Purpose
This study aimed to explore the imaging value of 68Ga-FAPI-04 PET/CT in synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome and compare it with that of 99mTc-MDP bone scan.
Methods
Nineteen participants with SAPHO syndrome underwent 68Ga-FAPI-04 PET/CT and 99mTc-MDP bone scan. Demographic data and clinical features were recorded, SAPHO imaging features were analyzed, and the osteoarticular lesion detection rate in both methods was calculated.
Results
This prospective study recruited 4 men and 15 women aged 52.4 ± 8.6 years. The anterior chest wall was involved in all participants (100%). Palmoplantar pustulosis was the most common (36.8%) skin symptom. 99mTc-MDP bone scan and 68Ga-FAPI-04 PET/CT together detected 84 osteoarticular lesions, of which 91.7% (77/84) were detected by the former and 96.4% (81/84) by the latter. Furthermore, 68Ga-FAPI-04 PET/CT detected 5 cases of knee and hip joint synovitis.
Conclusions
68Ga-FAPI-04 PET/CT was more sensitive than 99mTc-MDP bone scan when evaluating osteoarticular lesions in SAPHO syndrome and could also evaluate synovial lesions. 68Ga-FAPI-04 PET/CT could be a good imaging method for SAPHO syndrome but requires further verification in a more extensive research cohort.
Key Words: 68Ga-FAPI-04, 99mTc-MDP, bone scan, PET/CT, SAPHO syndrome
Synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome is a rare chronic aseptic inflammatory disease that involves multiple organs, including the bones, joints, and skin.1 Osteoarticular disorder is a characteristic feature of SAPHO syndrome and typically involves the anterior chest wall.2,3 Palmoplantar pustulosis is among the most common cutaneous features of SAPHO syndrome, followed by severe acne and psoriasis vulgaris.4 The high heterogeneity of the disease makes its diagnosis and management challenging, especially if presenting with bone and joint but no skin involvement.1,5
Radiography and CT are commonly used for osteoarticular lesions assessment in SAPHO syndrome.5 However, these conventional imaging modalities often fail to detect early-stage lesions.6 A whole-body bone scan is a practical first-line approach for systematically evaluating osteoarticular lesions in SAPHO syndrome.6 It is highly sensitive and can detect potential lesions without clinical manifestations.1 Besides, it can reduce unnecessary biopsies.7
Fibroblast activation protein (FAP) is a type II transmembrane serine protease expressed by activated fibroblasts.8 Fibroblast activation protein inhibitors (FAPIs) can specifically target and bind to FAP and could be used as probes to visualize FAP-expressing lesions when radiolabeled.9 68Ga-FAPI has been introduced for tumor imaging and has demonstrated promising results. However, FAP expression is not cancer-specific; it is overexpressed in tissue remodeling sites associated with inflammation, fibrosis, rheumatological diseases, atherosclerosis, and cardiac injury, among others.10,11 68Ga-FAPI PET has shown good application prospects in imaging benign bone, joint, or muscle diseases, including immunoglobulin G4–related disease,12,13 polymyositis,14 rheumatoid arthritis,15 osteoarthritis,11,16,17 bone cyst,18 fracture,19 myositis ossificans,20 Schmorl node,21 osteofibrous dysplasia,16,22 intraosseous meningioma,23 and bone tuberculous.24 The histopathological features of SAPHO syndrome are nonspecific osteomyelitis with inflammatory cell infiltration and a healing process with osteosclerosis and bone marrow fibrosis.4,25,26 Therefore, we hypothesized that it would be possible to use 68Ga-FAPI imaging for SAPHO syndrome. We recently reported 68Ga-FAPI-04 PET/CT imaging results in a woman with SAPHO syndrome, showing potential application prospects.27 This study aimed to explore the imaging value of 68Ga-FAPI-04 PET/CT in SAPHO syndrome and compare it with that of 99mTc-MDP bone scan.
