Abstract
The complete sequence of adeno-associated virus type 1 (AAV-1) was defined. Its genome of 4,718 nucleotides demonstrates high homology with those of other AAV serotypes, including AAV-6, which appears to have arisen from homologous recombination between AAV-1 and AAV-2. Analysis of sera from nonhuman and human primates for neutralizing antibodies (NAB) against AAV-1 and AAV-2 revealed the following. (i) NAB to AAV-1 are more common than NAB to AAV-2 in nonhuman primates, while the reverse is true in humans; and (ii) sera from 36% of nonhuman primates neutralized AAV-1 but not AAV-2, while sera from 8% of humans neutralized AAV-2 but not AAV-1. An infectious clone of AAV-1 was isolated from a replicated monomer form, and vectors were created with AAV-2 inverted terminal repeats and AAV-1 Rep and Cap functions. Both AAV-1- and AAV-2-based vectors transduced murine liver and muscle in vivo; AAV-1 was more efficient for muscle, while AAV-2 transduced liver more efficiently. Strong NAB responses were detected for each vector administered to murine skeletal muscle; these responses prevented readministration of the same serotype but did not substantially cross-neutralize the other serotype. Similar results were observed in the context of liver-directed gene transfer, except for a significant, but incomplete, neutralization of AAV-1 from a previous treatment with AAV-2. Vectors based on AAV-1 may be preferred in some applications of human gene therapy.
Adeno-associated viruses (AAV) are small, nonenveloped, single-stranded DNA viruses which require helper virus to facilitate efficient replication (3). The 4.7-kb genome of AAV is characterized by two inverted terminal repeats (ITRs) and two sets of open reading frames, which encode the Rep and Cap proteins. The Rep open reading frames encode four proteins with molecular masses of 78, 68, 52, and 40 kDa. These proteins function mainly in regulating AAV replication and integration. The Cap open reading frames encode three structural proteins with molecular masses of 85 kDa (VP1), 72 kDa (VP2), and 61 kDa (VP3) (3). The two ITRs are the only cis elements essential for all steps in the AAV life cycle.
AAV have been found in many animal species, including nonhuman primates, canines, fowl, and humans (18). A total of six serotypes of AAV, including AAV type 1 (AAV-1), have been isolated from primates, and two have been isolated from nonhuman primates; AAV-2, AAV-3, and AAV-5 are from humans, and AAV-6 is from a human adenovirus preparation. AAV-2 is the most characterized primate serotype, since its infectious clone was the first one made (24). The full sequences for AAV-3A, AAV-3B, AAV-4, and AAV-6 recently were determined (4, 17, 22). Generally, all primate AAV show more than 80% homology in nucleotide sequence.
A number of unique properties make AAV a very promising vector for human gene therapy (19). AAV are not associated with any known human diseases and are generally not considered pathogenic. Wild-type AAV are capable of integrating into the host chromosome in a site-specific manner (14, 26). Recombinant AAV (rAAV) vectors can integrate into tissue culture cells at chromosome 19 if the Rep proteins are supplied in trans (1, 29). The transduced genomes of AAV have been shown to confer long-term gene expression in a number of tissues, including muscle, liver, brain, and retina (8, 13, 27, 28, 30, 31). The development of new methods for producing high-titer rAAV has largely removed the hurdles which prevented AAV vectors from being tested in large-animal models of human diseases and in human clinical trials (5, 6, 11, 32).
Among AAV-1 to AAV-6, only AAV-1 and AAV-4 are considered to be simian viruses, since they were isolated from nonhuman primates and monospecific antibodies to the viruses have not been detected in human serum (20). They may have advantages for use in human gene therapy to replace or augment the use of AAV-2 vectors. For example, AAV-1 vectors could be used in patients who develop anti–AAV-2 neutralizing antibodies (NAB) due to a naturally acquired infection or previous treatment with AAV-2 vectors. To study the possibility of using AAV-1 as a gene therapy vector, we constructed an AAV-1 infectious clone and determined its full sequence. Vectors derived from this infected clone were evaluated in murine models of liver and muscle gene transfer.
MATERIALS AND METHODS
Murine studies.
C57BL/6 mice (6- to 8-week-old males) were obtained from Jackson Laboratory. AAV vectors were administered by either intramuscular or intrasplenic injection as described before (8, 30).
Nonhuman primates.
Wild-caught juvenile rhesus monkeys were purchased from Covance (Alice, Tex.) and LABS of Virginia (Yemassee, S.C.) and kept in full quarantine. The monkeys weighed approximately 3 to 4 kg. The nonhuman primates used in the Institute for Human Gene Therapy research program are purposefully bred in the United States from specific-pathogen-free closed colonies. All vendors are U.S. Department of Agriculture class A dealers. The rhesus macaques are therefore not infected with important simian pathogens, including the tuberculosis agent, major simian lentiviruses (simian immunodeficiency virus and simian retroviruses), and cercopithecine herpesvirus. The animals are also free of internal and external parasites. The excellent health status of these premium animals minimizes the potential for extraneous variables. The use of these monkeys in protocols was approved by the Infection Control Committee of the Hospital of the University of Pennsylvania and the Environmental Health and Safety Office, the Institutional Biosafety Committee, and the Institutional Animal Care and Use Committee of the University of Pennsylvania. For this study, serum was obtained from monkeys prior to initiation of any protocol.
Human subjects.
Normal volunteers (n, 77) were analyzed for immune reactivity to AAV. Individuals were members of the University of Pennsylvania community. The study was approved by the Institutional Review Board of the University of Pennsylvania. The median age was 27 years (range, 18 to 54 years), and the age distributions in the two groups were similar.
Cell culture and virus.
