Abstract
The purpose of this case report was to present the aesthetic result of the reconstruction of facial residual asymmetry after orthognathic surgery using a patient-specific three-dimensional (3D) mold and a custom-made polymethyl methacrylate implant. Through computer-aided design (CAD), the healthy contralateral side of the mandible was superimposed onto the side with the defect. Exocad Gallway (exocad GmbH, Darmstadt, Germany) was used to design the patient-specific implants (PSIs) of the right mandibular angle. Next, the implant mold was created using the Meshmixer software (Version 3.5, Autodesk Inc., San Rafael, CA, USA) and fabricated using additive manufacturing. During the surgical procedure, the patient-specific implant (PSI) was cast inside the resin mold using Simplex P bone cement (Stryker, Mahwah, NJ, USA). The implant was fixed using three screws. Combining both indirect (involving the dental laboratory) and direct (with surgical intervention) approaches, this innovative hybrid method, which incorporates both computer-aided design and additive manufacturing (AM), not only enhanced facial aesthetics, functional rehabilitation, and patient quality of life but also mitigated the potential risks linked to conventional grafting methods.
Keywords: orthognathic surgery, computer-assisted design, computer-aided manufacturing, polymethyl methacrylate, facial asymmetry
1. Introduction
The aim of orthognathic surgery (OGS) is to provide bone and soft tissues with a symmetrical and functional appearance. Kronmiller et al. [1] defined symmetry as the exact sameness of the right and left sides of the face. Numerous studies have shown that hard and soft tissues alter in the wake of orthognathic surgery [2,3,4]. However, investigators’ accounts of the restoration of bone symmetry frequently highlight a lack of sufficient soft tissue symmetry. The mandatory surgical separation of the facial muscles as a result of severe bone deformities influences this perception. Thus, there are substantial soft tissue volume deficits on both the right and left sides of the face [5]. In these instances, additional surgical procedures to change the amount of the skeletal and soft tissue components on both sides are required to achieve better symmetry [6].
The benefits of orthognathic surgery based on patient-specific implants (PSIs) are well explained, and this technique has been successfully used for the last few years [7,8]. Facial implants can also be employed in augmentative surgeries to appropriately restore bony abnormalities or malpositions [9,10].
There are two main categories of grafting methods used to address mandibular defects: autogenous and alloplastic. Due to postoperative infection, donor site morbidity, and unpredictable bone resorption results, autogenous bone grafts are discouraged.
Initially, commonly used preformed stock alloplastic materials like porous polyethylene, polytetrafluoroethylene, and silicone could be adjusted to match a defect during surgery, but their disadvantages included empty spaces, migration risks, and infection due to shape mismatch with the underlying bone [11,12].
Polymer powder and a liquid monomer are combined to produce polymethyl methacrylate [13]. Polymer powder is composed of re-polymerized polymethyl methacrylate beads, a radiopaque component (such as BaSO4 or ZrO2 particles), and an initiator (benzoyl peroxide) that starts the polymerization reaction. Along with an antibiotic, the powder also includes barium sulfate (10–15 weight percentage) as a radio-pacifying agent for improved vision during clinical usage [14]. A minor quantity of hydroquinone, an activator (N, dimethyl-para-toluidine) that encourages radical formation, and methyl methacrylate monomer make up the liquid component. To avoid early polymerization during storage, the liquid component also contains a reaction inhibitor or stabilizer [13].
The advantages of PMMA include stability, high biocompatibility, low radiopacity, strong resistance to functional stress, easy handling, and low costs [7,15,16]. Lye et al. [17] define PMMA as the most suitable option for potential use in a mandibular endoprosthetic system. Regardless of its benefits, however, it presents several challenges in terms of exothermic reactions, toxic gases created during the mixing process, and rare allergic reactions that must be addressed [18,19].
Despite the limited number of clinical reports on the application of PSIs for mandibular contour restorations [2,5,7,9,20], this paper aimed to present a fully digitized workflow to create template-molded PSIs using PMMA bone cement during surgery.