MATERIALS AND METHODS
Study Design and Patients
Nineteen participants diagnosed with SAPHO syndrome at our hospital were consecutively recruited from September 2020 to August 2022. This study was approved by the ethics committee of our hospital (AHSWMU-2020-035). Written informed consent was obtained from all participants. After enrollment, the participants underwent 68Ga-FAPI-04 PET/CT and 99mTc-MDP bone scan within 1 week. The recorded demographic data and clinical features included age, sex, age at onset of dermatological and osteoarticular symptoms, age at SAPHO syndrome diagnosis, disease duration, and dermatological manifestations (palmoplantar pustulosis, severe acne, or psoriasis vulgaris). Laboratory evaluations included erythrocyte sedimentation rate (ESR), high-sensitivity C-reactive protein (hs-CRP), rheumatoid factor, antinuclear antibody (ANA), and human leukocyte antigen B27 (HLA-B27), measured within 1 week of the PET/CT examination. Findings in joint ultrasonography, CT, and MRI were recorded. Pathological skeletal involvement results, if any, were also recorded. All participants were followed up for at least 3 months. The 68Ga-FAPI-04 PET/CT imaging results of 1 of the 19 participants were previously reported.27
Diagnostic and Exclusion Criteria
The diagnostic criteria for SAPHO syndrome included (1) bone and joint involvement associated with palmoplantar pustulosis, psoriasis vulgaris, or severe acne; (2) isolated sterile hyperostosis/osteitis (adults); (3) chronic recurrent multifocal osteomyelitis (children); and (4) bone and joint involvement associated with chronic bowel diseases.28 The exclusion criteria were septic osteomyelitis, infectious chest wall arthritis, bone tumor, diffuse idiopathic skeletal hyperostosis, osteoarticular manifestations of retinoid therapy, palmoplantar keratoderma, and infectious palmoplantar pustulosis.6,25
99mTc-MDP Bone Scan and 68Ga-FAPI-04 PET/CT Imaging
Whole-body bone scan was performed 3 to 4 hours after intravenous injection of 740 to 925 MBq (20–25 mCi) 99mTc-MDP. SPECT/CT of the symptomatic sites and/or sites of abnormal uptake was performed. The injection dose of 68Ga-FAPI-04 was 1.85 MBq (0.05 mCi)/kg. PET/CT scanning from head to foot (3 minutes per bed position) was performed 40 to 60 minutes after the tracer injection. The resulting images were corrected by attenuation and reconstructed iteratively to obtain the transverse, coronal, and sagittal views of the PET/CT scans.
Image Analysis
All images were evaluated by 3 experienced nuclear medicine physicians blinded to the clinical features and laboratory evaluations. Interobserver differences in image interpretation were resolved by discussion, and a diagnostic consensus was reached. We defined several major regions to classify the lesion sites: anterior chest wall, comprising the ribs, sternoclavicular joints, costosternal joints, anterior first rib, clavicles, and sternum; axial skeleton, comprising the spine (cervical, thoracic, and lumbosacral) and sacroiliac joints; pelvis; peripheral joints and bones; and craniofacial bone. The site and number of osteoarticular lesions with abnormal tracer uptake on PET/CT or bone scan, abnormal CT findings, and SUVmax of lesions with abnormal tracer uptake on PET/CT were recorded. The imaging features on PET/CT and bone scan were analyzed, and the osteoarticular lesion detection rates by the 2 methods were calculated.
Statistical Analysis
Data analysis was performed using IBM SPSS Statistics for Macintosh, Version 26.0 (IBM Corp, Armonk, NY). Descriptive data are shown as mean ± SD, median (range), or number (%).
RESULTS
Demographic and Clinical Characteristics
Table 1 presents the demographic and clinical characteristics of the 19 participants. The 4 men and 15 women had a mean age of 52.4 ± 8.6 years. Anterior chest wall involvement occurred in all participants (100%); 68.4% had skin symptoms, with palmoplantar pustulosis being the most common (36.8%). The hs-CRP and ESR were elevated in most participants. No participant had a positive HLA-B27 test, but 6 had a positive ANA test. Four participants underwent bone biopsies. The histopathology results indicated fibrous tissue hyperplasia accompanied by lymphocyte and plasma cell infiltration, suggesting chronic nonspecific inflammation.
TABLE 1.
Demographic and Clinical Features of the 19 Participants With SAPHO Syndrome
| Demographic and Clinical Features (N = 19) | |
|---|---|
| Age, mean ± SD, y | 52.4 ± 8.6 |
| Sex, male/female, n | 4/15 |
| Age at symptom onset, mean ± SD, y | 49.6 ± 8.0 |
| Age at osteoarticular symptoms onset, mean ± SD, y | 50.5 ± 8.2 |
| Age at skin lesion onset, mean ± SD, y | 49.9 ± 8.1 |
| Disease duration, mean ± SD, mo | 29.4 ± 14.5 |
| Osteoarticular symptoms, n (%) | |
| Anterior chest pain | 19 (100) |
| Spinal pain | 2 (10.5) |
| Sacroiliac pain | 3 (15.8) |
| Peripheral skeletal or joint pain | 2 (10.5) |
| Craniofacial pain | 1 (5.3) |
| Skin manifestations, n (%) | |
| Palmoplantar pustulosis | 7 (36.8) |
| Severe acne | 5 (26.3) |
| Psoriasis vulgaris | 2 (10.5) |
| Laboratory examinations | |
| ESR, mean ± SD, mm/h | 41.1 ± 21.4 |
| hs-CRP, mean ± SD, mg/L | 7.6 ± 5.2 |
| Elevated ESR, n (%) | 13 (68.4) |
| Elevated hs-CRP, n (%) | 7 (36.8) |
| ANA-positive, n (%) | 6 (31.6) |
| Rheumatoid factor–positive, n (%) | 0 |
| HLA-B27–positive, n (%) | 0 |
Imaging Characteristics of 99mTc-MDP Bone Scan
Bone scan detected 77 osteoarticular lesions (Table 2) and showed that anterior chest wall involvement occurred in all participants (100%), involving 65 lesions. A bull's-head sign was seen in 31.6% of the participants (6/19).