AAV-free 293 cells and 84-31 cells were obtained from the Human Applications Laboratory of the University of Pennsylvania. These cells were cultured in Dulbecco’s modified Eagle medium with 10% fetal bovine serum (Hyclone), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a moisturized environment supplied with 5% CO2. The 84-31 cell line constitutively expresses adenovirus E4 proteins and has been described previously (7). AAV-1 (ATCC VR-645) seed stock was purchased from the American Type Culture Collection (Rockville, Md.). AAV were propagated in 293 cells with wild-type adenovirus type 5 as a helper virus.
rAAV generation.
rAAV were generated by transfection with an adenovirus-free method described elsewhere (30a). Briefly, the cis plasmid (with AAV ITRs), the trans plasmid (with the AAV Rep gene and Cap gene), and a helper plasmid (pFΔ13, with an essential region from the adenovirus genome) were cotransfected into 293 cells at a ratio of 1:1:2 by calcium phosphate precipitation. The AAV vectors used in this study express murine erythropoietin or human α1-antitrypsin from a chicken β-actin promoter enhanced by sequences from cytomegalovirus. pFΔ13 has an 8-kb deletion in the adenovirus E2B region and most of the late genes. The cells were harvested 96 h posttransfection and subjected to two rounds of CsCl gradient purification. For the generation of rAAV based on AAV-2, p5E18 was used as the trans plasmid, since it greatly improved the rAAV yield. To make rAAV with AAV-1 virions, pAV1H or p5E18(2/1) was used as the trans plasmid to provide Rep and Cap functions.
DNA techniques.
Hirt DNA extraction was performed as described before with minor modifications (25).
To construct AAV-1 infectious clones, the Hirt DNA from AAV-1-infected 293 cells was repaired with the Klenow enzyme (New England BioLabs) to make sure that the ends were blunt. The treated AAV-1 Hirt DNA was then digested with BamHI and cloned into three vectors. The internal BamHI fragment was cloned into pBluescript II-SK(+) cut with BamHI to produce pAV1-BM. The left and right fragments were cloned into pBluescript II-SK(+) cut with BamHI and EcoRV to obtain pAV1-BL and pAV1-BR, respectively. The AAV sequences in these three plasmids were subsequently assembled into the same vector to produce AAV-1 infectious clone pAAV-1.
For sequencing, an ABI 373 automatic sequencer was used to determine the sequences for all plasmids and PCR fragments used in this study by fluorescence sequencing (FS) dye chemistry. They were confirmed by sequencing both plus and minus strands. For sequencing the AAV terminal repeats, the fragment containing AAV ITRs was amplified from pAAV-1, pAV1-BR, or pAV1-BL with M13F (GTAAAACGACGGCCAGT) and CAP1a (GAA CCG TGC TGG TAA GGT TAT T) or M13F and V1Rep1s (CCA TGC CGG GCT TCT ACG AGA TCG TTA TCA GGG TG). The PCR was performed with a GC-advantage kit (Clontech Laboratories, Inc.). The 50-μl reaction mixture contained 1× reaction buffer (40 mM Tricine-KOH [pH 9.02] at 25°C, 15 mM KOAc, 3.5 mM Mg(OAc)2, 5% dimethyl sulfoxide, 3.75 μg of bovine serum albumin per ml); 1× GC melt; 10 mM dATP, dCTP, 7-deazaGTP–dGTP (3:1), and dTTP; 10 ng of template; 500 ng of each primer; and 2.5 U of polymerase. The purified PCR product was sequenced as described above.
A plasmid expressing AAV-2 Rep and Cap, p5E18(2/2), was constructed to maximize vector production. This was accomplished by relocating the p5 promoter to a position 3′ to the Cap gene, thereby minimizing expression of Rep78 and Rep68. This strategy was initially described by Li et al. (15). p5E18(2/2) was constructed in the following way. The previously described pMMTV-trans vector (30a) (i.e., the mouse mammary tumor virus promoter substituted for the p5 promoter in an AAV-2-based vector) was digested with SmaI and ClaI, filled in with the Klenow enzyme, and then recircularized with DNA ligase. The resulting construct was digested with XbaI, filled in, and ligated to the blunt-ended BamHI-XhaI fragment from pCR-p5, constructed in the following way. The p5 promoter of AAV was amplified by PCR with the following oligonucleotides: TGT AGT TAA TTA ACC CGC CAT GCT ACT TAT C and GGC GGC TGC GCG TTC AAA CCT CCC GCT TCA AAA TG. The amplified fragment was subsequently cloned into pCR2.1 (Invitrogen) to yield pCR-p5. The helper plasmid pAV1H was constructed by cloning the BfaI fragment of pAAV-1 into pBluescript II-SK(+) at the BcorV and SmaI sites. The 3.0-kb XbaI-KpnI fragment from p5E18(2/2), the 2.3-kb XbaI-KpnI fragment from pAV1H, and the 1.7-kb KpnI fragment from p5E18(2/2) were incorporated into a separate plasmid, p5E18(2/1), which contains AAV-2 Rep, AAV-1 Cap, and the AAV-2 p5 promoter located 3′ to the Cap gene.
For Southern blot analysis, Hirt DNA was digested with DpnI to remove bacterium-borne plasmid and probed with the internal BamHI fragment of AAV-1. The membrane was then washed under high-stringency conditions: twice for 30 min each time with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at 65°C and twice for 30 min each time with 0.1× SSC–0.1% SDS at 65°C. The membrane was then analyzed by both phosphorimager analysis and X-ray autoradiography.
Western blot analyses.
Serum samples were analyzed for reactivity to various AAV-2 capsid proteins by Western blotting as described previously (8). Purified AAV-2 vectors were used as antigens for the capsid proteins, and a monoclonal antibody that recognizes shared epitopes on VP1, VP2, and VP3 (clone B1; American Research Products) was used as a positive control. AAV-2 antigens were electrophoresed by SDS–10% polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Hybond-ECL; Amersham). The reactivity of the serum was measured by incubating membrane containing AAV antigen with test serum samples, followed by peroxidase anti-human immunoglobulin G antibody. The reaction was detected with an ECL kit (Amersham).
Anti-AAV NAB.
NAB titers were analyzed by assessing the ability of serum antibody to inhibit the transduction of reporter virus (AAV1-GFP or AAV2-GFP) into 84-31 cells, which are subclones of 293 cells that stably express adenovirus E4 proteins, which render them permissive for AAV transduction. Various dilutions of antibodies preincubated with reporter virus for 1 h at 37°C were added to 90% confluent cell cultures. Cells were incubated for 48 h, and the expression of green fluorescent protein (GFP) was measured by FluoroImaging (Molecular Dynamics). NAB titers were calculated as the highest dilution at which 50% of the cells stained green.
Human α1-antitrypsin assay.