2. Case Report
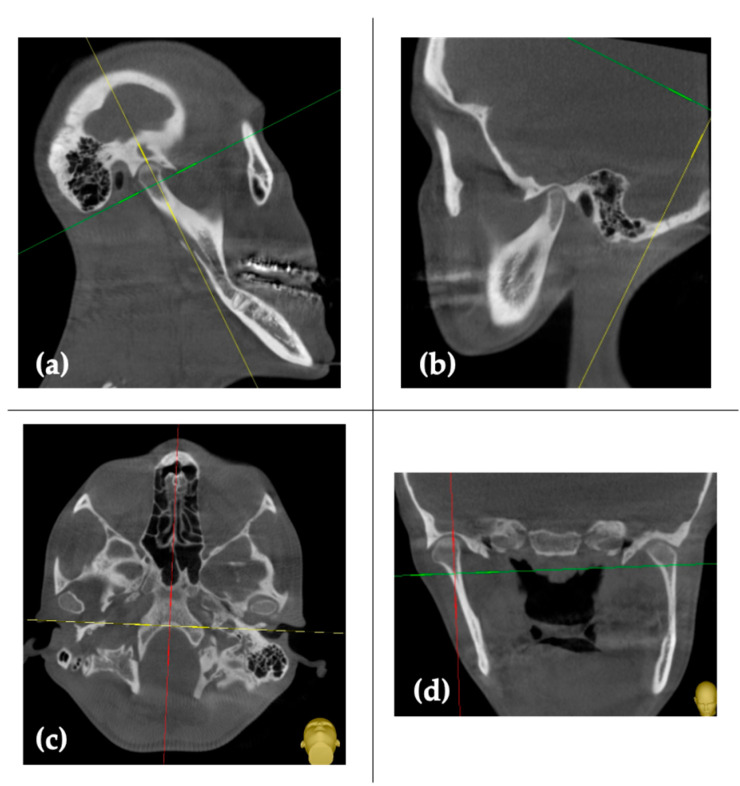
A 23-year-old woman presented with skeletal class III, associated with a condylar volume asymmetry between the left and right sides (Figure 1a–d).
Figure 1.
Pre-surgical CT scans: (a,b) sagittal sections; (c) axial section; and (d) coronal section.
She had undergone orthognathic surgery twelve months previously, which involved bilateral sagittal split osteotomy, genioplasty, and Le Fort I osteotomy. The surgery resulted in a significant improvement in the severe asymmetry in the lower third of her face. Despite the positive outcome achieved by our team, the patient returned to our dental office for further treatment (Figure 2a,b), as additional refinement was still required to address the remaining asymmetry. She had no major family history of illnesses or systemic conditions.
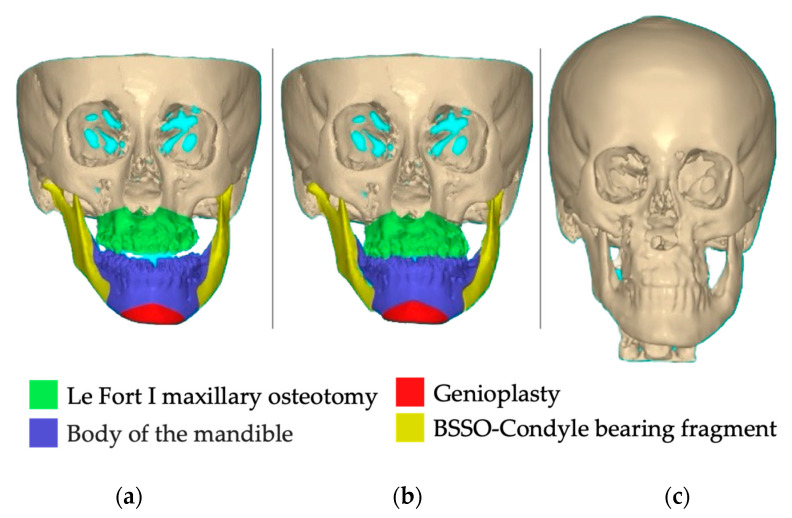
Figure 2.
Pre-surgical computer-assisted virtual simulation created using NemoFab (Nemotec, Madrid, Spain): (a) frontal view of patient skull; (b) frontal view of planned final reposition; and (c) 1-year follow-up CBCT control.
One year after OGS, three-dimensional face images were acquired using a computed tomography (CT) scanner (SOMATOM sensation 10, Siemens, Munich, Germany) with the following settings: 120 kVp tube voltage; 80 mAs tube current; and 0.6 mm slice thickness (Figure 2c).
Upon receiving a comprehensive explanation encompassing the diagnosis, prognosis with and without treatment, specific therapeutic measures, and the advantages, procedure-specific risks, and potential side effects associated with the treatment, the patient granted her informed consent.
