Background:
As the transmission of endemic respiratory pathogens returns to prepandemic levels, understanding the epidemiology of respiratory coinfections in children with SARS-CoV-2 is of increasing importance.
Methods:
We performed a retrospective analysis of all pediatric patients 0–21 years of age who had a multiplexed BioFire Respiratory Panel 2.1 test performed at Children’s Healthcare of Atlanta, Georgia, from January 1 to December 31, 2021. We determined the proportion of patients with and without SARS-CoV-2 who had respiratory coinfections and performed Poisson regression to determine the likelihood of coinfection and its association with patient age.
Results:
Of 19,199 respiratory panel tests performed, 1466 (7.64%) were positive for SARS-CoV-2, of which 348 (23.74%) also had coinfection with another pathogen. The most common coinfection was rhino/enterovirus (n = 230, 15.69%), followed by adenovirus (n = 62, 4.23%), and RSV (n = 45, 3.507%). Coinfections with SARS-CoV-2 were most commonly observed in the era of Delta (B.1.617.2) predominance (190, 54.60%), which coincided with periods of peak rhino/enterovirus and RSV transmission. Although coinfections were common among all respiratory pathogens, they were significantly less common with SARS-CoV-2 than other pathogens, with exception of influenza A and B. Children <2 years of age had the highest frequency of coinfection and of detection of any pathogen, including SARS-CoV-2. Among children with SARS-CoV-2, for every 1-year increase in age, the rate of coinfections decreased by 8% (95% CI, 6–9).
Conclusions:
Respiratory coinfections were common in children with SARS-CoV-2. Factors associated with the specific pathogen, host, and time period influenced the likelihood of coinfection.
Keywords: COVID-19, RSV, influenza, codetection, pediatric
As of March 2023, the coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was estimated to have caused more than 676 million cases and 6.8 million deaths worldwide.1 Nonpharmacologic interventions to mitigate SARS-CoV-2 transmission, including masking, social distancing, school closures, and limitations to global travel, also interrupted the circulation of other respiratory pathogens early in the pandemic.2 Relaxation of COVID-19 mitigation procedures, although variable over time and region, allowed for return to prepandemic transmission patterns of many respiratory pathogens.3 Now that these pathogens are circulating concurrently with SARS-CoV-2, understanding the epidemiology and pathogenesis of respiratory coinfections is important for guiding clinical prevention and management decisions in patients with COVID-19.
In adults, respiratory viral coinfections with SARS-CoV-2 have generally been found to be infrequent and not associated with increased clinical disease severity. Initial reports found the frequency of respiratory coinfections to be low,4 and this was supported by multiple studies5–7 including a meta-analysis performed in the early pandemic.8 While these early studies may have been confounded by the transient disruptions in transmission of respiratory pathogens related to COVID-19 mitigation procedures, more recent surveillance data have similarly identified a low percentage (0.9%) of respiratory viral coinfections in adults hospitalized with COVID-19.9 Interestingly, although respiratory viral coinfections have not been associated with worse clinical outcomes in adults with COVID-19,10,11 bacterial coinfections have been associated with increased mortality.9
Our understanding of the epidemiology of coinfections with SARS-CoV-2 in children, who experience more frequent exposures to and infections from seasonal respiratory pathogens, has evolved over time. Data from early in the pandemic found that respiratory coinfections were frequent among children with COVID-1912,13 and were associated with increased disease severity compared with children who had COVID-19 alone.14,15 More recent data from the COVID-19-Associated Hospital Surveillance Network (COVID-NET) found that SARS-CoV-2 coinfections with influenza were infrequent throughout the pandemic in hospitalized US children, whereas coinfections with rhino/enterovirus fluctuated and RSV increased in the Delta era. COVID-NET further found that SARS-CoV-2 coinfection with rhino/enterovirus was associated with increased risk of ICU admission and supplemental oxygen requirement in young children.16 Now that endemic pathogens are returning to prepandemic circulation patterns in the community, contemporary analyses of the epidemiology of coinfections in children are needed.
In this study, we aimed to describe the epidemiology of respiratory coinfections in children with SARS-CoV-2 compared with other pathogens by performing a retrospective analysis of all children presenting to Children’s Healthcare of Atlanta, Georgia, who had multiplexed RP testing performed in 2021. We hypothesized that respiratory coinfections were common in children with SARS-CoV-2 and that the likelihood of coinfection was influenced by multiple factors specific to the pathogen, host, and time period.
