Abstract
Secreted protein acidic and rich in cysteine (SPARC), also termed osteonectin or BM-40, is a matricellular protein which regulates cell adhesion, extracellular matrix production, growth factor activity, and cell cycle. Although SPARC does not perform a structural function, it, however, modulates interactions between cells and the surrounding extracellular matrix due to its anti-proliferative and anti-adhesion properties. The overexpression of SPARC at sites, including injury, regeneration, obesity, cancer, and inflammation, reveals its application as a prospective target and therapeutic indicator in the treatment and assessment of disease. This article comprehensively summarizes the mechanism of SPARC overexpression in inflammation and tumors as well as the latest research progress of functional nanomaterials in the therapy of rheumatoid arthritis and tumors by manipulating SPARC as a new target. This article provides ideas for using functional nanomaterials to treat inflammatory diseases through the SPARC target. The purpose of this article is to provide a reference for ongoing disease research based on SPARC-targeted therapy.
Keywords: SPARC, rheumatoid arthritis, target, tumors, functional nanomaterials
Introduction
Targeted therapy of inflammation and tumor is the main focus of the current research. To date, there is no perfect treatment plan for rheumatoid arthritis, which is a well-known immunodeficiency inflammatory disease (Watanabe et al., 2022). With the development and synthesis of functional nanomaterials, serum albumin, such as human serum albumin (HSA), has become a popular material used in the cure of rheumatoid arthritis. The powerful affinity between serum albumin and secreted protein acidic and rich in cysteine (SPARC) has become a huge driving force for drug-targeted therapy (Liu et al., 2019). In tumor treatment, functional nanomaterials have achieved certain results by manipulating SPARC-targeted therapy. The specific binding between HSA and nab-paclitaxel realizes the purpose of drug-dependent release and precise targeted therapy of cancer (Yardley, 2013). The high binding of HSA to SPARC realizes the targeted aggregation of paclitaxel in tumor lesions. The discovery of biomimetic drug delivery of functional nanomaterials in vivo by manipulating SPARC significantly alleviates rejection (Lin et al., 2016). This article will introduce the regulatory mechanism and research progress of SPARC in rheumatoid arthritis and tumors in detail. Based on the pathological phenomenon of high-level expression of SPARC in rheumatoid arthritis and tumors, it is more feasible for functional nanomaterials to deliver drugs directly to the lesion.
This article reviews the regulatory mechanism and expression sites of SPARC in inflammation and tumors. Understanding the structure of SPARC and the cause of its overexpression will help determine the pathogenesis of RA and facilitate the research on targeted therapy of late RA. In particular, the mechanism of SPARC as a potential target of RA and the latest research progress of functional nanomaterials to manipulate SPARC to treat inflammation and tumors were discussed.
Discovery, function, and expression of SPARC
Discovery and structure of SPARC
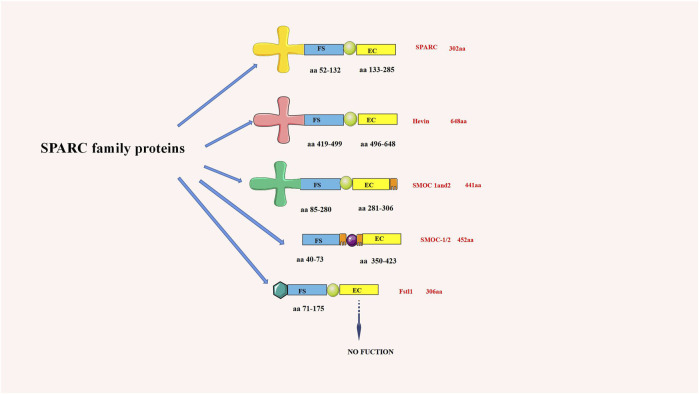
Termine JD first discovered that SPARC existed in the non-collagenous fetal bovine bone, which was the main component of its protein extract (Bradshaw, 2012). As an important part of the SPARC protein family, its domain comprises a cysteine-rich follistatin-like (FS) domain, an acidic N-terminal domain, and an a-helical extracellular (EC) calcium-binding domain with an EF-hand motif. Due to its structural properties, SPARC can bind various extracellular components (Gaudet et al., 2011). SPARC, equally known as BM-40 or 43K protein, has two calcium-binding sites on the protein, the N-terminal acidic domain, which binds 5 to 8 Ca2+ with less affinity, and the EF-hand motif located in the C-terminal domain, which binds Ca2+ ions with high affinity (Termine et al., 1981). The SPARC structure and SPARC protein family are shown in Figure 1.
FIGURE 1.
chematic diagram of the SPARC family structure (aa is the number of amino acids). SPARC is one of the most representative proteins in the SPARC family.
Physiological function of SPARC
SPARC is overexpressed during tissue renewal and repair. As a repair protein, SPARC plays an important role in manipulating cell proliferation, migration, and cytokine expression (Ng et al., 2013). Susceptibility of SKM-1 cells to Ara-C is enhanced with elevated levels of SPARC expression, accompanied by accelerated cell cycle restriction and apoptosis (Liang et al., 2022). Knockdown of the SPARC gene prolongs fibrosis during wound healing (Luo et al., 2019). Fibrotic changes in vitro and in vivo are effectively eliminated by specific suppression of SPARC (Wang et al., 2010). SPARC curbs apoptosis and inhibits the aggression and diversion of ovarian cancer cells (Chen et al., 2012). SPARC takes the center stage as ovarian cancer grows. The tumor suppressor gene, SPARC gene, is underexpressed in gastric cancer cell family (Zhou, 2018). SPARC inhibits the apoptosis of bone marrow stromal cells by inhibiting their proliferation (Deng, 2018). The activity of patients with ankylosing spondylitis can be assessed by detecting peripheral blood mononuclear cells and serum SPARC levels (Wang and Yang, 2018). As a matrix cell protein, SPARC is widely distributed in the eye and has the function of communicating cell and extracellular matrix signal transmission (Xiang and Tang, 2018). Silencing the SPARC gene reduces high fluorine-mediated cytotoxicity and inhibits apoptosis (Zhao et al., 2019). The degree of the expression level of SPARC protein can improve insulin resistance of adipocytes, thereby improving the insulin sensitivity of GK rats (Yao et al., 2020). As a stromal cell glycoprotein, SPARC is overexpressed in most inflammatory sites. SPARC has an irreplaceable role to play in prognosis and cure for cancer by regulating the proliferation, migration, and apoptosis of tumor cells. Knockdown or elimination of the SPARC gene can affect the speed of wound healing and reduce the effects due to cytotoxicity. SPARC protein can stimulate skin tissue fibrosis and increase fibroblast proliferation and collagen deposition.
Expression and regulation of SPARC
The expression of SPARC was significantly elevated during embryonic development as compared to normal adult tissues (Wong and Sukkar, 2017). SPARC expression levels are highly increased in epithelial cells during tissue injury, inflammation, and abnormal growth, such as tumors (Chiodoni et al., 2010). For some highly metastatic tumors, SPARC expression shows a high expression status, such as glioblastoma (Feng and Tang, 2014). SPARC, a potential indicator of inflammation and interferon responses, has the ability to shift anti-inflammatory macrophages into pro-inflammatory macrophages (Ryu et al., 2022). Huge expression levels of SPARC are usually detected at the sites of inflammation (Maltzman, 2022). Compared to normal cartilage, there are a large number of SPARC in the upper and middle areas of arthritic cartilage in RA patients, which can promote the decomposition of the ECM that is located on the surface area of arthritic cartilage through matrix metalloproteinases (MMPs). The proliferation of chondrocytes in the middle and deep layers can be regulated by SPARC. The unusual synthesis and degradation of SPARC might be ascribed to the destruction of the cartilage and bone (Li et al., 2023). Suppression of SPARC gene expression could inhibit the proliferation of human keloid fibroblasts (HKFs), block the process of the cell cycle, and promote apoptosis, and the downregulation of the TGF-β signaling pathway may have some relevance to this mechanism (Li, 2022). As an important factor, SPARC participates in the regulation of multiple signaling pathways, such as JAK/STAT and mTOR (Wang et al., 2019). SPARC regulates osteoblast mineralization through the p38 MAPK pathway (Zhu, 2020). Expression levels of SPARC and SPARCL-1 are substantially elevated in Glu-induced hippocampal neuronal injury (Agostina et al., 2021), and SPARC and SPARCL-1 regulated autophagy through the AKT–mTOR pathway to affect Glu-induced hippocampal neuron injury (Chen, 2020). SPARC is involved in VEGF-induced fibrosis in HTF cells, and it will be a new therapeutic opportunity for antifibrotic strategies after the completion of filtration surgery (Xiang and Tang, 2018). SPARC promotes the apoptosis of HUASMCs through the mitochondrial pathway, inhibits the proliferation rate of HUASMCs, thereby reducing their cell viability, promotes the secretion of gelatinase (MMP2 and MMP9), and leads to the degeneration of the extracellular matrix and internal elastic layer in the vessel wall (Ye, 2017). The expression and regulation mechanism of SPARC are shown in Figure 2. The following section introduces the latest application and research progress of SPARC as an underlying and efficient curative target for inflammatory diseases and tumors as well as functional nanomaterials to manipulate SPARC to treat diseases.
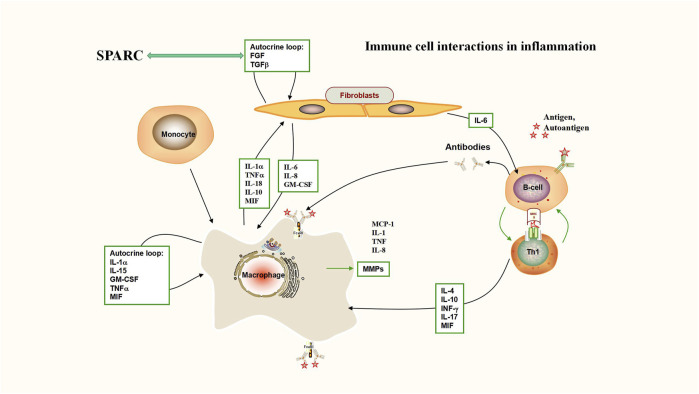
FIGURE 2.
