Abstract
Introduction
Patient and public involvement (PPI) in research emphasizes the importance of doing research with, rather than for people with lived health/illness experience(s). The purpose of this scoping review is to investigate the breadth and depth of scientific literature on PPI in cancer research and to identify how is PPI applied and reported in cancer research.
Methods
We searched MEDLINE, Embase, CINAHL, and PsycInfo up to March 2022. All titles/abstracts and full‐text results were screened by two reviewers. Data were analyzed and are presented in both narrative and tabular format.
Results
We screened 22,009 titles/abstract, reviewed 375 full‐text articles, of which 101 studies were included in this review. 66 papers applied PPI; 35 used co‐design methodologies. PPI in cancer research in published research has increased steadily since 2015 and often includes those with a past diagnosis of cancer or relatives/informal caregivers. The most common applied methods were workshops or interviews. PPI was generally used at the level of consultation/advisor and occurred mainly in early stages of research. Costs related to PPI were mentioned in 25 papers and four papers described training provided for PPI.
Conclusions
Results of our review demonstrate the nature and extent of PPI expansion in cancer research. Researchers and research organizations entering the fray of PPI should consider planning and reporting elements such as the stage, level, and role type of PPI, as well as methods and strategies put in place to assure diversity. Furthermore, a thorough evaluation of whether all these elements meet the stated PPI purpose will help to grasp its impact on research outcomes.
Patient or Public Contribution
Two patients participated in the stakeholder consultation as part of the scoping review methodology, contributed to the discussion on refining the results, and critically reviewed the manuscript. Both are co‐authors of this manuscript.
Keywords: cancer, patient and public involvement, PPI, research
1. INTRODUCTION
There is growing recognition of the value of patient and public involvement (PPI) in research, and specifically in health research, as a means of improving validity and relevance of research findings. 1 , 2 , 3 While many definitions of PPI exist, in this paper we draw on the definition from the National Institute for Health and Care Research (NIHR), which describes PPI as: “research conducted ‘with’ or ‘by’ members of the public rather than ‘for’ or ‘about’ them”. 4 PPI approaches recognize the experiential knowledge of people with lived health or illness experiences and posit that the incorporation of such voices in research will improve the effectiveness and value of health research. 5 Those with lived experience may also bring unique insights to health research by increasing the quality, transparency and relevance of research to patients, improving recruitment and retention rates of research participants, broadening the range of people represented in studies, and improving the dissemination of results beyond the academic setting. 6 , 7 , 8
Numerous models and theoretical frameworks exist to support PPI in research. 9 , 10 The incorporation of PPI in health research is particularly well established and supported in the United States, Canada, and the UK by organizations including the Patient‐Centered Outcomes Research Institute (PCORI), 11 the Strategy for Patient Oriented Research (SPOR), 12 or the National Institute for Health and Care Research (NIHR), 5 respectively. PPI conceptualization differs depending on the framework, but in general it is acknowledged as a continuum ranging from lower to higher levels of involvement including information, participation, consultation, collaboration, to (the highest level) (co‐)leading. 5 , 11 , 13 , 14 , 15 , 16 , 17 , 18 Overall, PPI in research is still a developing phenomenon in many countries, which can take on different forms, corresponds to diverse practices, and entails multiple rationales. 9
Different aspects of interest have been highlighted in the literature that need to be considered when implementing a PPI approach in research. First, tokenism (or “tick‐box”) approaches to participation are a major challenge. That is, the inclusion or naming of a patient without their authentic engagement. We use italics here to emphasis the problematic nature of the idea of authentic engagement, and how this can be determined given variable preferences of patient partners. 9 Second, representation of diverse patient profiles in terms of socio‐demographics, economics, education, or cultural and linguistic diversity, remains an issue in the PPI context. 19 While it is crucial to clarify the role of each individual and plan PPI according to available time and resources, it is important to think about multiple spaces of involvement so that people with diverse backgrounds are invited and feel welcome to participate. Third, the resources and training necessary for patients and researchers to engage in PPI. There is a need to conceive innovative training practices (for both researchers and patients) that can better take into account the variety of persons' experiential knowledge, pre‐existing skills and needs for establishing a common understanding between patients, members of the public, and researchers. 20 , 21 Fourth, along with outcomes assessment, any research project that includes PPI should be accompanied by an evaluation to determine the experiences of the PPI partners, and determine areas to strengthen or improve (i.e., degree of social diversity or the level of shared decision‐making at specific stages of research). 22 , 23 Furthermore, although tools such as the Guidance for Reporting Involvement of Patients and the Public (GRIPP2) 24 aim to facilitate systematic reporting in health and social care research, inconsistent reporting on PPI makes it difficult for the research community to assess and understand the different PPI activities, losing opportunities to learn from previous experiences.
There has been growing interest among decision makers, researchers, and patients to engage PPI work in this constantly evolving cancer care and treatment space. In the field of cancer research, two systematic reviews from 2008 25 and 2018 2 explore the use of PPI among people with cancer. Both studies note the increasing recognition of PPI in cancer research and explore specifically why and how patients are involved. Hubbard and colleagues 25 outline the need for infrastructure to support PPI, including formal recruitment and training to support involvement activities. Furthermore, Hoffmann Pii and colleagues 2 identified a lack of evidence related to the processes and reporting of PPI, specifically during the definition and prioritization of research topics and the development of recruitment strategies. Since this last review has been published in 2018, there has been significant expansion of research using PPI approaches, and we sought to understand the current state of the literature.
Given the increasing focus on PPI in cancer research, the aim of this review is to: (i) examine the current use of PPI in cancer research and (ii) identify key elements related to PPI and describe their application in cancer research.
2. METHODS
To understand the current use of PPI in cancer research and identify how it is being applied in cancer research, we conducted a scoping review using the framework recommended by Arksey and O′Malley 26 and revised by Levac et al. 27 The framework consists of six steps: (1) identifying the research question; (2) identifying relevant studies; (3) developing eligibility criteria for study selection; (4) charting the extracted data; and (5) collating, summarizing, and reporting the results; and (6) stakeholder consultation.
2.1. Research question
The aim of this scoping review was to review the published scientific literature in PPI in cancer research from 2005 to March 2022 and report specifically on the application and reporting of PPI among these publications. The research questions driving this review are:
What is the breadth and depth of scientific literature on PPI in cancer research?
How is PPI applied and reported in cancer research?
2.2. Identifying relevant studies
We collaborated with a health sciences librarian (UE) experienced in PPI research. Two notable related publications on PPI in cancer were consulted in the development of our search strategy: (1) A 2018 Danish publication 2 covering literature published between December 2006–April 2017; and (2) A 2008 UK review of the literature 25 which included searches from 1994–2004. Based on the search parameters and design of these two reviews we set our search dates as 2005 onward to include all recent literature, while also capturing studies that may have been missed in prior publications.
A search strategy (Table SS1, *) was developed by UE and adapted to the following four databases, chosen as information sources for this scoping review: MEDLINE (OVID interface), Embase (OVID interface), Cumulative Index to Nursing and Allied Health Literature (CINAHL; EBSCO interface), and PsycInfo (EBSCO interface). The four databases were initially searched on March 12, 2021, with an update run March 3, 2022. Additional relevant articles were identified using a snowballing approach. The PRISMA flow diagram of the search and selection process is depicted in Figure 1.
FIGURE 1.
