Abstract
Synthetic nonmethylated oligonucleotides containing CpG dinucleotides (CpG-ODNs) have been shown to exhibit immunostimulatory activity. CpG-ODNs have the capacity to directly activate B cells, macrophages, and dendritic cells, and we show here that this is reflected by cell surface binding of oligonucleotides to these cell subsets. However, T cells are not directly activated by CpG-ODNs, which correlates with the failure to bind to the T-cell surface. Efficient competition for CpG-induced B-cell activation by non-CpG-containing oligonucleotides suggests that oligonucleotides might bind to an as yet undefined sequence-nonspecific receptor prior to cellular activation. Induction of protective T-cell responses against challenge infection with lymphocytic choriomeningitis virus (LCMV) or with recombinant vaccinia virus expressing the LCMV glycoprotein was achieved by immunizing mice with the immunodominant major histocompatibility complex class I-binding LCMV glycoprotein-derived peptide gp33 together with CpG-ODNs. In these experiments, B cells, potentially serving as CpG-ODN-activated antigen-presenting cells (APCs), were not required for induction of protective immunity since CpG-ODN–gp33-immunized B-cell-deficient mice were equally protected against challenge infection with both viruses. This finding suggested that macrophages and/or dendritic cells were sufficiently activated in vivo by CpG-ODNs to serve as potent APCs for the induction of naive T cells. Furthermore, treatment with CpG-ODN alone induced protection against infection with Listeria monocytogenes via antigen-independent activation of macrophages. These data suggest that CpG activation of macrophages and dendritic cells may provide a critical step in CpG-ODN adjuvant activity.
Unmethylated CpG dinucleotides are found more frequently in genomes of bacteria and viruses than in vertebrate DNA (9, 22). In vitro these CpG motifs in a given base context (mimicking bacterial DNA) have been shown to activate antigen-presenting cells (APCs) to upregulate certain surface molecules such as CD69 and major histocompatibility complex class II as well as costimulatory molecules such as B-7.1 and B-7.2 (22, 30–34). In addition, CpG-containing oligonucleotides (CpG-ODNs) have been shown to induce cytokine secretion by activated APCs, including interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-1, IL-12, and gamma interferon (IFN-γ) (24, 31, 33, 34, 38, 39). For this activation process to occur, it has recently been shown that CpG-ODNs have to gain access to acidic endosomal compartments, which results in intracellular production of reactive oxygen species, eventually leading to activation of the transcription factor NF-κB, which in turn allows transcription of several proto-oncogenes as well as cytokine genes (40).
In vivo, administration of CpG-ODNs was shown to directly induce TNF-α production by macrophages (33) or IFN-γ production by NK cells (14). Coadministration of CpG-ODNs with soluble protein antigens in incomplete Freund adjuvant (IFA) was shown to promote Th1 responses, whereas without coadministration Th2 responses were obtained (12, 13, 23). Furthermore, infection of BALB/c mice with Leishmania major in the presence of CpG-ODNs shifted the T-cell response toward Th1 cytokines, resulting in protection from the normally lethal infection (41). In addition, it was demonstrated that CpG-ODNs are effective as immune adjuvants in tumor antigen immunization (36). However, whether CpG-ODNs can act as adjuvant to induce protective immunity against viral infections has not been addressed yet.
In this report, we show in vitro that binding of biotinylated CpG-ODNs (CpG-ODN-BIO) to the cell surface of B cells correlated with their ability to activate B cells. CpG-ODN-mediated cellular activation could be inhibited by addition of excess non CpG-containing oligonucleotides (non-CpG-ODNs), suggesting that oligonucleotides may bind in a competitive way to an as yet undefined cellular receptor. Furthermore, CpG-ODNs were found to be efficient adjuvants in the induction of protective T-cell immunity against challenge infection with lymphocytic choriomeningitis virus (LCMV) and recombinant vaccinia virus. Induction of protective T-cell immunity was achieved in the absence of B cells potentially acting as CpG-ODN-activated APCs, suggesting that in vivo activation of macrophages and dendritic cells plays a major role in at least some antiviral protective mechanisms.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6, SV129 (H-2b), and BALB/c (H-2d) mice were obtained from the breeding colony of the Institut für Zuchthygiene, Tierspital Zürich, Zurich, Switzerland. Generation of immunoglobulin M (IgM)-deficient mice (21) and the generation of the vesicular stomatitis virus (VSV) glycoprotein (VSV-G) transgenic mouse lines MONITOR (1) and KINDG (2) have been described previously. Mice were bred in a specific-pathogen-free mouse facility.
Viruses and bacteria.
The LCMV isolate WE was originally provided by F. Lehmann-Grube, Hamburg, Germany, and grown on L929 cells (ATCC CRL 1) with a low multiplicity of infection. VSV Indiana seeds (Mudd-Summers isolate), originally obtained from D. Kolakofsky, University of Geneva, were grown on BHK-21 cells (ATCC CRL 8544) infected at low multiplicity and plaqued on Vero cells.
Vaccinia virus expressing VSV-G (VV-INDG) was a generous gift of B. Moss (Laboratory of Viral Diseases, National Institutes of Health, Bethesda, Md.) (26). VV-G2, a recombinant vaccinia virus expressing LCMV glycoprotein (LCMV GP), has been described elsewhere (37). Recombinant viruses were grown at low multiplicity of infection on BSC40 cells and plaqued on BSC40 cells.
