Abstract
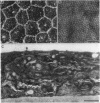
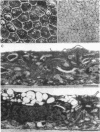
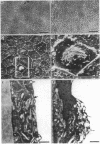
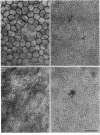
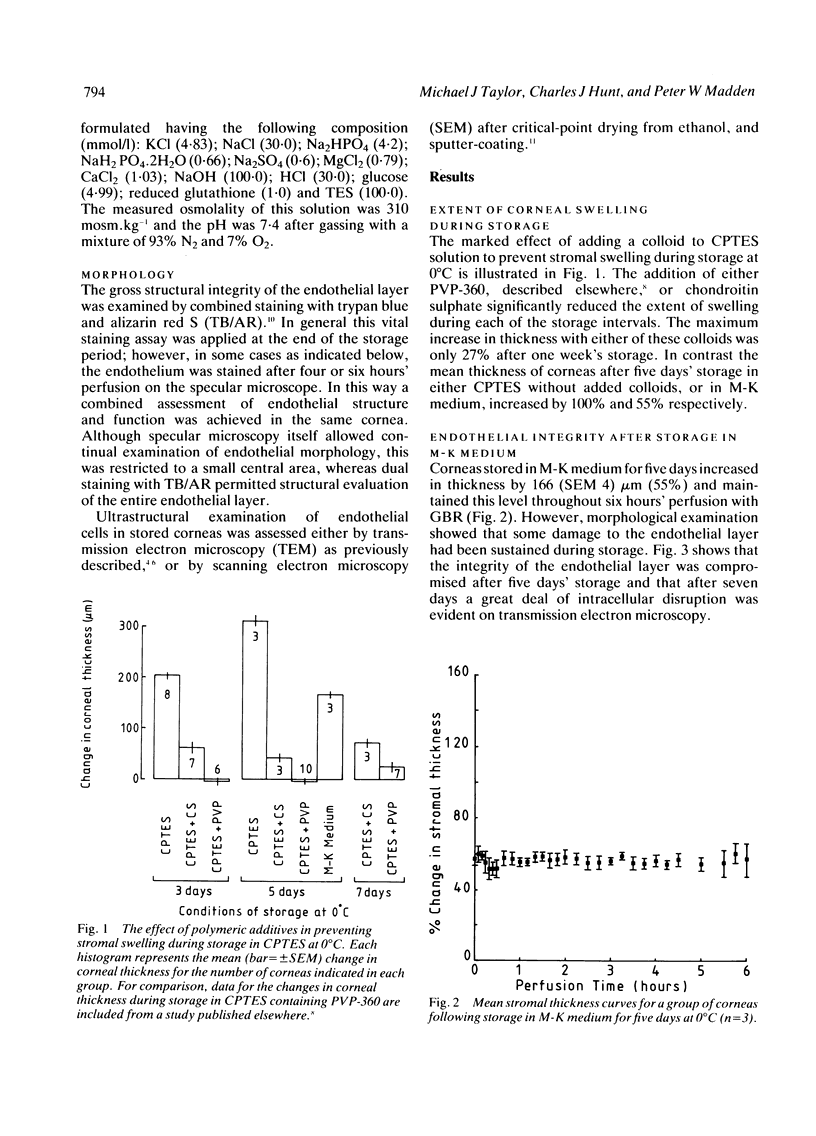
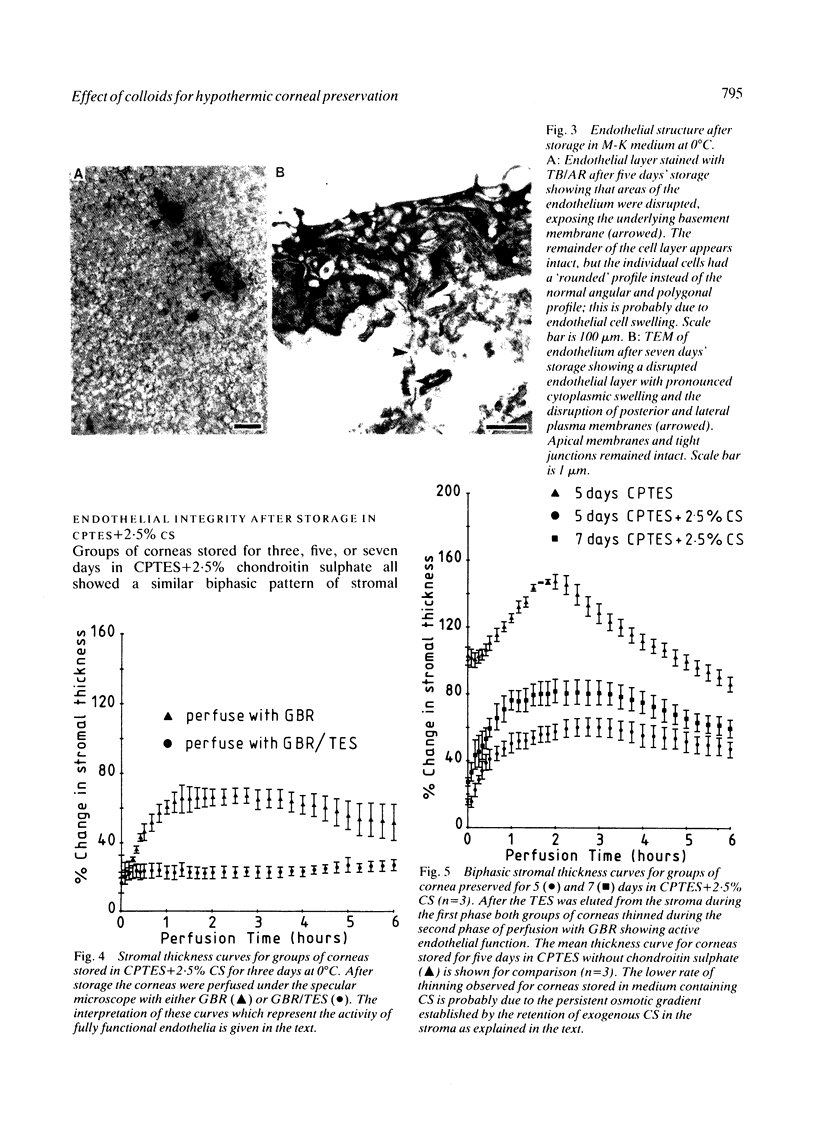
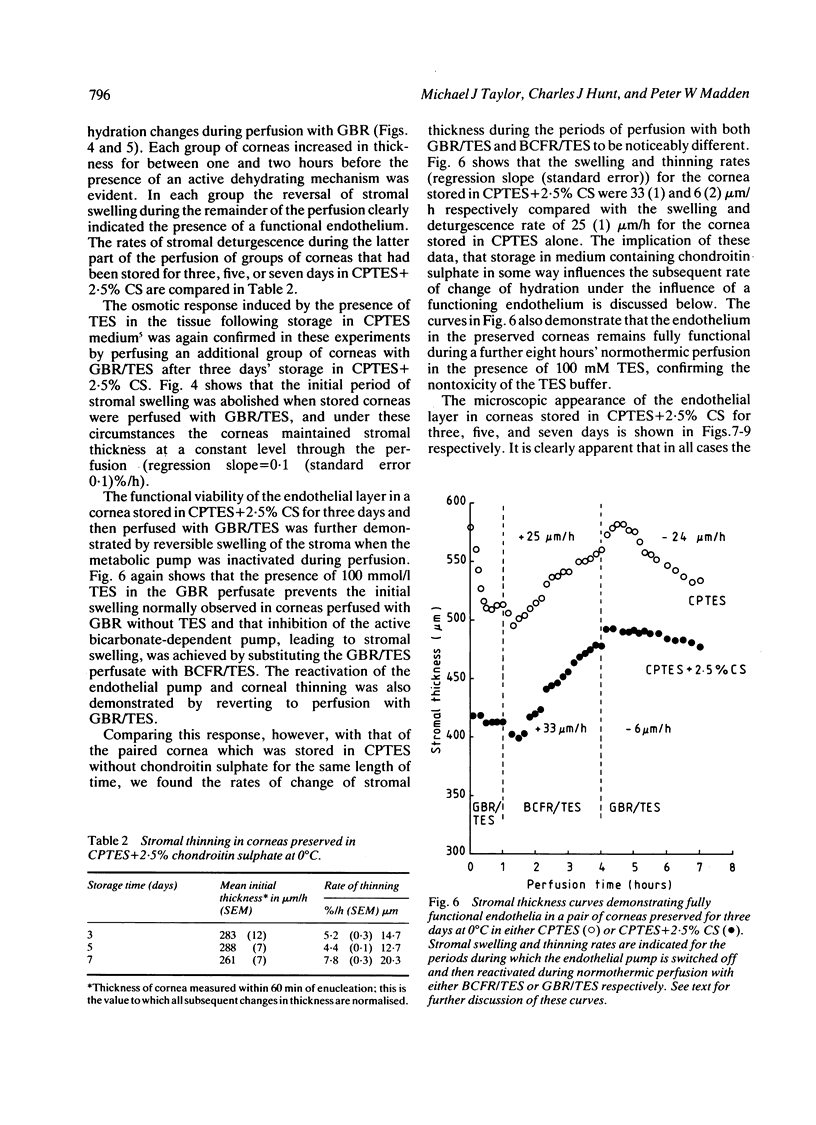
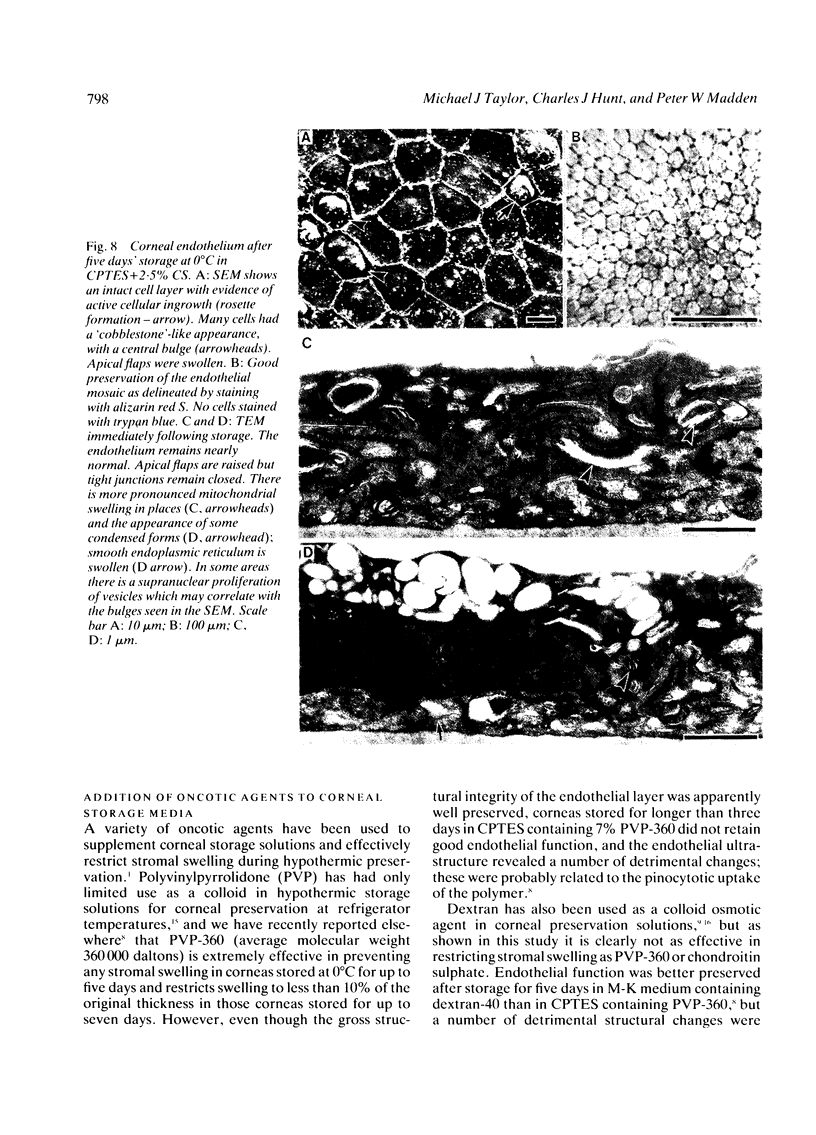
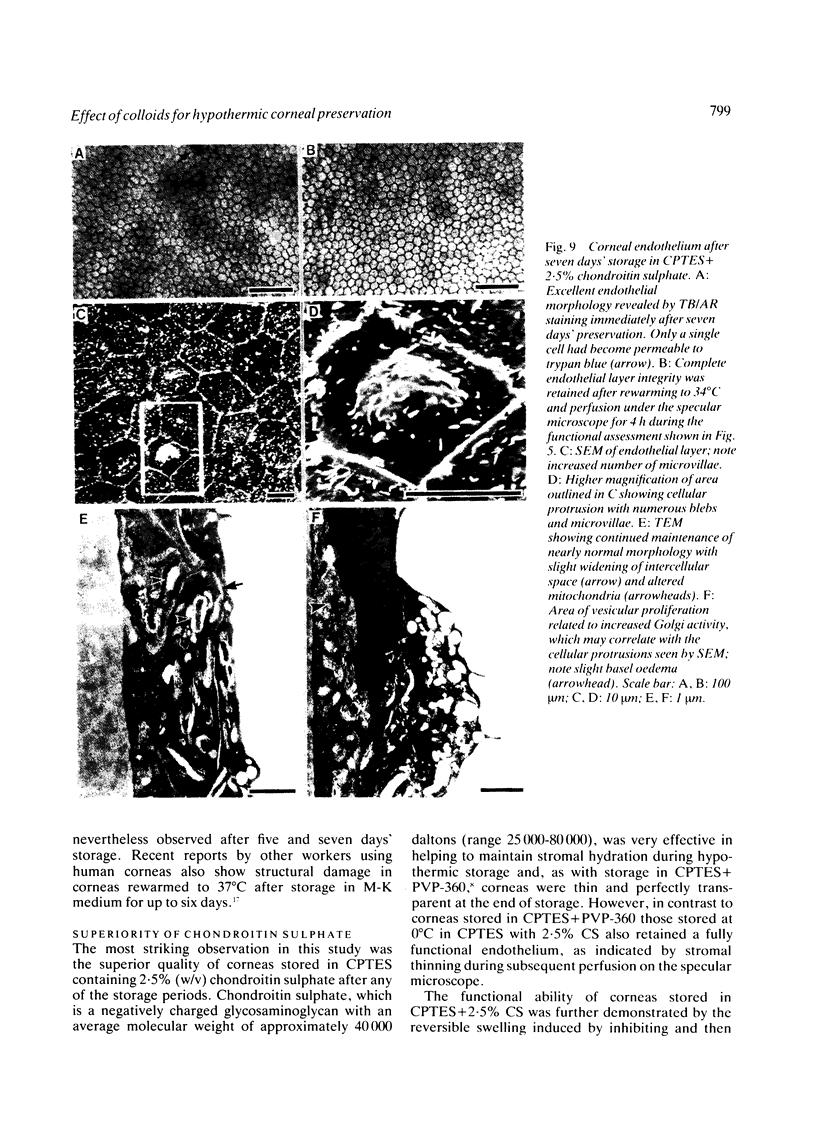
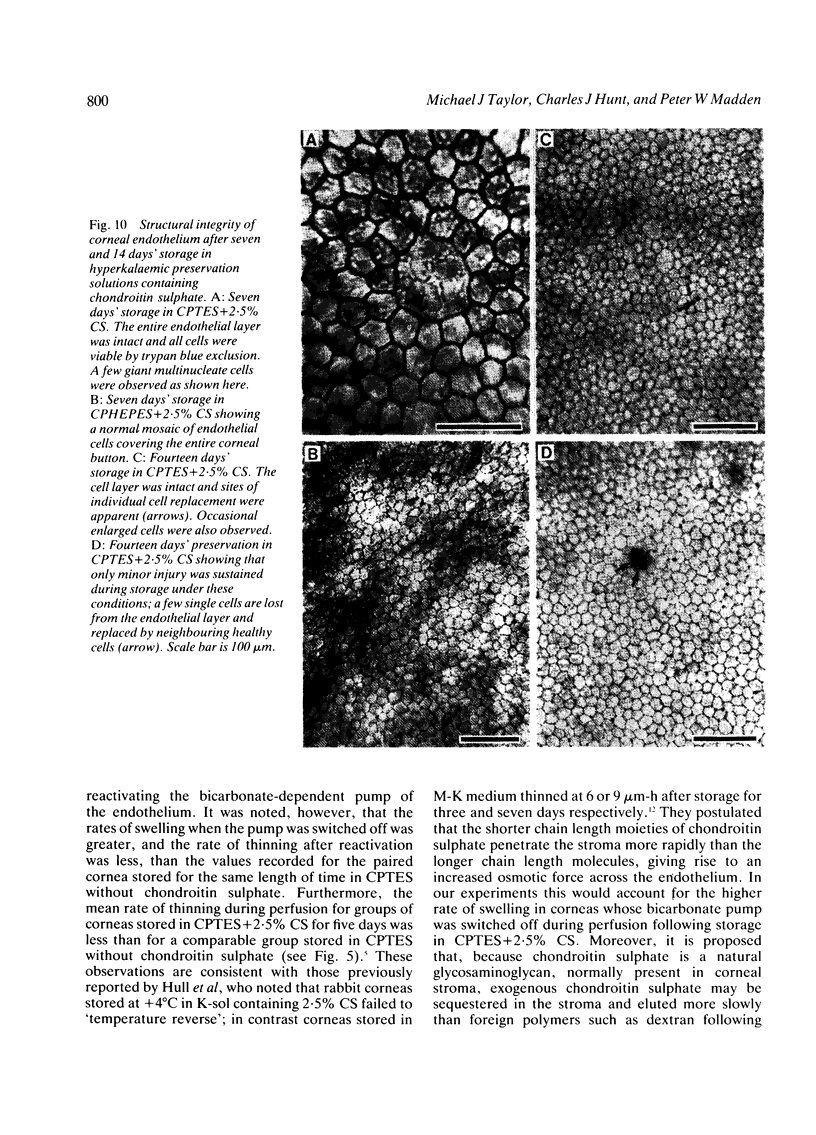
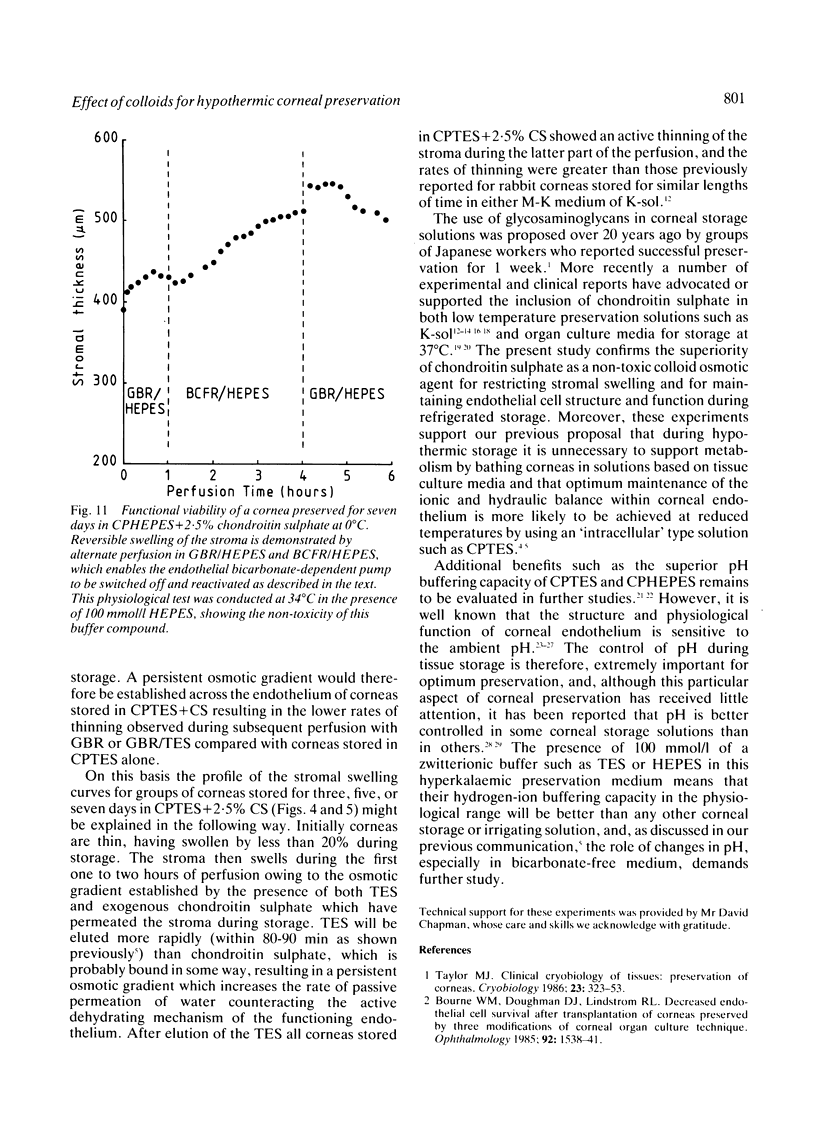
Periods of preservation for donor corneas, even for short times, are necessary to facilitate optimum conditions in penetrating keratoplasty. However, current techniques for corneal storage at low temperatures may not provide optimal conditions for maintaining tissue integrity. In particular, the ionic composition of the storage medium has received little attention since it has been assumed throughout that the normal complement of ions in tissue culture media will also be suitable for preservation at reduced temperatures. This study extends our previous investigations on the merits of using CPTES (corneal-potassium-TES), a potassium-rich balanced salt solution