TABLE 2.
Osteoarticular Lesions Revealed by 99mTc-MDP Bone Scan and 68Ga-FAPI-04 PET/CT in 19 Participants
| 99mTc-MDP Bone Scan | 68Ga-FAPI-04 PET/CT | Bone Scan + PET/CT | |||||
|---|---|---|---|---|---|---|---|
| Site | Patients | Lesions | Patients | Lesions | SUVmax Median (Range) |
Patients | Lesions |
| Anterior chest wall | 19 | 65 | 19 | 64 | 4.5 (1.5–8.7) | 19 | 67 |
| Cervical spine | 1 | 1 | 1 | 2 | 3.8 (3.3–4.3) | 1 | 2 |
| Thoracic spine | 0 | 0 | 1 | 1 | 2.1 | 1 | 1 |
| Lumbosacral spine | 2 | 5 | 4 | 7 | 3.8 (2.1–4.6) | 4 | 7 |
| Sacroiliac joint | 3 | 3 | 3 | 3 | 4.5 (1.9–5.8) | 3 | 3 |
| Pelvis | 1 | 1 | 1 | 1 | 2.6 | 1 | 1 |
| Peripheral joint and bone | 1 | 1 | 1 | 1 | 4.4 | 1 | 1 |
| Craniofacial bone | 1 | 1 | 2 | 2 | 4.1 (2.0–6.2) | 2 | 2 |
| Total | — | 77 | — | 81 | 4.3 (1.5–8.7) | — | 84 |
Imaging Characteristics of 68Ga-FAPI-04 PET/CT
PET/CT detected 81 osteoarticular lesions (Table 2) and showed that anterior chest wall involvement occurred in all participants (100%), involving 64 lesions. The median SUVmax of all lesions was 4.3. The main bone and joint involvement manifestations included hyperostosis, osteosclerosis, or osteolysis. PET/CT detected 5 synovial lesions in the knee and hip joints (Figs. 1 and 2). A right breast nodule with abnormal tracer uptake (Fig. 1) detected in one participant was confirmed as lobular carcinoma in situ via puncture.
FIGURE 1.
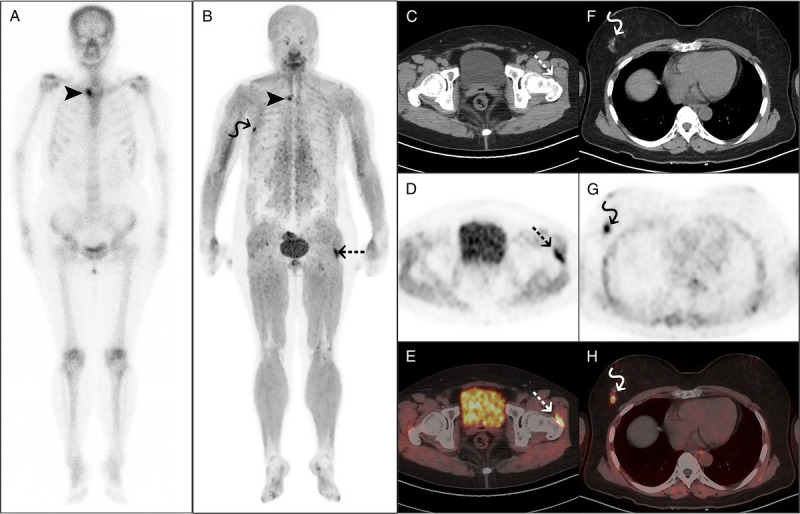
68Ga-FAPI-04 PET/CT detected left hip synovitis in a 57-year-old woman with SAPHO syndrome. The woman presented with anterior chest wall and left hip pain for 6 months and palmar pustules over the past 3 years. Laboratory examination showed elevated ESR (67 mm/h) and hs-CRP (13 mg/L) and a positive ANA test. 99mTc-MDP bone scan (A) and 68Ga-FAPI-04 PET/CT (B) both showed increased tracer uptake in the right sternoclavicular joint (arrowheads). PET/CT (B–E) also showed increased tracer uptake in the synovial sac of the left hip joint (dotted arrows). Subsequent ultrasonography of the left hip confirmed the presence of synovitis. Furthermore, a nodule in the right breast with abnormal tracer uptake (F–H, curved arrows) was subsequently confirmed as lobular carcinoma in situ via puncture.