Immune cell interactions in inflammation (interaction between SPARC and TGF-β). Deficiency of TGF-β signaling effectively protects against inflammatory joint erosion in the arthritis model. Interaction between SPARC and TGF-β plays a key role in inflammatory sites including rheumatoid arthritis.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease that occurs along with a phenomenon that influences both joints (Ainsworth et al., 2022). The typical pathology of RA is characterized by changes in inflammatory cells and deformation of small joints. Patients with severe RA have significant atherosclerosis (Wang Z. et al., 2022). Macrophages are key cells in the treatment of RA. Macrophage activation is the main feature (Xu Y. et al., 2022). SPARC acts as a multifunctional modulator of parenchymal cells with enhanced expression in smooth muscle cells and macrophages in the atherosclerotic lesion stage (Raines et al., 1992). The high representation level of SPARC in macrophages can be an effective way to treat RA. Macrophages and FLS became the main target cells for targeted therapy of RA (Tu et al., 2022). SPARC may play a role in arthritis by stimulating MMP synthesis in synovial fibroblasts (Tremble et al., 1993). Multiple research experiments have found that synovial inflammation in arthritic mouse models can significantly be inhibited by blocking the NF-κB pathway. Due to the increased amount of TGF-β expression in RA-FLS, it clearly promotes the destruction and erosion of cartilage and bones by FLS (Stanford et al., 2016). SPARC exhibits anti-inflammatory effects through negative regulatory mechanisms on TGF-β and NF-κB signaling (Puolakkainen et al., 2003). SPARC could be a promoter of ECM degradation through the action of MMPs.
Expression of SPARC in RA
Persistent synovitis and systemic inflammation are key features of RA (Giachi et al., 2022). A UK study found that the lowest prevalence of RA was much higher for women than that for men (Charles et al., 2013). There have been reports that the SPARC expression level in the ground and middle layer of articular cartilage in RA patients is significantly increased, and the level of synovial fluid and synoviocytes is increased (Nakamura et al., 1996). The discovery of SPARC provides new insights into it as a new target. IHC verified the high expression of SPARC in RA inflammatory joints (Liu et al., 2019), but SPARC staining is absent in normal cartilage. Synoviocytes from both RA and OA joints were found to have increased SPARC synthesis (Nakamura et al., 1996). At present, there are few reports on the high expression of SPARC in the RA joint synovium. Finding effective ways to treat RA is a major medical challenge. The discovery of SPARC, a potential target and therapeutic index, is expected to help advance the effective treatment of RA.
Regulatory mechanism of SPARC in RA
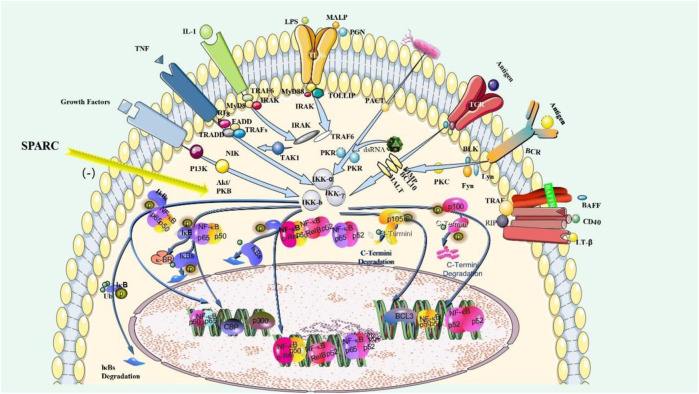
SPARC, as a matrix protein, participates in the remodeling of normal and abnormal tissues (Puolakkainen et al., 2003). SPARC exhibits anti-inflammatory effects through an adverse regulator of TGF-β and NF-κB signaling (Sangaletti et al., 2011). The negative regulation mechanism of SPARC on NF-κB is shown in Figure 3. Previous studies have shown that the concentration of TGF-β that is required for the maximal stimulation of SPARC synthesis is basically consistent with the level of TGF-β in RA joints (Fava et al., 1989). SPARC can regulate the apoptosis of immune cells and limit the apoptosis of B-cell precursors (Tripodo et al., 2012). By culturing rabbit articular chondrocytes, it was found that TGF-β significantly increased SPARC levels, whereas IL-1β significantly decreased SPARC (Nakamura et al., 1996). Modulation of inflammatory cells and cytokines is an important therapeutic target to control inflammation in RA. As an important factor, TGF-β has become a hotspot in the study of RA treatment (Cheng et al., 2022). The interplay of SPARC with TGF-β is shown in Figure 3. The level/activity of TGF-β in patients with RA was found to be markedly higher than that in healthy individuals. In a model of inflammatory arthritis, the deficiency of TGF-β signaling effectively prevents inflammatory joint erosion (Xia et al., 2022). Inhibiting the NF-𝜅B pathway can inhibit RA-FLS and RAW 264.7 cell proliferation, induce apoptosis, and improve RA inflammation (Yang Y. P. et al., 2022). For example, aspirin promotes apoptosis in HFLS by downregulating the NF-κB pathway (Zhang X. et al., 2018). Inflammation is an inescapable pathological feature of RA. A central regulatory role in the pathological process of RA is played by the activation of the NF-κB signaling path (Xu Y. J. et al., 2022). Pathological NF-κB activation assumes a significant task at the time of emergence and development of rheumatoid arthritis (Zhou and Zhang, 2012). SPARC is shown at high levels in RA joints and has regulatory effects on TGF-β and NF-κB signaling paths. At present, the research on the mechanism of RA is gradually deepening, and the research on TGF-β and NF-κB signaling pathways in RA is increasing. SPARC, as a matrix protein, plays an anti-inflammatory role through the negative regulatory mechanism of TGF-β and NF-κB pathways. Massive proliferation of HFLS is the basic pathological feature of RA. The character of SPARC in the promotion of HFLS-RA apoptosis is bound to provide an opportunity for SPARC to become a fresh target for the cure of RA. The regulation mechanism of SPARC in RA is shown in Figure 3.
FIGURE 3.
Negative regulation mechanism of SPARC on NF-κB. SPARC inhibits the proliferation of macrophages and HFLS-RA by negatively regulating the NF-κB pathway and promotes their apoptosis by playing an anti-inflammatory role.
Role of SPARC in RA
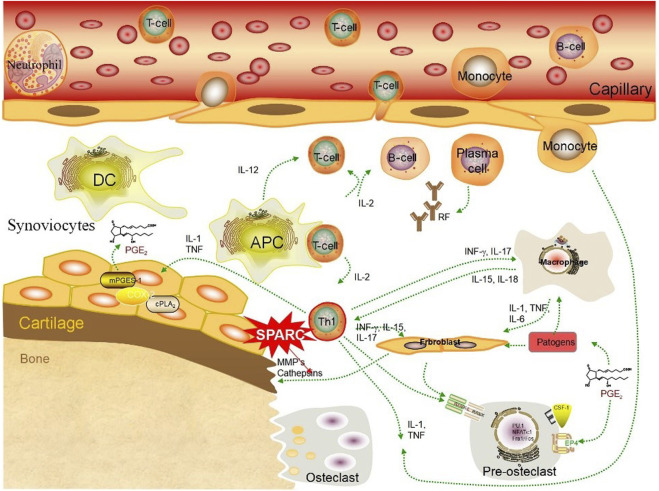
SPARC has a dual role in resorbing and regenerating arthritic cartilage. Numerous studies have demonstrated synovial tissue heterogeneity in RA joints (Ouboussad et al., 2019). HFLS forms the synovial intima, which has an important function in the destruction of RA joints (Firestein, 2004). FLS presents an aggressive phenotype in joint destruction and inflammation in patients with RA (Nygaard and Firestein, 2020). FLS-mediated excessive production of MMPs damages the collagen-rich structure of the joint tissue at the pannus–cartilage interface of rheumatoid joints and promotes FLS invasion (Nygaard and Firestein, 2020). MMPs play a key role in the remodeling of the ECM (Zhang et al., 2021). SPARC might promote the decomposition of the ECM through the action of MMPs on the surface area of arthritic cartilage (Tremble et al., 1993). SPARC promotes ECM degradation through MMPs, as shown in Figure 4. Irregular synthesis and degradation of SPARC may be associated with cartilage and bone deterioration. SPARC exudes a range of bioactive peptides containing the (K)GHK array that can affect angiogenesis and synovial hyperplasia to influence vascular remodeling (Lane et al., 1994). The pannus phenomenon is closely related to SPARC. FLS-RA is the main cell in RA joint destruction, and the excessive production of MMPs mediated by it will destroy the joint tissue structure.
FIGURE 4.
SPARC promotes ECM degradation through MMPs. SPARC promotes the apoptosis of HUASMCs through the mitochondrial pathway, inhibits the proliferation rate of HUASMCs cells, thereby reducing their cell viability, promotes the secretion of gelatinase (MMP2 and MMP9), and leads to the degeneration of the extracellular matrix and internal elastic layer in the vessel wall
As a chronic autoimmune disease caused by immune system dysfunction, RA is very important to find an active therapeutic marker for the growth of RA. As a hidden target of RA, SPARC can have excellent benign effects on RA joints based on the negative regulation of TGF-β and NF-κB pathways. Currently, the focus of RA curative research is on indicators such as MMPs and receptor activation of the NF-κB ligand (RANKL) (Meng et al., 2022). As an overexpressed substance in RA articular cartilage, SPARC participates not only in the ECM degradation process promoted by MMPs but also in negatively regulating TGF-β and NF-κB pathways. Therefore, SPARC becomes a dual possible target for the therapeutic cure of RA. In the pathogenesis of RA, SPARC can exhibit its full anti-inflammatory effect. The discovery of SPARC, a potential target, has significant implications for treating and prognosticating RA in the years to come.
