Abstract
Cataract is the leading cause of blindness worldwide. There is an increased incidence of cataract formation in the diabetic population due to several factors. Diabetes mellitus accelerates the development of cataract. Oxidative stress results in most of the diabetic complications including diabetic cataract. Oxidative stress leading to the expression of various enzymes has also been proven as crucial for cataractous changes in the lens in old age. A narrative review was undertaken to investigate the expression of different biochemical parameters as well as enzymes in diabetic and senile cataracts. Identification of these parameters is crucial for the prevention and treatment of blindness. Combinations of MeSH terms and key words were used to do literature search in PubMed. The search resulted 35 articles and among them, 13 were relevant to the topic and were included in synthesis of results. Seventeen different types of enzymes were identified in the senile and diabetic cataracts. Seven biochemical parameters were also identified. Alteration in biochemical parameters and expression of enzymes were comparable. Majority of the parameters were raised or altered in diabetic cataract compared to senile cataract.
Keywords: Age-related cataract, cataract, cataract enzymology, diabetic cataract, senile cataract
Cataract is a major cause of visual impairment in the general population[1,2] as well as in the diabetic population.[3-5] Development of cataract is accelerated in patients with diabetes compared to the normal population.[6] Diabetes mellitus (DM) is one of the major risk factors for cataract, and cataract occurs two to five times more frequently in patients with DM than in those without DM.[7,8]
Causal biochemical relationship between DM and cataract development has been proven by various researchers.[7] DM and cataracts, as independent diseases, are major health concerns and impose immense economic burden, especially in developing countries where treatment options for both of these are not optimal.[9] Aging is a known risk factor for development of both DM and cataract, which increases burden on health-care services.[10] For the prevention and treatment of blindness, risk factor identification is crucial. Oxidative stress culminates into most of the diabetic complications including diabetic cataract.[11] Oxidative stress leading to expression of various enzymes has also been proven to be crucial for the development of cataractous changes in lens in old age.[12-15]
To the best of our knowledge, there is paucity of published literature regarding comparison of expression of both enzymatic and biochemical parameters in diabetic cataract and senile cataract. Therefore, we undertook this narrative review to explore the expression of different biochemical parameters as well as enzymes in diabetic and senile cataract. Various enzymatic and biochemical parameters expressed in cataractous lens of both diabetic and age-related cataract were compared.
Methods
Extensive literature search and desk review was done by searching databases (Google Scholar, PubMed). Studies whose full text was available in English from the year 1958 to 2022 were included, and the following concepts were built to answer the review question on enzymatic and biochemical properties of lens in age-related cataract and diabetic cataract.
Concept 1 - Cataract: MeSH terms identified for this concept were “Cataract”[Mesh], “Cataract/enzymology”[Mesh], and “lens, crystalline”[Mesh].
Concept 2 - Age-related or senile cataract: MeSH terms used were “Cataract, Age-Related Nuclear,” “Cataract, Age-Related Cortical.” Key words identified were “age-related cataract” and “senile cataract.”
Concept 3 - Diabetic cataract: MeSH term “Diabetes Mellitus”[Mesh] and key word “Diabetic cataract” were identified.
The combination of the MeSH terms (Boolean operators) and keywords was used to search in PubMed. The following combinations were used: [((“Cataract”[Mesh] OR “Cataract/enzymology”[Mesh]) AND (“Age-related cataract” OR “senile cataract”)) AND (“diabetic cataract”)]. The search resulted in 35 articles published during the period 1985–2022 in English language. Three independent assessors thoroughly scrutinized all the abstracts. Among the abstracts, only 13 were relevant to the topic. The full texts of these that were available in English language were downloaded and included to arrive at a conclusion. The same methodology has been described in the flowchart [Fig. 1]. After screening for duplication and going through the titles and abstracts, we obtained 13 relevant articles, and the results of the literature search are given in Table 1.
Figure 1.
