Abstract
Synaptic configurations in precisely wired circuits underpin how sensory information is processed by the nervous system, and the emerging animal behavior. This is best understood for chemical synapses, but far less is known about how electrical synaptic configurations modulate, in vivo and in specific neurons, sensory information processing and context-specific behaviors. We discovered that INX-1, a gap junction protein that forms electrical synapses, is required to deploy context-specific behavioral strategies during C. elegans thermotaxis behavior. INX-1 couples two bilaterally symmetric interneurons, and this configuration is required for the integration of sensory information during migration of animals across temperature gradients. In inx-1 mutants, uncoupled interneurons display increased excitability and responses to subthreshold temperature stimuli, resulting in abnormally longer run durations and context-irrelevant tracking of isotherms. Our study uncovers a conserved configuration of electrical synapses that, by increasing neuronal capacitance, enables differential processing of sensory information and the deployment of context-specific behavioral strategies.
One-Sentence Summary:
Coupling of interneurons by electrical synapses reduces membrane resistance and filters sensory inputs to guide sensory-dependent behavioral choices.
Behavioral outputs rely on sensory information. Sensory information can be differentially processed based on the configurations of synapses in the circuit, enabling similar sensory stimuli to elicit different behavioral strategies in context-dependent manners (1–15). This action selection (16–18) enables animals to avoid deploying incompatible locomotory strategies in response to similar sensory stimuli at behavioral choice points. While the importance of action selection in behavioral choice strategies is well-recognized (16–18), the synaptic configurations that support action selection are not well understood.
Dissecting action selection mechanisms at a circuit level requires: 1) deriving predictable choice points for a given behavioral paradigm, 2) knowing the circuit substrates underlying the behavioral choice points and 3) understanding sensory input processing and locomotory strategy selection at the behavioral choice points. C. elegans thermotaxis behavior (19) provides a tractable model to interrogate the circuitry and synaptic bases of action selection. C. elegans does not have an innate preferred temperature, and instead learns to prefer the temperature at which it was cultivated in the presence of food (19). When in a temperature gradient, animals perform two behavioral strategies to reach and stay within their learned preferred temperature: migration across the gradient to arrive at the previously experienced temperature range (gradient migration), and tracking of isotherms upon encountering their preferred temperature (isothermal tracking) (19). Gradient migration and isothermal tracking are two behaviors that cannot be performed simultaneously. Because the action selection switch between gradient migration and isothermal tracking occurs within the temperature window at which the animal was cultivated, thermotaxis behavior provides an assay in which the behavioral choice point is both predictable and quantifiable. Importantly, the specific neurons that underlie thermotaxis behavior have been identified (11, 20–28). Laser-ablation studies of neurons in this circuit produces defects in both isothermal tracking and gradient migration (11, 20–24, 29, 30), indicating shared circuitry between the two strategies. How synaptic configurations in this circuit influences processing of thermosensory information to deploy context-specific behavioral strategies is not known.
To uncover circuits that underpin action selection mechanisms, we performed behavioral genetic experiments in C. elegans. We first adapted a thermotaxis assay to enrich for the quantification of isothermal tracking and gradient migration in a population of isogenic animals (Fig. 1 and Supp. Fig. 1). Animals were placed in separate regions of a temperature gradient with regards to their preferred temperature goal (20°C), and the locomotory strategies were recorded, segmented and quantified while they performed gradient migration and isothermal tracking (Fig. 1). Consistent with previous reports (31), under these conditions wild-type C. elegans spent about ~12% of their total time on the assaying arena performing isothermal tracking when within ±2°C of their preferred temperature, and with an average duration of 65 seconds per isothermal-oriented run (Fig. 1B, E). Distribution of the durations of isothermal track events followed an exponential decay with a time constant of 49.61 seconds and a half-life of 34.39 seconds (Fig. 1G and (31)).
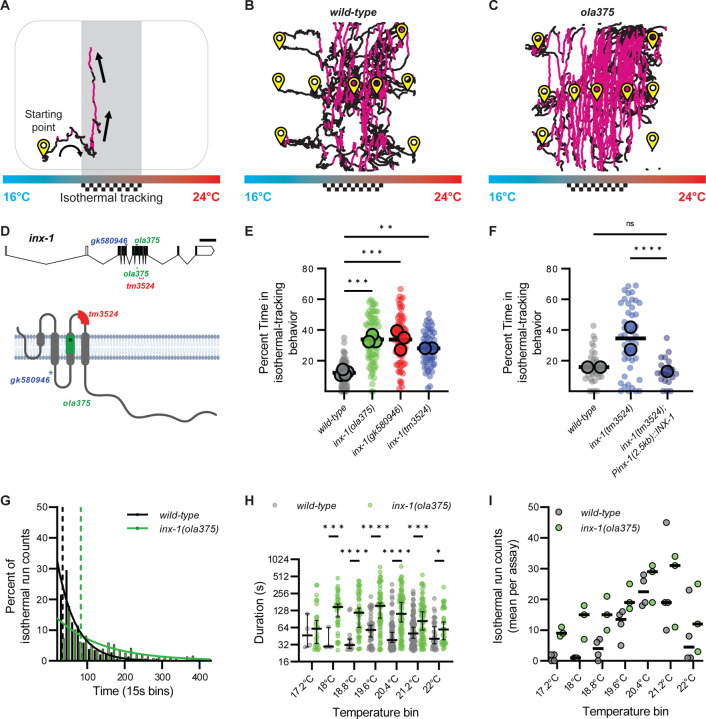
Figure 1. inx-1 mutants track isotherms at context irrelevant temperatures.
A. C. elegans track of a worm performing thermotaxis behavior. C. elegans perform two behavioral strategies during thermotaxis: gradient migration towards their preferred temperature and isothermal tracking at their preferred temperature. In the schematic, the preferred temperature region where animals are known to perform isothermal tracking is shaded and highlighted with a checkered goal pattern. Periods of isothermal tracking automatically recognized via a quantitative algorithm (see Methods) are highlighted in red. Arrows denote direction of travel. B. Representative image of wild-type worm tracks for animals trained at 20°C (checkered goal pattern). Animals start points denoted with yellow symbol. Animals were placed in an H-shape configuration in the gradient, as explained in Supplementary Figure 1 and Methods. Periods of isothermal tracking automatically recognized via a quantitative algorithm are highlighted in red C. As B, but for ola375 mutant worms isolated from a forward genetic screen. D. Molecular lesions present in inx-1 alleles, and their effects on the inx-1 gene and protein sequenc. The schematic uses inx-1a.1 isoform. Single nucleotide polymorphisms include A>G at position X:6,948,431 for inx-1(ola375) X; C>T at position X:6,949,062 for inx-1(gk580946) X). Insertion/deletions include a 16bp deletion at position X:6,948,406..6,948,421 for inx-1(ola375) X and 238bp deletion at position X:6,948,032..6,948,269 for inx-1(tm3524) X. Introduction of an early STOP codon in W127Opal for inx-1(gk580946) X, Y221Opal for inx-1(ola375) X. E. Percentage of time animals spend tracking isotherms (per worm track) for wild-type, ola375 mutants, and two independent inx-1 mutant alleles (inx-1(tm3524) X and inx-1(gk580946) X). Individual track values are presented by semi-transparent single-colored dots, while assay means are represented by bigger-size, slightly transparent circles with a black border. Colors denote genotypes. ** denotes P<0.005 and *** denotes P<0.0005 by Tukey’s multiple comparisons test after obtaining significance (P<0.0001) in a nested one-way ANOVA test. F. Percentage of time animals spend tracking isotherms, per worm track, for wild-type, inx-1(tm3524) X mutants, and inx-1(tm3524) X; olaEx2136 (inx-1 rescue). **** denotes P<0.0001 by Dunnetťs T3 multiple comparisons test after obtaining significance in both Brown-Forsythe (P<0.0001) and Welch's (P<0.0001) ANOVA tests on the individual tracks. Individual track values are presented by semi-transparent single-colored dots, while assay means are represeted with bigger-size, slightly transparent dots with a black borders. Colors denote genotypes. G. Histogram of the durations of wild-type (n = 288) and inx-1(ola375) X (n = 354) isothermal runs. Solid lines denote best-fit for one phase decay curves. Half-lives of the best-fit for one phase decay curves are 34.39 seconds for wild-type and 83.23 seconds for inx-1(ola375) X animals, denoted by the two vertical dotted lines. The time constants of the best-fit one phase decay curves are τ = 49.61 seconds for wild-type and τ = 120.1 seconds for inx-1(ola375) X animals, respectively. H. Semi-logarithmic (Y axis in log2) bee-swarm plot of isotherm-oriented run durations for wild-type and inx-1(ola375) X, per 0.5°C temperature bin. * Denotes q<0.05, *** denotes q<0.001, **** denotes q<0.00001 by multiple Mann-Whitney tests with a False Discovery Rate of 1% (using the Benjamini, Krieger, and Yekutieli method). I. Assay means of number of isotherm-oriented runs for wild-type and inx-1(ola375) X animals, per 0.5°C temperature bin.