MATERIALS AND METHODS
Patient Cohort
We performed a retrospective analysis of all pediatric patients 0–21 years of age who had a multiplexed respiratory panel (RP) test performed at Children’s Healthcare of Atlanta, Atlanta, Georgia, from January 1, 2021, to December 31, 2021. The RP (BioFire RP 2.1) was given emergency use authorization by the Federal Drug Administration in May 2020 and implemented at Children’s in November 2020. It simultaneously detects adenovirus, coronaviruses 229E, HKU1, NL63, OC43, SARS-CoV-2, human metapneumovirus (HMPV), human rhinovirus/enterovirus, influenza A (including subtypes H1, H3, and H1-2009), influenza B, parainfluenza viruses 1–4, RSV, and bacteria including Bordetella pertussis, Bordetella parapertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae from nasopharyngeal swab specimens. Patients were included in the analysis regardless of their clinical presentation or testing site [e.g., emergency department (ED), inpatient or intensive care units, outpatient, or procedure/operating rooms]. Two patients who did not have a known testing site were excluded from analyses. Analyses were performed for all visits, and sub-analyses were performed limited to the first RP for each patient. Time periods of variant circulation were classified based on regional data pertinent to Georgia from the US Centers for Disease Control and Prevention COVID-19 Data Tracker as follows: January 1 to July 3 was considered Alpha (B.1.1.7) predominant; July 4 to December 17 was considered Delta (B.1.617.2) predominant; and December 17 to December 31 was considered Omicron (B.1.1.529) predominant. The decision to perform an RP was made at the clinician’s discretion. Although detection of a pathogen may have represented either symptomatic infection or asymptomatic carriage or shedding, we were unable to distinguish these in the current analyses, and, therefore, termed a codetection to be a coinfection hereafter. This retrospective study was approved by the Institutional Review Board at Emory University with waiver of informed consent.
Statistical Analyses
Patients were categorized as having a positive or negative SARS-CoV-2 RP test and as having positive or negative result for other pathogens. The proportion of patients with coinfections, defined as having more than 1 pathogen detected, was determined, and demographic features were summarized using descriptive statistics. Categorical variables were displayed as frequencies and percentages, while continuous variables were presented as means and standard deviations. χ2 or Fisher’s test were used to determine if having SARS-CoV-2 was independent of having other pathogens. Some participants visited the site more than once. For this reason, sensitivity analyses were conducted limiting participants to their first visit (n = 15,287).
In addition to calculating the proportion of coinfections in SARS-CoV-2 participants, we also calculated proportion of coinfections in participants who were positive for another pathogen. χ2 test was used to determine if having a coinfection was independent of testing location. Using data from all visits, we compared the proportion of coinfections between individual pathogens and SARS-CoV-2. There were several participants who were positive for both SARS-CoV-2 and the pathogen of interest, making these groups dependent and invalid for a traditional test of difference in proportion. As such, we used a method developed by Derrick et al17 that allows for comparison of proportions in groups with partially overlapping samples through the package partially overlapping available in R.18 In some instances, there were not overlapping participants, which was often seen in small sample sizes. We then used an equality test for binomial proportions, where 95% confidence intervals and corresponding P values were calculated using the Wald method with a continuity correction.
To determine if SARS-CoV-2 positivity was associated with count of infections, we performed a simple Poisson regression with SARS-CoV-2 status as the sole predictor and the count of infections as the outcome. Likewise, to determine if age was significantly associated with number of coinfections in SARS-CoV-2-positive children, we performed a simple Poisson regression analysis including age as the predictor and number of coinfections as the outcome. The presence of overdispersion was checked through the dispersion parameter as calculated by the Pearson χ2 statistic divided by the degrees of freedom and a dispersion parameter close to 1 was considered to mean, and there was no overdispersion. The presence of zero-inflation was also checked through visual assessment of the count of infections. Overdispersion and zero-inflation were not present in either Poisson regression models.
All analyses were conducted in SAS 9.4 (Cary, NC) and R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). A P value below 0.05 was considered significant, and all tests were 2-tailed.