The concentration of human α1-antitrypsin in mouse serum was measured by an enzyme-linked immunosorbent assay (ELISA). The coating antibody was rabbit anti-human α1-antitrypsin (Sigma). We used goat anti-human α1-antitrypsin (Sigma) as the primary detection antibody (30). The sensitivity of the assay is 0.3 to 30 ng/ml.
Nucleotide sequence accession number.
The AAV-1 sequence is available through GenBank under accession no. AF063497.
RESULTS
Generation of an infectious clone of AAV-1.
The replicated form of AAV-1 was extracted from 293 cells infected with AAV-1 and wild-type adenovirus type 5. Analysis of Hirt DNA revealed three bands with apparent molecular sizes equivalent to a double-stranded dimer (9.4 kb), a double-stranded monomer (4.7 kb), and single-stranded DNA (1.7 kb). The monomer band was excised from the gel and digested with BamHI, revealing a pattern of three fragments (1.1, 0.8, and 2.8 kb), in accordance with the original description of AAV-1 by Bantel-Schaal and zur Hausen (2). The three fragments were subcloned into one plasmid-based construct to obtain pAAV-1.
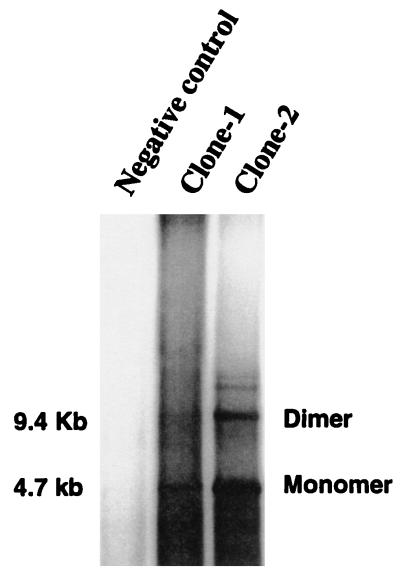
This clone was tested for its abilities to rescue AAV-1 genomes from the plasmid backbone and to replicate and package infectious virus. Plasmid pAAV-1 was transfected into 293 cells infected with adenovirus type 5 at a multiplicity of infection of 10, and the virus supernatant was used to reinfect 293 cells. DNA hybridization analysis of DpnI-digested Hirt DNA from infected 293 cells demonstrated the rescue and replication of AAV-1 genomes (Fig. 1).
FIG. 1.
DNA hybridization analysis of the rescue and replication of the AAV-1 infectious clone. Two independent clones of pAAV-1 (Clone-1 and Clone-2) were transfected into 293 cells, which were then superinfected with adenovirus type 5. Hirt DNA was extracted 48 h postinfection and digested with DpnI before electrophoresis in an 0.8% agarose gel. An internal BamHI fragment of pAAV-1 was used as a probe. The autoradiograph shows the locations of replicated dimers and monomers and their molecular sizes. The analysis included cells not infected (negative control) or infected with clone-1 or clone-2.
Genomic structure of AAV-1.
The entire AAV-1 genome was sequenced from pAAV-1 (Fig. 2). PCR products derived from the original AAV-1 seed stock were also sequenced to confirm the structure of the replicated form. No major differences were noted. The length of AAV-1 (4,718 nucleotides) is very close to that of AAV-3 (4,726 nucleotides), which is shorter than AAV-4 (4,774 nucleotides) but longer than AAV-2 (4,681 nucleotides) and AAV-6 (4,683 nucleotides).
