Abstract
Cystic echinococcosis is a zoonosis caused by the larvae of Echinococcus granulosus. Pulmonary disease may be asymptomatic until the cyst ruptures or becomes secondarily infected. We report a case of pulmonary cystic echinococcosis presenting in the United Kingdom, with discussion on management: optimum antihelminthic agent, length of treatment and type of operative intervention. Treatment should be individualized to the clinical scenario.
Keywords: cyst, hydatid, pulmonary
Hydatid disease, or human echinococcosis results from infection with the larval stage of the tapeworm Echinococcus, with Echinococcus granulosus causing cystic echinococcosis (CE) and Echinococcus multilocularis causing alveolar echinococcosis. Hydatid disease is found most in the Mediterranean region, South America, north/east Africa, southern/central Russia and central Asia). The definitive hosts for E. granulosus are dogs, with livestock (eg, sheep and goats) acting as intermediate hosts.1
Eggs released into the feces of the definitive host are ingested by the intermediate host, hatching in the small intestine and releasing hooked oncospheres which are able to penetrate the intestinal wall and move through the circulation to end organs, usually liver (65%) and lungs (25%).1 Once in the end organ, the oncosphere develops into an echinococcal (hydatid) cyst containing many protoscoleces. The definitive host ingests the cyst-filled organs of the intermediate host and becomes infected, with protoscoleces attaching to their intestinal mucosa and maturing into adult worms in 32–80 days.1 Humans are an aberrant intermediate host, with infection occurring after ingestion of eggs via the fecal-oral route.
CASE DESCRIPTION
A previously healthy 12-year-old boy presented to his local hospital with 24 hours history of fever and worsening right-sided lower chest pain. His oxygen saturations were 92% on room air, with tachypnoea of 36 breaths per minute at rest. His initial white cell count was 10.7 × 109/L; with neutrophils 9 × 109/L, lymphocytes 0.6 × 109/L, eosinophils 0.38 × 109/L and C-reactive protein (CRP) 68 mg/L. Chest radiograph (CXR) (Fig. 1A) findings were suggestive of a lung abscess. He was commenced on intravenous ceftriaxone and oral clindamycin to cover likely local pathogens for primary lung abscess (Streptococcus aureus, Streptococcus pneumoniae and Group A Streptococcus). By day 5 of admission, the child remained febrile. Additional history included that the child had moved to the United Kingdom from Botswana at 7 years of age. He had lived in a rural area of Botswana and had helped to look after goats at his family’s property.
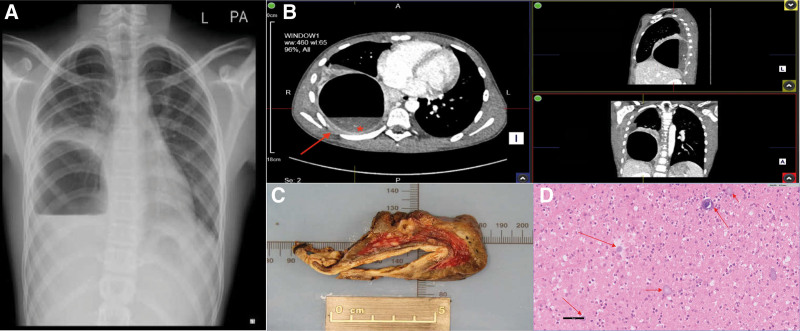
FIGURE 1.
Radiology and histopathology findings. A: Plain CXR demonstrating large right-sided pulmonary cyst containing an air-fluid level. B: CT thorax with multi-angle views demonstrating cystic/cavitating lesion of right lower lobe containing dependent fluid. The lesion is thin-walled, with an associated area of nodularity of the pleura (arrow). Calcifications are visible within the fluid (asterisk) and there is no marked hilar lymphadenopathy. C: Macroscopic appearance of resected lobe of lung. D: Hematoxylin and eosin-stained section demonstrating laminated layers of Echinococcus at varying stages of development (arrows). CT, computed tomography; CXR, chest radiograph.
Further investigations demonstrated CRP 270 mg/L, Erythrocyte sedimentation rate 117 mm/h, and alanine transaminase 143 IU/L. Hepatitis B, C and HIV serologies and interferon-gamma release assay (QuantiFERON-TB Gold, Quiagen, Germantown, MD) were negative. Computed tomography scan of the thorax (Fig. 1B) demonstrated a cystic/cavitating lesion in the right lower lobe with an associated area of nodularity of the pleura at the base of the lesion. There were calcifications noted within the cyst fluid, suggestive of a nonacute lesion. There was no marked hilar lymphadenopathy. Abdominal ultrasound did not demonstrate any other cysts.
