Abstract
Mutations in the tubulin-specific chaperon D (TBCD) gene, involved in the assembly and disassembly of the α/β-tubulin heterodimers, have been reported in early-onset progressive neurodevelopment regression, with epilepsy and mental retardation. We describe a rare homozygous variant in TBCD, namely c.881G>A/p.Arg294Gln, in a young woman with a phenotype dominated by distal motorneuronopathy and mild mental retardation, with neuroimaging evidence of corpus callosum hypoplasia. The peculiar phenotype is discussed in light of the molecular interpretation, enriching the literature data on tubulinopathies generated from TBCD mutations.
Keywords: tubulin-specific chaperon D, distal motoneuronopathy, tubulinopathy, corpus callosum hypoplasia
1. Introduction
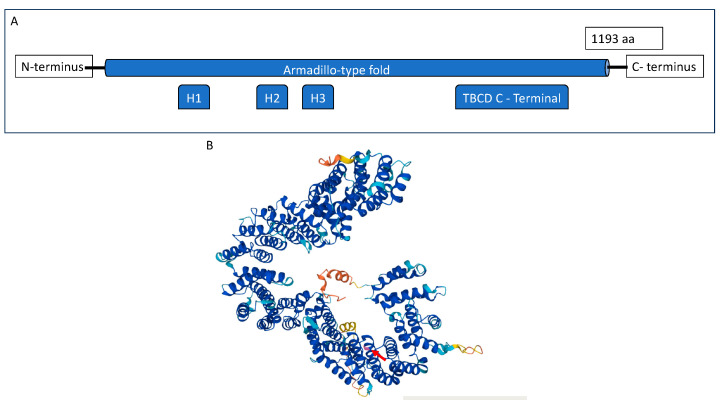
The TBCD gene is located in the chromosome 17q25.3 and codes for a protein of 1193 amino acids termed tubulin folding cofactor D. The TBCD protein is mainly composed of armadillo/HEAT motifs or multiple α-helices running in antiparallel directions linked by short loops and a TFCD-C C terminal. It contains three HEAT repeats (Figure 1).
Figure 1.
(A) Schematic representation of the TBCD protein. (B) Three-dimensional structure of the TBCD protein. The protein is mainly composed of α-helices in antiparallel configuration, typical of armadillo/HEAT motifs. Arg294 (pointed by the red arrow) is represented in light purple, and it is located in an alpha helix domain.
The family of tubulins reaches levels of ~5% of total cell protein content and are the constituent of microtubules, essential components of all eukaryotic cells as GTP-binding proteins, involved in a number of functions such as cell division, morphology, polarization, migration, and intracellular transport [1].
TBCD actively participates in the assembly–disassembly of the apical junctional complex and other intercellular and cellular–substratum binding of epithelial cells [2]. In vitro studies showed that the overexpression of cofactors D in cultured cells resulted in the destruction of the tubulin heterodimer and of the microtubules and in ectopic dendrite arborization, suggesting that an optimum level of TBCD is crucial for in vivo neuronal morphogenesis [3].
Defective TBCD function impairs soluble α/β-tubulin levels and accelerated microtubule polymerization in patient-derived primary cells [4]. Similarly, the hyperstabilization of microtubules has been shown to result in neurodegeneration, as in the autosomal-dominant spastic paraplegia type 4 (SPG4), which is associated with the degeneration of the corticospinal tracts and heterozygous mutations in SPAST, encoding a protein (spastin) implied in microtubule assembly and dynamics [5]. In vitro investigations examined the protein–protein interactions between the TBCD (wildtype and mutant) and other complex components (a/b-tubulin, TBCE, TBCC, and ARL2), suggesting that the TBCD mutations, which affect the folding of the a-solenoid repeat domains, may impair the proper formation of the microtubule chaperone complexes and consequently of the proper synaptic transmission in the developing brain [1,6,7].
Mutations in the genes encoding tubulins and microtubule-associated proteins cause different neurodevelopmental and early-onset neurodegenerative disorders [8,9,10]. They are more commonly described in patients with consanguineous parents, and it accounts for approximately 24% of early-onset neurodegenerative encephalopathies [8]. All the patients described in the literature presented during the first year of life with developmental regression, epilepsy, and microcephaly [8,9,10]. TBCD has been associated with atypical Spinal Muscular Atrophy (SMA) only recently, following a single case report [11].
Here, we report a rare homozygotic mutation in the TBCD gene in a woman with a phenotype resembling distal motorneuronopathy. We discuss the putative role of this gene in our case in light of the molecular and previous literature data.