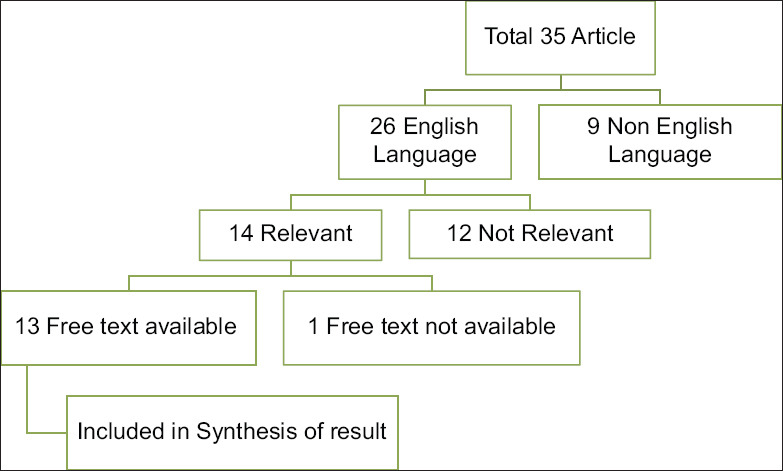
Methodology for selecting articles
Table 1.
Review of literature of enzymatic and biochemical properties of lens in ARC and diabetic cataract
| Authors | Year | Characteristics | Methodology | Results |
|---|---|---|---|---|
| Ozmen et al.[12] | 1997 | Enzymatic | LPO concentrations in senile and diabetic cataract lenses were determined as TBARS by a method modified from Satoh and Yagi, and GSH concentration was measured according to Beutler | Lens LPO levels were significantly higher in diabetic cataract lens (P<0.01). In the genesis of cataract, free radical damage as a causative factor was found more important in cases of diabetic cataract than in senile cataract |
| Balog et al.[13] | 2001 | Enzymatic | Absorption activity of mitochondrial enzyme cytochrome c oxidase | Activity of cytochrome c oxidase in the mitochondrial fraction of lens epithelial cells was found to be two times lower in smokers with senile cataract when compared to nonsmokers having senile cataract Mean cytochrome c oxidase activity was significantly less in senile (4.81±0.42) cataract than in diabetic cataract (6.76±0.61) |
| Andrzejewska- Buczko et al.[16] | 2001 | Enzymatic | Tryptophan, kynurenine, 3-hydroxykynurenine, kynurenic acid, and anthranilic acid were measured in aqueous humor and lenses obtained from nondiabetic patients with type II diabetes in the course of surgical cataract extraction | The concentrations of kynurenine, 3-hydroxykynurenine, and anthranilic acid in the aqueous humor were more in diabetic subjects The concentrations of tryptophan and kynurenic acid in the aqueous humor were similar in both groups Levels of tryptophan and its metabolites were found to be more in diabetic lenes |
| Ozmen et al.[12] | 2002 | Enzymatic | Activities of the antioxidant enzymes such as Cu- and Zn-SOD were measured by the enzymatic method and of catalase was measured by colorimetric method in the cataractous lenses | Both Cu- and Zn-SOD and catalase levels were significantly lower in the diabetic cataractous lenses than in senile cataractous lenses |
| Anthrayose et al.[17] | 2004 | Biochemical | Estimation of protein and taurine | The lens weight was found to be significantly increased (P<0.001) with development and maturation of cataract The concentrations of total lens protein (P<0.05) and taurine (P<0.01) were found to be significantly decreased in cataractous lens compared to normal lens |
| Raitelaitiene et al.[18] | 2005 | Ultrasonic and biochemical study | Mean lens thickness was more in cataractous lens compared to the normal lens (statistically insignificant) Mean attenuation coefficient (dB/cm MHz) and ultrasound attenuation coefficient were also more in cases of senile and diabetic cataract lens Amount of soluble protein was lesser in diabetic cataract when compared to senile cataractous lens | |
| Maurya et al.[15] | 2006 | Enzymatic | SOD and catalase | The mean serum levels of SOD and catalase were lower as age increased These enzymes were significantly lower in diabetic cataracts (9.13 and 16.42 units/ml, respectively) compared to senile cataracts (25.30 and 57.27 units/ml, respectively) |
| Chandrasena et al.[19] | 2006 | Enzymatic | Erythrocyte catalase, GPX, and SOD activities were assayed | Senile cataract was associated with significantly low levels of erythrocyte catalase, GPX, and SOD, when compared to diabetic cataract |
| Chitra et al.[20] | 2020 | Enzymatic | Plasma oxidative stress markers such as the lipid peroxidation end product MDA, protein oxidation products, protein carbonyls, and DNA oxidative damage marker 8-hydroxy-2-deoxyguanosine were evaluated Plasma advanced glycation end products index, erythrocyte aldose reductase activity, and sorbitol levels were evaluated | After adjusting for age, plasma MDA levels were significantly higher in diabetic cataracts (P<0.001) and nondiabetic cataract subjects (P<0.05), compared to nondiabetic subjects with clear lens Plasma advanced glycation end products index was significantly higher (P<0.05) only in diabetic cataracts Aldose reductase activity and sorbitol levels were significantly higher (P<0.001) in both diabetic and nondiabetic subjects with cataract compared to nondiabetic subjects with clear lens |