2.1. PSI’s Mold Design and Fabrication
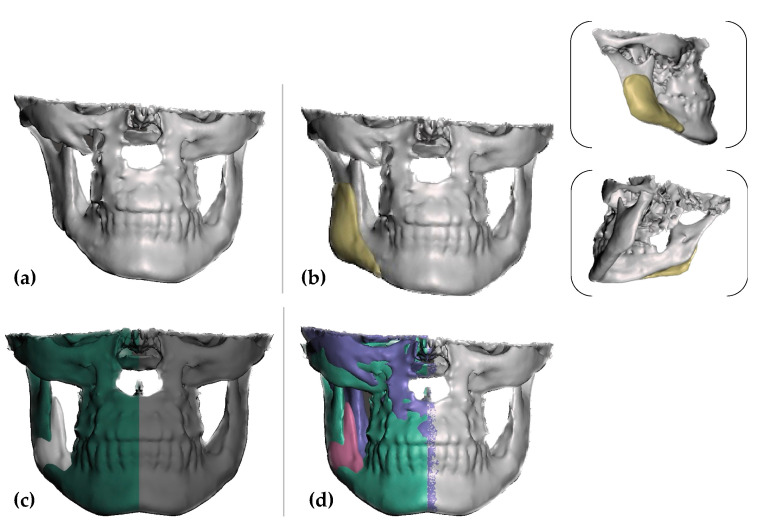
The data were exported in a Digital Imaging and Communications in Medicine format (DICOM) and then converted to standard tessellation files (STL) using the Exoplan 3.0 Dicom Viewer (exocad GmbH, Darmstadt, Germany). The PSI’s design was created using Exocad Gallway (exocad GmbH, Darmstadt, Germany) on the right mandibular angle, using the normal, healthy contralateral as the model (Figure 3a–c).
Figure 3.
Virtual images of the designed patient-specific implant using Exoplan 3.0 Dicom Viewer (exocad GmbH, Darmstadt, Germany): (a) initial situation, (b) PSI frontal view; and (c,d) mirroring the left side (healthy contralateral).
The Meshmixer software (Version 3.5, Autodesk Inc., San Rafael, CA, USA) was used to smooth the surface and create the implant mold (Figure 4).
Figure 4.
Virtual images of the designed mold using Meshmixer software (Version 3.5, Autodesk Inc., San Rafael, CA, USA).
The STL file of the 3D-designed mold was sent to an ASIGA 3D MAX UV printer (ASIGA, Alexandria, NSW, Australia) to be fabricated using Phrozen Water-Washable Dental Model 3D Printer Resin (Phrozen Technology, Hsin-chu, Taiwan). Prior to surgery, a 25 min immersion in Gigasept PAA (Schülke & Mayr GmbH, Norderstedt, Germany) sterilization was carried out.
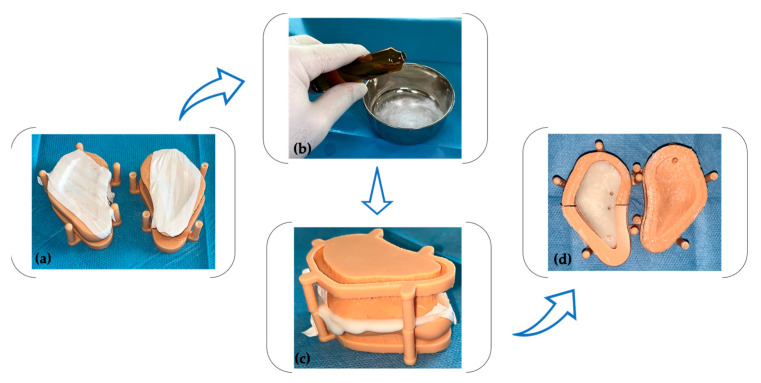
During the surgical procedure, the bone cement (Surgical Simplex P, Stryker, Mahwah, NJ, USA) was unpacked and utilized. The monomer and polymer components were mixed in accordance with the manufacturer’s instructions (Figure 5b), and the mixture was subsequently injected into a previously isolated mold using a sterile Teflon band (Figure 5a). Following an 8 min polymerization period (Figure 5c), the cast was opened, and the PSI (patient-specific implant) was extracted. Minor smoothing and drilling were conducted (Figure 5d) using a tungsten carbide bur (H251EQ, Komet Dental, Lemgo, Germany) and a 2.2 mm drill (Medicon eG, Tuttlingen, Germany).
Figure 5.
The PSI fabrication process involved the following steps: (a) isolation of the mold using a sterile Teflon band; (b) mixing the monomer and polymer components; (c) allowing for a setting period; and (d) trimming and drilling the implant.
To ensure complete cooling of the implant (to counteract the exothermic polymerization reaction effect), it was immersed in Betadine (Egis Pharmaceutical PLC, Budapest, Hungary) for an additional 10 min. The PMMA implant used in this case had a minimum thickness of 1 mm in the upper and anterior poles and a maximum thickness of 7 mm in the posterior and lower pole.