FIGURE 2.
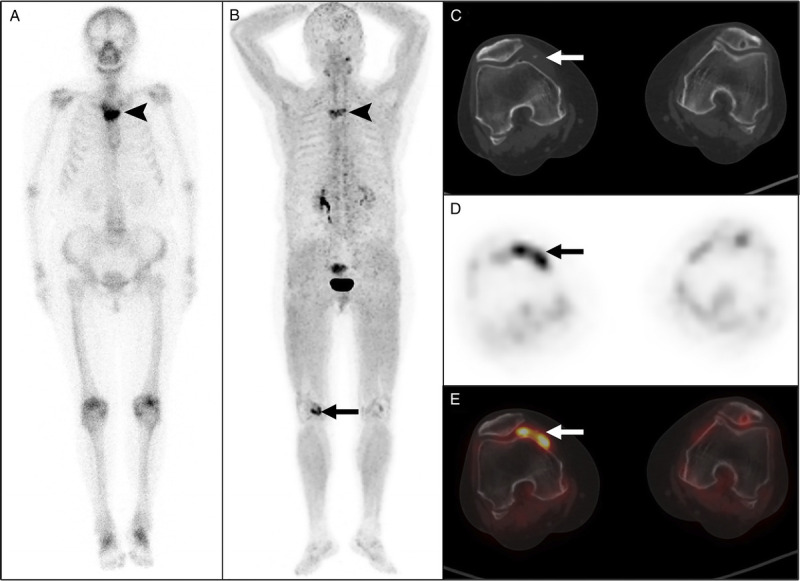
68Ga-FAPI-04 PET/CT detected right knee synovitis in a 66-year-old woman with SAPHO syndrome. The woman presented with anterior chest wall pain for 5 months and multiple intermittent pustules in both feet over the past 2 years. No abnormality was found in the laboratory examination. 99mTc-MDP bone scan (A) and 68Ga-FAPI-04 PET/CT (B) both showed increased tracer uptake in the sternal angle (arrowheads). PET/CT (B–E) also showed increased tracer uptake in the right knee joint (arrows). Subsequent ultrasonography of the right knee joint confirmed the presence of synovitis. A sternal biopsy showed signs of nonspecific chronic inflammation. This case was previously reported.27
99mTc-MDP Bone Scan and 68Ga-FAPI-04 PET/CT Osteoarticular Lesion Detection Rates
Bone scan and PET/CT together detected 84 osteoarticular lesions. Anterior chest wall involvement was the most common, with 67 lesions detected. The lumbosacral spine was the second most frequent site, with 7 lesions detected in 4 participants. The detection rates of bone scan and PET/CT were 91.7% (77/84) and 96.4% (81/84), respectively (Table 2).
DISCUSSION
Previous studies have shown that anterior chest wall involvement is most common in SAPHO syndrome and occurs in 60% to 95% of adult patients.3,6 In this study, anterior chest wall involvement occurred in all participants, with a slightly higher prevalence than previously reported. The coexistence of osteolysis and osteosclerosis was the main structural change observed in CT, especially in the anterior chest wall area. Some researchers believe that the radiographic features of SAPHO syndrome differ according to the disease stage.6 Early lesions tend to be osteodestructive, whereas later lesions tend to be osteoproliferative.6
Skin involvement is a typical manifestation of SAPHO syndrome. Previous studies found that most patients (94.9%) had dermatological manifestations,29 and palmoplantar pustulosis was among the most common cutaneous features.6 The incidence of skin involvement in our study (68.4%) was lower than previously reported.