| Zhu et al.[21] | 2021 | Biochemical | Comparison of racemization of Asp 58 residue, a hotspot position in αA-crystallin, from the cortex and nucleus of diabetic and age-matched senile cataractous lenses, by identifying l-Asp/l-isoAsp/d-Asp/d-isoAsp by mass spectrometry | Diabetic cataractous lenses showed a significantly increased cortex/nucleus ratio of d-Asp 58 and decreased protein solubility |
| Kaliaperumal et al.[22] | 2021 | Biochemical and enzymatic | Serum magnesium Serum MDA, an indicator of oxidative stress biomarker. Antioxidant status such as serum GSH and GPX-3 | Significantly decreased levels of magnesium, GSH, GPX-3 and increased level of MDA in diabetic cataractous lens compared to senile cataractous lens |
| Li et al.[23] | 2021 | Enzymatic | Anterior capsule specimens from diabetic cataract (DC) and age-related cataract (ARC) patients were obtained to compare the expression difference of autophagy-related genes The phosphorylation levels of AMPK-AMP activated protein kinase mTOR- mammalian target of rapamycin (AMPK, AKT, and mTOR) and the expression of FOXO3 and TFEB were measured | The expression of autophagy-related genes ATG5, FYCO1, ATG8, ATG12, Beclin1, and ULK1 in the anterior capsule LECs of diabetic cataractous lens was significantly downregulated when compared to senile cataractous lens AMPK and AMPK-dependent transcription factors, FOXO3 and TFEB, were also inhibited in cataractous lens |
| Chen et al.[24] | 2022 | Biochemical | Determining the role for TRPV2 in the development of diabetic cataracts using immunohistochemistry and western blotting analysis | TRPV2 expression levels were significantly increased (which, in turn, enhanced the reactive oxygen species-induced lens epithelial cell apoptosis) in the lens epithelial cells of patients with diabetic cataracts compared to those with senile cataract |
ARC=Age-related cataract, GPX-3=Glutathione peroxidase-3, GSH=Reduced glutathione, LPO=Lipid peroxide, MDA=Malondialdehyde, TBARS=Thiobarbituric acid-reactive substances, TRPV2=Transient receptor potential vanilloid 2
Results
We arrived at the results by reviewing all the articles found relevant during the literature search. The results are given under the following subheadings:
Enzymatic properties
The expression of lens lipid peroxide (LPO) levels was significantly (P < 0.01) higher in diabetic lens than in senile cataractous lens, as calculated using Student’s t-test. Mean level (mean 4.29 ± 2.05) of expression of reduced glutathione (GSH) was less in diabetic lens than in senile cataract lens (mean 4.68 ± 3.12).[12] Mean cytochrome c oxidase activity was significantly less in senile (4.81 ± 0.42) cataract than in diabetic cataract (6.76 ± 0.61). Significantly different activity of cytochrome c oxidase was found in active smokers and the nonsmoking patients with diabetic cataract.[13] Copper- and zinc-superoxide dismutase (Cu-SOD and Zn-SOD) and catalase were significantly lower in diabetic cataracts (9.13 and 16.42 units/ml, respectively) compared to senile cataracts (25.30 and 57.27 units/ml, respectively).[14,15,19]
Plasma advanced glycation end products (AGEP) index was significantly higher (P < 0.05) only in diabetic cataracts, but not in nondiabetic subjects with cataracts. Aldose reductase activity and sorbitol levels were significantly higher (P < 0.001) in both diabetic and nondiabetic subjects with cataract compared to nondiabetic subjects with clear lens. When adjusted for age, plasma malondialdehyde (MDA) levels were significantly higher in diabetic cataracts (P < 0.001) and nondiabetic cataract subjects (P < 0.05), compared to nondiabetic subjects with clear lens.[20] Significantly decreased levels of serum magnesium, GSH, and glutathione peroxidase-3 (GPX-3) and increased level of MDA were found in diabetic cataract patients compared to senile cataract patients. Significant negative correlation of serum magnesium with MDA and positive correlation with GPX-3 were observed.[22] A study from Poland reported accumulation of tryptophan and all of its assayed metabolites (tryptophan, kynurenine, 3-hydroxykynurenine, kynurenic acid, and anthranilic acid) in the lenses obtained from patients with diabetes.[16] In a study by Li et al.[23] on downregulation of activated protein kinase AMPK-dependent FOXO3 and TFEB involves in the inhibition of autophagy in diabetic cataract. As compared to age-related cataract patients, the expression of autophagy-related genes ATG5, FYCO1, ATG8, ATG12, Beclin1, and ULK1 in the anterior capsule lens epithelial cells (LECs) of diabetic cataract patients was significantly downregulated.
