Abstract
GB virus C (GBV-C), also known as hepatitis G virus, is a recently discovered flavivirus-like RNA agent with unclear pathogenic implications. To investigate whether human peripheral blood mononuclear cells (PBMC) are susceptible to in vitro GBV-C infection, we have incubated PBMC from four healthy blood donors with a human GBV-C RNA-positive serum. By means of (i) strand-specific reverse transcription-PCR, cloning, and sequencing; (ii) sucrose ultracentrifugation and RNase sensitivity assays; (iii) fluorescent in situ hybridization; and (iv) Western blot analysis, it has been demonstrated that GBV-C is able to infect in vitro cells and replicate for as long as 30 days under the conditions developed in our cell culture system. The concentration of GBV-C RNA increased during the second and third weeks of culture. The titers of the genomic strand were 10 times higher than the titers of the antigenomic strand. In addition, the same predominant GBV-C sequence was found in all PBMC cultures and in the in vivo-GBV-C-infected PBMC isolated from the donor of the inoculum. GBV-C-specific fluorescent in situ hybridization signals were confined to the cytoplasm of cells at different times during the culture period. Finally, evidence obtained by sucrose ultracentrifugation, RNase sensitivity assays, and Western blot analysis of the culture supernatants suggests that viral particles are released from in vitro-GBV-C-infected PBMC. In conclusion, our study has demonstrated, for the first time, GBV-C replication in human lymphoid cells under experimental in vitro infection conditions.
A novel flavivirus-like agent, named GB virus C (GBV-C) and also hepatitis G virus (HGV), has been recently isolated by two independent groups (17, 18, 31, 32). Due to their high degrees of nucleotide and amino acid sequence homology (86 and 96%, respectively), GBV-C and HGV are thought to be isolates of the same virus (36). An association between GBV-C infection and acute posttransfusional hepatitis as well as fulminant hepatitis of non-A to non-E etiology has been shown by epidemiological studies based on PCR technology (2, 9, 12, 19, 40). Furthermore, GBV-C infection is particularly prevalent in patients with chronic hepatitis C virus (HCV) infections (10 to 25%) (1, 3, 34, 38). GBV-C is capable of inducing persistent infection in about 5 to 10% of GBV-C-infected individuals (13, 21). GBV-C was found to infect chimpanzees, and the course of infection of the virus in this animal model mimicked that observed in humans, although these chimpanzees did not develop hepatitis (4). Despite these data, a direct relationship between GBV-C infection and the establishment of chronic hepatitis has not yet been clearly demonstrated, and the association with fulminant hepatitis has not been corroborated by subsequent studies. The recent development of a serologic assay for the identification of antibodies to the putative envelope 2 (E2) protein of GBV-C (7, 26, 33), a marker of past infection, has revealed differences in prevalence of anti-E2 in healthy individuals from different parts of the world, with the prevalence being relatively high in western Europe (10 to 16%) (24).
The GBV-C genome organization was found to be organized similarly to that of HCV; it is a positive-sense, single-stranded RNA (9.4 kb in length) which contains a single open reading frame flanked by 5′ and 3′ noncoding (NC) regions, with the structural and nonstructural (NS) proteins being encoded in the 5′ and 3′ ends of the open reading frame, respectively (36). By comparison of the GBV-C genomic sequence with those of other members of the Flaviviridae family, it has been determined that GBV-C encodes two putative envelope glycoproteins (E1 and E2) (14) as well as serine protease-RNA helicase (NS3) and RNA-dependent RNA polymerase (NS5) activities. It is noteworthy that a coding region for the putative core protein has not been confirmed to exist (27, 30, 39).
As for HCV, although its replication mechanism is unknown, it is suspected that the antigenomic GBV-C RNA strand may be the replicative intermediate. Surprisingly, the investigation of GBV-C replicative sites has led to very contradictory findings. Thus, it has not been clearly established whether the liver is the primary replication site for GBV-C and whether extrahepatic tissues (such as hematopoietic cells) support the replication of this virus (15, 19, 23). In vitro culture systems for GBV-C replication have not been extensively studied. In this regard, only MT-2C (a human T-cell leukemia virus type 1-infected human T-cell line) and PH5CH (a nonneoplastic human hepatocyte line immortalized with simian virus 40 large T antigen) cells have been found to support GBV-C replication (11).
In this study, we have investigated whether GBV-C can infect and replicate in human cells of hematopoietic origin in vitro, and our results have demonstrated (i) the existence of active GBV-C replication and (ii) the release of viral particles from GBV-C-infected cells into the culture supernatant.
MATERIALS AND METHODS
GBV-C inoculum.
The serum from a patient exhibiting long-term liver dysfunction after autologous bone marrow transplantation (GBV-C RNA positive in both serum and the liver, as demonstrated previously [37]) was used as the inoculum (PCR titer, 108 genome equivalents/ml). This patient was not infected by HCV, hepatitis B virus, human immunodeficiency virus, or related viruses.
Isolation and preparation of cells.
Peripheral blood mononuclear cells (PBMC) from four healthy blood donors (who were not infected by GBV-C, HCV, hepatitis B virus, human immunodeficiency virus, Epstein-Barr virus, or cytomegalovirus) were isolated from fresh, heparinized venous blood by centrifugation on Ficoll-Hypaque gradients (SEROMED; Biochrom KG, Berlin, Germany), washed twice with phosphate-buffered saline (PBS), and suspended in RPMI 1640 medium (Imperial Laboratories, Andover, United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum (Imperial), 20 mM HEPES, 2 mM glutamine, and antibiotics. Cell viability was assessed by the trypan blue exclusion test. PBMC were seeded at a density of 2 × 106 viable cells/ml of RPMI in petri dishes (Costar Corp., Cambridge, Mass.) and cultured for 48 h at 37°C in a humidified atmosphere containing 5% CO2, with stimulation by phytohemagglutinin (10 μg/ml; Sigma Chemical Co., St. Louis, Mo.) plus Escherichia coli lipopolysaccharide (10 μg/ml; Sigma). After stimulation, cells from each donor were counted and adjusted to a density of 107 viable cells/ml in RPMI 1640 supplemented with 20 U of interleukin-2 (IL-2) (Chiron-Cetus Corp., Emeryville, Calif.) per ml. In addition, a cell pool, obtained by mixing equal number of cells from each individual donor, was also adjusted to a density of 107 viable cells/ml in RPMI supplemented with 20 U of IL-2 per ml.
Incubation of cells with GBV-C inoculum.
Cells from each individual donor and the pool were aliquoted into 24-well cell culture clusters at a density of 106 viable cells/200 μl of RPMI supplemented with IL-2 and incubated with the GBV-C RNA-positive serum (10 μl/106 cells) for 4 h at 37°C in a humidified atmosphere with 5% CO2. After incubation, the cells were collected and washed five times with PBS, and the cultures were maintained at a density of 2 × 106 cells/ml in RPMI supplemented with IL-2. The culture medium was changed weekly for a 1-month period. At each medium change time point, cells from each culture (four donors and the pool) were counted; aliquots of the cells and their corresponding supernatants were stored at −80°C, and the remaining cells were subcultured 1:4 with fresh cells from each of the donors and the cell pool. As a negative control, a pool of the same fresh PBMC from the four healthy blood donors used for the GBV-C in vitro infection experiments was incubated under the same conditions with human serum from a healthy individual (GBV-C RNA negative) and maintained as described above.
RNA extraction.
Total RNA was extracted from 200 μl of each culture supernatant and cell wash, as well as from cells, by using two phenol-acid guanidinium thiocyanate extraction steps followed by a chloroform-isoamyl alcohol (29:1) step and precipitation with 2-propanol (5). Total RNA extracted from cells was quantitated, and 1 μg of PBMC-derived total RNA, or the entire quantity of supernatant-derived RNA, was used for cDNA synthesis.
