Abstract
Human immunodeficiency virus type 1 (HIV-1) Vpr is a virion-associated protein which facilitates HIV-1 infection of nondividing cells by contributing to the nuclear transport of the preintegration complex (PIC). Vpr was also shown to induce a cell cycle G2 arrest in infected proliferating cells that optimizes HIV-1 long terminal repeat (LTR)-directed gene expression and viral production. However, it is unclear whether this activity is mediated primarily early by virion-associated Vpr or alternatively late during infection when Vpr is de novo expressed. We report here that in the absence of de novo expression, virion-associated Vpr induces a transient G2 arrest that can subsequently lead to cell killing by apoptosis. Interestingly, the induction of both cell cycle G2 arrest and apoptosis by virion-associated Vpr requires viral entry but not viral replication, since reverse transcriptase and protease inhibitor treatments do not prevent these Vpr effects. These results raise the possibility that in vivo both infectious and noninfectious viruses contribute to the dysfunction and killing of CD4+ cells. In addition, our results reveal that virion-associated Vpr stimulates viral replication in proliferating cells after establishing a cell cycle G2 arrest by increasing LTR-directed gene expression. Importantly, this Vpr-mediated LTR activation appears to be a requirement for subsequent optimal Tat transactivation. Taken together, these results strongly suggest that in addition to participating in the HIV PIC nuclear transport in nondividing cells, virion-associated Vpr activates HIV-1 LTR-directed gene expression by manipulating the host cell cycle. From this, we conclude that Vpr functions as an immediate-early protein during HIV-1 infection.
Human immunodeficiency virus type 1 (HIV-1), the etiological agent of AIDS, infects and ultimately incapacitates critical cellular components of the immune system. Part of the explanation for the complex HIV-host interaction lies in the complex genetic organization of the viral genome. In addition to gag, pol, and env structural gene products, HIV encodes six regulatory or accessory proteins that are not found in the other classes of retroviruses. Some of these products (Tat and Rev) are essential for HIV replication, while others (Vif, Vpr, Vpu, and Nef) appear to optimally modulate the infection and replication processes (7, 9). Vpr is a 14-kDa, 96-amino-acid protein that is highly conserved among HIV-1, HIV-2, and simian immunodeficiency virus. The importance of Vpr during HIV infection and pathogenesis has been suggested by a number of in vitro and in vivo studies (reviewed in references 7 and 9). Vpr was shown to be packaged in significant quantities into viral particles (4, 47). These observations suggested that Vpr may play a role in early events during infection. Indeed, recent studies have demonstrated that Vpr participates in the active nuclear translocation of the HIV-1 preintegration complex (PIC) in nondividing cells by interacting with the nuclear transport pathway (12, 17, 29, 34, 41). This function of Vpr appears essential for HIV infection of macrophages under conditions that closely mimic the in vivo situation (39). A recently discovered second function of Vpr is to promote cell differentiation and growth arrest at the G2/M phase of the cell cycle (16, 21, 25, 35, 36). This Vpr-mediated cell cycle arrest activity is conserved among divergent HIV and simian immunodeficiency viruses (32). Recently, several studies have provided evidence that Vpr up-regulates HIV replication during infection of dividing T cells and primary macrophages as a result of its cell cycle-modulating activity (14, 39, 44). Thus, it is conceivable that Vpr may contribute to HIV persistence in the host by optimizing viral production during the short life span of infected cells in vivo (14).
Recent studies indicate that the turnover of both HIV and infected CD4+ cells is extremely rapid in HIV-infected individuals (18, 31, 42). However, whether active HIV-1 replication predominantly kills infected cells or induces the death of uninfected cells (bystander cell killing) remains controversial. While it has been shown that HIV replication directly kills CD4+ T cells, other studies have reported that a marked decrease of the CD4+ cell population occurs even if the frequency of HIV-infected cells detected in vivo is low (2, 11, 13). Although the mechanisms involved in HIV-mediated cytopathicity are not fully understood, many studies have indicated that apoptosis is involved in direct and indirect HIV-mediated CD4+ cell depletion in vivo (2, 11, 15, 19, 20). Interestingly, Vpr was shown to differentially regulate the occurrence of apoptosis. During active HIV replication, Vpr expression induces apoptosis of infected cells (38, 44). On the other hand, under certain conditions such as low-level expression, Vpr was shown to act as a negative regulator of T-cell-induced apoptosis (1, 6).
In this study, we examined the effect of virion-associated Vpr during the early stages of HIV infection in dividing T cells by using an efficient vesicular stomatitis virus G glycoprotein (VSV-G)-pseudotyped HIV infection system (3, 44). Our results indicate that virion-associated Vpr induces cell cycle arrest of infected cells in G2 and stimulates HIV expression. However, these effects are transient without de novo Vpr expression. In addition, we provide evidence indicating that after viral entry, virion-associated Vpr can induce cell killing by apoptosis even in the presence of anti-HIV agents.
MATERIALS AND METHODS
Cell lines, antisera, and chemicals.
The Jurkat T-lymphoid cell line and the human embryonic kidney 293T cell line were maintained in RPMI 1640 medium or Dulbecco modified Eagle medium containing 10% fetal calf serum. The rabbit polyclonal anti-Vpr serum and the monoclonal anti-HIV p24 antibody were described previously (24, 46). Propidium iodide, actinomycin D, Polybrene, and AZT (3′-azido-3′-deoxythymidine) were purchased from Sigma Chemical Inc. (Mississauga, Ontario, Canada). The annexin V-fluorescein isothiocyanate (FITC) kit (no. 1828681) was purchased from Boehringer Mannheim Inc. (Laval, Quebec, Canada).
HIV molecular clones and expressors.
The HIV proviral constructs HxBRUR+ or HxBRUR− and the envelope-defective HIV-1 proviral plasmids used in this study, including HxBRUR+/Env−, HxBRUR−/Env−, and HxBRURR80A/Env−, were described previously (39, 45). The Vpr expressors SVCMV-VPR, SVCMVRR80A, and the negative control plasmid SVCMVR− were constructed by PCR amplification of the HxBRU Vpr sequence as described elsewhere (45). The VSV-G expressor SVCMV–VSV-G was also described previously (44). The chloramphenicol acetyltransferase (CAT) expressor pCEP4IIICAT was constructed by inserting a XhoI-BamHI fragment containing the HIV long terminal repeat (LTR) and the CAT gene into an episomal plasmid pCEP4 polylinker (Invitrogen, Carlsbad, Calif.). This XhoI-BamHI fragment was derived from an HIV- LTR-driven CAT expressor plasmid (IIICAT) (37).