Biochemical properties
In diabetic cataract, the lens weight increased significantly (P < 0.001) with maturation of cataract. Diabetic lens also showed a reduction in total proteins in comparison to normal lenses, which was statistically significant (P < 0.05). The mean lens weight increased significantly (P < 0.001) with maturation of cataract. Total proteins in senile cataract lenses also decreased gradually from immature to hyper-mature cataract. The concentration of lens taurine decreased significantly (P < 0.01) in comparison to normal lens in senile and diabetic cataracts.[17] Study of ultrasonic and biochemical parameters of diabetic and senile cataract revealed that both in senile and diabetic cataracts, the mean lens thickness was more compared to the normal lens. Mean attenuation coefficient (dB/cm MHz) was also more in senile and diabetic cataract lens compared to normal lens. Amount of soluble protein was less in diabetic lens compared to the senile cataract lens.[18] Zhu et al.[21] studied and compared the racemization in cataractous lens from diabetic and aging individuals. Compared to nondiabetic cataractous lenses, diabetic cataractous lenses showed a significantly increased cortex/nucleus ratio of d-Asp 58, which originated primarily from an increased percentage of d-Asp 58 in the lens cortex of diabetic cataracts. Decreased protein solubility was seen in diabetic cataractous lenses. The role of transient receptor potential vanilloid 2 (TRPV2) in the development of diabetic cataracts was elicited by Chen et al.[24] in China, and they revealed that TRPV2 expression levels were significantly increased in the lens epithelial cells of patients with diabetic cataracts compared to those with senile cataract.
Comparison of enzymatic and biochemical parameters
GSH, superoxide dismutase (SOD), catalase and serum magnesium were found to be raised in senile cataract, while the other remaining factors were raised in diabetic cataract. A comparative list of the enzymatic and biochemical parameters in both cataracts has been presented in Table 2.
Table 2.
Comparison between enzymatic and biochemical parameters
| Characteristics | Nondiabetic (senile) cataract | Diabetic cataract |
|---|---|---|
| Enzymatic property | ||
| Lens lipid peroxide | Raised | |
| Reduced glutathione | More | |
| Cytochrome c oxidase | More | |
| Superoxide dismutase | More | |
| Catalase | More | |
| Erythrocyte catalase | More | |
| Plasma advanced glycation end products index | More | |
| Aldose reductase | More | |
| Sorbitol | More | |
| Plasma malondialdehyde | More | |
| Serum magnesium | More | |
| Serum GSH | More | |
| GPX-3 | More | |
| Tryptophan | More | |
| Autophagy-related genes | Downregulated | |
| Serum chromium | More | |
| Total antioxidant status and uric acid levels | More | |
| Biochemical parameters | ||
| Weight | Raised | Raised |
| Protein content | Decreased | Decreased |
| Taurine | Decreased | Decreased |
| Mean lens thickness | Increased | Increased |
| Soluble protein | Decreased | |
| Cortex-to-nucleus ratio of D-Asp 58 | Increased | |
| TRPV2 | Increased |
GPX-3=glutathione peroxidase-3, GSH=reduced glutathione, TRPV2=transient receptor potential vanilloid 2
Discussion
In this narrative review, after evaluating results from the 13 articles, seven biochemical parameters and 17 enzymatic parameters were identified, which were found to be increased or decreased in senile or diabetic cataract. The association of these parameters with various factors was discussed, and the results of all the studies were found to be comparable.
