Abstract
Intestinal trefoil factor 3 (TFF3) is a protein secreted by many cell types, and its serum and urine levels vary in patients with kidney disease. Therefore, the present study aimed to determine the diagnostic value of TFF3 in allogeneic kidney transplant patients included in the one-year follow-up. To analyze the influence of the diagnostic method used, we studied the type of biological material and the time elapsed since renal transplantation on the parameter’s value. The study also aimed to investigate the relationship between TFF3 levels and creatinine and estimated glomerular filtration rate (eGFR) values in the serum and urine of the patients studied. The study used blood and urine samples from adult patients (n = 19) 24–48 h, 6 months, and 12 months after kidney transplantation. We collected one-time blood and urine from healthy subjects (n = 5) without renal disease. We applied immunoenzymatic ELISA and xMap Luminex flow fluorimetry to determine TFF3 in serum and urine. There was a significant difference in TFF3 levels in the serum of patients collected on the first one or two days after kidney transplantation compared to the control group (determined by ELISA and Luminex) and six months and one year after kidney transplantation (ELISA). We observed a correlation between creatinine concentration and urinary TFF3 concentration (ELISA and Luminex) and a negative association between eGFR and urinary (ELISA) and serum (Luminex) TFF3 concentration in patients on the first and second days after kidney transplantation. We noted significant correlations between eGFR and TFF3 levels in the serum and urine of patients determined by the two methods six months and one year after transplantation. In women, we observed that urinary TFF3 concentration increased significantly with increasing creatinine and that with increasing eGFR, urinary TFF3 concentration determined by two methods decreased significantly. In the present study, the choice of diagnostic method for the determination of TFF3 in serum and urine significantly affected the concentration of this biomarker. The values of this parameter determined by ELISA were higher than those assessed using the Luminex assay. Based on the presented results, we can conclude that TFF3 has great potential to monitor renal transplant patients. Determination of this protein in parallel with creatinine and eGFR levels in serum and urine may provide helpful diagnostic information.
Keywords: transplantation, biomarkers, intestinal trefoil factor 3
1. Introduction
Indirect assessment of renal damage in patients after allogeneic kidney transplantation is mainly based on evaluating values of classical laboratory parameters, including serum electrolytes, urea, and creatinine, with an estimation of estimated glomerular filtration rate (eGFR) and a general urinalysis, which is insufficient. Therefore, the panel of these tests should be expanded to include new parameters, including protein biomarkers [1]. A biomarker of renal injury should be an indicator that can be measured and assessed as a component of a pathogenic process, biological process or pharmacological response [2].
Intestinal trefoil factor 3 (TFF3) is a member of the human trefoil factor family, along with the peptides TFF1 and TFF2 [3]. This protein is mainly secreted by mucosal cells of the small and large intestines [4]. It allows it to act as a biomarker in ulcerative colitis and correlates well with acute phase protein levels [5]. In addition, this protein may participate in glucose metabolism [6]. TFF3 is also secreted by nerve cells and regulates learning processes [7]. This peptide has neuroprotective effects, as it extinguishes caspase-3 activity, which damages microglial cells [8]. TFF3 also has anti-apoptotic and pro-proliferative functions and is thought to contribute to the progression of solid tumors [9]. In addition, TFF3 may influence the metastasis of cancer cells in epithelial tissues [10]. The effect of TFF3 on the regenerative capacity of the mucosa has led to ongoing attempts to use this protein in therapy [11]. We suggest that TFF3 expresses in all mucus-secreting tissues, including renal tubules.
The intestinal trefoil factor can be used as a biomarker in patients with kidney damage [12]. The results show that serum levels of TFF3 are significantly higher in patients with chronic kidney disease (CKD) than in controls. In addition, we observed that the level of this protein is higher in patients with CKD than in those with other lifestyle diseases [13]. TFF3 levels also increased in the urine of patients with worsening chronic kidney disease, and in combination with the presence of microalbuminuria, this protein may be a predictor of a worse prognosis [14]. The Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have recognized the determination of urinary TFF3 levels as a specific and sensitive biomarker for monitoring drug-induced kidney injury [15]. TFF3 has also been analyzed as a marker of the autoimmune process. Yan et al. [16] noted that plasma TFF3 levels were higher in systemic lupus erythematosus (SLE) patients with nephritis than in those with SLE without renal lesions. In addition, levels of this protein correlate with clinical features of dysfunction in lupus nephritis. TFF3 levels may increase in children with congenital renal and urinary tract abnormalities and may predict worsening renal function [17]. It had high serum levels of this protein immediately after kidney transplantation and a subsequent decrease, irrespective of delayed graft function (DGF) [18]. The role of TFF3 as a marker of renal allograft rejection is not yet well understood. Therefore, the present study aimed to determine the diagnostic value of TFF3 in allogeneic kidney transplant patients included in the one-year follow-up period and to analyze the influence of the diagnostic method used, the type of biological material and the time elapsed since kidney transplantation on the value of the parameter studied. In addition, we analyzed the relationship between TFF3 levels and creatinine and eGFR values in the patients studied.
2. Results
Table 1 shows TFF3 levels determined in the serum and urine of control subjects and patients one to two days, six months, and one year after kidney transplantation. The concentration of this protein was highest in the serum and urine of patients one day after kidney transplantation and then decreased six months and one year after surgery. Only for the Luminex urinary TFF3 assay were concentrations highest in patients one year after kidney transplantation. We found much lower TFF3 concentrations in the serum and urine of control subjects. In renal transplant patients, creatinine concentrations decreased with time while eGFR values increased.