containing an impermeant anionic pH buffer (TES), as a storage solution specifically designed to prevent the loss of intracellular potassium and minimise endothelial cell swelling during the time that the normal regulatory processes are switched off. The effect of adding the natural polymer chondroitin sulphate (CS) as a colloid osmotic agent to the hyperkalaemic storage medium is now examined. Corneas stored in CPTES containing 2.5% chondroitin sulphate retained a very high level of structural and functional integrity after three, five, and seven days storage at 0 degrees C; furthermore, stromal swelling was restricted to only 21%. All corneas stored in CPTES + 2.5% CS showed active endothelial function by thinning efficiently at rates that were greater than those previously reported for rabbit corneas stored for similar lengths of time in either M-K medium or K-sol. The zwitterionic buffers TES and HEPES were interchangeable in the hyperkalaemic solution and were non-toxic to corneal endothelium at a concentration of 100 mM. These compounds offer excellent pH buffering in bicarbonate-free medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourne W. M., Doughman D. J., Lindstrom R. L. Decreased endothelial cell survival after transplantation of corneas preserved by three modifications of corneal organ culture technique. Ophthalmology. 1985 Nov;92(11):1538–1541. doi: 10.1016/s0161-6420(85)33834-4. [DOI] [PubMed] [Google Scholar]
- Bourne W. M. Endothelial cell survival on transplanted human corneas preserved at 4 C in 2.5% chondroitin sulfate for one to 13 days. Am J Ophthalmol. 1986 Sep 15;102(3):382–386. doi: 10.1016/0002-9394(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Bourne W. M., Lindstrom R. L., Doughman D. J. Endothelial cell survival on transplanted human corneas preserved by organ culture with 1.35% chondroitin sulfate. Am J Ophthalmol. 1985 Dec 15;100(6):789–793. doi: 10.1016/s0002-9394(14)73368-9. [DOI] [PubMed] [Google Scholar]
- Bowman K. A., Elijah R. D., Cheeks K. E., Green K. Intracellular potential and pH of rabbit corneal endothelial cells. Curr Eye Res. 1984 Aug;3(8):991–1000. doi: 10.3109/02713688409011745. [DOI] [PubMed] [Google Scholar]