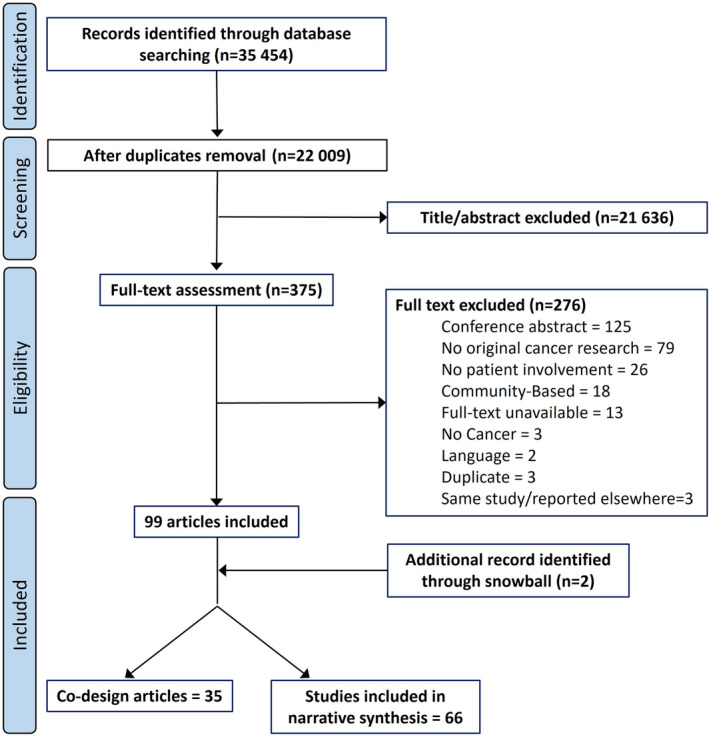
PRISMA flow diagram of the search and selection process.
2.3. Screening and eligibility criteria
Regarding the screening phase, peer‐reviewed original cancer studies describing the involvement of people affected by cancer (patients and/or relatives) or lay public were considered. There were no restrictions imposed on language within the search strategy; however, only articles that could be effectively translated into English, French, or Spanish were included in this review. We excluded opinion articles, discussion papers, editorials, commentaries, brief reports, protocols, and reviews.
In the eligibility phase (title/abstract screening), the inclusion of articles was restricted to the explicit mention of PPI or related terms. Papers using a Community‐Based Participatory Research approach (CBPR) were excluded. Although sharing similarities with PPI, CBPR approaches traditionally focus on behavioral or social change or inequalities and on community identity rather than individuals or small groups having a living experience with the disease. 28
2.4. Study selection
Studies were exported from the database interface and uploaded into Covidence software. 29 Following de‐duplication within Covidence, titles and abstracts were screened independently by two members of the review team (MS, SCL, or KRH). Discrepancies in eligibility screening were resolved by discussion to reach consensus. After title and abstract screening, full texts were reviewed (MS, SCL, KRH) for their eligibility. If two authors deemed a full‐text article to be ineligible for this review, the reason(s) were documented. 30
2.5. Charting the extracted data
An extraction grid was developed reflecting the elements related to the research question. The first and last authors piloted the extraction grid with three articles. Relevant data were collected in a tabular form including author, year, country, name of the study, type of disease, characteristics of the participants, characteristics of patients/relatives/public involved, stage of research where PPI was applied, level of involvement, role type, method for involvement, aim for applying PPI, and training and costs associated with PPI. Data extraction was completed independently by two reviewers (SCL and MS). Weekly meetings were conducted to discuss and resolve disagreements and assure reliability during the extraction process. Data were compiled in Microsoft Excel.
Articles describing the use of a co‐design approach (e.g., Experience‐Based Co‐Design, User Centered Design of digital applications) were kept in a separate group as these methodologies often imply active involvement as part of the participatory approach without the stated PPI aim. We identified co‐design studies where patients or members of the public were involved independent of the methodology (e.g., board members, steering committee).
2.6. Collating, summarizing, and reporting the results
Results were summarized using a descriptive and a narrative synthesis process. Descriptive results (frequency and percentages) are reported for countries, number of publications per year, methods, participants' characteristics, and cancer type. An iterative process was used to identify categories related to the purpose of PPI in cancer research, the level of involvement and role types, and the different stages of the research process. During the data abstraction phase, we categorized the levels of involvement based on established PPI frameworks (see Table S2) as participation, consultation, collaboration, or partnership. Next, we identified different role types of involvement, which we characterized as personal engagement, advisor/expert, or co‐researcher. Levels and role types were ultimately refined based on the iterative analysis. Definitions for each category are provided in the results section and in Table S5.
2.7. Stakeholder consultation
A consultation meeting was held including the research team and two patient partners (diverse in age and cancer experience) with experience in participating in research projects. The meeting aimed to gather input and refine the findings to increase the relevance of the results and confirm their validity in the specific context of PPI in cancer research. Preliminary results were presented by the first and last authors followed by an open discussion on the relevance of these results for the field, any important aspect missing, and how these findings might be disseminated to the stakeholders in the field. The main aspects of the discussion were collected, analyzed, and integrated in the manuscript.
3. FINDINGS
In total, 101 articles are included in this review: 66 cancer research studies applying PPI and 35 studies using a co‐design method (Figure 1). We first present an analysis of the 66 papers using a PPI approach, followed by a brief analysis of the 35 studies using a co‐design method.
3.1. Characteristics of the studies
Out of the 66 PPI articles, 16 originated in the United Kingdom, 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 14 in the United States, 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 12 in Australia, 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 7 in Canada, 73 , 74 , 75 , 76 , 77 , 78 , 79 2 in China, 80 , 81 and 1 in Japan. 82 European countries included: Switzerland (n = 2), 83 , 84 The Netherlands (n = 2), 85 , 86 and Spain (n = 1), 87 as well as two studies comprising several countries (n = 2). 88 , 89 Northern European countries included Norway (n = 3), 90 , 91 , 92 Denmark (n = 2), 93 , 94 Sweden (n = 2) 95 , 96 (Figure 2A). Overall, an increase in cancer research articles stating the involvement of patients or members of the public is observed from 2015 onwards with a sharp increase noted in 2020 (Figure 2B).
FIGURE 2.
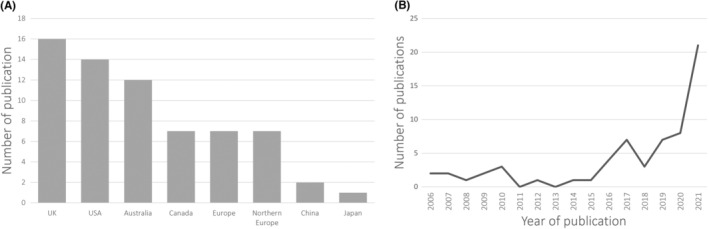
Characteristics of the selected studies based on country (A) and year (B) of publication.
3.2. Characteristics of the Patient/Public involved in the studies
Characteristics of participants involved in the research were extracted. Breast cancer was the type of cancer with the most articles reporting PPI (8/66), followed by pediatric (n = 6) and neuroendocrine cancers (n = 5). Most of the studies (17/66) did not specify the type of cancer of patients involved or included multiple types (9/66). Regarding the PPI target population, 4/66 included adolescents or young adults (AYAs) 41 , 44 , 68 , 94 and the rest consisted in adult population, of which 5/62 studies included parents of pediatric patients, 40 , 53 , 79 , 95 , 96 2/62 older adults, 32 , 76 and 1 study included patients with intellectual disability 90 (Table S3). People previously diagnosed with cancer not currently undergoing treatment, including survivors, were the most frequent type of PPI participants (39/66), followed by relatives or informal caregivers (36/66). Furthermore, 6 out of 66 studies incorporated the views of the public (those without cancer or caregiving experience) into cancer research (Table S3 and Figure 3). 32 , 34 , 42 , 58 , 90 , 93
FIGURE 3.