To identify molecules that underlie behavioral choice, we performed an unbiased forward-genetic screen. We selected mutants that moved toward the preferred temperature but displayed defects in deploying the context-dependent isothermal tracking strategy. From this screen we isolated the mutant ola375, which outperformed wild-type animals in isothermal tracking both within and outside the ±2°C range of their preferred temperature (20°C), at the expense of gradient migration performance (example tracks in Fig. 1C, quantified in Fig. 1E). ola375 mutant animals spent ~34% of their time tracking isotherms, almost three times more than their wild-type counterparts (Fig. 1E). Moreover, the average run duration in the isothermal track for ola375 mutant animals was 140.5 seconds, more than doubling the wild-type average. The distribution of their run time durations in the isothermal direction still followed an exponential decay (Fig. 1G), but the decay was two times slower than that of wild-type, with a time constant of 120.1 seconds and a half-life of 83.23 seconds. We observed that the isotherm-oriented distributions of run durations were consistently higher in ola375 mutant animals as compared to wild type animals across the gradient (Fig. 1H), with differences being more significant near the preferred temperatures. The number of isotherm-oriented runs initiated, both for wild-type and ola375 mutants, exhibited modulation based on the distance to their preferred temperature (Fig. 1I). Together, our data indicate that ola375 corresponds to an allele that displays increased persistence of run duration in isotherm-oriented runs as compared to wild type animals.
To identify the genetic lesion resulting in the behavioral defects of ola375 animals, we performed positional mapping and whole-genome sequencing (32–35). These strategies revealed a missense mutation and a small insertion-deletion, resulting in an early STOP codon in the fifth coding exon of the gene encoding for INX-1/Innexin 1 (Fig. 1D and Supp. Fig 1B). Three additional lines of evidence support that ola375 is a loss-of-function allele of INX-1: 1) inx-1(tm3524) and inx-1(gk580946) alleles, both loss-of-function alleles, phenocopied the ola375 allele in the behavioral defects during thermotaxis (Fig. 1E); 2) inx-1 (tm3524) failed to complement the ola375 allele (data not shown), suggesting that the tm3524 and ola375 alleles correspond to genetic lesions within the same gene, inx-1; and 3) transgenic expression of wild-type inx-1 genomic DNA rescued the thermotaxis behavioral phenotype of inx-1(tm3524) mutants (Fig. 1F). INX-1 is a member of the innexin family of proteins, which are functionally and topologically related to vertebrate connexins (36–41). While connexins can form gap junctions in vertebrates, innexins do so in invertebrates (37, 38, 42–49). In C. elegans, inx-1 is expressed in neurons and body wall muscle (50, 51). It contributes to electrical coupling of body wall muscle cells (52) and synchrony of neuronal activities during rhythmic behavior (53, 54).
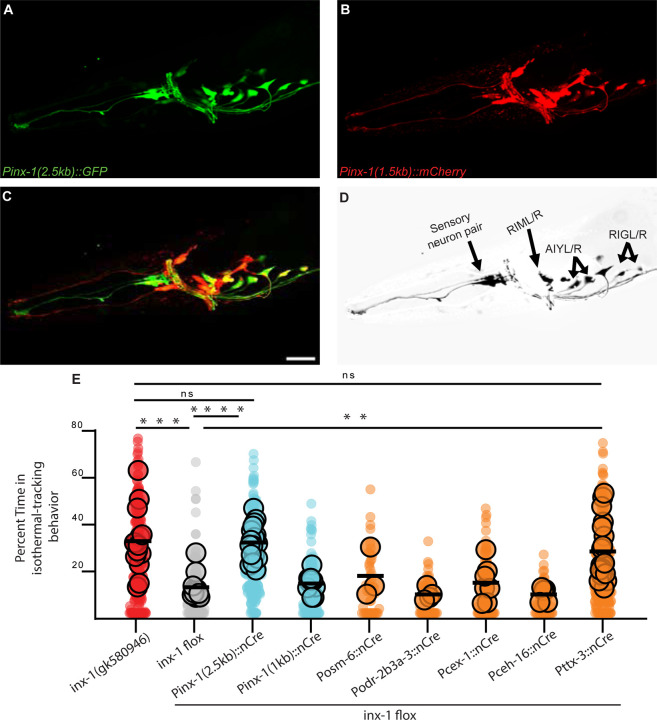
Rescue experiments with inx-1 cDNA using different lengths of the inx-1 promoter revealed that expression of wild-type inx-1 cDNA in inx-1(tm3524) mutants under the control of a 2.5-kb, but not a 1.5-kb promoter sequence (upstream of the inx-1 translation initiation site) could rescue the mutant behavior. To identify the neurons where INX-1 acts to regulate the thermotaxis behavior strategies, we expressed GFP under the control of the Pinx-1(2.5 kb) promoter fragment (Fig. 2A) and mCherry under the Pinx-1(1.5 kb) (Fig. 2B) fragment, respectively, and used a subtractive strategy to identify neurons in which inx-1 is required for rescue (Fig. 2C and Supp. Fig. 1C). This strategy led to the identification of four neuronal pairs (AIY, RIM, RIG, and an unidentified amphid neuron) that were detected with the longer (rescuing), but not the shorter Pinx-1 promoter fragment, consistent with the hypothesis that expression of INX-1 in (some or all) of these four neuronal pairs is necessary for rescue. To further examine this hypothesis, we then generated a conditional knockout strain by flanking the inx-1 gene with LoxP sites (Supp. Fig. 1E and (55)) and expressing Cre (56) in the candidate neurons by using specific promoters (Pttx-3 for AIY, Pcex-1 for RIM, and Pceh-16 for RIG). We observed that cell-specific knockout of inx-1 in AIY (but not in other neurons) recapitulated the aberrant action selection phenotype observed in inx-1 mutant animals (Fig. 2E). Consistently, AIY-specific expression of wild-type inx-1 abrogated the isothermal tracking phenotype of the inx-1(tm3524) mutants. The expression of wild-type inx-1 in AIY also caused an abnormal gradient migration phenotype, presumably from inx-1 overexpression (data not shown and (57)).