Production of pseudotyped viruses and infection.
VSV-G-pseudotyped HIV-1 virus stocks were generated by cotransfection of 293T cells (5 × 106) with 12.5 μg of envelope-defective HIV-1 proviral DNA and 25 μg of VSV-G expression plasmid SVCMV–VSV-G by using the calcium phosphate coprecipitation method. VSV-G-pseudotyped Vpr− HIV containing trans-incorporated Vpr protein were generated from 293T cells cotransfected with HxBRUR−/Env− (12.5 μg), SVCMV-VPR (18 μg), and SVCMV–VSV-G (25 μg) expressors. Vpr− HIV containing trans-incorporated Vpr protein were prepared as described above, except that HxBRUR− was used instead of HxBRUR−/Env−. The Vpr+ and Vpr− HIV stocks were produced by transfection of 293T cells with HxBRUR+ or HxBRUR− proviral plasmids. At 72 h posttransfection (p.i.), cell supernatants were collected, clarified, and ultracentrifuged at 45,000 rpm in a Beckman 60Ti rotor for 1 h to pellet pseudotyped or HIV virions. Each viral stock was resuspended in RPMI 1640 medium and filtered through a 0.45-μm-pore-size filter (Costar, Cambridge, Mass.). Virus stocks were subjected to titer determination by the multinuclear activation of galactosidase indicator (MAGI) assay (22).
To infect Jurkat cells, 0.5 × 106 cells were incubated with either different VSV-G pseudotyped viruses or wild-type HIV at multiplicities of infection (MOI) of 10 for 8 h in the presence of 10 μg of Polybrene per ml. Infected cells were then washed and cultured at a density of 0.5 × 106 cells/ml. To monitor viral production, infected-cell supernatants were collected at different time intervals. Virus levels in the supernatants were determined by the HIV reverse transcriptase (RT) assay, as described previously (44).
Immunoblot analysis.
To examine whether viruses incorporate Vpr at comparable levels, similar amounts of each virus preparation (with the same RT activity) were lysed in Laemmli buffer and viral proteins were separated on sodium dodecyl sulfate–12.5% polyacrylamide gels. After electrophoresis, the proteins were transferred to nitrocellulose filters (pore size, 0.45-μm; Schleicher & Schuell) by electroblotting. The blots were incubated first with monoclonal antibody against HIV p24 or the rabbit polyclonal anti-Vpr antibodies and then with a horseradish peroxidase-linked donkey anti-mouse or anti-rabbit antibody. After several washes, the blots were developed by using the 3,3′-diaminobenzidine detection system as recommended by the manufacturer (Sigma Chemical Inc.).
Cell cycle profile, cell growth, and apoptosis analyses.
Cells were harvested at several time points p.i. and tested for DNA content by flow cytometry analysis (44). Briefly, the cells were washed and resuspended in 80% ethanol on ice. Following additional washes, the cells were treated with 180 U of RNase A per ml in 1 ml of phosphate-buffered saline at 37°C for 30 min and subsequently stained with 30 μg of propidium iodide (PI) per ml. The cellular DNA content was then analyzed with a FACScan apparatus and Consort 30 software. In parallel, the viable cell number was determined every 12 h postinfection by trypan blue exclusion assay to monitor cell growth.
The occurrence of apoptotic cells was detected by the annexin V-FITC assay performed as recommended by the manufacturer (Boehringer Mannheim Inc.). Briefly, 0.25 × 106 infected cells were washed once with phosphate-buffered saline and then resuspended in annexin V binding buffer (2.5 μg of annexin V-FITC per ml, 10 mM HEPES–NaOH [pH7.4], 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 1 μg of PI per ml). After 10 to 15 min of incubation, stained cells were washed twice with binding buffer, resuspended in binding buffer containing 1% paraformaldehyde, and analyzed with a FACScan apparatus.
Effect of Vpr on LTR-directed CAT expression.
Jurkat T cells were transfected with the pCEP4IIICAT plasmid by the standard electroporation method. After 48 h, hygromycin (500 μg/ml) was added to the culture for positive selection. After 10 days, hygromycin-resistant cells were harvested and used for infection as described above. The CAT assay was performed as described previously (5).
RESULTS
A transient cell cycle G2 arrest is mediated by virion-associated Vpr during the early stage of infection.
To investigate the functional role of virion-associated Vpr in HIV infection of dividing T cells, we used a previously described VSV-G-pseudotyped HIV-1 one-cycle infection system (3, 44). This system allows a highly efficient infection of Jurkat T cells, achieving simultaneous infection of over 95% of cells in the culture (3, 44). The VSV-G-pseudotyped Vpr+ or Vpr− HIV particles were produced from 293T cells cotransfected with HIV proviral plasmid HxBRUR+/Env− expressing Vpr in cis or HxBRUR−/Env− and a VSV-G expressor, SVCMV–VSV-G. In parallel, Vpr− HIV containing trans-incorporated Vpr protein were generated by cotransfection of 293T cells with the Vpr− HIV proviral plasmid HxBRUR−/Env−, a wild-type Vpr expressor, SVCMV-VPR, and SVCMV–VSV-G. Since the resulting pseudotyped viruses do not encode a functional Vpr gene (the ATG initiation codon is mutated), infection with these viruses delivers trans-incorporated Vpr into cells without subsequent de novo Vpr expression. Therefore, these viruses were designated Vpr+-trans (Vpr+-T) viruses.