Chemical modification of RNA.
Chemical modification of the 3′ end of the RNA was performed by periodate oxidation followed by reduction with NaBH4 as described by Gunji et al. (10). Briefly, following denaturation of the RNA samples at 95°C for 5 min, 200 μl of 50 mM sodium acetate (pH 5.2) and 50 μl of 20 mM NaIO4 were added, and the mixtures were incubated at 30°C for 12 h. The reaction was stopped by the addition of 60 μl of 10% ethylene glycol, and the RNA was precipitated with ethanol. The RNA was redissolved in 300 μl of diethylpyrocarbonate-treated water and incubated with 100 μl of 100 mM NaBH4, dissolved in 50 mM NaOH, on ice for 1 h. The reaction was stopped by addition of 20 μl of ice-cold acetic acid; this was followed by ethanol precipitation of the RNA.
Amplification of genomic and antigenomic GBV-C RNA strands.
Genomic and antigenomic GBV-C RNA strands were amplified by strand-specific reverse transcription (RT) and PCR, using primers from the 5′ NC and NS3 regions of the GBV-C genome (Table 1). To reduce RNA secondary structure and to maximize the stringency of the cDNA synthesis, each RNA sample was preheated to 70°C for 3 min. Subsequent cDNA synthesis was carried out for 60 min at 42°C in a 20-μl reaction mixture containing 50 mM Tris-HCl (pH 8.3), 37.5 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 0.5 mM each deoxynucleoside triphosphate, RNasin (40 U), 100 U of SuperScript II RNase H reverse transcriptase (GIBCO BRL, Life Technologies, Inc., Gaithersburg, Md.), and 50 pmol of the corresponding polarity primer (sense for the detection of antigenomic GBV-C RNA and antisense for the detection of genomic GBV-C RNA). Prior to the amplification of the cDNA by nested PCR, cDNA samples were heated to 95°C for 45 min and then treated with 100 μg of RNase per ml. One-tenth of the cDNA was amplified for 30 cycles (94°C for 25 s, 50°C for 35 s, and 68°C for 2.5 min), followed by a final extension step at 68°C for 7 min, in a 50-μl reaction mixture consisting of 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 1 mM each deoxynucleoside triphosphate, 50 pmol of each of the primers, and 1.5 U of Taq DNA polymerase (GIBCO BRL). Furthermore, the presence of the antigenomic GBV-C RNA strand was confirmed by performing cDNA synthesis at a high temperature with the thermostable enzyme Tth (Pharmacia Biotech, Uppsala, Sweden) as recently described by Laskus et al. (16). The second PCR round was performed under the same conditions as described above, using 5 μl of the product of the first PCR and the specific 5′ NC internal primers. The expected PCR product was analyzed by agarose gel electrophoresis and Southern blot hybridization with a 32P-labelled internal probe (Table 1). Hybridization was performed at 45°C in 6× SSC buffer (90 mM sodium citrate, 0.9 M NaCl2; pH 7.0) containing 0.2% sodium dodecyl sulfate with 106 cpm of the 32P-5′-end-labelled oligonucleotide probe.
TABLE 1.
Primers and probes used in strand-specific RT-PCR for detection of GBV-C RNA
| Specificity and sense | Name | nt sequence (5′ to 3′) | nt positions | Size (bp) |
|---|---|---|---|---|
| 5′ NC region | ||||
| Outer sense | A1 | CGGCACTGGGTGCAAGCCCCA | 10–30 | |
| Outer antisense | A2 | CCGGCCCCCACTGGTCCTTG | 367–387 | 377 |
| Inner sense | A3 | CGACGCCTACTGAAGTAGACG | 36–56 | |
| Inner antisense | A4 | GTACGCCTATTGGTCAAGAGA | 336–356 | 320 |
| Tagged sensea | T1 | ATGCACATTCGCCTGCAAGACGACGCCTACTGAAGTAGACG | ||
| Tagged antisensea | T2 | ATGCACATTCGCCTGCAAGAGTACGCCTATTGGTCAAGAGA | ||
| T3 | ATGCACATTCGCCTGCAAGA | |||
| 5′ NC probe | P | TAAATCCCGGTCATCCTGGTA | 127–147 | |
| β-Actin | ||||
| Sense | B1 | AGCGGGAAATCGTGCGTG | 2278–2296 | |
| Antisense | B2 | CAGGGTACATGGTGGTGCC | 2570–2589 | 311 |
| NS3 | ||||
| Outer sense | NS3-1 | GCTCGCCTATGACTCAGCATC | 4194–9214 | |
| Outer antisense | NS3-2 | GTCACCTCAACGACCTCCTCC | 4504–4524 | 330 |
| Inner sense | NS3-3 | GAGACAAAGCTGGACGTTGGT | 4226–4246 | |
| Inner antisense | NS3-4 | CAACCCACAGTCGGTGACAGA | 4478–4498 | 272 |
Primers T1 and T2 were obtained by addition of primer T3 at the 5′ position of primers A3 and A4, respectively. The expected size of PCR products obtained by using tagged primers were as follows: A1-T2, 336 bp; A2-T1, 351 bp; A3-T3, 340 bp; and A4-T3, 340 bp.
Synthetic GBV-C RNA templates.
Synthetic genomic and antigenomic GBV-C RNA strands were generated from a vector, pCRII-TOPO (TOPO TA cloning kit; Invitrogen, Carlsbad, Calif.), containing the 5′ NC region (nucleotides [nt] 1 to 592). The plasmid DNA template was linearized with HindIII or EcoRV and transcribed with T7 and SP6 RNA polymerases (Riboprobe Transcription Systems; Promega, Madison, Wis.), producing genomic and antigenomic GBV-C RNA strands, respectively. The absence of residual plasmid DNA was effected by DNase digestion for 30 min at 37°C. The absence of residual DNA was verified by inclusion of a control PCR without the RT step.
Specificity of detection of genomic and antigenomic GBV-C RNA strands.
The specificity of the genomic and antigenomic GBV-C RNA amplifications was assayed as follows: (i) by synthesis of cDNA without adding reverse transcriptase, for exclusion of PCR product contamination; (ii) by genomic and antigenomic GBV-C RNA amplification without the specific primer during RT; (iii) by addition of total RNA only after heat inactivation (for 30 min at 95°C) of the RT and posterior cDNA synthesis and nested PCR steps; (iv) by RT-nested PCR using total RNA chemically modified at its 3′ end as previously described (10); and (v) by amplification of genomic and antigenomic 5′ NC regions of the GBV-C RNA, using tagged primers in the whole RT-PCR on unmodified and chemically modified total RNA.
Semiquantification of GBV-C RNA.
Endpoint titers of GBV-C RNA were estimated by testing 10-fold dilutions of both GBV-C RNA strands from biological samples as well as synthetic GBV-C RNA templates. Titers were normalized according to the β-actin mRNA level determined on the same specimen, using RT-PCR with specific primers for β-actin mRNA (Table 1).
Sucrose centrifugation.
Culture supernatants (25 μl each) were ultracentrifuged in 2.975 ml of 20% sucrose in a buffer containing 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 150 mM NaCl at 50,000 rpm and 10°C for 24 h in an SW60 rotor (Beckman Co., Palo Alto, Calif.).
RNase sensitivity of the genomic and antigenomic GBV-C RNA strands.
Twenty-five microliters of each culture supernatant was subjected to ultracentrifugation as described above. Pellets were suspended in 200 μl of PBS and divided into two aliquots. One of these aliquots was subjected to treatment with 0.1% Nonidet P-40 (NP-40) (4 h, room temperature). Afterward, half of each aliquot was treated with RNase A (1 mg/ml; 37°C, 30 min). Finally, all pellets were extracted as described above and subjected to strand-specific RT-PCR.