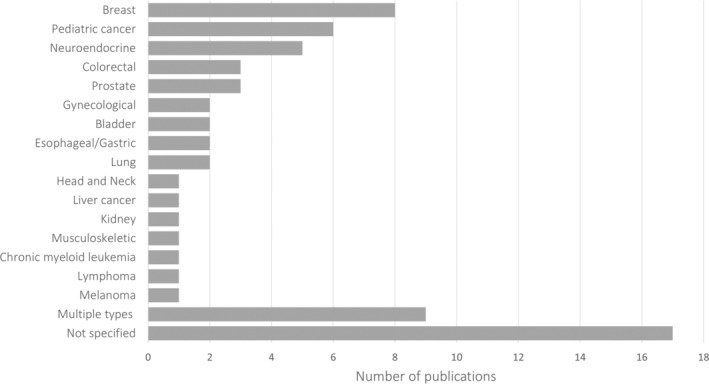
Selected studies distributed based on the main cancer type.
Half of the articles (35/66) included a mention to the PPI population diversity. Diversity referred to age, income, social status, rural/urban area, education, ethnicity, or under‐researched communities (Table S3). Based on the collected data, purposeful sampling was found to be the most commonly used method to ensure diversity among patient partners and members of the public. Various recruitment strategies were employed including hospital consultations, community organizations, charities, recruitment agencies, and even public spaces. Determining the exact number of patients, representatives, informal caregivers, or members of the public involved in each study was difficult, as many articles remained vague when reporting this information. Involvement ranged from one representative in the study steering committee to hundreds in the case of topic prioritization surveys (Table S3).
3.3. Purpose of PPI in cancer research
We identified four main purposes for PPI in cancer research (Table S4): (1) to assure that the research is relevant (e.g., defining research priorities or research questions); (2) to assure that the research is appropriate and the research documents are comprehensive (e.g., creating, defining or revising content elements in questionnaires and surveys, study documents, resources, electronic applications' interface); (3) to assure that the research is acceptable, feasible, or attainable (e.g., defining objectives, revising methods, helping and assuring recruitment) and (4) to assure actionability (e.g., defining strategies, next phases, implementation).
3.4. Methods used for PPI
The studies included a variety of qualitative and quantitative methods (Figure 4). Workshops and meetings (including steering/advisory board meetings) were the most frequently used methods followed by interviews. In order to reach consensus (i.e., prioritization) or establish ratings, several methods are used ranging from iterative processes (i.e., Delphi method), Nominal Group Techniques, to established methodologies such the James Lind Alliance process 42 , 44 , 77 , 78 , 79 or the Stakeholder Engagement in quEstion Development and Prioritization (SEED). 51
FIGURE 4.
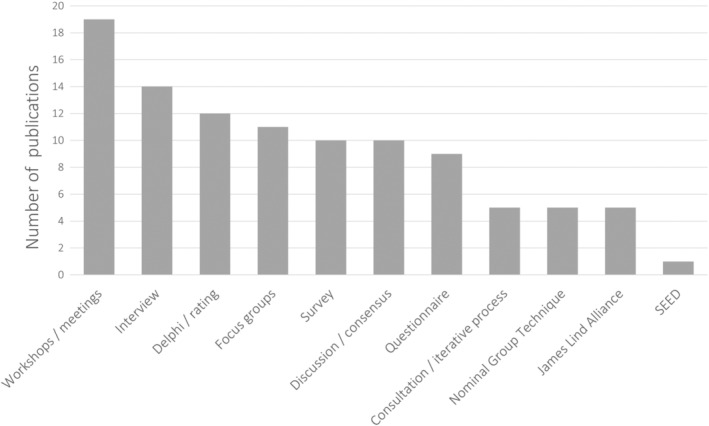
Overview on the different methods applied in PPI approaches. SEED (Stakeholder Engagement in quEstion Development and Prioritization).
3.5. Levels, role types, and research stages of PPI
Based on the continuum of involvement in PPI frameworks (Table S2), we identified four levels of involvement. These levels include: (i) Information/Participation: to obtain broad information, opinions, experience, concerning a one‐time or specific task question, or topic (i.e., for identification or validation of a topic via a survey); (ii) Consultation: to obtain feedback and advice on a defined research question or research activity (i.e., revise study documents, content relevance, ratings). Patients or the public take an active role in the research project; (iii) Collaboration: to work directly with patients throughout or at different moments of the research process to ensure that their expectations and concerns are understood and addressed; (iv) Partnership: to establish an equal and active co‐leadership between the patient and the researcher where decisions about the research process are shared (i.e., members of steering committee or study board).
Similarly, we identified three different role types, including: (a) Personal engagement: when patients provided a personal perspective and feedback based on their direct experience. This might include members of the public (no affected by cancer); (b) Advisor or Expert: when patients provide advice and guidance from the perspective of both individual and collective experience, bringing the views of a diverse range of patients. For instance, patients participate on associations or organizational boards and hold high‐level of expertise across a broad range of cancer care (i.e., patient representatives or advocates); (c) Co‐researcher: patient was considered as equal partner with essential knowledge necessary for a meaningful contribution to the research project. Involvement in the research process was categorized in 8 stages ranging from the identification or prioritization of research topics to publication of the results (Figure 5 and Table S5).
FIGURE 5.
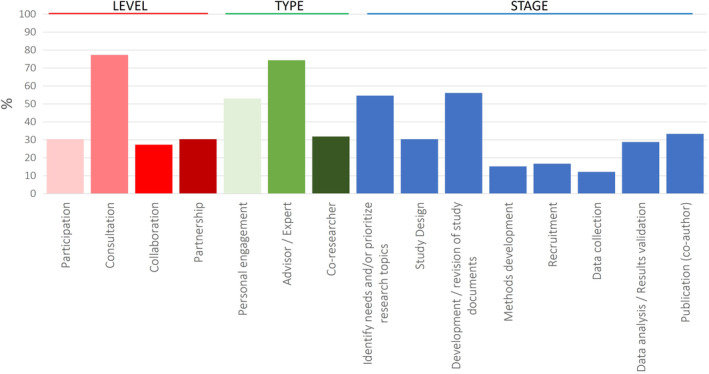
Overview on the level of involvement, role type, and stage of research where PPI is applied. Results are provided in percentages. Definitions and detailed information per study is provided in Suppl. Table 5.
Consultation and Advisor/Expert were the level of involvement and role most currently used in 77% and 74% of the studies respectively. Almost half of the studies (45%; 30/66) included two or more levels of involvement, and 48% combined two or more roles. PPI was mostly applied during the first stages of research including prioritization of research topics (55%) and development and/or revision of study documents (56%). PPI approaches were less used in the Recruitment and Data collection stages of research. One third of included studies had patients as co‐authors. A closer look into the data indicates a trend toward a wider PPI uptake encompassing more stages of the research process in recent years (Table S5).
3.6. Training, costs, and reporting
Costs related to PPI were mentioned in 38% (25/66) of the studies and referred to whether they paid participants or not, gave a stipend, vouchers/gift cards, covered lunch or travel costs, or were costs relative to the study (e.g., room rental, beverages). In four studies, 31 , 43 , 54 , 87 training was provided to the patients to collect data and conduct analysis or to co‐facilitate focus groups (Table S3). Regarding systematic reporting, three articles out of the 49 published from 2017 or later used the GRIPP2 tool 46 , 56 , 93 and two others mentioned it as reference. 36 , 54
3.7. Co‐design studies
In this review, co‐design studies were treated separately as these approaches involve patients as study subjects by collecting their own experience in a first step and to actively working together with healthcare professionals to ultimately improve care. Out of 35 studies identified using a co‐design approach, only 12 stated that they included patients or members of the public in a role other than study participant. More specifically, seven of those 12 studies involved patients in study committee or as co‐researcher, four involved patients in the design of the study, and two in the data collection process (Table S6).