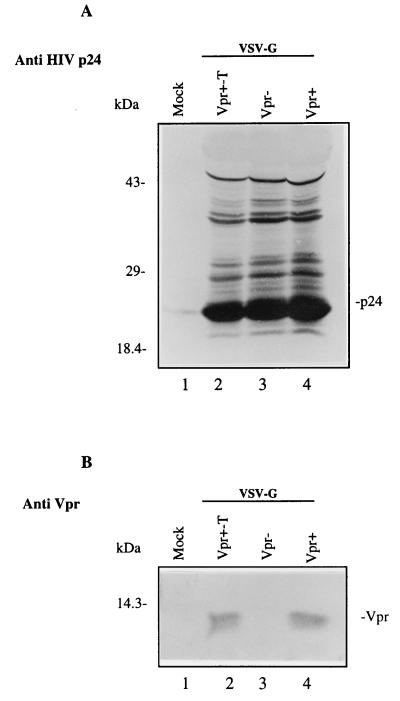
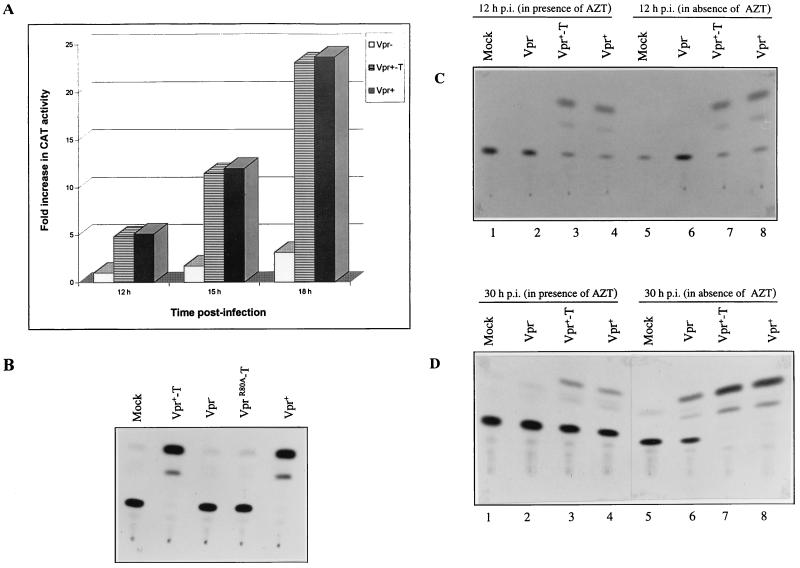
To determine the levels of virion-associated Vpr in Vpr+ and Vpr+-T viruses, the same amount of viral particles, as determined by virion-associated RT activity, was analyzed by Western blotting with an anti-HIV p24 monoclonal antibody and an anti-Vpr polyclonal antibody, as described in Materials and Methods. The results in Fig. 1A show that similar amounts of HIV p24gag were detected in the different virus samples (lanes 2 to 4). Moreover, abundant amounts of Vpr were detected in Vpr+-T and Vpr+ viral particles (Fig. 1B, lanes 2 and 4) but not in Vpr− viruses (lane 3). Densitometric analysis of p24gag and Vpr bands revealed that the relative amount of Vpr incorporated in Vpr+ and Vpr+-T viral particles was similar (data not shown).
FIG. 1.
Detection of HIV-1 Vpr and p24gag proteins in Vpr−, Vpr+, and Vpr+-T VSV-G-pseudotyped HIV-1. Each viral stock was produced from 293T cells cotransfected with the corresponding HIV provirus, SVCMV–VSV-G and SVCMV-VPR+ expressors, as described in Materials and Methods. Similar amounts of viral particles, as determined by virion-associated RT activity, were lysed in Laemmli buffer. Viral proteins were then run onto a sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose. The presence of HIV p24gag and Vpr in the viral particles was detected by immunoblotting with a monoclonal anti-HIV p24 antibody (A) or with a rabbit anti-Vpr serum (B).
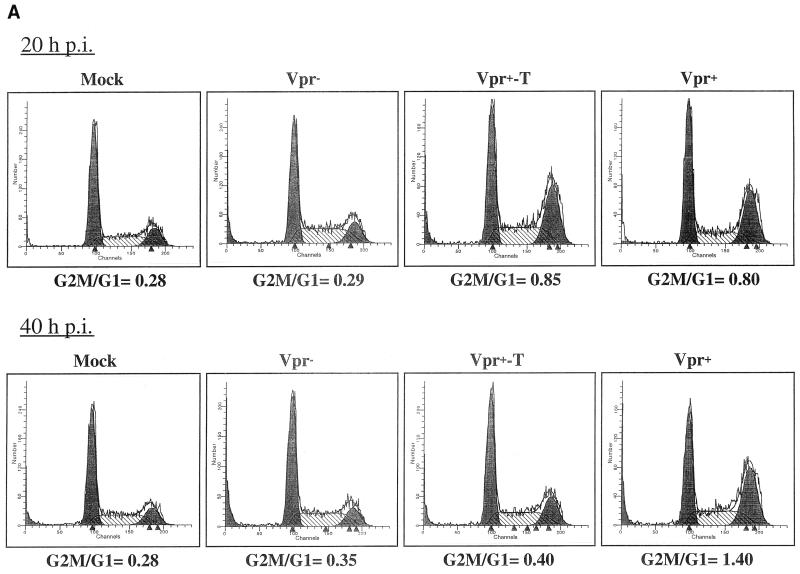
To investigate whether virion-associated Vpr could mediate a cell cycle G2 arrest, Jurkat T cells were infected with Vpr+, Vpr−, or Vpr+-T viruses at a multiplicity of infection (MOI) of 10. At different time points p.i., the cell cycle profile of infected cells was evaluated by PI staining of cellular DNA and flow cytometric analysis. The results reveal that at 20 h p.i., the cell cycle profile of cells infected with Vpr− virus was indistinguishable from that of mock-infected cells (G2-M/G1 ratio of 0.29 for Vpr− and 0.28 for mock-infected cells) (Fig. 2A). In contrast, Jurkat T cells infected with either Vpr+ or Vpr+-T pseudotyped viruses displayed a drastic redistribution of their cell cycle profile (G2-M/G1 ratio of 0.8 for Vpr+ and 0.85 for Vpr+-T) (Fig. 2A). Interestingly, at 40 h p.i., the G2-M/G1 ratio in Vpr+ HIV-infected cells had increased to 1.4 whereas the G2-M/G1 ratio in Vpr+-T HIV-infected cells had decreased to 0.4, a value comparable to that obtained in Vpr− HIV-infected cells or mock-infected cells (Fig. 2A). These results suggest that at early times following infection (20 h p.i.), virion-associated Vpr derived from both Vpr+ and Vpr+-T pseudotyped viruses induced a G2 arrest. However, unlike in the Vpr+ viral infection, the G2 arrest mediated by the virion-incorporated Vpr (Vpr+-T) was transient since, at 40 h, the G2 arrest phenotype diminished substantially. This effect is probably due to the relative stability of the Vpr protein, with a reported half-life of more than 20 h (28), and the lack of de novo expression of Vpr in these Vpr+-T HIV-infected cells.
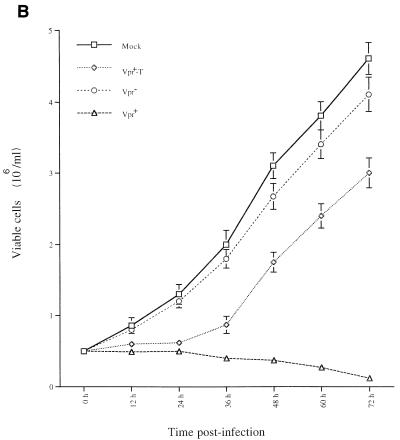
FIG. 2.