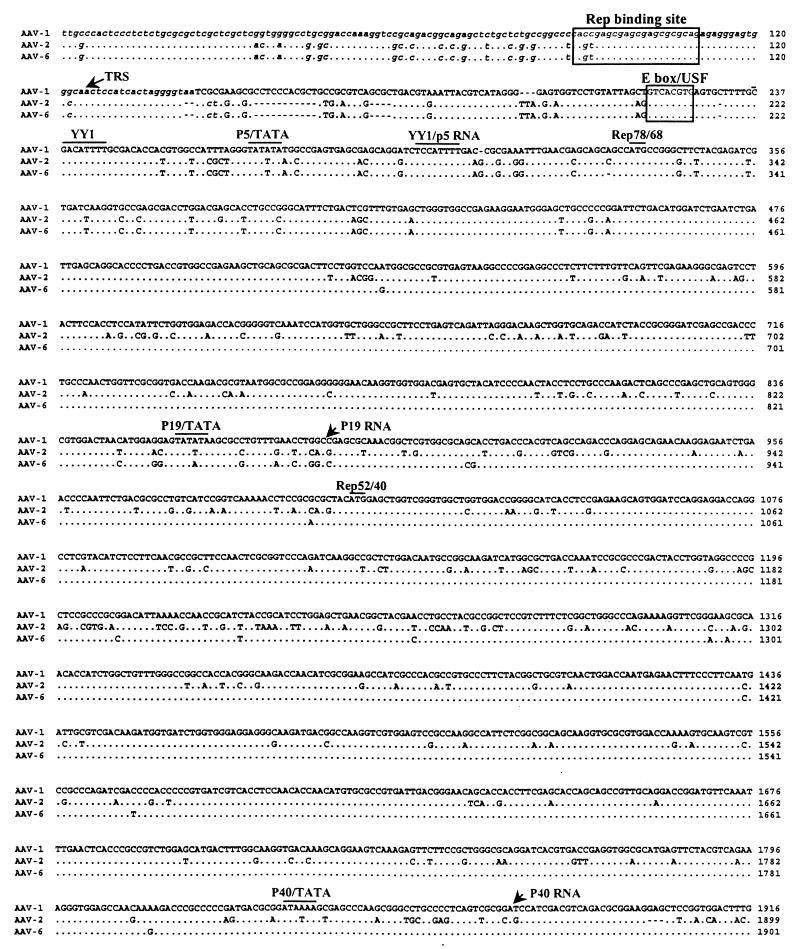
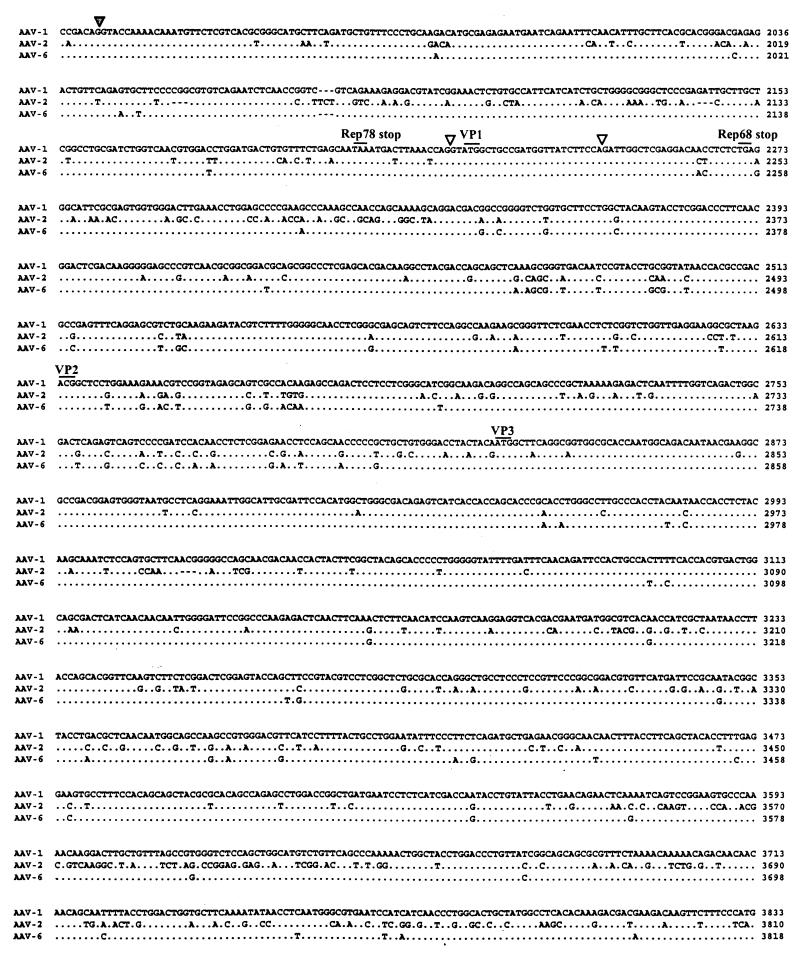
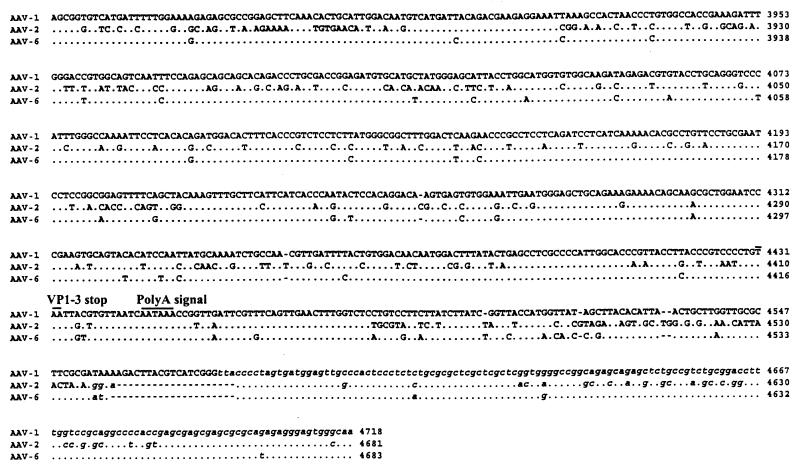
FIG. 2.
Alignment of nucleotides for AAV-1, AAV-2, and AAV-6. The full sequence of AAV-1 is indicated in the top line; the AAV-2 and AAV-6 sequences are shown below with nucleotide differences. Homology is indicated by dots. Critical landmarks in the structures of the AAV genomes are shown. Gaps are indicated by dashes. The 3′ ITR of AAV-1 is shown in the same configuration as in the published AAV-2 and AAV-6 sequences (22). TRS, terminal resolution site.
The AAV-1 genome shows more than 80% identity with other known AAV and contains the characteristic structural features. The ITRs of AAV-1 are predicted to form T-shaped hairpin structures (Fig. 3). The right and left ITRs of AAV-1 are identical and virtually the same as the right ITR of AAV-6, except for 1 nucleotide in the A and A′ sequences and the last nucleotide in the D sequence. The AAV-2 Rep binding motif (GCTCGCTCGCTCGCTG) found in the AAV-2 preintegration region in human chromosome 19 is well conserved in AAV-1.
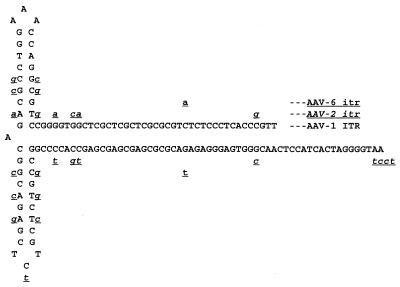
FIG. 3.
Potential secondary structure of AAV-1 ITR. Nucleotide differences in the corresponding sequences of AAV-2 and AAV-6 relative to AAV-1 are underlined. AAV-2 sequence differences are lowercase, underlined, and italicized, whereas AAV-6 sequence differences are lowercase and underlined.
Although the overall features of AAV terminal repeats are conserved among the different serotypes, their lengths differ slightly. The terminal repeats of AAV-1 are 143 nucleotides long, while those of AAV-2, AAV-3, and AAV-4 are 145 or 146 nucleotides long. The loop region of the AAV-1 ITR mostly resembles that of the AAV-4 ITR in that it also uses TCT instead of the TTT found in the AAV-2 and AAV-3 ITR. We ruled out the possibility of a sequencing error at this site by using restriction enzyme digestion of the replicative intermediate, since these 3 nucleotides are part of the SacI site (GAGCTC).
The p5 promoter region of AAV-1 shows some divergence from homologous regions of other AAV serotypes but maintains critical regulatory elements; the repeated YY1 sites are present throughout all known AAV serotypes, including AAV-1. In AAV-4, 56 additional nucleotides are inserted between YY1 and the E-box/USF site, while in AAV-1, 26 additional nucleotides are inserted before the E-box/USF site. The p19 promoter, the p40 promoter, and poly(A) can also be identified in the AAV-1 genome by homology to those in known AAV serotypes, which are also highly conserved (3).