Enzymatic characteristics
LPO levels were found to be higher in diabetic cataract lens compared to age-related cataractous lens. This can be explained by the fact that, lipid peroxidation has been recognized to be one among the major endogenous processes which cause injury to the membranous structures of cells and tissues. Similarly, GSH levels were found to be lower in age-related cataractous lens.[12] Smoking and cytochrome c oxidase activity were found to be related by Balog et al.,[13] who showed significant difference between active smokers and the nonsmoking patients with diabetic cataract. Also, mean cytochrome c oxidase activity was less in senile cataract than in diabetic cataract. This is in accordance with the fact that cytochrome c oxidase helps in adenosine triphosphate (ATP) production needed for lens nutrition and also has antioxidant activity. The activities of other enzymes like Cu- and Zn-SOD and catalase were significantly lower in the lens in diabetic cataract than in senile cataract.[14] Maurya et al.[15] observed a similar finding of low serum levels of SOD and catalase in diabetic cataract compared to senile cataract. Also, decreased levels of both the enzymes were observed with increase in age. The early cataractous changes in lens may be due to decrease in serum levels of the major antioxidant subjected to increased oxidative stress in diabetes. Oxidative stress has been implicated in cataractogenesis.[25] Plasma oxidative stress markers such as the lipid peroxidation end product MDA, protein oxidation products, protein carbonyls, and DNA oxidative damage marker 8-hydroxy-2-deoxyguanosine were found to be higher in diabetic cataract compared to the nondiabetic cataract and in patients with clear lens.[20] Indicators of oxidative stress such as serum magnesium were found to be reduced in diabetic cataract; also, antioxidants such as GSH and GPX-3 were decreased in diabetic cataract compared to senile cataract. Increased level of serum MDA was observed in diabetic cataract. Hypomagnesemia may be a significant causative factor for increased oxidative stress and it triggers earlier cataractogenesis in patients with type 2 DM.[22]
The levels of erythrocyte catalase, GPX, and SOD were significantly low in senile cataract compared to diabetic cataract.[19] Accumulation of tryptophan and all of its assayed metabolites was reported in diabetic cataractous lenes.[16] Significant downregulation of expression of autophagy-related genes was observed in diabetic cataract than senile cataract. This may be the underlying mechanism of diabetic cataract formation.[23] Few researchers had shown association of aqueous humor (AH) parameters and cataract formation in diabetics and senile cataract. The chromium (Cr) level in the serum and AH was found to be lower in senile cataract than in diabetic cataract by a few researchers.[26] Total antioxidant status and uric acid levels were lower in the AH from diabetic cataract than in nondiabetic subjects with cataract.[27] Mitrovic et al.[28] demonstrated disturbed AH microenvironment in diabetic cataract, with significant changes in vascular endothelial growth factor (VEGF), interleukin (IL)-10, and Fas ligand (FasL).
Biochemical characteristics
In both diabetic and senile cataracts, weight increases with maturation of cataract. Protein content in cataractous lens decreases both in diabetic cataract and senile cataract when compared to the normal lens. Also, the taurine content decreases in both diabetic and senile cataracts.[17] Proteins and taurine content in lenses were altered with maturity of cat aract and were not related to age or sex. Similar finding of decreased protein solubility in diabetic cataractous lenses was reported by Zhu et al.[21] Also, the amount of soluble protein was less in diabetic lens compared to the senile cataractous lens. Compared to the normal lens, ultrasound attenuation coefficient was higher in senile and diabetic cataracts.[18]
Qianqian et al.[29] compared the lens proteomic profiles between diabetic cataract (type-1) and age-related cataract and concluded that the aB- and bB1-crystallin may accelerate the development of diabetic cataracts, particularly in type-1 diabetes. Zhu et al.[21] compared racemization of Asp 58 residue, a hotspot position in aA-crystallin, from the cortex and nucleus of diabetic and age-matched senile cataractous lenses, where they reported diabetic cataractous lenses had increased cortex/nucleus ratio of D-Asp 58, compared to nondiabetic cataract. Chen et al.[24] studied the role of TRPV2 in the development of diabetic cataracts immunohistochemically and by western blotting analyses and showed that TRPV2 expression levels were increased in the lens epithelial cells of patients with diabetic cataracts compared to those with senile cataract. The mechanism suggested was enhanced reactive oxygen species–induced lens epithelial cell apoptosis by upregulating TRPV2 expression and TRPV2-mediated Ca2+ overload in a high-glucose environment.