Table 1.
Creatinine, estimated glomerular filtration rate (eGFR), and trefoil factor 3 (TFF3) concentrations in serum and urine of study patients collected one to two days, six months, and one year after kidney transplantation were assessed using ELISA and Luminex (Med., median; Q1, lower quartile; Q2, upper quartile).
| Group | Time after Kidney Transplantation | Creatinine (in mg/dL) Med (Q1–Q2) |
eGFR (in mL/min/1.73) Med (Q1–Q2) |
Concentration of TFF3 (in ng/mL) | |||
|---|---|---|---|---|---|---|---|
| ELISA | Luminex | ||||||
| Serum Med (Q1–Q2) |
Urine Med (Q1–Q2) |
Serum Med (Q1–Q2) |
Urine Med (Q1–Q2) |
||||
| control | 0.78 (0.65–0.93) |
94 (92–112) |
7.84 (6.11–8.17) |
89.26 (79.72–90.78) |
3.18 (2.10–3.86) |
31.95 (23.88–37.78) |
|
| patients after kidney transplantation | One to two days | 4.05 (2.58–5.94) |
13 (9–21) |
63.65 (31.51–82.58) |
210.4 (66.62–273.9) |
9.79 (8.24–21.46) |
35.39 (14.85–72.29) |
| Six months | 1.44 (1.14–1.53) |
52 (43–68) |
15.73 (12.76–24.89) |
106.5 (43.38–268.1) |
3.52 (2.71–6.78) |
34.90 (16.13–76.40) |
|
| One year | 1.32 (1.18–1.58) |
53 (47–63) |
16.22 (10.54–20.94) |
95.16 (54.80–290.8) |
4.87 (2.76–6.97) |
39.11 (19.64–83.27) |
|
We used the nonparametric Mann-Whitney U test (Table 2) to compare patients’ serum and urine TFF3 concentration values at different times after kidney transplantation obtained using ELISA and Luminex with the control group. There was a significant difference in the concentration of TFF3 determined in patients’ serum collected one to two days after kidney transplantation compared to the control group determined by ELISA (p = 0.001) and Luminex (p = 0.004). Additionally, TFF3 levels in patient serum were determined using ELISA six months (p = 0.009) and one year (p = 0.013) after kidney transplantation.
Table 2.
Comparison of TFF3 values in serum and urine of patients of the study group at different times after kidney transplantation and the control group obtained using ELISA and Luminex (SG, study group; CG, control group; A, one to two days after kidney transplantation; B, six months after kidney transplantation; C, one year after kidney transplantation; U, Mann-Whitney test value for small-size groups; p, significance level p = 0.05).
| TFF3 Level | Time after Kidney Transplantation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One to Two Days | Six Months | One Year | ||||||||||
| Sum of Ranks SG | Sum of Ranks CG | U Mann-Whitney | Sum of Ranks SG |
Sum of Ranks CG |
U Mann-Whitney | Sum of Ranks SG |
Sum of Ranks CG | U Mann-Whitney | ||||
| U | p | U | p | U | p | |||||||
| ELISA | ||||||||||||
| in serum | 284 | 16 | 1 | 0.001 | 275 | 25 | 10 | 0.009 | 273 | 27 | 12 | 0.013 |
| in urine | 256 | 44 | 29 | 0.201 | 239 | 61 | 46 | 0.943 | 243 | 57 | 42 | 0.722 |
| Luminex | ||||||||||||
| in serum | 279 | 21 | 6 | 0.004 | 253 | 47 | 32 | 0.286 | 258 | 42 | 27 | 0.155 |
| in urine | 242 | 58 | 43 | 0.776 | 239 | 61 | 46 | 0.943 | 246 | 54 | 39 | 0.570 |
There was a correlation between creatinine levels and TFF3 levels in urine collected from patients on the first and second days after renal transplantation, as determined by ELISA (p = 0.022) and Luminex (p = 0.006) (Table 3). In women after renal transplantation, we observed that urinary TFF3 levels determined by ELISA (p = 0.013) and Luminex (p = 0.030) increased significantly with increasing creatinine (Table 4). Significant correlations between creatinine levels and TFF3 levels in serum and urine determined by the two methods were noted in all patients studied six months and one year after renal transplantation.
Table 3.
Spearman’s rank correlation coefficients between creatinine, estimated glomerular filtration rate (eGFR), and trefoil factor 3 (TFF3) levels in patients (n = 19), including men (n = 10) and women (n = 9) one to two days, six months, and one year after kidney transplantation (R, rho, ρ; significance level p = 0.05).