Cloning and sequencing of genomic GBV-C RNA strands.
The amplified products in the 5′ NC region of the genomic GBV-C RNA strand, obtained from (i) cells of the individual donors and the cell pool at the end of the culture period (day 30), (ii) the inoculum used for experimental infection of PBMC from donors, and (iii) PBMC isolated from heparinized blood collected at the same time, and from the same patient, as the serum that was used as the inoculum, were studied. Cloning was performed in E. coli (TA cloning kit; Invitrogen, San Diego, Calif.), and the resultant nucleic acids were sequenced with the ALF-1 Express automatic sequencer (Amersham-Pharmacia). Sequences were aligned by using Clustal X software (36a). Genetic distances between all sequences obtained were calculated by the Kimura two-parameters modification method, using the PHYLIP package (version 3.5c). Statistical analysis of mean genetic distances between groups of sequences was performed with Student’s t test for comparison of means.
Western blot analysis of culture supernatants.
Twenty-five microliters of each culture supernatant was ultracentrifuged in a sucrose gradient as described above. Pellets were suspended in 100 μl of 50 mM Tris, pH 7.5, and the suspensions were aliquoted into three portions. One aliquot was left untreated; the other two aliquots were treated with 0.1% NP-40 for 3 h at room temperature. After detergent treatment, one of the two NP-40-treated aliquots was immediately frozen and the other was centrifugated again under the conditions described above. Subsequently, all samples were analyzed by polyacrylamide gel electrophoresis and Western blotting, using as primary antibodies (i) a 1:200 dilution in PBS-Tween 20 of a human serum with detectable circulating antibodies to the putative E2 protein of GBV-C (as detected with the Anti-HGenv Kit; Boehringer GmbH, Mannheim, Germany) and negative for the GBV-C RNA, (ii) a 1:200 dilution in PBS-Tween 20 of a human serum without detectable antibodies to the E2 protein of GBV-C and negative for the GBV-C RNA, and (iii) a dilution of a monoclonal antibody (MAb) produced against the E2 protein of GBV-C (27) (kindly provided by A. M. Engel, Roche Diagnostics, Penzberg, Germany) at a final concentration of 1 μg/ml. As the secondary antibody, peroxidase-conjugated rabbit anti-human immunoglobulin G (IgG; Dako A/S, Glostrup, Denmark) was used in the first two cases and peroxidase-conjugated rabbit anti-mouse IgG (Dako A/S) was used in the third case. Finally, detection was performed with a chemiluminescent substrate (SuperSignal; Pierce, Rockford, Ill.). As a positive control in the Western blot analysis, 100 ng of a recombinant putative E2 protein of GBV-C (kindly provided by I. K. Mushahwar, Virus Discovery Group, Abbott Laboratories, North Chicago, Ill.) was included.
FISH.
For fluorescent in situ hybridization (FISH), 106 mononuclear cells were centrifuged at 1,200 rpm in a Beckman F-2402 (model GS-15R) for 10 min, resuspended in freshly prepared 4% paraformaldehyde in PBS, and then fixed for 10 min at 4°C. After fixation, the cells were pipetted onto dehydrated slides (105 per slide). After air drying, the slides were washed three times in PBS and dehydrated through a graded series of ethanol dilutions (30 to 70%). The slides were stored in 70% ethanol at 4°C.
To obtain the probe, the complete 5′ NC region of the GBV-C genome (cloned in the pCR II-TOPO vector [Invitrogen]) was excised from the plasmid by EcoRI digestion and the fragment was gel purified by using a Geneclean Kit (Bio 101, Vista, Calif.). The purified DNA was labelled with digoxigenin–11-dUTP (Boehringer) by using a nick translation kit (GIBCO BRL). After ethanol precipitation, the labelled probe was redissolved in hybridization mixture (50% deionized formamide, 10% dextran sulfate, 100 μg of sonicated salmon sperm DNA per ml, and 250 μg of tRNA per ml in 2× SSC) to a final concentration of 100 ng per 20 μl and stored at −20°C.
Prior to FISH, slides were dehydrated by successive incubations in 70, 90, and 100% ethanol and then rehydrated by being put through a series of ethanol dilutions. Afterward, the slides were rinsed in PBS and postfixed in freshly prepared 4% paraformaldehyde in PBS for 20 min at room temperature. Then the cells were digested with 1 μg of proteinase K (GIBCO BRL) per ml in 20 mM Tris HCl (pH 7.4)–2 mM CaCl2 at 37°C for 7 min. After the digestion, the slides were rinsed in PBS for 5 min, refixed in 4% paraformaldehyde for 5 min, dipped in distilled water, dehydrated through a series of ethanol dilutions (30 to 100%) at −20°C, and allowed to dry for at least 2 h.
The probe was denatured for 5 min at 90°C, quenched on ice, and then applied to the slides under coverslips sealed with a rubber solution. The hybridization was carried out at 50°C for 16 h in a humidified chamber.
After the hybridization, the slides were washed in 2× SSC at 42°C for 15 min and the RNA on them was digested with RNase A (20 μg/ml; Boehringer) for 30 min at 37°C. After being washed consecutively at 42°C in 2× SSC, 0.5× SSC, and then 0.1× SSC (15 min each), the digoxigenin-labelled hybrids were detected with a fluorescein isothiocyanate conjugate (Boehringer). The signals were amplified with three antibodies (mouse antidigoxigenin, anti-mouse Ig–digoxigenin, and an antidigoxigenin-fluorescein isothiocyanate conjugate), using the Fluorescent Antibody Enhancer Set for DIG Detection Kit (Boehringer). The slides were counterstained with 4′,6-diamidino-2-phenyllindole (0.6 μg/ml) (ONCOR; Appligene, Heidelberg, Germany).
The specificity of the hybridization signals was assessed by pretreatment of the slides with RNase A (20 μg/ml) before hybridization and by hybridization with the pCR II-TOPO vector alone labelled with digoxigenin and omission of the probe in the hybridization mixture.
Image visualization of in situ-hybridized PBMC was performed with a Nikon Elipse E400 microscope. Images were acquired with a charge-coupled device camera (model DIC-N; Ward Precision Instruments, Cambridge, United Kingdom). The capture of the fluorescent signals was performed with Visiolog 5.0 image analysis software (Noesis Vision, Inc., Quebec City, Quebec, Canada).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences presented in this article are AF125468 through AF125505.
RESULTS
Sensitivity and strand specificity of the RT-PCR.
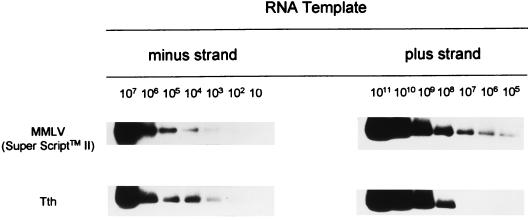
To determine the sensitivity of the RT-PCR assay developed in this study, amplification of synthetic GBV-C RNA transcripts of positive and negative polarity was performed under specific conditions for each template. Synthetic viral RNA templates corresponding to the 5′ NC genomic and antigenomic GBV-C strands were analyzed using 10-fold serial dilutions. Moloney murine leukemia virus Super Script II was able to detect 100 copies of the respective template per reaction. However, it also unspecifically detected 105 template copies of the plus strand. When Tth polymerase was used, the assay detected 100 template copies of the correct strand and unspecifically detected only 108 template copies of the plus strand (Fig. 1). The sensitivity of the assay was not affected by the presence of cellular RNA, regardless of the GBV-C RNA strand tested (data not shown). Self-priming of RNA templates was never observed, even in the presence of cellular RNA. The absence of residual plasmid DNA was confirmed by the achievement of negative results in the PCR analysis when the RT step was omitted.