2.2. Surgical Procedure
The blood test results of the individual were within the expected parameters. During the initial phase, the individual was administered a non-steroidal analgesic (Ketorol, Dr. Reddy’s Laboratories, București, Romania) through an intravenous route. Additionally, 2 g of amoxicillin was administered as a loading dose.
To ensure the proper sterilization of the surgical area, the patient performed a 1 min rinse using Curasept (Curaden AG, Kriens, Switzerland). Then, a sterile surgical drape was utilized to disinfect and cover the surgical site.
Inducing local anesthesia involved the utilization of 4% articaine with epinephrine 1:100,000 (Ubistein, 3M ESPE, St. Paul, MN, USA). To locate the issue, customary vestibular incision and subperiosteal dissection were utilized.
The PSI was firmly seated after sufficient inferior and posterior side dissection. The PSI was applied directly and fastened with three 2.0/9 mm mini-screws obtained from Medicon (Medicon eG, Tuttlingen, Germany). The first two screws were positioned in the retromolar area, approximately 1 cm apart, and inserted with a tilted trajectory in the buccal–lingual direction, with a distal orientation, effectively securing the implant in the oblique ridge region. The third screw was placed in the lower border, specifically on the mesial aspect of the second molar, with as much of a horizontal orientation as possible.
During the postoperative period, the patient’s treatment plan included a prescription for amoxicillin and clavulanic acid (Augmentin, Glaxo Wellcome, Mayenne, France). The prescribed dosage was 2 g (1 g taken orally twice a day) to be taken for six days. Furthermore, the patient was advised not to brush the surgical site for a period of three weeks, to consume soft foods, and to maintain good oral hygiene practices.
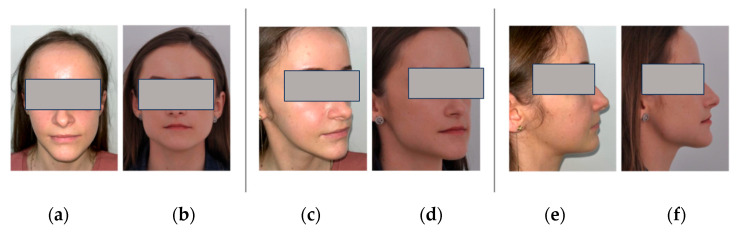
During the first month, the patient attended weekly examinations, followed by monthly follow-ups. No instances of exposure or infection were observed. Following the procedure, the patient did not experience any particular discomfort, such as pain or increased numbness in the jaw and lower lip, and she was satisfied with the significant improvement in facial appearance (Figure 6a–c).
Figure 6.
The extraoral images of patient frontal, semi-profile, and profile: (a,c,e) preoperative; (b,d,f) 1 month postoperative.
3. Discussion
The link between condylar and craniofacial asymmetries is an intriguing subject in the field of dentistry and maxillofacial surgery. Recent studies have explored the relationships between various asymmetries and how they might be connected [21,22].
Modern CAD software facilitates the act of mirroring, enhancing symmetry, and enabling the precise alignment of implants with the contralateral side [18,19,23,24]. Nevertheless, it should be noted that this mirroring process has been associated with overcorrection. Despite this challenge, we considered it a reasonable compromise in our approach.
In recent years, PSI bone augmentations have emerged as a beneficial approach for treating facial asymmetries following orthognathic surgery [25,26]. Compared with patients with non-laterognathic dysgnathia and symmetrical craniofacial deformities, achieving symmetry in patients with laterognathic disorders, whether syndromic or not, is far more difficult [27].
The use of PSIs for maxillofacial reconstruction features predictable outcomes, eliminates the usual complications seen in non-custom-made implants, and boasts excellent patient satisfaction. Its main drawback is the high cost.
PSIs are a proven method of facial skeleton rebuilding, and their usage in orthognathic surgery is gradually gaining popularity. The repeatability, simplicity, and low complication rates of PSIs in nonsyndromic craniofacial patients have been acknowledged in a number of studies; however, data on orthognathic patients are rather restricted due to the recent use of PSIs. In our case report, we observed no complications related to custom-made PSIs.
It is important to note that while orthognathic surgery only requires an accuracy of 1.0 mm in linear distance deviation and a 2–4° angular deviation, occlusal fine tuning is achieved postoperatively through orthodontic treatment [28]. Occlusal rehabilitation necessitates a precision of 20–40 µm [29]. Since no viscerocranium is entirely symmetrical in nature [30], facial asymmetry of 1.0–2.0 mm is barely noticeable. When it comes to individuals with laterognathy, especially those with severe craniofacial abnormalities, the question arises of whether contemporary surgical planning and execution methods can accomplish such symmetry.