2. Case Presentation
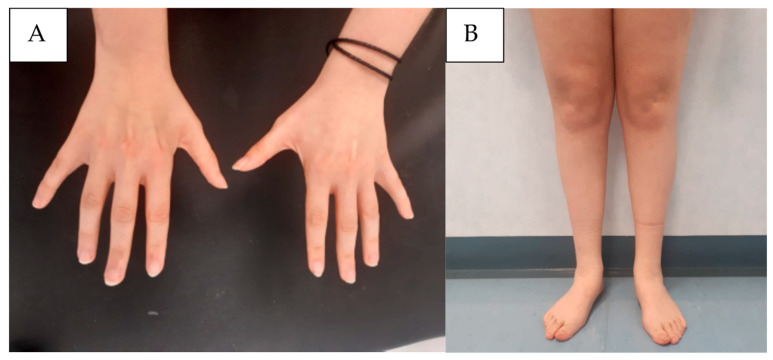
A 22-year-old woman presented with early fatigue and low exercise tolerance since childhood. She was born from a full-term eutocic birth. On personal history, she reported a slight intellectual disability with an Intelligence Quotient at age 17 measured by WAIS-IV of 61, mainly driven by defective linguistic (63) and comprehension (63) skills, childhood social anxiety disorder with selective mutism and behavioral disorder, and pavor nocturnus until age 8. Her history was negative for epilepsy, and her EEG record was normal. The motor and developmental stages of development were normally achieved. Upon orthopedic assessment, initially the symptoms were ascribed to the feet deformity (flatfeet), leading to bilateral corrective surgery when she was 13. Given the persistence of symptoms after surgery and physical rehabilitation, a neurological examination at 17 years old was performed, showing mild distal weakness, without visible fasciculations, associated with hypotrophy in the intrinsic muscles of the hand and posterior–anterior distal leg muscle lodges (Figure 2A,B). The reflexes were globally normal in the upper limbs, whereas they were absent in lower limbs. The Babinski and Hoffman reflexes were absent. Slight muscle hypotonia at the lower limbs was detected. No sensory or coordination impairment was detected. A mild low-amplitude distal hand tremor was noted.
Figure 2.
Proband’s clinical picture, showing atrophy at the level of the intrinsic hand muscles (A) and an inverted “champagne-bottle” appearance to the lower extremities (B).
Neurophysiological study showed sporadic fibrillations potentials and positive sharp waves in the left I dorsal interosseous and right anterior tibial. The motor unit potentials were globally of large amplitude and long duration, with sporadic polyphasic potentials. Decreased motor unit recruitment was revealed, thus revealing a reduced number of motor units. The motor evoked potentials revealed a prolongation in the peripheral motor conduction time.
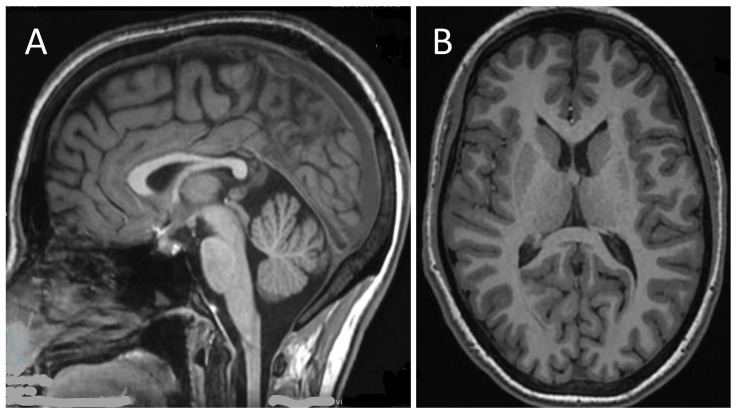
The cervical Magnetic Resonance Imaging (MRI) was normal, whereas a brain MRI repeated at age 21 revealed a thin corpus callosum (Figure 3), which was previously suspected but could not be definitely determined at age 17, with normal representation of cerebellar morphology and ventricular system.
Figure 3.
Brain magnetic resonance imaging (MRI) of the patient at 17 years of age: sagittal T1-weighted image revealed thinned corpus callosum (A); axial T1-weighted image shows normal morphology of the ventricular system (B).
Slightly elevated creatine kinase (CK) values (217 UI/mL) were reported in the serum. An extensive autoimmune screening was uninformative. In looking for a systemic involvement, the abdomen and thoracic ultrasound and eye examination revealed no abnormalities. The transthoracic heart ultrasound showed a slight left ventricular wall thickening without any dilatation or alterations in segmental kinetics. The ascending aorta and all the valves were normal.