- Fischer F. H., Wiederholt M. The pH dependency of sodium and chloride transport in the isolated human cornea. Invest Ophthalmol Vis Sci. 1978 Aug;17(8):810–813. [PubMed] [Google Scholar]
- Fischer F., Voigt G., Liegl O., Wiederholt M. Effect of pH on potential difference and short circuit current in the isolated human cornea. Pflugers Arch. 1974 Jun 11;349(2):119–131. [PubMed] [Google Scholar]
- Gonnering R., Edelhauser H. F., Van Horn D. L., Durant W. The pH tolerance of rabbit and human corneal endothelium. Invest Ophthalmol Vis Sci. 1979 Apr;18(4):373–390. [PubMed] [Google Scholar]
- Green K., Simon S., Kelly G. M., Jr, Bowman K. A. Effects of [Na+], [Cl-], carbonic anhydrase, and intracellular pH on corneal endothelial bicarbonate transport. Invest Ophthalmol Vis Sci. 1981 Oct;21(4):586–591. [PubMed] [Google Scholar]
- Hull D. S., Green K., Bowman K., Csukas S., Riley M. V. Intracellular pH and glutathione levels in rabbit corneal endothelium following storage in moist chamber and MK medium. Invest Ophthalmol Vis Sci. 1983 Feb;24(2):214–217. [PubMed] [Google Scholar]
