Abstract
Infection by human herpesvirus 8 (HHV-8) is associated with the development of Kaposi’s sarcoma (KS). Since regression of KS can be achieved by treatment of the patients with alpha interferon (IFN-α), we analyzed the effects of IFN-α or anti-IFN-α antibodies (Ab) on HHV-8 latently infected primary effusion lymphoma-derived cell lines (BCBL-1 and BC-1) and on peripheral blood mononuclear cells (PBMC) from patients with all forms of KS and from at-risk subjects. IFN-α inhibited in a dose-dependent manner the amplification of HHV-8 DNA in BCBL-1 cells induced to lytic infection with tetradecanoyl phorbol acetate (TPA). This effect was associated with the inhibition of the expression of HHV-8 nut-1 and kaposin genes that are induced early and several hours, respectively, after TPA treatment. In addition, IFN-α inhibited virus production and/or release from BCBL-1 cells. Inhibition of nut-1 and kaposin genes by IFN-α was also observed in BC-1 cells induced with n-butyrate. Conversely, the addition of anti-IFN-α Ab to TPA-induced BCBL-1 cells resulted in a larger number of mature enveloped particles and in a more extensive cytopathic effect due to the neutralization of the endogenous IFN produced by these cells. IFN was also produced by cultured PBMC from HHV-8-infected individuals, and this was associated with a loss of viral DNA during culture. However, the addition of anti-IFN-α Ab or anti-type I IFN receptor Ab promoted the maintenance of HHV-8 DNA in these cells that was associated with the detection of the latency-associated kaposin RNA. Finally, the addition of IFN-α reduced the HHV-8 load in PBMC. Thus, IFN-α appears to have inhibitory effects on HHV-8 persistent infection of PBMC. These results suggest that, in addition to inhibiting the expression of angiogenic factors that are key to KS development, IFN-α may induce KS regression by reducing the HHV-8 load and/or inhibiting virus reactivation.
Kaposi’s sarcoma (KS) is a tumor of vascular origin that is particularly common and aggressive in human immunodeficiency virus (HIV)-infected individuals (AIDS-KS) but is also found as other epidemiologic forms including African or endemic KS, classic KS (C-KS) and posttransplantation KS (PT-KS) (9, 25, 67). KS develops as multiple lesions arising at independent sites in the skin and, in the most aggressive forms, also in visceral and lymphatic organs (26). The lesions are characterized by a rich inflammatory-cell infiltrate (particularly evident in early lesions), angiogenesis, slit-like vascular spaces, extravasated erythrocytes, and the presence of perivascular and interstitial spindle-shaped cells that are considered to be the tumor cells of KS (KS cells) (22, 30, 48, 49, 63, 75).
All epidemiologic forms of KS have the same histological features, and are all associated with infection by human herpesvirus 8 (HHV-8) (3, 11, 14, 20, 53, 71). HHV-8 is a novel herpesvirus that is also found in primary effusion lymphoma (PEL) and Castelman’s disease (13, 77). In individuals at risk for KS, HHV-8 infection is highly predictive of disease development (33, 55, 65, 88).
HHV-8 is present in a latent form in KS spindle cells and lesional endothelial cells but yields a lytic infection in lymphocytes and monocytes infiltrating KS lesions (10, 17, 61, 79, 81). In addition, HHV-8 can infect circulating B cells, monocytes/macrophages, T cells, and KS-like spindle cell progenitors that are increased in number in the blood of patients with all forms of KS (34, 38, 51, 74, 76). These cells infiltrate KS lesions and can recruit the virus into tissues (10, 30, 61, 75, 87). Although restricted to a small proportion of the inflammatory cells infiltrating the lesions, HHV-8 lytic infection may play an important role in KS initiation or progression by producing or inducing factors with paracrine chemotactic and growth effects (6, 12, 40, 54, 59, 80) and/or by transmitting the virus to the spindle and endothelial cells present in the lesions (10, 30, 75, 80). However, therapy with antiherpetic drugs, which blocks HHV-8 lytic infection (39, 50), has had variable results and has failed to induce remission in late-stage KS (15, 56).
Systemic administration of alpha-interferon (IFN-α) to KS patients has been used in the therapy of C-KS and in patients with AIDS-KS and relatively normal CD4+ T-cell counts (16, 21, 36, 43, 62, 86). Clinical responses to IFN-α alone have been observed in up to 46% of the treated HIV-infected subjects, particularly in patients with little or no visceral involvement (reviewed in reference 57). IFN-α is known to inhibit the proliferation of KS spindle cells by down regulation of c-myc expression (41) and to inhibit the production of basic fibroblast growth factor (73), an angiogenic factor highly expressed in all forms of KS that plays a key role in KS cell growth and angiogenesis (24, 27, 68, 69). However, another possible target of IFN-α is HHV-8. In other in vitro models, the IFN-γ and tumor necrosis factor alpha combination can block herpes simplex virus and cytomegalovirus productive infection through induction of IFN-α/β (28, 29, 45). However, nothing is known about the effects of IFN-α/β on HHV-8 infection and on its life cycle.
Here we show that IFN-α inhibits reactivation of latent HHV-8 in PEL-derived cells and reduces the viral load in cultured peripheral blood mononuclear cells (PBMC) from KS patients and individuals at risk for KS.
MATERIALS AND METHODS
Patients.
Sixteen homosexual men with AIDS-KS, six asymptomatic HIV-infected homosexual men (HIV+), three patients with C-KS, one patient with PT-KS, and two post-kidney-transplant (PT) patients were included in the study. The patients with AIDS-KS were treated with combinations of zidovudine (AZT), D4T, 3TC, ddC, ddI, granulocyte-monocyte colony-stimulating factor, IFN-α, vincristine, bleomycin, Saquinavir, Indinavir, Ritonavir, or Taxol. All patients gave informed consent to participate in the study.
Cell cultures.
BCBL-1 cells were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, and were cultured in RPMI 1640 containing 10% fetal calf serum (FCS), 1 mM sodium pyruvate, and 50 μM β-mercaptoethanol. BC-1 cells (obtained from Patrick Moore, Columbia University, New York, N.Y.) were cultured in RPMI 1640 containing 15% FCS. The cells were split at a density of 3 × 105 cells/ml twice a week. PBMC were isolated by Ficoll-Hypaque density gradient centrifugation, seeded in six-well culture plates (3 × 106 to 4 × 106 cells/well), and cultured in RPMI 1640 containing 15% FCS. Fresh medium was added on day 3. Human lung A549 cells were cultured in Dulbecco’s minimal essential medium containing 10% FCS.
Induction of PEL cells.
Exponentially growing BCBL-1 and BC-1 cells (about 106 cells/ml) were collected and suspended in growth medium (5 × 105 cells/ml), and BCBL-1 were grown for an additional 24 h. The BCBL-1 and BC-1 cells were then induced with 20 ng of tetradecanoyl phorbol acetate (TPA) or 3 mM sodium n-butyrate (both from Sigma, Milan, Italy), respectively, for 2 days. For a 5-day induction of BCBL-1 cells, fresh culture medium containing TPA was added on day 3. Cells and supernatants were then harvested and immediately processed or frozen at −80°C.
Treatment with IFN-α or IFN-α neutralizing Ab.
BCBL-1 cells were split (day 0) at a density of 500,000 cells/ml to obtain identical cultures, which were then treated with human recombinant IFN-α2b (Schering-Plough, Milan, Italy) or IFN-α neutralizing antibody (Ab) (sheep, polyclonal Ab; BioSource) and TPA or left untreated. IFN-α2b and Abs were added to BCBL-1 cells 24 h prior to TPA induction (day 1). For a 5-day induction, IFN-α2b was added again on day 3. PBMC were plated (day 0) at a density of 106 cells/ml and received culture medium with or without IFN-α2b, anti-IFN-αAb, or 64G12, a monoclonal Ab (MAb) that acts as an antagonist of the type I IFN receptor (23) (a courtesy of F. Belardelli, Istituto Superiore di Sanità, Rome, Italy) on days 1 and 3 of culture.
HHV-8 plasmid construction.
HHV-8 DNA sequences corresponding to the viral open reading frame (ORF) K12 (T0.7 RNA, kaposin), ORF 26 (capsid protein [VP23]), and sequences encompassing the HHV-8 U-RNA-like nuclear transcript (nut-1 [T1.1 RNA]) (66) were amplified by long-range PCR from a C-KS lesion with the XL PCR kit (Perkin-Elmer), and the PCR products were cloned in the pCRII vector with the TA cloning kit (Invitrogen) to generate plasmids pCRII-T.07, p1.8Kb, and pCRII-T1.1, respectively.
Southern blot and Northern blot hybridization.
Genomic DNA and total RNA were extracted from BCBL-1 cells with the QIAamp blood kit and the RNeasy Mini Kit (both from Qiagen), respectively, as specified by the manufacturer. Equal amounts of total RNA (5 to 20 μg) were suspended in 30 μl of a buffer containing 40 mM morpholinepropanesulfonic acid (MOPS) (pH 7.0), 10 mM sodium acetate (pH 4.5), 10 mM EDTA (pH 8.0), 50% (vol/vol) formamide, and 2.2 M formaldehyde; incubated at 65°C for 15 min; and chilled on ice. To the RNA samples were then added 3 μl of a 50% (vol/vol) glycerol solution containing 0.25% (wt/vol) bromophenol blue and 1 μg of ethidium bromide. Equal amounts of genomic DNA (5 to 10 μg) were digested with BamHI (Boehringer, Mannheim, Germany). RNA and DNA samples were electrophoresed onto formaldehyde-agarose gels or native agarose gels, respectively, transferred onto nylon membranes (Hybond N; Amersham), and hybridized with probes radiolabelled by nick translation or random primer extension with the nick translation kit or the random-primed DNA labelling kit, respectively (Boehringer).
PCR analysis.
