao X., Xu J., Wang W., Liang C., Hua J., Liu J., Zhang B., Meng Q., Yu X., Shi S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davidson S., Coles M., Thomas T., Kollias G., Ludewig B., Turley S., Brenner M., Buckley C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021;21:704–717. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 86.Harper J., Sainson R.C. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin. Cancer Biol. 2014;25:69–77. doi: 10.1016/j.semcancer.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Liu T., Han C., Wang S., Fang P., Ma Z., Xu L., Yin R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaudhary M., Gadbail A.R., Vidhale G., Mankar M.P., Gondivkar S.M., Gawande M., Patil S. Comparison of myofibroblasts expression in oral squamous cell carcinoma, verrucous carcinoma, high risk epithelial dysplasia, low risk epithelial dysplasia and normal oral mucosa. Head Neck Pathol. 2012;6:305–313. doi: 10.1007/s12105-012-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta K., Metgud R., Gupta J. Evaluation of stromal myofibroblasts in oral leukoplakia, oral submucous fibrosis, and oral squamous cell carcinoma--an immunohistochemical study. J. Cancer Res. Ther. 2015;11:893–898. doi: 10.4103/0973-1482.147700. [DOI] [PubMed] [Google Scholar]
- 90.Angadi P.V., Kale A.D., Hallikerimath S. Evaluation of myofibroblasts in oral submucous fibrosis: Correlation with disease severity. J. Oral. Pathol. Med. 2011;40:208–213. doi: 10.1111/j.1600-0714.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- 91.Kapse S.C., Rathod N., Baad R., Mandlik J., Sharma A.S., Bommanavar S. Quantitative assessment of myofibroblast in severe dysplasia, microinvasion and oral squamous cell carcinoma: An immunohistochemical study. J. Contemp. Dent. Pract. 2013;14:34–38. doi: 10.5005/jp-journals-10024-1265. [DOI] [PubMed] [Google Scholar]
- 92.Vered M., Allon I., Buchner A., Dayan D. Stromal myofibroblasts accompany modifications in the epithelial phenotype of tongue dysplastic and malignant lesions. Cancer Microenviron. 2009;2:49–57. doi: 10.1007/s12307-009-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jayaraj G., Sherlin H.J., Ramani P., Premkumar P., Natesan A. Stromal myofibroblasts in oral squamous cell carcinoma and potentially malignant disorders. Indian. J. Cancer. 2015;52:87–92. doi: 10.4103/0019-509X.175580. [DOI] [PubMed] [Google Scholar]
- 94.de-Assis E.M., Pimenta L.G., Costa-e-Silva E., Souza P.E., Horta M.C. Stromal myofibroblasts in oral leukoplakia and oral squamous cell carcinoma. Med. Oral. Patol. Oral. Cir. Bucal. 2012;17:e733–e738. doi: 10.4317/medoral.17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Etemad-Moghadam S., Khalili M., Tirgary F., Alaeddini M. Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J. Oral. Pathol. Med. 2009;38:639–643. doi: 10.1111/j.1600-0714.2009.00768.x. [DOI] [PubMed] [Google Scholar]
- 96.Parajuli H., Teh M.-T., Abrahamsen S., Christoffersen I., Neppelberg E., Lybak S., Osman T., Johannessen A.C., Gullberg D., Skarstein K., et al. Integrin alpha11 is overexpressed by tumour stroma of head and neck squamous cell carcinoma and correlates positively with alpha smooth muscle actin expression. J. Oral. Pathol. Med. 2017;46:267–275. doi: 10.1111/jop.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coletta R.D., Salo T. Myofibroblasts in oral potentially malignant disorders: Is it related to malignant transformation? Oral. Dis. 2018;24:84–88. doi: 10.1111/odi.12694. [DOI] [PubMed] [Google Scholar]
- 98.Dayan D., Salo T., Salo S., Nyberg P., Nurmenniemi S., Costea D.E., Vered M. Molecular crosstalk between cancer cells and tumor microenvironment components suggests potential targets for new therapeutic approaches in mobile tongue cancer. Cancer Med. 2012;1:128–140. doi: 10.1002/cam4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kellermann M.G., Sobral L.M., da Silva S.D., Zecchin K.G., Graner E., Lopes M.A., Kowalski L.P., Coletta R.D. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: Induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral. Oncol. 2008;44:509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 100.Kawashiri S., Tanaka A., Noguchi N., Hase T., Nakaya H., Ohara T., Kato K., Yamamoto E. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Head Neck. 2009;31:1346–1353. doi: 10.1002/hed.21097. [DOI] [PubMed] [Google Scholar]