Figure 2. INX-1 is required in AIY interneurons to suppress context-irrelevant isothermal tracking.
A. Fluorescent micrograph of the head of an animal expressing GFP under the control of the rescuing 2.5kb inx-1 promoter. B. Fluorescent micrograph of the head of an animal expressing mCherry under the control of the 1.5kb inx-1 promoter. C. Composite of panels A-B. Scale bar is 50μm and applied to A, B and C. D. Neuronal pairs present under the 2.5kb inx-1 promoter but not the 1.5kb inx-1 promoter (for strategy, see Supplementary Figure 1C). E. Percentage of time animals spend tracking isotherms, per worm track, for inx-1(gk580946) or for inx-1(ola278). inx-1(ola278) is an engineered inx-1 floxed allele for conditional knockdowns using Cre recombinase (see Supplementary Figure 1E). Cre recombinase was expressed in the inx-1 floxed allele under the indicated promoters. ** denotes P<0.01, *** denotes P<0.001, **** denotes P<0.0001 by Kruskal-Wallis test. Individual track values are presented by semi-transparent single-colored dots, while assay means are represented by bigger-size, slightly transparent dots with a black border.
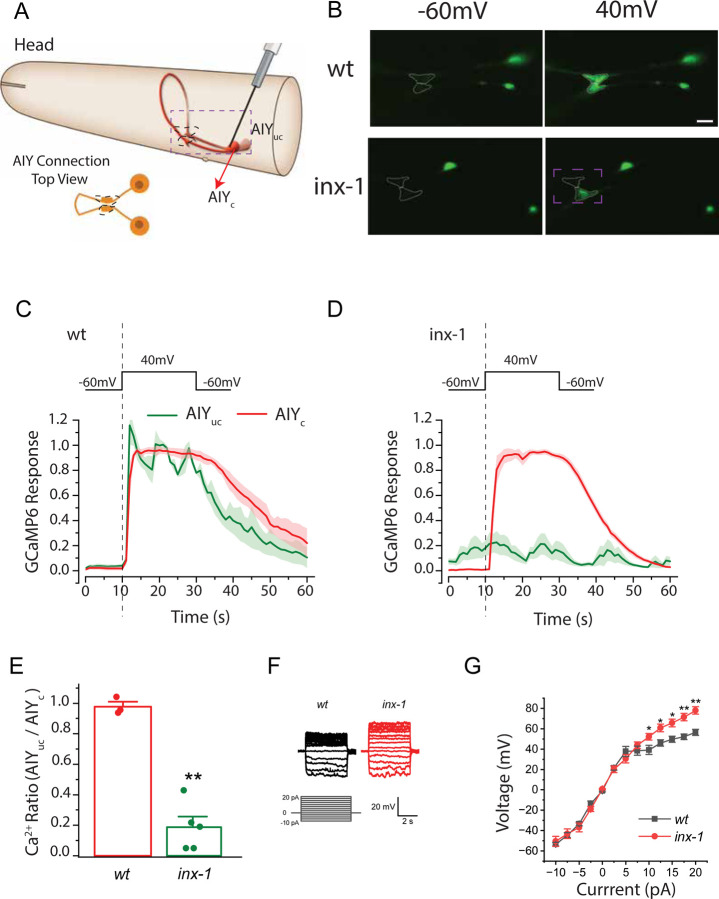
The AIY neuron class consists of two bilaterally symmetric interneurons that are necessary for proper thermal gradient migration and for isothermal tracking (11, 20, 21, 28, 30, 31, 58, 59). They are the only known postsynaptic partners to the bilateral pair of thermosensory neurons, AFDs ((60, 61) and Supp. Fig. 1D). Electron microscopy studies of the C. elegans connectomes have predicted a putative electrical synapse between the two AIYs at their synaptic regions (60), but the physiological function and molecular compositions of these structures are unknown. Since INX-1 is a gap junction protein, the identification of AIYs as the site of INX-1 function promoted us to investigate whether the AIY pair is electrically coupled, and whether this coupling is dependent on INX-1. To address this, we used transgenic animals expressing the genetically-encoded calcium indicator GCaMP6 in AIY and tested the effect of depolarizing one AIY (from −60 mV to +40 mV, for 20 seconds) on the calcium dynamics of both AIYs (Fig 3A). We analyzed the calcium dynamics at the AIY synaptic terminals known as Zone 2 (62), where the two AIYs have been shown to respond (24, 57) and where electrical synapses identified by EM studies were previously reported (60).
Figure 3. The bilateral pair of AIY interneurons are electrically coupled by INX-1 gap junctions.
A. Schematic of the C. elegans head and the bilaterally symmetric pair of AIY interneurons, with the clamped AIY (AIYc) and unclamped AIY (AIYuc). Dashed box represents imaged region in B. Images in B are from dorso-ventral views of the AIY pairs, schematized in lower left cartoon, with the synaptic region (called Zone 2) highlighted with dashed lines (as also in seen in B). B. Sample images of GCaMP6 fluorescence in the two AIYs before and during the 40-mV voltage step in wild type (wt) and inx-1(gk580946) mutants. Synaptic region (called Zone 2) of the two AIYs are marked by dotted lines (also in cartoon in A). Cell bodies are also visible to the right of the synaptic region. C. GCaMP6 signal strength over time in clamped AIY (AIYc) and unclamped AIY (AIYuc) of wild type animals. For results of individual animals normalized by the peak fluorescent signal of AIYc, see Supplementary Figure 2. D. As in (C), but for inx-1(gk580946) mutant animals. E. Comparison of the ratio (AIYuc/AIYc) of GCaMP6 signal. The ratio is the difference between the averages of AIYuc and AIYc over the 20 sec depolarizing period, and the preceding 10 sec hyperpolarizing period. wt (n = 3 animals) and inx-1 mutant (n = 5 animals). F. Sample membrane voltage traces in response to current injections for the indicated genotypes. G. Voltage versus current relationships of wild type (wt) and inx-1 mutants (n = 7 animals in both groups). The averaged membrane voltage over the last 4 sec of each current injection step (5 sec in duration) was used for quantification. The asterisks * and ** indicate statistically significant differences at p < 0.05 and p < 0.01, respectively (unpaired t-test).
In response to the voltage step, calcium signals increased in both AIYs of wild-type animals (Fig 3B–C and Supp Fig 2). In contrast, in inx-1(gk580946) mutant animals, only the clamped AIY responded (Fig. 3B, D, Supp Fig. 2 and Supplementary Movies 1–2). The calcium signal ratio of the AIY pair (unclamped over clamped) during the depolarizing voltage step (+40 mV) was 0.977 ± 0.032 in wild-type and 0.187 ± 0.070 in inx-1 mutants (Fig. 3E), indicating that INX-1 is required for the activation of the unclamped AIY. Calcium signal remained quiet prior to the voltage step in both AIYs of wild-type animals. In contrast, calcium signal often oscillated in the unclamped AIY of the inx-1(gk580946) mutant, and the oscillations appeared to be unrelated to the membrane voltage of the clamped AIY (Fig. 3F). Our data indicate that the hyperpolarizing voltage (−60 mV) could effectively silence the calcium activity of both AIYs in wild-type worms but not in inx-1 mutants. Importantly, our data indicate the AIY pair is electrically coupled via INX-1.