Cell pellets were resuspended in a lysis buffer containing 10 mM Tris-HCl (pH 8.0), 1% (vol/vol) polyoxyethylene-10 lauryl ether (Sigma), and 0.1 mg of proteinase K (Boehringer) per ml; incubated at 65°C for 2 h; and heat inactivated at 94°C for 15 min. Amounts of lysates corresponding to 105 cells were amplified with AmpliTaq-Gold or, for β-globin amplification (see below), AmpliTaq polymerase (Perkin-Elmer). Primers KS1/KS2 (nucleotides 987 to 1006 and 1200 to 1219 in the KS 330 BAM sequence [14]) were used to amplify DNA sequences from HHV-8 ORF 26 (VP23), and primers v-cycA and v-cycB (5′-ATC CTG CGG AAT GAC GTT GG-3′ and 5′-CCT GTT AGT GGC CAG TAA GC-3′, respectively) were used to amplify DNA sequences within HHV-8 ORF 72 (v-cycD). β-Globin sequences were amplified with primers GLA1 and GR2 (5′-CAA CTT CAT CCA CGT TCA CC-3′ and 5′-GAA GAG CCA AGG ACA GGT AC-3′, respectively). PCR conditions with primers v-cycA and v-cycB were 10 min at 94°C followed by 45 cycles of denaturation (94°C for 1 min), annealing (57°C for 1 min), and extension (72°C for 1 min); for β-globin the conditions were 10 min at 95°C followed by 35 cycles of denaturation (95°C for 30 s), annealing (65°C for 30 s), and extension (72°C for 1 min). The conditions used with primers KS1 and KS2 (45 cycles) were as described previously (14). The reaction mixtures contained 1.5 mM MgCl2. Oligonucleotides internal to the amplified sequences were used as probes for the detection of the PCR products from ORF 26 (14), ORF 72 (5′-AGG AAC CAA CAG CGC ACA GC-3′), or β-globin (5′-TTT AGT GAT GGC CTG GCT CAC CTG G-3′). PCR products were blotted onto Hybond N membranes and hybridized by standard techniques.
For semiquantitative PCR analysis, cell extracts or supernatants were serially diluted in a buffer containing 10 mM Tris-HCl (pH 7.8), 0.1 mM EDTA, and highly purified sonicated salmon sperm DNA (50 μg/ml). Aliquots of 5 μl were used for PCR amplification with primers KS1 and KS2 (BCBL-1 supernatants, 30 to 35 cycles) or primers v-cycA and v-cycB (PBMC extracts, 45 cycles). To ensure that the same relative amount of cells was analyzed, the same extract dilutions were amplified with primers for β-globin or β-actin (see below).
RT-PCR analysis.
Total RNA purified with the RNeasy Mini Kit was digested at 37°C three times in a buffer (100 μl) containing 10 mM sodium acetate (pH 4.8), 5 mM MgSO4, 5 mM MgCl2, and 20 U of RNase-free DNase (Boehringer) at 37°C and further purified with the RNeasy Kit as suggested by the manufacturer. cDNA was synthesized from total RNA (105 to 106 cells) with the reverse transcription system kit (Promega) by incubating the reaction mixtures with hexanucleotide random primers for 10 min at room temperature, 30 min at 42°C, and 30 min at 53°C. After heat inactivation of reverse transcriptase (RT), 1/3 of each reaction mixture was subjected to 45 cycles of PCR for VP23 or T0.7 whereas amplification of β-actin was performed with 1/15 of the RT reaction mixtures and 40 PCR cycles. Primers KS1 and KS2 were used for VP23 amplification, and primers RT-22A and RT-22B (5′-CAC CAT TCC TCT CCG CATTA-3′ and 5′-GTC TGC CGA AGT CAG TGC CA-3′, respectively) were used for T0.7 amplification with the same cycling conditions. To ensure the integrity of the samples, β-actin sequences were amplified with the primers BA1 and BA4 (5′-CAT GTG CAA GGC CGG CTT CG-3 and 5′-GAA GGT GTG GTG CCA GAT TT-3′, respectively) with a 10-min denaturation step at 95°C followed by 35 cycles of denaturation (94°C for 1.5 min), annealing (55°C for 30 s), and extension (72°C for 2 min). β-Actin PCR products were detected by ethidium bromide staining.
For MxA RT-PCR, cDNA underwent MxA-specific amplification by using a recently established assay (4). MxA-specific primers corresponding to nucleotides 1626 to 1914 of the published sequence were used (1). The amplified 289-bp product was identified by ethidium bromide staining of agarose gels.
Analysis of free virus in BCBL-1 supernatants.
BCBL-1 cells were cultured for 24 h in the presence or absence of IFN-α2b (25 IU/ml) and induced with TPA (20 ng/ml) for 5 days. Aliquots of supernatants corresponding to 3 × 104 cells were adjusted to 60 μl and digested twice with 20 U of RNase-free DNase at 37°C for 90 min in a buffer containing 40 mM Tris-HCl (pH 7.9), 10 mM NaCl, 6 mM MgCl2, and 10 mM CaCl2. The reaction was ended by adding EDTA (pH 8.0) to a final concentration of 50 mM and by rapid heating at 95°C for 10 min. The supernatants were adjusted to 100 μl by adding 40 μl of water containing polyoxyethylene 10-lauryl ether (1%, vol/vol) and digested with proteinase K (0.1 mg/ml) for 2 h at 65°C. After heat inactivation at 95°C for 10 min, the supernatants were diluted 1:100 in a buffer containing 10 mM Tris-HCl (pH 7.8), 0.1 mM EDTA, and highly purified sonicated salmon sperm DNA (50 μg/ml), and 5 μl was used as starting dilution for semiquantitative PCR analysis as described above.
IFN titration in supernatants of BCBL-1 cells and cultured PBMC.
IFN was titrated by measuring the virus yield reduction. Briefly, A549 cells were seeded in 96-well microtiter plates and incubated overnight with serially diluted (1:3.2) cell supernatants. The cells were then infected with the encephalomyocarditis virus (EMCV) at a high multiplicity of infection (>10) and incubated overnight. The antiviral activity was determined by assessing the end-point protection against the EMCV-induced cytopathic effect after staining the cell monolayers with crystal violet. The internal laboratory standard containing 100 IU of IFN-α2b per ml was calibrated against a reference standard of IFN-α (NIH Ga 023-902-530) and was included in each titration (37).
Anti-HHV-8 serologic testing.
BCBL-1 cells were treated for 48 h with 20 ng of TPA per ml. A 10-μl volume of suspension (4 × 106 cells/ml) was smeared on the coverslips, rapidly air dried, and fixed in acetone-methanol solution for 10 min. Fixed smears were incubated successively in two steps of 30 min each at 37°C with the serum samples diluted 1:5 (in duplicate) and with fluorescein-labeled, affinity-purified goat Ab to human immunoglobulin G (Kierkegaard & Perry Laboratories). Titer determinations were done by fivefold serial dilutions. All the microscopic examinations were conducted by two different investigators on coded samples in a blinded fashion. An inverse titer of 5 was considered positive in the presence of a bright cytoplasmic staining. No correlation was found between Epstein-Barr virus and HHV-8 Ab titers by this assay (65). Serum samples from 8- to 12-month old babies and HIV-seronegative KS patients were used as negative and positive controls, respectively.
ISH.
Cultured PBMC were harvested, centrifuged, washed twice, resuspended in phosphate-buffered saline, seeded onto Silan-coated slides, air dried, and fixed in 4% buffered paraformaldehyde as described previously (81). In situ hybridization (ISH) was performed as described previously, under high-stringency conditions (10) with strand-specific 35S-labeled VP23 RNA hybridization probes (specific activity, 109 cpm/μg) transcribed from plasmid p557-19 encompassing HHV-8 ORF 26 (10) or plasmid pBluescript-T0.7 encompassing the kaposin gene (81).
Transmission electron microscopy.
BCBL-1 cells were washed in phosphate-buffered saline, fixed in 2.5% glutaraldehyde in cacodylate buffer (0.1 M; pH 7.2) for 20 min at room temperature, and postfixed in 1% OsO4 in cacodylate buffer for 1 h at room temperature. Fixed cells were dehydrated through a graded series of ethanol solutions and embedded in Agar 100 (Agar Aids, Cambridge, United Kingdom). Serial ultrathin sections were collected on 200-mesh grids and then counterstained with uranyl acetate and lead citrate. Sections were analyzed with a Zeiss 902 electron microscope at 80 kV.
FACS analysis.
PBMC from a healthy volunteer were cultured as described above in the presence or absence of phytohemagglutinin (PHA) (3 μg/ml) and interleukin-2 (10 U/ml), anti-IFN-α Ab (100 IU/ml), or MAb 64G12 (2.5 μg/ml). Floating and adherent cells were harvested after 16 h and 2 and 7 days of culture and analyzed by fluorescence-activated cell sorting (FACS) with mouse MAbs conjugated with fluorescein isothiocyanate or phycoerythrin. Ungated cells were analyzed for size and antigen expression. Cells stained with fluorescein isothiocyanate- or phycoerythrin-conjugated isotype-matched Ab directed against irrelevant epitopes served as negative controls.
RESULTS
Induction of early and late viral genes by TPA treatment of BCBL-1 cells.
The effect of IFN-α on the HHV-8 life cycle was initially evaluated in BCBL-1 cells. These cells are latently infected with HHV-8 and can be induced to undergo viral lytic replication by treatment with TPA (64).