FIG. 1.
Sensitivity and specificity of the RT-PCR assay for the detection of genomic and antigenomic GBV-C RNA strands. Synthetic GBV-C RNA transcripts (corresponding to the 5′ NC region) of positive and negative polarity were generated by in vitro transcription, and 10-fold dilutions were performed in polyethylene glycol- and diethylpyrocarbonate-treated water. cDNA synthesis was performed in the presence of the sense primer, and afterward the reverse transcriptase was inactivated by heating the product at 95°C for 45 min (Moloney murine leukemia virus [MMLV] Super Script II) or chelation (Tth). The number of target template copies was determined from the optical density measurement and confirmed by electrophoresis in an agarose gel. Assays included amplification of 0 to 106 RNA copies per reaction. Subsequently, the products of the nested PCRs were analyzed by Southern hybridization with a 32P-labelled probe.
When RT was performed with chemically modified RNA, the presence of antigenomic GBV-C RNA was confirmed in the cells in which antigenomic RNA had been detected by using unmodified total RNA. No amplification was obtained when the RT step was omitted or when total RNA was added to the RT reaction after reverse transcriptase inactivation. Furthermore, no antigenomic GBV-C RNA was detected when RT was performed without the corresponding primers. RT-PCR was also performed with tagged primers, using both modified and unmodified total RNA, and the sensitivity was comparable to that of conventional RT-PCR (data not shown).
Detection of genomic and antigenomic GBV-C RNA strands in cell cultures.
The presence of genomic and antigenomic GBV-C RNA strands in supernatants and cells was investigated by amplification of the 5′ NC region sequences at 4 h postinfection and at days 7, 14, 21, and 30 of culture in the five independent experiments (donors 1, 2, 3, and 4 and the cell pool). These results are shown in Table 2. Amplification of the NS3 region gave identical results. After GBV-C inoculation, and prior to cell culture, the cells were washed five times, and the last two washes were negative for GBV-C RNA (data not shown).
TABLE 2.
Detection of genomic and antigenomic GBV-C RNA strands in cells and culture supernatants by RT-PCR after in vitro GBV-C inoculation
| Time postinfection | HGV RNA type | RNA strands detected fora:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor 1
|
Donor 2
|
Donor 3
|
Donor 4
|
Pool
|
|||||||
| S | C | S | C | S | C | S | C | S | C | ||
| 4 h | Genomic | + | + | + | + | + | + | + | + | + | + |
| Antigenomic | − | − | − | − | − | − | − | − | − | − | |
| Day 7 | Genomic | + | + | + | + | + | + | + | + | + | + |
| Antigenomic | − | − | − | + | − | + | − | − | − | − | |
| Day 14 | Genomic | + | + | + | + | + | + | + | + | + | + |
| Antigenomic | − | + | + | − | − | + | − | + | − | + | |
| Day 21 | Genomic | + | + | + | + | + | + | + | + | + | + |
| Antigenomic | − | − | + | + | − | + | − | + | + | + | |
| Day 30 | Genomic | + | + | + | + | + | + | + | + | + | + |
| Antigenomic | − | − | + | − | + | + | + | + | + | + | |
S, culture supernatant; C, cultured cells. +, specified RNA type detected; −, specified RNA type not detected.
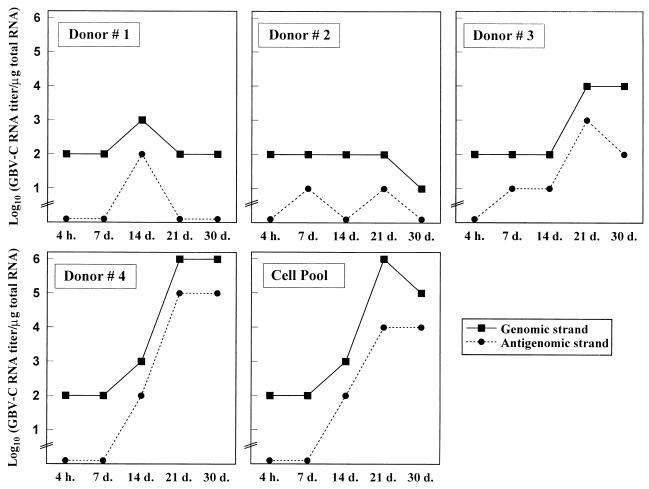
Four hours after GBV-C inoculation, genomic GBV-C RNA strands were detected in all supernatants and cells. The genomic GBV-C RNA strands were continuously detected for up to 30 days in cells (Table 2), and they were also detected in the supernatants of cell cultures. Intracellular antigenomic GBV-C RNA strands appeared after the 7th day of culture and were detected both sporadically (donors 1 and 2) and continuously (donors 3 and 4 and the cell pool) during the culture period (Table 2). Concerning the titration of intracellular GBV-C RNA, the level of the genomic GBV-C RNA strand increased during the 2nd or 3rd week of culture (donors 3 and 4 and the cell pool) and the antigenomic GBV-C RNA strand levels were always 1 log unit lower than those of the genomic strands (Fig. 2). Measurements were normalized by β-actin mRNA coamplification. In supernatants, antigenomic GBV-C RNA strands were sporadically detected at days 21 and 30 (Table 2).
FIG. 2.
Determination of GBV-C RNA content in experimentally GBV-C-infected PBMC. Results for each individual healthy blood donor and the cell pool are expressed as the log10 of the genomic and antigenomic GBV-C RNA contents per microgram of total RNA from cells, as determined with successive 10-fold dilutions at the end of the 4-h infection period and after 7, 14, 21, and 30 days of culture.
Intracellular β-actin mRNA was always detected by single RT-PCR, showing that the lack of antigenomic-strand GBV-C RNA detection was not the result of cellular RNA degradation.
Since genomic GBV-C RNA strands, and in some cases also antigenomic strands, were detected in the supernatants of cell cultures, we attempted to investigate the nature of these GBV-C strands. After sucrose ultracentrifugation, the RNase sensitivities of the genomic and antigenomic strands were evaluated before and after removal of the viral envelope by NP-40 treatment (Table 3). The results obtained showed that the antigenomic strand was sensitive to RNase after NP-40 treatment but the genomic strand remained detectable. These results suggest that the antigenomic strand detected in supernatants was not protected by a viral nucleocapsid.
TABLE 3.
RNase sensitivity of the genomic and antigenomic GBV-C RNA strands in pellets from culture supernatants of PBMC experimentally infected with GBV-C
| Treatment used on pellet derived from culture supernatanta
|
GBV-C RNA strand-specific RT-PCR resultb
|
|||
|---|---|---|---|---|
| NP-40 | RNase A | RNase inhibitor | Genomic strand | Antigenomic strand |
| − | − | − | + | + |
| − | + | − | + | + |
| + | + | − | + | − |
| + | − | − | + | + |
| + | + | + | + | + |
+, present in reaction mixture; −, absent from reaction mixture.
+, product obtained; −, no product obtained.
Sequence analysis.