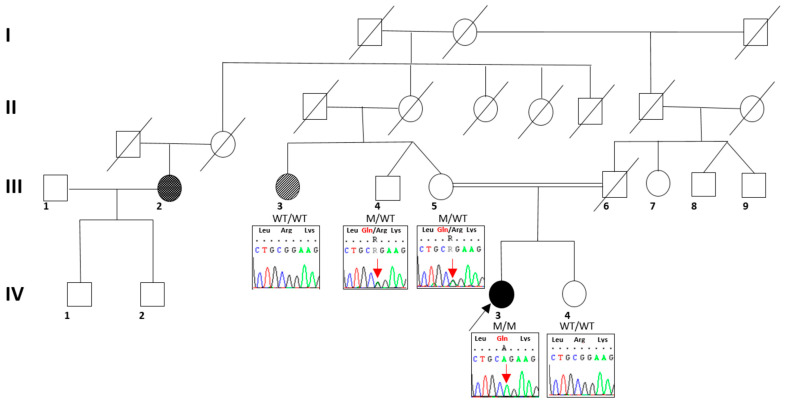
The disease slowly progressed over the years, and the patient mainly complains of fatigue in several daily actions, including climbing the stairs and walking for long distances, without shortness of breath. She did not report any difficulties with the fine movements with her hands. The family history (Figure 4) revealed that her parents were consanguineous, being first cousins, without any intellectual disability or motor signs; their past medical history was overall negative for neurological conditions. Her 25-year-old sister presented a long history of isolated muscle cramps. Her father died from pulmonary artery intimal sarcoma when he was 60 years old. Her mother’s sister was affected by a severe intellectual disability and motor impairment since birth, with development delay, not better investigated. A mother’s first cousin had autism and pes planus.
Figure 4.
Proband’s pedigree (indicated by an arrow, IV—3). The filled symbols indicate individuals affected by neurological disorders. The grey color indicates the proband’s aunt, presenting with intellectual disability since birth and motor impairment since 8 months old; the black color indicates the proband’s second-degree cousin presenting with autism and pes planus; no segregation analysis was available for him.
Informed consent for genetic analysis was obtained from the patient. Genomic DNA was extracted from the peripheral lymphocytes obtained from the patient as standard procedures [12]. The MLPA analysis to search for multiexon rearrangements in the SPAST gene and the direct sequencing of the SMN (Survival Motor Neuron) gene did not show any deletions or mutations.
A multigene panel including 271 genes explored by Next Generation Sequencing and involved in hereditary spastic paraparesis, motor neuronopathies, and peripheral nerve involvement (see Appendix A) revealed a homozygous point mutation c.881G>A/p.Arg294Gln in TBCD. The variant was confirmed by Sanger sequencing, and it was heterozygous in the healthy parents and sister. The mutation was classified as Likely Pathogenic according to the ACMG nomenclature (https://varsome.com/ accessed on November 2021).
3. Discussion
Altered TBCD protein levels affect microtubule formation leading to abnormal microtubule trafficking in the human brain, and impairing dendrites’ arborization. Microtubule dynamics are complex tightly regulated processes at the basis of cell viability, architecture, and division. Tubulin alpha and beta assemble in heterodimers following the concerted action of chaperone molecules as TBCA to TBCE; in particular, TBCD-β tubulin and TBCE-α tubulin interact with each other, and together with TBCC they form a supercomplex that releases native tubulin heterodimers upon E-site GTP hydrolysis [13]. Moreover, TBCD activity is modulated by the small GTPase Arl2, which is constitutionally bound to TBCD often forming a trimer of TBCD-β tubulin-Arl2 [14].
Given their fundamental role in regulating cell growth and dendrites formation, tubulinopathies are a heterogenous group of conditions characteristically presenting with cortical malformations and dysmorphic basal ganglia [15,16]. From a review of the literature, different mutations are associated with a broad spectrum of neurological disorders, mainly involving infants with initial normal development followed by neuroregression, epilepsy, and brain atrophy (for complete details, please refer to Table 1). In particular, several compound heterozygous mutations were reported in Chinese and Japanese children presenting with early-onset developmental regression, epilepsy of infancy with migrating focal seizures, and hypotonia. On the brain MRI, cerebral atrophy with secondary microcephaly and brain atrophy with thin corpus callosum were reported [8].
Homozygous mutations were also described in several Israeli, Japanese, Faroese, Indian-Jewish, and Egyptian-Jewish children [1,9], with an overlapping phenotype characterized by muscle weakness, absent visual tracking, and postnatal microcephaly. More than half of the affected individuals had postnatal growth failure, seizure, respiratory failure, developmental regression, optic nerve atrophy, hypotonia, and muscle atrophy [1].
Interestingly, two siblings were diagnosed with atypical SMA at a very early age during childhood, with hypotonia and muscle weakness indicating lower motor unit dysfunction, and progressive complicated central nervous system dysfunctions, axonal-dominant degeneration or motor unit reduction, and pes equinus of both feet was noted. In this case, two different mutations in two different genes were reported, but the homozygous mutation in TBCD gene (Arg942Gln) was considered to be the causative variant [11].