Recombinant baculoviruses expressing the LCMV nucleoprotein or VSV-G have been previously described (3, 7). Each recombinant baculovirus was derived from nuclear polyhedrosis virus and was grown at 28°C in Spodoptera frugiperda cells in spinner cultures in TC-100 medium. Recombinant proteins were produced as previously described (27).
Listeria monocytogenes was originally obtained from B. Blanden (Canberra, Australia). It was cultured in Trypticase soy broth (BBL Microbiology Systems, Cockeysville, Md.), and overnight cultures were titrated on tryptose blood agar plates (Difco Laboratories, Detroit, Mich.).
Cell culture and cell purification.
If not stated differently, in vitro ODN activation experiments were performed in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 1.5 mM l-glutamine, 50 μM 2-mercaptoethanol, penicillin (100 U/ml), and streptomycin (100 U/ml). B cells and CD4+ T cells were purified from single-cell spleen suspensions by magnetic bead cell sorting according to the protocol of the supplier (Miltenyi Biotec, Bergisch Gladbach, Germany).
Peptides and oligonucleotides.
Phosphorothioate-modified ODN were custom synthesized by Microsynth (Balgach, Switzerland). The following oligonucleotide sequences were used (bold letters indicate the proposed active motif): 1668pt (5′-TCC ATG ACG TTC CTG AAT AAT-3′), CD40pt (5′-GAG ATG AGA AGG AAG AAT GGG AAA AC-3′), VαDOpt (5′-TGG GGC TGA CTG ATA CCA-3′), and VβUPpt (5′-GCT GGC AAC CTT CAA ATA-3′). 1668pt-ODN, which served as the activating oligonucleotide, has been experimentally used and described previously (22, 24, 30–34, 38, 39). CD40pt-ODN, VαDOpt-ODN, and VβUPpt-ODN served as negative control oligonucleotides.
Peptides (purchased from Neosystem, Strasbourg, France) used were GP33 (KAVYNFATM; LCMV GP, Db), LLO (listeriolysin O) 91-99 (GYKDGNGYI; LLO, Kd), and p8 (SSKAQVFEHPHIQDAASQL; VSV-G, I-Ab).
Proliferation.
B220+ cells and CD4+ T cells were purified from whole spleen cell suspensions by magnetic bead cell sorting (Miltenyi Biotec) according to the protocol of the supplier. The purity of the cells was 97 to 98%, as determined by fluorescence-activated cell sorting. A total of 3 × 105 purified lymphocytes were stimulated with serial threefold dilutions of 1668pt-ODN (highest concentration, 2 μM), CD40pt-ODN (highest concentration, 2 μM), or lipopolysaccharide (LPS; highest concentration, 10 μg/ml); 24 h later, proliferation was determined by [3H]thymidine incorporation (1 μCi/well).
Cytofluorometric analyses.
The following monoclonal antibodies were used for analysis. Fluorescein isothiocyanate (FITC)-conjugated anti-B220, anti-Mac-1, anti-CD4, and anti-CD8 and biotinylated anti-CD69, anti-CD44, and anti-I-Ab were purchased from Pharmingen. 1668pt-ODN-BIO (5′-TCC ATG ACG TTC CTG ATG CTT TT-biotin-3′) was custom synthesized by Microsynth. Tricolor-conjugated streptavidin was purchased from Caltag Laboratories, Burlingame, Calif.). For human experiments, cells were either (i) peripheral blood mononuclear cells (PBMCs) separated by centrifugation over Ficoll at 2200 rpm for 22 min and washed twice in RPMI 1640 or (ii) Epstein-Barr virus-transformed B-cell lines. The following antibodies were used: anti-CD3-FITC (Becton Dickinson, Oxford, England), anti-CD4-FITC (Serotec, Oxford, England), anti-CD8-tricolor (Caltag), anti-CD21-FITC (Dako, Glastrup Denmark), and streptavidin-phycoerythrin (Sigma, St. Louis, Mo.). Flow cytometry was performed on a FACSstar Plus flow cytometer (Becton Dickinson).
Protection of mice from replication of LCMV.
Mice were immunized subcutaneously (s.c.) with 10 nmol of 1668pt-ODN or 10 nmol CD40pt-ODN together with 100 μg of gp33, with 100 μg of gp33 alone, or with 10 nmol of 1668pt-ODN alone. Seven days later, mice were boosted by the same protocol. Seven days after boosting, mice were challenged intravenously (i.v.) with 200 PFU of LCMV. LCMV titers in the spleen were determined 4 days later as described previously (6) and are reported as log10 PFU per organ.
Protection of mice from replication of recombinant vaccinia virus.
Mice were immunized s.c. with 10 nmol of 1668pt-ODN or 10 nmol CD40pt-ODN together with 100 μg of gp33, with 100 μg of gp33 alone, or with 10 nmol of 1668pt-ODN alone. Seven days later, mice were challenged intraperitoneally (i.p.) with 2 × 106 PFU of VV-G2. Alternatively mice were immunized subcutaneously with 10 nmol of 1668pt-ODN or 10 nmol of CD40pt-ODN together with 200 μg of peptide p8. Seven days later, mice were boosted by the same protocol. Seven days after boosting, mice were challenged i.p. with 2 × 106 PFU of VV-INDG. Vaccinia virus titers in ovaries were determined 5 days later as described previously (8) and are reported as log10 PFU in both ovaries.
Determination of bacterial titers.