| TFF3 Level in | Creatinine | eGFR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients after Kidney Transplantation | Patients after Kidney Transplantation | |||||||||||
| Total | Men | Women | Total | Men | Women | |||||||
| R | p | R | p | R | p | R | p | R | p | R | p | |
| One to two days after kidney transplantation | ||||||||||||
| serum(ELISA) | 0.22 | 0.367 | 0.44 | 0.200 | −0.30 | 0.433 | −0.19 | 0.438 | −0.48 | 0.159 | 0.48 | 0.194 |
| urine (ELISA) | 0.52 | 0.022 | 0.61 | 0.060 | 0.78 | 0.013 | −0.63 | 0.004 | −0.59 | 0.072 | −0.88 | 0.002 |
| serum (Luminex) | 0.61 | 0.006 | 0.59 | 0.074 | 0.30 | 0.433 | −0.63 | 0.004 | −0.63 | 0.052 | −0.41 | 0.273 |
| urine (Luminex) | 0.34 | 0.156 | 0.42 | 0.229 | 0.72 | 0.030 | −0.45 | 0.053 | −0.40 | 0.249 | −0.83 | 0.006 |
| Six months after kidney transplantation | ||||||||||||
| serum (ELISA) | 0.27 | 0.268 | 0.14 | 0.700 | 0.47 | 0.205 | −0.34 | 0.152 | −0.22 | 0.533 | −0.45 | 0.222 |
| urine (ELISA) | 0.23 | 0.340 | 0.10 | 0.789 | 0.45 | 0.224 | −0.21 | 0.387 | −0.10 | 0.777 | −0.58 | 0.104 |
| serum (Luminex) | 0.21 | 0.385 | −0.01 | 0.973 | 0.67 | 0.050 | −0.50 | 0.031 | −0.22 | 0.533 | −0.71 | 0.032 |
| urine (Luminex) | 0.11 | 0.657 | −0.02 | 0.947 | 0.28 | 0.460 | −0.25 | 0.295 | −0.02 | 0.960 | −0.45 | 0.222 |
| One year after kidney transplantation | ||||||||||||
| serum (ELISA) | 0.15 | 0.528 | −0.19 | 0.603 | 0.57 | 0.112 | −0.27 | 0.260 | 0.41 | 0.244 | −0.80 | 0.010 |
| urine (ELISA) | 0.24 | 0.314 | 0.18 | 0.627 | 0.25 | 0.516 | −0.21 | 0.387 | −0.02 | 0.960 | −0.25 | 0.516 |
| serum (Luminex) | 0.23 | 0.349 | −0.22 | 0.533 | 0.73 | 0.025 | −0.18 | 0.459 | 0.43 | 0.214 | −0.85 | 0.004 |
| urine (Luminex) | 0.19 | 0.437 | −0.05 | 0.881 | 0.30 | 0.433 | −0.13 | 0.598 | 0.19 | 0.603 | −0.28 | 0.460 |
Table 4.
Creatinine, estimated glomerular filtration rate (eGFR), and trefoil factor 3 (TFF3) concentrations in serum of men (n = 10) and women (n = 9) one to two days, six months, and one year after kidney transplantation assessed using ELISA and Luminex ((Med., median; Q1, lower quartile; Q2, upper quartile).
| Level in | TFF3 (ng/mL) Med (Q1–Q2) |
||||||
|---|---|---|---|---|---|---|---|
| Men | Women | ||||||
| Time after Transplantation | |||||||
| One to Two Days | Six Months | One Year | One to Two Days | Six Months | One to Two Years | ||
| ELISA | serum | 69.22 (30.48–87.76) |
14.80 (13.29–24.89) |
12.69 (8.18–17.81) |
63.65 (52.04–76.98) |
18.34 (12.76–22.89) |
18.72 (15.32–20.92) |
| urine | 137.0 (66.62–266.0) |
138.5 (34.91–333.5) |
89.27 (49.52–290.8) |
213.8 (172.6–273.9) |
53.47 (45.21–212.5) |
184.4 (68.60–212.9) |
|
| Luminex | serum | 14.04 (9.63–22.28) |
3.24 (2.71–5.98) |
3.90 (2.23–6.02) |
9.56 (8.24–13.95) |
4.61 (2.89–8.11) |
5.54 (3.26–6.97) |
| urine | 28.78 (14.01–41.06) |
50.69 (12.77–64.59) |
36.43 (13.87–83.27) |
57.25 (25.14–72.29) |
22.20 (18.24–76.40) |
52.67 (24.02–78.24) |
|
| Level in |
Creatinine (mg/dL) in serum
Med (Q1–Q2) |
||||||
| Men | Women | ||||||
| Time after transplantation | |||||||
| One to two days | Six months | One year | One to two days | Six months | One year | ||
| serum | 5.36 (3.31–9.04) |
1.47 (1.18–1.63) |
1.40 (1.22–1.54) |
3.81 (2.58–4.05) |
1.35 (1.03–1.48) |
1.21 (1.17–1.58) |
|
|
GFR (in mL/min/1.73)
Med (Q1–Q2) |
|||||||
| Men | Women | ||||||
| Time after transplantation | |||||||
| One to two days | Six months | One year | One to two days | Six months | One year | ||
| estimated | 11 (7–19) | 58 (47–69) | 59 (52–66) | 14 (12–21) | 47 (41–57) | 49 (39–54) | |
The correlation analysis between eGFR and TFF3 concentration was in line with the relationships discussed above (Table 3). On the first and second days after renal transplantation, all study patients had a negative correlation between eGFR and urine TFF3 concentration determined by ELISA (p = 0.004) and serum TFF3 concentration determined by Luminex (p = 0.004). In the female renal transplant patients studied, the urinary TFF3 concentration determined by the two methods decreased significantly with increasing eGFR. In all study patients six months and one year after transplantation, significant correlations were observed between eGFR and TFF3 concentrations in both serum and urine, determined by two methods.