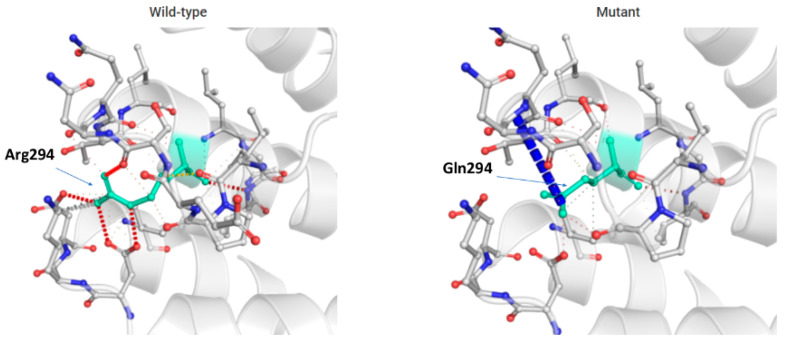
The mutation found in our case is likely to be harmful, upon in silico prediction using PolyPhen-2 (Polymorphism Phenotyping at http://genetics.bwh.harvard.edu/pph2/ accessed on November 2021). Upon 3D modeling, we observed that the amino acid change from arginine to glutamine at residue 294 caused a destabilization in the binding site to the complex Arl2/beta tubulin with other residuals in the evolutionary conserved alpha helices structure, altering the physiologic protein function (Figure 5).
Figure 5.
In silico prediction of the functional consequence of c.881G>A in the TBCD gene, with substitution of arginine with glutamine at position 294. Wildtype and mutant residues are colored in light green and are also represented as sticks alongside with the surrounding residues, which are involved in any type of interactions.
Our patient presented a milder phenotype compared to most cases reported in the literature, with clinical findings limited to slowly progressive muscle weakness and slight intellectual retardation along with a neurophysiological pattern of axonal-dominant motor neuron degeneration and motor unit reduction [11]. Interestingly, a similar slight delay in intellectual development was described by Tian et al. [17], although our patient did not manifest symptoms attributable to autism spectrum disorder (ASD) or treatable epilepsy. Curiously, although a mild elevation of CK was recorded in a patient by Chen et al. [18], the clinical picture of neurological involvement was more serious than our case. Additionally, the cerebral MRI revealed thin corpus callosum, similar to the cases of the Chinese and Japanese children described by Zhang and Chen [8,18], confirmed by the data on tubulinopathies association with corpus callosum hypoplasia [15].
In our case, the phenotype was, however, dominated by motor symptoms with neurophysiological evidence of a selective motor neuronopathy at four limbs like SMA. Only recently has the pathogenic link between SMN deficiency and altered microtubule stability been elucidated, pointing to a mitochondrial mislocalization in motor neurons driven by SMN loss [19]. Miyake et al. reported in their case series that patients with a homozygous missense mutation may present a milder phenotype possibly because binding of the altered TBCD protein with β-tubulin was only mildly affected, whereas the patients with most severe phenotype carried truncated or missense variants, which significantly impaired the TBCD binding to other crucial cellular scaffold players [1,6,7]. To this end, Flex et al. confirmed by functional studies in fibroblasts that TBCD mutants may differently impact the complex stabilization with ARL2, TBCE, and β-tubulin, suggesting that the severity of the TBCD loss of function shapes the phenotype along the spectrum of tubulinopathies [4]. In a recent study from South America, two subjects presented with a severe phenotype characterized by developmental encephalopathy and SMA with a novel homozygous missense mutation in TBCD close to a previously described site, which resulted in perturbed TBCD function and microtubule dynamics [20]. To the best of our knowledge, the compound heterozygous mutation c.881G>A in the TBCD gene has been only associated with continuous epileptic spasms as a severe form of status epilepticus [21], without mentioning a peripheral nervous system involvement.
Therefore, it could be hypothesized that some TBCD missense mutations, such as the one here described, c.881G>A, may only partially disrupt the protein capabilities to bind β tubulin-Arl2 and consequently affect tubulin dynamics more selectively in motor neurons compared to cortical neurons during the developmental stages.
Our case well illustrates how atypical SMA presentations with only mild intellectual impairment should be further inquired for developmental disorders of infancy with central nervous system neuroimaging and extensive genetic panels taking into consideration TBCD and other proteins involved in microtubule dynamics and mitochondrial trafficking. For these selected cases, antisense therapy currently available for SMN1-defective SMA would not be feasible, and other molecules with microtubule-stabilizing functions could be considered. The wide range of neurological effects of TBCD gene alterations is complex and growing with the literature data involving mainly neurodevelopment regression and epilepsy. In this context, the molecular basis of these disorders involving the complex tubulin machinery could be better understood through the detection of differential gene variants and their effective clinical impacts.