- Hull D. S., Green K., Frey N. Rabbit corneal endothelial physiologic and morphologic characteristics following storage in MK medium and K-Sol. Acta Ophthalmol (Copenh) 1986 Dec;64(6):649–656. doi: 10.1111/j.1755-3768.1986.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Lindstrom R. L., Doughman D. J., Skelnik D. L., Mindrup E. A. Minnesota system corneal preservation. Br J Ophthalmol. 1986 Jan;70(1):47–54. doi: 10.1136/bjo.70.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden P. W. The evaluation of endothelial damage following corneal storage: a comparison of staining methods and the value of scanning electron microscopy. Curr Eye Res. 1987 Dec;6(12):1441–1451. doi: 10.3109/02713688709044508. [DOI] [PubMed] [Google Scholar]
- McCarey B. E., Kaufman H. E. Improved corneal storage. Invest Ophthalmol. 1974 Mar;13(3):165–173. [PubMed] [Google Scholar]
- Rootman D. S., Hasany S. M., Basu P. K. A morphometric study of endothelial cells of human corneas stored in MK media and warmed at 37 degrees C. Br J Ophthalmol. 1988 Jul;72(7):545–549. doi: 10.1136/bjo.72.7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. M., Bourne W. M., Campbell R. J. Chondroitin sulfate for corneal preservation at 4 degrees C. Evaluation by electron microscopy. Arch Ophthalmol. 