There was a significantly higher concentration of TFF3 (p < 0.0001) in the serum of renal transplant patients tested at three time points combined using ELISA (Me = 20.35 ng/mL) relative to the TFF3 value determined by Luminex (Me = 6.02 ng/mL). It was confirmed in a detailed analysis one to two days, six months, and one year after kidney transplantation. Serum TFF3 levels in renal transplant patients assessed using ELISA were significantly higher one to two days (p < 0.0001), six months (p < 0.0001), and one year after renal transplantation (p < 0.0001) relative to values obtained using the Luminex method. When TFF3 levels were averaged in the urine of renal transplant patients collected at the three time points together, we found significantly higher levels of this biomarker (p < 0.0001) determined by ELISA (Me = 122.33 ng/mL) relative to values determined by Luminex (Me = 35.39 ng/mL). Detailed analyses separately for each of the three intakes also confirmed it.
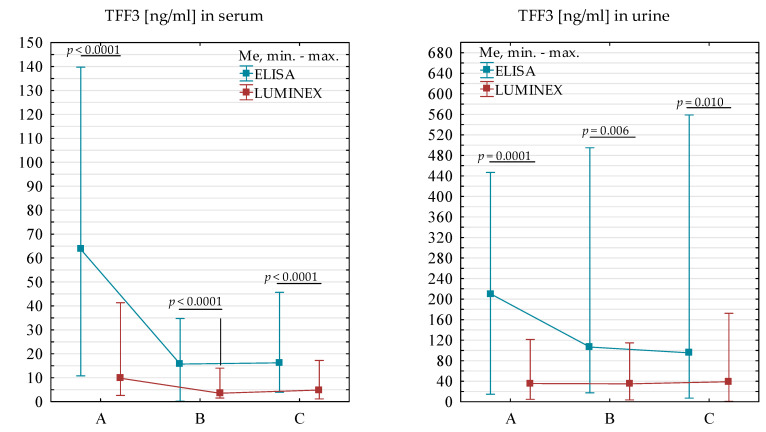
The concentration of TFF3 in the urine of patients determined by ELISA was significantly higher one to two days (p < 0.0001), six months (p = 0.006), and one year (p = 0.010) after kidney transplantation compared to the values of this biomarker determined by Luminex (Figure 1). The differences between the values obtained by the two methods may be due to the test itself. ELISA is a technique to detect relative mass values for naturally occurring human TFF3, but Luminex is used to evaluate many parameters at once. Along with this marker, other proteins are also found in the standard cocktail. The same diluent and optimal pH must be used for all tested parameters, which may cause discrepancies in the values obtained between methods.
Figure 1.
Comparison of TFF3 values in serum and urine of patients collected one to two days (A), six months (B), and one year (C) after kidney transplantation using ELISA and Luminex methods.
Due to the large scatter in the data, the change in values between the measurements was additionally analyzed. This analysis showed differences between the methods only in the measurements on the first day after transplantation and after six months and between measurements on the first day after transplantation and one year after transplantation in serum (Table 5).
Table 5.
Differences in values in serum and urine between two measurements (A–B, one to two days to six months; A–C, one to two days to one year; B–C, six months to one year; U, Mann-Whitney test value for small-size groups; p, significance level p = 0.05).
| Measurements | Sum of Ranks (ELISA) | Sum of Ranks (Luminex) |
U Mann-Whitney | |
|---|---|---|---|---|
| U | p | |||
| A–B TFF3 in serum | 495 | 246 | 56 | <0.0001 |
| A–C TFF3 in serum | 477 | 264 | 74 | 0.002 |
| B–C TFF3 in serum | 381 | 360 | 170 | 0.770 |
| A–B TFF3 in urine | 397 | 344 | 154 | 0.448 |
| A–C TFF3 in urine | 408 | 333 | 143 | 0.280 |
| B–C TFF3 in urine | 386 | 355 | 165 | 0.661 |
The relationship between glucose and TFF3 was also checked by Spearman’s rank method six months and a year after kidney transplantation by ELISA and Luminex methods, but no significant correlations were found (p > 0.05).
3. Discussion
The results presented here extend the knowledge regarding the usefulness of testing serum and urine TFF3 levels in renal transplant patients. It appears that analysis of this parameter may reflect renal function after transplantation.
Various cells synthesis TFF3 and have many functions, including involvement in wound healing, mucosal protection, cell proliferation and migration. However, the role of TFF3 in these processes is not fully understood. Clinical and experimental findings indicate that TFF3 is also involved in many pathological processes, including mucosal disorders and cancer [19,20]. Increased expression of TFF3 has been observed in some cancers, including breast, lung, liver, prostate, gastric, and endometrial cancers [21,22,23,24,25,26,27]. This peptide has potential value as a biomarker, including cancer metastasis [28,29].