4. DISCUSSION
In this paper, we report the findings of an extensive review of the literature to understand the current use of PPI in cancer research and identify how it is being applied. The most salient finding of our review is that involvement of patients and the public in cancer research has increased since 2015 with a burst of publications occurring from 2020 onwards. The UK, United States, Australia, and Canada have been publishing the majority of work in this area. We identified, combined, and synthesized similar key PPI elements across the studies that helped to reframe the purpose, level, role types, and stages of patient involvement in cancer research. Our review exposes several challenges regarding PPI, including the lack of diversity and costs associated with PPI approaches. The expansion of PPI in cancer research points to the need for additional scrutiny on the nature of published PPI.
Results from our review indicate that women with breast cancer remains the population most involved in cancer research. This is not surprising given the highly engaged breast cancer community. 97 The literature shows that people affected by cancer, particularly women with breast cancer, have been involved in a range of research programs, projects and initiatives especially in the USA, UK, Canada, and Australia. 2 , 25 Concern toward the lack of diversity or the presence of overrepresentation of privileged people remains one of the barriers when applying PPI. 98 , 99 Several studies have shown that PPI projects involve a majority of persons with higher education and socioeconomic background, while people with learning difficulties, older people, or people from minority ethnic groups are underrepresented. 9 This underrepresentation in PPI activities by groups with distinct needs warrants reflection, particularly considering the aim of most PPI research to enhance the applicability of the work to the target population. We can echo this concern from our review, wherein only four studies focused on the specific needs of the adolescent/young adults, two on older adults, and one on individuals with intellectual disabilities. Nevertheless, half of the studies mention the characteristics of the PPI participants, acknowledging the awareness for diversity.
Levels of involvement ranged from participation to partnership (from a passive to a higher level of involvement) and role types from personal engagement to co‐researcher (sharing an individual experience to essential knowledge for decision‐making). PPI was mostly used at the level of “consultation” to obtain advice or feedback. “Advisor/Expert,” providing guidance from the perspective of both individual and collective experience, was the role type of involvement most applied. Interestingly, half of the studies included two or more levels and role types of involvement reflecting the engagement in research as part of a continuum as described by several models. 100 , 101
Similarly to McCarron et al, 102 who analyzed the engagement practices of patient partners in health research, we also found that workshops and meetings were the methods used most often followed by interviews. Our observation supports what Greenhalgh 9 and Langley 103 describe as a shift toward practical workshops in knowledge creation or “collective making” rather than static “one‐size fits all” frameworks or linear models of research production. This tendency might also explain the difficulty to describe PPI in research dissociated from co‐development, co‐design, or participatory approaches, as we can observe more often that patient and members of the public are involved in a range of different activities, stages, roles, and levels along the same research project.
Furthermore, similar to previous reports, 1 , 2 , 104 , 105 , 106 we also observed that PPI practices in cancer research tend to concentrate on the early phase of research to prioritize or design research agendas, while involvement in data analysis and dissemination is uncommon. These observations may point to concerns around an absence of support to fully adopt a PPI approach and whereby PPI is implemented in the “easiest” way possible, leading to the risk of tokenism. Nevertheless, an overview on the involvement at the different stages of research across studies indicates a trend to extend PPI to later stages as seen from studies published after 2020. This escalation coincides with the increase of publications applying PPI approaches observed around this period.
The lack of PPI implementation may also point to patient and researcher needs for greater resources and support. A recent review of the literature on barriers and enablers identified both financial compensation and resources, and training as key factors in PPI. 107 Interestingly, only one third of papers in our review (38%) included mention of costs associated with the PPI‐related efforts (remuneration, stipends, travel costs, etc.). In addition, only four papers out of the 66, mentioned patients receiving training. Given the complex demands of applying PPI in research, and the expert knowledge of researchers, providing training for patients may allow them to fully understand their role and may begin to address the power differential between researcher and patient.
A final point of reflection relates to the evaluation and reporting of PPI in cancer research. To date, there is still a gap in understanding about PPI's impact on research outcomes and how it is achieved. 108 On their review, Bird and colleagues aimed to understand this impact. 109 From the 14 studies they identified, the impact of partnership was mainly evaluated by the research team itself or together with patient partners or other stakeholders, and only two included external reviewers. The authors highlight the urgent need of standardization and transparency when applying a PPI approach to avoid ambiguity in definitions, methods, and reporting of results. 109 Researchers might consider undertaking initial, midterm, and end‐of‐project evaluations of evolving relationships to increase their understanding of patient involvement. 110 Furthermore, our results show that systematic and standardized reporting of PPI approaches remain limited. Some efforts have been made in this direction to develop tools to evaluate 111 , 112 or report the impact of PPI 24 on their study. However, standardization in reporting guided by static checklists (e.g., GRIPP2) can also lead to certain limitations or constraints given the broad spectrum of PPI approaches. 113
5. STRENGTHS AND LIMITATIONS
This study has several limitations and notable strengths. The inclusion of studies was restricted to mention of PPI in titles and abstracts or stated methodology from the first articles selection (title/abstract). However, this may have resulted in missed papers. We did not search the gray literature, given our goal of understanding the current deployment of PPI in published cancer research. A final limitation relates to the inclusion of details related to PPI in written reports. We relied upon the authors' accounts of PPI, and it is possible that not all PPI endeavors were included in the manuscripts when they did involve PPI. This study also has notable strengths that include the comprehensive nature of the search and analysis, the extensive data visualization, and both tabular and narrative synthesis. Together these strengths create an important output and resource material to guide future PPI in cancer research.
6. CONCLUSION AND RECOMMENDATIONS
This paper serves as a map to illustrate where we are in the implementation of PPI in cancer research. We note the increasing volume of cancer research using a PPI approach, alongside distinct patterns in the role type of involvements. However, we also note limitations in how PPI is being implemented and reported. We recommend that to enhance future cancer research using a PPI approach, authors should report in a reflexive and precise way how and at which level PPI was employed. This may be guided (but not limited to) the use of standardized checklists such as the GRIPP2. Based on our work, we also argue that there is a need for increased evaluation of PPI approaches in cancer research. This may require that publications using PPI are given additional space to justify and explain PPI considering article length restrictions.
AUTHOR CONTRIBUTIONS
Sara Colomer‐Lahiguera: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (equal); investigation (lead); methodology (lead); project administration (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Matthieu Steimer: Data curation (equal); formal analysis (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Ursula Ellis: Data curation (equal); methodology (equal); resources (equal); writing – original draft (equal); writing – review and editing (equal). Manuela Eicher: Conceptualization (equal); funding acquisition (lead); resources (equal); validation (equal); writing – original draft (equal); writing – review and editing (equal). Margaret Tompson: Validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Tourane Corbière: Validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Kristen Haase: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); methodology (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING STATEMENT
Part of this work has been supported by the Foundation BRYN TURNER‐SAMUELS.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS APPROVAL STATEMENT
Not applicable.