Table 1.
The TBCD variants published in the previous literature associated with phenotypes of early-onset neurodegenerative encephalopathy. Abbreviations: ASD: autism spectrum disorders; CC: corpus callosum; CSE: convulsive status epilepticus; EEG: electroencephalogram; F: female; Het: heterozygous; Homo, homozygous; M; male; MRI, magnetic resonance imaging; NCS, nerve conduction studies; NCSE: non convulsive status epilepticus. NR: not reported. WM: white matter. The * indicated the predicted consequence at the protein level of the variant to translation termination codon.
| Number of Patients | Sex | Ethnicity | Age at Onset | Familiarity | Zygosity (Het/Homo) | Allele 1 Variant | Allele 2 Variant | Amino Acid Change |
Reference | Perinatal History | Neurological Symptoms | Neurological Assessments | Extra-Neurological Manifestations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epilepsy | Peripheral Neuropathy | Mental Retardation | Hypotonia | Bulbar Involvement | Others | Imaging/MRI | NCS | EEG | ||||||||||||
| 1 | M | Chinese | neonatal period | NR | compound heterozogous | c.881G>A | c.22801C>A | R294Q | Liao, 2020 [21] | NR | Intractable epilepsy, focal seizures | NR | At 20 months: significant atrophy | NR | spared | NR | significant atrophy | NR | Low voltage on EEG, NCSE, CSE | NR |
| 1 | M | Chinese | 10 months | point mutation in the father, deletion in the mother (affected) | compound heterozygous | 230A>G | deletions of exons 28 to 39 | H77R | Zhang, 2018 [8] | NR | Intractable epilepsy, generalized seizures | NR | development regression after 5 months of age | yes | spared | microcephaly, hyperreflexia, reduced motor activity, bilateral Babinski reflexes | diffuse cortical atrophy with thinned CC | NR | high-amplitude delta wave background, multi- focal interictal spikes | bilateral hip dislocation at 8 months of age. |
| 1 | M | Japanese | at birth | parents were carrier | compound heterozygous | c.1564-12C>G (splicing) | C.2314C>T | R772C | Miyake, 2016 [1] | normal | no | NR | development regression | yes | spared | respiratory failure, muscle atrophy | cortical atrophy | NR | NR | NR |
| 1 | F | Japanese | at birth | parents were carrier | compound heterozygous | c.1564-12C>G (splicing) | C.2314C>T | R772C | normal | no | NR | development regression | yes | spared | respiratory failure, muscle atrophy | cortical atrophy | NR | NR | NR | |
| 1 | F | Japanese | 1 month | parents were carrier | compound heterozygous | c.1160T>G | c.2761G>A | M387R | normal | west syndrome | NR | development regression | yes | spared | respiratory failure, muscle atrophy | NR | NR | NR | NR | |
| 1 | M | Japanese | 1 month | parents were carrier | compound heterozygous | c.1160T>G | c.2761G>A | A921T | normal | cataplexy | NR | development regression | yes | spared | respiratory failure, muscle atrophy | NR | NR | NR | NR | |
| 1 | M | Chinese | 5 months | parents were carrier | compound heterozygous | c.2280C>A | c.3365C>T | Y760 * | normal | generalized seizures | NR | development regression | yes | spared | respiratory failure | NR | NR | NR | NR | |
| 1 | F | Chinese | 5 months | parents were carrier | compound heterozygous | c.2280C>A | c.3365C>T | P1122L | normal | generalized seizures | NR | development regression | yes | spared | NR | NR | NR | NR | NR | |
| 1 | F | Israelian | 9 months | parents were carrier | homozigous | c.2810C>G | P937R | normal | generalized seizures | NR | development regression | no | spared | NR | NR | NR | NR | NR | ||
| 1 | F | Israelian | 9 months | parents were carrier | homozigous | c.2810C>G | P937R | normal | generalized seizures | NR | development regression | no | spared | NR | NR | NR | NR | NR | ||
| 1 | F | Japanese | 5 months | parents were carrier | homozigous | c.2825G4A | R942Q | Ikeda, 2016 [11] | normal | partial seizure | NR | development regression | yes | spared | NR | cortical atrophy | NR | NR | NR | |
| 8 | M | Faroese | 6 months | parents of 2 patients were carrier | homozigous | 3099C>G | N1033K | Grønborg, 2018 [10] | normal | generalized treatment resistant epilepsy | NR | development regression | yes | spared | respiratory failure, spasticity, | cortical and global cerebral atrophy | NR | NR | Bilateral hip luxation | |