Virion-associated Vpr induces a transient cell cycle arrest at G2. Jurkat T cells were infected with VSV-G-pseudotyped Vpr− Vpr+ or Vpr+-T HIV. At 20 and 40 h p.i., the cell-associated DNA content of mock-infected and virus-infected Jurkat T cells was analyzed by PI staining as described in Materials and Methods. (A) Histograms of flow cytometric analysis of DNA content in mock-infected cells as well as in all pseudotyped virus-infected cells. The ability of each virus to induce G2 arrest was determined by calculating the G2-M/G1 ratio. (B) In parallel, at different times, p.i., the number of viable cells was counted by the trypan blue exclusion assay. The values represent means of duplicated samples. Similar results were obtained in three independent experiments.
To further evaluate the cell cycle-interfering effect of Vpr, we also monitored the number of viable cells at different time points after infection by a trypan blue exclusion assay. As shown in Fig. 2B, the number of mock-infected and Vpr− HIV-infected cells increased throughout the time course of the experiment. In contrast, a cell growth arrest was observed in both Vpr+ and Vpr+-T HIV-infected cells during the first 24 h p.i. After 24 h p.i., the Vpr+ HIV infection resulted not only in cell growth arrest but also in cell killing, since the number of viable cells continue to decrease with time. However, the Vpr+-T HIV-infected cells started to grow at a rate similar to that of the Vpr− HIV-infected cells. Taken together, these results indicate that the growth arrest mediated by virion-associated Vpr is transient and lasts for only approximately 24 h. However, sustained de novo expression of Vpr in HIV-infected cells is required to maintain the G2 arrest state and ultimately leads to cell killing.
Virion-associated Vpr-mediated cell cycle G2 arrest requires viral entry but is not dependent on viral replication.
To investigate whether Vpr must be translocated in cells to mediate this G2 arrest, we infected Jurkat T cells with Vpr+/VSV-G− or Vpr+-T/VSV-G− viral particles that do not contain VSV-G or HIV envelope glycoproteins. In contrast to infection with the VSV-G pseudotyped Vpr+ viruses, which induced a G2 arrest, incubation of Jurkat T cells with Vpr+/VSV-G− or Vpr+-T/VSV-G− viral particles did not reveal any cell cycle G2 arrest phenotype (Table 1). Hence, cell cycle arrest requires both Vpr and viral entry into cell. These results also rule out the possibility that the observed cell cycle arrest resulted from contaminating soluble Vpr proteins present in our virion preparations.
TABLE 1.
Effect of AZT treatment on virion-associated Vpr-mediated cell cycle arrest at G2a
| Pseudotyped virus | G2-M/G1 ratio at:
|
|
|---|---|---|
| 20 h | 40 h | |
| Mock | 0.18 | 0.16 |
| Vpr+/VSV-G− | 0.2 | NDc |
| Vpr+-T/VSV-G− | 0.23 | ND |
| Vpr−/VSV-G+ | 0.22 | 0.20 |
| Vpr+/VSV-G+ | 1.01 | 1.25 |
| Vpr+-T/VSV-G+ | 0.90 | 0.32 |
| Vpr−/VSV-G+ + AZTb | 0.21 | 0.34 |
| Vpr+/VSV-G+ + AZT | 0.99 | 0.4 |
| Vpr+-T/VSV-G+ + AZT | 0.92 | 0.38 |
| HxBRUR−/Env+ + AZT | 0.23 | ND |
| HxBRUR+/Env+ + AZT | 0.9 | ND |
| HxBRUR+-T/Env+ + AZT | 0.87 | ND |
The cell-associated DNA content of infected Jurkat cells was analyzed by PI DNA staining as described in Materials and Methods. The G2 arrest ability of each pseudotyped virus was determined by calculating the G2-M/G1 ratio.
+ AZT, AZT (5 μM) was added to Jurkat cell cultures 2 h prior to infection and was maintained during the course of infection.
ND, not determined.
We also tested whether viral replication is required for virion-associated Vpr-mediated cell cycle arrest. Jurkat T cells pretreated with 5 μM AZT (for 2 h) were infected with pseudotyped Vpr+, Vpr+-T, or Vpr− viruses and cultured in the presence of AZT. The 5 μM AZT treatment was shown to efficiently inhibit viral production in this system, since viral RT activity was not detected in the supernatants of infected cells (data not shown). Analysis of the cell cycle profile revealed that even in the presence of AZT, Vpr+ and Vpr+-T viral infection still induced a G2 arrest at 20 h p.i. and that the extent of cell cycle arrest was comparable to that in the absence of AZT (Table 1). Similar results were obtained with viruses produced from transfected cells treated with a protease inhibitor, Palinavir (data not shown). However, at 40 h p.i., the G2 arrest phenotype induced in both Vpr+ and Vpr+-T virus-infected cells was not maintained in the presence of AZT. To rule out the possibility that the cell cycle arrest phenotype occurs only with VSV-G pseudotyped HIV, we infected Jurkat T cells with wild-type, Vpr+-T/Env+, Vpr+/Env+, and Vpr−/Env+ HIV in the presence and absence of AZT. The cell cycle measurements were performed at 20 h p.i. before the occurrence of HIV-1 envelope glycoprotein-mediated syncytium formation. The results revealed that the infection mediated by Vpr+-T/Env+ and Vpr+/Env+ viruses also efficiently induced cell cycle G2 arrest at 20 h p.i. even in the presence of AZT (Table 1). From these results, we conclude that virion-associated Vpr can induce a G2 arrest as long as viral entry is not prevented. Inhibition of subsequent steps of the infection cycle does not inhibit the G2 cell cycle arrest mediated by virion-associated Vpr.
Virion-associated Vpr induces apoptosis in infected Jurkat T cells in the presence of AZT.