BALB/c mice were immunized s.c. with 10 nmol of 1668pt-ODN or 10 nmol of CD40pt-ODN together with 100 μg of LLO peptide. Seven days later mice were challenged i.v. with 300 CFU of L. monocytogenes. Five days after inoculation, the whole spleen or one lobe of the liver was harvested and homogenized. Bacterial titers were determined by plating out four serial 10-fold dilutions of organ suspensions on tryptose blood agar plates.
RESULTS
CpG-ODN bind to the surface of B cells and macrophages but not of T cells.
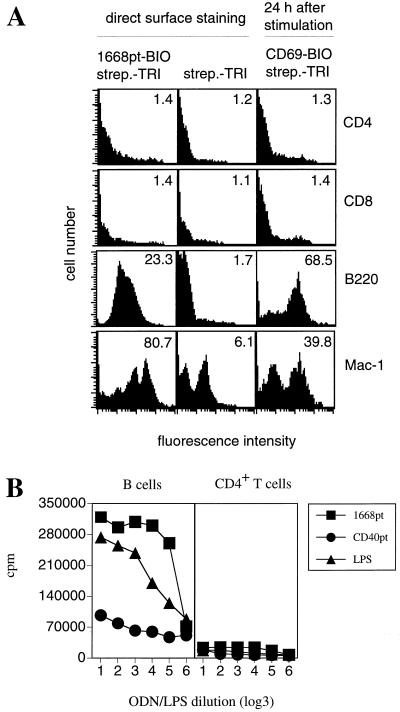
To characterize the lymphocyte subsets to whose surface synthetic CpG-ODN could bind, biotinylated CpG-ODNs were synthesized. Whole spleen cells suspensions were incubated with 1668pt-ODN-BIO in addition to antibodies specific for several selected markers which identified lymphocyte subsets. Gating on B220+, Mac-1+, CD4+, and CD8+ lymphocytes revealed that 1668pt-ODN-BIO bound to B cells and macrophages but not to CD4+ or CD8+ T cells (Fig. 1A; Table 1). Neither prior activation of T cells with phorbol myristate acetate-ionomycin nor prior activation of B cells with LPS enhanced binding of 1668pt-ODN-BIO to the cell surface (data not shown).
FIG. 1.
(A) Surface binding and cellular activation by 1668pt-ODN. Surface staining with 1668pt-ODN-BIO gated on CD4+ T cells, CD8+ T cells, B cells, or macrophages is shown in the left-hand panels; background control stainings with streptavidin-tricolor (strep.-TRI) are shown in the middle panels; 1668pt-ODN-induced cellular activation of CD4+ T cells, CD8+ T cells, B cells, and Mac-1+ cells was measured by upregulation of CD69 expression and is shown in the right-hand panels. Numbers in the upper right corners indicate median values. One of four equivalent experiments is presented. (B) 1668pt-induced proliferation. Purified B cells and CD4+ T cells were incubated with serial dilutions of 1668pt-ODN, CD40pt-ODN, and LPS. Proliferation was assessed by [3H]thymidine incorporation. One of three equivalent experiments is presented.
TABLE 1.
Cell surface staining of 1668pt-ODN-BIO
| Condition | % 1668pt-ODN+ spleen cells gated ona:
|
|||
|---|---|---|---|---|
| B220+ | Mac-1+ | CD4+ | CD8+ | |
| With FCS | 97 | 80 | 10 | 9 |
| Without FCS | 98 | 90 | 15 | 10 |
Background staining without 1668pt-ODN-BIO reagent was 5 to 10%.
The binding properties of 1668pt-ODN-BIO correlated with its capacity to activate the cell subsets to which it can bind: 1668pt-ODN induced blast formation, proliferation, and upregulation of activation markers such as CD69 and CD25 in B cells but not in CD4+ or CD8+ T cells (Fig. 1A, right panels, and Fig. 1B; Table 2).
TABLE 2.
In vitro activation of splenic B cells, CD4+ or CD8+ T cells, and Mac-1.1+ cells with 1668pt-ODN
| Cell type | % of spleen cell populationa
|
|||
|---|---|---|---|---|
| B220+ | Mac-1+ | CD4+ | CD8+ | |
| CD69+ | 96 (2) | 52 (18) | 5 (5) | 8 (6) |
| IL-2R+ | 90 (3) | ND | 9 (10) | 10 (9) |
Percentage of CD69+ or IL-2R+ B cells, CD4+ or CD8+ T cells, or Mac-1+ cells after activation with 1668pt-ODN. Values in parentheses indicate numbers of CD69+ or IL-2R+ nonactivated cells. Background staining values were 7% for CD69 staining and 9% for IL-2R staining. ND, not determined.
To further analyze whether surface binding of 1668pt-ODN-BIO required prior binding to serum protein components as was shown to be the case for LPS, which binds to the plasma protein LPS binding protein prior to binding to its surface receptor CD14 (35), surface stainings were performed in presence or absence of FCS. Surface staining analysis revealed that 1668pt-ODN-BIO bound to the surface of purified B cells equally well in the presence or absence of FCS (Table 1). Nevertheless, the lymphocytes capable of binding 1668pt-ODN-BIO may have bound an undefined protein to the cell surface which was not removed by intensive washing and which could mediate 1668pt-ODN-BIO binding.
Similar experiments were performed with human PBMCs from healthy donors. Again surface binding of biotinylated CpG-ODNs was observed for B cells (>95%) and was virtually absent for CD4+ and CD8+ T cells (<10%) (data not shown; background staining, <5%).