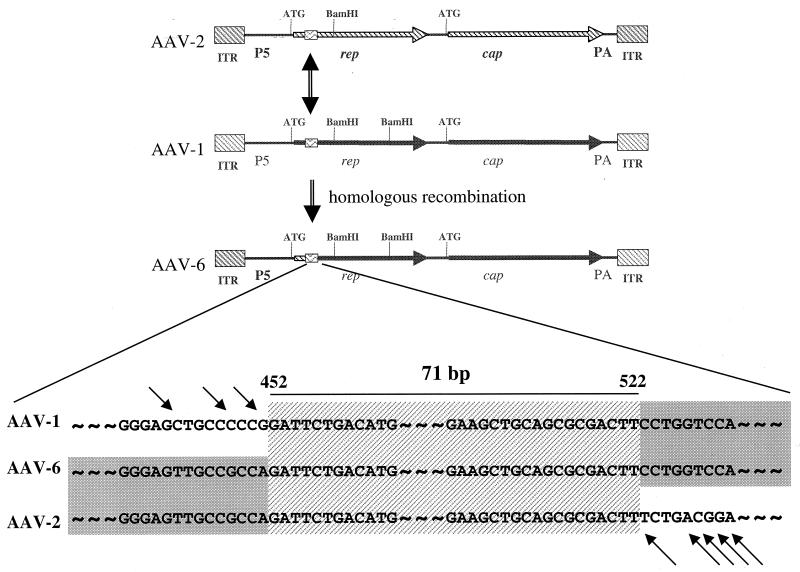
AAV-6 is a hybrid of AAV-1 and AAV-2.
The sequence of the AAV-1 coding region is virtually identical to that of AAV-6 from position 452 to the end of the coding region (99% homology). The first 508 nucleotides of AAV-6 were shown to be identical to those of AAV-2 (22). AAV-6 is organized in the following manner: AAV-2 left ITR, AAV-2 p5 promoter, AAV-1 coding region, and AAV-1 right ITR. This organization suggests that AAV-6 is a naturally occurring hybrid of AAV-1 and AAV-2 (Fig. 4).
FIG. 4.
Hypothesis for the creation of AAV-6 from homologous recombination between AAV-1 and AAV-2. The first line represents the AAV-2 genome, with Rep and Cap sequences demarcated by hatched arrows. The second line represents the AAV-1 genome, with the Rep and Cap sequences depicted as solid arrows. The hypothesis suggests homologous recombination between AAV-2 and AAV-1 at the region of the box containing wavy lines; this region contains a highly homologous sequence of the Rep gene shared by AAV-1 and AAV-2. PA, poly(A). A more detailed illustration of the common region of these three viruses is shown at the bottom. Arrows indicate nucleotides that differ.
Recombinant vector based on AAV-1.
Our initial goal was to create a recombinant vector which uses the Cap proteins of AAV-1 together with a vector genome comprised of AAV-2 ITR. We wanted to take advantage of the well-characterized and favorable biology of AAV-2 ITR while developing a recombinant virus whose capsid proteins are derived entirely from simian-based AAV-1. This vector, referred to here as the AAV-1 vector, should be serologically distinct from AAV-2.
To create the desired recombinant vector, we developed plasmids that could transcomplement vector production with AAV-1 Cap. The first plasmid is a homolog of the psub201 vector of AAV-2 in which the ITRs were deleted from pAAV-1, leaving behind Rep and Cap expressed from endogenous promoters (pAV1H). A recombinant vector was produced by transfecting into 293 cells three plasmids: an AAV-2-based vector (i.e., AAV-2 ITR with a reporter gene), pAV1H, and a plasmid containing adenovirus type 5 helper functions. Lysates were purified on CsCl gradients, yielding low titers of the recombinant vector (i.e., 5 × 1010 genomes/50 15-cm2 plates).
The AAV helper vector was revised in two ways to improve yield. First, the AAV-1 Rep gene in pAV1H was substituted for the corresponding gene in AAV-2. Second, the p5 promoter was relocated behind the Rep and Cap open reading frames to reduce the expression of the p78 and p68 forms; this modification has been used to enhance the titers of AAV-2 vectors (15,300). The resulting plasmid (p5E182/1) produced 10- to 20-fold higher quantities of the vector than pAV1H (i.e., 1012 genomes/50 15-cm2 plates).
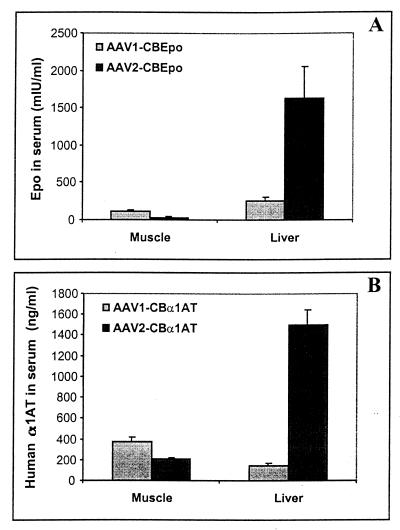
The performance of AAV-1 vectors was evaluated with immunodeficient mice (i.e., RAG-1 KO) injected intramuscularly or in the portal circulation to target the liver (Fig. 5). Direct comparisons were made to equivalent quantities of AAV-2 vectors containing the same vector genomes encoding secreted proteins easily measured in blood (i.e., murine erythropoietin and human α1-antitrypsin). Early experiments indicated similar in vivo performances of AAV-1 vectors produced with pAV1H and p5E18(2/1) (data not shown). All subsequent studies used AAV-1 vectors derived from p5E18(2/1) because of the increased yield.
FIG. 5.
In vivo activities of AAV-1 and AAV-2 vectors. Recombinant stocks of virus were generated by transfection and purified through cesium chloride gradients. (A) AAV-1 and AAV-2 vectors expressing murine erythropoietin (Epo) from a cytomegalovirus-enhanced β-actin promoter (CB). Equivalent stocks of these two vectors were injected into muscle (5 × 1010 genomes) or liver via the portal circulation (1 × 1011 genomes), and the animals (four groups) were analyzed on day 30 for the presence of erythropoietin in blood. Data for the groups were combined. Error bars show standard errors. (B) An identical experiment was performed with AAV-1 and AAV-2 vectors expressing human α1-antitrypsin (α1AT) from the same promoter. Serum harvested from four groups of animals 30 days after gene transfer was analyzed for the presence of α1-antitrypsin by an ELISA. Data are reported as in panel A.