Primary differentials included a congenital cystic adenomatoid malformation or CE (cystic hydatid disease) with superadded bacterial infection, or pulmonary amoebiasis. Amoebic and hydatid serologies were sent and metronidazole was added to cover for amoebic disease.
MEDICAL AND SURGICAL MANAGEMENT
Hydatid serology was positive (Echinococcus IgG enzyme-linked immunosorbent assay (ELISA) positive at optical density of 0.517). This was confirmed with species-specific immunoblot testing, which was positive for E. granulosus. Albendazole (15 mg/kg/day) and praziquantel (40 mg/kg/day) were commenced, and plans were made for surgical removal of the lesion. Prednisolone and chlorphenamine were given respectively at 24 and 12 hours preoperatively to reduce the risk of anaphylaxis, which can occur with operative spillage of hydatid contents.2
Right lower lobectomy was completed 3 weeks after the initial presentation, allowing the child to have several days’ treatment with praziquantel preoperatively. Intraoperatively, significant adhesions and inflammatory changes of the pleura were noted, and cyst contents were described as “gelatinous/caseous” material. Macroscopic appearance of the excised lobe is shown in Figure 1C. Histology demonstrated markedly thickened pleura and laminated eosinophilic retractile layer of Echinococcus in varying stages of development (Fig. 1D).
The child was well postoperatively and defervesced. He received 2 further weeks of praziquantel and 3 months of albendazole. He had full blood count and liver function test monitoring every 2 weeks while on albendazole, with no neutropenia or disturbance of his liver enzymes. Postoperative CXR showed good expansion of the right lung and almost complete resolution of the pleural thickening. One year postoperatively, the child remained well and had been able to resume playing football.
DISCUSSION
Diagnosis of Cystic Echinococcosis
Pulmonary CE appears to be more frequent in children than in adults, most commonly presenting with chest pain, cough and/or dyspnea, with hemoptysis or urticarial/anaphylactic symptoms if rupture into the bronchial tree occurs.3 Spillage of cyst material into the bronchial tree or pleural cavity can cause pneumothorax, pleural effusion, empyema or pulmonary abscess with secondary infection.3 The growth of the cyst is slow enough (around 1–5 cm/year)1 that most children and adolescents with lung lesions remain asymptomatic despite large lesions, which may be picked up incidentally on CXR.
A diagnosis of hydatid disease would be supported by eosinophilia, associated with leakage of cyst contents, but this is only present in up to 15% of cases.3 Serologic tests are the mainstay of diagnosis in the absence of histopathology and are also useful for monitoring posttreatment. A positive screening test can be followed by immunoblot testing using species-specific antigens to confirm a diagnosis of CE.4 Serology is more likely to be positive in liver disease (with 80%–90% sensitivity reported for IgG ELISA) than lung disease (60%–85% sensitivity for IgG ELISA),4 therefore negative serology does not necessarily rule out disease.
IMAGING IN CYSTIC ECHINOCOCCOSIS
Pulmonary CE is likely to be unilateral in children, occurring most frequently in the right lower lobe, as in this case.5 Uncomplicated cysts are classically described as being well-circumscribed, fluid-filled lesions with homogenous content and “hyperdense” walls and calcifications are noted to be rare.6 Cysts that have ruptured into the bronchial tree can develop an air-fluid level or “Cumbo’s sign.” Superinfected cysts may also have the appearance of an air-fluid level,6 and 1 retrospective pediatric study noted that CXR findings of children with pulmonary echinococcosis appeared very similar to those of a lung abscess in 65% of cases.5
MANAGEMENT OF CYSTIC ECHINOCOCCOSIS
Given the wide range of presentations with pulmonary CE, there is no uniform treatment recommendation, rather the location, size and stage of the cyst and available resources dictate disease management.
Albendazole acts by inhibiting the assembly of microtubules, preventing the germinal layer of the cyst from absorbing glucose. Some studies have shown efficacy of albendazole alone on small and uncomplicated cysts, including 1 solely pediatric study, but for more complicated lesions (eg, larger or infected cysts, or lesions causing compression of the parenchyma),5 a conservative surgical approach has been recommended with adjunctive albendazole treatment. This latter approach was supported by a systematic review and meta-analysis, which noted greater treatment success with the combination of surgery and albendazole over albendazole alone.7
Praziquantel is thought to have both protoscolecidal action and reduce viability of early cysts, with suggestion that preoperative treatment may reduce the risk of recurrence and seeding with spillage of cyst contents. This was supported by a study of 22 patients with pulmonary CE; in the group treated with praziquantel for 5–6 days preoperatively, 43%–64% protoscoleces were nonviable at surgery, versus 9% in untreated patients.8
A number of pharmacological studies also noted increased bioavailability of albendazole when co-administered with praziquantel, and a small safety study recorded only mild, infrequent and reversible side effects such as nausea and loose stools. There are no randomized controlled trials of longer-term praziquantel treatment in cases not amenable to surgery, or in severe disease.