Competition for 1668pt-ODN induced in vitro activation of naive splenic B cells by non-CpG-ODNs.
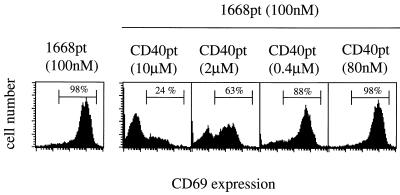
Since it still not known whether oligonucleotides mediate cellular activation via binding to a cellular receptor, we assessed whether 1668pt-ODN-induced activation of purified B cells could be competed by the addition of excess non-CpG-ODNs (CD40pt-ODN, VαDOpt-ODN, and VβUPpt-ODN). Therefore, purified naive splenic B cells were incubated with 100 nM 1668pt-ODN in the presence of graded amounts of non-CpG-ODNs. Cell blast formation as well as CD69 upregulation on B cells could be completely inhibited by excess administration of all three non-CpG-ODNs. As shown in Fig. 2, CD40pt-ODN completely inhibited CD69 upregulation at a 100-fold excess and reduced CD69 expression to 40% at a 20-fold excess. These findings suggest that the discrimination between CpG-ODN and non-CpG-ODN might occur after binding to an as yet undefined sequence-unspecific saturable ODN receptor.
FIG. 2.
Competition for 1668pt-ODN-mediated B-cell activation by addition of CD40pt-ODN. Purified splenic B cells from C57BL/6 mice were incubated in the presence of 100 nM 1668pt-ODN (left-hand panel) or in addition to graded amounts of CD40pt-ODN (four panels on the right). CD69 upregulation was determined 24 h later. Numbers above marker lines indicate percentages of CD69+ B cells. One of three similar experiments is presented.
To determine whether ODNs bound to the same surface receptor CD14 as LPS does (35), purified B cells were incubated with graded amounts of LPS in the presence or absence of excess CD40pt-ODN (10 μM). Equivalent concentration thresholds (at 80 ng/ml) for LPS-induced B-cell activation were observed in the presence or absence of CD40pt-ODN, suggesting that ODNs do not bind to the LPS receptor CD14 (35) (not shown). To further determine whether 1668pt-ODN can activate B cells originating from different mouse strains, purified B cells from C57BL/6, BALB/c, and SV129 mice were incubated with 1668pt-ODN, and all of them were induced to blast, proliferate, and upregulate CD69 (not shown). The same results were obtained with B cells originating from different mutant mouse strains deficient either in TNF receptor I (TNFRI), TNFRII, IFN-γR, and IFN-αβR, suggesting that these cytokines and/or the respective cytokine receptors were not involved in 1668pt-ODN-induced B-cell activation (not shown).
To exclude the possibility that the lymphocyte-activating capacity of 1668pt-ODN and the nonactivating properties of CD40pt-ODN are due to different degradation kinetics in mouse serum, 1668pt-ODN and CD40pt-ODN were incubated for different time periods ranging from 5 min to 26 h in mouse serum at 37°C, and resulting oligonucleotide lengths were analyzed by polyacrylamide gel electrophoresis. No degradation of either 1668pt-ODN or CD40pt-ODN was observed during this time period (not shown).
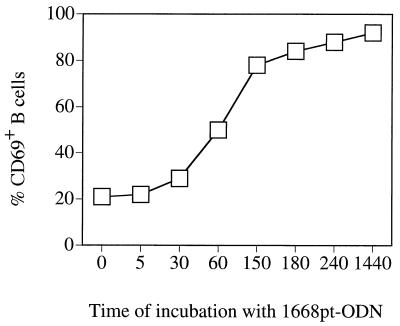
To define the time period of incubation with 1668pt-ODN required for activation, purified B cells were incubated for different length of time with 1668pt-ODN, intensively washed, and cultured in medium alone for up to 24 h. Analysis of CD69 expression showed that an incubation period of at least 1 to 2 h was required for 1668pt-ODN-induced B-cell activation (Fig. 3).
FIG. 3.
Kinetics of 1668pt-ODN-mediated B-cell activation. Purified B cells from C57BL/6 mice were incubated for the indicated time periods (in minutes) with 1668pt-ODN, washed three times, and then cultured in medium only. After a total culture time of 24 h, CD69 upregulation was determined. One of two equivalent experiments is presented.
Induction of protective immunity against challenge infection with LCMV.