AAV-2 vectors consistently produced 10- to 50-fold more serum erythropoietin or α1-antitrypsin when injected into liver compared to muscle. This result was very different from that for AAV-1 vectors, with which muscle expression was equivalent to or greater than liver expression. In fact, AAV-1 outperformed AAV-2 in muscle when equivalent titers based on genomes were administered.
NAB to AAV-1 and AAV-2 in nonhuman and human primates.
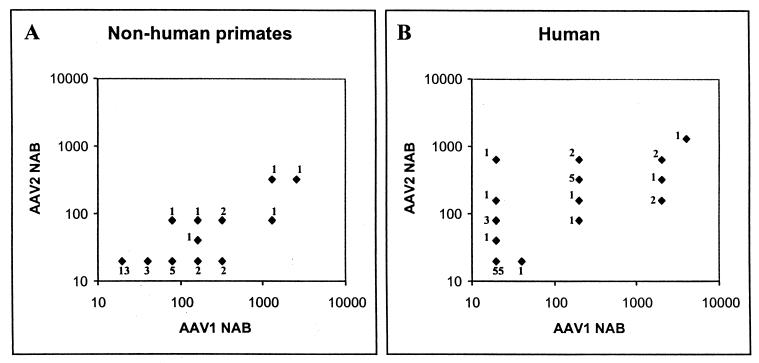
Simple and quantitative assays for NAB to AAV-1 and AAV-2 were developed with recombinant vectors. A total of 33 rhesus monkeys and 77 normal human subjects were screened. Figure 6 summarizes the results. A reciprocal dilution of NAB equal to 20 is the baseline (i.e., no detectable NAB).
FIG. 6.
NAB to AAV-1 and AAV-2 in nonhuman and human primates. Sera were analyzed for neutralizing activity against rAAV-1 and rAAV-2 as described in Materials and Methods. The reciprocal dilution of the NAB was plotted for AAV-2 versus AAV-1. Sera were titrated in sequential twofold dilutions. A titer of 1:20 represents the background (i.e., no detectable NAB). The number next to each datum point represents the total number of animals or humans with a particular NAB profile. In panel A, 33 adolescent rhesus monkeys were analyzed. In panel B, 77 normal human volunteers were evaluated.
Analysis of NAB in rhesus monkeys showed that 61% of animals tested positive for AAV-1; a minority (24%) had NAB to AAV-2. Over one-third of animals had antibodies to AAV-1 but not AAV-2 (i.e., were monospecific for AAV-1), whereas no animals were positive for AAV-2 without reacting to AAV-1. These data support the hypothesis that AAV-1 is endemic in rhesus monkeys. The presence of true AAV-2 infections in this group of nonhuman primates is less clear, since we cannot rule out cross-neutralizing activity of an AAV-1 response to AAV-2. It is interesting that there is a linear relationship between AAV-2 NAB and AAV-1 NAB in animals that had both.
Analysis of sera from normal human subjects was remarkable for a relative lack of NAB to either virus, with 71% of subjects scoring negative for both AAV-1 and AAV-2. Only 1 of 77 individuals was positive for AAV-1 and not AAV-2 (the titer in this person was only twofold higher than the baseline), whereas 6 individuals (8%) were positive for AAV-2 but not AAV-1. Overall, more patients had NAB to AAV-2 (27%) than to AAV-1 (20%), and the overall titers against AAV-2 appeared to be higher. These data support the hypothesis that AAV-2 infections are present in the human population, although independent primary infections with AAV-1 cannot be ruled out. The lack of monospecific antibodies to AAV-1 argues against this virus infecting humans to any appreciable degree.
Applications of AAV-1 vectors in animal models of gene therapy.
AAV-1 could have a role in human gene therapy in situations in which AAV-2 is rendered ineffective because of preexisting NAB, such as in patients with a prior history of AAV-2 infection or those who received gene therapy with an AAV-2 vector. This hypothesis presumes that there is little cross-neutralization in vivo between AAV-1 and AAV-2. Experiments were designed to test this hypothesis in the context of AAV-mediated gene transfer to murine liver and muscle. The basic paradigm is to inject AAV-1 or AAV-2 expressing human α1-antitrypsin and to follow up with a second vector of the same or different serotype and expressing murine erythropoietin. The efficiency of gene transfer is quantitatively measured by ELISA analysis of erythropoietin and α1-antitrypsin in blood.
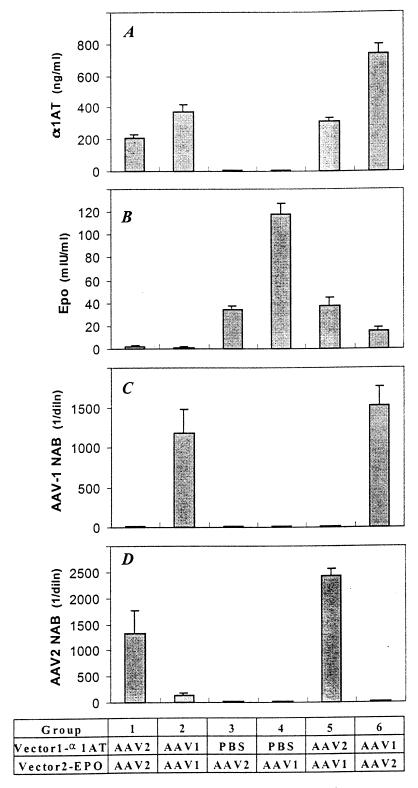
An analysis of vector readministration in muscle is presented in Fig. 7. Administration of AAV-2 vectors led to high levels of AAV-2 NAB that blocked the readministration of AAV-2 in vivo (group 1). The NAB in these animals did not neutralize AAV-1 in vitro, although there was a modest diminution (i.e., fivefold) in the transduction of an AAV-1 vector in vivo (group 5) to a level below that achieved in naive animals (group 4). Similarly, AAV-1 vectors resulted in high levels of AAV-1 NAB that blocked AAV-1 readministration in vivo (group 2) but did not interfere with AAV-2 vector transduction in vivo (group 6), compared to the results for naive animals that received AAV-2 alone (group 3).