To then determine the effect of current injections on the membrane voltage of the AIY neurons, we performed current-clamp experiments on single AIYs. In wild-type, the relationship between current and membrane voltage was linear over the current range from −10 to +5 pA, but exhibited a reduced slope at larger positive currents (Fig. 3G). In inx-1(gk580946) mutants, the slope of the membrane voltage versus current relationship was identical to that in wild-type at the −10pA to +5 pA range, but was substantially steeper than wild-type AIYs at +5 pA to +20 pA range (Fig. 3G). These findings suggest that the changes in membrane permeability of the AIYs of the inx-1 mutants are different from those of wild-type animals, resulting in greater changes in membrane voltage at the current range of +5 pA to +20 pA. Collectively, these results indicate that the two AIYs are electrically coupled by gap junctions containing INX-1, and that this coupling might alter the electrophysiological properties of the two AIY neurons.
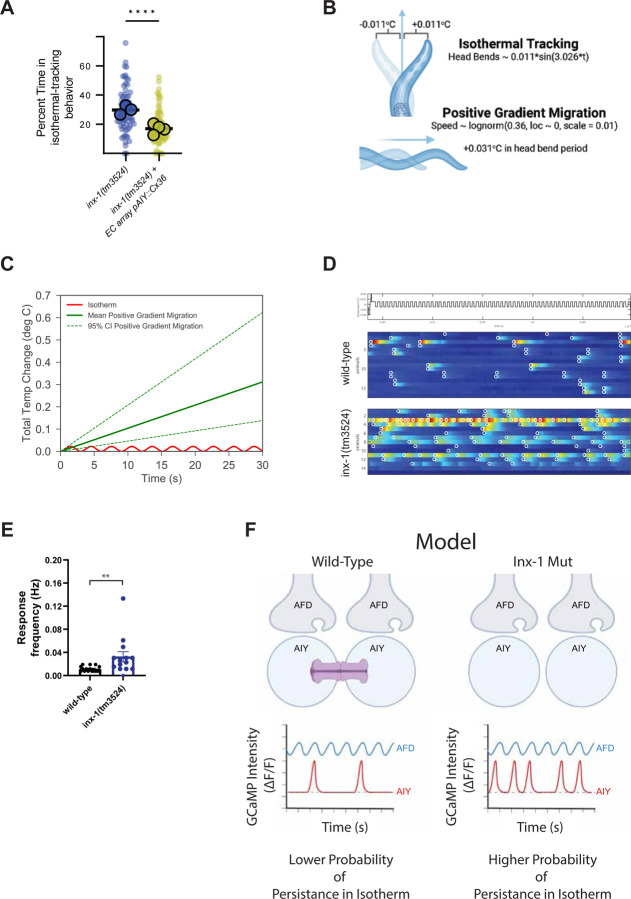
The gap junctions could serve to dampen the response of AIY to sensory inputs by shunting excitatory currents, similar to how amacrine cells in the retina are coupled via electrical synapses to achieve noise reduction during light sensory processing (63–65). But the observed phenotypes in inx-1 mutants could also be influenced by uncharacterized interactions with other innexins in neighboring cells, or by undetermined inx-1 signaling roles in AIY. To examine if the observed action selection phenotype emerged due to its specific role in electrically coupling the two AIY interneurons, we used heterologous expression of mammalian Connexin 36 (Cx36) and specifically expressed it in AIYs of inx-1(tm3524) animals. Transgenic animals expressing Cx36 specifically in the AIY interneurons exhibited a dramatic decrease in the time spent on isothermal tracking compared to the original inx-1(tm3524) mutants (Fig. 4A). These results suggest that a loss of electrical coupling between the two AIYs underlies the aberrant thermotaxis behavior of the inx-1 mutants, and that wild-type thermotaxis behaviors rely on the electrical coupling of the AIY interneurons via INX-1 gap junctions.
Figure 4. AIY sensitization in inx-1 mutant animals increases response frequency to small temperature changes.
A. Connexin36 was expressed in AIYs of inx-1(tm3524) under the control an AIY-specific promoter (Pttx-3), and percent time isothermal tracking was calculated for inx-1(tm3524) animals, or for inx-1(tm3524) animals expressing the Pttx-3::connexin36 transgene. Each small circle represents a single track, and large circles represent assays. Boxes represent median and quartiles, and whiskers represent minimum and maximum points. Results of the transgenic group were obtained for three independent lines. ** Denotes p < 0.005 (nested t test). B. Diagram depicting modeled temperature changes induced by head bends in isothermal run (top) and forward movement directly up temperature gradient (bottom). C. Quantification of total temperature changes evoked by models presented in D as a function of run duration. D. Calcium responses of wild-type (middle) and inx-1 mutants (bottom) immobilized animals when stimulated by an isotherm (+/− 0.01°C oscillations around Tc 20°C, schematic on top of plots). Color scale indicates delta F/F GCaMP intensity. Responses crossing threshold are circled in white. E. Frequency of individual AIY calcium transients in wild-type animals (8 animals, 16 AIYs) and inx-1(tm3524) mutants (7 animals, 14 AIYs). Values are shown as mean ± SE and the asterisks ** denote p < 0.005 by two-tailed Mann-Whitney test. F. Schematic model of AFD to AIY signaling, and resulting behavior, in wild type versus inx-1 mutants. In wild type animals, coupled AIYs have lower resistance and dampened responses to thermosensory stimuli coming from AFD. These dampened responses enable AIYs to integrate larger changes of thermosensory information as animals perform gradient migration. In inx-1 mutants, uncoupled AIYs are hyperexcitable due to a change in their electrophysiological properties resulting from the uncoupling. This hyperexcitability results in AIYs responding to subthreshold sensory signals. Activation of AIYs AIY initiate and sustain a forward-moving run (66–71), and their hyperactivation to subthreshold stimuli would result in a higher probability of animals abnormally persisting in isotherms.
We hypothesized that the increased sensitivity of AIY observed in the inx-1 mutants may preferentially increase the response rate of AIY to the small-scale changes in temperatures associated with isothermal tracking. To test this hypothesis, we modeled (1) head-bends by fitting a sinusoidal function to positional measurements of the nose of an animal as it freely navigates a temperature gradient (Fig. 4B and Supp. Fig. 3A), and (2) bouts of forward movement by recording the speed of animals as they move directly up a temperature gradient and fitting the data with a lognormal distribution (Fig. 4C and Supp. Fig. 3B). Our estimates suggest that animals performing isothermal tracking in a gradient similar to our experimental conditions (described in Methods and in Fig. 1A) experience oscillations, with each head bend, of + and − 0.011°C around the absolute temperature being tracked, while in the same head bend period of 3.026 seconds, animals moving up the gradient (at median speed) experience an increase in temperature of 0.031°C (Fig. 4B). We hypothesized that the smaller temperature changes experienced during isothermal tracking might result in a lower probability of activation of AIY in wild type animals, but in a higher probability of activation in the uncoupled and sensitized AIYs of inx-1 mutant animals.