Limitations of the study
Only PubMed articles with free access were included for the review.
Conclusion
The review explored various biochemical and enzymatic parameters expressed in age-related cataract and diabetic cataract. These parameters provide a better insight and in-depth understanding of the pathogenesis of age-related cataract and diabetic cataract, thus enabling ophthalmologists to take appropriate decisions and helping them in management of these conditions. Majority of the parameters were raised or altered in diabetic cataract compared to senile cataract. Nonetheless, further research is mandated in this regard.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Khairallah M, Kahloun R, Bourne R, Limburg H, Flaxman SR, Jonas JB, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Investig Ophthalmol Vis Sci. 2015;56:6762–9. doi: 10.1167/iovs.15-17201. [DOI] [PubMed] [Google Scholar]
- 3.Khanna RC, Murthy GVS, Giridhar P, Krishnaiah S, Pant HB, Palamaner Subash Shantha G, et al. Cataract, visual impairment and long-term mortality in a rural cohort in India: The Andhra Pradesh eye disease study. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0078002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein BEK, Klein R, Moss SE. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985;92:1191–6. doi: 10.1016/s0161-6420(85)33877-0. [DOI] [PubMed] [Google Scholar]
- 5.Drinkwater JJ, Davis WA, Davis TME. A systematic review of risk factors for cataract in type 2 diabetes. Diabetes Metab Res Rev. 2019;35 doi: 10.1002/dmrr.3073. doi:10.1002/dmrr. 3073. [DOI] [PubMed] [Google Scholar]
- 6.Kato S, Oshika T, Numaga J, Kawashima H, Kitano S, Kaiya T. Influence of rapid glycemic control on lens opacity in patients with diabetes mellitus. Am J Ophthalmol. 2000;130:354–5. doi: 10.1016/s0002-9394(00)00546-8. [DOI] [PubMed] [Google Scholar]
- 7.Pollreisz A, Schmidt-Erfurth U. Diabetic cataract-pathogenesis, epidemiology and treatment. J Ophthalmol. 2010;2010:1–8. doi: 10.1155/2010/608751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Wan XH, Zhao GH. Meta-analysis of the risk of cataract in type 2 diabetes. BMC Ophthalmol. 2014;14:94. doi: 10.1186/1471-2415-14-94. doi:10.1186/1471-2415-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabin G, Chen M, Espandar L. Cataract surgery for the developing world. Curr Opin Ophthalmol. 2008;19:55–9. doi: 10.1097/ICU.0b013e3282f154bd. [DOI] [PubMed] [Google Scholar]
- 10.Olafsdottir E, Andersson DKG, Stefánsson E. The prevalence of cataract in a population with and without type 2 diabetes mellitus. Acta Ophthalmol. 2012;90:334–40. doi: 10.1111/j.1755-3768.2011.02326.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Yan H, Lou MF. Does oxidative stress play any role in diabetic cataract formation?-Re-evaluation using a thioltransferase gene knockout mouse model. Exp Eye Res. 2017;161:36–42. doi: 10.1016/j.exer.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Ozmen D, Mutaf I, Ozmen B, Mentes J, Bayindir O. Lens lipid peroxides and glutathione concentrations in diabetic cataract. Ann Clin Biochem. 1997;34:190–2. doi: 10.1177/000456329703400211. [DOI] [PubMed] [Google Scholar]
- 13.Balog Z, Sikic J, Vojniovic B, Balog S. Senile cataract and the absorption activity of cytochrome C oxidase. Coll Antropol. 2001;25(Suppl 1):33–6. [PubMed] [Google Scholar]