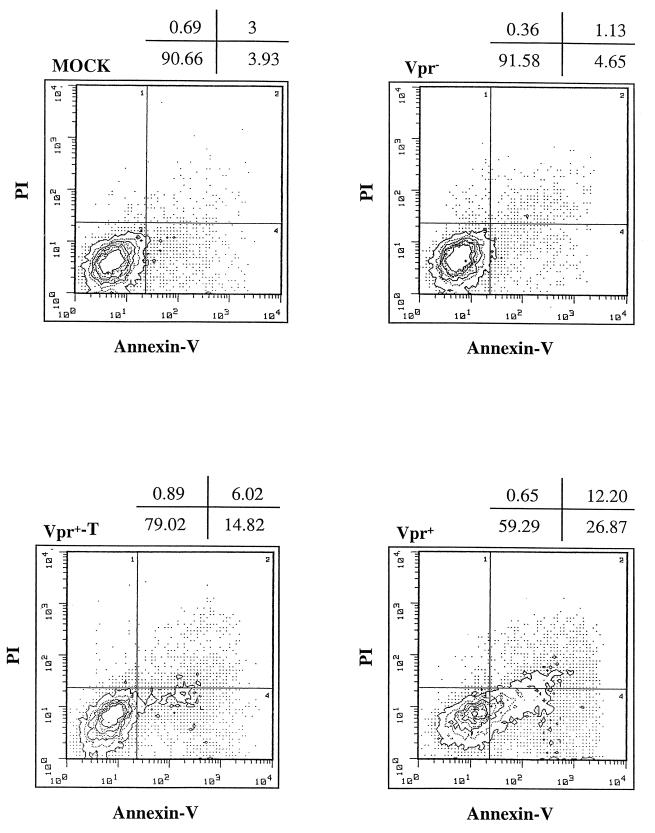
We next examined whether virion-associated Vpr induces apoptosis of infected cells. After 24 and 48 h p.i., double staining of Jurkat T cells infected with VSV-G-pseudotyped Vpr+, Vpr−, or Vpr+-T viruses was performed with annexin V-FITC and propidium iodide, as described in Materials and Methods. At 24 h p.i., no obvious apoptotic cells were detected in any infected cultures compared with the mock-infected culture (data not shown). At 48 h p.i., a significant number of apoptotic cells were detected in Jurkat T cells infected with VSV-G-pseudotyped Vpr+ and Vpr+-T viruses. Mock-infected as well as Vpr− HIV-infected cultures exhibited detectable apoptosis in a small proportion of cells (less than 5%). Interestingly, in contrast to the Vpr+ HIV infection, Vpr+-T HIV infection resulted in a significant but smaller proportion of cells becoming apoptotic (Fig. 3) (15%, compared to 27% in Vpr+ viral infection). These results suggest that virion-associated Vpr indeed induces apoptosis in a small percentage of infected Jurkat T cells late during infection (48 h p.i.). The lower percentage of apoptotic cells in Vpr+-T virus-infected Jurkat cells than of Vpr+ virus-infected cells probably reflects the lack of Vpr de novo expression.
FIG. 3.
Virion-associated Vpr induces apoptosis of Jurkat T cells during single-cycle infection. Jurkat T cells were infected with VSV-G pseudotyped Vpr−, Vpr+, or Vpr+-T HIV. At 48 h p.i., mock-infected and virus-infected cells were costained with annexin V and PI and analyzed by flow cytometry. The axes represent the cell-associated fluorescence intensity of annexin V (x) and PI (y). The percentage of cells in each quadrant is indicated above each graph. Similar results were obtained in two independent experiments.
To test whether AZT treatment could block this virion-associated Vpr-mediated apoptosis, Jurkat T cells were pretreated with 5 μM AZT (for 2 h) and infected with pseudotyped Vpr+, Vpr+-T, or Vpr− viruses and cultured in the presence of AZT. After 48 h p.i., double-staining analysis with annexin V-FITC and propidium iodide showed that in the presence of AZT, approximately 14% of apoptotic cells were detected in both Vpr+ and Vpr+-T HIV infected cultures while only 4 to 5% of apoptotic cells were found in mock-infected and Vpr− HIV-infected culture (Table 2). These results indicate that AZT does not prevent apoptosis mediated by virion-associated Vpr after virus entry. However, it appears that a threshold level of Vpr is required to induce apoptosis, since the expression of de novo Vpr appears to increase the number of cells becoming apoptotic.
TABLE 2.
Effect of AZT on Vpr-induced apoptosis
| Pseudotyped virus | % of apoptotic cellsa |
|---|---|
| Mock | 4 |
| Vpr−/VSV-G+ | 5 |
| Vpr+-T/VSV-G+ | 14 |
| Vpr+-T/VSV-G+ + AZTb | 15 |
| Vpr+/VSV-G+ | 25 |
| Vpr+/VSV-G+ + AZT | 13 |
The percentage of apoptotic cells in each infected culture was determined by costaining of infected cells with annexin V- FITC and PI at 48 h p.i. and analysis by flow cytometry.
+ AZT, AZT (5 μM) was added to Jurkat cell cultures 2 h prior to infection and maintained during the course of infection.
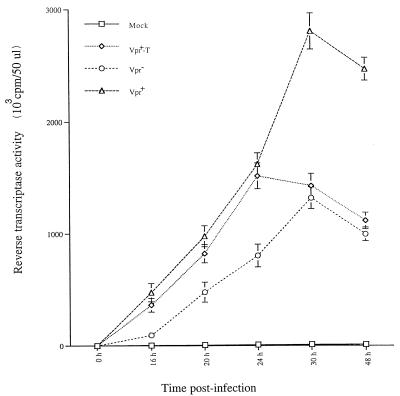
Virion-associated Vpr elevates viral production early in HIV infection.
Recent studies have reported that the presence of Vpr stimulates viral production by a mechanism which is functionally related to Vpr cell cycle G2 arrest activity (14, 44). Since virion-associated Vpr induces a transient G2 arrest, we tested whether virion-associated Vpr stimulated viral production. Jurkat T cells were infected with different VSV-G-pseudotyped Vpr+, Vpr+-T, or Vpr− viruses. At different times p.i., supernatants from each infected culture were collected and viral production was evaluated by measuring virion-associated RT activity. As shown in Fig. 4, at early times p.i. (within 24 h), Jurkat T cells infected with Vpr+ pseudotyped viruses produced approximately twice as much virus as did Jurkat T cells infected with Vpr− pseudotyped virus. Interestingly, similar levels of virus were produced in Vpr+-T HIV-infected cells to those in Vpr+ HIV-infected cultures (Fig. 4). After 24 h p.i., the RT activity in Vpr+ HIV-infected culture continued to increase and reached a plateau at 30 to 48 h p.i. whereas the RT activity from the Vpr+-T HIV-infected culture declined to a level similar to that detected in the Vpr− HIV-infected culture. This data clearly indicate that after viral entry, virion-associated Vpr has a stimulating effect on viral production. However, in the absence of de novo expression of Vpr, this early activation of viral production cannot be sustained.