RESULTS
Patient Cohort
Of 19,199 unique visits with RP testing performed from January 1 to December 31, 2021, 1466 (7.64%) were positive and 17,733 (92.36%) were negative for SARS-CoV-2 (Table 1). Of those positive for SARS-CoV-2, 348 (23.74%) had a coinfection by another pathogen on the RP, the most common of which was rhino/enterovirus (n = 230, 15.69%), followed by adenovirus (n = 62, 4.23%), and RSV (n = 45, 3.07%) (Table 2). Of those testing negative for SARS-CoV-2, 10,361 (58.43%) had detection of another pathogen, the most common of which was rhino/enterovirus (n = 6055, 58.44%), RSV (n = 2428, 23.43%), and adenovirus (n = 1215, 11.73%). Regardless of SARS-CoV-2 status, those who were positive for another pathogen were significantly younger (mean age in years [SD] 4.33 [4.60] and 3.66 [4.09] for SARS-CoV-2 negative and positive, respectively) than those who did not have another pathogen (mean age in years [SD], 7.30 [6.35] and 7.35 [6.31] for SARS-CoV-2 negative and positive; P < 0.05 for all comparisons). Most of the RPs were performed during the Delta-predominant era from July 4 to December 17, 2021. Most positive tests for SARS-CoV-2 and for other respiratory pathogens also occurred during this time period. Coinfections with SARS-CoV-2 were most observed in the Delta era (n = 190, 54.60%), followed by the Omicron era (n = 83, 23.85%) and the Alpha era (n = 75, 21.55%).
TABLE 1.
Patient Characteristics of All Visits (n = 19,199)
| SARS-CoV-2 Negative | SARS-CoV-2 Positive | |||
|---|---|---|---|---|
| + Other Pathogen | – Other Pathogen | + Other Pathogen | – Other Pathogen | |
| Total number, N (%) | 10,361 (58.43) | 7372 (41.57) | 348 (23.74) | 1118 (76.26) |
| Age, mean (SD) | 3.66 (4.09) | 7.35 (6.31) | 4.33 (4.60) | 7.30 (6.35) |
| Era | ||||
| Alpha: January 1–July 3, N (%) | 3278 (31.64) | 3137 (42.55) | 75 (21.55) | 233 (20.84) |
| Delta: July 4–December 17, N (%) | 6515 (62.88) | 3857 (52.32) | 190 (54.60) | 502 (44.90) |
| Omicron: December 18–31, N (%) | 568 (5.48) | 378 (5.13) | 83 (23.85) | 383 (34.26) |
| Location | ||||
| Emergency department | 7590 (73.26) | 3532 (47.91) | 261 (75.00) | 817 (73.08) |
| Intensive care unit | 1065 (10.28) | 1513 (20.52) | 45 (12.93) | 94 (8.41) |
| Inpatient floor | 1392 (13.43) | 2011 (27.28) | 30 (8.62) | 171 (15.30) |
| Outpatient | 248 (2.39) | 258 (3.50) | 11 (3.16) | 30 (2.68) |
| Procedure/operating room | 66 (0.64) | 58 (0.79) | 1 (0.29) | 6 (0.54) |
TABLE 2.
Summary of Coinfection with SARS-CoV-2 by Pathogen (N = 19,199)
| Number of Participants With Pathogen | SARS-CoV-2 Positive, N = 1466 | SARS-CoV-2 Negative, N = 17,733 | P * | |
|---|---|---|---|---|
| Rhino/enterovirus | 6285 | 230 (15.69%) | 6055 (34.15%) | <0.001 |
| RSV | 2473 | 45 (3.07%) | 2428 (13.69%) | <0.001 |
| Influenza A | 193 | 5 (0.34%) | 188 (1.06%) | 0.008 |
| Influenza B | 6 | 2 (0.14%) | 4 (0.02%) | 0.071 |
| Parainfluenza 1 | 11 | 0 (0.00%) | 11 (0.06%) | >0.999 |
| Parainfluenza 2 | 284 | 13 (0.89%) | 271 (1.53%) | 0.051 |
| Parainfluenza 3 | 1,071 | 26 (1.77%) | 1045 (5.89%) | <0.001 |
| Parainfluenza 4 | 210 | 7 (0.48%) | 203 (1.14%) | 0.018 |
| Coronavirus 229E | 51 | 2 (0.14%) | 49 (0.28%) | 0.434 |
| Coronavirus HKU1 | 11 | 1 (0.07%) | 10 (0.06%) | 0.583 |
| Coronavirus NL63 | 187 | 4 (0.27%) | 183 (1.03%) | 0.004 |
| Coronavirus OC43 | 371 | 6 (0.41%) | 365 (2.06%) | <0.001 |
| HMPV | 1110 | 24 (1.64%) | 1086 (6.12%) | <0.001 |
| Mycoplasma pneumoniae | 2 | 0 (0.00%) | 2 (0.01%) | >0.999 |
| Adenovirus | 1277 | 62 (4.23%) | 1215 (6.85%) | <0.001 |
Chi-square or Fisher’s tests for independence between SARS-CoV-2 detection and detection of the other pathogen.