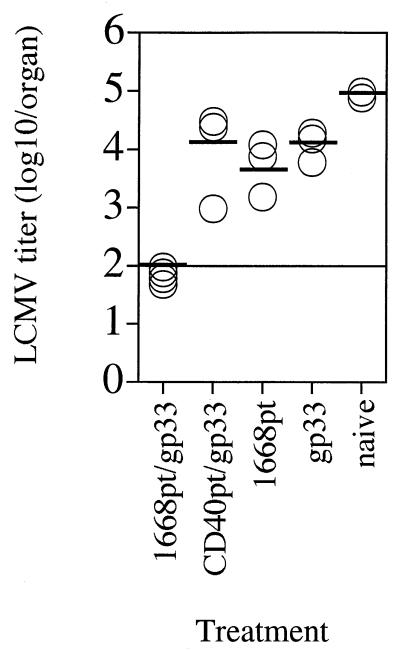
To achieve protective immunity against infections by using defined peptide T-cell epitopes as vaccines, the use of adjuvants such as IFA or complete Freund adjuvant (CFA) is normally required. Unfortunately, those effective adjuvants generally cause side effects which preclude their use in humans. To analyze whether immunostimulatory ODNs such as 1668pt-ODN could be used as adjuvants for induction of protective T-cell immunity against viral infections, murine infection with LCMV was chosen as the experimental system. LCMV is a noncytopathic virus, and infection is controlled by LCMV-specific CD8+ T cells via perforin-mediated lysis of infected cells (10, 18, 42). The immunodominant epitope within LCMV GP in the H-2b haplotype spans amino acids 33 to 41 (gp33) (29). Thus, C57BL/6 mice were immunized and boosted s.c. with an aqueous solution of gp33 together with 1668pt-ODN or the control CD40pt-ODN. As a control, mice were immunized and boosted with gp33 peptide alone or with 1668pt-ODN alone. Seven days after boosting, the mice were challenged i.v. with 200 PFU of LCMV; 4 days later, viral titers in the spleen were determined (Fig. 4). Mice immunized with gp33 plus 1668pt-ODN were completely protected against LCMV infection, whereas gp33–CD40pt-ODN-treated mice were not. Mice immunized with gp33 alone or with 1668pt-ODN alone were not protected against LCMV challenge, suggesting that peptide-induced antigen-specific activation could be achieved only in the presence of an adjuvant.
FIG. 4.
1668pt-ODN as adjuvant for s.c. peptide vaccination for protection against LCMV challenge. C57BL/6 mice were immunized s.c. with 1668pt-ODN plus gp33, CD40pt-ODN plus gp33, gp33 alone, or 1668pt-ODN alone or were left untreated; 7 days later the mice were boosted by the same protocol; 7 days after boosting, mice were challenged i.v. with 200 PFU of LCMV; 4 days after virus challenge, LCMV titers in the spleen were determined. Each symbol represents a single mouse. One of three identical experiments is shown.
The route of immunization with gp33 plus 1668pt-ODN was crucial for efficient induction of protective T-cell immunity since i.v. and i.p. immunization with equivalent doses of gp33 plus 1668pt-ODN did not confer protection against LCMV challenge infection (not shown).
Induction of protective T-cell immunity against challenge infection with recombinant vaccinia virus.
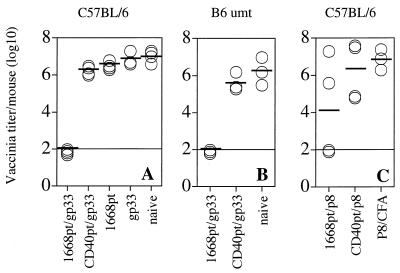
To analyze whether 1668pt-ODN-assisted peptide immunization could also induce protective immunity against vaccinia virus infection, C57BL/6 mice were immunized s.c. with gp33 plus 1668pt-ODN, gp33 plus CD40pt-ODN, gp33 alone, and 1668pt-ODN alone. Mice were challenged i.p. 7 days later with VV-G2. Protection against VV-G2 is known to be T-cell mediated via release of the type 1 cytokines IFN-γ and TNF-α (8, 19). Five days after VV-G2 challenge, vaccinia virus titers in the ovaries were assessed (Fig. 5A). Mice immunized with gp33 plus 1668pt-ODN were completely protected against VV-G2 challenge infection, in contrast to mice immunized with gp33 plus CD40pt-ODN, with gp33 alone, or with 1668pt-ODN alone. Since 1668pt-ODN is an efficient B-cell activator, it was of interest to determine whether B cells were required as potential APCs for induction of protective T-cell immunity. Therefore, B-cell-deficient mice were immunized and challenged by the same experimental protocol (Fig. 5B). B-cell-deficient mice and normal control mice were equally protected against challenge infection with VV-G2, indicating that B cells serving as APCs were, at least in this experimental setup, not required for induction of protective T-cell immunity. Thus, it is likely that 1668pt-ODN locally activated dendritic cells and/or macrophages to become mature professional APCs presenting the exogenously provided gp33 and able to induce naive gp33-specific T cells.
FIG. 5.
1668pt-ODN as adjuvant for peptide vaccination for protection against challenge infection with recombinant vaccinia virus. (A) C57BL/6 mice were immunized s.c. with 1668pt-ODN plus gp33, CD40pt-ODN plus gp33, gp33 alone, or 1668pt-ODN alone or were left untreated; 7 days later, mice were challenged i.p. with 2 × 106 PFU of VV-G2; 5 days after virus challenge, vaccinia virus titers in the ovaries were determined. Each symbol represents a single mouse. (B) The same experiment was performed in IgM-deficient mice (B6 umt). (C) C57BL/6 mice were immunized s.c. with 1668pt-ODN plus p8, with CD40pt-ODN plus p8, or with p8 emulsified in CFA; 7 days later, mice were boosted by the same protocol with except with IFA used instead of CFA; 7 days after boosting, mice were challenged i.p. with 2 × 106 PFU of VV-INDG; 5 days after virus challenge, vaccinia virus titers in the ovaries were determined. One of two identical experiments is shown.
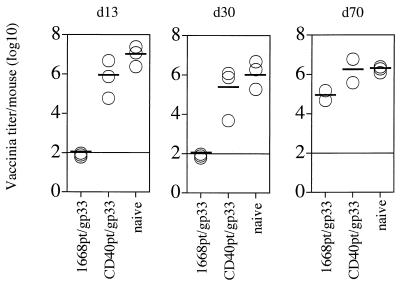
In further experiments, we determined the duration of antiviral protection. Mice were challenged with VV-G2 at 13, 30, and 70 days after immunization with gp33 plus 1668pt-ODN or with gp33 plus CD40pt-ODN. Complete protection against recombinant vaccinia virus challenge was observed up to 30 days after immunization but was no longer detectable 70 days after immunization (Fig. 6).