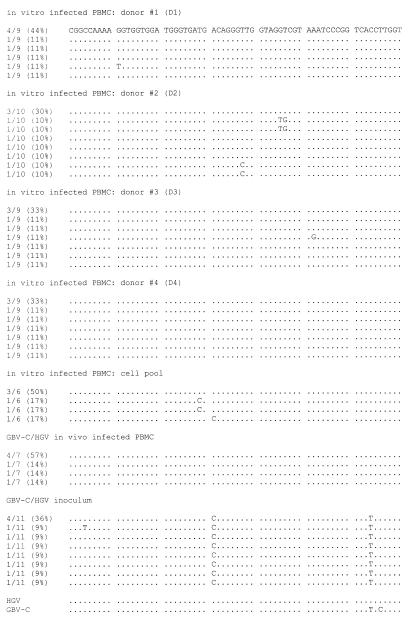
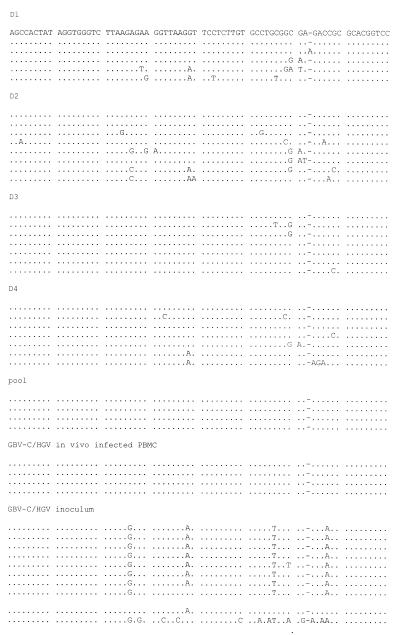
The amplified 5′ NC region products corresponding to genomic GBV-C RNA strands obtained from cells at the end of the culture period (day 30), as well as those corresponding to the inoculum used for experimental infection and to PBMC isolated from heparinized blood collected at the same time and from the same patient as the serum used as the inoculum, were cloned and sequenced. A fragment of 255 bp (from nt −632 to −378 with respect to the sequence of HGV isolate R10291 [18]) was used for sequence comparisons, and a total of 61 clones were analyzed and compared with the prototype HGV (18) and GBV-C (17) strains. As shown in Fig. 3, complexities for the in vitro-infected PBMC (individual donors and cell pool), the in vivo-infected PBMC from the patient whose serum was used as the inoculum and, the inoculum were similar (ranging from 0.57 to 0.80). A predominant sequence was found in all of the cases. However, the predominant sequences obtained for each individual donor and the cell pool, as well as for the in vivo-infected PBMC, were identical (representing between 30 and 57% of the total spectrum of sequences) and differed from the predominant sequence or any other sequence obtained from the inoculum (Fig. 3).
FIG. 3.
Alignment of the GBV-C/HGV 5′ NC sequences (255 bp; nt −632 to −378, according to R10291 isolate numbering [18]) amplified from in vitro-infected PBMC (D1, D2, D3, D4, and cell pool), in vivo-infected PBMC isolated from the patient whose serum was used as the inoculum, and the GBV-C/HGV-positive serum used as the inoculum. Sequence identity with the predominant sequence found in the in vitro-infected cells is indicated by dots, and insertions are indicated by dashes. Comparisons with HGV (R10291 [18]) and GBV-C (U36380 [17]) prototypes are shown. The pair of numbers on the left of each line, one before and one after the shill, indicates the number of clones analyzed and the percentages of each sequence within the spectrum obtained, respectively.
In addition, a comparison of the mean genetic distances between all sequences obtained in each individual donor or the cell pool, those sequences obtained from in vivo-infected PBMC or the inoculum, and those of the HGV (18) and GBV-C (17) prototypes was performed. This type of statistical analysis showed that sequences from donors and the cell pool were more closely related to those from in vivo-infected PBMC than to those derived from the inoculum (P < 0.001 [Student’s t test] in all five cases) (Table 4) but also that they were more closely related to the sequences of the inoculum than to those of the GBV-C prototype (P < 0.001 in the five cases) (Table 4).
TABLE 4.
Mean genetic distances between sequences obtained in cells from individual donors or the cell pool and the in vivo-infected PBMC or the GBV-C prototype
| Sample | Mean genetic distance ± SEM fora:
|
||
|---|---|---|---|
| Inoculum | In vivo PBMC from GBV-C-infected inoculum donor | GBV-C prototype | |
| Donor 1 | 0.055 ± 0.010 | 0.013 ± 0.017 (P < 0.001) | 0.116 ± 0.009 (P < 0.001) |
| Donor 2 | 0.058 ± 0.010 | 0.020 ± 0.018 (P < 0.001) | 0.117 ± 0.007 (P < 0.001) |
| Donor 3 | 0.053 ± 0.007 | 0.008 ± 0.005 (P < 0.001) | 0.112 ± 0.006 (P < 0.001) |
| Donor 4 | 0.051 ± 0.008 | 0.008 ± 0.006 (P < 0.001) | 0.114 ± 0.004 (P < 0.001) |
| Cell pool | 0.049 ± 0.007 | 0.006 ± 0.006 (P < 0.001) | 0.113 ± 0.003 (P < 0.001) |
The P values, obtained by using Student’s t test, refer to the comparison of the mean genetic distances between each sample and the inoculum with those between the same sample and the in vivo PBMC or the GBV-C prototype.
FISH.
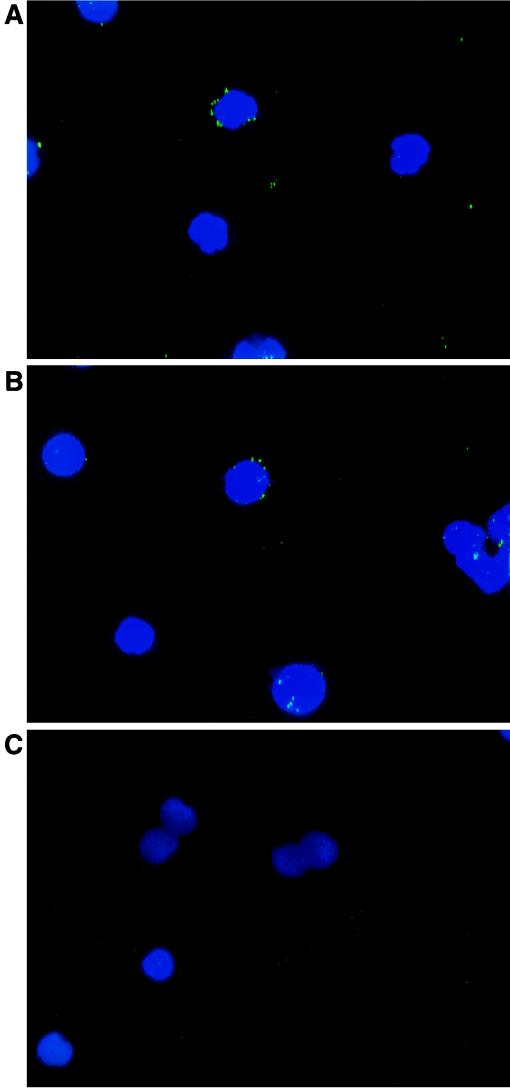
The FISH technique was applied to donor 4 and pooled cells inoculated with GBV-C-positive serum at days 7 and 30 of culture and to pooled cells inoculated with GBV-C-negative serum (negative control) on the same days. Specific hybridization signals were detected in the cytoplasm of the GBV-C-inoculated donor 4 and pool cells (Fig. 4A and B). No nuclear signals were observed. The specificity of the in situ hybridization was demonstrated by the lack of signal when the slides were hybridized with the unrelated plasmid (vector alone) and when the probe was omitted from the hybridization mixture. Furthermore, the positive signals were abolished by pre-FISH RNase treatment. No hybridization signal was observed either in cultured cells not subjected to proteinase K treatment prior to incubation with the probe (ruling out the possibility of viral particles adhering to the cell membrane) or in cells from the uninfected culture used as a negative control (Fig. 4C).
FIG. 4.
FISH in experimentally GBV-C-infected cells maintained in culture for 30 days. The fluorescent signals were always located in the cytoplasm of the cells. Shown are positive signals obtained in cells from donor 4 at days 7 (A) and 30 (B) of culture, as well as the absence of a fluorescent signal in cells from a negative-control culture (cell pool inoculated with GBV-C-negative serum at day 30) (C). Cells were counterstained with propidium iodide. Original magnifications, ×650.