HMPV indicates human metapneumovirus; RSV, respiratory syncytial virus.
Many RPs were performed in the ED (n = 12,200), followed by the inpatient floors (n = 3604), ICU (n = 2717), outpatient (n = 547), and procedure/operating rooms (n = 131) (Table 1). Compared with other testing locations, the ED had the highest percentage of SARS-CoV-2 infections (n = 1078, 8.84%), the highest percentage of other respiratory infections (n = 7,590, 62.21%), the highest percentage of coinfections (n = 2074, 17.00%), and the highest percentage of coinfections with SARS-CoV-2 (n = 817, 2.14%). In contrast, many patients in the ICU and inpatient floors had completely negative RPs (n = 1513/2717, 55.69% and 2011/3604, 55.80%, respectively). Coinfections were observed in the procedure/operating rooms (9.92%), the ICU (9.42%), outpatient (9.32%), and inpatient floors (7.99%), but all at significantly lower percentages than in the ED (P < 0.001 for all comparisons).
When the analysis was limited to the first RP for each patient, there were 15,287 unique patients with RP testing performed, of whom 1253 (8.20%) were positive and 14,034 (91.80%) were negative for SARS-CoV-2 on their first RP (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/F79). Of those positive for SARS-CoV-2 at the first RP, 289 (23.06%) also had coinfection of another pathogen. Of those testing negative for SARS-CoV-2 at the first RP, 8588 (61.19%) had detection of another pathogen. Similar trends were observed for age, time period, and testing location when the analysis was limited to the first RP in comparison to all RPs.
Pathogen Detection by Month and Age
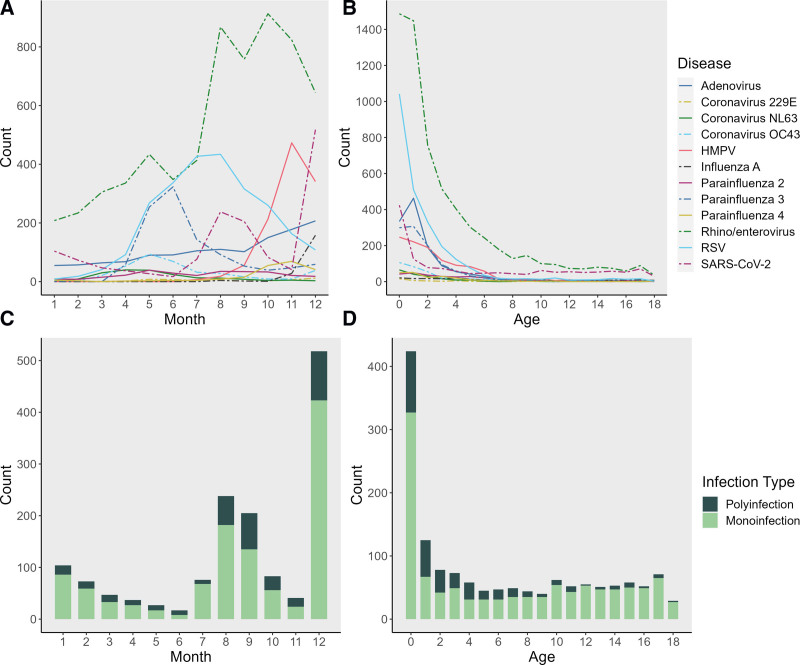
Rhino/enterovirus was the most detected pathogen, with peak detection in October 2021 (see Figure, Supplemental Digital Content 2, http://links.lww.com/INF/F80; Fig. 1). RSV was the second most detected pathogen, with a summer surge that peaked in August 2021 and trended down thereafter. SARS-CoV-2 was detected most in the second half of 2021, with peaks in August and December, coinciding with peak Delta and Omicron circulation, respectively. Coinfection also rose in proportion during the second half of 2021, although infection with a single pathogen was consistently more common throughout the year. Children <2 years of age had the highest frequency of detection of any pathogen, of each individual pathogen on the RP, and of coinfection. This was similarly true of SARS-CoV-2, as the most infected population was <1 year of age. Furthermore, we found that age and the number of coinfections correlated in SARS-CoV-2-positive participants. For every 1-year increase in age in SARS-CoV-2-positive participants, the rate of coinfections decreased by 8% (95% CI: 6–9, P < 0.001) in all visits and by 7% (95% CI: 5–9, P < 0.001) in the first visits.