Tumors
The microenvironment of the tumor is a vital regulator of tumor initiation, progression, and migration. SPARC is covered in interactions between the tumor and stroma and influences cancer growth by influencing tumor invasion (Camacho et al., 2020). High level of SPARC expression is typically linked to aggressive and highly metastatic tumors. Based on this feature, SPARC is often used as a prognostic marker for different cancers. Interestingly, further clinical exploration found that SPARC could be used as a candidate aim for the control of cancer (Podhajcer et al., 2008). SPARC is categorized as a promising target and indicator for treating various cancers.
Gastric cancer
Risk factors for stomach cancer, which is one of the extremely common and high-mortality cancers, include dietary habits and bacterial infections (Smyth et al., 2020). Gastric cancer affects dietary life for a long time for patients, bringing great changes and troubles. Discovering new potential therapeutic or prognostic targets will bring good news to patients with gastric cancer.
Regulatory mechanisms of SPARC in gastric cancer
Through network pharmacology analysis, SPARC expression has been shown to be strongly correlated with the bad prognosis of GC. SPARC can be used as a marker of its bad prognosis (Li et al., 2019). Subsequently, clinical studies have found that SPARC is a possible marker for diagnosing and prognosticating gastric cancer and an important factor regulating various signaling pathways (Wang et al., 2019). The SPARC gene acts to suppress epigenetic tumorigenesis (Shao, 2018). SPARC brings new targets and therapies to treat gastric cancer (Zhou, 2018). SPARC may support the invasion, metastasis, and angiogenesis of gastric cancer cells (Ma Y. S. et al., 2017). Genome-scale analysis reveals that SPARC is a marker for the diagnosis and prognosis of gastric cancer (Xu et al., 2018). A meta-analysis was performed to elucidate that SPARC was a prospective clinical predictor of survival in gastric cancer patients (Wang et al., 2014). The impact of SPARC on the proliferation and migration of gastric cancer cells was induced by regulating developmental factor arrival (Phan et al., 2007). SPARC levels are inversely correlated with GC clinicopathological factors (Zhang et al., 2014). Clinical data showed that the proficiency of SPARC was significantly reduced in a group of patients with gastric cancer after treatment (Gao et al., 2015). Early network pharmacology provided data support for the discovery of SPARC, a new prognostic indicator, and later, a clinical research further confirmed this point of view.
Role of SPARC in gastric cancer
The investigators revealed that the level of SPARC in GC may be related to the induction of GC cells (Li Z. et al., 2016). SPARC positivity in gastric cancer tissue was higher than in non-cancerous gastric tissue (Gao et al., 2017). SPARC and VEGF jointly promote angiogenesis in tumor sites (Wang et al., 2012). Human gastric cancer cell invasion and growth may be inhibited by the downregulation of SPARC (Yin et al., 2010). In tumor tissues lacking SPARC, the tumor growth rate and volume were significantly reduced (Ma et al., 2021). SPARC was upregulated in gastric tumor samples and had a strong relationship with poorer overall survival. The prognostic significance of risk score models for gastric cancer prediction can be refined by analyzing differentially expressed genes (DEGs) using SPARC (Shan et al., 2021). The PCR method reveals the important role of SPARC epistasis in gastric carcinogenesis (Chen et al., 2018). Downregulating the expression of VGEF can block the angiogenesis of gastric cancer (Zhang J. L. et al., 2012). Increased levels of SPARC expression are closely related to poor outcomes in GC in Kaplan–Meier survival analysis (Liao et al., 2018). The level of expression of SPARC is strongly correlative to the level of GC cells (Zhu et al., 2015). SPARC may participate in gastric cancer by affecting the tumor microenvironment transfer (Mo et al., 2017). As a target gene for treating GC, SPARC has the exclusive function of detecting drug sensitivity (Zhao et al., 2021). The discovery of SPARC will lead the way for further research on the role of circRNA in the GC body (Tian et al., 2020). SPARC is a resource for conducting a survey on the potential biomarkers and targets for the diagnosis, prognosis, and treatment of GC (Hao et al., 2019). SPARC is a key growth factor in gastric cancer (Zhang J. L. et al., 2012). SGC7901 cells expressing high levels of SPARC are more active and resistant to anticancer drugs than MGC803 cells expressing low levels of SPARC (Li A. et al., 2015). The period for the greatest disease response in cancer patients is generally in the sophisticated stage of cancer. The discovery of a novel predictor is essential for gastric cancer patients. In patients with stage II/III gastric cancer, high levels of SPARC gene expression may be a valuable prognostic factor (Suzuki et al., 2018). The Gene Expression Omnibus (GEO) network database found that SPARC overexpression levels were linked to reduced overall GC survival rates (Yan et al., 2018). SPARC is located in peritumoral fibroblasts and rarely observed in cancer cells’ cytoplasm (Nakajima et al., 2018). Moving forward, the clinical detection of the prognosis of GC patients can be more targeted at HFLS around CG cells. SPARC, as a prognostic indicator, will bring convenience to the detection of GC prognosis.
Network pharmacology brings data support for SPARC as a GC prognostic indicator, and clinical pharmacology experiments such as WB continue to verify that SPARC and GC are inseparable. As an effective prognostic indicator for GC, the high expression of SPARC was accompanied by the rapid growth of GC cells.
Hepatocellular carcinoma
One of the most lethal cancers worldwide is hepatocellular carcinoma (HCC) (Piñero et al., 2020). As the sixth most common cancer worldwide, the overexpression of SPARC reduces HCC proliferation during spheroid cell culture. SPARC improves patient survival by inhibiting the tumorigenic ability of host macrophages. Additionally, SPARC leads to its reduced tumorigenicity by inducing mesenchymal–epithelial transition (MET) (Atorrasagasti et al., 2010). The dual regulatory effect of SPARC on HCC has become the biggest breakthrough in the targeted therapy of HCC.
Regulatory mechanism of SPARC in hepatocellular carcinoma
When HCC patients develop disease, the mRNA of SPARC is expressed at a high standard in vivo (Gao et al., 2021). The overexpression of SPARC was shown to strengthen the cytotoxic effect of sorafenib in Hep3B and HepG2 cells, significantly elicited LDH release, and induced oxidative stress compared with parental cells (Hua et al., 2021).
Role of SPARC in hepatocellular carcinoma
A study found that HCC cells in SPARC knockout mice were significantly affected (A et al., 2021). As the mechanism of SPARC inactivation in HCC, SPARC methylation becomes a biological indicator of HCC prognosis (Zhang Y. et al., 2012). A meta-analysis was conducted and revealed that an elevated SPARC level was correlative with the poor survival of HCC (Yang X. et al., 2022). SPARC affects HCC cell viability through the regulation of the ERK1/2-MMP2/9 pathway (Liu et al., 2020). The growth rate and angiogenesis of HCC are tightly correlated with the high expression level of SPARC (Lau et al., 2006). MuiR-29a-overexpressing HCC cells inhibited phosphorylation of AKT/mTOR downturn of SPARC (Zhu et al., 2012). The identification of this new target of SPARC has made a historic leap forward in the targeted care of HCC (Hua et al., 2015). SPARC is extremely expressive in severe HCC tissue sections without abnormalities found in normal tissues (Le Bail et al., 1999). SPARC has been confirmed to be a bona fide target of miR-211 (Deng et al., 2016). Sorafenib is the first-line drug to treat advanced HCC, and SPARC is considered a potential drug target (Goyal et al., 2019). The fluorescent probe method detected that SPARC was significantly higher in HCC patients than in healthy individuals (Segat et al., 2009). Increased tumor volume of HCC in nude mice is supported by the overexpression of SPARC (Jiang et al., 2019). SPARC was also a central gene with significant diagnostic value (Ye et al., 2020). The follistatin-like domain of the SPARC protein decreased the level of E-cadherin expression (Sun et al., 2018). SPARC has the driving force to regulate the progression of HCC (Wan et al., 2015). SPARC has been entangled in the progress of fibrosis in the liver (Nakatani et al., 2002). Based on the lack of effective therapeutic targets for HCC, SPARC will play a prominent role as a new target (Ang et al., 2016).
SPARC will be a potent target for the management of HCC. Looking ahead, strong and effective treatment can be achieved using functional nanomaterials by manipulating SPARC, reducing the pain of patients.
Breast cancer
Breast cancer, the most prevalent cancer in females globally, is characterized by bone metastases. SPARC has the ability to inhibit osteoclast activation in the microenvironment besides inhibiting breast cancer cell migration and invasion. SPARC can be a viable curative target for treating breast cancer metastasized to the bone (Ma J. et al., 2017). Compared with tumor cells, SPARC appears at higher levels in the stromal cells of the tumor (Shi et al., 2022). After stage treatment, the expression of SPARC in the stromal cells will be used as a predictive pointer for breast cancer.
Regulatory mechanism of SPARC in breast cancer
Triple-negative breast cancer occurs in up to 20% of breast cancer families. In triple-negative breast cancer, SPARC is widely expressed (Lindner et al., 2015). The high affinity between nal-paclitaxel’s functional structural protein, HSA, and SPARC is responsible for its excellent efficacy in the management of breast cancer (Yardley, 2013). There is an urgent need for alternative therapeutic strategies in TNBC on the basis of tumor-specific molecular targets. Studies have demonstrated a novel crosstalk between proteases and stromal cellular proteins in the tumor microenvironment through the limited proteolysis of SPARC and have found that SPARC may serve as a possible therapeutic target in TNBC (Alcaraz et al., 2021). SPARC expression in CAF is an exclusive prognostic factor for poor prognosis in TNBC (Alcaraz et al., 2022). Breast cancer afflicting women must be addressed well. The emergence of a good target and prognostic indicator, SPARC, is going to open the door to a whole new world in the treatment of breast cancer.