FIG. 4.
Virion-associated Vpr stimulates HIV production during the early stage of infection. At each time interval after Jurkat T cells were infected with VSV-G-pseudotyped Vpr−, Vpr+ or Vpr+-T HIV (as indicated), supernatant from each infected culture was collected and the amount of virion-associated RT activity was determined. Values represent means of duplicate samples. Similar results were obtained in three independent experiments.
Stimulation of LTR-driven CAT gene expression.
To investigate whether the Vpr stimulation of viral production resulted from protein transactivation activity, we tested the effect of virion-associated Vpr on the expression of a CAT reporter gene driven by the HIV-1 LTR. Briefly, Jurkat T cells were transfected with an HIV-1 LTR-CAT expressor (pCEP4IIICAT), which contains a hygromycin resistance gene. After 10 days of hygromycin selection, cells were infected with VSV-G pseudotyped Vpr+-T, Vpr+, or Vpr− virus. At 12, 15, and 18 h p.i., infected cells were lysed and the CAT activity in these cell lysates was measured. The results in Fig. 5A show that, at 12 h p.i., indicator cells infected with Vpr+ and Vpr+-T virus exhibited a five fold increase in CAT activity, as compared to Vpr− virus infected cells. The CAT activity continued to increase in Vpr+ and Vpr+-T virus-infected cells by twofold at 15 h p.i. and by fourfold at 18 h p.i. This continued increase in CAT activity most probably results from initiation of Tat expression, since it was not observed upon treatment with AZT (data not shown). Interestingly, CAT activity increased only by about twofold between 12 and 18 h p.i. in Vpr− virus-infected cells. At 18 h p.i., CAT activity was ninefold higher in Vpr+ and Vpr+-T virus-infected cells than in Vpr− virus-infected cells. We next tested a Vpr C-terminal mutant, R80A, which was previously shown to be incapable of inducing a cell cycle G2 arrest. As shown in Fig. 5B, pseudotyped virus containing the R80A mutant (VprR80A-T) could not stimulate LTR-directed CAT activity upon infection of the indicator cell line.
FIG. 5.
Virion-associated Vpr stimulates HIV LTR-directed CAT gene expression. Jurkat T cells transfected with pCEP4III LTR-CAT were selected with hygromycin (500 μg/ml). After 10 days of selection, hygromycin-resistant Jurkat T cells were infected with VSV-G-pseudotyped Vpr−, Vpr+, or Vpr+-T HIV (as indicated). (A) At 12, 15, and 18 h p.i., CAT activity was determined. The transactivation level in each VSV-G pseudotyped HIV-infected cell sample is expressed as fold increase in CAT activity. The CAT activity value obtained in Vpr−-infected Jurkat T cells at 12 h p.i. was arbitrarily set at 1 (A). (B) CAT activity detected in pCEP4III LTR-CAT-transfected Jurkat cells following infection with VSV-G-pseudotyped Vpr−, Vpr+, Vpr+-T, and VprR80A-T viruses. (C and D) Hygromycin-resistant Jurkat cells were infected with VSV-G-pseudotyped Vpr−, Vpr+, or Vpr+-T HIV in the presence (lanes 1 to 4) or absence (lanes 5 to 8) or 5 μM AZT. At 12 h p.i. (C) and 30 h p.i. (D), CAT activity in each VSV-G-pseudotyped, HIV-infected cell sample was measured.
To confirm that virion-associated Vpr itself was responsible for the early stimulation of HIV LTR-directed gene expression, we performed similar experiments in the presence or absence of AZT (5 μM). Figure 5C and D reveals that in the presence of AZT, LTR-directed CAT activity was detected in Vpr+-T and Vpr+ virus-infected cells (lanes 3 and 4) but not in cells infected with Vpr− virus (lane 2). At 12 h p.i. the levels of CAT activity detected in Vpr+-T and Vpr+ virus-infected cells in the presence or absence of AZT were similar (Fig. 5C, compare lanes 3 and 4 with lanes 7 and 8), confirming that at early time points p.i., virion-associated Vpr itself stimulated LTR-directed CAT expression. At 30 h p.i. and in the absence of AZT, an increase in CAT activity was detected in Vpr− virus-infected cells (Fig. 5D, lane 6), presumably through the activating effect of de novo-expressed Tat. Interestingly, a more pronounced stimulation of CAT activity was detected in Vpr+-T and Vpr+ virus-infected cells (Fig. 5D, lanes 7 and 8), suggesting that virion-associated Vpr may exert a positive effect on Tat transactivation. Overall, these results indicate that virion-associated Vpr can stimulate LTR-directed gene expression. This early stimulation of gene expression, which appears to potentiate Tat transactivation, correlates with the ability of the protein to mediate cell cycle arrest in G2.
DISCUSSION
Like structural gag and env gene products, the HIV accessory protein Vpr is expressed late in the HIV replication cycle and is efficiently packaged into progeny viral particles. The virion-associated Vpr actively participates in the nuclear transport of the HIV PIC in nondividing cells. In addition, the expression of Vpr, even in the absence of any other viral proteins, induces a cell cycle arrest in G2 and increases gene expression from a variety of viral promoters (4, 21, 36). One central question is why a protein like Vpr, that stimulates LTR-directed gene expression by manipulating the host cell cycle, is expressed in the late stages of viral replication and packaged in substantial amounts in progeny viral particles. Is this function of the protein required only late in infection, or, alternatively, is it possible that, in addition to participating in the nuclear translocation of the HIV PIC, virion-associated Vpr has a positive effect on viral gene expression early in infection? Until now, the molecular mechanism(s) regulating the basal transcriptional activity of integrated HIV LTR is still not fully resolved. Whether a viral factor(s) contributes to the immediate-early activation of the LTR before de novo expression is initiated remains to be determined.
Our results clearly demonstrate that virion-associated Vpr induces a G2 arrest in host cells following viral entry. This effect was observed at a MOI as high as 10 (as described in the present study) but also at a MOI as low as 0.01 (data not shown). Indeed, titer determination studies of VSV-G-pseudotyped HIV particles indicate that (i) the G2-M/G1 ratio of cells infected at a MOI of 1 is comparable to that of cells infected at a MOI of 10, suggesting that once a threshold level of Vpr is translocated into a target cell, an efficient cell cycle G2 arrest is initiated (data not shown); (ii) infection of cells at MOIs ranging from 0.5 to 0.01 still induced a detectable cell cycle arrest in G2, although not as efficiently as infection at MOIs ranging from 1 to 10 (data not shown). These results support data recently reported by Poon et al. (33) showing that at a MOI of 0.15, the level of arrest induced by virion-associated Vpr alone is lower than that observed with wild-type virus capable of de novo Vpr production, probably because higher levels of Vpr are expressed in cells de novo.