Recurrence is one of the major complications of hydatid cysts, with reported rates of postoperative recurrence as high as 18% in some studies, postulated to be because of spillage of cyst contents and dissemination, or additional small cysts being missed intraoperatively.3 Conservative surgical methods, such as cystotomy and capitonnage (emptying the cyst and closing its cavity with sutures) have been recommended where possible, as they conserve the maximum amount of lung parenchyma, with reduced morbidity, shorter hospital stay and low risk of recurrence. The largest case series of 643 children who had surgical treatment of lung hydatid demonstrated good outcomes with 3% postoperative morbidity and 0.5% mortality.9
There is debate about the optimum length of perioperative therapy; an extensive review of data on albendazole use from multiple sources suggested that initial treatment should be for 3 months, with some consideration of continuation beyond this being balanced with the possibility of side effects.10 The most common side effects of albendazole treatment are reversible hepatotoxicity, cytopenias and alopecia, hence the need for regular full blood count and liver function monitoring while on treatment. Timing of relapse in CE is very variable and has been reported between 3 months and 20 years posttreatment,1 therefore patients are followed up for 10 years with repeated imaging and serology to check for signs of recurrence.
SUMMARY
This case demonstrates the importance of exposure history in a child with fever and pulmonary cyst; this brought hydatid disease into the differential diagnosis, allowing preoperative antiparasitic therapy to be initiated. The review of the literature demonstrates some of the issues and uncertainties surrounding diagnosis and treatment of echinococcal disease, the lack of randomized controlled trials on treatment and the need to individualize management to the clinical scenario.
ACKNOWLEDGMENTS
Consent for the use of the anonymized images contained in this report was kindly provided by the patient and his legal guardian.
Footnotes
The authors have no funding or conflicts of interest to disclose.
P.C. and S.K. contributed equally to the work.
Contributor Information
Andrew Ives, Email: andyives69@hotmail.com.
Darren Fowler, Email: darren.fowler@ouh.nhs.uk.
Kokila Lakhoo, Email: kokila.lakhoo@ouh.nhs.uk.
David Grant, Email: david.grant@ouh.nhs.uk.
Dominic Kelly, Email: dominic.kelly@ouh.nhs.uk.
Stéphane Paulus, Email: stephane.paulus@msd.ox.ac.uk.
Shelley Segal, Email: shelley.segal@ouh.nhs.uk.
James J. Gilchrist, Email: james.gilchrist@paediatrics.ox.ac.uk.
Alex Kew, Email: a.kew@nhs.net.
Peter Chiodini, Email: p.chiodini@nhs.net.
Seilesh Kadambari, Email: seilesh.kadambari@paediatrics.ox.ac.uk.
REFERENCES
- 1.Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125–133. [DOI] [PubMed] [Google Scholar]
- 2.Neumayr A, Troia G, de Bernardis C, et al. Justified concern or exaggerated fear: the risk of anaphylaxis in percutaneous treatment of cystic echinococcosis—a systematic literature review. PLoS NeglTrop Dis. 2011;5:e1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morar R, Feldman C. Pulmonary echinococcosis. Eur Respir J. 2003;21:1069–1077. [DOI] [PubMed] [Google Scholar]
- 4.Moro P. Clinical manifestations and diagnosis of echinococcosis. Available at: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-echinococcosis. Published 2021. Updated 1st June 2021. Accessed 3 April 2023.
- 5.Anadol D, Gocmen A, Kiper N, et al. Hydatid disease in childhood: a retrospective analysis of 376 cases. Pediatr Pulmonol. 1998;26:190–196. [DOI] [PubMed] [Google Scholar]
- 6.Garg MK, Sharma M, Gulati A, et al. Imaging in pulmonary hydatid cysts. World J Radiol. 2016;8:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velasco-Tirado V, Alonso-Sardon M, Lopez-Bernus A, et al. Medical treatment of cystic echinococcosis: systematic review and meta-analysis. BMC Infect Dis. 2018;18:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YH. Hydatid cysts in children. Diagnostic and therapeutic aspects. Apropos of 1195 cases praziquantel in the treatment of helminthiasis. Zhonghua Yi Xue Za Zhi. 1984;64:243–247. [PubMed] [Google Scholar]
- 9.Chaouachi B, Ben Salah S, Lakhoua R, et al. Hydatid cysts in children. Diagnostic and therapeutic aspects. Apropos of 1195 cases. Ann Pediatr (Paris). 1989;36:441–444, 447-9. [PubMed] [Google Scholar]
- 10.Horton RJ. Albendazole in treatment of human cystic echinococcosis: 12 years of experience. Acta Trop. 1997;64:79–93. [DOI] [PubMed] [Google Scholar]