- 14.Ozmen B, Ozmen D, Erkin E, Guner I, Habif S, Baymdir O. Lens superoxide dismutase and catalase activities in diabetic cataract. Clin Biochem. 2002;35:69–72. doi: 10.1016/s0009-9120(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 15.Maurya OPS, Mohanty L, Bhaduri G, Chandra A. Role of anti-oxidant enzymes superoxide dismutase and catalase in the development of cataract: Study of serum levels in patients with senile and diabetic cataracts. J Indian Med Assoc. 2006;104:394–7. [PubMed] [Google Scholar]
- 16.Andrzejewska-Buczko J, Pawlak D, Tankiewicz A, Matys T, Buczko W. Possible involvement of kynurenamines in the pathogenesis of cataract in diabetic patients. Med Sci Monit. 2001;7:742–5. [PubMed] [Google Scholar]
- 17.Anthrayose C V, Shashidhar S. Studies on protein and taurine in normal, senile and diabetic cataractous human lenses. Indian J Physiol Pharmacol. 2004;48:357–60. [PubMed] [Google Scholar]
- 18.Raitelaitiene R, Paunksnis A, Ivanov L, Kurapkiene S. Ultrasonic and biochemical evaluation of human diabetic lens. Medicina (Kaunas) 2005;41:641–8. [PubMed] [Google Scholar]
- 19.Chandrasena LG, Chackrewarthy S, Perera PTMJ, De Silva D. Erythrocyte antioxidant enzymes in patients with cataract. Ann Clin Lab Sci. 2006;36:201–4. [PubMed] [Google Scholar]
- 20.Chitra PS, Chaki D, Boiroju NK, Mokalla TR, Gadde AK, Agraharam SG, et al. Status of oxidative stress markers, advanced glycation index, and polyol pathway in age-related cataract subjects with and without diabetes. Exp Eye Res. 2020;200 doi: 10.1016/j.exer.2020.108230. doi:10.1016/J. EXER.2020.108230. [DOI] [PubMed] [Google Scholar]
- 21.Zhu XJ, Zhang KK, He WW, Qi J, Lu Y. Racemization in cataractous lens from diabetic and aging individuals: Analysis of Asp 58 residue in aA-crystallin. Aging (Albany NY) 2021;13:15255–68. doi: 10.18632/aging.203086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaliaperumal R, Venkatachalam R, Nagarajan P, Sabapathy SK. Association of serum magnesium with oxidative stress in the pathogenesis of diabetic cataract. Biol Trace Elem Res. 2021;199:2869–73. doi: 10.1007/s12011-020-02429-9. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Sun Q, Qiu X, Zhang J, Zheng Y, Luo L, et al. Downregulation of AMPK dependent FOXO3 and TFEB involves in the inhibition of autophagy in diabetic cataract. Curr Eye Res. 2021;47:555–64. doi: 10.1080/02713683.2021.2009516. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Chen Y, Ding W, Zhan T, Zhu J, Zhang L, et al. Oxidative stress-induced TRPV2 expression increase is involved in diabetic cataracts and apoptosis of lens epithelial cells in a high-glucose environment. Cells. 2022;11:1196. doi: 10.3390/cells11071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halliwell B. Antioxidants: The basics-what they are and how to. Antioxidants Dis Mech Ther Antioxidants Dis Mech Ther Strateg. 1996;38:1–20. [Google Scholar]
- 26.Cumurcu T, Mendil D, Erkorkmaz U. Aqueous humor and serum levels of chromium in cataract patients with and without diabetes mellitus. Ophthalmologica. 2008;222:324–8. doi: 10.1159/000144076. [DOI] [PubMed] [Google Scholar]
- 27.Aksoy H, Keles S, Koçer I, Akçay F. Diabetic cataract and the total antioxidant status in aqueous humor. Clin Chem Lab Med. 2001;39:143–5. doi: 10.1515/CCLM.2001.024. [DOI] [PubMed] [Google Scholar]
- 28.Mitrovic S, Kelava T, Sucur A, Grcevic D. Levels of selected aqueous humor mediators (IL-10, IL-17, CCL2, VEGF, FasL) in diabetic cataract. Ocul Immunol Inflamm. 2016;24:159–66. doi: 10.3109/09273948.2014.949779. [DOI] [PubMed] [Google Scholar]
- 29.Qianqian Y, Yong Y, Zhaodong C, Yonghui T, Jun S, Yuzheng H. Differential protein expression between type 1 diabetic cataract and age-related cataract patients. Folia Biol (Praha) 2015;61:74–80. doi: 10.14712/fb2015061020074. [DOI] [PubMed] [Google Scholar]