- 101.Kelner N., Rodrigues P.C., Bufalino A., Fonseca F.P., dos Santos-Silva A.R., Miguel M.C.C., Pinto C.A.L., Leme A.F.P., Graner E., Salo T., et al. Activin A immunoexpression as predictor of occult lymph node metastasis and overall survival in oral tongue squamous cell carcinoma. Head Neck. 2015;37:479–486. doi: 10.1002/hed.23627. [DOI] [PubMed] [Google Scholar]
- 102.Cavallo F., De Giovanni C., Nanni P., Forni G., Lollini P.L. 2011: The immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–326. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Albini A., Tosetti F., Benelli R., Noonan D.M. Tumor inflammatory angiogenesis and its chemoprevention. Cancer Res. 2005;65:10637–10641. doi: 10.1158/0008-5472.CAN-05-3473. [DOI] [PubMed] [Google Scholar]
- 104.Duray A., Demoulin S., Hubert P., Delvenne P., Saussez S. Immune suppression in head and neck cancers: A review. Clin. Dev. Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ries J., Agaimy A., Vairaktaris E., Kwon Y., Neukam F.W., Strassburg L.H., Nkenke E. Evaluation of MAGE-A expression and grade of dysplasia for predicting malignant progression of oral leukoplakia. Int. J. Oncol. 2012;41:1085–1093. doi: 10.3892/ijo.2012.1532. [DOI] [PubMed] [Google Scholar]
- 106.Young M.R., Neville B.W., Chi A.C., Lathers D.M., Boyd Gillespie M., Day T.A. Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma. Cancer Immunol. Immunother. 2007;56:1077–1086. doi: 10.1007/s00262-006-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saini R., Lee N.V., Liu K.Y., Poh C.F. Prospects in the Application of Photodynamic Therapy in Oral Cancer and Premalignant Lesions. Cancers. 2016;8:83. doi: 10.3390/cancers8090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poh C.M., Zheng J., Channappanavar R., Chang Z.W., Nguyen T.H.O., Rénia L., Kedzierska K., Perlman S., Poon L.L.M. Multiplex Screening Assay for Identifying Cytotoxic CD8(+) T Cell Epitopes. Front. Immunol. 2020;11:400. doi: 10.3389/fimmu.2020.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Simoni Y., Becht E., Fehlings M., Loh C.Y., Koo S.L., Teng K.W.W., Yeong J.P.S., Nahar R., Zhang T., Kared H., et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 110.de Visser K.E., Eichten A., Coussens L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 111.He X., Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elmusrati A., Wang J., Wang C.Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral. Sci. 2021;13:24. doi: 10.1038/s41368-021-00131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weber M., Lutz R., Olmos M., Glajzer J., Baran C., Nobis C.P., Möst T., Eckstein M., Kesting M., Ries J. Beyond PD-L1-Identification of Further Potential Therapeutic Targets in Oral Cancer. Cancers. 2022;14:1812. doi: 10.3390/cancers14071812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cirillo N., Wu C., Prime S.S. Heterogeneity of Cancer Stem Cells in Tumorigenesis, Metastasis, and Resistance to Antineoplastic Treatment of Head and Neck Tumours. Cells. 2021;10:3068. doi: 10.3390/cells10113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hsu J.M., Xia W., Hsu Y.H., Chan L.C., Yu W.H., Cha J.H., Chen C.T., Liao H.W., Kuo C.W., Khoo K.H., et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat. Commun. 2018;9:1908. doi: 10.1038/s41467-018-04313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee Y., Shin J.H., Longmire M., Wang H., Kohrt H.E., Chang H.Y., Sunwoo J.B. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin. Cancer Res. 2016;22:3571–3581. doi: 10.1158/1078-0432.CCR-15-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lechner A., Schlößer H., Rothschild S.I., Thelen M., Reuter S., Zentis P., Shimabukuro-Vornhagen A., Theurich S., Wennhold K., Garcia-Marquez M., et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget. 2017;8:44418–44433. doi: 10.18632/oncotarget.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zheng Y., Zhang J., Huang W., Zhong L.L.D., Wang N., Wang S., Yang B., Wang X., Pan B., Situ H., et al. Sini San Inhibits Chronic Psychological Stress-Induced Breast Cancer Stemness by Suppressing Cortisol-Mediated GRP78 Activation. Front. Pharmacol. 2021;12:714163. doi: 10.3389/fphar.2021.714163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ponandai-Srinivasan S., Lalitkumar P.G., Garcia L., Varghese S.J., Carlson J.W., Gemzell-Danielsson K., Floter Radestad A. Mifepristone mediates anti-proliferative effect on ovarian mesenchymal stem/stromal cells from female BRCA1−/2− carriers. Acta Obstet. Gynecol. Scand. 2019;98:250–261. doi: 10.1111/aogs.13485. [DOI] [PubMed] [Google Scholar]