Supporting information
Table S1. Search strategies
Table S2. Established frameworks and their different levels of involvement
Table S3. Characteristics of PPI participants in cancer research studies
Table S4. Purpose of PPI
Table S5. Description of the level, type and stages of research where PPI was applied per study
Table S6. Co‐design studies identified
ACKNOWLEDGMENTS
The authors wish to acknowledge assistance from Steven Hall.
Colomer‐Lahiguera S, Steimer M, Ellis U, et al. Patient and public involvement in cancer research: A scoping review. Cancer Med. 2023;12:15530‐15543. doi: 10.1002/cam4.6200
Margaret Tompson and Tourane Corbière is patient partner.
Endnote
The complete strategy can also be found in https://osf.io/audqy/?view_only=e66742b5b89e4a22a815adf489c2037b
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Boote J, Wong R, Booth A. ‘Talking the talk or walking the walk?’ A bibliometric review of the literature on public involvement in health research published between 1995 and 2009. Health Expect. 2015;18(1):44‐57. doi: 10.1111/hex.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pii KH, Schou LH, Piil K, Jarden M. Current trends in patient and public involvement in cancer research: a systematic review. Health Expect. 2019;22(1):3‐20. doi: 10.1111/hex.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skovlund PC, Nielsen BK, Thaysen HV, et al. The impact of patient involvement in research: a case study of the planning, conduct and dissemination of a clinical, controlled trial. Research Involvement and Engagement. 2020;6(1):43. doi: 10.1186/s40900-020-00214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute for Health Research . Glossary ‐ Patient and public involvement. Accessed 18 April, 2022. https://www.nihr.ac.uk/about‐us/glossary.htm?letter=P&postcategory=‐1#:~:text=Patient%20and%20public%20involvement,as%20'subjects'%20of%20research
- 5. National Institute for Health Research (NIHR) . Briefing Notes for Researchers ‐ Public Involvement in NHS, Health and Social Care Research. National Institute for Health Research. Updated 05/04/2021. Accessed 15 April, 2022. https://www.nihr.ac.uk/documents/briefing‐notes‐for‐researchers‐public‐involvement‐in‐nhs‐health‐and‐social‐care‐research/27371 [Google Scholar]
- 6. Crocker JC, Ricci‐Cabello I, Parker A, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta‐analysis. BMJ. 2018;363:k4738. doi: 10.1136/bmj.k4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. South A, Hanley B, Gafos M, et al. Models and impact of patient and public involvement in studies carried out by the Medical Research Council clinical trials unit at University College London: findings from ten case studies. Trials. 2016;17(1):376. doi: 10.1186/s13063-016-1488-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boote J, Telford R, Cooper C. Consumer involvement in health research: a review and research agenda. Health Policy. 2002;61(2):213‐236. doi: 10.1016/S0168-8510(01)00214-7 [DOI] [PubMed] [Google Scholar]
- 9. Greenhalgh T, Hinton L, Finlay T, et al. Frameworks for supporting patient and public involvement in research: systematic review and co‐design pilot. Health Expect. 2019;22(4):785‐801. doi: 10.1111/hex.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jull JE, Davidson L, Dungan R, Nguyen T, Woodward KP, Graham ID. A review and synthesis of frameworks for engagement in health research to identify concepts of knowledge user engagement. BMC Med Res Methodol. 2019;19(1):211. doi: 10.1186/s12874-019-0838-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frank L, Forsythe L, Ellis L, et al. Conceptual and practical foundations of patient engagement in research at the patient‐centered outcomes research institute. Qual Life Res. 2015;24(5):1033‐1041. doi: 10.1007/s11136-014-0893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manafo E, Petermann L, Mason‐Lai P, Vandall‐Walker V. Patient engagement in Canada: a scoping review of the ‘how’ and ‘what’ of patient engagement in health research. Health Research Policy and Systems. 2018;16(1):5. doi: 10.1186/s12961-018-0282-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Canadian Institutes of Health Research . Strategy for Patient‐Oriented Research ‐ Patient Engagement Framework. Canadian Institutes of Health Research. Accessed 15 April, 2022. https://cihr‐irsc.gc.ca/e/48413.html [Google Scholar]
- 14. Cancer Australia and Cancer Voices Australia . National Framework, for Consumer Involvement in Cancer Control . 2011. https://www.canceraustralia.gov.au/sites/default/files/publications/national_consumer_framework_web_504af020f2184.pdf
- 15. Cleemput I, Dauvrin M, Kohn L, Mistiaen P, Christiaens W, Léonard C. Position of KCE on Patient Involvement in Health Care Policy Research. Belgian Health Care Knowledge Centre (KCE); 2019. KCE Reports 320 D/2019/10273/57. https://kce.fgov.be/en/position‐of‐kce‐on‐patient‐involvement‐in‐health‐care‐policy‐research [Google Scholar]
- 16. Pomey M‐P, Dumez V, Boivin A, et al. The participation of patients and relatives in Quebec's health system: the Montréal model. In: Pomey M‐P, Denis J‐L, Dumez V, eds. Patient Engagement: how Patient‐Provider Partnerships Transform Healthcare Organizations. Springer International Publishing; 2019:17‐61. [Google Scholar]
- 17. Oncode Institute . Patient Engagement Programme. Accessed 04 August, 2021. https://www.oncode.nl/research/programs/patient‐engagement‐programme
- 18. Cancer Research UK . Patient involvement toolkit for researchers. Accessed 15 April, 2022. https://www.cancerresearchuk.org/funding‐for‐researchers/patient‐involvement‐toolkit‐for‐researchers
- 19. Staniszewska S, Denegri S, Matthews R, Minogue V. Reviewing progress in public involvement in NIHR research: developing and implementing a new vision for the future. BMJ Open. 2018;8(7):e017124. doi: 10.1136/bmjopen-2017-017124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parkes JH, Pyer M, Wray P, Taylor J. Partners in projects: preparing for public involvement in health and social care research. Health Policy. 2014;117(3):399‐408. doi: 10.1016/j.healthpol.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 21. Staley K, Cockcroft E, Shelly A, Liabo K. ‘What can I do that will most help researchers?’ A different approach to training the public at the start of their involvement in research. Research Involvement and Engagement. 2019;5(1):10. doi: 10.1186/s40900-019-0144-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deane K, Delbecque L, Gorbenko O, et al. Co‐creation of patient engagement quality guidance for medicines development: an international multistakeholder initiative. BMJ Innovations. 2019;5(1):43‐55. doi: 10.1136/bmjinnov-2018-000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institute for Health Research (NIHR), Research design service (RDS) . Patient and Public Involvement in Health and Social Care Research. National Institute for Health and Care Research; 2018:p 30. [Google Scholar]
- 24. Staniszewska S, Brett J, Simera I, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. doi: 10.1136/bmj.j3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hubbard G, Kidd L, Donaghy E. Involving people affected by cancer in research: a review of literature. Eur J Cancer Care. 2008;17(3):233‐244. doi: 10.1111/j.1365-2354.2007.00842.x [DOI] [PubMed] [Google Scholar]
- 26. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19‐32. doi: 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 27. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dawson S, Campbell SM, Giles SJ, Morris RL, Cheraghi‐Sohi S. Black and minority ethnic group involvement in health and social care research: a systematic review. Health Expect. 2018;21(1):3‐22. doi: 10.1111/hex.12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Covidence systematic review software . 2022. Available at www.covidence.org.