FIG. 6.
Longevity of antiviral protection. C57BL/6 mice were immunized s.c. with 1668pt-ODN plus gp33 or CD40pt-ODN plus gp33 and were challenged with 2 × 106 PFU of VV-G2 at 13, 30, or 70 days (d13, d30, or d70) after priming; 5 days after virus challenge, vaccinia virus titers in the ovaries were determined. One of two identical experiments is shown.
To test whether 1668pt-ODN could also serve as an adjuvant for peptide-mediated activation of virus-specific CD4+ T cells, C57BL/6 mice were immunized s.c. with 1668pt-ODN plus the VSV-G-derived I-Ab-binding peptide p8 in saline (11), with CD40pt-ODN plus p8, or with p8 emulsified in CFA. Seven days later mice were boosted by the same procedure except with IFA used in the place of CFA. Seven days after boosting, mice were challenged i.p. with VV-INDG. Five days later, vaccinia virus titers in the ovaries were determined (Fig. 5C). Two of four mice receiving the p8 peptide together with 1668pt-ODN were protected against challenge infection with VV-INDG, whereas none of the p8–CD40pt-ODN- or p8–CFA-immunized mice were protected. This finding suggests that CpG-ODNs may be at least as efficient if not better adjuvants than the commonly used CFA.
1668pt-ODN induces protection against infection with L. monocytogenes.
It has been shown that in the early phase of primary L. monocytogenes infection in mice, neutrophils and macrophages play an important role in restricting bacterial replication. In addition, the cytokines IFN-γ and TNF-α as well as reactive oxygen intermediates produced by IFN-γ-activated macrophages are essential for protection (5, 15, 20). However, later phases of primary infections as well as secondary infections are mainly controlled by specific (memory) CD8+ T-cell responses (4, 17).
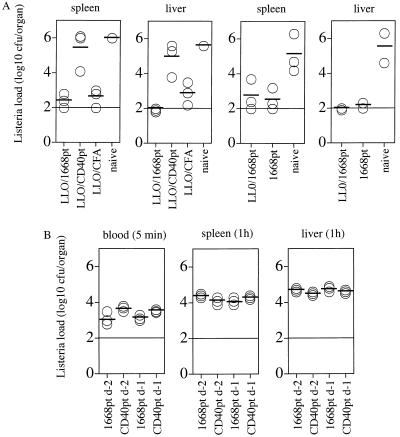
Since it is known that CpG-ODNs are able to directly activate macrophages, it was of interest to determine whether this capacity was sufficient to induce macrophage-mediated protection against infection with the facultative intracellular bacterium L. monocytogenes or whether specific T-cell priming had to occur in addition. BALB/c mice were immunized s.c. with the listeriolysin (LLO)-derived CTL epitope LLO 91-99 (28) in association with 1668pt-ODN, with LLO 91-99 plus CD40pt-ODN, with LLO 91-99 in CFA, or with 1668pt-ODN alone. Mice were challenged i.v. 7 days later with 3,000 CFU of L. monocytogenes; 5 days later, Listeria titers in the spleen and liver were determined (Fig. 7A). Immunization with 1668pt-ODN with or without the LLO 91-99 peptide conferred protection, in contrast to CD40pt-ODN plus LLO 91-99 peptide, suggesting that the protective mechanism in this experimental infection was mediated not by LLO-specific CD8+ T cells but rather by in vivo activation of macrophages. 1668pt-ODN-mediated protection could also be achieved in B-cell-deficient mice (not shown).
FIG. 7.
1668pt-ODN as adjuvant in induction of protection against infection with L. monocytogenes. (A) BALB/c mice were immunized s.c. with 1668pt-ODN plus LLO 91-99 peptide, CD40pt-ODN plus LLO 91-99 peptide, or LLO 91-99 peptide in CFA or were left untreated (left two panels). In addition, BALB/c mice were immunized s.c. with 1668pt-ODN plus LLO 91-99 peptide or 1668pt-ODN alone or were left untreated (right two panels); 7 days later, mice were challenged i.v. with 3,000 CFU of L. monocytogenes. After 5 days, bacterial titers in the spleen and liver were determined. Each symbol represents a single mouse. One of two identical experiments is represented (B) BALB/c mice were immunized s.c. with 1668pt-ODN or with CD40pt-ODN and 1 or 2 days later challenged i.v. with 2 × 105 CFU of L. monocytogenes. Bacterial titers were determined in the blood after 5 min and in the spleen and liver after 1 h. Each symbol represents a single mouse. One of two identical experiments is shown.
To rule out the possibility that the observed differences in bacterial titers 5 days after infection were due to a reduced ability of 1668pt-ODN-treated mice to be infected with L. monocytogenes, 1668pt-ODN-treated and CD40pt-ODN-treated mice were inoculated i.v. 1 or 2 days later with 2 × 105 bacteria, and bacterial titers were determined in the blood 5 min later and in the spleen and liver 1 h after inoculation. At these very early time points after inoculation, 1668pt-ODN-treated and CD40pt-ODN-treated mice revealed comparable bacterial titers (Fig. 7B). These results suggested that the 1668pt-ODN-mediated macrophage activation, rather than differences in initial bacterial spread and cellular uptake, is most likely to be responsible for the protection observed.