TFF3 is synthesized in the urinary tract epithelia, mainly the proximal and distal tubules and collecting ducts [30]. Elevated urinary TFF3 levels have been found in people of African descent, patients with diabetes and those taking blood pressure-lowering drugs [31]. We concluded that TFF3 might influence the regenerative capacity of the kidney, possibly through restitution after injury, effects on differentiation, or both [31]. Tanaka et al. [32] found that increased TFF3 mRNA expression in renal biopsy specimens from patients with tubulointerstitial fibrosis in IgA nephropathy (IgAN) was associated with increased urinary TFF3 levels and that examination of urinary TFF3 levels may reflect interstitial tubular fibrosis in IgAN patients. We found elevated serum and urine TFF3 levels in patients with CKD, which may be due to the secretion of this peptide by damaged renal tubular epithelial cells [13]. Increased levels of TFF3 have been associated with excess mortality risk, which traditional markers of kidney disease may overlook. Endre [18] described a study by Pianta et al. (unpublished data), which noted an increase in TFF3 levels in patients (n = 75) after transplantation and a subsequent decrease, irrespective of the presence of DGF. The present study confirmed this relationship and showed that serum TFF3 levels determined by ELISA decrease significantly after renal transplant surgery. In contrast, no such relationship was shown in serum as determined by the Luminex method or in urine by both methods. In contrast, Pianta et al. [33], based on an analysis of serum and urine results from kidney recipients (n = 81), concluded that urinary TFF3 concentration testing is not a promising biomarker for the early diagnosis of delayed graft function.
The study presented here extends the knowledge of this biomarker in renal function after transplantation, as seen by the significant correlations between TFF3 and creatinine and eGFR at different times. We found the choice of diagnostic method for the determination of TFF3 in serum and urine to have a significant effect on the concentration of this biomarker. The values of this parameter determined with ELISA are higher than those assessed using the Luminex assay.
There are several limitations worth noting in this analysis. A small sample size and the single center approach suggest the need for validation in larger, multi-center populations. It would be judicious to plan a biopsy prior performed at time points corresponding to blood and urine collections. Furthermore, our findings need to be validated through other studies to ensure consistency of observed associations and their subsequent impact on clinical trial design. Monitoring patients for only 24 h, 6 and 12 months after kidney transplantation may not have captured all variables which contribute to ongoing renal injury in this group. However, a notable strength of the study is the prospective design which enabled access to urine output and serum creatinine values for 24 h, 6 and 12 months after kidney transplantation in all patients. Thus, it is likely that this marker will show clinically relevant performance. Further research efforts are certainly needed for the pursuit of data on each patients clinical course, such that it is understood whether or not the TFF3 levels that are being obtained might possibly also reflect other recent events in the patients clinical course.
Based on the results of our and other authors’ studies, TFF3 is a promising marker for monitoring the status of renal transplant patients; however, these data should be approached with caution, and further studies are needed.
4. Materials and Methods
We conducted the study between 2018 and 2022. The study included adult kidney transplant patients from deceased donors in the region of northwestern Poland. They were patients of the Transplant Clinic of the Independent Public Clinical Hospital No. 2 PUM in Szczecin. The exclusion criterion for the study was the recipient’s age below 18 years, and the inclusion criterion was preserved graft function one year after surgery. The study group comprised 19 patients, including nine women and 10 men, aged 26 to 71 years (mean age 51.9 ± 12.1 years) and weighing 61 to 114 kg (mean 78.32 ± 13.33 kg). Their mean time on dialysis was 2.79 ± 3.60 years (0 to 16 years). Demographic data of the study group are presented in Table 6. We collected fasting blood from the patients in the morning and urine at intervals according to the schedule: 1–2 days, 6 months, and 12 months. Samples were collected at these time points to determine the dynamics of changes in concentrations of selected biomarkers in short- and long-term post-transplant evaluation. We collected 57 blood samples and 57 urine samples in the study group. In addition, analogous material was also collected once from five healthy subjects (three women and two men) aged between 28 and 44 years (mean subject age 32.6 ± 6.69 years) and weighing between 58 and 90 kg (mean 75.6 ± 14.47 kg) without renal disease, who constituted the control group. Table 7 shows the demographic dataof the control group. Characteristics of patients in the study and control groups are presented in Table 8.
Table 6.
Demographic data of the study group (M, men; W, women).
| Patient Number | Gender | Age at tx (in Years) |
Weight (in kg) | Total Dialysis Time (in Years) |
Place of Residence | Diagnosis of the Disease |
|---|---|---|---|---|---|---|
| 1 | M | 49 | 66 | 16 | village | Primary glomerulopathies without renal biopsy |
| 2 | W | 40 | 61 | 5 | village | Secondary glomerulopathies—in systemic lupus erythematosus |
| 3 | W | 58 | 80 | 2 | city with a population of over 100,000 | Diabetic nephropathy—in type I diabetes |
| 4 | M | 55 | 72 | 2 | village | Primary glomerulopathies with renal biopsy |
| 5 | M | 50 | 82 | 0 | village | Primary glomerulopathies with renal biopsy |
| 6 | W | 63 | 71 | 1 | city with a population of over 100,000 | Primary glomerulopathies with renal biopsy |
| 7 | M | 60 | 80 | 3 | town with less than 20,000 inhabitants | Hypertensive nephropathy |
| 8 | M | 54 | 91 | 3 | city with a population of over 100,000 | Hypertensive nephropathy |
| 9 | M | 36 | 105 | 1 | city with a population of over 100,000 | Cystic kidney disease—polycystic kidney disease |
| 10 | W | 71 | 68 | 0 | city of 20,000–100,000 inhabitants | Condition after right nephrectomy due to roponephrosis |
| 11 | W | 57 | 68 | 3 | town with less than 20,000 inhabitants | Hypertensive nephropathy |
| 12 | W | 47 | 78 | 0 | city of 20,000–100,000 inhabitants | Interstitial non-bacterial nephritis—other or unspecified |
| 13 | M | 61 | 88 | 0 | town with less than 20,000 inhabitants | Hypertensive nephropathy |
| 14 | W | 49 | 70 | 2 | city of 20,000–100,000 inhabitants | Hypertensive nephropathy |
| 15 | W | 63 | 75 | 2 | town with less than 20,000 inhabitants | Interstitial bacterial nephritis—with bladder dysfunction |
| 16 | M | 49 | 114 | 2 | city of 20,000–100,000 inhabitants | Cystic kidney disease—polycystic kidney disease |
| 17 | M | 26 | 70 | 5 | town with less than 20,000 inhabitants | Primary glomerulopathies with renal biopsy—(FSGS) focal glomerular vitrification/sclerosis |
| 18 | M | 67 | 76 | 5 | city with a population of over 100,000 | Cystic kidney disease—polycystic kidney disease |
| 19 | W | 31 | 73 | 1 | village | Primary glomerulopathies with renal biopsy |
Table 7.