- 30. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corner J, Wright D, Hopkinson J, Gunaratnam Y, McDonald JW, Foster C. The research priorities of patients attending UK cancer treatment centres: findings from a modified nominal group study. Br J Cancer. 2007;96(6):875‐881. doi: 10.1038/sj.bjc.6603662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Forbes LJ, Nicholls CM, Linsell L, Graham J, Tompkins C, Ramirez AJ. Involving users in the design of a randomised controlled trial of an intervention to promote early presentation in breast cancer: qualitative study. BMC Med Res Methodol. 2010;10:110. doi: 10.1186/1471-2288-10-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holch P, Warrington L, Bamforth LCA, et al. Development of an integrated electronic platform for patient self‐report and management of adverse events during cancer treatment. Ann Oncol. 2017;28(9):2305‐2311. doi: 10.1093/annonc/mdx317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huddy JR, Weldon SM, Ralhan S, et al. Sequential simulation (SqS) of clinical pathways: a tool for public and patient engagement in point‐of‐care diagnostics. BMJ Open. 2016;6(9):e011043. doi: 10.1136/bmjopen-2016-011043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kelly L, Caldwell K, Henshaw L. Involving users in service planning: a focus group approach. Eur J Oncol Nurs. 2006;10(4):283‐293. doi: 10.1016/j.ejon.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 36. Mayland CR, Gerlach C, Sigurdardottir K, et al. Assessing quality of care for the dying from the bereaved relatives' perspective: using pre‐testing survey methods across seven countries to develop an international outcome measure. Palliat Med. 2019;33(3):357‐368. doi: 10.1177/0269216318818299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meads DM, O'Dwyer JL, Hulme CT, Chintakayala P, Vinall‐Collier K, Bennett MI. Patient preferences for pain Management in Advanced Cancer: results from a discrete choice experiment. The Patient. 2017;10(5):643‐651. doi: 10.1007/s40271-017-0236-x [DOI] [PubMed] [Google Scholar]
- 38. Melnychuk M, Vindrola‐Padros C, Aitchison M, et al. Centralising specialist cancer surgery services in England: survey of factors that matter to patients and carers and health professionals. BMC Cancer. 2018;18(1):226. doi: 10.1186/s12885-018-4137-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nicholas OJ, Joseph O, Keane A, et al. Patient and public involvement refines the design of ProtOeus: a proposed phase II trial of proton beam therapy in Oesophageal cancer. The Patient. 2021;14(5):545‐553. doi: 10.1007/s40271-020-00487-8 [DOI] [PubMed] [Google Scholar]
- 40. Phillips B, Depani S, Morgan J. What do families want to improve in the management of paediatric febrile neutropenia during anti‐cancer treatment? Report of a patient/public involvement group. BMJ Paediatr Open. 2019;3(1):e000398. doi: 10.1136/bmjpo-2018-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor RM, Mohain J, Gibson F, Solanki A, Whelan J, Fern LA. Novel participatory methods of involving patients in research: naming and branding a longitudinal cohort study, BRIGHTLIGHT. BMC Med Res Methodol. 2015;15:20. doi: 10.1186/s12874-015-0014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wan YL, Beverley‐Stevenson R, Carlisle D, et al. Working together to shape the endometrial cancer research agenda: the top ten unanswered research questions. Gynecol Oncol. 2016;143(2):287‐293. doi: 10.1016/j.ygyno.2016.08.333 [DOI] [PubMed] [Google Scholar]
- 43. Wright D, Foster C, Amir Z, Elliott J, Wilson R. Critical appraisal guidelines for assessing the quality and impact of user involvement in research. Health Expect. 2010;13(4):359‐368. doi: 10.1111/j.1369-7625.2010.00607.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gibson F, Fern LA, Phillips B, et al. Reporting the whole story: analysis of the 'out‐of‐scope' questions from the James Lind Alliance teenage and young adult cancer priority setting partnership survey. Health Expect. 2021;24(5):1593‐1606. doi: 10.1111/hex.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kanapathy M, Burentuvshin O, Varghese J, Naderi N, Canny R, Mosahebi A. Priority setting in breast reconstructive surgery: a DELPHI consensus. J Plast Reconstr Aesthet Surg. 2022;75(4):1297‐1315. doi: 10.1016/j.bjps.2021.11.068 [DOI] [PubMed] [Google Scholar]
- 46. Cavers D, Cunningham‐Burley S, Watson E, Banks E, Campbell C. Setting the research agenda for living with and beyond cancer with comorbid illness: reflections on a research prioritisation exercise. Research Involvement and Engagement. 2020;6(1):17. doi: 10.1186/s40900-020-00191-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crawford SY, Boyd AD, Nayak AK, et al. Patient‐centered design in developing a mobile application for oral anticancer medications. J Am Pharm Assoc (2003). 2019;59(2s):S86‐S95.e1. doi: 10.1016/j.japh.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 48. Head BA, Studts JL, Bumpous JM, et al. Development of a telehealth intervention for head and neck cancer patients. Telemed J E Health. 2009;15(1):44‐52. doi: 10.1089/tmj.2008.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Javid SH, Lawrence SO, Lavallee DC. Prioritizing patient‐reported outcomes in breast cancer surgery quality improvement. Breast J. 2017;23(2):127‐137. doi: 10.1111/tbj.12707 [DOI] [PubMed] [Google Scholar]
- 50. Payne JB, Dance KV, Farone M, et al. Patient and caregiver perceptions of lymphoma care and research opportunities: a qualitative study. Cancer. 2019;125(22):4096‐4104. doi: 10.1002/cncr.32401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rafie CL, Zimmerman EB, Moser DE, Cook S, Zarghami F. A lung cancer research agenda that reflects the diverse perspectives of community stakeholders: process and outcomes of the SEED method. Res Involv Engagem. 2019;5:3. doi: 10.1186/s40900-018-0134-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith AB, Chisolm S, Deal A, et al. Patient‐centered prioritization of bladder cancer research. Cancer. 2018;124(15):3136‐3144. doi: 10.1002/cncr.31530 [DOI] [PubMed] [Google Scholar]
- 53. Snaman JM, Helton G, Baker JN, et al. Engaging parents of children who died from cancer in research on the early grief experience. J Pain Symptom Manage. 2021;61(4):781‐788. doi: 10.1016/j.jpainsymman.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 54. Treiman K, McCormack L, Olmsted M, et al. Engaging patient advocates and other stakeholders to design measures of patient‐centered communication in cancer care. The Patient. 2017;10(1):93‐103. doi: 10.1007/s40271-016-0188-6 [DOI] [PubMed] [Google Scholar]
- 55. Handley NR, Binder AF, Heyer A, et al. Development of the oncology opportunity cost assessment tool: item generation and content validity testing. JCO Oncol Pract. 2022;18(3):e360‐e371. doi: 10.1200/op.21.00288 [DOI] [PubMed] [Google Scholar]
- 56. Smith AB, Lee JR, Lawrence SO, et al. Patient and public involvement in the design and conduct of a large, pragmatic observational trial to investigate recurrent, high‐risk non‐muscle‐invasive bladder cancer. Cancer. 2022;128(1):103‐111. doi: 10.1002/cncr.33897 [DOI] [PubMed] [Google Scholar]