The etiology of SAPHO syndrome remains elusive.2 Two hypotheses were proposed to explain its possible pathogenesis.25 One is that Propionibacterium acnes infection activates the innate immunity and T cell–mediated immune process.25 The other hypothesis is the genetic theory, which suggests that SAPHO syndrome is possibly associated with HLA-B27.25 However, some studies found no or only a few (2.5%–13%) HLA-B27–positive patients with SAPHO syndrome.2,29–31 All participants in our study were negative for HLA-B27, suggesting that SAPHO syndrome may not be related to HLA-B27. The serum inflammatory markers hs-CRP and ESR were elevated in most participants, and 6 had a positive ANA test, suggesting that SAPHO syndrome was an inflammatory disease associated with autoimmunity.
Figures 3 and 4 show that 68Ga-FAPI-04 PET/CT detected more bone and joint lesions than 99mTc-MDP bone scan, regardless of whether these lesions had significant density changes on CT. Our pilot study found that 68Ga-FAPI-04 PET/CT may be more sensitive than 99mTc-MDP bone scan in detecting bone and joint lesions in SAPHO syndrome, even though it missed 3 anterior chest wall lesions in 2 participants (Fig. 4). Moreover, 68Ga-FAPI-04 PET/CT revealed knee and hip joint synovitis in 5 participants (Figs. 1 and 2), compensating for the limitation of 99mTc-MDP bone scan in imaging soft tissue lesions. Dorst et al15 found that arthritic joints of patients with rheumatoid arthritis showed clear 68Ga-FAPI-04 accumulation along the synovial membrane. Their in vitro experiments showed stable FAP gene expression in human synovial fibroblasts, indicating that inflamed synovium could be visualized using FAP-targeting tracers. Although there is no direct evidence to confirm the overexpression of FAP in bone joints or synovial lesions in our study, we speculate that 68Ga-FAPI-04 accumulation resulted from uptake by activated fibroblasts.
FIGURE 3.
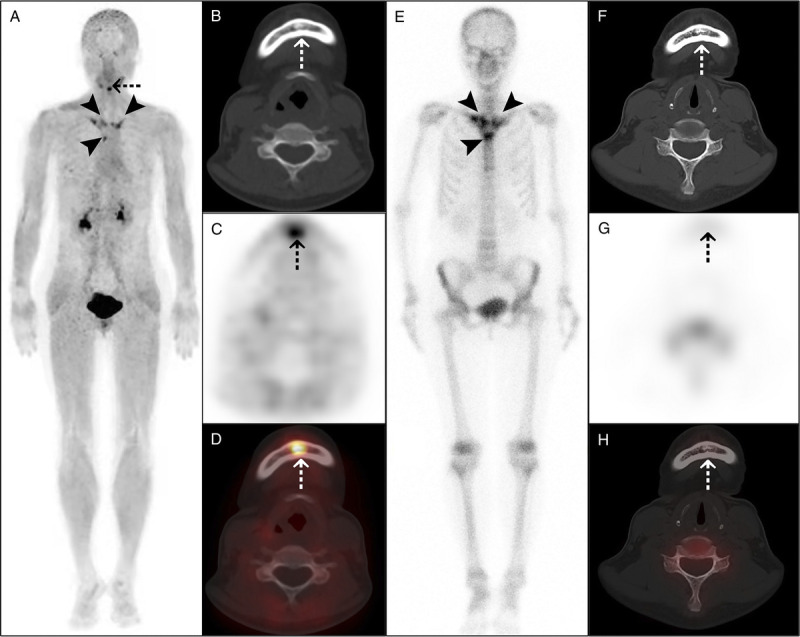
68Ga-FAPI-04 PET/CT detected more osteoarticular lesions than 99mTc-MDP bone scan in a 53-year-old woman with SAPHO syndrome. The woman presented with anterior chest wall pain for 3 years. Laboratory examination showed elevated ESR (32 mm/h) and a positive ANA test. The maximum intensity projection (A) of 68Ga-FAPI-04 PET/CT showed increased tracer uptake in the bilateral sternoclavicular joints, bilateral anterior first ribs, and sternal angle (arrowheads). Moreover, PET/CT (A–D) showed osteosclerosis in the mandible, with increased tracer uptake (dotted arrows). 99mTc-MDP bone scan (E) showed increased tracer uptake in the above sites of anterior chest wall (arrowheads), resembling the bull's-head sign. However, 99mTc-MDP bone scan showed no abnormal bone metabolism in the mandible (F–H, dotted arrows).
FIGURE 4.