FIGURE 1.
Pathogen detection by multiplexed respiratory PCR panel (BioFire) at Children’s Healthcare of Atlanta, 2021. A: Pathogen detection by month. B: Pathogen detection by age. C: Respiratory coinfections with SARS-CoV-2 by month. D: Respiratory coinfections with SARS-CoV-2 by age.
Coinfections in Children With SARS-CoV-2
Most infections were not independent of SARS-CoV-2 status. Rhino/enterovirus, RSV, influenza A, parainfluenza 3, parainfluenza 4, coronavirus NL63, coronavirus OC43, HMPV, and adenovirus were each significantly more likely to be seen in those without SARS-CoV-2 versus those with SARS-CoV-2 (P < 0.05 for all comparisons) (Table 2). When we limited participants to their first visit only, we found similar results (see Table, Supplemental Digital Content 3, http://links.lww.com/INF/F81). The incident rate of detecting another pathogen in SARS-CoV-2-positive participants was 0.39 (95% CI: 0.36–0.43) times the incident rate for SARS-CoV-2 negative individuals (P < 0.001) in all visits.
Of the 1426 patients who tested positive for SARS-CoV-2 at any point, 159 had repeat RPs performed at a subsequent visit after testing positive for SARS-CoV-2. Thirty (18.9%) remained positive for SARS-CoV-2 at their first return visit (median days after initial test = 16, IQR 7–37.5), while 67 (42.1%) had acquired a new pathogen (median days after initial test = 91, IQR 55.5–128). There were 46 patients who returned within 28 days, of whom 6 (13.0%) had acquired a new infection, 21 (45.7%) continued to test positive for SARS-CoV-2, and 4 (8.7%) continued to test positive for SARS-CoV-2 as well as for a new pathogen.
Coinfections in Children With Other Respiratory Pathogens
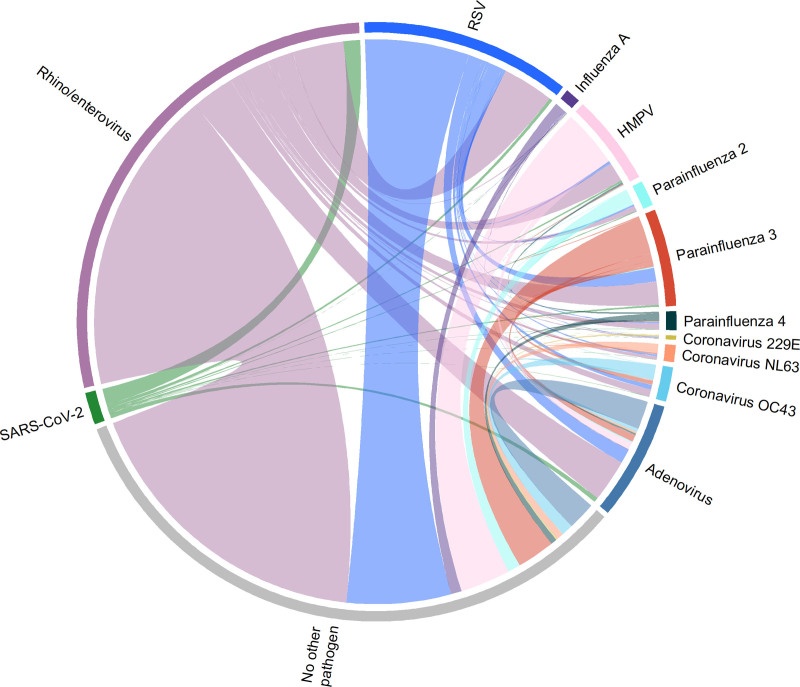
We then determined the proportion of coinfections in participants who tested positive for other respiratory pathogens (Table 3). In pathogen groups with more than 50 observations, coinfections were most observed with adenovirus (n = 885, 69.3%), followed by parainfluenza virus 4 (n = 125, 59.52%) and coronavirus 229E (n = 30, 58.82%). Coinfections were least frequently observed among children with influenza A (n = 45, 23.32%). The most common coinfection observed overall was rhino/enterovirus with RSV (n = 713) (Fig. 2). Coinfections were significantly less common among children with SARS-CoV-2 compared with all other pathogens except for influenza A (difference in proportion: 0.42, 95% CI: –5.96 to 6.80, P = 0.897) and influenza B (difference in proportion: –9.60, 95% CI: –55.75 to 36.55, P = 0.949). Similar trends were seen when examining only the first RP for each patient (see Table, Supplemental Digital Content 4, http://links.lww.com/INF/F82). Coinfections stratified by testing site were also determined (see Tables, Supplemental Digital Content 5, http://links.lww.com/INF/F83, and 6, http://links.lww.com/INF/F84).