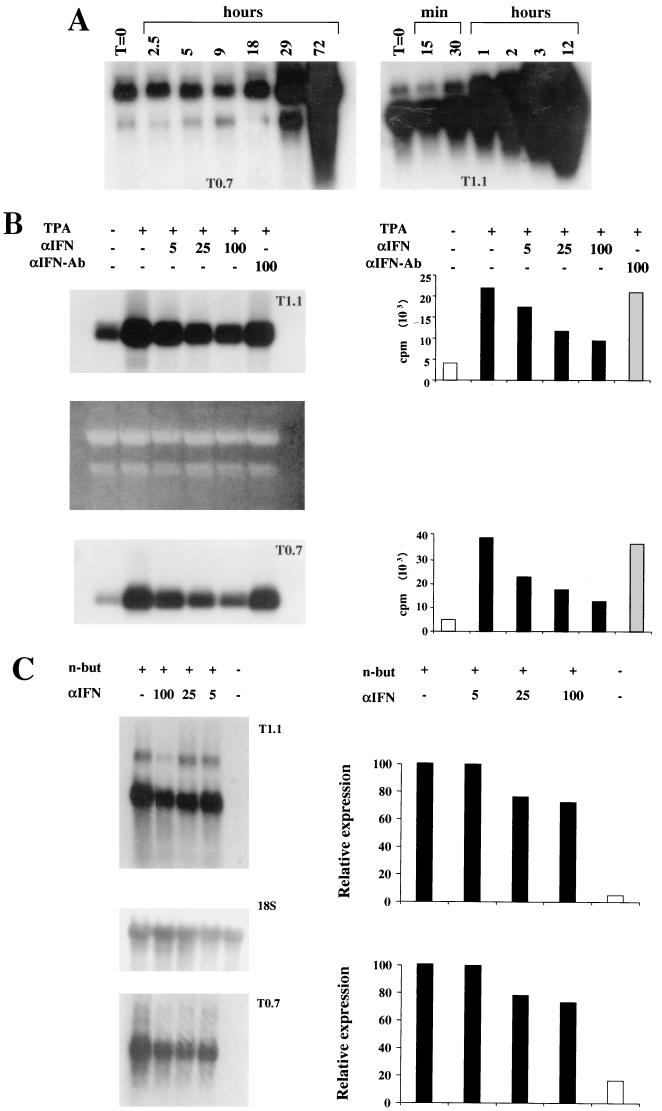
Before analyzing the effect of IFN-α on HHV-8 reactivation in these cells, the studies focused on identifying the viral genes induced early or late after TPA treatment. To this end, the kinetics of expression of HHV-8 genes including ORFs K12 and K7, encoding kaposin and nut-1 RNA, respectively (66), were examined by Northern blot hybridization. Kaposin RNA is expressed in latently infected cells but is upregulated upon induction of the HHV-8 lytic cycle, while nut-1 is a lytic viral nuclear RNA (70, 81, 82, 91). Both Kaposin RNA (T0.7) and nut-1 nuclear RNA (T1.1) were induced by TPA in BCBL-1 cells. Specifically, the levels of T0.7 RNA were unchanged in the first 18 h and increased at 29 h and up to 72 h after induction (Fig. 1A). By contrast, expression of T1.1 RNA was induced very early (at 30 to 60 min postinduction) and continued to rise during the following 12 h (Fig. 1A). Thus, T1.1 RNA was induced very early by TPA whereas T0.7 RNA, which behaves as a latency gene in uninduced cells, was up regulated by TPA with kinetics consistent with that of a late gene.
FIG. 1.
Inhibition of HHV-8 gene expression and DNA amplification by IFN-α in TPA-induced BCBL-1 or n-butyrate-induced BC-1 cells. (A) Kinetics of induction of kaposin (T0.7) and nut-1 (T1.1) genes in BCBL-1 cells. Cells were induced with TPA (20 ng/ml) and harvested at the indicated time points, and total RNA (10 μg) was analyzed by Northern blot hybridization with the probes pCRII-T0.7 and pCRII-T1.1 (B and C) Inhibition of T1.1 and T0.7 RNA expression by human recombinant IFN-α2b in BCBL-1 (B) and BC-1 (C) cells. The cells were cultured for 24 h in the presence or absence of the indicated amounts of IFN-α2b and subsequently induced with TPA (20 ng/ml; BCBL-1) or n-butyrate (3 mM; BC-1). After 48 h, the cells were harvested and total RNA (5 μg) was analyzed by Northern blot hybridization with the probes pCRII-T1.1 and pCRII-T0.7. (B) Dose-dependent inhibition of T1.1 and T0.7 gene expression by IFN-α2b. Total RNA was quantitated spectrophotometrically, and 28S and 18S rRNA were stained after electrophoresis with ethidium bromide as a loading control. The histograms show quantitation of T1.1 or T0.7 hybridization bands by Instant Imager (Packard, Meriden, Conn.). No or few effects were observed with anti-IFN-α Ab at 100 IU/ml. (C) Dose-dependent inhibition of HHV-8 gene expression in BC-1 cells by IFN-α2b. T0.7 and T1.1 hybridization bands were normalized by Instant Imager to the corresponding 18S rRNA hybridization bands (18S). Histograms represent the percentages of (normalized) counts per minute calculated by assuming that the signal from cells induced in the absence of IFN-α is equal to 100%. n-but, n-butyrate. (D) Southern blot hybridization of DNA extracted from the same number of BCBL-1 cells cultured for 48 h in the presence or absence of various amounts of IFN-α2b as indicated. DNA (10 μg) was digested with BamHI and hybridized with the pCRII-T0.7 probe. The right panel shows a quantitation of the hybridization bands by Instant Imager. No or few effects were observed with anti-IFN-α Ab at 100 IU/ml (data not shown).
Effects of IFN-α2b on HHV-8 latent and lytic infection of BCBL-1 cells.
To analyze the effect of IFN-α on HHV-8 infection, the same number of BCBL-1 cells was induced with TPA in the presence or absence of IFN-α2b or anti-IFN-αAb and analyzed for T1.1 and T0.7 gene expression by Northern blot hybridization. Expression of both T1.1 and T0.7 was inhibited in a dose-dependent manner by IFN-α2b, whereas anti-IFN-αAb had little or no effect (Fig. 1B).
To confirm that this effect was not due to interference of IFN-α with the TPA activation pathway, similar experiments were performed with another PEL cell line (BC-1) that was induced to undergo HHV-8 lytic infection by treatment with n-butyrate, as described previously (70). Expression of T1.1 and T0.7 was also inhibited by IFN-α2b in these cells as evaluated by normalizing the levels of T0.7 and T1.1 RNA to the hybridization bands obtained by reprobing the membranes to an 18S rRNA probe (Fig. 1C).
The effect of IFN-α was then determined on viral DNA amplification in BCBL-1 cells by Southern blot hybridization. As shown in Fig. 1C, IFN-α2b, but not anti-IFN-α Ab, inhibited viral DNA amplification by 70 to 80%. Specifically, upon TPA induction, cells cultured in the absence of IFN-α2b showed a two- to fourfold increase in viral DNA load (Fig. 1C). Since under these conditions only 10 to 15% of the cells express lytic antigens (reference 61 and data not shown) or yield viral progeny (see below), this corresponds to about a 20- to 40-fold amplification of viral DNA in the responsive cells, most of which is blocked by IFN-α. Thus, IFN-α inhibits the early phases of HHV-8 reactivation and impairs downstream steps of viral lytic replication, including late T0.7 gene expression and viral DNA amplification.
To evaluate the effect of IFN-α on latent HHV-8 infection, the viral DNA load in uninduced BCBL-1 cells cultured in the presence or absence of 5 or 50 IU of IFN-α2b per ml was analyzed. Cell cultures maintained in the presence or absence of IFN-α2b were counted and plated at the same cell density (3 × 105 cells/ml) for seven passages. At various passages, the cells were harvested, viable cells were counted by trypan blue dye exclusion, and equal amounts of DNA and total RNA were analyzed by Southern or Northern blotting for HHV-8 load and expression, respectively. No significant inhibitory effects of IFN-α on viral DNA load were observed. However, it should be noted that any change in viral DNA load in the small fraction of cells undergoing spontaneous reactivation would probably be masked by the viral DNA present in the large number of latently infected cells. Nevertheless, a mild dose-dependent inhibition of T1.1 gene expression, particularly at late cell passages, and a mild dose-dependent inhibition of cell growth were observed (data not shown).
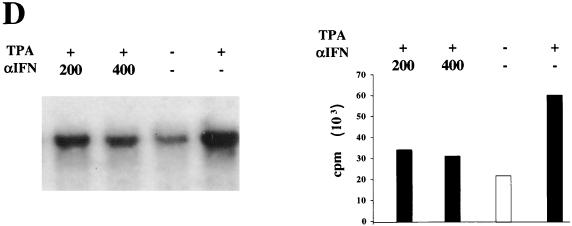
Responsiveness of latently and lytically infected BCBL-1 cells to IFN-α was determined by RT-PCR analysis of the MxA gene, whose expression is triggered specifically by type I IFNs and is associated with a strong antiviral response (5, 72, 78) (Fig. 2). MxA expression was undetectable in cells cultured in the absence of IFN-α but was induced at low levels by 1 IU of IFN-α2b per ml and efficiently stimulated at higher IFN-α concentrations (Fig. 2). Therefore, IFN-α did not inhibit latent HHV-8 infection in BCBL-1 cells, although the cells were responsive to exogenous IFN-α.
FIG. 2.
Induction of MxA gene expression by IFN-α2b in BCBL-1 cells. RT-PCR of total RNA from BCBL-1 cells not induced (A) or induced (B) with TPA and cultured for 24 h in the absence or presence of the indicated amounts of IFN-α2b is shown. MW, 50-bp DNA ladder; PC, positive control made with RNA from K562 cells induced with 100 IU of IFN-α per ml. A faint band that was difficult to reproduce photographically was observed in TPA-induced cells in the absence of IFN-α2b.
In addition, a faint RT-PCR band was evident in cultures induced by TPA. When increasing doses of IFN-α2b were added to TPA-induced cells, MxA expression was stimulated in a synergistic fashion and strong RT-PCR signals were observed at all IFN-α concentrations used (Fig. 2). These results suggest that the transduction pathways activated by IFN-α and TPA are not antagonistic and that HHV-8 reactivation and replication is inhibited by the strong antiviral response elicited by IFN-α.
Inhibition of virus production and release from BCBL-1 cells by IFN-α.