Western blot analysis of culture supernatants.
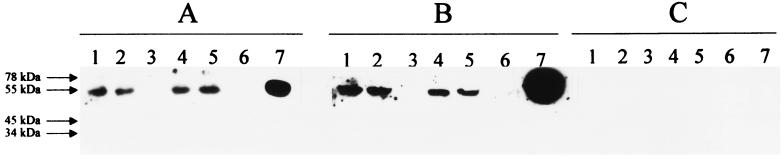
To investigate whether viral particles were present in the culture supernatants, pellets resulting from sucrose ultracentrifugation were analyzed by polyacrylamide gel electrophoresis and Western blotting before and after NP-40 treatment. In all cases tested, the use of the anti-E2 MAb, as well as the use of a dilution of a human serum with detectable anti-E2 antibodies, revealed the presence of this viral envelope protein in the culture supernatants (Fig. 5); no signal was observed when a dilution of an anti-E2-negative and GBV-C RNA-negative serum was used as the primary antibody in the Western blot analysis. Furthermore, NP-40-treated samples were ultracentrifuged again and analyzed. In those cases, neither the anti-E2 MAb nor the human serum with anti-E2 antibodies revealed the presence of the viral envelope protein in the resulting pellets (Fig. 5). The size of the protein visualized before and after NP-40 treatment was consistent with the expected size for the putative E2 protein of GBV-C.
FIG. 5.
Western blot analysis of culture supernatants. Culture supernatants of cells from donor 4 at days 21 (lanes 1 to 3) and 30 (lanes 4 to 6), and 100 ng of a recombinant putative E2 protein of GBV-C (lane 7), were subjected to polyacrylamide gel electrophoresis and Western blot analysis, using as primary antibodies a MAb prepared against the E2 protein of GBV-C (27), at a final concentration of 1 μg/ml (A); a 1:200 dilution of a GBV-C RNA-negative human serum with detectable circulating antibodies to the putative E2 protein of GBV-C (B); and a 1:200 dilution of a GBV-C RNA-negative human serum without detectable antibodies to the E2 protein of GBV-C (C). Lanes: 1 and 4, pellets frozen immediately after ultracentrifugation; 2 and 5, pellets treated with 0.1% NP-40 for 3 h at room temperature and immediately frozen; 3 and 6, pellets treated with NP-40 and ultracentrifuged again.
DISCUSSION
In this study, we investigated whether human PBMC support GBV-C replication after in vitro experimental infection. Under the conditions developed in our cell culture system, GBV-C persisted as long as 30 days in cells. We have studied the GBV-C replication status in cell cultures by four different methods: (i) strand-specific RT-PCR, (ii) sucrose ultracentrifugation and GBV-C RNA RNase sensitivity assays, (iii) FISH, and (iv) Western blot analysis.
As for HCV (29), the detection of the antigenomic GBV-C RNA strand has been questioned recently due to a lack of strand specificity in RT-PCR assays, attributed to false priming of the incorrect strand, self-priming due to secondary structure, and random priming by cellular nucleic acids. In this study, we have developed a strand-specific RT-PCR which has been extensively studied by the use of conventional and tagged primers, as well as by chemical modification of RNA. Furthermore, the presence of the antigenomic GBV-C RNA strand was confirmed by performing cDNA synthesis at a high temperature with the thermostable enzyme Tth (16). RT-PCR was performed without addition of primers during the RT step, and the lack of detection of the GBV-C RNA showed that nonspecific priming by cellular RNA or self-priming did not occur under these experimental conditions. Furthermore, the presence of the antigenomic GBV-C RNA strand was confirmed by performing cDNA synthesis at a high temperature to further ensure strand specificity and by its detection on total cellular RNA chemically modified at its 3′ end to avoid cellular RNA priming. These experimental approaches confirmed the specific detection of the antigenomic GBV-C RNA strand.
Intracellular genomic GBV-C RNA strands were detected soon after infection and were present during the entire culture period, although there were differences among donors. However, intracellular antigenomic GBV-C RNA strands were detected both intermittently at the end of the 1st and 3rd weeks and continuously from the 1st or 2nd week postinfection until the end of the culture period. The intermittent detection of the antigenomic strand was also reported by Shimizu et al. (28) for experimental HCV infection in HPB-Ma cells and by Cribier et al. (6) for PBMC infected with HCV in vitro. It is noteworthy that the concentrations of GBV-C RNA increased during the 2nd or 3rd week of culture. Moreover, the levels of genomic strands were 10 times higher than those of antigenomic strands. This ratio is similar to that reported for HCV (28). Our finding that different donors’ cells differed in their production of the virus and in the presence of the antigenomic strand has also been reported in the case of HCV (6), and this may suggest the existence of specific host factors related to the different susceptibilities to GBV-C/HGV infection. By contrast, the sequence analysis of the amplified 5′ NC genomic GBV-C RNA products in all samples (the cells experimentally infected in vitro at day 30, the inoculum, and PBMC isolated at the same time and from the same patient as the serum that was used as the inoculum) has demonstrated that (i) a predominant GBV-C sequence was found; (ii) this predominant GBV-C sequence was the same, irrespective of the culture (donor 1 to 4 or pool cells); (iii) this GBV-C variant was not found in the spectrum of sequences of the inoculum; and (iv) the predominant sequence obtained in the in vivo-infected PBMC isolated from the GBV-C-infected donor was the same GBV-C variant as that found after 30 days of culture in the in vitro experimentally infected PBMC. Taken together, these results suggest that in addition to specific host factors, the efficiency of experimental in vitro infection might also depend on the nature of the inoculum and the existence or lack of lymphotropic variants.
Genomic GBV-C RNA strands were found in all culture supernatants, while antigenomic strands were detected only in some cases. The RNase sensitivity of the antigenomic strand after NP-40 treatment of supernatants suggested that the antigenomic strand was present separately from the genomic strand and that it was not encapsidated. This observation had been previously reported for HCV (28). In addition, comparison of the results obtained in the Western blot analyses of the pellets obtained after two successive ultracentrifugations and NP-40 treatment of culture supernatants with those obtained for the pellet resulting from the first ultracentrifugation, treated or not treated with NP-40, indicated that the protein visualized was not present in the pellet after NP-40 treatment and the second ultracentrifugation. These facts, together with the observation that the size of the protein visualized before and after NP-40 treatment was consistent with the expected size for the putative E2 protein of GBV-C, suggest that viral particles have been released from PBMC experimentally infected with GBV-C.
By performing FISH, we have also provided evidence for the presence of GBV-C RNA in cells. Interestingly, these signals were confined to the cytoplasmic compartment, and nuclear signals were not observed in any case. The proportion of positive cells ranged from 0.1 to 3.5%. The possibility that the fluorescent signals were caused by GBV-C attached to the cell membrane was also ruled out. Application of our FISH technique to PBMC directly recovered from GBV-C RNA-positive patients showed the same cytoplasmic pattern (unpublished data), suggesting that the intracellular localization of the GBV-C RNA in PBMC after in vitro experimental infection with GBV-C is similar to that observed in freshly recovered PBMC from GBV-C-infected patients.
An important issue of this study is that the inoculum used for the experimental infection of cells is itself a GBV-C RNA-positive serum. Therefore, our results are not influenced by the presence of other, related viruses, such as HCV, that are usually found concomitantly in GBV-C-infected patients. To our knowledge, this is the first study with the goal of experimentally infecting human hematopoietic cells with GBV-C. As is the case with HCV, there is no a reliable in vitro culture system for GBV-C replication. Recently, Ikeda et al. (11) have found that a human T-cell line (MT-2C) and a hepatocyte cell line (PH5CH) support GBV-C infection, although their results are limited due to the nature of the serum inoculum used (a GBV-C RNA-positive serum coinfected with high levels of HCV RNA). Therefore, the possibility that HCV supports GBV-C replication in MT-2C and PH5CH cannot be excluded.