To test this hypothesis, we imaged calcium dynamics of both AIYs in immobilized wild-type and inx-1 mutant animals after conditioning them at 20°C for several hours to create an internal state favoring isothermal tracking, and presenting them with oscillating temperature stimuli (every ~4 seconds) centered around 20°C (with an amplitude of ± 0.01°C) (Fig 4D). In wild-type, calcium transients occurred at a low rate (0.01145 Hz, or one response every ~87 seconds, after the animal had experienced ~22 stimuli). These responses in wild type were often concurrent in the two AIYs, with a Pearson’s correlation coefficient of 0.8 (Fig. 4D–E). In contrast, calcium transients occurred twice more frequently in inx-1(tm3524) mutant animals (0.03244 Hz, or one response every ~31 seconds) but they were asynchronous between the two AIYs (Pearson’s correlation coefficient of 0.4) (Fig. 4D–E). These results indicate that INX-1 plays a major role in the synchronous responses between the two AIYs. Our findings also support a model in which the uncoupled AIYs in inx-1 mutants are sensitized, and respond more frequently than wild type animals to small magnitude changes in temperature (±0.02°C), like those seen during isothermal tracking.
AIY activity is known to suppress turns to induce and sustain bouts of forward movement (‘runs’) (66–71). In inx-1 mutant animals, uncoupled AIYs display increased excitability and abnormally respond to small changes in temperature. Our data therefore supports a model in which AIY hyperexcitability ‘traps’ animals in long runs of minor temperature changes, decreasing the efficiency with which they move up the temperature gradient and can perform context-relevant behaviors (Fig 4F).
Discussion
We uncovered a specific in vivo behavioral role played by a configuration of electrical synapses formed between bilaterally-symmetric interneurons. To differentiate between two behavioral strategies, these electrical synapses increase the effective membrane permeability of this neuronal pair to filter out sensory information of subthreshold magnitude. By coupling a pair of bilaterally symmetrical neurons, electrical synapses decrease the membrane resistance of the interneurons and dampen the effect of subthreshold excitatory synaptic inputs. This effect of electrical synapses in the circuit activity states supports context-dependent deployment of complementary behavioral strategies in the nematode C. elegans.
In multiple behavioral contexts, AIY calcium activity is required to initiate and sustain a forward-moving run (66–71). Our findings are consistent with a model in which the probability of AIY activation account for the behavioral differences observed between wild-type and inx-1 mutants. In wild-type animals, AIYs have a low level of activity at the initiation of an isotherm-oriented run far away from their preferred temperature. The absence of activity would result in the probabilistic exit from the isothermal orientation via the execution of a reversal or pirouette (31, 70), thereby ending the run and reorienting the animal on a different direction. However, hyperexcitable AIYs in inx-1 mutants would be activated by stimuli that are subthreshold for AIY activation of the in wild-type animals. This hyperexcitability of AIYs in inx-1 mutants leads to the persistence of isotherm-oriented runs, and for animals to be ‘trapped’ in this behavioral state.
Wild-type animals also track isotherms, but unlike inx-1 mutants, this behavioral strategy is restricted to a temperature context near (±2°C) of their cultivation temperature (11, 19, 31). We posit that INX-1 could also serve as a regulatory switch for modulating action selection in wild-type animals. Regulation of the open/close states of the gap junctions by other molecules or post-translational modifications (44, 47, 72–76) may be a molecular substrate that enables a plastic uncoupling of the AIY pair. In this model, a change in the mode of sensory processing would ultimately affect action selection. Our findings also support a model whereby signal gains in the AFD→AIY synapse to smaller temperature derivatives could similarly impact isothermal tracking performance. Importantly, our findings reveal a role for electrical synapses in decreasing responses in coupled AIY interneuron pairs, which in turn are necessary to facilitates migration across the temperature gradient towards their cultivation temperature.
The organization of electrical synapses between the two AIYs might be a conserved and important configuration in sensory processing. The configuration is reminiscent of the gap junctions organization between amacrine cells in the retina. Amacrine cells are coupled by gap junctions that dampen their responses to visual sensory stimuli (63–65). Dampening of the gain of amacrine cells is critical for coincidence detection by photoreceptors, noise reduction and sensory processing during light adaptation (63–65). Thus, this configuration might confer circuits the ability to deploy context-dependent plastic responses by dynamically modulating sensory information processing, thereby increasing the versatility of neural circuits during sensory stimuli.
Materials and Methods
Reagents and Resources
Molecular biology
Plasmids were generated using Gibson Assembly (New England Biolabs) or the multi-site Gateway cloning system (Invitrogen). Either Phusion or Q5 High-Fidelity DNA-polymerase (NEB) were used for cloning or subcloning elements into the Gateway entry vectors. Cell-specific promoter fragments were amplified from genomic DNA or preexisting plasmids and introduced into pENTR41 or pENTR 50-TOPO vectors (Invitrogen); CDS of interest were inserted into pDONR221[1-2] (Invitrogen); and preexisting 3’UTR regions of commonly used genes (unc-54, let-858) into pDONR221[2-3] were used. Every insert was sequenced in their respective entry vector prior to the four-component LR recombination to generate the final expression plasmid (78).
Generation of transgenic strains
Transgenic C. elegans strains were generated by microinjection of the plasmids of interest into the gonad syncytia following standard approaches (79). Transgenic lines were selected and maintained based on the expression one or multiple of the following co-injection markers: Punc-122::GFP, Punc-122::RFP, Punc-122::dsRed Pmyo-3::mCherry, Pelt-7::GFP::NLS or Pelt-7::mCherry::NLS. Extrachromosomal arrays were integrated into the nematode genome via UV-activated trimethylpsoralen (TMP, Sigma, T6137), following standard methods. For a full list of strains used and generated by this work, please refer to the Supplemental Strain Table.
Generation of “floxed” inx-1(ola278) for conditional Knock-Out experiments
We inserted LoxP sites flanking the endogenous inx-1 genomic coding locus via the CRISPR-based, genomic edition protocol detailed in Dickinson et al., 2015 (55). This strain also carries an inserted tagRFP sequence and a Hygromycin B resistance gene after the 3’ LoxP insertion (Supplementary Fig 1E). Complete inserted sequence can be found in Supplemental Information.
Nematode Strains and maintenance
Nematodes were regularly maintained at room temperature (20–23°C) or inside Precision 815 (Thermo Scientific) or I-36NL (Percival Scientific) incubators at 20°C, grown on bacterial lawns of Escherichia coli strain OP50 seeded onto Nematode Growth Medium, according to husbandry standards (80). One-day adult hermaphrodite worms were used in all experiments unless otherwise noted. The N2 Bristol strain was used as the wild-type background.
Genotyping of mutant strains
Adult worms were lysed following standard protocols and PCRs were performed using GoTaq Green Master Mix (Promega, REF-M7123). Mutant alleles were distinguished from wild-type by imaging Restriction Fragment Length Polymorphisms (RFLP) on an agarose gel or by Sanger Sequencing performed by GENEWIZ (Azenta Life Sciences). The full set of genotyping primers and PCR conditions can be found in Supplemental Information.
Thermotaxis Behavioral Assays
For all behavior experiments, the animals’ developmental stage was synchronized by either allowing gravid adults to lay eggs in a seeded plate for two hours, three days prior to the assay or by picking L4 animals – identified by the clear half-moon patch in the midsection of the animal – the day before the experiment. The plates were then kept in Precision 815 (Thermo Scientific) or I-36NL (Percival Scientific) incubators at 20°C up to the time of the experiment, for experiments with a cultivation temperature of 20°C, or shifted to the appropriate temperature 4–6 hours prior to testing, in the case of temperature shift assays.