DISCUSSION
Synthetic CpG-ODNs imitating bacterial DNA show in vitro and in vivo the capacity to activate and to induce maturation of several cell subsets, including B cells, macrophages, NK cells, and dendritic cells (22, 24, 30–34, 38, 39). Surface staining analysis using biotinylated ODNs revealed that ODNs bound to the cell surface of those cell subsets which could be activated by CpG-ODNs but not to the surfaces of CD4+ and CD8+ T cells which were not directly activated by CpG-ODNs. The possible existence of a surface receptor able to bind ODNs has already been postulated and suggested by experiments where cellular uptake of antisense oligonucleotides was analyzed (25).
1668pt-ODN-mediated cellular activation could be efficiently blocked in the presence of excess non-CpG-ODNs. Thus, there may exist a saturable sequence-unspecific ODN receptor at some stage of the cellular activation process, possibly after cellular uptake, which is able to discriminate between CpG-and non-CpG-ODNs. This observation suggests that during in vivo infection there may be competition between pathogen-derived activating and nonactivating DNA sequences. The overall effect may depend on the balance of activating versus nonactivating sequences in a particular genome. Theoretically, some pathogens may have evolved sequences which compete or even block this CpG-ODN-mediated activation process.
During the 1668pt-ODN-mediated cellular activation process, CpG-ODN is bound to the surface and then taken up by endocytosis (22). Endocytosis as well as subsequent acidification of CpG-ODNs is required for the downstream cellular activation processes involving generation of reactive oxygen species, finally leading to NF-κB activation (40).
The excellent in vitro and in vivo immunostimulatory capacities of CpG-ODNs prompted us to test those as adjuvants in peptide-mediated T-cell vaccination against viral infections. Induction of protective cytotoxic T-lymphocyte (CTL) responses against many viral infections can be achieved by immunizing naive mice with immunodominant CTL epitopes. However, such peptide immunizations are completely dependent on the use of adjuvants; in the murine system, mainly IFA and CFA are used. Since these adjuvants possess side effects, it is of great interest to find equally potent but less harmful adjuvants that can be used in humans. CpG-ODNs seem to efficiently fulfill these requirements.
Protective immunity against infection with noncytopathic LCMV is mediated by LCMV-specific CTLs (16, 42). Subcutaneous but not i.v. administration of an immunodominant CTL epitope (gp33) together with CpG-ODN conferred complete protection against LCMV challenge infection. Similarly, gp33–CpG-ODN immunization induced protective immunity against challenge infection with a cytopathic recombinant vaccinia virus expressing the LCMV GP. In this case it is known that the protective mechanism involves T-cell-mediated secretion of the type 1 cytokines IFN-γ and TNF-α (8). These examples demonstrated that peptide-induced vaccinations for the generation of protective CTL responses were successfully achieved with CpG-ODNs as adjuvants, and notably, vaccination was at least as efficient if not more efficient than with CFA as the adjuvant. B cells were not required for induction of protective CTL responses by peptide–CpG-ODN immunization in the two experimental systems analyzed in this report. Thus, the local application of CpG-ODN induced probably a strong activation of either resident APCs such as immature dendritic cells (e.g., Langerhans cells) or tissue macrophages, or the CpG-ODN were transported to the draining lymph node where lymphoid tissue APCs were activated. This CpG-ODN-induced APC activation and the maturation (in the case of dendritic cells) probably greatly enhanced the efficiency with which the APCs activated naive T cells.
Furthermore, s.c. immunization with CpG-ODNs conferred protection against infection with the facultative intracellular bacterium L. monocytogenes. To achieve this protection, immunization with CpG-ODNs alone was sufficient; addition of L. monocytogenes-derived CTL epitopes was not required. Protection was achieved independently of the presence of B cells, suggesting that in vivo activation of macrophages contributed to the protective mechanism. Since control and clearance of primary L. monocytogenes infection is mediated by activated macrophages via intracellular nitric oxide synthase production (5, 15, 20), CpG-ODN treatment probably induced a listeriocidal activation status in the macrophages.
In conclusion, the data presented in this report indicate that CpG-ODNs are potent adjuvants for peptide-mediated T-cell vaccination to achieve antiviral protection. Whether these findings can be extrapolated to other viral infections remains to be demonstrated. However, the implications for eliciting protective CTL and T helper cell-mediated immunity in human vaccine strategies are potentially exciting. Furthermore, it will be of interest to elucidate the mechanism responsible for the potent adjuvant effect and to possibly enhance its longevity.
ACKNOWLEDGMENTS
We thank Martin Bachmann, Urs Karrer, Weldy Bonilla, Constantino Lopez, Stephan Oehen, and Burkhard Ludewig for helpful suggestions and discussions, Alana Althage and Edit Horvath for excellent technical assistance, and Elisabeth Hörhager and Roswitha Gampp for excellent secretarial assistance.
This work was supported by grants from the Swiss National Science Foundation, the Kanton of Zürich, the Human Frontier Science Program, and the Wellcome Trust.