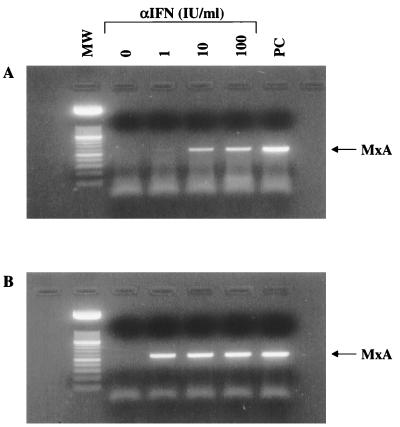
BCBL-1 cells induced for 5 days with TPA release large numbers of HHV-8 particles into the medium (39, 44). To analyze the effect of IFN-α on virus production, BCBL-1 cells were cultured for 24 h with or without IFN-α (25 IU/ml) and subsequently induced for 5 days with TPA. At the end of the induction, supernatants were harvested and extensively digested with pancreatic DNase I. After digestion, the supernatants were analyzed by limiting-dilution PCR to determine the relative amount of DNase-resistant (encapsidated) HHV-8 DNA. DNase-resistant DNA was about 25 times more abundant in TPA-induced BCBL-1 cells than in uninduced cells (Fig. 3f and e, respectively) and about 5 times less abundant in TPA-induced cells cultured in the presence of IFN-α (Fig. 3g and f, respectively). Thus, IFN-α caused a 80% reduction of virus release and/or production.
FIG. 3.
Inhibition of the production and/or release of free virus in supernatants from TPA-induced BCBL-1 cells by IFN-α2b. The cells were induced for 5 days and analyzed for cell viability and numbers by trypan blue exclusion. The proportions of viable (blue trypan-excluding) cells were 83% in the control (uninduced) culture, 72% in the TPA-induced culture, and 86% in the culture induced with TPA in the presence of αIFN. (a and b) Supernatants from TPA-induced BCBL-1 cells before (a) or after (b) DNase treatment. (c) DNase treatment of culture medium containing 100 ng of plasmid p1.8Kb encompassing the HHV-8 PCR target sequences. (d) Positive-control PCR performed with the indicated amounts of plasmid p1.8Kb. (e to g) DNase-treated supernatants from BCBL-1 cells uninduced (e) or induced (f and g) with TPA in the absence (e and f) or presence (g) of IFN-α2b (25 IU/ml). Lanes: 1, starting dilution corresponding to supernatants derived from 15 cells; 2 to 5, serial dilutions (1:5). DNase I digestion resulted in a fivefold decrease of HHV-8 DNA in both TPA-induced and -uninduced cell cultures (data not shown). Cells cultured for 24 h in the presence or absence of IFN-α2b (25 IU/ml) were induced with TPA (20 ng/ml) for 5 days, and supernatants were analyzed by semiquantitative PCR. Shown is the autoradiogram of the HHV-8 PCR products (primers KS1 and KS2) hybridized with a specific oligonucleotide probe.
Production of endogenous IFN-α by BCBL-1 cells and improvement of viral particle morphogenesis and virus yield in the presence of anti-IFN-α Ab.
Since most virally infected cells produce IFN activity, BCBL-1 cells were analyzed for the production of endogenous IFN. Serially diluted supernatants from BCBL-1 cells were added to human lung A549 cells, and after 24 h the cells were infected with EMCV to determine the capability of supernatants to inhibit viral infection (37). A small but measurable antiviral activity, ranging from 3 to 10 IU/ml and potentially including type I and II IFNs, was detected in the supernatants from both TPA-induced and uninduced BCBL-1 cells.
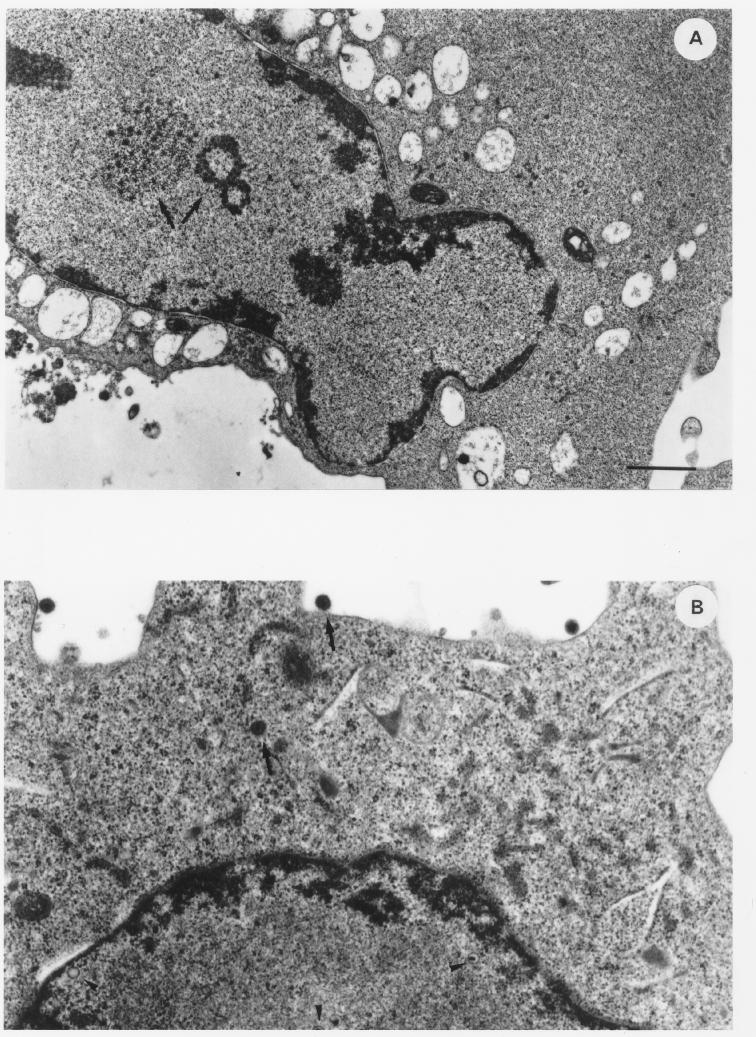
To evaluate the effect of this endogenous IFN-α on viral particle assembly and virus yield, BCBL-1 cells were cultured for 24 h in the presence or absence of anti-IFN-α Ab (100 IU/ml). The cells were then induced with TPA for 48 h in the presence of anti-IFN-α Ab and analyzed by transmission electron microscopy. Viral particles were found in both Ab-treated and untreated cells, and the percentage of cells undergoing viral lytic replication was similar under the two experimental conditions (10 of 92 and 15 of 92 cells in the absence and presence of anti-IFN-α Ab, respectively). However, considerable differences were observed in viral particle maturation, cytopathic effect, and number of viral particles per cell (Fig. 4). Specifically, numerous productively infected cells from untreated cultures showed only the early events of viral maturation, i.e., intranuclear aggregation of virus-specific electron-opaque material (Fig. 4A). These features were never observed in anti-IFN-α Ab-treated cultures, in which viral morphogenesis was in a more advanced phase (Fig. 4C and D), although individual viral particles in every stage of maturation were observed in both untreated (Fig. 4B) and treated (Fig. 4C and D) cells. Moreover, extracellular virions were more numerous in treated cells (Fig. 4C) than in untreated cells (Fig. 4B) and viral replication was frequently associated with a more extensive cytopathic effect in treated cells (Fig. 4D).
FIG. 4.
Thin-section electron micrographs of BCBL-1 cells induced with TPA (20 ng/ml) for 48 h in the absence (A and B) or presence (C and D) of anti-IFN-α Ab (100 IU/ml). Cells in various stages of particle maturation and cytopathic effects are shown. (A) Cell induced with TPA in the absence of anti-IFN-α Ab, showing intranuclear aggregation of virus-specific electron-opaque material (arrows). (B) Cell induced with TPA in the absence of anti-IFN-α Ab, showing HHV-8 particles in different stages of maturation (nucleocapsids [arrowheads] and complete virions [arrows]). (C) Cell induced with TPA in the presence of anti-IFN-α Ab, revealing capsids in various stages of packaging of viral DNA (arrowheads) and numerous complete virions at the cell surface. (D) Extensively lysed cell induced with TPA in the presence of anti-IFN-α Ab, in which nucleocapsids with typical hexagonal outlines and variable DNA cores in the nucleus and in the cytoplasm can still be observed. Bars, 1 μm.
Since anti-IFN-α Ab had no effects on HHV-8 gene expression (Fig. 1) or DNA replication, these results suggest that endogenous production of IFN-α can inhibit viral particle morphogenesis.
Production of IFN-α by cultured PBMC from HHV-8-infected individuals causes a decrease in the viral load that is counteracted by anti-IFN-α Ab.
To analyze the effect of IFN-α on HHV-8-infected primary cells, sera and PBMC from 28 patients with KS or at risk for KS (16 with AIDS-KS, 3 with C-KS, 1 with PT-KS, 2 PT, and 6 HIV+) were analyzed by an immunofluorescence assay or by PCR for the presence of anti-HHV-8 Ab or HHV-8 DNA, respectively (Table 1). All KS patients (15 of 15) and PT patients (2 of 2), and 80% of the HIV+ men (4 of 5) had anti-HHV-8 Ab, whereas a total of 31% of these patients (8 of 26) also contained detectable levels of HHV-8 DNA (Table 1). However, culture of these PBMC for 6 to 7 days resulted in the loss of viral DNA from these cells (see below). To verify whether this was associated with the production of endogenous IFN, supernatants of PBMC from 12 individuals (6 with AIDS-KS, 1 with C-KS, and 5 HIV+) were analyzed for the production of endogenous IFNs. All supernatants protected A549 cells from EMCV infection (IFN titers of 4 [range, 3 to 10] IU/ml), indicating that PBMC from these patients produced measurable levels of IFNs during culture.
TABLE 1.