There is a wide range of materials available for bone regeneration and as substitutes for repairing bone defects, particularly in cranial bones. Commonly utilized materials for this purpose include metals like titanium, tantalum, and their alloys, as well as magnesium and its alloys. In addition to metals, ceramics, polymethyl methacrylate, poly-L-lactic acid (PLA), polylactide (PLLA), and polyetheretherketone (PEEK) are also frequently employed as bone substitutes.
Titanium (Ti), which may be created utilizing milling or three-dimensional (3D) printing technologies to generate a patient-specific implant (PSI), is one of the most biocompatible materials. Titanium implants have been utilized for internal maxillomandibular fixations and as dental implants for several decades since this biocompatible inert material has an inherent tendency toward osseointegration with the surrounding bone. Its proven biocompatibility makes it the material of choice for internal fixation and reconstruction in the craniofacial region. As a result, titanium 3D PSIs provide hope for the future of patients with this issue [31].
Another prominent alloplastic substance is PEEK, which has a number of appealing qualities such as strength, durability, resilience to environmental conditions, and a decreased infection rate [32]. PEEK bone augmentations have been found to be useful in the treatment of facial asymmetry in recent years. Because of this, additional improvements in facial symmetry following orthognathic surgery have been accomplished [25,33,34], including significant enhancements in soft-tissue symmetry [35]. PEEK augmentations have no morbidity at the donor site and are always an option [36], which sets them apart from autologous replacement procedures. The benefits of a PSI-based orthognathic operation with the insertion of PEEK bone augmentations are undeniable, but the technique is not without its drawbacks. The introduction of a foreign body can lead to infection [37]. Antibiotic shielding techniques, however, can mitigate this threat [38]. Larger augmentations increase the risk of complications such as infection and implant exposure or recurrence [39].
Polymethylmethacrylate (PMMA) cement reconstruction has demonstrable durability and a manageable learning curve when it comes to restoring the natural contour and shape of the skull [12,15]. According to Slimani et al. [16] this method is preferable since it is easy to implement, efficient, and flexible enough to accommodate defects of varying sizes. Initially tested on monkeys, the procedure was eventually used on humans in 1941 to correct cranial bone abnormalities [40]. Besides its use in neurosurgery, PMMA cement is extensively employed in dentistry, maxillofacial surgery, traumatology, and orthopedics. Due to its capacity to promote cell ingrowth, it can serve as a substitute for natural bone. The attachment of cells to this substance and their growth within its pores would result in good integration with biological tissues [40,41,42].
To ensure consistent graft robustness without thin sections that could lead to potential fracture sites, it is crucial to achieve the proper thickness of PMMA cement. This provides visually pleasing results and reduces the risk of complications such as wound dehiscence, infection, and the need for revision procedures resulting from tensionless wound closure. Previous studies have reported cytotoxic damage and thermal tissue damage caused by PMMA implants directly molded on the lesion due to the exothermic polymerization reaction during hardening [25]. However, the procedure described in this paper, utilizing indirect and remote molding, poses no harm to sensitive tissues.
To generate maximum surface friction and maintain long-term stability, precise positioning of the targeted PSI is of the highest significance [18]. Improper positioning of the PSI may damage the inferior alveolar nerve during screw fixation and can result in the implant’s edge being perceptible [43]. In cases where the buccal vestibule has significant fibrosis or scarring from prior surgery, a submandibular approach may be considered [18]. However, intraoral techniques are generally recommended to minimize the risk of extraoral scarring in aesthetic cases [23,44]. Based on our clinical experience, with a proper incision and sufficient reflection of the periosteum, the implant can be inserted with ease, avoiding unnecessary contact with the oral mucosa. By employing bimanual manipulation—intraorally with forceps and extra-orally with the fingers of the left hand behind the posterior border of the ascending ramus and lower border of the mandible—the implant is smoothly seated and effortlessly finds its proper position.
Mechanical fixation with screws is the most common method used to maintain PSI stability after surgery. The size of the implant might affect the total number of screws used. However, further clinical studies involving larger patient cohorts and finite element analysis are necessary to determine the optimal number of screws. In the case presented, the PSI was fixed using three screws.
Despite the relatively short follow-up period (6 months), it is important to note that the patient did not experience any complications associated with PSI reconstruction, and there was no evidence of infection. The wound healing was uneventful. Consistent with these results, earlier research has determined that infection rates during maxillofacial PSI repair are extremely low (between 7.7 and 14.3%) [6,10,24]. Based on Alasseri et al.’s [45] experience with non-custom-made implants, postoperative infections are typically seen within the first few weeks and rarely occur after one month.