Role of SPARC in breast cancer
Breast cancer tissues and most breast cancer cells have a higher level of SPARC (Li X. L. et al., 2022). A high SPARC level correlated with breast cancer cell differentiation becomes a new therapeutic approach (Bawazeer et al., 2018). SPARC is downregulated during breast cancer development (Nagai et al., 2011). As a 32 kDa secreted glycoprotein, the elevated level of SPARC was related to the overall survival of patients (Watkins et al., 2005). SPARC acts as a prognostic indicator. After chemotherapy, the overall survival of patients with senior SPARC expression was markedly shortened (Zhu et al., 2016). Network pharmacology found that SPARC can be used as the basis for early breast cancer diagnosis (Azim et al., 2013). SPARC is an inhibitor of the migration and invasion of breast cancer, while resisting the platelet deficiency caused by it (Koblinski et al., 2005). Breast cancer progression to the S phase was slowed down by SPARC (Dhanesuan et al., 2002). SPARC might be a helpful marker for aggressive, metastatic-prone tumors (Wong et al., 2008). SPARC is able to induce the activation of MMP-2 in breast cancer cell lines (Gilles et al., 1998). Appealing targets for anti-metastatic therapy in breast cancer are SPARC and its secondary effectors (Güttlein et al., 2017). SPARC is accompanied by a reduced extracellular matrix (Hellinger et al., 2020). SPARC was found to be 69.3% positive in cancerous tissues (Guo et al., 2017). SPARC upregulation is a system whereby PTEN controls the deposition of collagen in the mammary stroma (Jones et al., 2021). SPARC-mediated degradation of the ECM and its potential link to MMPs could lead to breast cancer development (Kim et al., 2017). Compared to those with poor SPARC expressions, those with positive SPARC expressions had a 2.34-fold increased risk of death (Hsiao et al., 2010). Plasma concentration of SPARC was less in normal females, suggesting an anti-adhesive effect of circulating SPARC (Maroni et al., 2015). A high expression of bone remodeling protein-SPARC in a highly malignant phenotype can be observed (Lukianova et al., 2022). Screening of aggressive cancer-associated DEGs and important pathways revealed that SPARC could be a promising target gene in metastatic breast cancer (Chen et al., 2015). The upregulation of SPARC was observed after neoadjuvant chemotherapy is correlated with chemotherapy resistance in breast cancer patients (Wang et al., 2016). A high expression of bone remodeling protein-SPARC in a highly malignant phenotype can be observed (Szynglarewicz et al., 2016). The inhibition of SPARC secretion by oleic acid ultimately favors T-cell activation as an underlying mechanism for the reduction of breast cancer aggressiveness (Bellenghi et al., 2022). The vast majority of stromal cells showed abundant cytoplasmic SPARC reactivity (Barth et al., 2005). Patients with a high level of SPARC have increased survival rate (Beck et al., 2008).
Triple-negative breast cancer has been plagued clinically due to the absence of a suitable target. SPARC, as a target protein that inhibits the cell cycle of breast cancer, is bound to increase the target of chemotherapy medicines. The combination of functional nanomaterials and SPARC will bring good news to patients in the future.
Non-small-cell lung cancer
Investigations have shown that SPARC promotes pathological reactions in non-small-cell lung cancer (NSCLC) and idiopathic pulmonary fibrosis by encouraging microvascular remodeling and overproduction of ECM proteins (Wong and Sukkar, 2017). The primary reason of cancer death worldwide is NSCLC Mirhadi et al., 2022). SPARC was a latent prognostic biomarker in NSCLC (Fabrizio et al., 2020).
Regulatory mechanism of SPARC in NSCLC
Lung cancer growth may be inhibited by the elevated level of SPARC mRNA in lung cancer tissues (Wang B. et al., 2018). Network pharmacology analysis shows that SPARC is expressed at a highly increased level in NSCLC tissues (Ma G. Y. et al., 2022). SPARC is a biologically relevant mechanism that is upregulated in the development of lung cancer as a specific site that regulates collagen binding (Kehlet et al., 2018). SPARC overexpression in A549 and H1299 cells drives migration and EMT, respectively (Sun et al., 2018). The downregulation of SPARC expression in H322 and A549 cells leads to the inhibition of cell invasion (Zhou et al., 2010). SPARC has the advantage of promoting, invading, and migrating lung cancer cells, making it a novel therapeutic target.
Role of SPARC in NSCLC
In two NSCLC cell lines, CL1-5 and H1299, SPARC treatment increased cell growth, migration, and the mesenchymal phenotype (Hung et al., 2017). SPARC can be a prognostic overall survival indicator (Huang et al., 2012). The upregulation of SPARC helps define the pathogenesis of NSCLC (Grant et al., 2014). Plasma of lung cancer patients contains overexpressed SPARC (Andriani et al., 2018). SPARC promotes growth and inhibits the apoptosis of lung cancer cells (Xu et al., 2019). SPARC produced by stromal cells supports a high degree of vascular maturation (Koukourakis et al., 2003). The positive representation rate of SPARC in stages I and II lung cancer was significantly lower than that in stages III and IV lung cancer (Zhang Z. et al., 2012). SPARC expression was upregulated in lung cancer cells via promoter demethylation and is correlative with a decreased DNA methyltransferase (DNMT) activity (Pan et al., 2008). SPARC increased the permeability of macromolecules through the integrin/focal adhesion/cell-promoted persistent activation of the alveolar epithelial junction axis (Conforti et al., 2020). The SPARC gene is a tumor-promoting gene (Yang et al., 2019). The SPARC gene is also a prognostic gene (Zhong et al., 2022). SPARC was significantly downregulated in hypermethylated tumors (Ito et al., 2005).
Network pharmacology and experimental studies have continuously confirmed the strong presence of SPARC in lung cancer. As a novel therapeutic target, the high-level expression of SPARC is mediated by promoter demethylation. Clinical cancer samples confirmed that SPARC can be a candidate therapeutic marker and prognostic indicator for the diagnosis of NSCLC.
Melanoma
Melanoma significantly affects the work and study of patients. The emergence of novel targets and prognostic indicators is critical for the entire therapeutic community.
Regulatory mechanism of SPARC in melanoma
Cell migration, adhesion, cytoskeletal features, and cell shape are influenced by the inhibition of SPARC expression in human melanoma cells (Salvatierra et al., 2015). Fibroblast-like morphology in normal human melanocytes overexpressed SPARC (Haber et al., 2008). SPARC stimulates cathepsin B-mediated melanoma invasion through this mechanism using collagen I and α2β1 integrin as mediators (Girotti et al., 2011). SPARC can enhance the blocking effect of Pf4 on the phosphorylation of ERK and the metastasis of melanoma cells, and the synergistic effect of SPARC provides the possibility of targeting the secretome of cancer cells for therapeutic development (Zeng et al., 2021). SPARC is bound to become a novel target for the development of melanoma therapies.
Role of SPARC in melanoma
The growth of melanoma is not regulated by exogenous SPARC or by stromal tissue, but solely by the amount of SPARC that is yield by the malignant cells themselves (Prada et al., 2007). SPARC could become a new target to stop the progression of melanoma (Maloney et al., 2009). SPARC limits p53 levels by activating Akt and MDM2, and gaining SPARC expression in the development of melanoma confers a survival advantage by the inhibition of p53-dependent apoptotic signaling pathways (Fenouille et al., 2011). SPARC maintains a high-level expression in melanoma (Fenouille et al., 2012). Interactions between melanoma and other cells are facilitated in a SPARC-dependent form (Defresne et al., 2011). Normal melanocytes do not express SPARC (Ledda et al., 1997). SPARC is a possible leader for the treatment of melanoma (An et al., 2013). SPARC is a stromal cell protein associated with melanoma invasiveness (Rocco et al., 2011). SPARC overexpression enhances vascular extravasation (Tichet et al., 2015). Serum levels of SPARC were significantly higher in individuals with melanoma than those in healthy donors (Ikuta et al., 2005). SPARC is found to be strongly expressed in paraffin-embedded samples of melanoma (Pieniazek et al., 2016). The proliferation of melanoma is associated with the regulation of the expression of proteins involved in the epithelial–mesenchymal transition and with the suppression of MMP-2 and activation of MMP-9 (Cerezo et al., 2013). SPARC plays an important role in the tumor evasion of immune surveillance by reducing anti-tumor PMN action (Alvarez et al., 2005). Invasive melanoma cells may invade intraepidermal regions and produce epidermal melanoma metastases (EMM) (Meling et al., 2021). SPARC aids cell expansion and could be a possible target for melanoma treatment (Horie et al., 2010). Desmoplastic melanoma (DM) is a unique form of melanoma in which SPARC expression is extremely high (Garrido et al., 2014). The SPARC gene is a key gene affecting the migration of melanoma (Kaochar et al., 2018). SPARC is one of the indicators that helps identify the progression of melanoma (Chakraborty et al., 2009). SPARC-enriched tumors had a substantially higher percentage of vascular occupancy (Ordonez et al., 2005). The utilization of therapeutic genes that are directed by the SPARC driver could be a useful approach for the treatment of cancer (Lopez et al., 2006). The high level of SPARC in acidic media is considered to be an essential potential for the invasive activity of tumors (Kato et al., 2000). SPARC promotes metastasis and vascular extravasation in melanoma.
The high expression of SPARC was found by conducting a study on melanoma specimens. SPARC was found to be a key target for treating melanoma. SPARC can enhance the permeability of blood vessels, its high expression in acidic media provides motivation, and it is a leader for treating melanoma.
Esophageal cancer
Due to its highly aggressive nature and poor survival rate, esophageal cancer is one of the most aggressive cancers worldwide (Domper Arnal et al., 2015). Esophageal cancer greatly afflicts patients and produces many complications.