Detection of HHV-8 DNA and HHV-8 serologic test results in PBMC from patients with KS or at risk for KS
| Diagnosis | No. of patients | HHV-8 DNA (no. positive/no. analyzed) (%)a | Anti-HHV-8 Ab (no. positive analyzed) (%)b |
|---|---|---|---|
| AIDS-KS | 16 | 5/16 (30) | 15/15 (100) |
| C-KS | 2 | 2/2 (100) | 1/1 (100) |
| PT-KS | 1 | 0/1 (0) | NDc |
| HIV+ | 6 | 1/6 (15) | 4/5 (80) |
| PT | 3 | 0/2 (0) | 2/2 (100) |
| Total | 28 | 8/27 (31) | 22/23 (95) |
PCR for HHV-8 detection was performed with primers specific for HHV-8 ORF 26 (VP 23) or ORF 13 (v-cycD) amplification.
Serological analysis by immunofluorescence was done as described in Materials and Methods with TPA-induced BCBL-1 cells.
ND, not done.
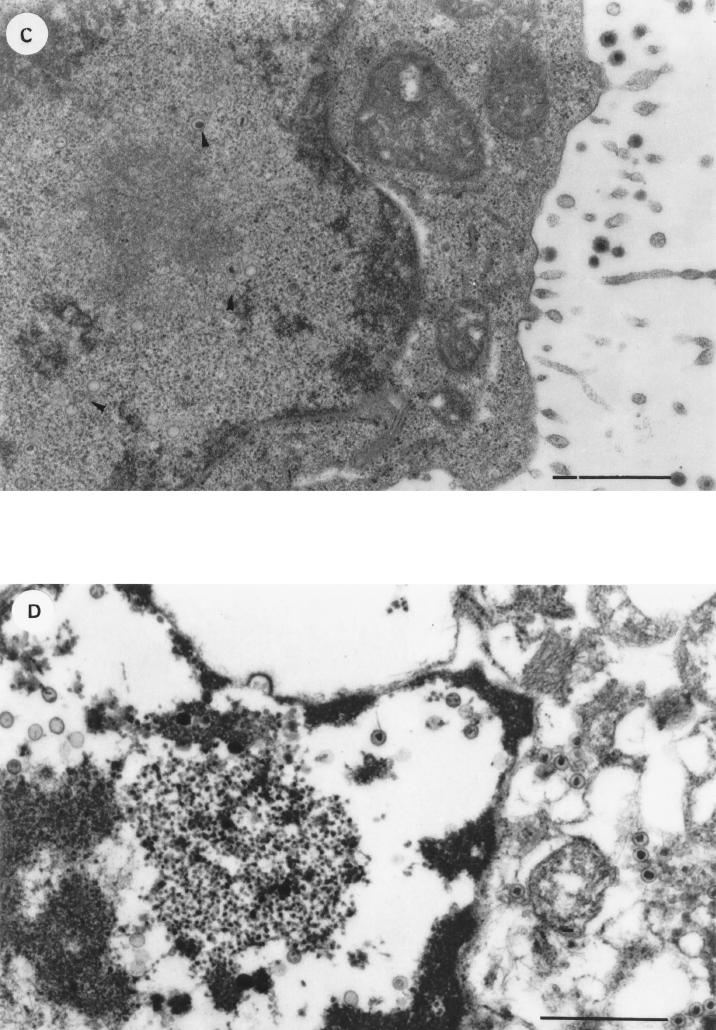
To determine whether endogenous IFN-α could account for the loss of viral DNA, PBMC from six PCR-positive patients (four with AIDS-KS, one with C-KS, and one HIV+) were cultured in the presence or absence of anti-IFN-α Ab (100 IU/ml). Anti-IFN-α Ab maintained or increased the viral DNA load in PBMC from five of these patients (Fig. 5 and data not shown). In particular, in the absence of anti-IFN-α Ab, PBMC from four patients showed a loss or dramatic reduction in the HHV-8 DNA load after culture. In contrast, in the presence of anti-IFN-α Ab, they maintained the viral DNA or load (Fig. 5A and B and data not shown). Two patients positive in both serologic tests and PCR in previous bleedings (one C-KS patient and one AIDS-KS patient) tested PCR negative at the time of this analysis. However, in the C-KS patient, culture with anti-IFN-α Ab increased the DNA load to levels detectable by PCR (Fig. 5C). In addition, PBMC from the other PCR-positive C-KS patient were cultured in the presence or absence of MAb 64G12, a MAb that acts as an antagonist for the type I IFN receptor (23). Similarly to anti-IFN-α Ab, MAb 64G12 was able to maintain the HHV-8 DNA load at levels higher than those obtained with the culture medium alone (Fig. 5D). Maintenance of viral DNA was observed in both floating and adherent cells from one AIDS-KS patient (Fig. 5A) and in the floating cells from the HIV+ patient (Fig. 5B). PBMC from the other patients were collected as bulk cells.
FIG. 5.
Effect of anti-IFN-α Ab and MAb 64G12 on the HHV-8 DNA load in PBMC cultured from patients with AIDS-KS (A), HIV infection (B), or C-KS (C and D). Cells were analyzed prior to culture (PBMC) or after culture for 3 days (A) or 6 days (B to D) in the absence (RPMI) or presence of anti-αIFN Ab (αIFN-Ab; 100 IU/ml) or MAb 64G12. (A and B) Floating and adherent cells were harvested separately. (C and D) Cells were harvested in bulk. Aliquots of cell lysates corresponding to 105 or 104 cells were analyzed by PCR with primers specific for HHV-8 ORF 26 or β-globin (data not shown), respectively. Panel D shows a serial-dilution PCR analysis of PBMC from a C-KS patient, cultured in the absence (RPMI) or presence of the indicated amounts of MAb 64G12. Numbers above the lanes represent dilution factors. UND, undiluted. The β-actin gene was also amplified from the same cell extracts by serial dilution PCR to ensure that the same number of cells from all culture conditions was analyzed (data not shown). HHV-8 PCR products were hybridized with an oligonucleotide probe internal to the amplified sequences. NC, negative control made with salmon sperm DNA; PC, positive-control PCR performed with the indicated number of molecules of plasmid p1.8Kb or with DNA (1 ng) from a KS lesion. All specimens were positive for β-globin amplification visualized by ethidium bromide staining (data not shown).
PBMC from two of the above patients yielded enough cells to allow a parallel RT-PCR analysis and were therefore analyzed for the expression of the MxA gene. Both patients gave positive results for MxA RNA, further supporting the idea that the effects elicited by the Abs were due to the neutralization of endogenous IFN-α.
To rule out the possibility that the effects of the anti-IFN-α Ab on HHV-8 DNA load were due to the activation of PBMC by the antibody preparations, cells from a healthy donor were cultured in the presence or absence of anti-IFN-α Ab, MAb 64G12, or PHA and interleukin-2 and analyzed by FACS for the expression of activation markers. As expected, PHA induced a dramatic up regulation of all the activation markers analyzed, including HLA-DR, CD25, CD30, and CD86. By contrast, no difference in the pattern of expression of the above markers was detected in PBMC cultured for either 16 h or 2 or 7 days in the presence of either Ab preparation compared to PBMC cultured with medium alone (data not shown).
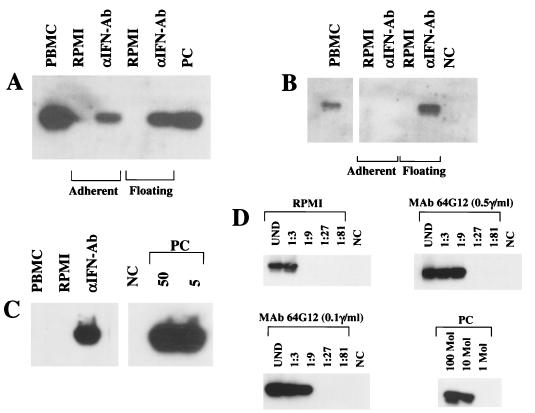
To determine the effect of IFN-α on the HHV-8 load, PBMC from two AIDS-KS patients were cultured for 3 to 6 days in the presence or absence of IFN-α2b (100 IU/ml). A 10-fold reduction in the viral load was observed in PBMC from one patient after 6 days of culture, and another 2-fold reduction was induced by IFN-α, as determined by serial dilution PCR (Fig. 6). The other patient showed a reduction of the PCR signal only after culture with 500 IU of IFN-α2b per ml (data not shown). These data suggested that, due to the production of endogenous IFN, relatively high concentrations of IFN-α2b are required to reduce the HHV-8 load in cultured PBMC.
FIG. 6.
HHV-8 DNA load in pBMC from an AIDS-KS patient before (day 0) or after (day 6) culture in the presence (αIFN) or absence (RPMI) of IFN-α2b (100 IU/ml). Shown is the autoradiogram of a serial-dilution PCR experiment performed with primers specific for HHV-8 ORF 72 (v-cycD) (A) or for the human β-globin gene (B). PCR products were hybridized with specific oligonucleotide probes. Numbers above the lanes represent dilution factors (A) or aliquots of cell extracts corresponding to the indicated number of cells (B). NC, negative control made with salmon sperm DNA; PC, positive-control PCR performed with the indicated number of the control plasmid pCRII-v-cycD (A) or 250 pg of human DNA from a uterine cervical carcinoma cell line (B).
T0.7 gene expression in PBMC cultured in the presence of anti-IFN-α Ab.