TABLE 3.
Difference in Proportion of Coinfection in SARS-CoV-2-positive Individuals Versus Individuals Positive for Other Pathogens (N = 19,199)
| Pathogen | Number of Participants With Pathogen | Participants With Any Coinfection, N (%) | Difference in Proportion With SARS-CoV-2, * (95% CI) | P † |
|---|---|---|---|---|
| Rhino/enterovirus | 6285 | 2032 (32.33%) | –8.59 (–11.22 to –5.97) | <0.001 |
| RSV | 2473 | 1019 (41.21%) | –17.47 (–20.54 to –14.39) | <0.001 |
| Influenza A | 193 | 45 (23.32%) | 0.42 (–5.96 to 6.80) | 0.897 |
| Influenza B | 6 | 2 (33.33%) | –9.60 (–55.75 to 36.55) | 0.949 |
| Parainfluenza 1 | 11 | 8 (72.73%) | –48.99 (–79.98 to –18) | 0.001 |
| Parainfluenza 2 | 284 | 112 (39.44%) | –15.70 (–21.29 to –10.11) | <0.001 |
| Parainfluenza 3 | 1071 | 543 (50.70%) | –26.96 (–30.72 to –23.20) | <0.001 |
| Parainfluenza 4 | 210 | 125 (59.52%) | –35.79 (–42.29 to –29.28) | <0.001 |
| Coronavirus 229E | 51 | 30 (58.82%) | –35.09 (–47.16 to –23.01) | <0.001 |
| Coronavirus HKU1 | 11 | 9 (81.82%) | –58.08 (–85.56 to –30.6) | <0.001 |
| Coronavirus NL63 | 187 | 91 (48.66%) | –24.93 (–31.65 to –18.20) | <0.001 |
| Coronavirus OC43 | 371 | 207 (55.80%) | –32.06 (–37.29 to –26.83) | <0.001 |
| HMPV | 1110 | 405 (36.49%) | –12.75 (–16.30 to –9.20) | <0.001 |
| Mycoplasma pneumoniae | 2 | 2 (100.00%) | –76.26 (–100 to –49.05) | <0.001 |
| Adenovirus | 1277 | 885 (69.30%) | –45.56 (–49.30 to –41.83) | <0.001 |
Proportion of coinfection in SARS-CoV-2-positive participants was 23.74%
Tests if there was a significant difference in the proportion of codetection in positive SARS-CoV-2 samples and proportion of codetection in other pathogen positive samples.
HMPV indicates human metapneumovirus; RSV, respiratory syncytial virus.
FIGURE 2.
Chord diagram showing respiratory coinfections within the study cohort for all pathogens detected >50 times.
DISCUSSION
We performed a retrospective cohort analysis of 19,199 unique visits from children with RP testing performed at Children’s Healthcare of Atlanta from January 1 to December 31, 2021. We found that SARS-CoV-2 infections were detected in 1466 (7.64%) of the visits, and 348 (23.74%) of these were associated with respiratory coinfections, the most common of which was rhino/enterovirus, followed by adenovirus and RSV. Coinfections with SARS-CoV-2 occurred most commonly in the era of Delta predominance, which coincided with the periods of peak rhino/enterovirus and RSV transmission. Although coinfections were common among all respiratory pathogens, they were significantly less common with SARS-CoV-2 than other pathogens, with exception of influenza A and influenza B, which were detected infrequently throughout the study.
Regarding the epidemiology of respiratory viral infections in our study, SARS-CoV-2 was associated with peaks in August and December 2021,19 coinciding with peak Delta and Omicron variant transmission, respectively. The most commonly detected pathogens were rhino/enterovirus and RSV, which were associated with summer surges.20,21 Rhino/enterovirus was also the most commonly detected copathogen among those with coinfections, which is consistent with previous studies,6,22–24 although adenovirus was associated with the highest proportion of coinfections (69.29%) among those with sufficient sample size for comparison. Coinfections were most observed in children <2 years of age, who had the highest frequency of detection of any pathogen, of each individual pathogen on the RP, including SARS-CoV-2, and of coinfection. Thus, factors associated with the specific pathogen, host, and time period influenced the likelihood of coinfection.