One limitation of this technique lies in the requirement for expertise in utilizing the software that generates both the PSI and the PSI molded cast. Our case report confirmed the excellent viability and low cost of using a PSI molded cast during surgery. This approach has the potential to extend accessibility to a broader range of patients. Furthermore, a PSI acquired intraoperatively from a molded cast ensures an accurate fit and optimal functional and aesthetic outcome.
4. Conclusions
In conclusion, our case demonstrates that intraoperative-template-molded osteoplasty using PMMA is a safe, sustainable, efficient, and cost-effective technique for accurately recreating a patient’s natural bone thickness. By employing a single-stage repair method, we observed enhanced functional, aesthetic, and psychological results while also minimizing discomfort for a substantial number of patients. Our findings highlight the potential benefits and effectiveness of this approach in the specific context of our case.
Author Contributions
Conceptualization, N.O., C.A.O. and M.B.; methodology N.O., C.A.O. and M.B.; validation, A.M.; investigation, N.O., C.A.O. and M.B.; resources, G.L.G. and D.G.B.; writing—original draft preparation, N.O., C.A.O., M.B. and E.-R.B.; writing—review and editing, D.G.B., G.L.G. and E.-R.B.; visualization, M.P.; supervision, N.O., C.A.O. and M.B.; project administration, M.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study did not require ethical approval; the surgical procedures were performed in accordance with appropriate medical standards.
Informed Consent Statement
Informed consent was obtained from the subject involved in this study. Written informed consent was obtained from the patient prior to the publication of this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kronmiller J.E. Development of asymmetries. Semin. Orthod. 1998;4:134–137. doi: 10.1016/S1073-8746(98)80014-5. [DOI] [PubMed] [Google Scholar]
- 2.Bailey L.J., Collie F.M., White R.P., Jr. Long-term soft tissue changes after orthognathic surgery. Int. J. Adult Orthod. Orthognath. Surg. 1996;11:7–18. [PubMed] [Google Scholar]
- 3.Betts N.J., Dowd K.F. Soft tissue changes associated with orthognathic surgery. Atlas Oral Maxillofac. Surg. Clin. 2000;8:13–38. doi: 10.1016/S1061-3315(18)30030-1. [DOI] [PubMed] [Google Scholar]
- 4.Jung J., Lee C.H., Lee J.W., Choi B.J. Three dimensional evaluation of soft tissue after orthognathic surgery. Head Face Med. 2018;14:21. doi: 10.1186/s13005-018-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinser M.J., Mischkowski R.A., Sailer H.F., Zöller J.E. Computer-assisted orthognathic surgery: Feasibility study using multiple CAD/CAM surgical splints. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;113:673–687. doi: 10.1016/j.oooo.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Cheong Y.W., Lo L.J. Facial asymmetry: Etiology, evaluation, and management. Chang. Gung Med. J. 2011;34:341–351. [PubMed] [Google Scholar]
- 7.Suojanen J., Leikola J., Stoor P. The use of patient-specific implants in orthognathic surgery: A series of 32 maxillary osteotomy patients. J. Cranio-Maxillofac. Surg. 2016;44:1913–1916. doi: 10.1016/j.jcms.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Suojanen J., Leikola J., Stoor P. The use of patient-specific implants in orthognathic surgery: A series of 30 mandible sagittal split osteotomy patients. J. Cranio-Maxillofac. Surg. 2017;45:990–994. doi: 10.1016/j.jcms.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Rammos C.K., Cayci C., Castro-Garcia J.A., Feiz-Erfan I., Lettieri S.C. Patient-specific polyetheretherketone implants for repair of craniofacial defects. J. Craniofacial Surg. 2015;26:631–633. doi: 10.1097/SCS.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 10.Jalbert F., Boetto S., Nadon F., Lauwers F., Schmidt E., Lopez R. One-step primary reconstruction for complex craniofacial resection with PEEK custom-made implants. J. Cranio-Maxillofac. Surg. 2014;42:141–148. doi: 10.1016/j.jcms.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Binder W.J. Custom-designed facial implants. Facial Plast. Surg. Clin. North Am. 2008;16:133–146. doi: 10.1016/j.fsc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Ridwan-Pramana A., Wolff J., Raziei A., Ashton-James C.E., Forouzanfar T. Porous polyethylene implants in facial reconstruction: Outcome and complications. J. Craniomaxillofac. Surg. 2015;43:1330–1334. doi: 10.1016/j.jcms.