Several studies have reported that GBV-C is not hepatotropic (15, 22), whereas other authors have recently demonstrated that GBV-C indeed replicates in the human liver (21). The genomic GBV-C RNA strand has been detected in PBMC from a subset of HCV- and GBV-C-coinfected patients (20, 23); however, while the antigenomic GBV-C RNA strand has been detected in liver (16, 19), spleen (16), and bone marrow (16) samples, it has not been detected in PBMC from infected individuals, except in one case reported by Saito and coworkers (25). Ellenrieder et al. (8) have recently demonstrated an increased prevalence of GBV-C (16.3%) in patients with low-grade non-Hodgkin’s lymphoma. This fact, together with the reported association of GBV-C with mixed cryoglobulinemia (35), may be indicative of a relationship between GBV-C infection and lymphoid disorders.
In conclusion, our study has demonstrated, for the first time, GBV-C replication in human lymphoid cells after experimental in vitro infection. Extensive sequence analysis of this lymphotropic GBV-C genome(s), as well as further characterization of the cell subsets able to support GBV-C replication and the release of viral particles into the culture supernatants, needs to be performed in future investigations.
ACKNOWLEDGMENTS
This work was supported by the Fundacion Estudio Hepatitis Virales (Madrid, Spain). M.F. and M.C. are research fellows for the Fundación Conchita Rábago (Madrid, Spain). S.N., J.M. and E.R. are supported by research grants from the Fundacion Estudio Hepatitis Virales.
We thank I. K. Mushahwar (Virus Discovery Group, Abbott Laboratories, North Chicago, Ill.) for kindly providing the recombinant putative GBV-C E2 protein and A. M. Engel (Roche Diagnostics, Penzberg, Germany) for the monoclonal antibody against the putative GBV-C E2 protein. We also thank M. Rodriguez de Alba and R. Sanz (Department of Cytogenetics, Fundación Jiménez Díaz, Madrid) for support in performing the FISH technique. We are very grateful to the healthy blood donors for their kind cooperation and blood donations.
REFERENCES
- 1.Aikawa T, Sugay Y, Okamoto H. Hepatitis G infection in drug abusers with chronic hepatitis C. N Engl J Med. 1996;334:195. doi: 10.1056/NEJM199601183340316. [DOI] [PubMed] [Google Scholar]
- 2.Alter H J, Nakatsuyi Y, Melpolder J, Wages J, Wesley R, Shih J W K, Kim J P. The incidence of transfusion-associated hepatitis G virus infection and its relation to liver disease. N Engl J Med. 1997;336:747–754. doi: 10.1056/NEJM199703133361102. [DOI] [PubMed] [Google Scholar]
- 3.Berenguer M, Terrault N A, Piatak M, Yun A, Kim J P, Lau J Y N, Lake J R, Roberts J R, Ascher N, Ferrell L, Wright T. Hepatitis G virus infection in patients with hepatitis C virus infection undergoing liver transplantation. Gastroenterology. 1996;111:1569–1575. doi: 10.1016/s0016-5085(96)70019-7. [DOI] [PubMed] [Google Scholar]
- 4.Bukh J, Kim J P, Govindarajan S, Apgar C L, Foung S K H, Wages J, Jr, Yun A J, Shapiro M, Emerson S U, Purcell R H. Experimental infection of chimpanzees with hepatitis G virus and genetic analysis of the virus. J Infect Dis. 1998;177:855–862. doi: 10.1086/515255. [DOI] [PubMed] [Google Scholar]
- 5.Chomzynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Cribier B, Schmitt C, Bingen A, Kirn A, Keller F. In vitro infection of peripheral blood mononuclear cells by hepatitis C virus. J Gen Virol. 1995;76:2485–2491. doi: 10.1099/0022-1317-76-10-2485. [DOI] [PubMed] [Google Scholar]
- 7.Dille B J, Surowy T K, Gutierrez R A, Coleman P F, Knigge M F, Carrick R J, Aach R D, Hollinger F B, Stevens C E, Barbosa L H, Nemo G J, Mosley J W, Dawson G J, Mushahwar I K. An ELISA for detection of antibodies to the E2 protein of GB virus C. J Infect Dis. 1997;175:458–461. doi: 10.1093/infdis/175.2.458. [DOI] [PubMed] [Google Scholar]
- 8.Ellenrieder V, Weidenbach H, Frickhofen N, Michel D, Prummer O, Klatt S, Bernas O, Mertens T, Adler G, Beckh K. HCV and HGV in B-cell non-Hodgkin’s lymphoma. J Hepatol. 1998;28:34–39. doi: 10.1016/s0168-8278(98)80199-2. [DOI] [PubMed] [Google Scholar]
- 9.Fiordalisi G, Zanella I, Mantero G, Bettinardi A, Stellini R, Paraninfo G, Cadeo G, Primi D. High prevalence of GB virus C infection in a group of Italian patients with hepatitis of unknown etiology. J Infect Dis. 1996;174:181–183. doi: 10.1093/infdis/174.1.181. [DOI] [PubMed] [Google Scholar]
- 10.Gunji T, Kato N, Hayashi M, Saitoh S, Shimotohno K. Specific detection of positive and negative stranded hepatitis C viral RNA using chemical RNA modification. Arch Virol. 1994;134:293–302. doi: 10.1007/BF01310568. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda M, Sugiyama K, Mizutani T, Tanaka T, Tanaka K, Shimotohno K, Kato N. Hepatitis G virus replication in human cultured cells displaying susceptibility to hepatitis C virus infection. Biochem Biophys Res Commun. 1997;235:505–508. doi: 10.1006/bbrc.1997.6818. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis L M, Davidson F, Hanley J P, Yap P L, Ludlam C A, Simmonds P. Infection with hepatitis G virus among recipients of plasma products. Lancet. 1996;348:1352–1355. doi: 10.1016/s0140-6736(96)04041-x. [DOI] [PubMed] [Google Scholar]
- 13.Karayiannis P, Hadziyannis S J, Kim J, Pickering J M, Piatak M, Hess G, Yun A, McGarvey M J, Wages J, Thomas H C. Hepatitis G virus infection: clinical characteristics and response to interferon. J Viral Hepatol. 1997;4:37–44. doi: 10.1046/j.1365-2893.1997.00128.x. [DOI] [PubMed] [Google Scholar]
- 14.Kato T, Mizokami M, Nakano T, Orito E, Ohba K, Kondo Y, Tanaka Y, Ueda R, Mukaide M, Fujita K, Yasuda K, Iino S. Heterogeneity in E2 region of GBV-C/hepatitis G virus and hepatitis C virus. J Med Virol. 1998;55:109–117. [PubMed] [Google Scholar]
- 15.Laskus T, Radkowski M, Wang L-F, Vargas H, Rakela J. Lack of evidence for hepatitis G virus replication in the livers of patients coinfected with hepatitis C and G viruses. J Virol. 1997;71:7804–7806. doi: 10.1128/jvi.71.10.7804-7806.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laskus T, Radkowski M, Wang L-F, Vargas H, Rakela J. Detection of hepatitis G virus replication sites by using highly strand-specific Tth-based reverse transcriptase PCR. J Virol. 1998;72:3072–3075. doi: 10.1128/jvi.72.4.3072-3075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leary T P, Muerhoff A S, Simons J N, Pilot-Matias T J, Erker J C, Chalmers M L, Schlauder G G, Dawson G J, Desai S M, Mushahwar I K. Sequence and genomic organization of GBV-C: a novel member of the flavivirus associated with human non-A-E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Linnen J, Wages J, Zhank-Keck Z-Y, Fry K E, Krawezynski K Z, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, Karayiannis P, Fung K, Nakatsuji Y, Shih J W K, Young L, Piatak M, Jr, Hoover C, Fernandez J, Chen S, Zou J-C, Morris T, Hyams K C, Ismay S, Lifson J D, Hess G, Foung S K H, Thomas H, Bradley D, Margolis H, Kim J P. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 19.Madejón A, Fogeda M, Bartolomé J, Pardo M, González C, Cotonat T, Carreño V. GB virus C RNA in serum, liver, and peripheral blood mononuclear cells from patients with chronic hepatitis B, C, and D. Gastroenterology. 1997;113:573–578. doi: 10.1053/gast.1997.v113.pm9247478. [DOI] [PubMed] [Google Scholar]