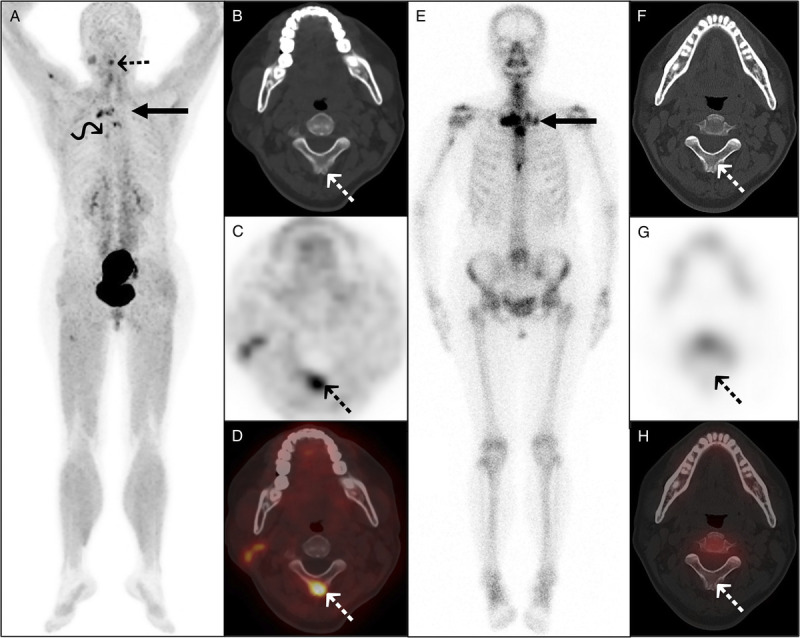
Complementarity of 68Ga-FAPI-04 PET/CT and 99mTc-MDP bone scan in imaging a 52-year-old woman with SAPHO syndrome. The woman presented with facial acne for 3 years and whole-body bone pain for 1 year. Laboratory examination showed elevated ESR (48 mm/h) and hs-CRP (11.63 mg/L). The maximum intensity projection (A) of 68Ga-FAPI-04 PET/CT showed increased tracer uptake in the multiple bones and joints. Axial PET/CT (B–D) showed abnormal tracer uptake in the second cervical vertebra spinous process (dotted arrows), without apparent bone density changes. In contrast to PET/CT, 99mTc-MDP bone scan additionally showed increased tracer uptake in the left sternoclavicular and sternocostal joints (E, long arrow), but not detected the lesions of the second cervical vertebra spinous process (F–H, dotted arrows) and the right sixth costal vertebra joint (curved arrow).
Moreover, 68Ga-FAPI-04 and 99mTc-MDP are nonspecific tracers. Both might show abnormal uptake in various benign bone diseases, infectious diseases, and malignant bone tumors. SAPHO syndrome is often diagnosed after excluding other disease processes.6 The bull's-head sign seen in whole-body bone scans is a characteristic feature of SAPHO syndrome.3 In our study, 99mTc-MDP bone scan showed the bull's-head sign in 31.6% (6/19) of the participants, higher than previously reported (10%–22.9%).6,29,32 Because 68Ga-FAPI-04 PET/CT might not show the typical bull's-head sign seen in a whole-body bone scan, this reduces its diagnostic specificity. Moreover, some studies showed 18F-FDG could be used to distinguish active from inactive inflammatory lesions in SAPHO syndrome.33–36 18F-FDG PET/CT was not included in this study as it is not routinely used for this disease in clinical practice.
The limitations of this study include the following. First, the number of cases included was insufficient for a more detailed statistical analysis. Second, biopsy results were available only for some participants, possibly introducing bias into our data. Third, this study did not evaluate the value of repetitive imaging in evaluating the curative effect because of the short follow-up and treatment heterogeneity. Fourth, no control group was included. A control group could help improve the interpretation of the SAPHO syndrome findings. This should be improved in future studies.
In summary, 68Ga-FAPI-04 PET/CT was a good evaluation approach for SAPHO syndrome. It was more sensitive than 99mTc-MDP bone scan and could detect early-stage bone and joint lesions. It can also evaluate synovial lesions and assess the disease involvement more comprehensively than 99mTc-MDP bone scan. However, these findings need to be verified in a larger research cohort.
Acknowledgments
The authors thank the members of the Department of Nuclear Medicine, The Affiliated Hospital, Southwest Medical University, and Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, for their technical guidance, cooperation, and assistance in completing this study.
Footnotes
Conflicts of interest and sources of funding: The authors declare that they have no competing interests. This work was supported by the school-level scientific research project of the Southwest Medical University (grant 2021ZKQN066) and Luzhou Science and Technology Plan Project (2022-JYJ-118).
T.X., H.D., and D.F. contributed equally to this article and shared joint first authorship. S.Z. and Y.C. contributed equally to this article and shared joint corresponding authorship.