1986 Sep;104(9):1358–1361. doi: 10.1001/archopht.1986.01050210112035. [DOI] [PubMed] [Google Scholar]
- Tamaki K., Yamaguchi T., Varnell E. D., Kaufman H. E. Histological study of corneas preserved in two new media. Br J Ophthalmol. 1987 Aug;71(8):570–577. doi: 10.1136/bjo.71.8.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J. Clinical cryobiology of tissues: preservation of corneas. Cryobiology. 1986 Aug;23(4):323–353. doi: 10.1016/0011-2240(86)90038-6. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Hunt C. J. A new preservation solution for storage of corneas at low temperatures. Curr Eye Res. 1985 Sep;4(9):963–973. doi: 10.3109/02713689509000003. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Hunt C. J. Dual staining of corneal endothelium with trypan blue and alizarin red S: importance of pH for the dye-lake reaction. Br J Ophthalmol. 1981 Dec;65(12):815–819. doi: 10.1136/bjo.65.12.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Hunt C. J. Hypothermic preservation of corneas in a hyperkalaemic solution (CPTES): I. Short-term storage in the absence of colloid osmotic agents. Br J Ophthalmol. 1989 Oct;73(10):781–791. doi: 10.1136/bjo.73.10.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Hunt C. J. Tolerance of corneas to multimolar dimethyl sulfoxide at 0 degrees C. Implications for cryopreservation. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):400–412. [PubMed] [Google Scholar]
- Taylor M. J., Pignat Y. Practical acid dissociation constants, temperature coefficients, and buffer capacities for some biological buffers in solutions containing dimethyl sulfoxide between 25 and -12 degrees C. Cryobiology. 1982 Feb;19(1):99–109. doi: 10.1016/0011-2240(82)90129-8. [DOI] [PubMed] [Google Scholar]
- Taylor M. J. The role of pH and buffer capacity in the recovery of function of smooth muscle cooled to -13 degrees C in unfrozen media. Cryobiology. 1982 Dec;19(6):585–601. doi: 10.1016/0011-2240(82)90188-2. [DOI] [PubMed] [Google Scholar]
- Yau C. W., Kaufman H. E. A medium-term corneal preserving medium (K-Sol). Arch Ophthalmol. 1986 Apr;104(4):598–601. doi: 10.1001/archopht.1986.01050160154032. [DOI] [PubMed] [Google Scholar]