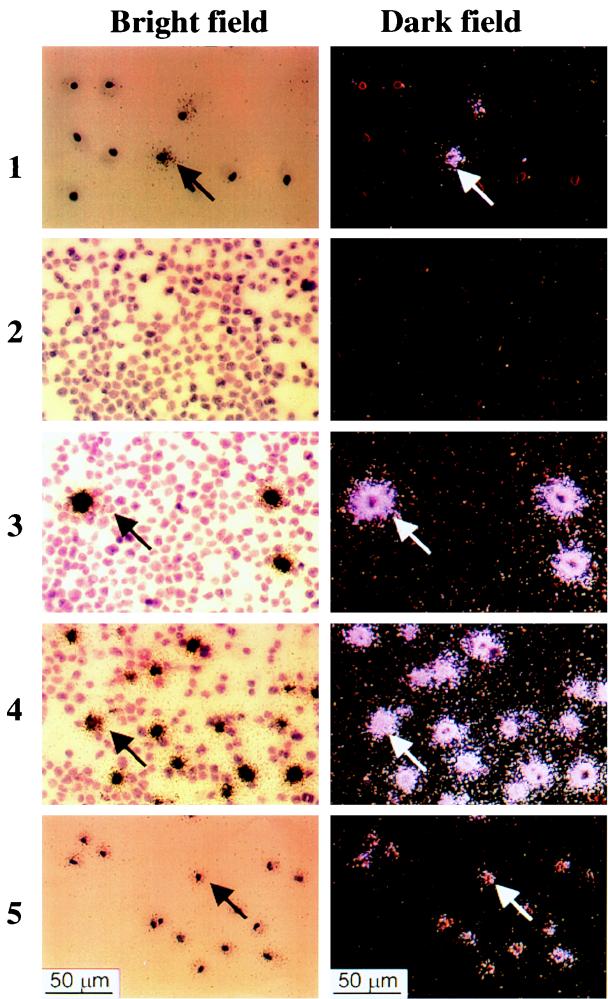
To verify the effect of anti-IFN-α Ab on HHV-8 gene expression, PBMC from two of the PCR-positive AIDS-KS patients were analyzed by RT-PCR and ISH for the expression of T0.7 and VP23 mRNA after culture with or without anti-IFN-α Ab (100 IU/ml). Expression of T0.7 but not VP23 was detected by RT-PCR and, in one patient, also by ISH (Fig. 7 and data not shown). By contrast, no T0.7 or VP23 expression was detected in cells cultured without anti-IFN-α Ab (data not shown). The same PBMC showed a dramatic decrease in or complete loss of HHV-8 DNA upon culture, which was counteracted by anti-IFN-α Ab. These results suggest that neutralization of endogenous IFN-α results in an increase in the viral load that may be associated with HHV-8 latent infection in cultured PBMC.
FIG. 7.
ISH with an antisense riboprobe for T0.7 mRNA (bright-field results on the left, dark-field results on the right) of PBMC from an AIDS-KS patient after culture with anti-IFN-α Ab. (Panels 1) PBMC from the AIDS-KS patient cultured for 7 days in the presence of anti-IFN-α. The arrow points to a cell showing T0.7 signals. Cells were negative after ISH with a VP23 antisense riboprobe (data not shown). (Panels 2). Negative control made with Jurkat cells. (Panels 3). TPA-induced BCBL-1 cells hybridized with a VP23 antisense probe as a control of the ability of ISH to detect VP23 expression under the conditions used. (Panels 4) TPA-induced BCBL-1 hybridized with the T0.7 probe. (Panels 5) PBMC from the AIDS-KS patient hybridize with a probe for β-actin mRNA. No T0.7 signals were observed in the adherent cells from the patient after culture without anti-IFN-α Ab (data not shown). PBMC were negative after ISH with a VP23 antisense riboprobe (data not shown).
DISCUSSION
IFN-α has been successfully used in the therapy of various clinical forms of KS, including AIDS-KS and C-KS, that are associated with HHV-8 infection (16, 36, 42, 57, 86). Type I IFNs are known to inhibit the replication of alpha-, beta-, and gammaherpesviruses, including herpesvirus saimiri and Epstein-Barr virus that are closely related to HHV-8 (2, 7, 35, 47, 83, 85, 89). However, no information is available on the effect of IFN-α on the life cycle of HHV-8. The results of this study show that IFN-α inhibits HHV-8 reactivation in PEL-derived cells and reduces the HHV-8 load in PBMC from patients with KS or at risk of contracting KS.
IFN-α had strong inhibitory effects on HHV-8 gene expression, DNA amplification, and viral particle release from PEL cells induced with TPA or n-butyrate. In particular, it inhibited the expression in BCBL-1 cells of the HHV-8 nut-1 gene, whose activation appears to be an early event triggered by TPA. In addition, blocking of endogenous IFN-α with neutralizing Ab resulted in a more extensive cytopathic effect and in a more efficient viral particle morphogenesis in these cells. Since anti-IFN-α Ab did not increase late (kaposin) viral gene expression or DNA amplification, these data suggest that endogenous IFN-α produced by BCBL-1 cells had inhibitory effects on virus particle maturation. In addition, HHV-8 gene expression was inhibited in n-butyrate-induced BC-1 cells. Since TPA and butyrate are known to activate different pathways (31, 46), it is likely that the effects of IFN-α were specifically directed against HHV-8 replication. Thus, as for other herpesviruses, both early and late stages of the HHV-8 life cycle may be targets of IFN-α antiviral activity (19, 52, 58, 60). Consistent with these data, low doses of human recombinant IFN-α2b induced the expression of the gene encoding MxA, which displays strong antiviral effects and is specifically triggered by type I IFNs. IFN-α, however, did not inhibit HHV-8 latent infection in BCBL-1 cells.
Anti-IFN-α Ab also had striking effects on HHV-8 infection of cultured PBMC from KS patients or individuals at risk for KS. Cultured PBMC from these individuals produced endogenous IFN-α that caused a reduction in the viral load. In fact, anti-IFN-α Ab and MAb 64G12 maintained or increased viral load during culture. This was associated with the expression of the latency-associated kaposin gene but not with the lytic VP23, suggesting that IFN-α may be able to inhibit latent HHV-8 infection in cultured PBMC. In addition, exogenous IFN-α reduced the viral DNA load in cultured PBMC. Further studies are required to determine whether these mechanisms are also operative in vivo.
HHV-8 encodes a homolog of the interferon regulatory factor (IRF) family members (32, 66, 90). Expression of cloned HHV-8 IRF (v-IRF) inhibits IRF-mediated gene expression (32, 90), suggesting that HHV-8 may use this homolog as a decoy to escape IFN-mediated antiviral effects. However, the ability of IFN to inhibit HHV-8 infection in BCBL-1 cells and PBMC indicates that, in the context of the viral genome, v-IRF may have other, as yet unknown functions.
KS onset is associated with a high HHV-8 load in PBMC (18, 38), suggesting that HHV-8-infected circulating cells play an important role in KS development (10, 30, 34, 75). This may reflect the ability of circulating cells, including B cells, T cells, monocytes, and circulating spindle cell progenitors, to infiltrate or localize into tissues and to transmit the virus to other cell types (30, 34, 76). The inhibitory effects of IFN-α on the HHV-8 load in PBMC, its known immunomodulatory effects (reviewed in references 8 and 84), and its ability to inhibit angiogenic factors with a key role in KS development may explain its efficacy as monotherapy for all forms of KS. Since lymphocytes and monocytes infiltrating KS lesions are productively infected by HHV-8 (10, 61) and since antiherpetic drugs have strong inhibitory effects on herpesvirus lytic infection, the use of IFN-α in combination with these antiviral drugs may be an important tool for the control of productive and latent HHV-8 infection in vivo and for the clinical management of patients with all forms of KS.
ACKNOWLEDGMENTS
This work was supported by grants from the Progetto Sangue and the IX AIDS Project from the Italian Ministry of Health and by a grant from the “Associazione Italiana per la Ricerca sul Cancro” (AIRC, Milan) to B. Ensoli, by a grant from the Italian Ministry of Education and Research (MURST 40%) to F. Dianzani, by a grant from the Deutsche Forschungsgemeinschaft (SFB 464) and the German Ministry of Education, Science, Research and Technology (BMBF) (BioFuture program) to M. Stürzl, and by the European Concerted Action “Pathogenesis of AIDS-KS.”
We thank A. Wunderlich (GSF, Neuherberg) for technical help and Angela Lippa, Federica Maria Regini, and Alessandra Neri for editorial assistance.