Regulatory mechanism of SPARC in esophageal cancer
In the epithelial cells of esophageal cancer, SPARC is extremely expressed (Brabender et al., 2005). The expression of the SPARC gene is found to be increased in esophageal cancer (Luo et al., 2004). SPARC is highly shown in the specimen (Botelho et al., 2010). The protein expression pattern of SPARC is useful for developing reasonable strategies for precocious detection of high-risk features and for the prevention and management of ESCC (Xue et al., 2006). SPARC becomes a prominent target for esophageal cancer imaging (Zhao et al., 2022). SPARC is linked to unfavorable prognosis in patients with ESCC (Wu et al., 2017). SPARC has a strong correlation with MMP-2 expression, and this correlation could play a critical role in the growth of esophageal cancer (Yamashita et al., 2003). Laminin-5γ2 chain and SPARC might participate in esophageal SCC progression, and their concurrent expression is associated with adverse prognosis (Xue et al., 2011). Compared to control mucosa, SPARC is highly overexpressed in tumor tissues (Porte et al., 1998). The downregulation of SPARC may reduce cell movement and invasion involved in EMT via the p-FAK/p-ERK pathway, which could represent a novel therapeutic approach for ESCC (Zhang et al., 2020).
Role of SPARC in esophageal cancer
Peritumoral stromal cells (tumor-associated fibroblasts and macrophages) were the principal source of SPARC in the TME, and exogenous SPARC increased tumor cell invasion (Ma W. et al., 2022). SPARC is a cytoplasmic and nuclear protein found in ESCC cells (Che et al., 2006). A high SPARC expression in ESCC parenchyma with IHC is combined with lymph node metastasis and bad prognosis (Chen et al., 2017).
During the procedure of esophageal cancer, SPARC could be deployed as a potential medicinal target for endoscopic detection due to its extremely high expression in IHC. In areas with high incidence, early diagnosis is more important for esophageal cancer, which has resulted in the emergence of endoscopic techniques. The high expression of SPARC provides development potential and convenience for detection. Through literature search and analysis of clinical cancer samples, it is found that SPARC is located in the nucleus and cytoplasm of ESCC and SPARC can be considered as a prognostic indicator.
Ovarian cancer
Ovarian cancer (OvCa) is currently the fifth most frequent cause of cancer-related mortality among females in the United States, and approximately 140,000 females globally die from ovarian cancer each year (Penny, 2020). SPARC has a therapeutic effect by inhibiting OvCa cell metabolism (Naczki et al., 2018).
Regulatory mechanism of SPARC in ovarian cancer
SPARC inhibits the NF-κB pathway mediating macrophage-induced ovarian cancer cell invasion (Said et al., 2008). SPARC suppresses the differentiation of ovarian cancer cells and transition of adipocytes to cancer (John et al., 2019). SPARC promoter methylation is an essential element in the occurrence and survival of ovarian cancer, and SPARC can be used as a therapeutic target and predictive marker for ovarian cancer (Socha et al., 2009). SPARC markedly reduced the proliferative, chemotactic, and invasive effects of LPA on ovarian cancer cell lines (Said N. A. et al., 2007). Knocking down SPARC production clearly reduced ovarian cancer cell proliferation, induced the apoptosis, and prevented the cells from invading and metastasizing. SPARC becomes a key factor in ovarian cancer development as it is overexpressed in aggressive subclones and ovarian cancer samples (Chen et al., 2012).
Role of SPARC in ovarian cancer
SPARC helps normalize the ovarian cancer malignant ascites microenvironment by downregulating the VEGF–integrin–MMP axis, reducing the levels and activation of bioactive lipids, and ameliorating downstream inflammatory (Said et al., 2007b). The expression of SPARC in ovarian cancer cells was negatively associated with the level of malignancy (Yiu et al., 2001). In the ovarian stroma containing malignant cells, particularly at the tumor–stroma interface in invasive tumors, elevated levels of SPARC mRNA and protein expression were found (Brown et al., 1999). SPARC has limitations on normal ovaries in premenopausal patients (Paley et al., 2000). SPARC may work as a tumor suppressor to reduce angiogenesis and lymphangiogenesis in ovarian cancer by decreasing the amount of VEGF-C and VEGF-D expression (Peng et al., 2017). SPARC modulates TGF-β1 fibrillar ECM deposition through a novel mechanism to influence cancer cell biology (Tumbarello et al., 2016). SPARC inhibits the αv- and β1-integrin-mediated attachment of ovarian cancer cells to the ECM (Said et al., 2007a).
Malignant ascites is the most common cause of death from ovarian cancer, and SPARC normalizes malignant ascites in the ovarian cancer microenvironment and improves inflammation through downregulating the VEGF–integrin–MMP axis. Due to the negative correlation among SPARC and ovarian cancer and the regulation of the microenvironment, SPARC could be a possible drug target for curing ovarian cancer.
Other cancers
Neoadjuvant chemoradiotherapy can improve the tumor resection rate (Li Y. et al., 2016) and SPARC was found to be its central gene (Sun et al., 2020). Rectal cancer patients with high SPARC expression have poor prognosis. SPARC emerges as a prognostic indicator for patients with colorectal cancer undergoing chemoradiotherapy (Kurtul et al., 2017).
Bladder cancer (BCa) is the most prevalent malignancy of the urinary tract and remains one of the most frequent forms of cancer in the world (Dobruch and Oszczudłowski, 2021). The frequency and strength of SPARC expression negatively correlated with disease-specific survival in human bladder tumor tissues (Said et al., 2013). SPARC shows potential as a prognostic indicator of tumor recurrence or progression during prostate cancer theranostics but is affected by hematuria (Critselis et al., 2019). The level of the SPARC gene has a significant relationship with the histological type, pathological status, and prognosis of bladder cancer (Yamanaka et al., 2001). The SPARC level in human bladder cancer cell lines is negatively associated with the proliferation rate (Larson et al., 2010; Makridakis et al., 2010; Said, 2016).
Head and neck cancer is one of the most prevalent cancers globally (Siegel et al., 2017). In cDNA array analysis, the expression of SPARC in tumor regions was senior compared with adjacent normal regions. SPARC can enhance the proliferation and relocation of head and neck cancer cells (Chang et al., 2017).
SPARC can be used as a prospective therapeutic index and prognostic biomarker for cancers including rectal cancer, bladder cancer, and head and neck cancer. SPARC was negatively correlated with tumor development. As a candidate therapeutic approach for tumors, future research can focus on the highly expressed sites of SPARC in cancer cells. As a prognostic indicator and potential target for various tumors, it is shown in Figure 5.
FIGURE 5.
SPARC as a candidate target and as a therapeutic prognostic indicator for the adjuvant treatment of cancer. As a new therapeutic target, SPARC is extremely expressed in most tumors and will surely provide greater convenience for the effective treatment of tumors with functional nanomaterials in the future.
Diabetes
SPARC is considered an important factor in diabetes (Kos and Wilding, 2010). SPARC-null mice are profoundly diabetic. The cross-sectional SPARC levels of women with gestational diabetes mellitus were greater than those of normal controls (Recinella et al., 2020). SPARC might serve as a promising and intriguing protein target for potential therapeutic interventions or as a biomarker to track the progression of diseases (Atorrasagasti et al., 2022). SPARC is found to be highly expressed in adipocytes, as well as in parenchymal and non-parenchymal hepatocytes, and pancreatic cells. SPARC suppresses adipogenesis and promotes insulin resistance. SPARC dramatically exacerbates diabetes in mice fed a high-fat diet (Atorrasagasti et al., 2019).
Regulatory mechanism of SPARC in diabetes
SPARC is reduced in the islets from diabetic donors (Harries et al., 2013). The occurrence of diabetes-related kidney growth was related to the reduction of mRNA and protein of SPARC. In the pathogenesis of diabetes-associated renal growth, SPARC is involved in its pathogenesis (Gilbert et al., 1995). Diabetes-associated mesenteric vascular hypertrophy is relevant to increased SPARC expression in vessel walls (Jandeleit-Dahm et al., 2000). SPARC deficiency inhibits the rise in superoxide production elicited by diabetes stimulation, avoiding the induced hepatocyte damage (Aseer et al., 2017). SPARC downregulates RGS4 protein in pancreatic beta cells to enhance insulin secretion (Hu et al., 2020). MiR-29 directly targets SPARC and adversely modulates glucose metabolism by suppressing SPARC expression. SPARC could be an ideal target for treating diabetes (Song et al., 2018).
Role of SPARC in diabetes
SPARC is significantly associated with inflammation in late pregnancy (Xu et al., 2013). SPARC is highly expressed in obesity and type 2 diabetes mellitus (T2DM), and levels of SPARC in plasma are relatively high in T2DM patients (Wu et al., 2011). For diabetic rats, SPARC is significantly upregulated in the liver and downregulated in the pancreas (Aseer et al., 2015). In the plasma of diabetic patients, the content of SPARC is extremely elevated (Lee et al., 2013). The serum SPARC level in the diabetic nephropathy group was the highest (Li L. et al., 2015). SPARC is highly expressed in the islets of NOD mice and is highest in the islets of young mice. SPARC identifies novel regulators of islet survival and ß-cell growth, a new target for treating diabetes (Ryall et al., 2014). Serum SPARC levels in T2DM patients who have coronary heart disease were elevated (Wang et al., 2015). SPARC expression correlates with fat mass in human adipose tissues (Kos et al., 2009). SPARC can be identified as potential central biomarkers for future drug development in the SD rat model of DMED (Wang Y. et al., 2022). In proliferative diabetic retinopathy (PDR), SPARC is highly expressed and can be a valid therapeutic target for disease treatment (Boneva et al., 2021).
As one of the diseases with the most complications, diabetes continues to plague lives. Functional nanomaterials manipulate SPARC to treat diabetes, which will definitely become a hot topic in the future.
Glaucoma
Glaucoma, a disease affecting the optic neuron, is the second most prevalent cause of irreversible blindness. One of the primary hazardous factors for the development of glaucoma is elevated intraocular pressure (IOP) (Wallace et al., 2014). SPARC has been detected in different ocular tissues, including the cornea, ciliary epithelium, and other tissues.