- 20.Mellor J, Haydon G, Blair C, Livingstone W, Simmonds P. Low level or absent in vivo replication of hepatitis C virus and hepatitis G virus/GB virus C in peripheral blood mononuclear cells. J Gen Virol. 1998;79:705–714. doi: 10.1099/0022-1317-79-4-705. [DOI] [PubMed] [Google Scholar]
- 21.Mushahwar I K, Zuckerman J N. Clinical implications of GB virus C. J Med Virol. 1998;56:1–3. doi: 10.1002/(sici)1096-9071(199809)56:1<1::aid-jmv1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Pessoa M G, Terrault N A, Detmer J, Kolberg J, Collins M, Hassoba H M, Wright T L. Quantitation of hepatitis G and C viruses in the liver: evidence that hepatitis G virus is not hepatotropic. Hepatology. 1998;27:877–880. doi: 10.1002/hep.510270335. [DOI] [PubMed] [Google Scholar]
- 23.Radkowski M, Wang L-F, Vargas H, Rakela J, Laskus T. Lack of evidence for GB virus C/hepatitis G virus replication in peripheral blood mononuclear cells. J Hepatol. 1998;28:179–183. doi: 10.1016/0168-8278(88)80002-3. [DOI] [PubMed] [Google Scholar]
- 24.Ross R S, Viazov S, Schmitt U, Schmolke S, Tacke M, Ofenloch-Haehnle B, Holtmann M, Muller N, Villa G D, Yoshida C F, Oliveira J M, Szabo A, Paladi N, Kruppenbacher J P, Philipp T, Roggendorf M. Distinct prevalence of antibodies to the E2 protein of GB virus C/hepatitis G virus in different parts of the world. J Med Virol. 1998;54:103–106. doi: 10.1002/(sici)1096-9071(199802)54:2<103::aid-jmv6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Saito S, Tanaka K, Kondo M, Morita K, Kitamura T, Kiba T, Numata K, Sekihara H. Plus- and minus-stranded hepatitis G virus RNA in liver tissue and in peripheral blood mononuclear cells. Biochem Biophys Res Commun. 1997;237:288–291. doi: 10.1006/bbrc.1997.7103. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Tanaka T, Okamoto H, Miyakawa Y, Mayumi M. Association of circulating hepatitis G virus with lipoproteins for a lack of binding with antibodies. Biochem Biophys Res Commun. 1996;229:719–725. doi: 10.1006/bbrc.1996.1871. [DOI] [PubMed] [Google Scholar]
- 27.Schmolke S, Tacke M, Schmitt U, Engel A M, Ofenloch-Haehnle B. Identification of hepatitis G virus particles in human serum by E2-specific monoclonal antibodies generated by DNA immunization. J Virol. 1998;72:4541–4545. doi: 10.1128/jvi.72.5.4541-4545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu Y K, Purcell R H, Yoshikura H. Correlation between the infectivity of hepatitis C virus in vivo and its infectivity in vitro. Proc Natl Acad Sci USA. 1993;90:6037–6041. doi: 10.1073/pnas.90.13.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shindo M, Di Bisceglie A M, Akatsuka T, Fong T-L, Scaglione L, Donets M, Hoofnagle J H, Feinstone S M. The physical state of the negative strand of hepatitis C virus RNA in serum of patients with chronic hepatitis C. Proc Natl Acad Sci USA. 1994;91:8719–8723. doi: 10.1073/pnas.91.18.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons J N, Desai S M, Schultz D E, Lemon S M, Mushahwar I K. Translation initiation in GB viruses A and C: evidence for internal ribosome entry and implications for genome organization. J Virol. 1996;70:6126–6135. doi: 10.1128/jvi.70.9.6126-6135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons J N, Leary T P, Dawson G J, Pilot-Matias T J, Muerhoff A S, Schlauder G G, Desai S M, Mushahwar I K. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med. 1995;1:564–569. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 32.Simons J N, Pilot-Matias T J, Leary T P, Dawson G J, Desay S M, Schlauder G G, Muerhoff A S, Erker J C, Buijk S L, Chalmers M L, Van Sant C L, Mushahwar I K. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc Natl Acad Sci USA. 1995;92:3401–3405. doi: 10.1073/pnas.92.8.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tacke M, Kiyosawa K, Stark K, Schlueter V, Ofenloch-Haehnle B, Hess G, Engel A M. Detection of antibodies to a putative hepatitis G virus envelope protein. Lancet. 1997;349:318–320. doi: 10.1016/S0140-6736(96)06461-6. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka E, Alter J, Nakatsuji Y, Shih W-K, Kim J P, Matsumoto A, Kobayashi M, Kiyosawa K. Effect of hepatitis G virus infection on chronic hepatitis C. Ann Intern Med. 1996;125:772–773. doi: 10.7326/0003-4819-125-9-199611010-00007. [DOI] [PubMed] [Google Scholar]
- 35.Tepper J L, Feinman S V, D’Costa L, Sooknannan R, Pruzanski W. Hepatitis G and hepatitis C RNA viruses coexisting in cryoglobulinemia. J Rheumatol. 1998;25:925–928. [PubMed] [Google Scholar]
- 36.Thomas H C, Pickering J, Karayiannis P. Identification, prevalence and aspects of molecular biology of hepatitis G virus. J Viral Hepatol. 1997;4(Suppl. 1):51–54. doi: 10.1111/j.1365-2893.1997.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 36a.Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G. The CLUSTAL-X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomás J F, Rodríguez E, Bartolomé J, Madejón A, Fogeda M, Oliva H, Moreno A, Fernández J M, Carreño V. Detection of hepatitis G virus from serum and liver of a patient with long-term liver dysfunction after autologous bone marrow transplantation. Bone Marrow Transplant. 1997;19:1053–1057. doi: 10.1038/sj.bmt.1700782. [DOI] [PubMed] [Google Scholar]
- 38.Wang J T, Tsai F C, Lee C-Z, Chen P-J, Sheu J C, Wang T H, Chen D-S. A prospective study of transfusion associated GB virus C infection: similar frequency but different clinical presentation compared with hepatitis C virus. Blood. 1996;88:1881–1886. [PubMed] [Google Scholar]
- 39.Xiang J, Klinzman D, McLinden J, Schmidt W N, LaBrecque D R, Gish R, Stapleton J T. Characterization of hepatitis G virus (GB-C virus) particles: evidence for a nucleocapsid and expression of sequences upstream of the E1 protein. J Virol. 1998;72:2738–2744. doi: 10.1128/jvi.72.4.2738-2744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshiba M, Okamoto H, Mishiro S. Detection of the GBV-C hepatitis virus genome in serum from patients with fulminant hepatitis of unknown aetiology. Lancet. 1995;346:1131–1132. doi: 10.1016/s0140-6736(95)91802-7. [DOI] [PubMed] [Google Scholar]