| 1 | M | Indian-Jewish | 20 months | parents were heterozigous carrier | homozigous | c.1423G>A | A475T | Pode-Shakked, 2016 [9] | normal | generalized seizures | NR | development regression | yes | spared | microcephaly and right-sided plagiocephaly, | dilatated ventricles and subarachnoid spaces with diffuse thinning of the WM and CC, mild secondary hypomyelination | NR | disorganized high amplitude delta wave background and multi- focal polyspike discharges at moderate rate. | low anterior hairline, large ears, pectus excavatum, right hand single transverse palmar crease, lateral deviation of the first toes | |
| 1 | F | Egyptian-Jewish | 24 months | consanguineous, carrier | homozigous | c.2810C>G | P937R | normal | generalized seizures | NR | development regression | no | spared | NR | mild cortical atrophy, moderately thin corpus callosu | normal | high-amplitude delta wave background, multi- focal interictal spikes | |||
| 1 | F | Egyptian-Jewish | 24 months | consanguineous, carrier | homozigous | c.2810C>G | P937R | normal | generalized treatment resistant epilepsy | NR | development regression | no | spared | NR | cortical atrophy and moderately thin CC | NR | NR | NR | ||
| 1 | M | German/Sicilian/Cajun-Hungarian/Irish | 6 months | parents were heterozigous carrier | compound heterozigous | c.1757C>T | c.3192-2A>G | A586V | normal | generalized treatment resistant epilepsy | severe motor axonal neuropathy | development regression | yes | spared | NR | cortical atrophy and moderately thin CC | severe motor axonal neuropathy | NR | NR | |
| 1 | M | German/Sicilian/Cajun-Hungarian/Irish | 6 months | parents were heterozigous carrier | compound heterozigous | c.1757C>T | c.3192-2A>G | A586V | normal | generalized treatment resistant epilepsy | NR | development regression | no | spared | NR | cortical atrophy and moderately thin CC | NR | NR | several tendon lengthening orthopedic surgeries | |
| 1 | F | Chinese | 12 months | NR | compound heterozigous | c.3365C>T | c.1739G>A | P1122L, R580Q | Tian, 2019 [17] | NR | generalized tonic-clonic seizures | NR | slight delay of intellectual development | no | NR | dystonia | myelination delay reflected by abnormal signal in the occipital WM | NR | Interictal EEG: large number of spike waves | NR |
| 1 | F | Chinese | 6 months | NR | compound heterozigous | c.230A>G | c.907C>T | H77R, R303 * | NR | generalized tonic-clonic seizures | NR | nearly normal intellectual development | no | NR | NR | myelination delay reflected by abnormal signal in the occipital WM | NR | Interictal EEG: low amplitudespike waves in midline | NR | |
| 1 | M | Chinese | - | NR | compound heterozigous | c.2953C>T | c.3550C>T | R979C, Q1184 * | NR | generalized tonic-clonic seizures | absent | ASD | no | NR | NR | normal | NR | NR | NR | |
| 1 | F | Chinese | 12 months | parents were heterozigous carrier | compound heterozigous | c.1340C>T | c.817+2T>C | A447V | Chen, 2021 [18] | normal | generalized tonic-clonic seizures | NR | early-onset neurodegeneration, failure to thrive | yes | failure to thrive | respiratory failure | thinning of the CC, diffuse cerebral atrophy involving both gray and WM, dilatated ventricles | NR | NR | severe scoliosis, thrombocytopenia, presence of accessory spleen |
| 1 | F | Chinese | 18 months | parents were heterozigous carrier | compound heterozigous | c.1340C>T | c.817+2T>C | A447V | normal | focal to generalized tonic-clonic seizures | NR | early-onset neurodegeneration | yes | spared | respiratory failure | hypoplasia of CC, prominent enlargement of cerebral cortical sulci and ventricles | NR | slow wave activities | mild elevation of aspartate aminotransferase and CK (335 IU/L). | |
4. Conclusions
Our findings extend the phenotypic traits of tubulinopathy generated from TBCD mutations, by the identification of a rare homozygous TBCD variant associated with a predominant distal motoneuronopathy, slight mental retardation, and corpus callosum atrophy, a clinical picture distinct from the literature reports. In this setting, computational modeling methods and accurate genotype–phenotype correlations may better clarify the impact of the secondary and tertiary structure alteration following amino acid substitution, to better understand the mechanism of neurodegeneration related to tubulinopathies, leading to an early diagnosis and appropriate genetic counseling.
Acknowledgments
We acknowledge support from Rete RIN Ist.Virtuale MR.
Appendix A. Methods for Genetic Analysis
Genomic DNA was obtained using MagPurix Automated Nucleic Acid Purification System (Zinexts, Zhonghe, Taiwan).