Regulatory mechanism of SPARC in glaucoma
The influence of SPARC on TGF-β2-mediated ocular hypertension offers a violent disease connection to primary open-angle glaucoma (POAG) pathogenesis. SPARC may be a curative target for certain eye diseases, for instance POAG (Scavelli et al., 2015). In the iris of PACG, SPARC was markedly elevated. By modifying the organization of the ECM to affect the biomechanical properties of the iris, SPARC could have a role in the generation of PACG (Chua et al., 2008). The lack of SPARC reduces intraocular pressure in a glaucoma mouse model (Wallace et al., 2015). The survival of postoperative surgical wounds was significantly improved in a SPARC-null mouse model of chronic glaucoma filtration (Seet et al., 2010). A key regulator of the TGF-β2-mediated ocular hypertension node is SPARC. By limiting the expression of collagen IV and fibronectin, loss of SPARC markedly attenuates the effect of TGF-β2 (Swaminathan et al., 2014). TGF-β2-mediated IOP elevation: SPARC may be a downstream regulator (Kang et al., 2013). Network pharmacology identified SPARC as a gene associated with glaucoma pathogenesis (Moazzeni et al., 2019). SPARC is involved in IOP dysregulation and is a potential therapeutic target (Rhee et al., 2009). The outflow of the aqueous humor across the TM pathway may be impaired by SPARC.
Role of SPARC in glaucoma
SPARC has a major role in aqueous humor outflow across the trabecular meshwork (TM) (Fu et al., 2021). For the treatment of glaucoma, SPARC knockout has emerged as an attractive option (Seet et al., 2012). SPARC was significantly elevated in idiopathic open-angle glaucoma and angle-closure glaucoma. SPARC may be considered a potential target for treating glaucoma (NikhalaShree et al., 2019). SPARC levels in aqueous humor are prognostic factors for the trabeculectomy surgical outcome (Zhang Z. et al., 2018). SPARC remains a prospective index for the treatment of glaucoma (Kang et al., 2011). In comparison with the cataract group, the level of SPARC was evidently higher in the APAC group and was positively correlated with IOP (Wang J. et al., 2018). SPARC is extremely issued in the fetal cornea and TM (Carnes et al., 2018).
SPARC has become a good target for the treatment of a series of ophthalmic diseases, such as glaucoma, and will bring good news to patients in the future. Scarless treatment will be preferred by everyone in the future.
Manipulation of SPARC by functional nanomaterials
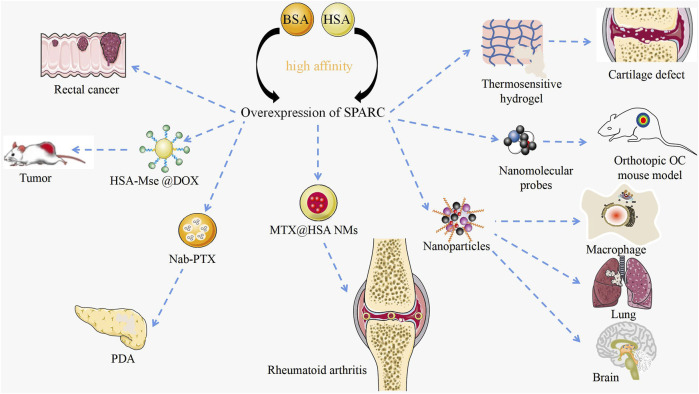
As a potential diagnostic marker and prognostic factor for disease, SPARC has achieved certain therapeutic results in its targeted agents as shown in Figure 6. The manipulation of SPARC by functional nanomaterials plays a role in further targeted treatment of diseases.
FIGURE 6.
Targeted therapy with SPARC as a potential target. Current research on manipulating SPARC to treat diseases with novel nanomaterials. Functional nanomaterials can achieve targeted therapy of tumors by manipulating SPARC.
Manipulation of SPARC by functional nanomaterials in RA and OA
RA and OA are bone degenerative diseases that have been plaguing clinics. Li et al. designed a thermosensitive hydrogel with negative charge targeting fibrocartilage for the continued supply of docetaxel to promote fibrocartilage clearing in a cartilage defect pattern. Fibrocartilage was stabilized by microtubule stabilization. The mechanism of clearing was confirmed to inhibit SPARC (Li J. et al., 2022). In RA and CIA joints, SPARC is highly overexpressed in the synovial membrane and synovial fluid. Given the major appetency between SPARC and HSA, MTX@HSA NMs have become effective potential nanomedicines for the cure of RA. Functional nanomaterial HSA might significantly improve the therapeutic effect and anti-inflammatory activity of MTX (Liu et al., 2019). Probes carrying functional nanomaterials determine the pathogenesis of OA by manipulating SPARC (Ceppi et al., 2019). RA and OA significantly affect the mobility of patients. Manipulating SPARC with functional nanomaterials to treat RA is bound to result in a series of problems.
Manipulation of SPARC by functional nanomaterials in tumors
Dual-targeted therapy for metastatic rectal cancer was carried out by developing lactoferrin-mediated liposomes that bind to the LRP-1 receptor. Lactoferrin modification and endogenous albumin adsorption realize the advantages of functional nanomaterial manipulation of SPARC for rectal cancer (Xu Y. et al., 2022). A bioresponsive micro–nano (MTN) system was used for the effective clearance of Cryptococcus neoformans in vivo. The strategy is built on the excessive expression of matrix metalloproteinase 3 (MMP-3) in the infectious microenvironment (IME) and several associated target cells based on the overexpression of SPARC. Using BSA as a carrier, which is the natural carrier of SPARC, based on the overexpression of SPARC, NPs were deeper targeted to lung tissue, brain, and infected macrophages (Cheng et al., 2021). Albumin-based nanoparticles have been shown to be a useful drug delivery mechanism due to the intrinsic targeting of albumin through SPARC-mediated acceptor endocytosis (Hassanin and Elzoghby, 2020). Fibrotic stroma and tumor-promoting pancreatic stellate cells (PSCs) are key features in the microenvironment of pancreatic ductal adenocarcinoma (PDA). Nab-PTX is an HSA PTX nanoparticle. The enhanced matrix penetration may be due to its small in vivo size and the high affinity of HSA for SPARC (Wei et al., 2017). Based on the large avidity between HSA and SPARC, precise targeting of cancer sites can be achieved and the concentration of PDA drugs can be enhanced. HSA-coated MSe @ DOX forms redox-responsive nanoparticles (HSA-MSe @ DOX) on the surface, enhancing tumor targeting through nanoparticle interactions with SPARC in MCF-7 cells (Zhao et al., 2017). As a source of amino acids and energy for fast-growing cancer cells, SPARC is overexpressed in many tumors and its role is to transport albumin (Lin et al., 2016). Based on the high expression of SPARC in cancer and inflammatory sites, manipulating SPARC with functional nanomaterials to treat cancer will become an efficient and feasible strategy.
Using HSA or BSA as a carrier, the drug can precisely be targeted to the lesion, which avoids not only adverse reactions but also long treatment cycle and multiple biological disadvantages of side effects. The research of new nanomaterial manipulation on SPARC has gradually deepened.
Future perspectives
The emergence of SPARC as a potential target and prognostic indicator is good news for the entire medical community. The investigation and research on the relationship between SPARC and cancer has been very comprehensive, but there are very limited articles on SPARC and RA for reference, which also brings limitations to the development and utilization of SPARC-targeted drug delivery systems. The effective treatment of RA has always been a difficult problem in the medical field, and the discovery of SPARC potential targets provides a bright spot for our future research. Summarizing the SPARC therapeutic mechanism and potential targets will provide impetus for the targeted drug delivery system controlled by functional nanomaterials or good prognosis of diseases.
Summary
As an albumin-binding protein, SPARC was originally derived from human and fetal bovine bone and has high affinity with HSA and BSA. Multiple studies have found that SPARC is highly expressed in RA joint inflammation sites, various cancers, glaucoma, obese patients, diabetic patients, and melanoma patients as shown in Table 1. With the rapid development of the nano industry, targeted drug delivery systems are gradually emerging. Functional nanomaterials manipulate SPARC to produce anti-inflammatory therapeutic effects. RA and cancer are diseases that have plagued human beings for a long time. When alleviating the adverse reactions of patients, it is very critical to find a precise target for the effective treatment of cancer and RA. As a potential target for disease treatment, SPARC mostly uses HSA and BSA as carriers. When utilizing the affinity of the two, it can also efficiently and accurately load the drug for disease treatment into target cells. At present, based on several nanoparticles for the treatment of RA and tumors, for the treatment of RA, SPARC as a prospective target not only has an anti-inflammatory effect in the joint synovium but also realizes the precise targeting of drugs, achieving the effect of killing two birds with one stone.
TABLE 1.
Summary of SPARC as a potential target and prognostic indicator.
| Disease name | Mechanism | Reference |
|---|---|---|
| Rheumatoid arthritis | Negative regulation of NF-κB and TGF-β pathways | Mechanism of SPARC and SPARCL-1 regulating autophagy in glutamate-induced hippocampal neuron injury (xxx); Cheng et al. (2021); Critselis et al. (2019) |
| Gastric cancer | SPARC expression can promote gastric cancer cell invasion, metastasis, and angiogenesis | Haber et al. (2008); Hung et al. (2017) |
| Hepatocellular carcinoma | Overexpression of SPARC in HCC cells leads to reduced tumorigenicity in part by inducing MET | Ikuta et al. (2005) |
| Breast cancer | SPARC curbs the mobility and invasion of breast cancer, while resisting the platelet deficiency caused by SPARC | Lin et al. (2016); Lopez et al. (2006) |
| Non-small-cell lung cancer | By stimulating microvascular remodeling and excessive deposition of ECM proteins, SPARC induces pathological changes in non-small-cell lung cancer and idiopathic pulmonary fibrosis | Ng et al. (2013); NikhalaShree et al. (2019) |
| Melanoma | SPARC promotes cathepsin B-mediated melanoma invasion using collagen I and α2β1 integrin as mediators. SPARC helps in cell growth | Piñero et al. (2020); Said et al. (2007c) |
| Esophageal cancer | Downregulation of SPARC can reduce cell migration and invasion involved in EMT through the p-FAK/p-ERK pathway | Swaminathan et al. (2014); Termine et al. (1981) |
| Ovarian cancer | SPARC inhibits the NF-κB pathway mediating the macrophage-induced invasion of ovarian cancer cells | Wan et al. (2015); Wang et al. (2012) |
| Rectal cancer | Cox regression analysis identified the central genes of prognosis, and SPARC was the central gene | Wang et al. (2022a) |
| Bladder cancer | SPARC expression in human bladder cancer cell family negatively correlates with its proliferation rate and inhibits cell cycle progression by decelerating down the G1/S cell cycle | Wei et. (2017) |
| Head and neck cancer | SPARC strengthens cell proliferation, migration, and mesenchymal phenotype | Wu et al. (2017) |
| Diabetes | SPARC is expressed in adipocytes, parenchymal and non-parenchymal hepatocytes, and pancreatic cells. SPARC suppresses adipogenesis and promotes insulin resistance | Xu et al. (2022b); Xue et al. (2011); Ye (2017) |
| Glaucoma | The influence of SPARC on TGF-β2-mediated ocular hypertension gives a unique insight into the pathogenesis of glaucoma | Zhang et al. (2021); Zhao et al. (2022) |
Funding Statement
This research was financially supported by the National Natural Science Foundation of China (project nos. 82274178 and 81803811), the 2020 Hunan Province Science and Technology Innovation Key Projects (project no. 2020SK1020), the open project of the State Key Laboratory of Quality Research in Chinese Medicine (Macau University of Science and Technology, MUST-SKL-2021-012) funded by the Macao Science and Technology Development Fund, Macau Special Administrative Region, the Wuxi Young Talent Program of Huaihua City, and the Hunan University of Medicine high-level talent introduction startup funds.