REFERENCES
- 1.Aebi M, Fab J, Hurt N, Samuel C E, Thomis D, Bazzigher L, Pavlovoc J, Haller O, Staeheli P. cDNA structures and regulation of two interferon-induced Mx proteins. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altinkilic B, Brandner G. Interferon inhibits herpes simplex virus-specific translation: a reinvestigation. J Gen Virol. 1988;69:3107–3112. doi: 10.1099/0022-1317-69-12-3107. [DOI] [PubMed] [Google Scholar]
- 3.Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- 4.Antonelli G, Simeoni E, Solmone M, Antonelli L, Iaiani G, Dianzani F. In vitro and in vivo detection of IFN-induced MxA and MxB mRNA by RT-PCR. Eur Cytokine Net. 1996;7:494. [Google Scholar]
- 5.Arnhetier H, Skuntz S, Noteborn M, Chang S, Meier E. Transgenic mice with intracellular immunity to influenza virus. Cell. 1990;62:51–61. doi: 10.1016/0092-8674(90)90239-b. [DOI] [PubMed] [Google Scholar]
- 6.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Ceserman E, Gershengorn M C, Mesri E A. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 7.Beilharz M W, McDonald W, Watson M W, Heng J, McGeachie J, Lawson C M. Low-dose oral type I interferons reduce early virus replication of murine cytomegalovirus in vivo. J Interferon Cytokine Res. 1997;17:725–630. doi: 10.1089/jir.1997.17.625. [DOI] [PubMed] [Google Scholar]
- 8.Belardelli F, Gresser I. The neglected role of type I interferon in the T-cell response: implications for its clinical use. Immunol Today. 1996;17:369–372. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 9.Beral V. Epidemiology of Kaposi’s sarcoma. Cancer Surv. 1991;10:5–22. [PubMed] [Google Scholar]
- 10.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer N H, Tschachler E, Colombini S, Ensoli B, Stürzl M. Monocytes in Kaposi’s sarcoma lesions are productively infected by human herpesvirus-8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boshoff C, Whitby D, Hatzioannou T, Fisher C, van der Walt J, Hatzakis J, Weiss R, Schulz T. Kaposi’s sarcoma-associated herpesvirus in HIV-negative Kaposi’s sarcoma. Lancet. 1995;345:1043–1044. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- 12.Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Scweickart V L, Siani M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–292. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 13.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 15.Cordero E, Lopez-Cortes F L, Viciana P, Alarcon A, Pachon J. Foscarnet and AIDS-associated Kaposi’s sarcoma. AIDS. 1997;11:1787–1788. [PubMed] [Google Scholar]
- 16.Costa da Cunha C S, Lebbe C, Rybojad M, Agbalika F, Ferchal F, Rabian C, Vignon-Pennamen M D, Calvo F, Morel P. Long-term follow-up of non-HIV Kaposi’s sarcoma treated with low-dose recombinant interferon alfa-2b. Arch Dermatol. 1996;132:285–290. doi: 10.1001/archderm.132.3.285. [DOI] [PubMed] [Google Scholar]
- 17.Davis M A, Stürzl M, Blasig C, Schreier A, Guo H G, Reitz M, Opalenik S R, Browning P J. Expression of human herpesvirus 8-encoded cylin D in Kaposi’s sarcoma spindle cells. J Natl Cancer Inst. 1997;89:1868–1871. doi: 10.1093/jnci/89.24.1868. [DOI] [PubMed] [Google Scholar]
- 18.Decker L L, Shankar P, Khan G, Freeman R B, Dezube B J, Lieberman J, Thorley-Lawson D A. The Kaposi’s sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184:283–288. doi: 10.1084/jem.184.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delcayre A X, Lotz M, Lernhardt W. Inhibition of Epstein-Barr virus-mediated capping of CD21/CR2 by alpha interferon (IFN-α): immediate antiviral activity of IFN-α during the early phase of infection. J Virol. 1993;67:2918–2921. doi: 10.1128/jvi.67.5.2918-2921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Lellis L, Fabris M, Cassai F, Corallini A, Giraldo G, Feo C, Monini P. Herpesvirus-like DNA sequences in non-AIDS Kaposi’s sarcoma. J Infect Dis. 1995;172:1605–1607. doi: 10.1093/infdis/172.6.1605. [DOI] [PubMed] [Google Scholar]
- 21.De Wit R, Shattenkerk J K M E, Boucher C A B, Bakker P J M, Veenhof K H N, Danner S A. Clinical and virological effects of high-dose recombinant inteferon-alpha in disseminated AIDS-related Kaposi’s sarcoma. Lancet. 1988;ii:1214–1217. doi: 10.1016/s0140-6736(88)90810-0. [DOI] [PubMed] [Google Scholar]
- 22.Dorfman R F, Path F R C. The histogenesis of Kaposi’s sarcoma. Lymphology. 1984;17:76–77. [PubMed] [Google Scholar]
- 23.Eid P, Tovey M G. Characterization of a domain of a human type I interferon receptor protein involved in ligand binding. J Interferon Cytokine Res. 1995;15:295–211. doi: 10.1089/jir.1995.15.205. [DOI] [PubMed] [Google Scholar]
- 24.Ensoli B, Markham P, Kao V, Barillari G, Fiorelli V, Gendelman R, Raffeld M, Zon G, Gallo R C. Block of AIDS-Kaposi’s sarcoma (KS) cell growth, angiogenesis and lesion formation in nude mice by antisense oligonucleotides targeting basic fibroblast growth factor: a novel strategy for the therapy of KS. J Clin Investig. 1994;94:1736–1746. doi: 10.1172/JCI117521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ensoli B, Stürzl M. Kaposi’s sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998;9:63–83. doi: 10.1016/s1359-6101(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 26.Ensoli B, Gallo R C. Growth factors in AIDS-associated Kaposi’s sarcoma: cytokines and HIV-1 Tat protein. AIDS Updates. 1994;7:1–12. [Google Scholar]
- 27.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld R, Cafaro A, Chang H K, Brady J N, Gallo R C. Synergy between basic fibroblast growth factor and human immunodeficiency virus type 1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 28.Feduchi E, Carrasco L. Mechanism of inhibition of HSV-1 replication by tumor necrosis factor and inteferon-γ. Virology. 1991;180:822–825. doi: 10.1016/0042-6822(91)90100-p. [DOI] [PubMed] [Google Scholar]
- 29.Feduchi E, Alonso A M, Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989;63:1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorelli V, Gendelman R, Sirianni M C, Chang H K, Colombini S, Markham P D, Monini P, Sonnabend J, Pintus A, Gallo R C, Ensoli B. γ-Interferon produced by CD8+ T cells infiltrating Kaposi’s sarcoma induces spindle cells with angiogenic phenotype and synergy with HIV-1 Tat protein: an immune response to HHV-8 infection? Blood. 1998;91:956–967. [PubMed] [Google Scholar]
- 31.Flemington E, Speck S H. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1217–1226. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao S, Boshoff C, Javachandra S, Weiss R A, Chang Y, Moore P S. KSHV ORF K9 (vIRF) in an oncogene which inhibits the interferon signaling pathway. Oncogene. 1997;15:1979–1985. doi: 10.1038/sj.onc.1201571. [DOI] [PubMed] [Google Scholar]
- 33.Gao S, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Eng J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 34.Goletti, D., P. Monini, S. Colombini, P. Leone, A. Cafaro, M. Stürzl, M. Franco, P. Leone, F. Citterio, R. Little, and B. Ensoli. Expanded tropism of Human Herpesvirus-8 for hematopoietic-derived cells upon Kaposi’s sarcoma development. Submitted for publication.
- 35.Gribaudo G, Ravaglia S, Gaboli M, Gariglio M, Cavallo R, Landolfo S. Interferon-alpha inhibits the murine cytomegalovirus immediate-early gene expression by down-regulating NF-kappa B activity. Virology. 1995;211:251–260. doi: 10.1006/viro.1995.1398. [DOI] [PubMed] [Google Scholar]
- 36.Gridelli C, Palmieri G, Airoma G, Incoronato P, Pepe R, Barra E, Bianco A R. Complete regression of laryngeal involvement by classic Kaposi’s sarcoma with low-dose alpha-2b interferon. Tumori. 1990;76:292–293. doi: 10.1177/030089169007600318. [DOI] [PubMed] [Google Scholar]
- 37.Hankel H, Capobianchi M R, Frezza F, Castilletti C, Dianzani F. Interferon induction by HIV-1-infected cells: a possible role of sulfatides or related glycolipids. Virology. 1996;221:113–119. doi: 10.1006/viro.1996.0357. [DOI] [PubMed] [Google Scholar]
- 38.Harrington W J, Bagasra O, Sosa C E, Bobroski L E, Baum M, Wen X L, Cabral L, Byrne G E, Pomerantz R J, Wood C. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi’s sarcoma patients. J Infect Dis. 1996;174:1101–1105. doi: 10.1093/infdis/174.5.1101. [DOI] [PubMed] [Google Scholar]
- 39.Kedes D H, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. J Clin Investig. 1997;9:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kledal T N, Rosenkilde M M, Coulin F, Simmons G, Johnsen A H, Alouani S, Power C A, Luttichau H R, Gerstoft J, Clapham P R, Clark-Lewis I, Wells T N C, Schwartz T W. A broad-spectrum chemokine antagonist encoded by Kaposi’s sarcoma-associated herpesvirus. Science. 1997;277:1656–1659. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 41.Köster R, Blatt L M, Streubert M, Zietz C, Hermeking H, Brysch W, Stürzl M. Consensus-interferon and platelet-derived growth factor adversely regulate proliferation and migration of Kaposi’s sarcoma cells by control of c-myc expression. Am J Pathol. 1996;149:1871–1885. [PMC free article] [PubMed] [Google Scholar]
- 42.Krown S E. Interferon-alpha: evolving therapy for AIDS-associate Kaposi’s sarcoma. J Interferon Cytokine Res. 1998;18:209–214. doi: 10.1089/jir.1998.18.209. [DOI] [PubMed] [Google Scholar]
- 43.Krown S E, Real F X, Cunningham-Rundles S, Myskowski P, Koziner B, Fein S, Mittelman A, Oettgen H F, Safai B. Preliminary observations on the effect of recombinant leukocyte A interferon in homosexual men with Kaposi’s sarcoma. N Engl J Med. 1983;308:1071–1076. doi: 10.1056/NEJM198305053081806. [DOI] [PubMed] [Google Scholar]
- 44.Lagunoff M, Ganem D. The structure and coding organization of the genomic termini of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) Virology. 1997;236:147–154. doi: 10.1006/viro.1997.8713. [DOI] [PubMed] [Google Scholar]
- 45.Lucin P, Jonjic S, Messerle M, Polic B, Hengel H, Koszinowski U H. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumor necrosis factor. J Gen Virol. 1994;75:101–110. doi: 10.1099/0022-1317-75-1-101. [DOI] [PubMed] [Google Scholar]
- 46.Luka J, Kallin B, Klein G. Induction of Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979;94:228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- 47.Lvovsky E, Levine P H, Fuccillo D, Ablashi D V, Bengali Z H, Armstrong G R, Levy H B. Epstein-Barr virus and herpesvirus saimiri: sensitivity to interferons and interferon inducers. JNCI. 1981;66:1013–1019. doi: 10.1093/jnci/66.6.1013. [DOI] [PubMed] [Google Scholar]