Demographic data of the control group (M, men; W, women).
| Number of Participants |
Gender | Age (in Years) | Weight (in kg) | Place of Residence |
|---|---|---|---|---|
| 1 | M | 28 | 58 | city with a population of over 100,000 |
| 2 | W | 28 | 90 | |
| 3 | M | 33 | 90 | |
| 4 | W | 30 | 65 | |
| 5 | W | 44 | 75 |
Table 8.
Characteristics of patients in the study and control groups (AM, arithmetic mean; SD, standard deviation; Med., median; Min, minimum value; Max, maximum value; Q1, lower quartile; Q2, upper quartile).
| Study Group | Control Group | ||||
|---|---|---|---|---|---|
| Age at tx (in Years) |
Weight (in kg) | Total Dialysis Time (in Years) |
Age (in Years) | Weight (in kg) |
|
| AM ± SD | 51.90 ± 12.06 | 78.32 ± 13.33 | 2.79 ± 3.60 | 32.60 ± 6.69 | 75.60 ± 14.47 |
| Med | 54.00 | 75.00 | 2.00 | 30.00 | 75.00 |
| Min | 26.00 | 61.00 | 0.00 | 28.00 | 58.00 |
| Max | 71.00 | 114.0 | 16.00 | 44.00 | 90.00 |
| Q1 | 47.00 | 70.00 | 1.00 | 28.00 | 65.00 |
| Q2 | 61.00 | 82.00 | 3.00 | 33.00 | 90.00 |
The Bioethics Committee of the Pomeranian Medical University in Szczecin (resolution no. KB-0012/114/12) approved the study. The study was conducted following the Declaration of Helsinki.
We used two methods to determine TFF3 concentrations: enzyme-linked immunosorbent assay immunoenzymatic (ELISA) and xMap Luminex flow fluorimetry. Both assays were from R&D Systems (Minneapolis, MN, USA): the Human TFF3 Quantikine ELISA Kit and the Human Kidney Biomarker Premixed Magnetic Luminex® Performance Assay. We performed the assays according to the protocols provided by the manufacturer. Serum creatinine was determined using a colorimetric assay based on the Jaffé method on a Cobas C 501 instrument from Roche Diagnostics (Mannheim, Germany), while eGFR was calculated according to the CKD-EPI formula.
Statistical results were analyzed using Statistica 13.3 (Statistica PL, StatSoft). We examined the distribution of the data using the Shapiro-Wilk test, taking into account the division into test and control groups and separately for the values of the variables obtained on the first day, six months, and one year after renal transplantation. A nonnormal distribution characterized the variables assessed, so data were presented in tables and graphs in the form of the median, minimum, and maximum values and lower and upper quartiles, and the tests used in the analyses were nonparametric.
5. Conclusions
In conclusion, TFF3 in serum and urine may be a promising biomarker for diagnosing renal function and prognosis, mainly on the first and second days after kidney transplantation. Simultaneous determination of this biomarker and creatinine and eGFR levels in the patient’s serum and urine may provide helpful diagnostic information. Furthermore, large-scale studies are warranted to investigate the diagnostic and prognostic value of serum and urine TFF3 levels in renal transplant patients.
Author Contributions
K.R.: literature search and review; manuscript draft preparation; writing manuscript; conceptualization; formal analysis; investigation; resources and preparation of manuscript revision; and formal analysis. I.W.-K.: methodology; preparation of manuscript revision; supervision; and final acceptance of the manuscript. B.K.-S.: validation of methodology. P.K.: methodology. P.R.: methodology. B.D.: conceptualization; participation in writing the manuscript. K.Ł.: methodology; participation in writing the manuscript. B.M.: methodology; participation in writing the manuscript. D.K.-B.: writing the manuscript; preparation of manuscript revision; supervision; and final acceptance of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Biometric Committee of the Pomeranian Medical University in Szczecin (KB-0012/114/12 from 29 October 2012).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the statutory budget of the Department of Diagnostic Immunology, Pomeranian Medical University in Szczecin (WMS-136/S/2023).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Anglicheau D., Malone A., Chon J. Kidney Transplantation in Adults: Investigational Methods in the Diagnosis of Acute Kidney Allograft Rejection—UpToDate. [(accessed on 29 November 2021)]. Available online: https://www.uptodate.com/contents/kidney-transplantation-in-adults-investigational-methods-in-the-diagnosis-of-acute-kidney-allograft-rejection?topicRef=7352&source=see_link.