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Soleymani Eil Bakhtiari S., Bakhsheshi-Rad H.R., Karbasi S., Tavakoli M., Razzaghi M., Ismail A.F., RamaKrishna S., Berto F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers. 2020;12:1469. doi: 10.3390/polym12071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dukle A., Murugan D., Nathanael A.J., Rangasamy L., Oh T.H. Can 3D-Printed Bioactive Glasses Be the Future of Bone Tissue Engineering? Polymers. 2022;14:1627. doi: 10.3390/polym14081627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jean W.C., Huang M.C., Felbaum D.R. Optimization of skull base exposure using navigation-integrated, virtual reality templates. J. Clin. Neurosci. 2020;80:125–130. doi: 10.1016/j.jocn.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Slimani M., Baus A., Bich C.S., de Rousiers A., Duhoux A., Brachet M., Duhamel P., Bey E. Methylmetacrylate (PMMA) cranioplasty technique: Technical interest of intraoperative modeling and review of the literature. Ann. Chir. Plast. Esthet. 2023;68:99–105. doi: 10.1016/j.anplas.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Lye K.W., Tideman H., Merkx M.A., Jansen J.A. Bone cements and their potential use in a mandibular endoprosthesis. Tissue Eng. Part. B Rev. 2009;15:485–496. doi: 10.1089/ten.teb.2009.0139. [DOI] [PubMed] [Google Scholar]
- 18.Mommaerts M.Y. Guidelines for patient-specific jawline definition with titanium implants in esthetic, deformity, and malformation surgery. Ann. Maxillofac. Surg. 2016;6:287–291. doi: 10.4103/2231-0746.200325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldsmith D., Horowitz A., Orentlicher G. Facial skeletal augmentation using custom facial implants. Atlas Oral. Maxillofac. Surg. Clin. 2012;20:119–134. doi: 10.1016/j.cxom.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Möllmann H.L., Apeltrath L., Karnatz N., Wilkat M., Riedel E., Singh D.D., Rana M. Comparison of the Accuracy and Clinical Parameters of Patient-Specific and Conventionally Bended Plates for Mandibular Reconstruction. Front. Oncol. 2021;11:719028. doi: 10.3389/fonc.2021.719028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty S.R., Al-Bayatti S., AlKawas S., Talaat W., Narasimhan S., Gaballah K., Al-Rawi N., Alsaegh M., Madiyal A., Balan P., et al. Analysis of the Volumetric Asymmetry of the Mandibular Condyles Using CBCT. Int. Dent. J. 2022;72:797–804. doi: 10.1016/j.identj.2022.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shetty S., Guddadararangiah S. Case Report: Unilateral condylar hyperplasia. F1000Research. 2021;10:46. doi: 10.12688/f1000research.48499.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo J.M., Baek S.H., Kim J.C., Choi J.Y. Contour Restoration of Over-Resected Mandibular Angle and Lower Border by Reduction Mandibuloplasty Using Three-Dimensional Planning and Computer-Aided Design and Manufacturing Custom-Made Titanium Implants. J. Craniofac. Surg. 2018;29:e340–e343. doi: 10.1097/SCS.0000000000004283. [DOI] [PubMed] [Google Scholar]
- 24.Yim H.W., Nguyen A., Kim Y.K. Facial Contouring Surgery with Custom Silicone Implants Based on a 3D Prototype Model and CT-Scan: A Preliminary Study. Aesthet. Plast. Surg. 2015;39:418–424. doi: 10.1007/s00266-015-0482-z. [DOI] [PubMed] [Google Scholar]
- 25.Staal F., Pluijmers B., Wolvius E., Koudstaal M. Patient-specific implant for residual facial asymmetry following orthognathic surgery in unilateral craniofacial microsomia. Craniomaxillofacial Trauma. Reconstr. 2016;9:264–267. doi: 10.1055/s-0036-1581061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neovius E., Engstrand T. Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J. Plast. Reconstr. Aesthet. Surg. 2010;63:1615–1623. doi: 10.1016/j.bjps.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Kerkfeld V., Schorn L., Depprich R., Lommen J., Wilkat M., Kübler N., Rana M., Meyer U. Simultaneous PSI-Based Orthognathic and PEEK Bone Augmentation Surgery Leads to Improved Symmetric Facial Appearance in Craniofacial Malformations. J. Pers. Med. 2022;12:1653. doi: 10.3390/jpm12101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu S.S.-P., Gateno J., Bell R.B., Hirsch D.L., Markiewicz M.R., Teichgraeber J.F., Zhou X., Xia J.J. Accuracy of a computer-aided surgical simulation protocol for orthognathic surgery: A prospective multicenter study. J. Oral. Maxillofac. Surg. 2013;71:128–142. doi: 10.1016/j.joms.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sannino G., Gloria F., Schiavetti R., Ottria L., Barlattani A. Dental Wings CAD/CAM system precision: An internal and marginal fit sperimental analisys. Oral Implant. 2009;2:11–20. [PMC free article] [PubMed] [Google Scholar]