Author Contributions: T.X., H.D., and D.F. contributed to the study design, and T.X. wrote the manuscript. T.X., H.D., D.F., and Q.S. collected and analyzed the clinical data of patients. G.L. is responsible for image processing of all patients. S.Z. and Y.C. was responsible for revising for important intellectual content. S.Z. and Y.C. contributed equally to this article and shared joint corresponding authorship. All authors read and approved the final manuscript.
Contributor Information
Tingting Xu, Email: xutingting1420@126.com.
Haoyuan Ding, Email: 315699969@qq.com.
Dongmei Fan, Email: 104652905@qq.com.
Qingxue Shu, Email: 337324547@qq.com.
Guangfu Liu, Email: 1191015778@qq.com.
Shumao Zhang, Email: zhangshumao1127@126.com.
REFERENCES
- 1.Shao S, Wang W, Qian Y. SAPHO syndrome involving the mandible treated with 99Tc-MDP. J Craniofac Surg. 2020;31:510–512. [DOI] [PubMed] [Google Scholar]
- 2.Wang M Li Y Cao Y, et al. Mandibular involvement in SAPHO syndrome: a retrospective study. Orphanet J Rare Dis. 2020;15:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu M Cao Y Li J, et al. Anterior chest wall in SAPHO syndrome: magnetic resonance imaging findings. Arthritis Res Ther. 2020;22:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun X Li C Cao Y, et al. F-18 FDG PET/CT in 26 patients with SAPHO syndrome: a new vision of clinical and bone scintigraphy correlation. J Orthop Surg Res. 2018;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C Wang L Wu N, et al. A retrospective study of bone scintigraphy in the follow-up of patients with synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome: is it useful to repeat bone scintigraphy for disease asses sment? Clin Rheumatol. 2020;39:1305–1314. [DOI] [PubMed] [Google Scholar]
- 6.Schaub S, Sirkis HM, Kay J. Imaging for synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO) syndrome. Rheum Dis Clin North Am. 2016;42:695–710. [DOI] [PubMed] [Google Scholar]
- 7.Sallés M Olivé A Perez-Andres R, et al. The SAPHO syndrome: a clinical and imaging study. Clin Rheumatol. 2011;30:245–249. [DOI] [PubMed] [Google Scholar]
- 8.Backhaus P Burg MC Roll W, et al. Simultaneous FAPI PET/MRI targeting the fibroblast-activation protein for breast cancer. Radiology. 2022;302:39–47. [DOI] [PubMed] [Google Scholar]
- 9.Qin C Shao F Gai Y, et al. 68Ga-DOTA-FAPI-04 PET/MR in the evaluation of gastric carcinomas: comparison with 18F-FDG PET/CT. J Nucl Med. 2022;63:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giesel FL Kratochwil C Schlittenhardt J, et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68Ga-FAPI and 18F-FDG PET/CT in cancer patients. Eur J Nucl Med Mol Imaging. 2021;48:4377–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erol Fenercioğlu Ö Beyhan E Ergül N, et al. 18F-FDG PET/CT and 68Ga-FAPI-4 PET/CT findings of bilateral knee osteoarthritis in a patient with uveal malignant melanoma. Clin Nucl Med. 2022;47:e144–e146. [DOI] [PubMed] [Google Scholar]
- 12.Pan Q, Luo Y, Zhang W. Recurrent immunoglobulin G4–related disease shown on 18F-FDG and 68Ga-FAPI PET/CT. Clin Nucl Med. 2020;45:312–313. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y Pan Q Yang H, et al. Fibroblast activation protein–targeted PET/CT with 68Ga-FAPI for imaging IgG4-related disease: comparison to 18F-FDG PET/CT. J Nucl Med. 2021;62:266–271. [DOI] [PubMed] [Google Scholar]
- 14.Zheng J Chen H Lin K, et al. [68Ga]Ga-FAPI [18F]FDG PET/CT images in a patient with juvenile polymyositis. Eur J Nucl Med Mol Imaging. 2021;48:2051–2052. [DOI] [PubMed] [Google Scholar]
- 15.Dorst DN Rijpkema M Buitinga M, et al. Targeting of fibroblast activation protein in rheumatoid arthritis patients: imaging and ex vivo photodynamic therapy. Rheumatology. 2022;61:2999–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin C Song Y Liu X, et al. Increased uptake of 68Ga-DOTA-FAPI-04 in bones and joints: metastases and beyond. Eur J Nucl Med Mol Imaging. 2022;49:709–720. [DOI] [PubMed] [Google Scholar]