Behavioral analyses were performed as described previously (30, 57). A population of synchronized one-day adult hermaphrodites were picked onto an unseeded plate and washed in M9 (81). 3–5 worms were then transferred by micropipette on a 3µl M9 droplet to the respective starting points on the assay plates (82), equilibrated for 5–10 min, and they were allowed to freely crawl on the arena for 30–60 min, acquiring images at 2fps with a MightEx BCE-B050-U camera. Nematode tracks were analyzed using the MagatAnalyzer software package with modifications as previously indicated (30, 57, 83) and additional custom MATLAB (MathWorks) scripts.
Sensitized forward-genetic screens
To unbiasedly find new genes that might regulate or modulate the distinct thermotaxis gradient migration and isothermal tracking behaviors, forward-genetic screens were performed on pkc-1(nj1) loss-of-function mutants (strain IK105), which perform constitutively thermophilic behaviors. This screen resulted in recovery of ola375. In addition to suppressing the pkc-1(nj1) mutant phenotype of migrating up a shallow temperature gradient regardless of their preferred trained temperature (84), these animals tracked isotherms more often, and further away from their preferred temperature. We mapped the causative lesion to a 5 Mb region in Chromosome X (genomic position ~3Mb to ~8Mb) by Hawaiian SNP mapping (32) and Whole-Genome Sequencing (WGS) (33, 34) making use of the CloudMap pipeline (35). Whole-Genome Sequencing (WGS) was performed by the Yale Center for Genome Analysis (YCGA).
Identification of ola375 causative lesions
We further characterized the causative molecular lesion of ola375 by fine mapping using SNPs present in the divergent, Hawaiian wild-type strain (CB4856) (32) and outcrossing SNPs with the reference N2 wild-type strain. When recombinants with wild-type DNA regions within the 5Mb previously-mapped region were recovered, from either the 3Mb or the 8Mb flank, both the suppressing pkc-1(nj1) phenotype and the isothermal “hypertracking” phenotype were greatly diminished. Further analysis of the behavioral phenotypes, in combination with underlying molecular lesions in that region, led us to identify a novel mutant allele for gap junction innexin gene inx-1(ola375), which was exclusively responsible for the isothermal “hypertracking” phenotype under a wild-type background. This genetic lesion consists on both a missense SNP and a small indel in the fifth coding exon, resulting in an early STOP codon (see Fig.1D, Supplementary Figure 1 and Supp. Information). We established that inx-1(ola375) is the causative lesion to the isothermal tracking defects detected in this screen via four approaches: 1) examining additional alleles of inx-1, namely tm3524 and gk580946, and determining that they phenocopy the behavioral phenotypes (persistent isothermal tracking) observed for inx-1(ola375); 2) performing complementation tests to allele tm3524 and determining that inx-1(ola375) fails to completement the observed behavioral phenotypes; 3) performing genetic rescue experiments with a genomic region of inx-1 and observing that is sufficient to rescue the behavioral phenotypes and 4) performing conditional knock-out experiments and observing that cell-specific knockouts of inx-1 in the AIY interneurons are sufficient to reconstitute the observed behavioral phenotype for inx-1(ola375).
Major Types of Behavioral Paradigms Used
Shallow gradients for Gradient Migration Quantification
The original suppressor screen was performed on equipment previously described (30, 57), monitoring nematode gradient migration in the presence of a shallow temperature gradient (0.18°C/cm). Briefly, two pairs of thermoelectric components controlled by two Accuthermo FTC100D PID controllers sit at either side of an aluminum slab, and generate a defined linear temperature gradient. The system is cooled by a closed refrigeration system connected to a liquid cooling radiator in contact with dry ice. The aluminum slab in turn contacts a square assay plate (Corning®) with a 224 x 224 mm internal arena where the worms will perform. To ensure efficient heat transfer between the slab and the arena, either a volume of glycerol was used or a fitted, smaller aluminum sheet was intercalated between the aluminum slab and the assay plate. Red LEDs parallel to the plate generate a dark background image with bright outlines of the nematodes, captured by a MightEx camera (BCE-B050-U) above, at 2 frames per second, for 30–60 min. The whole system is encased in a modified cabinet. Unless otherwise explicitly noted, the gradient of the arena goes from 18°C to 22°C, and animals are placed in the middle of the arena, near 20°C. 24–33 animals are tested per assay.
Moderate and steep gradients for Isothermal Tracking Quantification
C. elegans perform maximal isothermal tracking behavior at ~0.6°C/cm gradients or higher (31). To generate these gradients, we used a modified, smaller version of the equipment described above and previously (30, 57), kindly gifted to us by Aravi Samuel (Harvard University). Unless otherwise explicitly noted, the gradient on the arena is centered on 20°C and goes from 17°C to 23°C for 0.6°C/cm gradient and 16°C to 24°C for 0.8°C/cm gradient. To quantitatively assess and adequately quantify isothermal tracking across the full gradient, a population of animals is assayed by starting in an H configuration, as shown in Supplementary Figure 1A. For a qualitative assessment of animals performing more isothermal tracking or under the wrong context, four starting droplets at each respective edge of the gradient were used. 24–27 animals are tested per assay.
Imaging
Confocal imaging
Young adults or L4 animals were mounted in 2% agarose dissolved in M9 buffer pads and anaesthetized with 10mM levamisole (Sigma). Confocal images were acquired with dual Hamamatsu ORCA-FUSIONBT SCMOS cameras on a Nikon Ti2-E Inverted Microscope using a confocal spinning disk CSU-W1 System, 488nm and 561nm laser lines and a CFI PLAN APO LAMBDA 60X OIL objective. Images were captured using the NIS-ELEMENTS software, with 2048px x 2048px, 16-bit depth, 300nm step size, 300ms of exposure time and enough sections to cover the whole worm depth.
Calcium Imaging
Imaging calcium dynamics was performed as previously described (57), with some modifications. The sample mounting protocol was modified to enrich the samples with animals positioned dorsoventrally, allowing for imaging of both AIY neurons simultaneously. Temperature control elements and most microscopy elements remain identical to (57), with a Leica DM6B being used in addition of Leica DM5500. Image acquisition was performed using MicroManager (85).
Quantification and statistical analysis
Quantification of isothermal tracking
Worm tracks were first analyzed and segmented by a modified MAGATAnalyzer software package (30, 83). These trajectories were then filtered into periods of isothermal tracking, defined as forward motion events in which at least 90% of the displacement occurred in the vertical, isotherm orientation, for a minimum of 25 seconds; and periods of non-isothermal tracking in which the movement of the worm did not pass the isothermal tracking filter. The segmented isothermal tracking periods were further analyzed by their duration in seconds, temperature at which the period started and number of events.
Quantification of behavior
Quantifications of turns, thermotaxis indices and other parameters relevant to gradient migration were automatically scored per worm track by an adapted MAGATAnalyzer software package, previously described (30, 83).
Quantification of calcium imaging in AIY
Segmentation into regions of interest and downstream data processing was performed using FIJI (86), and custom scripts written in MATLAB (MathWorks) as detailed previously (57). For analyses of AIY calcium dynamics, we generated and quantified a ROI at the synaptic subcellular Zone 2 region (62). Responses were scored as the initial rise of the AIY calcium signal as determined by a human observer and an automated response calling based on signal intensity and its derivative, as previously described (57).