In order to exclude large rearrangements in the SPAST gene, we performed MLPA analyses using SALSA MLPA P165, following the manufacturer’s instructions (MRC Holland; Amsterdam, The Netherlands), and Coffalyser.Net software (MRC-Holland) was employed to analyze the MLPA results.
The massive parallel sequencing of 271 genes (list of genes available on request) involved in hereditary spastic paraparesis, motor neuronopathies, and peripheral nerve was performed using the NextSeq500 (Illumina, San Diego, CA, USA) sequencer. Variants with possible pathogenetic significance were validated by the Sanger method on the sequencer 3500 Genetic Analyzer.
Raw data alignment to the reference human genome sequence was carried out using SureCall (Agilent Technologies, Santa Clara, CA, USA), while Ingenuity Variant Analysis (Qiagen, Venlo, The Netherlands) was used for the variant calling process. Single nucleotide variations and small insertions and deletions were selected using the following criteria: (i) quality score > 30; (ii) at least 30X of coverage; and (iii) MAF (minor allele frequency) < 1% in the dbSNP (https://www.ncbi.nlm.nih.gov/projects/SNP/ accessed on November 2021), 1000 Genome (browser.1000genomes.org), EVS database (evs.gs.washington.Edu), gnomAD (https://gnomad.broadinstitute.org/ accessed on November 2021).
The variant classification was based on the American College of Medical Genetics and Genomics’ published guidelines.
Author Contributions
Conceptualization, M.C., E.Z. and F.M.S.; methodology, A.T., C.S., R.T. and F.M.S.; formal analysis, R.T., A.T. and F.M.S.; investigation, M.C., E.Z., and I.M.; resources, E.Z., G.G. and I.M.; data curation, M.C., G.G., C.S., N.F., E.Z., I.M. and J.M.; writing—original draft preparation, M.C. and I.M.; writing—review and editing, E.Z., F.M.S., A.T. and J.M; visualization, M.C.; supervision, F.M.S. and J.M.; project administration, I.M.; funding acquisition, A.T. and F.M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of Area Vasta Emilia Nord (Protocol 2730/18).
Informed Consent Statement
Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
Further data concerning the subject clinical presentation are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
Research in AT and FMS laboratories was supported by Italian Ministry of Health, Ricerca Corrente 2023 and RC 5X1000.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Miyake N., Fukai R., Ohba C., Chihara T., Miura M., Shimizu H., Kakita A., Imagawa E., Shiina M., Ogata K., et al. Biallelic TBCD Mutations Cause Early-Onset Neurodegenerative Encephalopathy. Am. J. Hum. Genet. 2016;99:950–961. doi: 10.1016/j.ajhg.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanarraga M.L., Bellido J., Jaén C., Villegas J.C., Zabala J.C. TBCD links centriologenesis, spindle microtubule dynamics, and midbody abscission in human cells. PLoS ONE. 2010;5:e8846. doi: 10.1371/journal.pone.0008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okumura M., Sakuma C., Miura M., Chihara T. Linking cell surface receptors to microtubules: Tubulin folding cofactor D mediates Dscam functions during neuronal morphogenesis. J. Neurosci. 2015;35:1979–1990. doi: 10.1523/JNEUROSCI.0973-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flex E., Niceta M., Cecchetti S., Thiffault I., Au M.G., Capuano A., Piermarini E., Ivanova A.A., Francis J.W., Chillemi G., et al. Biallelic Mutations in TBCD, Encoding the Tubulin Folding Cofactor D, Perturb Microtubule Dynamics and Cause Early-Onset Encephalopathy. Am. J. Hum. Genet. 2016;99:962–973. doi: 10.1016/j.ajhg.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarrade A., Fassier C., Courageot S., Charvin D., Vitte J., Peris L., Thorel A., Mouisel E., Fonknechten N., Roblot N., et al. A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum. Mol. Genet. 2006;15:3544–3558. doi: 10.1093/hmg/ddl431. [DOI] [PubMed] [Google Scholar]
- 6.Tian G., Bhamidipati A., Cowan N.J., Lewis S.A. Tubulin folding cofactors as GTPase-activating proteins. GTP hydrolysis and the assembly of the alpha/beta-tubulin heterodimer. J. Biol. Chem. 1999;274:24054–24058. doi: 10.1074/jbc.274.34.24054. [DOI] [PubMed] [Google Scholar]