Author contributions
J-XL and J-SC conceptualized the idea and designed the experiment; SJ and H-FS collected and analyzed the literature; SJ and SL prepared the manuscript; J-XL and NZ revised the manuscript; and J-XL, J-SC, and H-FS reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| SIN | Sinomenine |
| SPARC | Secreted protein acidic and rich in cysteine |
| NF-κB | Nuclear factor-κB |
| HSA | Human serum albumin |
| BSA | Bovine serum albumin |
| FLS | Fibroblast-like synovial cells |
| VEGF | Vascular endothelial growth factor |
| MMPs | Matrix metalloproteinases |
| FS | Follistatin-like |
| EC | Extracellular |
| ISG | Interferon-stimulated gene |
| RA | Rheumatoid arthritis |
| IHC | Immunohistochemistry |
| ELISA | Enzyme-linked immunosorbent assay |
| OA | Osteoarthritis |
| TGF-β | Transforming growth factor-β |
| ECM | Extracellular matrix |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| GC | Gastric cancer |
| DEGs | Differentially expressed genes |
| PPI | Protein–protein interaction |
| GDM | Gestational diabetes mellitus |
| ADR | Doxorubicin |
| 5-FU | 5-Fluorouracil |
| DEGs | Differentially expressed genes |
| TME | Tumor microenvironment |
| PPI | Protein–protein interaction |
| GEO | Gene Expression Omnibus |
| MET | Mesenchymal–epithelial transition |
| ROS | Reactive oxygen species |
| LDH | Lactate dehydrogenase |
| NAFLD | Non-alcoholic fatty liver disease |
| AMO | Anti-miR-29a oligonucleotide |
| NCRT | Neoadjuvant chemoradiotherapy |
| SNPs | Single-nucleotide polymorphisms |
| HSCs | Hepatic stellate cells |
| TNBC | Triple-negative breast cancer |
| IRS | Immune response scoring |
| MDSCs | Myeloid-derived suppressor cells |
| CTGF | Connective tissue growth factor |
| PT | Phyllodes tumors |
| ISH | In situ hybridization |
| NSCLC | Non-small-cell lung cancer |
| ET-3 | Endothelin-3 |
| EMM | Epidermal melanoma metastases |
| CSM | Common skin metastasis |
| ESCC | Esophageal squamous cell carcinoma |
| FFPE | Formalin-fixed paraffin-embedded |
| BE | Barrett’s esophagus |
| siRNA | Small interfering RNA |
| OvCa | Ovarian cancer |
| LARC | Locally advanced rectal cancer |
| T2DM | Type 2 diabetes mellitus |
| NOD | Non-obese diabetic |
| SAAT | Subcutaneous abdominal adipose tissue |
| DMED | Diabetic erectile dysfunction |
| TM | Trabecular meshwork |
References
- Agostina M. O., Fiore E., Bayo J., Casali C., Fernandez-Tomé M., Rodríguez M., et al. (2021). SPARC inhibition accelerates NAFLD-associated hepatocellular carcinoma development by dysregulating hepatic lipid metabolism. Liver Int. 41 (7), 1677–1693. 10.1111/liv.14857 [DOI] [PubMed] [Google Scholar]
- Ainsworth R. I., Hammaker D., Nygaard G., Ansalone C., Machado C., Zhang K., et al. (2022). Systems-biology analysis of rheumatoid arthritis fibroblast-like synoviocytes implicates cell line-specific transcription factor function. Nat. Commun. 13 (1), 6221. 10.1038/s41467-022-33785-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz L. B., Mallavialle A., David T., Derocq D., Delolme F., Dieryckx C., et al. (2021). A 9-kDa matricellular SPARC fragment released by cathepsin D exhibits pro-tumor activity in the triple-negative breast cancer microenvironment. Theranostics 11 (13), 6173–6192. 10.7150/thno.58254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz L. B., Mallavialle A., Mollevi C., Boissière-Michot F., Mansouri H., Simony-Lafontaine J., et al. (2022). SPARC in cancer-associated fibroblasts is an independent poor prognostic factor in non-metastatic triple-negative breast cancer and exhibits pro-tumor activity. Int. J. Cancer 152, 1243–1258. 10.1002/ijc.34345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M. J., Prada F., Salvatierra E., Bravo A. I., Lutzky V. P., Carbone C., et al. (2005). Secreted protein acidic and rich in cysteine produced by human melanoma cells modulates polymorphonuclear leukocyte recruitment and antitumor cytotoxic capacity. Cancer Res. 65 (12), 5123–5132. 10.1158/0008-5472.Can-04-1102 [DOI] [PubMed] [Google Scholar]
- An X. J., Li Y. Q., Qu X. Y., Zhang J., Zhang L. Y., Wang M., et al. (2013). Silencing endothelin-3 expression attenuates the malignant behaviors of human melanoma cells by regulating SPARC levels. J. Huazhong Univ. Sci. Technol. Med. Sci. 33 (4), 581–586. 10.1007/s11596-013-1162-3 [DOI] [PubMed] [Google Scholar]
- Andriani F., Landoni E., Mensah M., Facchinetti F., Miceli R., Tagliabue E., et al. (2018). Diagnostic role of circulating extracellular matrix-related proteins in non-small cell lung cancer. BMC Cancer 18 (1), 899. 10.1186/s12885-018-4772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang C., Miura J. T., Gamblin T. C., He R., Xiu J., Millis S. Z., et al. (2016). Comprehensive multiplatform biomarker analysis of 350 hepatocellular carcinomas identifies potential novel therapeutic options. J. Surg. Oncol. 113 (1), 55–61. 10.1002/jso.24086 [DOI] [PubMed] [Google Scholar]
- Aseer K. R., Kim S. W., Choi M. S., Yun J. W. (2015). Opposite expression of SPARC between the liver and pancreas in streptozotocin-induced diabetic rats. PLoS One 10 (6), e0131189. 10.1371/journal.pone.0131189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseer K. R., Silvester A. J., Kumar A., Choi M. S., Yun J. W. (2017). SPARC paucity alleviates superoxide-mediated oxidative stress, apoptosis, and autophagy in diabetogenic hepatocytes. Free Radic. Biol. Med. 108, 874–895. 10.1016/j.freeradbiomed.2017.05.011 [DOI] [PubMed] [Google Scholar]
- Atorrasagasti C., Malvicini M., Aquino J. B., Alaniz L., Garcia M., Bolontrade M., et al. (2010). Overexpression of SPARC obliterates the in vivo tumorigenicity of human hepatocellular carcinoma cells. Int. J. Cancer 126 (11), 2726–2740. 10.1002/ijc.24966 [DOI] [PubMed] [Google Scholar]
- Atorrasagasti C., Onorato A., Gimeno M. L., Andreone L., Garcia M., Malvicini M., et al. (2019). SPARC is required for the maintenance of glucose homeostasis and insulin secretion in mice. Clin. Sci. (Lond) 133 (2), 351–365. 10.1042/cs20180714 [DOI] [PubMed] [Google Scholar]
- Atorrasagasti C., Onorato A. M., Mazzolini G. (2022). The role of SPARC (secreted protein acidic and rich in cysteine) in the pathogenesis of obesity, type 2 diabetes, and non-alcoholic fatty liver disease. J. Physiol. Biochem. 10.1007/s13105-022-00913-5 [DOI] [PubMed] [Google Scholar]
- Azim H. A., Jr., Singhal S., Ignatiadis M., Desmedt C., Fumagalli D., Veys I., et al. (2013). Association between SPARC mRNA expression, prognosis and response to neoadjuvant chemotherapy in early breast cancer: A pooled in-silico analysis. PLoS One 8 (4), e62451. 10.1371/journal.pone.0062451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. J., Moll R., Ramaswamy A. (2005). Stromal remodeling and SPARC (secreted protein acid rich in cysteine) expression in invasive ductal carcinomas of the breast. Virchows Arch. 446 (5), 532–536. 10.1007/s00428-005-1256-9 [DOI] [PubMed] [Google Scholar]
- Bawazeer S., Sabry D., Mahmoud R. H., Elhanbuli H. M., Yassen N. N., Abdelhafez M. N. (2018). Association of SPARC gene polymorphisms rs3210714 and rs7719521 with VEGF expression and utility of Nottingham Prognostic Index scoring in breast cancer in a sample of Egyptian women. Mol. Biol. Rep. 45 (6), 2313–2324. 10.1007/s11033-018-4394-2 [DOI] [PubMed] [Google Scholar]
- Beck A. H., Espinosa I., Gilks C. B., van de Rijn M., West R. B. (2008). The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab. Invest. 88 (6), 591–601. 10.1038/labinvest.2008.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellenghi M., Talarico G., Botti L., Puglisi R., Tabolacci C., Portararo P., et al. (2022). SCD5-dependent inhibition of SPARC secretion hampers metastatic spreading and favors host immunity in a TNBC murine model. Oncogene 41 (34), 4055–4065. 10.1038/s41388-022-02401-y [DOI] [PubMed] [Google Scholar]