Moreover, our results indicate that the establishment of a cell cycle arrest at G2 stimulates the activity of the HIV LTR since virion-associated Vpr mutant R80A, which has lost its cell cycle-modulating activity, was unable to activate CAT expression from the LTR. This early effect of Vpr on LTR-directed gene expression was shown to lead to an increase in viral production. Interestingly, in our experimental system, the positive effect of Vpr on the HIV-1 LTR appears to potentiate Tat transactivation. It is likely that Vpr-mediated transactivation, by increasing LTR-directed Tat expression, stimulated Tat transactivation. Alternatively, but without excluding the former possibility, optimal Tat transactivation may require a minimum level of LTR basal transcriptional activity. Such activation of basal transcription from the integrated LTR may be provided early in the infection by virion-associated Vpr via its cell cycle arrest at G2. Indeed, a recent study indicates that Tat functions after the formation of a specific transcription initiation complex and that Tat transactivation is accompanied by a remodeling of chromatin structure of integrated LTR (8). Interestingly, we have recently reported that Vpr, via its cell cycle arrest activity at G2, cooperates with p300/CBP, a transcriptional coactivator that regulates the activity of NF-κB as well as of a variety of transcription factors presumably through its ability to regulate chromatin structure by histone acetylation (10). Overall, our results support the notion that virion-associated Vpr, in addition to being involved in the nuclear translocation of the PIC, actively participates in the immediate-early activation of the HIV LTR by a molecular mechanism that is not fully understood. This newly identified activity of Vpr is reminiscent of that of VP16, a herpes simplex virus immediate-early gene product, even though the molecular mechanisms of their respective transactivation activity are likely to be distinct. VP16 is a transcription factor found in herpes simplex virus particles that selectively transactivates a class of viral promoters that control the expression of early gene products (40).
Apoptosis is one of the main cell-killing mechanisms involved in HIV-mediated direct and/or indirect CD4+ cell depletion in vivo (2, 11, 15, 19, 20). Several gene products, such as Tat, Nef, gp120, and Vpr, induce apoptosis of HIV-infected cells in different systems (16, 23, 38, 43, 44). Our results clearly indicate that virion-associated Vpr by itself can induce apoptosis of HIV infected cells. However, this Vpr-induced apoptosis occurs in a relatively small number of infected cells (15% at 48 h p.i.), suggesting that only a subpopulation of infected Jurkat cells is susceptible to Vpr-induced apoptosis. Interestingly, Ayyavoo et al. reported that the Vpr-modulated T-cell receptor triggered apoptosis in a manner similar to that of glucocorticoids (1). In the absence of T-cell receptor-mediated activation, Vpr induced apoptosis, whereas in the presence of such stimuli, Vpr interrupted the expected induction of apoptosis. By analogy to the latter results, it is possible that a subpopulation of cells in the Jurkat cell line are in a state that makes them more susceptible to Vpr-induced apoptosis. Our data also show that Vpr de novo expression increases the frequency of apoptotic cells in the culture (27% at 48 h), suggesting that a sustained threshold level of Vpr may be required for efficient induction of apoptosis.
The results obtained with Vpr+-T virus or Vpr+ virus in presence of AZT indicate that the effect of virion-associated Vpr on the cell cycle and on viral production is transient and lasts up to 24 h. This probably reflects the relative stability of the Vpr protein, which has been reported to have a half-life of more than 20 h (28). It is clear from our results that the maintenance of a G2 cell cycle arrest and the resulting positive effect on viral replication requires Vpr de novo expression. However, as discussed above, once a threshold level of Vpr is reached, cell killing by apoptosis may occur late during infection, at least in our in vitro system. Vpr is expressed late in the infection cycle and is packaged into viral particles presumably via an interaction with the p6 domain of the Gag precursor polyprotein (Pr55gag) (27, 30). It is tempting to speculate that the binding of Vpr to Pr55gag and the protein targeting to the site of viral assembly may regulate the amount of free Vpr in the cell and thereby control the delicate balance between the optimization of viral gene expression and production and the induction of cytopathic effects.
Previous studies have indicated that in plasma, the ratio of noninfectious to infectious virus particles is approximately 100,000:1 (26). However, the relevance of the presence of such a large number of noninfectious virus particles in vivo is not clear. During the preparation of this paper, Poon et al. demonstrated that HIV-1 Vpr packaged in virions, including those rendered defective for infection by RT or protease inhibitors, were capable of inducing cell cycle arrest (33). Interestingly, our results further indicate that virion-associated Vpr not only mediated cell cycle arrest at G2 but also could induce apoptosis in some cells. In agreement with the observation by Poon et al., our results indicate that virion-associated Vpr still induced a transient G2 arrest as well as apoptosis during HIV infection of Jurkat T cells in the presence of AZT as well as in Jurkat cells exposed to noninfectious virus particles produced from transfected cells treated with a protease inhibitor, Palinavir (Fig. 4 and Table 1; data not shown for Palinavir). Thus, these results raise the possibility that Vpr incorporated in infectious as well as noninfectious or defective virus particles contributes to the host immune system suppression in vivo by disturbing the cell cycle progression and/or by inducing apoptosis of HIV target cells. This could also at least partly account for the “bystander cell-killing” effect reported during HIV infection (11, 13).
Overall, in this study, we provided biological evidence that in addition to its PIC nuclear targeting activity in nondividing cells, virion-associated Vpr plays an important role in the immediate-early transcription of the HIV-1 genome during HIV infection. Moreover, the induction of cell cycle arrest at G2 and apoptosis by virion-associated Vpr, independent of viral replication, strongly suggest a role of Vpr in HIV-mediated CD4+-T-cell depletion and immune system dysfunction. Thus, therapeutic approaches directed against virion-associated Vpr function may strongly attenuate HIV infection and replication and reduce HIV-mediated CD4+-T-cell dysfunction.
ACKNOWLEDGMENTS
We thank Serge Senechal for the flow cytometric analysis. We also thank Daniel Lamarre and BioMega-Boehringer Ingelheim for the generous gift of the HIV protease inhibitor Palinavir.
E.A.C. is a recipient of a Medical Research Council of Canada (MRC) scientist award. This work was supported by grants from MRC and from the Fonds pour la Formation de Chercheurs et l’Aide à la Recherche (FCAR).