- 30.Villavisanis D.F., Workman C.I., Cho D.Y., Zapatero Z.D., Wagner C.S., Blum J.D., Bartlett S.P., Swanson J.W., Chatterjee A., Taylor J.A. Associations of Facial Proportionality, Attractiveness, and Character Traits. J. Craniofac. Surg. 2022;33:1431–1435. doi: 10.1097/SCS.0000000000008662. [DOI] [PubMed] [Google Scholar]
- 31.Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Maxillofac. Surg. 2014;4:9–18. doi: 10.4103/2231-0746.133065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller E.E., Triplett W.W. Iliac bone grafting: Review of 160 consecutive cases. J. Oral. Maxillofac. Surg. 1987;45:11–14. doi: 10.1016/0278-2391(87)90079-6. [DOI] [PubMed] [Google Scholar]
- 33.Olate S., Huetequeo-Molina C., Requena R., Uribe F. Patient Specific Implants to Solve Structural Facial Asymmetry after Orthognathic Surgery. J. Craniofac. Surg. 2021;32:e269–e271. doi: 10.1097/SCS.0000000000007113. [DOI] [PubMed] [Google Scholar]
- 34.Scolozzi P., Martinez A., Jaques B. Complex orbito-fronto-temporal reconstruction using computer-designed PEEK implant. J. Craniofac. Surg. 2007;18:224–228. doi: 10.1097/01.scs.0000249359.56417.7e. [DOI] [PubMed] [Google Scholar]
- 35.Saponaro G., Doneddu P., Gasparini G., Staderini E., Boniello R., Todaro M., D’Amato G., Pelo S., Moro A. Custom made onlay implants in peek in maxillofacial surgery: A volumetric study. Childʹs Nerv. Syst. 2020;36:385–391. doi: 10.1007/s00381-019-04307-9. [DOI] [PubMed] [Google Scholar]
- 36.Thrivikraman G., Athirasala A., Twohig C., Boda S.K., Bertassoni L.E. Biomaterials for Craniofacial Bone Regeneration. Dent. Clin. N. Am. 2017;61:835–856. doi: 10.1016/j.cden.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalmar C.L., Humphries L.S., Zimmerman C.E., Vu G.H., Swanson J.W., Bartlett S.P., Taylor J.A. Orthognathic Hardware Complications in the Era of Patient-Specific Implants. Plast. Reconstr. Surg. 2020;146:609e–621e. doi: 10.1097/PRS.0000000000007250. [DOI] [PubMed] [Google Scholar]
- 38.Deng L., Deng Y., Xie K. AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf. B Biointerfaces. 2017;160:483–492. doi: 10.1016/j.colsurfb.2017.09.061. [DOI] [PubMed] [Google Scholar]
- 39.Thien A., King N.K., Ang B.T., Wang E., Ng I. Comparison of polyetheretherketone and titanium cranioplasty after decompressive craniectomy. World Neurosurg. 2015;83:176–180. doi: 10.1016/j.wneu.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Bakhtiari S.E., Bakhsheshi-Rad H.R., Karbasi S., Tavakoli M., Hassanzadeh Tabrizi S.A., Ismail A.F., Seifalian A., RamaKrishna S., Berto F. Poly(methyl methacrylate) bone cement, its rise, growth, downfall and future. Polym. Int. 2021;70:1182–1201. doi: 10.1002/pi.6136. [DOI] [Google Scholar]
- 41.Zamorano L., Kadi A., Dong A. Computer-assisted neurosurgery: Simulation and automation. Stereot. Funct. Neuros. 1992;59:115–122. doi: 10.1159/000098927. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-de Thomas R.J., Munakomi S., De Jesus O. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2023. Craniotomy. [Google Scholar]
- 43.Aiache A.E. Mandibular angle implants. Aesthet. Plast. Surg. 1992;16:349–354. doi: 10.1007/BF01570699. [DOI] [PubMed] [Google Scholar]
- 44.Olate S., Uribe F., Huentequeo-Molina C., Goulart D.R., Sigua-Rodriguez E.A., Alister J.P. Mandibular Angle Contouring Using Porous Polyethylene Stock or PEEK-based Patient Specific Implants. A Critical Analysis. J. Craniofac. Surg. 2021;32:242–246. doi: 10.1097/SCS.0000000000006926. [DOI] [PubMed] [Google Scholar]
- 45.Alasseri N., Alasraj A. Patient-specific implants for maxillofacial defects: Challenges and solutions. Maxillofac. Plast. Reconstr. Surg. 2020;42:15. doi: 10.1186/s40902-020-00262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.