- 7.Bhamidipati A., Lewis S.A., Cowan N.J. ADP ribosylation factor-like protein 2 (Arl2) regulates the interaction of tubulin-folding cofactor D with native tubulin. J. Cell Biol. 2000;149:1087–1096. doi: 10.1083/jcb.149.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Zhang L., Zhou S. Developmental Regression and Epilepsy of Infancy with Migrating Focal Seizures Caused by TBCD Mutation: A Case Report and Review of the Literature. Neuropediatrics. 2020;51:68–71. doi: 10.1055/s-0039-1698423. [DOI] [PubMed] [Google Scholar]
- 9.Pode-Shakked B., Barash H., Ziv L., Gripp K.W., Flex E., Barel O., Carvalho K.S., Scavina M., Chillemi G., Niceta M., et al. Microcephaly, intractable seizures and developmental delay caused by biallelic variants in TBCD: Further delineation of a new chaper-one-mediated tubulinopathy. Clin. Genet. 2017;91:725–738. doi: 10.1111/cge.12914. [DOI] [PubMed] [Google Scholar]
- 10.Grønborg S., Risom L., Ek J., Larsen K.B., Scheie D., Petkov Y., Larsen V.A., Dunø M., Joensen F., Østergaard E. A Faroese founder variant in TBCD causes early onset, progressive encephalopathy with a homogenous clinical course. Eur. J. Hum. Genet. 2018;26:1512–1520. doi: 10.1038/s41431-018-0204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda T., Nakahara A., Nagano R., Utoyama M., Obara M., Moritake H., Uechi T., Mitsui J., Ishiura H., Yoshimura J., et al. TBCD may be a causal gene in progressive neurodegenerative encephalopathy with atypical infantile spinal muscular atrophy. J. Hum. Genet. 2017;62:473–480. doi: 10.1038/jhg.2016.149. [DOI] [PubMed] [Google Scholar]
- 12.D’Amore A., Tessa A., Casali C., Dotti M.T., Filla A., Silvestri G., Antenora A., Astrea G., Barghigiani M., Battini R., et al. Next Generation Molecular Diagnosis of Hereditary Spastic Paraplegias: An Italian Cross-Sectional Study. Front. Neurol. 2018;9:981. doi: 10.3389/fneur.2018.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian G., Cowan N.J. Tubulin-specific chaperones: Components of a molecular machine that assembles the α/β heterodimer. Methods Cell Biol. 2013;115:155–171. doi: 10.1016/B978-0-12-407757-7.00011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis J.W., Newman L.E., Cunningham L.A., Kahn R.A. A Trimer Consisting of the Tubulin-specific Chaperone D (TBCD), Regulatory GTPase ARL2, and β-Tubulin Is Required for Maintaining the Microtubule Network. J. Biol. Chem. 2017;292:4336–4349. doi: 10.1074/jbc.M116.770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahi-Buisson N., Poirier K., Fourniol F., Saillour Y., Valence S., Lebrun N., Hully M., Bianco C.F., Boddaert N., Elie C., et al. The wide spectrum of tubulinopathies: What are the key features for the diagnosis? Brain. 2014;137:1676–1700. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- 16.Hoff K.J., Neumann A.J., Moore J.K. The molecular biology of tubulinopathies: Understanding the impact of variants on tubulin structure and microtubule regulation. Front. Cell Neurosci. 2022;16:1023267. doi: 10.3389/fncel.2022.1023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian D., Rizwan K., Liu Y., Kang L., Yang Y., Mao X., Shu L. Biallelic pathogenic variants in TBCD-related neurodevelopment disease with mild clinical features. Neurol. Sci. 2019;40:2325–2331. doi: 10.1007/s10072-019-03979-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen C.L., Lee C.N., Chien Y.H., Hwu W.L., Chang T.M., Lee N.C. Novel Compound Heterozygous Variants in TBCD Gene Associated with Infantile Neurodegenerative Encephalopathy. Children. 2021;8:1140. doi: 10.3390/children8121140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bora G., Hensel N., Rademacher S., Koyunoğlu D., Sunguroğlu M., Aksu-Mengeş E., Balcı-Hayta B., Claus P., Erdem-Yurter H. Microtubule-associated protein 1B dysregulates microtubule dynamics and neuronal mitochondrial transport in spinal muscular atrophy. Hum. Mol. Genet. 2021;29:3935–3944. doi: 10.1093/hmg/ddaa275. [DOI] [PubMed] [Google Scholar]
- 20.Ocampo-Chih C., Dennis H., Lall N., Pham N., Liang B., Verma S., Neira Fresneda J. PEBAT, an Intriguing Neurodegenerative Tubulinopathy Caused by a Novel Homozygous Variant in TBCD: A Case Series and Literature Review. Pediatr. Neurol. 2023;139:59–64. doi: 10.1016/j.pediatrneurol.2022.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Liao J., Huang T., Srour M., Xiao Y., Chen Y., Lin S., Chen L., Hu Y., Men L., Wen J., et al. Status Epilepticus Manifested as Continuous Epileptic Spasms. Front. Neurol. 2020;11:65. doi: 10.3389/fneur.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further data concerning the subject clinical presentation are available upon reasonable request to the corresponding author.