REFERENCES
- 1.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, William W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 2.Baltimore D, Gandhi R T, Chen B K, Strauss S E, Dale J K, Lenardo J. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. Identification of HIV-1 Vpr product and function. J Acquired Immune Defic Syndr. 1990;1:11–18. [PubMed] [Google Scholar]
- 6.Conti L, Rainaldi G, Matarrese P, Varano B, Rivabene R, Columba S, Sato A, Belardelli F, Malorni W, Gessani S. The HIV-1 Vpr protein acts as a negative regulator of apoptosis in human lymphoblastoid T cell line: possible implications for pathogenesis of AIDS. J Exp Med. 1998;187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullen B R. HIV-1 auxiliary proteins: making connections in dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 8.El Kharroubi A, Piras G, Zensen R, Martin M A. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol Cell Biol. 1998;18:2535–2544. doi: 10.1128/mcb.18.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1883. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 10.Felzien L K, Woffendin C, Hottiger M O, Subbramanian R A, Cohen E A, Nabel G J. HIV transcriptional activation by the accessory protein, Vpr, is mediated by the p300 co-activator. Proc Natl Acad Sci USA. 1998;95:5281–5286. doi: 10.1073/pnas.95.9.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 12.Fouchier R A, Meyer B E, Simon J H, Fisher U, Albright A V, Gonzalez-Scarano F, Malim M H. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynn J M, McElligott D L, Mosier D E. Apoptosis induced by HIV infection in H9 T cells is blocked by ICE-family protease inhibition but not by a Fas (CD95) antagonist. J Immunol. 1996;157:2754–2758. [PubMed] [Google Scholar]
- 14.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 15.Gougeon M L, Laurent-Crawford A G, Hovanessian A G, Montagnier L. Direct and indirect mechanisms mediating apoptosis during HIV infection: contribution to in vivo CD4 T cell depletion. Semin Immunol. 1993;5:187–194. doi: 10.1006/smim.1993.1022. [DOI] [PubMed] [Google Scholar]
- 16.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho D H, Neumann A U, Perelson A P, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nat Med. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 19.Hovanessian A G, Laurent-Crawford A G, Krust B, Muller S, Riviere Y, Rey-Cuille M A, Bechet J M, Montagnier L. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 20.Hovanessian A G, Jacotot E, Callebaut C, Blanco J, Riviere Y, Krust B. HIV envelope glycoprotein-induced cell killing by apoptosis is enhanced with increased expression of CD26 in CD4+ T cells. Virology. 1995;223:318–330. doi: 10.1006/viro.1996.0483. [DOI] [PubMed] [Google Scholar]
- 21.Jowett J B, Planelles V, Poon B, Shah N P, Chen M L, Chen I S. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolesnitchenko V, Wahl L M, Tian H, Sunila I, Tani Y, Hartmann D P, Cossman J, Raffeld M, Orenstein J, Samelson L E. Human immunodeficiency virus 1 envelope-initiated G2 phase programmed cell death. Proc Natl Acad Sci USA. 1995;92:11889–11893. doi: 10.1073/pnas.92.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavallée C, Yao X-J, Ladha A, Göttlinger H, Haseltine W A, Cohen E A. Requirement of the Pr55gag precursor for incorporation of the Vpr product into human immunodeficiency type 1 viral particles. J Virol. 1994;68:1926–1934. doi: 10.1128/jvi.68.3.1926-1934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy J A. HIV and the pathogenesis of AIDS. Washington, D.C: ASM Press; 1998. [Google Scholar]
- 27.Lu Y L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam S, Khan S A, Jabbar M A, Monken C E, Collman R G, Srinivasan A. Identification of residues in the N-terminal acidic domain of HIV-1 Vpr essential for virion incorporation. Virology. 1995;207:297–302. doi: 10.1006/viro.1995.1081. [DOI] [PubMed] [Google Scholar]
- 29.Nie Z, Bergeron D, Subbramanian R, Yao X-J, Checroune F, Rougeau N, Cohen E A. The putative alpha helix 2 of human immunodeficiency virus type 1 Vpr contains a determinant which is responsible for the nuclear translocation of the proviral DNA in growth-arrested cells. J Virol. 1998;72:4104–4115. doi: 10.1128/jvi.72.5.4104-4115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxton W, Connor R I, Landau N R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of Gag and mutational analysis. J Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perelson A S, Neuman A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 32.Planelles V, Jowett J B, Li Q X, Xie Y, Hahn B, Chen I S. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon B, Ferbas K G, Stewart S A, Chen I S Y. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiviral agents. Science. 1998;281:266–268. doi: 10.1126/science.281.5374.266. [DOI] [PubMed] [Google Scholar]
- 34.Popov S, Rexach M, Zybarth G, Reiling N, Lee M-A, Ratner L, Lane C M, Moore M S, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 Vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sodroski J, Rosen C, Wong-Staal F. Trans-acting transcriptional regulation of human T-cells leukemia virus type III long terminal repeat. Science. 1985;227:171–173. doi: 10.1126/science.2981427. [DOI] [PubMed] [Google Scholar]
- 38.Stewart S A, Poon B, Jowett J B M, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subbramanian R A, Kessous-Elbaz A, Lodge R, Forget J, Yao X-J, Bergeron D, Cohen E A. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J Exp Med. 1998;187:1103–1111. doi: 10.1084/jem.187.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Triezenberg S J, Lamarco K L, McKnight S L. Evidence of DNA:protein interactions that mediate HSV-1 immediate early gene activation by VP16. Genes Dev. 1998;2:730–742. doi: 10.1101/gad.2.6.730. [DOI] [PubMed] [Google Scholar]
- 41.Vodicka M A, Koepp D M, Silver P S, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nat Med. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 43.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp 120. Nat Med. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 44.Yao X-J, Mouland A J, Subbramanian R A, Forget J, Rougeau N, Bergeron D, Cohen E A. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1 infected dividing Jurkat T cells. J Virol. 1998;72:4686–4693. doi: 10.1128/jvi.72.6.4686-4693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao X-J, Subbramanian R A, Rougeau N, Boisvert F, Bergeron D, Cohen E A. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao X-J, Garzon S, Boisvert F, Haseltine W A, Cohen E A. The effect of Vpu-induced syncytia formation. J Acquired Immune Defic Syndr. 1993;6:135–141. [PubMed] [Google Scholar]
- 47.Yu X F, Matsuda M, Essex M, Lee T H. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J Virol. 1990;64:5688–5693. doi: 10.1128/jvi.64.11.5688-5693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]