REFERENCES
- 1.Bachmann M F, Hoffmann-Rohrer U, Bürki K, Skuntz S, Arnheiter H, Hengartner H, Zinkernagel R M. T helper cell unresponsiveness: rapid induction in antigen-transgenic and reversion in non-transgenic mice. Eur J Immunol. 1994;24:2966–2973. doi: 10.1002/eji.1830241207. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M F, Hoffmann-Rohrer U, Kündig T M, Bürki K, Hengartner H, Zinkernagel R M. The influence of antigen organisation on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 3.Bailey M J, McLeod D A, Kang C Y, Bishop D. Glycosylation is not required for the fusion activity of the G protein of vesicular stomatitis virus in insect cells. Virology. 1989;169:323–331. doi: 10.1016/0042-6822(89)90157-8. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft G J, Schreiber R D, Bosma G C, Bosma M J, Unanue E R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987;139:1104–1107. [PubMed] [Google Scholar]
- 5.Bancroft G J, Schreiber R D, Unanue E R. Natural immunity: a T cell independent pathway of macrophage activation defined in scid mice. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 6.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel R M. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 or 96 well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 7.Battegay M, Moskophidis D, Waldner H, Bründler M A, Fung-Leung W P, Mak T W, Hengartner H, Zinkernagel R M. Impairment and delay of neutralizing antiviral antibody responses by virus specific cytotoxic T cells. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 8.Binder D, Kündig T M. Antiviral protection by CD8+ versus CD4+ T cells: CD8+ T cells correlating with cytotoxic activity in vitro are more efficient in antivaccinia virus protection than CD4-dependent interleukins. J Immunol. 1991;146:4301–4307. [PubMed] [Google Scholar]
- 9.Bird A P. CpG islands as gene markers in the vertebrate nucleus. Trends Genet. 1987;3:342. [Google Scholar]
- 10.Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 11.Burkhart C, Freer G, Castro R, Adorini L, Wiesmüller K H, Zinkernagel R M, Hengartner H. Characterization of T-helper epitopes of the glycoprotein of vesicular stomatitis virus. J Virol. 1994;68:1573–1580. doi: 10.1128/jvi.68.3.1573-1580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carson D A, Raz E. Oligonucleotide adjuvants for T helper 1 (Th1)-specific vaccination. J Exp Med. 1997;186:1621–1622. doi: 10.1084/jem.186.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu R S, Targoni O S, Krieg A M, Lehmann P V, Harding C V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowdery J S, Chace J H, Yi A K, Krieg A M. Bacterial DNA induces NK cells to produce IFNγ in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 15.Fehr T, Schoedon G, Odermatt B, Holtschke T, Schneemann M, Bachmann M F, Mak T W M, Horak I, Zinkernagel R M. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J Exp Med. 1997;185:921–931. doi: 10.1084/jem.185.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kägi D, Lederman B, Bürki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 17.Kägi D, Ledermann B, Bürki K, Hengartner H, Zinkernagel R M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 18.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 19.Kägi D, Seiler P, Pavlovic J, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. The roles of perforin- and fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:2356–2362. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura D, Roes J, Kühn R, Rajewski K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin m chain gene. Nature. 1992;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 22.Krieg A M, Yi A-K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 23.Lipford G B, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 24.Lipford G B, Sparwasser T, Bauer M, Zimmermann S, Koch E S, Heeg K, Wagner H. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997;27:3420–3426. doi: 10.1002/eji.1830271242. [DOI] [PubMed] [Google Scholar]
- 25.Loke S L, Stein C A, Zhang X H, Mori K, Nakanishi M, Subasinghe C, Cohen J S, Neckers L M. Characterization of oligonucleotide transport into living cells. Proc Natl Acad Sci USA. 1989;86:3474–3478. doi: 10.1073/pnas.86.10.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackett M, Yilma T, Rose J K, Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985;227:433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura Y, Possee R D, Overton H A, Bishop D H L. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987;68:1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- 28.Pamer E G, Harty J T, Bevan M J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pircher H P, Hoffmann R U, Moskophidis D, Zinkernagel R M, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 30.Pisetsky D S. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5:303–310. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 31.Sparwasser T, Koch E S, Ramunas M V, Heeg K, Lipford G B, Ellwart J W, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Sparwasser T, Miethke T, Lipford G B, Borschert K, Häcker H, Heeg K, Wagner H. Bacterial DNA cause septic shock. Nature. 1997;386:336–337. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- 33.Sparwasser T, Miethke T, Lipford G B, Erdmann A, Häcker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motifs: induction of TNF-alpha-mediated shock. Eur J Immunol. 1997;27:1671–1679. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 34.Stacey K J, Sweet M J, Hume D A. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2122. [PubMed] [Google Scholar]
- 35.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 36.Weiner G J, Liu H M, Wooldridge J E, Dahle C E, Krieg A M. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitton J L, Gebhard J R, Lewicki H, Tishon A, Oldstone M B. Molecular definition of a major cytotoxic T-lymphocyte epitope in the glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1988;62:687–695. doi: 10.1128/jvi.62.3.687-695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Katakoa T, Tokunaga T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992;36:983–997. doi: 10.1111/j.1348-0421.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 39.Yi A K, Klinman D M, Martin T L, Matson S, Krieg A M. Rapid immune activation by CpG motifs in bacterial DNA: systemic induction of IL-6 transcription through an antioxidant-sensitive pathway. J Immunol. 1996;157:5394–5402. [PubMed] [Google Scholar]
- 40.Yi A K, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg A M. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–4761. [PubMed] [Google Scholar]
- 41.Zimmermann S, Egeter O, Hausmann S, Lipford G B, Röcken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3726–3630. [PubMed] [Google Scholar]
- 42.Zinkernagel R M, Althage A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977;145:644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]