The majority of RP testing in our study was performed in the ED, which also had the highest percentage of SARS-CoV-2 infections, the highest percentage of other respiratory infections, the highest percentage of all coinfections, and the highest percentage of coinfections with SARS-CoV-2 when compared with other testing sites. Interestingly, most RPs performed in the ICU and inpatient floors were completely negative, and coinfections were less commonly observed when testing was performed in these locations compared with the ED. One possible explanation for this may be differences in the indication for testing of children in the ED versus later during their clinical course in the ICU or inpatient floors.25 Overall, these data suggest that respiratory coinfections in children with SARS-CoV-2 are common, and they are most often observed in young children presenting to the ED.
Recent data from COVID-NET surveillance found a similar frequency of respiratory viral coinfections among children hospitalized with SARS-CoV-2 infection (21%) as in our study but also found coinfections with SARS-CoV-2 and rhino/enterovirus or RSV to be associated with increased ICU admission and oxygen requirement in young children.16 Interestingly, the frequency of rhino/enterovirus coinfection with SARS-CoV-2 in our study was also higher in the ICU (n = 36/139, 25.90%) versus the inpatient floors (n = 15/201, 7.46%) and ED (n = 171/1078, 15.86%), but the rates of RSV coinfections were similar among the testing locations. Future studies are needed to understand pathophysiologic mechanisms that underlie these potential clinical associations to better inform patient management.
Limitations of our study include that it was a retrospective analysis within a single hospital system, which encompassed 3 freestanding children’s hospitals. Testing was performed at the clinician’s discretion, and we did not control for the clinical symptoms, site or indication for testing, or immune or vaccination status of the patient. Singleplex and fourplex PCR tests were also available for SARS-CoV-2 diagnosis in the hospital system, but these were not included in our analysis, which may have led to an underestimation of the true frequency of coinfections during the time period. Our initial analysis was performed for all visits with RP testing performed, which had the potential to overestimate coinfections if RPs were repeated with subsequent visits during a time period when the participant was still shedding the initial respiratory pathogen. The duration of SARS-CoV-2 shedding from the respiratory tract in children has been reported to be an average of 11.1 ± 5.8 days, although this may persist longer.26 Nevertheless, a sensitivity analysis limited to the first visit from each participant demonstrated similar results. Although we termed all RP codetections of multiple pathogens as coinfections, we were unable to distinguish the extent to which either pathogen was contributing to clinical disease. While early data following emergence of the Omicron variant were included in this analysis, we did not capture the peak of Omicron transmission27 or data about Omicron subvariants. Finally, we lacked data describing clinical disease severity or outcomes, which are important subjects of future research.
In conclusion, respiratory coinfections were not infrequent among children with SARS-CoV-2, and the most common were rhino/enterovirus and RSV. Coinfections were most common in the Delta-predominant era and in children <2 years of age whose testing was performed in the ED. Thus, factors associated with the specific pathogen, host, and time period influenced the likelihood of coinfection. Additional studies are needed to understand the role of coinfections in disease pathogenesis and patient outcomes to guide clinical management of children with SARS-CoV-2 infection.
ACKNOWLEDGMENTS
We thank Dr. Robert Jerris, Dr. Beverly Rogers, and the laboratory staff at Children’s Healthcare of Atlanta for their contributions to clinical testing for SARS-CoV-2 and other respiratory pathogens.
Supplementary Material
Footnotes
This work was supported by the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health under award Numbers U54 EB027690-03S1 and U54 EB027690-03S2 and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
C.A.R.’s institution has received funds to conduct clinical research unrelated to this manuscript from the National Institutes of Health, BioFire Inc, GSK, MedImmune, Micron, Janssen, Merck, Moderna, Novavax, PaxVax, Pfizer, Regeneron, Sanofi-Pasteur. She is co-inventor of patented respiratory syncytial virus vaccine technology unrelated to this manuscript, which has been licensed to Meissa Vaccines, Inc. The remaining authors have no conflicts of interest to disclose.
The information contained herein has not been previously published or presented at any meetings.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Adrianna Westbrook, Email: adrianna.lynn.westbrook@emory.edu.
Tingyu Wang, Email: wty_qiantu@163.com.
Kushmita Bhakta, Email: kushmita.bhakta@emory.edu.
Julie Sullivan, Email: jsulliv@emory.edu.
Mark D. Gonzalez, Email: Mark.Gonzalez@choa.org.
Wilbur Lam, Email: wilbur.lam@emory.edu.
REFERENCES
- 1.The Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). COVID-19 Dashboard. Available at: https://coronavirus.jhu.edu/map.html. Accessed April 10, 2023.