- 120.Chimal-Ramírez G.K., Espinoza-Sánchez N.A., Fuentes-Pananá E.M. Protumor activities of the immune response: Insights in the mechanisms of immunological shift, oncotraining, and oncopromotion. J. Oncol. 2013;2013:835956. doi: 10.1155/2013/835956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kondoh N., Mizuno-Kamiya M. The Role of Immune Modulatory Cytokines in the Tumor Microenvironments of Head and Neck Squamous Cell Carcinomas. Cancers. 2022;14:2884. doi: 10.3390/cancers14122884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lewis C.E., Pollard J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 123.Hassona Y., Cirillo N., Heesom K., Parkinson E.K., Prime S.S. Senescent cancer-associated fibroblasts secrete active MMP-2 that promotes keratinocyte dis-cohesion and invasion. Br. J. Cancer. 2014;111:1230–1237. doi: 10.1038/bjc.2014.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hassona Zhang P., Chua N.Q.E., Dang S., Davis A., Chong K.W., Prime S.S., Cirillo N. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents-Does Fibroblast Senescence Play a Role? Int. J. Mol. Sci. 2022;23:1637. doi: 10.3390/ijms23031637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amin M.B., Edge S., Greene F., Byrd D.R., Brookland R.K., Washington M.K., Gershenwald J.E., Compton C.C., Hess K.R., Sullivan D.C., et al. AJCC Cancer Staging Manual. Springer International Publishing; New York, NY, USA: 2018. [Google Scholar]
- 126.Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 127.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 128.Kather J.N., Suarez-Carmona M., Charoentong P., Weis C.A., Hirsch D., Bankhead P., Horning M., Ferber D., Kel I., Herpel E., et al. Topography of cancer-associated immune cells in human solid tumors. eLife. 2018;7:e36967. doi: 10.7554/eLife.36967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Troiano G., Rubini C., Togni L., Caponio V.C.A., Zhurakivska K., Santarelli A., Cirillo N., Lo Muzio L., Mascitti M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020;9:8333–8344. doi: 10.1002/cam4.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Almangush A., De Keukeleire S., Rottey S., Ferdinande L., Vermassen T., Leivo I., Mäkitie A.A. Tumor-Infiltrating Lymphocytes in Head and Neck Cancer: Ready for Prime Time? Cancers. 2022;14:1558. doi: 10.3390/cancers14061558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Troiano G., Caponio V.C.A., Adipietro I., Tepedino M., Santoro R., Laino L., Lo Russo L., Cirillo N., Lo Muzio L. Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral. Oncol. 2019;93:66–75. doi: 10.1016/j.oraloncology.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 132.Mariani P., Russo D., Maisto M., Troiano G., Caponio V.C.A., Annunziata M., Laino L. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis. J. Oral. Pathol. Med. 2022;51:39–51. doi: 10.1111/jop.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Riley R.D., Hayden J.A., Steyerberg E.W., Moons K.G., Abrams K., Kyzas P.A., Malats N., Briggs A., Schroter S., Altman D.G., et al. Prognosis Research Strategy (PROGRESS) 2, prognostic factor research. PLoS Med. 2013;10:e1001380. doi: 10.1371/journal.pmed.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steyerberg E.W., Moons K.G., van der Windt D.A., Hayden J.A., Perel P., Schroter S., Riley R.D., Hemingway H., Altman D.G., PROGRESS Group Prognosis Research Strategy (PROGRESS) 3, prognostic model research. PLoS Med. 2013;10:e1001381. doi: 10.1371/journal.pmed.1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Russo D., Mariani P., Caponio V.C.A., Lo Russo L., Fiorillo L., Zhurakivska K., Lo Muzio L., Laino L., Troiano G. Development and Validation of Prognostic Models for Oral Squamous Cell Carcinoma: A Systematic Review and Appraisal of the Literature. Cancers. 2021;13:5755. doi: 10.3390/cancers13225755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Almangush A., Bello I.O., Heikkinen I., Hagström J., Haglund C., Kowalski L.P., Coletta R.D., Mäkitie A.A., Salo T., Leivo I. Improving Risk Stratification of Early Oral Tongue Cancer with TNM-Immune (TNM-I) Staging System. Cancers. 2021;13:3235. doi: 10.3390/cancers13133235. [DOI] [PMC free article] [PubMed] [Google Scholar]