- 57. Shojaie D, Hoffman AS, Amaku R, et al. Decision making when cancer becomes chronic: needs assessment for a web‐based medullary thyroid carcinoma patient decision aid. JMIR Form Res. 2021;5(7):e27484. doi: 10.2196/27484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yan A, Hooyer K, Asan O, Flower M, Whittle J. Engaging veteran stakeholders to identify patient‐centred research priorities for optimizing implementation of lung cancer screening. Health Expect. 2022;25(1):408‐418. doi: 10.1111/hex.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stover AM, Urick BY, Deal AM, et al. Performance measures based on how adults with cancer feel and function: stakeholder recommendations and feasibility testing in six cancer centers. JCO Oncol Pract. 2020;16(3):e234‐e250. doi: 10.1200/jop.19.00784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Perry LM, Sartor O, Malhotra S, et al. Increasing readiness for early integrated palliative oncology care: development and initial evaluation of the EMPOWER 2 intervention. J Pain Symptom Manage. 2021;62(5):987‐996. doi: 10.1016/j.jpainsymman.2021.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carey M, Schofield P, Jefford M, Krishnasamy M, Aranda S. The development of audio‐visual materials to prepare patients for medical procedures: an oncology application. Eur J Cancer Care. 2007;16(5):417‐423. doi: 10.1111/j.1365-2354.2006.00772.x [DOI] [PubMed] [Google Scholar]
- 62. Janssen A, Donnelly C, Kay J, et al. Developing an intranet‐based lymphedema dashboard for breast cancer multidisciplinary teams: design research study. J Med Internet Res. 2020;22(4):e13188. doi: 10.2196/13188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Juraskova I, Butow P, Lopez A, et al. Improving informed consent: pilot of a decision aid for women invited to participate in a breast cancer prevention trial (IBIS‐II DCIS). Health Expect. 2008;11(3):252‐262. doi: 10.1111/j.1369-7625.2008.00498.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Robotin MC, Jones SC, Biankin AV, et al. Defining research priorities for pancreatic cancer in Australia: results of a consensus development process. Cancer Causes Control. 2010;21(5):729‐736. doi: 10.1007/s10552-010-9501-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saunders C, Crossing S. Towards meeting the research needs of Australian cancer consumers. BMC Res Notes. 2012;5:667. doi: 10.1186/1756-0500-5-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saunders C, Gooden H, Robotin M, Mumford J. As the bell tolls: a foundation study on pancreatic cancer consumer's research priorities. BMC Res Notes. 2009;2:179. doi: 10.1186/1756-0500-2-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Segelov E, Chan D, Lawrence B, et al. Identifying and prioritizing gaps in neuroendocrine tumor research: a modified Delphi process with patients and health care providers to set the research action plan for the newly formed commonwealth neuroendocrine tumor collaboration. J Glob Oncol. 2017;3(4):380‐388. doi: 10.1200/jgo.2016.006916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schilstra CE, Sansom‐Daly UM, Schaffer M, et al. "we have all this knowledge to give, so use us as a resource": partnering with adolescent and young adult cancer survivors to determine consumer‐led research priorities. J Adolesc Young Adult Oncol. 2022;11(2):211‐222. doi: 10.1089/jayao.2021.0052 [DOI] [PubMed] [Google Scholar]
- 69. Taggart J, Chin M, Liauw W, et al. Challenges and solutions to sharing a cancer follow‐up e‐care plan between a cancer service and general practice. Public Health Res Pract. 2021;31(2):31122108. doi: 10.17061/phrp31122108 [DOI] [PubMed] [Google Scholar]
- 70. Mazariego C, Jefford M, Chan RJ, et al. Priority recommendations for the implementation of patient‐reported outcomes in clinical cancer care: a Delphi study. J Cancer Surviv. 2022;16(1):33‐43. doi: 10.1007/s11764-021-01135-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dunn J, Green A, Ralph N, et al. Prostate cancer survivorship essentials framework: guidelines for practitioners. BJU Int. 2021;128(Suppl 3(Suppl 3)):18‐29. doi: 10.1111/bju.15159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crawford‐Williams F, Koczwara B, Chan RJ, et al. Defining research and infrastructure priorities for cancer survivorship in Australia: a modified Delphi study. Support Care Cancer. 2022;30(5):3805‐3815. doi: 10.1007/s00520-021-06744-2 [DOI] [PubMed] [Google Scholar]
- 73. Jibb LA, Stacey D, Carley M, et al. Research priorities for the pan‐Canadian oncology symptom triage and remote support practice guides: a modified nominal group consensus. Curr Oncol. 2019;26(3):173‐182. doi: 10.3747/co.26.4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schneider PJ, Evaniew N, McKay P, Ghert M. Moving forward through consensus: a modified Delphi approach to determine the top research priorities in Orthopaedic oncology. Clin Orthop Relat Res. 2017;475(12):3044‐3055. doi: 10.1007/s11999-017-5482-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Adams SC, Smith‐Turchyn J, Santa Mina D, et al. The exercise oncology knowledge mobilization initiative: an international modified Delphi study. Original research. Frontiers in Oncology. 2021;11:713199. doi: 10.3389/fonc.2021.713199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Haase KR, Tompson MA, Hall S, Sattar S, Ahmed S. Engaging older adults with cancer and their caregivers to set research priorities through cancer and aging research discussion sessions. Oncol Nurs Forum. 2021;48(6):613‐622. doi: 10.1188/21.Onf.613-622 [DOI] [PubMed] [Google Scholar]
- 77. Zhong T, Mahajan A, Cowan K, et al. Identifying the top research priorities in postmastectomy breast cancer reconstruction: a James Lind Alliance priority setting partnership. BMJ Open. 2021;11(8):e047589. doi: 10.1136/bmjopen-2020-047589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jones J, Bhatt J, Avery J, et al. The kidney cancer research priority‐setting partnership: identifying the top 10 research priorities as defined by patients, caregivers, and expert clinicians. Can Urol Assoc J. 2017;11(12):379‐387. doi: 10.5489/cuaj.4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Flegg K, Gelkopf MJ, Johnson SA, Dimaras H. The top 10 retinoblastoma research priorities in Canada as determined by patients, clinicians and researchers: a patient‐oriented priority‐setting partnership. CMAJ Open. 2020;8(2):E420‐E428. doi: 10.9778/cmajo.20190221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang S, Ye Z, Pan Z, et al. "shared decision making assistant": a smartphone application to meet the decision‐making needs of patients with primary liver cancer. Comput Inform Nurs. 2021;39(12):984‐991. doi: 10.1097/cin.0000000000000775 [DOI] [PubMed] [Google Scholar]
- 81. Wong CHL, Wong W, Lin WL, et al. Prioritizing Chinese medicine clinical research questions in cancer palliative care from patient and caregiver perspectives. Health Expect. 2021;24(4):1487‐1497. doi: 10.1111/hex.13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Furukawa Y, Saito H, Hasegawa K, Ichikawa M. Assessing the quality of cancer screening leaflets using the international patient decision aids standards instrument: a cross‐sectional content analysis. Patient Educ Couns. 2021;104(12):3100‐3103. doi: 10.1016/j.pec.2021.03.034 [DOI] [PubMed] [Google Scholar]
- 83. Schmidt F, Ribi K, Haslbeck J, Urech C, Holm K, Eicher M. Adapting a peer‐led self‐management program for breast cancer survivors in Switzerland using a co‐creative approach. Patient Educ Couns. 2020;103(9):1780‐1789. doi: 10.1016/j.pec.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 84. Da Silva Lopes AM, Colomer‐Lahiguera S, Mederos Alfonso N, et al. Patient‐reported outcomes for monitoring symptomatic toxicities in cancer patients treated with immune‐checkpoint inhibitors: a Delphi study. Eur J Cancer. 2021;157:225‐237. doi: 10.1016/j.ejca.2021.08.026 [DOI] [PubMed] [Google Scholar]
- 85. Gerritsen A, Jacobs M, Henselmans I, et al. Developing a core set of patient‐reported outcomes in pancreatic cancer: a Delphi survey. Eur J Cancer. 2016;57:68‐77. doi: 10.1016/j.ejca.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 86. Ector GI, Verweij L, Hermens RP, Blijlevens NM. Filling the gaps of patient information needs and information perception in chronic myeloid leukemia with the patient‐physician co‐produced web‐based platform CMyLife. Patient Educ Couns. 2022;105(3):686‐694. doi: 10.1016/j.pec.2021.06.025 [DOI] [PubMed] [Google Scholar]