- 2.Haddadin Z, Schuster JE, Spieker AJ, et al. Acute respiratory illnesses in children in the SARS-CoV-2 pandemic: prospective multicenter study. Pediatrics. 2021;148:e2021051462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic-United States, 2020-2021. Am J Transplant. 2021;21:3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peci A, Tran V, Guthrie JL, et al. Prevalence of co-infections with respiratory viruses in individuals investigated for SARS-CoV-2 in Ontario, Canada. Viruses. 2021;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazra A, Collison M, Pisano J, et al. Coinfections with SARS-CoV-2 and other respiratory pathogens. Infect Control Hosp Epidemiol. 2020;41:1228–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, et al. ; COVID-19 Researchers Group. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah MM, Patel K, Milucky J, et al. ; CDC COVID‐NET Surveillance Team. Bacterial and viral infections among adults hospitalized with COVID-19, COVID-NET, 14 states, March 2020-April 2022. Influenza Other Respir Viruses. 2023;17:e13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Hingrat Q, Bouzid D, Choquet C, et al. Viral epidemiology and SARS-CoV-2 co-infections with other respiratory viruses during the first COVID-19 wave in Paris, France. Influenza Other Respir Viruses. 2021;15:425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chekuri S, Szymczak WA, Goldstein DY, et al. SARS-CoV-2 coinfection with additional respiratory virus does not predict severe disease: a retrospective cohort study. J Antimicrob Chemother. 2021;76:iii12–iii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146:e20200961. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Wang H, Wang F, et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected children: a retrospective study. Medicine (Baltim). 2021;100:e24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garazzino S, Lo Vecchio A, Pierantoni L, et al. ; Italian SITIP-SIP Pediatric Infection Study Group. Epidemiology, clinical features and prognostic factors of pediatric SARS-CoV-2 infection: results from an Italian multicenter study. Front Pediatr. 2021;9:649358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvares PA. SARS-CoV-2 and respiratory syncytial virus coinfection in hospitalized pediatric patients. Pediatr Infect Dis J. 2021;40:e164–e166. [DOI] [PubMed] [Google Scholar]
- 16.Agathis NT, Patel K, Milucky J, et al. ; CDC COVID-NET Surveillance Team. Codetections of other respiratory viruses among children hospitalized with COVID-19. Pediatrics. 2023;151:e2022059037. [DOI] [PubMed] [Google Scholar]
- 17.Derrick B, Dobson-McKittrick A, Toher D, et al. Test statistics for comparing two proportions with partially overlapping samples. J Appl Quant Methods. 2015;10:1–14. [Google Scholar]
- 18.Partiallyoverlapping: Partially Overlapping Samples Tests. R package version 2.0. Version 2.0. 2018. Available at: https://CRAN.R-project.org/package=Partiallyoverlapping
- 19.Lambrou AS, Shirk P, Steele MK, et al. ; Strain Surveillance and Emerging Variants Bioinformatic Working Group. Genomic surveillance for SARS-CoV-2 variants: predominance of the delta (B.1.617.2) and omicron (B.1.1.529) variants - United States, June 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics. 2021;148:e2021052089. [DOI] [PubMed] [Google Scholar]
- 21.McNab S, Ha Do LA, Clifford V, et al. Changing epidemiology of respiratory syncytial virus in Australia-Delayed re-emergence in Victoria compared to Western Australia/New South Wales (WA/NSW) after prolonged lock-down for coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2021;73:2365–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Quinn J, Pinsky B, et al. Rates of Co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boschiero MN, Duarte A, Palamim CVC, et al. Frequency of respiratory pathogens other than SARS-CoV-2 detected during COVID-19 testing. Diagn Microbiol Infect Dis. 2022;102:115576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouafi M, Dubos F, Engelman I, et al. Rapid syndromic testing for respiratory viral infections in children attending the emergency department during COVID-19 pandemic in Lille, France, 2021-2022. J Clin Virol. 2022;153:105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayotte A, Mariani-Kurkdjian P, Boizeau P, et al. Viral identification using multiplex polymerase chain reaction testing does not reduce antibiotic prescribing in paediatric intensive care units. Microorganisms. 2023;11:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu CLH, Raval M, Schnall JA, et al. Duration of respiratory and gastrointestinal viral shedding in children with SARS-CoV-2: a systematic review and synthesis of data. Pediatr Infect Dis J. 2020;39:e249–e256. [DOI] [PubMed] [Google Scholar]
- 27.Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.