Electrophysiological analyses
Electrophysiological analyses were performed with transgenic strains expressing Pmod-1::GCaMP6s and Pttx-3::mCherry in wild-type and inx-1(gk580946) mutant genetic backgrounds. In each experiment, a young adult hermaphrodite animal was immobilized on a Sylgard-coated circular coverglass by applying Vetbond Tissue Adhesive (3M Company, St. Paul, MN) along the anterior dorsal region. After a longitudinal cut (~ 200 µm) was made by a diamond dissecting tool in the glued area, the cuticle above the cut line was pulled back and glued onto the coverglass to expose head neurons. The coverglass was then transferred to a recording chamber containing the extracellular solution, which contained (in mM) NaCl 140, KCl 5, CaCl2 5, MgCl2 5, dextrose 11 and HEPES 5 (pH 7.2). Following identification of the two AIYs based on mCherry fluorescence, one of them was used for voltage- or current-clamp recording in the classical whole-cell configuration. In the voltage-clamp experiments, AIY was held at −60 mV and stepped to 40 mV for 20 seconds before returning to the holding voltage. Meanwhile, calcium transients of both AIYs before (10 sec), during (20 sec), and after (30 sec) the voltage step were imaged at 1-sec intervals using an electron-multiplying CCD camera (iXonEMþ885, Andor Technology, Belfast, Northern Ireland), a FITC filter set (59222, Chroma Technology Corp.), a light source (Lambda XL, Sutter Instrument), and the NIS-Elements software (Nikon). TTL signals from the camera were used to synchronize the recordings of calcium transients with the voltage-clamp protocol. In the current-clamp experiments, negative and positive currents over the range of −10 pA to +20 pA at 2.5-pA intervals were injected into the clamped AIY for 5 sec per step. Borosilicate glass pipettes with a tip resistance of 3B5MO were used as electrodes for current- and voltage-clamp recordings with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), a digitizer (Digidata 1440A, Molecular Devices), and the Clampex software (version 11, Molecular Devices). Data were sampled at a rate of 10 kHz after filtering at 2 kHz. The pipette solution contained (in mM) KCl 120, KOH 20, Tris 5, CaCl2 0.25, MgCl2 4, sucrose 36, EGTA 5 and Na2ATP 4 (pH 7.2). A Nikon FN-1 microscope equipped with a 40X water-immersion objective was used in the electrophysiological and calcium imaging experiments.
Statistical analyses
All statistical tests were performed using GraphPad Prism version 9 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com Chosen statistical tests are described in the relevant figure legends.
Supplementary Material
Acknowledgements.
We thank the members of the Colón-Ramos lab for their thoughtful comments on the project. We thank the Caenorhabditis Genetics Center (supported by the National Institutes of Health (NIH), Office of Research Infrastructure Programs (P40 OD010440)); the C. elegans Reverse Genetics Core Facility at the University of British Columbia which is part of the International C. elegans Gene Knockout Consortium; and Shohei Mitani (Tokyo Women’s Medical University, Tokyo, Japan) for nematode strains. We thank Bill Schafer (MRC Laboratory of Molecular Biology) for kindly providing us with their Connexin36 construct (77) and Z. Altun (www.wormatlas.org) for diagrams used in figures. We thank Aravi Samuel (Harvard University) for generous assistance with technical knowledge in the development of behavioral rigs and calcium imaging platforms. We thank Hari Shroff (Janelia Research Campus) and Andrew Lauziere (University of Maryland) for image processing codes used for movies of freely moving animals. Research in the D.A.C.-R. lab was supported by NIH R01NS076558, National Science Foundation (NSF IOS 1353845), DP1NS111778 and by an HHMI Scholar Award.
REFERENCES
- 1.Murakami H., Bessinger K., Hellmann J., Murakami S., Aging-dependent and -independent modulation of associative learning behavior by insulin/insulin-like growth factor-1 signal in Caenorhabditis elegans. J Neurosci 25, 10894–10904 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faumont S., Lindsay T. H., Lockery S. R., Neuronal microcircuits for decision making in C. elegans. Curr Opin Neurobiol 22, 580–591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins K. M., Koelle M. R., Postsynaptic ERG potassium channels limit muscle excitability to allow distinct egg-laying behavior states in Caenorhabditis elegans. J Neurosci 33, 761–775 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satoh Y. et al. , Regulation of experience-dependent bidirectional chemotaxis by a neural circuit switch in Caenorhabditis elegans. J Neurosci 34, 15631–15637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins K. M. et al. , Activity of the C. elegans egg-laying behavior circuit is controlled by competing activation and feedback inhibition. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guillermin M. L., Carrillo M. A., Hallem E. A., A Single Set of Interneurons Drives Opposite Behaviors in C. elegans. Curr Biol 27, 2630–2639 e2636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampel S., McKellar C. E., Simpson J. H., Seeds A. M., Simultaneous activation of parallel sensory pathways promotes a grooming sequence in Drosophila. Elife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin H., Lin A. C., Neuronal mechanisms underlying innate and learned olfactory processing in Drosophila. Curr Opin Insect Sci 36, 9–17 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Grunwald Kadow I. C., State-dependent plasticity of innate behavior in fruit flies. Curr Opin Neurobiol 54, 60–65 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Wang Y. et al. , Flexible motor sequence generation during stereotyped escape responses. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda M. et al. , Context-dependent operation of neural circuits underlies a navigation behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A 117, 6178–6188 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dal Bello M., Perez-Escudero A., Schroeder F. C., Gore J., Inversion of pheromone preference optimizes foraging in C. elegans. Elife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao L., Bhandawat V., Mechanisms of Variability Underlying Odor-Guided Locomotion. Front Behav Neurosci 16, 871884 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiroki S. et al. , Molecular encoding and synaptic decoding of context during salt chemotaxis in C. elegans. Nat Commun 13, 2928 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W. et al. , Redundant neural circuits regulate olfactory integration. PLoS Genet 18, e1010029 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huda R., Goard M. J., Pho G. N., Sur M., Neural mechanisms of sensorimotor transformation and action selection. Eur J Neurosci 49, 1055–1060 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takagi S., Nose A., Circuit architecture for somatotopic action selection in invertebrates. Neurosci Res 140, 37–42 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Hulse B. K. et al. , A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and context-dependent action selection. Elife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedgecock E. M., Russell R. L., Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 72, 4061–4065 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori I., Ohshima Y., Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376, 344–348 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Hobert O. et al. , Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron 19, 345–357 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Satterlee J. S. et al. , Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron 31, 943–956 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Chung S. H., Clark D. A., Gabel C. V., Mazur E., Samuel A. D., The role of the AFD neuron in C. elegans thermotaxis analyzed using femtosecond laser ablation. BMC Neurosci 7, 30 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark D. A., Biron D., Sengupta P., Samuel A. D., The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci 26, 7444–7451 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biron D., Wasserman S., Thomas J. H., Samuel A. D., Sengupta P., An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc Natl Acad Sci U S A 105, 11002–11007 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhara A. et al. , Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 320, 803–807 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Beverly M., Anbil S., Sengupta P., Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J Neurosci 31, 11718–11727 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuyama H. J., Mori I., Neural Coding of Thermal Preferences in the Nematode Caenorhabditis elegans. eNeuro 7, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhara A., Ohnishi N., Shimowada T., Mori I., Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat Commun 2, 355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo L. et al. , Bidirectional thermotaxis in Caenorhabditis elegans is mediated by distinct sensorimotor strategies driven by the AFD thermosensory neurons. Proc Natl Acad Sci U S A 111, 2776–2781 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo L., Clark D. A., Biron D., Mahadevan L., Samuel A. D., Sensorimotor control during isothermal tracking in Caenorhabditis elegans. J Exp Biol 209, 4652–4662 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Davis M. W. et al. , Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6, 118 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doitsidou M., Poole R. J., Sarin S., Bigelow H., Hobert O., elegans C. mutant identification with a one-step whole-genome-sequencing and SNP mapping strategy. PLoS One 5, e15435 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuryn S., Le Gras S., Jamet K., Jarriault S., A strategy for direct mapping and identification of mutations by whole-genome sequencing. Genetics 186, 427–430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minevich G., Park D. S., Blankenberg D., Poole R. J., Hobert O., CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192, 1249–1269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodenough D. A., Goliger J. A., Paul D. L., Connexins, connexons, and intercellular communication. Annu Rev Biochem 65, 475–502 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Phelan P. et al. , Innexins: a family of invertebrate gap-junction proteins. Trends Genet 14, 348–349 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao L. et al. , Innexins form two types of channels. FEBS Lett 581, 5703–5708 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung G., Chever O., Rouach N., Connexons and pannexons: newcomers in neurophysiology. Front Cell Neurosci 8, 348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palacios-Prado N., Huetteroth W., Pereda A. E., Hemichannel composition and electrical synaptic transmission: molecular diversity and its implications for electrical rectification. Front Cell Neurosci 8, 324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curti S., Davoine F., Dapino A., Function and Plasticity of Electrical Synapses in the Mammalian Brain: Role of Non-Junctional Mechanisms. Biology (Basel) 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starich T., Sheehan M., Jadrich J., Shaw J., Innexins in C. elegans. Cell Commun Adhes 8, 311–314 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Bauer R. et al. , Intercellular communication: the Drosophila innexin multiprotein family of gap junction proteins. Chem Biol 12, 515–526 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Starich T. A., Xu J., Skerrett I. M., Nicholson B. J., Shaw J. E., Interactions between innexins UNC-7 and UNC-9 mediate electrical synapse specificity in the Caenorhabditis elegans locomotory nervous system. Neural Dev 4, 16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oshima A., Matsuzawa T., Nishikawa K., Fujiyoshi Y., Oligomeric structure and functional characterization of Caenorhabditis elegans Innexin-6 gap junction protein. J Biol Chem 288, 10513–10521 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall D. H., Gap junctions in C. elegans: Their roles in behavior and development. Dev Neurobiol 77, 587–596 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang H. et al. , Dissection of neuronal gap junction circuits that regulate social behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A 114, E1263–E1272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin E. J., Park S., Lyu X., Jin Y., Gap junctions: historical discoveries and new findings in the C aenorhabditis elegans nervous system. Biol Open 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker D. S., Schafer W. R., Distinct roles for innexin gap junctions and hemichannels in mechanosensation. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altun Z. F., Chen B., Wang Z. W., Hall D. H., High resolution map of Caenorhabditis elegans gap junction proteins. Dev Dyn 238, 1936–1950 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharya A., Aghayeva U., Berghoff E. G., Hobert O., Plasticity of the Electrical Connectome of C. elegans. Cell 176, 1174–1189 e1116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu P. et al. , Six innexins contribute to electrical coupling of C. elegans body-wall muscle. PLoS One 8, e76877 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi U., Wang H., Hu M., Kim S., Sieburth D., Presynaptic coupling by electrical synapses coordinates a rhythmic behavior by synchronizing the activities of a neuron pair. Proc Natl Acad Sci U S A 118, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang J. et al. , C. elegans enteric motor neurons fire synchronized action potentials underlying the defecation motor program. Nat Commun 13, 2783 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickinson D. J., Pani A. M., Heppert J. K., Higgins C. D., Goldstein B., Streamlined Genome Engineering with a Self-Excising Drug Selection Cassette. Genetics 200, 1035–1049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hubbard E. J., FLP/FRT and Cre/lox recombination technology in C. elegans. Methods 68, 417–424 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hawk J. D. et al. , Integration of Plasticity Mechanisms within a Single Sensory Neuron of C. elegans Actuates a Memory. Neuron 97, 356–367 e354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narayan A., Laurent G., Sternberg P. W., Transfer characteristics of a thermosensory synapse in Caenorhabditis elegans. Proc Natl Acad Sci U S A 108, 9667–9672 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez M. et al. , Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron 30, 241–248 (2001). [DOI] [PubMed] [Google Scholar]
- 60.White J. G., Southgate E., Thomson J. N., Brenner S., The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314, 1–340 (1986). [DOI] [PubMed] [Google Scholar]
- 61.Witvliet D. et al. , Connectomes across development reveal principles of brain maturation. Nature 596, 257–261 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colon-Ramos D. A., Margeta M. A., Shen K., Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318, 103–106 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bloomfield S. A., Dacheux R. F., Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res 20, 351–384 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Masland R. H., The fundamental plan of the retina. Nat Neurosci 4, 877–886 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Hanson L., Ravi-Chander P., Berson D., Awatramani G. B., Hierarchical retinal computations rely on hybrid chemical-electrical signaling. Cell Rep 42, 112030 (2023). [DOI] [PubMed] [Google Scholar]
- 66.Tsalik E. L., Hobert O., Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol 56, 178–197 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Gray J. M., Hill J. J., Bargmann C. I., A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A 102, 3184–3191 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chalasani S. H. et al. , Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Kocabas A., Shen C. H., Guo Z. V., Ramanathan S., Controlling interneuron activity in Caenorhabditis elegans to evoke chemotactic behaviour. Nature 490, 273–277 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Z., Liu J., Zheng M., Xu X. Z., Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell 159, 751–765 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H. et al. , Cholinergic Sensorimotor Integration Regulates Olfactory Steering. Neuron 97, 390–405 e393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouhours M. et al. , A co-operative regulation of neuronal excitability by UNC-7 innexin and NCA/NALCN leak channel. Mol Brain 4, 16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Correa P. A., Gruninger T., Garcia L. R., DOP-2 D2-Like Receptor Regulates UNC-7 Innexins to Attenuate Recurrent Sensory Motor Neurons during C. elegans Copulation. J Neurosci 35, 9990–10004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu P., Chen B., Mailler R., Wang Z. W., Antidromic-rectifying gap junctions amplify chemical transmission at functionally mixed electrical-chemical synapses. Nat Commun 8, 14818 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voelker L. et al. , INX-18 and INX-19 play distinct roles in electrical synapses that modulate aversive behavior in Caenorhabditis elegans. PLoS Genet 15, e1008341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi M. K., Liu H., Wu T., Yang W., Zhang Y., NMDAR-mediated modulation of gap junction circuit regulates olfactory learning in C. elegans. Nat Commun 11, 3467 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabinowitch I., Chatzigeorgiou M., Zhao B., Treinin M., Schafer W. R., Rewiring neural circuits by the insertion of ectopic electrical synapses in transgenic C. elegans. Nat Commun 5, 4442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merritt C., Seydoux G., Transgenic solutions for the germline. WormBook, 1–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mello C., Fire A., DNA transformation. Methods Cell Biol 48, 451–482 (1995). [PubMed] [Google Scholar]
- 80.Brenner S., The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stiernagle T., Maintenance of C. elegans. WormBook, 1–11 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goodman M. B. et al. , Thermotaxis navigation behavior. WormBook, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gershow M. et al. , Controlling airborne cues to study small animal navigation. Nat Methods 9, 290–296 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okochi Y., Kimura K. D., Ohta A., Mori I., Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J 24, 2127–2137 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edelstein A. D. et al. , Advanced methods of microscope control using muManager software. J Biol Methods 1, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schindelin J. et al. , Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.