- 17.Xu T Zhao Y Ding H, et al. [68Ga]Ga-DOTA-FAPI-04 PET/CT imaging in a case of prostate cancer with shoulder arthritis. Eur J Nucl Med Mol Imaging. 2021;48:1254–1255. [DOI] [PubMed] [Google Scholar]
- 18.Gungor S, Selcuk NA. Benign bone cyst mimicking bone metastasis demonstrated on 68Ga-FAPI. Clin Nucl Med. 2022;47:e95–e97. [DOI] [PubMed] [Google Scholar]
- 19.Lv Y, Lan X, Qin C. Incidental detection of sacral insufficiency fracture on 68Ga-FAPI PET/MR. Clin Nucl Med. 2021;46:1032–1033. [DOI] [PubMed] [Google Scholar]
- 20.Gong W Chen S He L, et al. Intense 68Ga-FAPI uptake in a patient with myositis ossificans: mimicking bone malignancy. Clin Nucl Med. 2022;47:638–639. [DOI] [PubMed] [Google Scholar]
- 21.Wu J Tang W Wang Y, et al. Schmorl node can cause increased 68Ga-FAPI activity on PET/CT. Clin Nucl Med. 2022;47:537–538. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y Wu J Liu L, et al. 68Ga-FAPI-04 PET/CT imaging for fibrous dysplasia of the bone. Clin Nucl Med. 2022;47:e9–e10. [DOI] [PubMed] [Google Scholar]
- 23.Gong W Yang X Li L, et al. Elevated 68Ga-FAPI uptake by primary benign intraosseous meningioma. Clin Nucl Med. 2022;47:994–995. [DOI] [PubMed] [Google Scholar]
- 24.Gong W Yang X Mou C, et al. Bone tuberculous granulomatous inflammation mimicking malignancy on 68Ga-FAPI PET/CT. Clin Nucl Med. 2022;47:348–349. [DOI] [PubMed] [Google Scholar]
- 25.Gao S Deng X Zhang L, et al. The comparison analysis of clinical and radiological features in SAPHO syndrome. Clin Rheumatol. 2021;40:349–357. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K Yamaguchi T Ozawa H, et al. Diagnosing active inflammation in the SAPHO syndrome using 18FDG-PET/CT in suspected metastatic vertebral bone tumors. Ann Nucl Med. 2007;21:477–580. [DOI] [PubMed] [Google Scholar]
- 27.Xu T Huang Y Zhao Y, et al. 68Ga-DOTA-FAPI-04 PET/CT imaging in a case of SAPHO syndrome. Clin Nucl Med. 2022;47:246–248. [DOI] [PubMed] [Google Scholar]
- 28.Govoni M Colina M Massara A, et al. SAPHO syndrome and infections. Autoimmun Rev. 2009;8:256–259. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y Li C Yang Q, et al. Three patterns of osteoarticular involvement in SAPHO syndrome: a cluster analysis based on whole body bone scintigraphy of 157 patients. Rheumatology. 2019;58:1047–1055. [DOI] [PubMed] [Google Scholar]
- 30.Aljuhani F Tournadre A Tatar Z, et al. The SAPHO syndrome: a single-center study of 41 adult patients. J Rheumatol. 2015;42:329–334. [DOI] [PubMed] [Google Scholar]
- 31.Cianci F Zoli A Gremese E, et al. Clinical heterogeneity of SAPHO syndrome: challenging diagnose and treatment. Clin Rheumatol. 2017;36:2151–2158. [DOI] [PubMed] [Google Scholar]
- 32.Fu Z Liu M Li Z, et al. Is the bullhead sign on bone scintigraphy really common in the patient with SAPHO syndrome? A single- center study of a 16-year experience. Nucl Med Commun. 2016;37:387–392. [DOI] [PubMed] [Google Scholar]
- 33.McGauvran AM Kotsenas AL Diehn FE, et al. SAPHO syndrome: imaging findings of vertebral involvement. AJNR Am J Neuroradiol. 2016;37:1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong A Bai Y Cui Y, et al. FDG PET/CT in early and late stages of SAPHO syndrome: two case reports with MRI and bone scintigraphy correlation. Clin Nucl Med. 2016;41:e211–e215. [DOI] [PubMed] [Google Scholar]
- 35.Pichler R Weiglein K Schmekal B, et al. Bone scintigraphy using Tc-99m DPD and F18-FDG in a patient with SAPHO syndrome. Scand J Rheumatol. 2003;32:58–60. [DOI] [PubMed] [Google Scholar]
- 36.Kohlfuerst S, Igerc I, Lind P. FDG PET helpful for diagnosing SAPHO syndrome. Clin Nucl Med. 2003;28:838–839. [DOI] [PubMed] [Google Scholar]