FIG. 7.
Readministration of AAV vectors in muscle. C57BL/6 mice were evaluated for AAV-mediated gene transfer following introduction into naive animals as well as 30 days following the first vector administration. The different experimental groups are shown below the graphs. In each case, vector 1 expressed α1-antitrypsin (α1AT) from a cytomegalovirus-enhanced β-actin promoter, while vector 2 expressed murine erythropoietin (EPO) from the same promoter. Group 1, AAV-2 followed by AAV-2; group 2, AAV-1 followed by AAV-1; group 3, phosphate-buffered saline (PBS) followed by AAV-2; group 4, PBS followed by AAV-1; group 5, AAV-2 followed by AAV-1; group 6, AAV-1 followed by AAV-2. (A) Serum α1-antitrypsin 30 days after vector 1 administration. (B) Serum erythropoietin (Epo) measured by an ELISA 30 days after vector 2 administration. (C) Reciprocal dilution (diln) of NAB to AAV-1 at day 30. (D) Reciprocal dilution of NAB to AAV-2 at day 30.
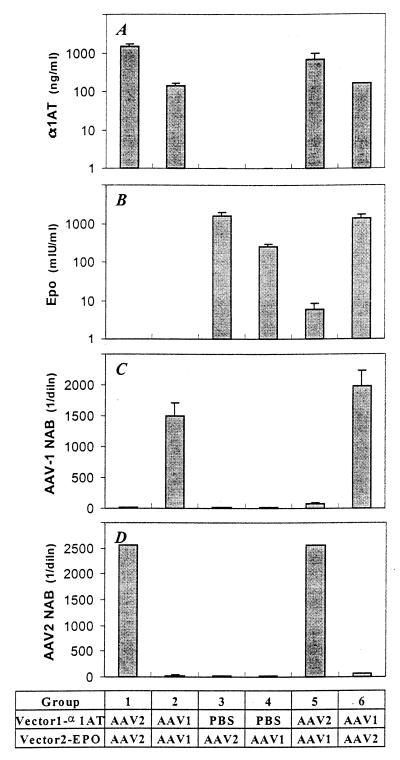
Similar studies performed with a model of liver-directed gene transfer, with vectors being injected into the portal circulation via the spleen, gave mixed results (Fig. 8). Previous exposure to AAV-1 does not interfere with AAV-2 gene transfer. However, the opposite is not true. Initial treatment with AAV-2 elicited high levels of NAB to AAV-2 and low levels of NAB to AAV-1 sufficient to suppress the gene transfer of AAV-1 20-fold.
FIG. 8.
Readministration of AAV vectors in the liver. These experiments are identical to those described in the legend to Fig. 7, except that animals received vectors in the portal circulation to target the liver.
DISCUSSION
AAV vectors have been based primarily on serotype 2, a human-derived parvovirus (19, 23). The early availability of an infectious clone of AAV-2 stimulated work on the development of replication-defective vectors. The utility of AAV-2-based vectors for achieving long-term, stable, and safe gene transfer has been demonstrated with small-animal models; a number of studies with large animals, including humans, are either planned or under way (9, 10, 21, 27). The tropism of replication-defective AAV-2 for efficient in vivo gene therapy is narrow.
The goal of this study was to isolate an infectious clone of AAV-1, which would be sequenced and used to develop a recombinant vector. The hypothesis was that AAV-1 vectors would have distinct biological profiles with advantages over AAV-2 vectors in some applications. Furthermore, AAV-1 and AAV-2 vectors should not cross-neutralize, providing important advantages when used in the context of preexisting immunity due to a natural infection or prior gene therapy treatment.
An infectious clone of AAV-1 was generated from a replication intermediate. Sequencing of the AAV-1 genome confirmed the presence of the structural components that characterize the AAV family. There is approximately 80% homology in nucleotide sequence between AAV-1 and AAV-2. Delineating the entire sequence of AAV-1 has clarified a potential etiology of AAV-6, which was previously isolated as a contaminant in a human adenovirus preparation. AAV-6 appears to be a hybrid of AAV-1 and AAV-2 formed by homologous recombination between highly conserved regions spanning 452 to 552 nucleotides. There has been minimal divergence from the precursor genomes since this homologous event occurred. The implications of this observation for gene therapy are unclear. The homologous sequences at the site of potential recombination are located at the 5′ end of the Rep gene, which is deleted in the AAV vectors. Recombination of this kind may lead to additional diversity within the AAV family, although it is not clear if AAV-6 resulted from recombination in vitro or occurred in vivo during naturally acquired infections.
The initial strategy for exploiting the biology of AAV-1 for vectors was to create actual chimeras in which the vector genome was formed from AAV-2 ITRs, whereas the Rep and Cap sequences used to generate the virions were derived from AAV-1. Our goal was to retain the favorable biology of AAV-2 in terms of efficient and stable transduction while modifying the entry pathways and potentially diverting humoral immune responses (8, 31). Analysis of these vectors with murine models of liver and lung gene therapy demonstrated some important differences. When normalized for equivalent doses of administered vector genomes, AAV-2 performs better in liver than in muscle, whereas the opposite is true of AAV-1. This finding presumably is due to differences in the efficiency of entry, although postentry events that differ between the two vectors cannot be ruled out.
Another important advantage of alternative serotypes is to avoid neutralization due to preexisting humoral immunity. The existence of monospecific NAB to AAV-1 in nonhuman primates and to AAV-2 in humans supports the same serospecificity of these AAV. It should be noted, however, that seropositivity to AAV-1 and AAV-2, which is not neutralizing, is detected at higher frequencies with Western assays or ELISAs (data not shown). Administration of the vectors into murine muscle demonstrated the predicted result, in that previous treatment with AAV-2 blocks the subsequent administration of AAV-2 while having only a modest impact on the efficiency of AAV-1 treatment. The opposite is also true, in that AAV-1 administered to muscle blocks a second treatment with AAV-1 but does not interfere with gene transfer mediated by AAV-2. The murine model of AAV gene transfer to liver yielded mixed results. Clearly, AAV-2 and AAV-1 completely blocked readministration of the same serotype. Initial treatment with AAV-1 did not affect a follow-up administration of AAV-2, although the opposite was not true. Specifically, initial treatment with AAV-2 diminished retreatment with AAV-1 approximately 20-fold, a value which will likely be significant in therapeutic applications. We conclude that AAV-2 generates cross-neutralizing antibodies to AAV-1 that have an impact on AAV-1-mediated gene transfer. This effect is more significant in the context of intravascular administration. There is no evidence that antibodies to AAV-1 cross-neutralize AAV-2-mediated gene transfer to muscle or liver.