- 87. Badia X, Aguarón A, Fernández A, et al. Patient involvement in reflective multicriteria decision analysis to assist decision making in oncology. Int J Technol Assess Health Care. 2019;35(1):56‐63. doi: 10.1017/s0266462318003641 [DOI] [PubMed] [Google Scholar]
- 88. Boelens PG, Taylor C, Henning G, et al. Involving patients in a multidisciplinary European consensus process and in the development of a 'patient summary of the consensus document for colon and rectal cancer care'. The Patient. 2014;7(3):261‐270. doi: 10.1007/s40271-014-0061-4 [DOI] [PubMed] [Google Scholar]
- 89. Beyer K, Moris L, Lardas M, et al. Updating and integrating Core outcome sets for localised, locally advanced, metastatic, and non‐metastatic castration‐resistant prostate cancer: an update from the PIONEER consortium. Eur Urol. 2022;81(5):503‐514. doi: 10.1016/j.eururo.2022.01.042 [DOI] [PubMed] [Google Scholar]
- 90. Skorpen S, Larsen FK, Holthe T. User participation is important for the creation of adapted cancer information material. In: Satgé D, MerrickInt J, eds. Cancer in Children and Adults with Intellectual Disabilities: Current Research Aspects. Nova Science Publishers, Inc; 2010:13‐19 [Google Scholar]
- 91. Sekse RJT, Nordgreen T, Flobak E, Lystrup M, Braathen E, Werner HMJ. Development of a framework and the content for a psychoeducational internet‐delivered intervention for women after treatment for gynecological cancer. Nurs Rep. 2021;11(3):640‐651. doi: 10.3390/nursrep11030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bergerød IJ, Braut GS, Fagerdal B, Gilje B, Wiig S. Developing a next‐of‐kin involvement guide in cancer care‐results from a consensus process. Cancer Nurs. 2021;44(6):E447‐e457. doi: 10.1097/ncc.0000000000000869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Birkeland S, Pedersen SS, Haakonsson AK, Barry MJ, Rottmann N. Men's view on participation in decisions about prostate‐specific antigen (PSA) screening: patient and public involvement in development of a survey. BMC Med Inform Decis Mak. 2020;20(1):65. doi: 10.1186/s12911-020-1077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sperling CD, Petersen GS, Hølge‐Hazelton B, et al. Being young and getting cancer: development of a questionnaire reflecting the needs and experiences of adolescents and young adults with cancer. J Adolesc Young Adult Oncol. 2017;6(1):171‐177. doi: 10.1089/jayao.2015.0063 [DOI] [PubMed] [Google Scholar]
- 95. Wikman A, Kukkola L, Börjesson H, et al. Development of an internet‐administered cognitive behavior therapy program (ENGAGE) for parents of children previously treated for cancer: participatory action research approach. J Med Internet Res. 2018;20(4):e133. doi: 10.2196/jmir.9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Woodford J, Farrand P, Hagström J, Hedenmalm L, von Essen L. Internet‐administered cognitive behavioral therapy for common mental health difficulties in parents of children treated for cancer: intervention development and description study. JMIR Form Res. 2021;5(7):e22709. doi: 10.2196/22709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. King S. Pink ribbons Inc: breast cancer activism and the politics of philanthropy. International Journal of Qualitative Studies in Education. 2004;17(4):473‐492. doi: 10.1080/09518390410001709553 [DOI] [Google Scholar]
- 98. Beresford P, Russo J. Patient and public involvement in research. In: Anell A, Nolte E, Merkur S, eds. Achieving Person‐Centred Health Systems: Evidence, Strategies and Challenges. Cambridge University Press, European Observatory on Health Systems and Policies; 2020:145‐172. [Google Scholar]
- 99. Reynolds J, Ogden M, Beresford R. Conceptualising and constructing ‘diversity’ through experiences of public and patient involvement in health research. Research Involvement and Engagement. 2021;7(1):53. doi: 10.1186/s40900-021-00296-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carman KL, Workman TA. Engaging patients and consumers in research evidence: applying the conceptual model of patient and family engagement. Patient Educ Couns. 2017;100(1):25‐29. doi: 10.1016/j.pec.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 101. Pomey MP, Flora L, Karazivan P, Dumez V, Lebel P, Vanier MC, Débarges B, Clavel N, Jouet E [The Montreal model: the challenges of a partnership relationship between patients and healthcare professionals]. Sante Publique. 2015;27(1 Suppl):S41‐50. Le <<Montreal model>> : enjeux du partenariat relationnel entre patients et professionnels de la sante. [PubMed] [Google Scholar]
- 102. McCarron TL, Clement F, Rasiah J, et al. Patients as partners in health research: a scoping review. Health Expect. 2021;24(4):1378‐1390. doi: 10.1111/hex.13272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Langley J, Wolstenholme D, Cooke J. ‘Collective making’ as knowledge mobilisation: the contribution of participatory design in the co‐creation of knowledge in healthcare. BMC Health Serv Res. 2018;18(1):585. doi: 10.1186/s12913-018-3397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Forsythe L, Heckert A, Margolis MK, Schrandt S, Frank L. Methods and impact of engagement in research, from theory to practice and back again: early findings from the Patient‐Centered Outcomes Research Institute. Qual Life Res. 2018;27(1):17‐31. doi: 10.1007/s11136-017-1581-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14(1):89. doi: 10.1186/1472-6963-14-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. 2015;18(5):1151‐1166. doi: 10.1111/hex.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ocloo J, Garfield S, Franklin BD, Dawson S. Exploring the theory, barriers and enablers for patient and public involvement across health, social care and patient safety: a systematic review of reviews. Health Research Policy and Systems. 2021;19(1):8. doi: 10.1186/s12961-020-00644-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pizzo E, Doyle C, Matthews R, Barlow J. Patient and public involvement: how much do we spend and what are the benefits? Health Expect. 2015;18(6):1918‐1926. doi: 10.1111/hex.12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bird M, Ouellette C, Whitmore C, et al. Preparing for patient partnership: a scoping review of patient partner engagement and evaluation in research. Health Expect. 2020;23(3):523‐539. doi: 10.1111/hex.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Staley K. 'Is it worth doing?' measuring the impact of patient and public involvement in research. Res Involv Engagem. 2015;1:6. doi: 10.1186/s40900-015-0008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Boivin A, L'Esperance A, Gauvin FP, et al. Patient and public engagement in research and health system decision making: a systematic review of evaluation tools. Health Expect. 2018;21(6):1075‐1084. doi: 10.1111/hex.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mrklas KJ, Boyd JM, Shergill S, et al. Tools for assessing health research partnership outcomes and impacts: a systematic review. Health Res Policy Syst. 2023;21(1):3. doi: 10.1186/s12961-022-00937-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Scholz B, Bevan A. Toward more mindful reporting of patient and public involvement in healthcare. Research Involvement and Engagement. 2021;7(1):61. doi: 10.1186/s40900-021-00308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategies
Table S2. Established frameworks and their different levels of involvement
Table S3. Characteristics of PPI participants in cancer research studies
Table S4. Purpose of PPI
Table S5. Description of the level, type and stages of research where PPI was applied per study
Table S6. Co‐design studies identified
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