- 2.Rogulska K., Wojciechowska-Koszko I., Dołȩgowska B., Kwiatkowska E., Roszkowska P., Kapczuk P., Kosik-Bogacka D. The Most Promising Biomarkers of Allogeneic Kidney Transplant Rejection. J. Immunol. Res. 2022;2022:6572338. doi: 10.1155/2022/6572338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thim L., May F.E.B. Structure of Mammalian Trefoil Factors and Functional Insights. Cell. Mol. Life Sci. 2005;62:2956–2973. doi: 10.1007/s00018-005-5484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podolsky D.K., Lynch-Devaney K., Stow J.L., Oates P., Murgue B., DeBeaumont M., Sands B.E., Mahida Y.R. Identification of Human Intestinal Trefoil Factor. Goblet Cell-Specific Expression of a Peptide Targeted for Apical Secretion. J. Biol. Chem. 1993;268:6694–6702. doi: 10.1016/S0021-9258(18)53305-6. [DOI] [PubMed] [Google Scholar]
- 5.Nakov R. New Markers in Ulcerative Colitis. Clin. Chim. Acta. 2019;497:141–146. doi: 10.1016/j.cca.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Ge H., Gardner J., Wu X., Rulifson I., Wang J., Xiong Y., Ye J., Belouski E., Cao P., Tang J., et al. Trefoil Factor 3 (TFF3) Is Regulated by Food Intake, Improves Glucose Tolerance and Induces Mucinous Metaplasia. PLoS ONE. 2015;10:e0126924. doi: 10.1371/journal.pone.0126924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold P., Rickert U., Helmers A.K., Spreu J., Schneppenheim J., Lucius R. Trefoil Factor 3 Shows Anti-Inflammatory Effects on Activated Microglia. Cell Tissue Res. 2016;365:3–11. doi: 10.1007/s00441-016-2370-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu S.Q., Roberts D., Zhang B., Ren Y., Zhang L.Q., Wu Y.H. Trefoil Factor 3 as an Endocrine Neuroprotective Factor from the Liver in Experimental Cerebral Ischemia/Reperfusion Injury. PLoS ONE. 2013;8:e77732. doi: 10.1371/journal.pone.0077732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao S., Zheng Q., Gao J., Yao Y., Ren S., Liu Y., Xu Y. Trefoil Factor 3 Contributes to the Malignancy of Glioma via Regulating HIF-1α. Oncotarget. 2017;8:76770. doi: 10.18632/oncotarget.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer zumBüschenfelde D., Tauber R., Huber O. TFF3-Peptide Increases Transepithelial Resistance in Epithelial Cells by Modulating Claudin-1 and -2 Expression. Peptides. 2006;27:3383–3390. doi: 10.1016/j.peptides.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Taupin D., Podolsky D.K. Trefoil Factors: Initiators of Mucosal Healing. Nat. Rev. Mol. Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 12.George B., Wen X., Mercke N., Gomez M., O’Bryant C., Bowles D.W., Hu Y., Hogan S.L., Joy M.S., Aleksunes L.M. Time-Dependent Changes in Kidney Injury Biomarkers in Patients Receiving Multiple Cycles of Cisplatin Chemotherapy. Toxicol. Rep. 2020;7:571. doi: 10.1016/j.toxrep.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du T.Y., Luo H.M., Qin H.C., Wang F., Wang Q., Xiang Y., Zhang Y. Circulating Serum Trefoil Factor 3 (TFF3) Is Dramatically Increased in Chronic Kidney Disease. PLoS ONE. 2013;8:80271. doi: 10.1371/journal.pone.0080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamanari T., Sugiyama H., Tanaka K., Morinaga H., Kitagawa M., Onishi A., Ogawa-Akiyama A., Kano Y., Mise K., Ohmoto Y., et al. Urine Trefoil Factors as Prognostic Biomarkers in Chronic Kidney Disease. Biomed Res. Int. 2018;2018:3024698. doi: 10.1155/2018/3024698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin B.R., Faubel S., Edelstein C.L. Biomarkers of Drug-Induced Kidney Toxicity. Ther. Drug Monit. 2019;41:213. doi: 10.1097/FTD.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan C., Yu L., Zhang X.L., Shang J.J., Ren J., Fan J., Feng X.Q., Zhang R.W., Xia Z.B., Duan X.W. Cytokine Profiling in Chinese SLE Patients: Correlations with Renal Dysfunction. J. Immunol. Res. 2020;2020:8146502. doi: 10.1155/2020/8146502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand S., Bajpai M., Khanna T., Kumar A. Urinary Biomarkers as Point-of-Care Tests for Predicting Progressive Deterioration of Kidney Function in Congenital Anomalies of Kidney and Urinary Tract: Trefoil Family Factors (TFFs) as the Emerging Biomarkers. Pediatr. Nephrol. 2021;36:1465–1472. doi: 10.1007/s00467-020-04841-8. [DOI] [PubMed] [Google Scholar]
- 18.Endre Z.H. Recovery from Acute Kidney Injury: The Role of Biomarkers. Nephron Clin. Pract. 2014;127:101–105. doi: 10.1159/000363678. [DOI] [PubMed] [Google Scholar]