- 48.MacPhail L A, Dekker N P, Regezi J A. Macrophages and vascular adhesion molecules in oral Kaposi’s sarcoma. J Cutaneous Pathol. 1996;23:464–472. doi: 10.1111/j.1600-0560.1996.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 49.McNutt N S, Fletcher V, Conant M A. Early lesions of Kaposi’s sarcoma in homosexual men. An ultrastructural comparison with other vascular proliferations in skin. Am J Pathol. 1983;111:62–77. [PMC free article] [PubMed] [Google Scholar]
- 50.Medveczky M M, Horvath E, Lund T, Medveczky P G. In vitro antiviral drug sensitivity of the Kaposi’s sarcoma-associated herpesvirus. AIDS. 1997;11:1327–1332. doi: 10.1097/00002030-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Mesri E A, Cesarman E, Arvanitakis L, Rafii S, Moore M A, Posnett D N, Knowles D M, Asch A S. Human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med. 1996;183:2385–2390. doi: 10.1084/jem.183.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mittnacht S, Straub P, Kirchner H, Jacobsen H. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology. 1988;164:201–210. doi: 10.1016/0042-6822(88)90637-x. [DOI] [PubMed] [Google Scholar]
- 53.Moore P S, Chang Y. Detection of herpesvirus-like sequences in Kaposi’s sarcoma in patients with and those without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 54.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 55.Moore P S, Kingsley L, Holmberg S D, Spira T, Gupta P, Hoover D R, Parry J P, Conley L J, Jaffe H W, Chang Y. Kaposi’s sarcoma-associated herpesvirus infection prior to onset of Kaposi’s sarcoma. AIDS. 1996;10:175–180. doi: 10.1097/00002030-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Morfeldt L, Torssander J. Long-term remission of Kaposi’s sarcoma following Foscarnet treatment in HIV-infected patients. Scand J Infect Dis. 1994;26:749–752. doi: 10.3109/00365549409008645. [DOI] [PubMed] [Google Scholar]
- 57.Morris A, Valley A W. Overview of the management of AIDS-related Kaposi’s sarcoma. Ann Pharmacother. 1996;30:1150–1163. doi: 10.1177/106002809603001015. [DOI] [PubMed] [Google Scholar]
- 58.Munoz A, Carrasco L. Formation of non-infective herpesvirus particles in cultured cells treated with human interferon. J Gen Virol. 1984;65:1069–1078. doi: 10.1099/0022-1317-65-6-1069. [DOI] [PubMed] [Google Scholar]
- 59.Neipel F, Albrecht J C, Fleckenstein B. Cell-homologous genes in the Kaposi’s sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oberman F, Panet A. Inhibition of transcription of herpes simplex virus immediate early genes in interferon-treated human cells. J Gen Virol. 1988;69:1167–1177. doi: 10.1099/0022-1317-69-6-1167. [DOI] [PubMed] [Google Scholar]
- 61.Orenstein J M, Alkan S, Blauvelt A, Jeang K T, Weinstein M D, Ganem D, Herndier B. Visualization of human herpesvirus type 8 in Kaposi’s sarcoma by light and transmission electron microscopy. AIDS. 1997;11:35–45. doi: 10.1097/00002030-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Real F X, Oettgen H F, Krown S E. Kaposi’s sarcoma and the acquired immunodeficiency syndrome: treatment with high and low doses of recombinant leukocyte A interferon. J Clin Oncol. 1986;4:544–551. doi: 10.1200/JCO.1986.4.4.544. [DOI] [PubMed] [Google Scholar]
- 63.Regezi S A, MacPhail L A, Daniels T E, De Souza Y G, Greenspan J S, Greenspan D. Human immunodeficiency virus-associated oral Kaposi’s sarcoma: a heterogeneous cell population dominated by spindle-shaped endothelial cells. Am J Pathol. 1993;43:240–249. [PMC free article] [PubMed] [Google Scholar]
- 64.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 65.Rezza, G., M. Andreoni, M. Dorrucci, P. Pezzotti, P. Monini, R. Zerboni, B. Salassa, V. Colangeli, L. Sarmati, E. Nicastri, A. Sinicco, R., Pristerà, G. Angarano, F. Aiuti, L. Ortona, and B. Ensoli. Human herpesvirus-8 seropositivity and risk of developing Kaposi’s sarcoma and other AIDS-related diseases among individuals with known dates of HIV seroconversion. J. Natl. Cancer Inst., in press. [DOI] [PubMed]
- 66.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y. Nucleotide sequence of the Kaposi’s sarcoma-associated herpesvirus (HHV-8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Safai B, Good R A. Kaposi’s sarcoma: a review and recent developments. Clin Bull. 1980;10:62–69. [PubMed] [Google Scholar]
- 68.Samaniego F, Markham P D, Gendelman R, Gallo R C, Ensoli B. Inflammatory cytokines induce endothelial cells to produce and release basic fibroblast growth factor and to promote Kaposi’s sarcoma-like lesions in nude mice. J Immunol. 1997;158:1887–1894. [PubMed] [Google Scholar]
- 69.Samaniego F, Markham P D, Gendelman R, Watanabe Y, Kao V, Kowalski K, Sonnabend J A, Pintus A, Gallo R C, Ensoli B. Vascular endothelial growth factor and basic fibroblast growth factor are expressed in Kaposi’s sarcoma and synergize to induce angiogenesis, vascular permeability and KS lesion development: induction by inflammatory cytokines. Am J Pathol. 1998;152:1433–1443. [PMC free article] [PubMed] [Google Scholar]
- 70.Sarid R, Fiore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schalling M, Ekman M, Kaaya E E, Linde A, Biberfeld P. A role for a new herpesvirus (KSHV) in different forms of Kaposi’s sarcoma. Nature Med. 1995;1:707–708. doi: 10.1038/nm0795-707. [DOI] [PubMed] [Google Scholar]
- 72.Sen G C, Ransohoff R M. Interferon-induced antiviral actions and their regulation. Adv Virus Res. 1993;42:57–102. doi: 10.1016/s0065-3527(08)60083-4. [DOI] [PubMed] [Google Scholar]
- 73.Singh R K, Gutman M, Bucana C D, Sanchez R, Llansa N, Fidler I J. Interferons alpha and beta down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Nat Acad Sci USA. 1995;92:4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sirianni M C. Human herpesvirus-8 DNA sequences in CD8+ T cells. J Infect Dis. 1997;176:541. doi: 10.1086/514072. [DOI] [PubMed] [Google Scholar]
- 75.Sirianni M C, Vincenzi L, Fiorelli V, Topino S, Scala E, Uccini S, Angeloni A, Faggioni A, Cerimele D, Cottoni F, Aiuti F, Ensoli B. γ-Interferon production in peripheral blood mononuclear cells (PBMC) and tumour infiltrating lymphocytes from Kaposi’s sarcoma patients: correlation with the presence of human herpesvirus-8 in PBMC and lesional macrophages. Blood. 1998;91:968–976. [PubMed] [Google Scholar]
- 76.Sirianni M C, Uccini S, Angeloni A, Faggioni A, Cottoni F, Ensoli B. Circulating spindle cells: correlation with human herpesvirus-8 (HHV-8) infection and Kaposi’s sarcoma. Lancet. 1997;349:225. doi: 10.1016/s0140-6736(05)64866-0. [DOI] [PubMed] [Google Scholar]
- 77.Soulier J, Grollet L, Oksenhendler E, Miclea J M, Cacoub P, Baruchel A, Brice P, Clauvel J P, d’Agay M F, Raphel M, Degos L, Sigaux F. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 78.Staeheli P, Pitossi F, Pavlovic J. Mx proteins: GTPases with antiviral activity. Trends Cell Biol. 1993;3:268–272. doi: 10.1016/0962-8924(93)90055-6. [DOI] [PubMed] [Google Scholar]
- 79.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stürzl, M., A. Schreir, G. Ascherl, C. Hohenadl, P. Monini, C. Zietz, P. J. Browning, F. Neipel, P. Biberfeld, and B. Ensoli. Human herpesvirus-8 (HHV-8) gene expression in Kaposi’s sarcoma (KS) primary lesions: an in situ hybridization study. Leukemia, in press. [DOI] [PubMed]
- 81.Stürzl M, Blasig C, Schreier A, Neipel F, Hohenadl C, Cornali E, Ascherl G, Esser S, Brockmeyer N H, Ekman M, Kaaya E E, Tschachler E, Biberfeld P. Expression of HHV-8 latency-associated T0.7 RNA in spindle cells and endothelial cells of AIDS-associated, classical and African Kaposi’s sarcoma (KS) Int J Cancer. 1997;72:68–71. doi: 10.1002/(sici)1097-0215(19970703)72:1<68::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 82.Sun R, Lin S F, Gradoville L, Miller G. Plyadenylylated nuclear RNA encoded by Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1996;93:11883–11888. doi: 10.1073/pnas.93.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thiele K, Kirchner H. Effects of different interferons on the replication of herpes simplex virus in human T lymphocytes. J Interferon Res. 1988;8:507–515. doi: 10.1089/jir.1988.8.507. [DOI] [PubMed] [Google Scholar]
- 84.Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112:1017–1021. doi: 10.1053/gast.1997.v112.pm9041265. [DOI] [PubMed] [Google Scholar]
- 85.Toraldo R, D’Avanzo M, Tolone C, Canino G, Iafusco F, Notarangelo L D, Ugazio A, Cirillo C. Effect of interferon-alpha therapy in a patient with common variable immunodeficiency and chronic Epstein-Barr virus infection. Pediatr Hematol Oncol. 1995;12:489–493. doi: 10.3109/08880019509009480. [DOI] [PubMed] [Google Scholar]
- 86.Tur E, Brenner S, Michalevicz R. Low dose recombinant interferon alfa treatment for classic Kaposi’s sarcoma. Arch Dermatol. 1993;129:1297–1300. [PubMed] [Google Scholar]
- 87.Uccini S, Sirianni M C, Vincenzi L, Topino S, Stoppacciaro A, Lesnoni La Parola I, Capuano M, Masini C, Cerimele D, Cella M, Lanzavecchia A, Allavena P, Mantovani A, Baroni C D, Ruco L P. Kaposi’s sarcoma cells express the macrophage-associated antigen mannose receptor and develop in peripheral blood cultures of Kaposi’s sarcoma patients. Am J Pathol. 1997;150:929–938. [PMC free article] [PubMed] [Google Scholar]
- 88.Whitby D, Howard M R, Tenant-Flowers M, Brink N S, Copas A, Boshoff C, Hatzioannou T, Suggett F E, Aldam D M, Denton A S, Miller R F, Weller I V D, Weiss R A, Tedder R S, Schulz T F. Detection of Kaposi’s sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–803. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 89.Yeow W S, Lcawson C M, Beilharz M W. Antiviral activities of individual murine IFN-alpha subtypes in vivo: intramuscular injection of IFN expression constructs reduces cytomegalovirus replication. J Immunol. 1998;160:2932–2939. [PubMed] [Google Scholar]
- 90.Zimring J C, Goodbourn S, Offerman M K. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J Virol. 1998;72:701–707. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi’s sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]