One should consider these results in the context of human applications of AAV vectors. The majority of humans do not have NAB to either AAV-1 or AAV-2. In fact, the absence of monospecific antibodies to AAV-1 in humans, together with the fact that it was isolated from monkeys, argues against it being a human virus. What is the relevance of neutralizing activity to AAV-1 in 20% of humans? Does this value reflect primary infection or cross-neutralization in the context of infection with wild-type AAV-2? Will this neutralizing activity have an impact on the efficiency of AAV-1 uptake? The data presented here suggest that the AAV-1 vector would be the preferred initial vector for muscle-directed gene therapy. It is more efficient than AAV-2 and does not preclude follow-up treatment with AAV-2. The situation for the liver is less clear. The AAV-1 vector is less efficient in the context of naive animals, although initial treatment with AAV-2 partially inhibits a second administration of AAV-1.
The impact of humoral immune responses to AAV vectors on human applications of gene therapy is an important consideration for chronic diseases. It appears that AAV expression will persist for a prolonged period of time in most target organs. What is less clear is how long the humoral immune response will persist. The use of vectors based on different serotypes should allow at least two treatments. A requirement for more frequent readministration of vectors over short time periods may require other strategies which blunt the initial humoral immune response (12, 16).
ACKNOWLEDGMENTS
We thank the Vector, Cell Morphology and Immunology Cores of the Institute for Human Gene Therapy and Wei Cao, Marcia Houston-Leslie, Rosalind Barr, Ruth Qian, George Qian, and Sarah Ehlen-Haecker for technical support.
This work was supported by grants from the NIH (P30 DK47757-06 and P01 HD32649-04), the Muscular Dystrophy Association, and Genovo, Inc., a company that J. M. Wilson founded and has equity in.
REFERENCES
- 1.Balague C, Kalla M, Zhang W W. Adeno-associated virus Rep78 protein and terminal repeats enhance integration of DNA sequences into the cellular genome. J Virol. 1997;71:3299–3306. doi: 10.1128/jvi.71.4.3299-3306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantel-Schaal U, zur Hausen H. Characterization of the DNA of a defective human parvovirus isolated from a genital site. Virology. 1984;134:52–63. doi: 10.1016/0042-6822(84)90271-x. [DOI] [PubMed] [Google Scholar]
- 3.Berns K I. Parvoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 1007–1041. [Google Scholar]
- 4.Chiorini J A, Yang L, Liu Y, Safer B, Kotin R M. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark K R, Voulgaropoulou F, Fraley D M, Johnson P R. Cell lines for the production of recombinant adeno-associated virus. Hum Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 6.Clark K R, Voulgaropoulou F, Johnson P R. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 1996;3:1124–1132. [PubMed] [Google Scholar]
- 7.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 9.Flotte T, Carter B, Conrad C, Guggino W, Reynolds T, Rosenstein B, Taylor G, Walden S, Wetzel R. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- 10.Flotte T R, Carter B J. In vivo gene therapy with adeno-associated virus vectors for cystic fibrosis. Adv Pharmacol. 1997;40:85–101. doi: 10.1016/s1054-3589(08)60138-6. [DOI] [PubMed] [Google Scholar]
- 11.Gao G, Qu G, Faust L Z, Engdahl R K, Xiao W, Hughes J V, Zoltick P W, Wilson J M. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 12.Halbert C L, Standaert T A, Wilson C B, Miller A D. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 14.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Samulski R J, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning W C, Zhou S, Bland M P, Escobedo J A, Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 17.Muramatsu S, Mizukami H, Young N S, Brown K E. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 18.Murphy F A, Fauquet C M, Mayo M A, Jarvis A W, Ghabrial S A, Summers M D, Martelli G P, Bishop D H L. Classification and nomenclature of viruses: sixth report of the International Committee on Taxonomy of Viruses. Arch Virol. 1995;1995:169–175. [Google Scholar]
- 19.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 20.Parks W P, Boucher D W, Melnick J L, Taber L H, Yow M D. Seroepidemiological and ecological studies of the adenovirus-associated satellite viruses. J Virol. 1970;2:716–722. doi: 10.1128/iai.2.6.716-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubenstein R C, McVeigh U, Flotte T R, Guggino W B, Zeitlin P L. CFTR gene transduction in neonatal rabbits using an adeno-associated virus (AAV) vector. Gene Ther. 1997;4:384–392. doi: 10.1038/sj.gt.3300417. [DOI] [PubMed] [Google Scholar]
- 22.Rutledge E A, Halbert C L, Russell D W. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samulski R J. Adeno-associated virus: integration at a specific chromosomal locus. Curr Opin Genet Dev. 1993;3:74–80. doi: 10.1016/s0959-437x(05)80344-2. [DOI] [PubMed] [Google Scholar]
- 24.Samulski R J, Berns K I, Tan M, Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci USA. 1982;79:2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samulski R J, Srivastava A, Berns K I, Muzyczka N. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell. 1983;33:135–143. doi: 10.1016/0092-8674(83)90342-2. [DOI] [PubMed] [Google Scholar]
- 26.Samulski R J, Zhu X, Xiao X, Brook J D, Housman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. . (Erratum, 11:1228, 1992.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 28.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne B J, Atkinson M, Flotte T R. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA. 1998;95:14384–14388. doi: 10.1073/pnas.95.24.14384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surosky R T, Urabe M, Godwin S G, McQuiston S A, Kurtzman G J, Ozawa K, Natsoulis G. Adeno-associated virus Rep proteins target DNA sequences to a unique locus in the human genome. J Virol. 1997;71:7951–7959. doi: 10.1128/jvi.71.10.7951-7959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao W, Berta S C, Lu M M, Moscioni A D, Tazelaar J, Wilson J M. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Xiao, W., and J. M. Wilson. Unpublished data.
- 31.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao X, Li J, Samulski R J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]