- 19.Kjellev S. The Trefoil Factor Family—Small Peptides with Multiple Functionalities. Cell. Mol. Life Sci. 2009;66:1350–1369. doi: 10.1007/s00018-008-8646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry J.K., Kannan N., Grandison P.M., Mitchell M.D., Lobie P.E. Are Trefoil Factors Oncogenic? Trends Endocrinol. Metab. 2008;19:74–81. doi: 10.1016/j.tem.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Al-Salam S., Sudhadevi M., Awwad A., Al Bashir M. Trefoil Factors Peptide-3 Is Associated with Residual Invasive Breast Carcinoma Following Neoadjuvant Chemotherapy. BMC Cancer. 2019;19:135. doi: 10.1186/s12885-019-5316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garraway I.P., Seligson D., Said J., Horvath S., Reiter R.E. Trefoil Factor 3 Is Overexpressed in Human Prostate Cancer. Prostate. 2004;61:209–214. doi: 10.1002/pros.20096. [DOI] [PubMed] [Google Scholar]
- 23.Kosriwong K., Menheniott T.R., Giraud A.S., Jearanaikoon P., Sripa B., Limpaiboon T. Trefoil Factors: Tumor Progression Markers and Mitogens via EGFR/MAPK Activation in Cholangiocarcinoma. World J. Gastroenterol. 2011;17:1631. doi: 10.3748/wjg.v17.i12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu Y., Yang Y., Ma D., Xiao W. Increased Trefoil Factor 3 Levels in the Serum of Patients with Three Major Histological Subtypes of Lung Cancer. Oncol. Rep. 2012;27:1277–1283. doi: 10.3892/or.2012.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oue N., Hamai Y., Mitani Y., Matsumura S., Oshimo Y., Aung P.P., Kuraoka K., Nakayama H., Yasui W. Gene Expression Profile of Gastric CarcinomaIdentification of Genes and Tags Potentially Involved in Invasion, Metastasis, and Carcinogenesis by Serial Analysis of Gene Expression. Cancer Res. 2004;64:2397–2405. doi: 10.1158/0008-5472.CAN-03-3514. [DOI] [PubMed] [Google Scholar]
- 26.Pandey V., Zhang M., Chong Q.Y., You M., Raquib A.R., Pandey A.K., Liu D.X., Liu L., Ma L., Jha S., et al. Hypomethylation Associated Enhanced Transcription of Trefoil Factor-3 Mediates Tamoxifen-Stimulated Oncogenicity of ER+ Endometrial Carcinoma Cells. Oncotarget. 2017;8:77268–77291. doi: 10.18632/oncotarget.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You M.L., Chen Y.J., Chong Q.Y., Wu M.M., Pandey V., Chen R.M., Liu L., Ma L., Wu Z.S., Zhu T., et al. Trefoil Factor 3 Mediation of Oncogenicity and Chemoresistance in Hepatocellular Carcinoma Is AKT-BCL-2 Dependent. Oncotarget. 2017;8:39323. doi: 10.18632/oncotarget.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bignotti E., Ravaggi A., Tassi R.A., Calza S., Rossi E., Falchetti M., Romani C., Bandiera E., Odicino F.E., Pecorelli S., et al. Trefoil Factor 3: A Novel Serum Marker Identified by Gene Expression Profiling in High-Grade Endometrial Carcinomas. Br. J. Cancer. 2008;99:768. doi: 10.1038/sj.bjc.6604546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takano T., Yamada H. Trefoil Factor 3 (TFF3): A Promising Indicator for Diagnosing Thyroid Follicular Carcinoma. Endocr. J. 2009;56:9–16. doi: 10.1507/endocrj.K08E-105. [DOI] [PubMed] [Google Scholar]
- 30.Rinnert M., Hinz M., Buhtz P., Reiher F., Lessel W., Hoffmann W. Synthesis and Localization of Trefoil Factor Family (TFF) Peptides in the Human Urinary Tract and TFF2 Excretion into the Urine. Cell Tissue Res. 2010;339:639–647. doi: 10.1007/s00441-009-0913-8. [DOI] [PubMed] [Google Scholar]
- 31.Astor B.C., Köttgen A., Hwang S.J., Bhavsar N., Fox C.S., Coresh J. Trefoil Factor 3 Predicts Incident Chronic Kidney Disease: A Case-Control Study Nested within the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Nephrol. 2011;34:291–297. doi: 10.1159/000330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka K., Sugiyama H., Yamanari T., Mise K., Morinaga H., Kitagawa M., Onishi A., Ogawa-Akiyama A., Tanabe K., Eguchi J., et al. Renal Expression of Trefoil Factor 3 MRNA in Association with Tubulointerstitial Fibrosis in IgA Nephropathy. Nephrology. 2018;23:855–862. doi: 10.1111/nep.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pianta T.J., Peake P.W., Pickering J.W., Kelleher M., Buckley N.A., Endre Z.H. Evaluation of Biomarkers of Cell Cycle Arrest and Inflammation in Prediction of Dialysis or Recovery after Kidney Transplantation. Transpl. Int. 2015;28:1392–1404. doi: 10.1111/tri.12636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.