- Boneva S. K., Wolf J., Hajdú R. I., Prinz G., Salié H., Schlecht A., et al. (2021). In-depth molecular characterization of neovascular membranes suggests a role for hyalocyte-to-myofibroblast transdifferentiation in proliferative diabetic retinopathy. Front. Immunol. 12, 757607. 10.3389/fimmu.2021.757607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho N. K., Schneiders F. I., Lord S. J., Freeman A. K., Tyagi S., Nancarrow D. J., et al. (2010). Gene expression alterations in formalin-fixed, paraffin-embedded Barrett esophagus and esophageal adenocarcinoma tissues. Cancer Biol. Ther. 10 (2), 172–179. 10.4161/cbt.10.2.12166 [DOI] [PubMed] [Google Scholar]
- Brabender J., Marjoram P., Lord R. V., Metzger R., Salonga D., Vallböhmer D., et al. (2005). The molecular signature of normal squamous esophageal epithelium identifies the presence of a field effect and can discriminate between patients with Barrett's esophagus and patients with Barrett's-associated adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 14 (9), 2113–2117. 10.1158/1055-9965.Epi-05-0014 [DOI] [PubMed] [Google Scholar]
- Bradshaw A. D. (2012). Diverse biological functions of the SPARC family of proteins. Int. J. Biochem. Cell Biol. 44 (3), 480–488. 10.1016/j.biocel.2011.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. J., Shaw P. A., Karp X., Huynh M. H., Begley H., Ringuette M. J. (1999). Activation of SPARC expression in reactive stroma associated with human epithelial ovarian cancer. Gynecol. Oncol. 75 (1), 25–33. 10.1006/gyno.1999.5552 [DOI] [PubMed] [Google Scholar]
- Camacho D., Jesus J. P., Palma A. M., Martins S. A., Afonso A., Peixoto M. L., et al. (2020). SPARC-p53: The double agents of cancer. Adv. Cancer Res. 148, 171–199. 10.1016/bs.acr.2020.05.004 [DOI] [PubMed] [Google Scholar]
- Carnes M. U., Allingham R. R., Ashley-Koch A., Hauser M. A. (2018). Transcriptome analysis of adult and fetal trabecular meshwork, cornea, and ciliary body tissues by RNA sequencing. Exp. Eye Res. 167, 91–99. 10.1016/j.exer.2016.11.021 [DOI] [PubMed] [Google Scholar]
- Ceppi L., Bardhan N. M., Na Y., Siegel A., Rajan N., Fruscio R., et al. (2019). Real-time single-walled carbon nanotube-based fluorescence imaging improves survival after debulking surgery in an ovarian cancer model. ACS Nano 13 (5), 5356–5365. 10.1021/acsnano.8b09829 [DOI] [PubMed] [Google Scholar]
- Cerezo M., Tichet M., Abbe P., Ohanna M., Lehraiki A., Rouaud F., et al. (2013). Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol. Cancer Ther. 12 (8), 1605–1615. 10.1158/1535-7163.Mct-12-1226-t [DOI] [PubMed] [Google Scholar]
- Chakraborty A. K., Funasaka Y., Ichihashi M., Pawelek J. M. (2009). Upregulation of alpha and beta integrin subunits in metastatic macrophage-melanoma fusion hybrids. Melanoma Res. 19 (6), 343–349. 10.1097/CMR.0b013e32832fe121 [DOI] [PubMed] [Google Scholar]
- Chang C. H., Yen M. C., Liao S. H., Hsu Y. L., Lai C. S., Chang K. P., et al. (2017). Secreted protein acidic and rich in cysteine (SPARC) enhances cell proliferation, migration, and epithelial mesenchymal transition, and SPARC expression is associated with tumor grade in head and neck cancer. Int. J. Mol. Sci. 18 (7), 1556. 10.3390/ijms18071556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles J., Britt H., Pan Y. (2013). Rheumatoid arthritis. Aust. Fam. Physician 42 (11), 765. [PubMed] [Google Scholar]
- Che Y., Luo A., Wang H., Qi J., Guo J., Liu Z. (2006). The differential expression of SPARC in esophageal squamous cell carcinoma. Int. J. Mol. Med. 17 (6), 1027–1033. 10.3892/ijmm.17.6.1027 [DOI] [PubMed] [Google Scholar]
- Chen J., Wang M., Xi B., Xue J., He D., Zhang J., et al. (2012). SPARC is a key regulator of proliferation, apoptosis and invasion in human ovarian cancer. PLoS One 7 (8), e42413. 10.1371/journal.pone.0042413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Z., He C. Y., Su X., Peng J. L., Chen D. L., Ye Z., et al. (2018). SPP1 rs4754 and its epistatic interactions with SPARC polymorphisms in gastric cancer susceptibility. Gene 640, 43–50. 10.1016/j.gene.2017.09.053 [DOI] [PubMed] [Google Scholar]
- Chen W. Y., Wu F., You Z. Y., Zhang Z. M., Guo Y. L., Zhong L. X. (2015). Analyzing the differentially expressed genes and pathway cross-talk in aggressive breast cancer. J. Obstet. Gynaecol. Res. 41 (1), 132–140. 10.1111/jog.12495 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang Y., Tan Y., Liu Z. (2017). Clinical significance of SPARC in esophageal squamous cell carcinoma. Biochem. Biophys. Res. Commun. 492 (2), 184–191. 10.1016/j.bbrc.2017.08.043 [DOI] [PubMed] [Google Scholar]
- Cheng L., Chen J., Rong X. (2022). Mechanism of emodin in the treatment of rheumatoid arthritis. Evid. Based Complement. Altern. Med. 2022, 9482570. 10.1155/2022/9482570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Niu M. M., Yan T., Ma Z., Huang K., Yang L., et al. (2021). Bioresponsive micro-to-nano albumin-based systems for targeted drug delivery against complex fungal infections. Acta Pharm. Sin. B 11 (10), 3220–3230. 10.1016/j.apsb.2021.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodoni C., Colombo M. P., Sangaletti S. (2010). Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 29 (2), 295–307. 10.1007/s10555-010-9221-8 [DOI] [PubMed] [Google Scholar]
- Chua J., Seet L. F., Jiang Y., Su R., Htoon H. M., Charlton A., et al. (2008). Increased SPARC expression in primary angle closure glaucoma iris. Mol. Vis. 14, 1886–1892. [PMC free article] [PubMed] [Google Scholar]
- Conforti F., Ridley R., Brereton C., Alzetani A., Johnson B., Marshall B. G., et al. (2020). Paracrine SPARC signaling dysregulates alveolar epithelial barrier integrity and function in lung fibrosis. Cell Death Discov. 6, 54. 10.1038/s41420-020-0289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critselis E., Rava M., Marquez M., Lygirou V., Chatzicharalambous D., Liapi P., et al. (2019). Diagnostic and prognostic performance of secreted protein acidic and rich in cysteine (SPARC) assay for detecting primary and recurrent urinary bladder cancer. Proteomics Clin. Appl. 13 (2), e1800148. 10.1002/prca.201800148 [DOI] [PubMed] [Google Scholar]
- Defresne F., Bouzin C., Grandjean M., Dieu M., Raes M., Hatzopoulos A. K., et al. (2011). Preconditioned endothelial progenitor cells reduce formation of melanoma metastases through SPARC-driven cell-cell interactions and endocytosis. Cancer Res. 71 (14), 4748–4757. 10.1158/0008-5472.Can-10-2449 [DOI] [PubMed] [Google Scholar]
- Deng B., Qu L., Li J., Fang J., Yang S., Cao Z., et al. (2016). MiRNA-211 suppresses cell proliferation, migration and invasion by targeting SPARC in human hepatocellular carcinoma. Sci. Rep. 6, 26679. 10.1038/srep26679 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Deng l. l. (2018). Expression of SPARC in MDS and its effect on bone marrow stromal cells. master. Chongqing, China: Chongqing Medical University. [Google Scholar]
- Dhanesuan N., Sharp J. A., Blick T., Price J. T., Thompson E. W. (2002). Doxycycline-inducible expression of SPARC/Osteonectin/BM40 in MDA-MB-231 human breast cancer cells results in growth inhibition. Breast Cancer Res. Treat. 75 (1), 73–85. 10.1023/a:1016536725958 [DOI] [PubMed] [Google Scholar]
- Dobruch J., Oszczudłowski M. (2021). Bladder cancer: Current challenges and future directions. Med. Kaunas. 57 (8), 749. 10.3390/medicina57080749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domper Arnal M. J., Ferrández Arenas Á., Lanas Arbeloa Á. (2015). Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 21 (26), 7933–7943. 10.3748/wjg.v21.i26.7933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio F. P., Sparaneo A., Fontana A., Mazza T., Graziano P., Pantalone A., et al. (2020). Potential prognostic role of SPARC methylation in non-small-cell lung cancer. Cells 9 (6), 1523. 10.3390/cells9061523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R., Olsen N., Keski-Oja J., Moses H., Pincus T. (1989). Active and latent forms of transforming growth factor beta activity in synovial effusions. J. Exp. Med. 169 (1), 291–296. 10.1084/jem.169.1.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Tang L. (2014). SPARC in tumor pathophysiology and as a potential therapeutic target. Curr. Pharm. Des. 20 (39), 6182–6190. 10.2174/1381612820666140619123255 [DOI] [PubMed] [Google Scholar]
- Fenouille N., Puissant A., Tichet M., Zimniak G., Abbe P., Mallavialle A., et al. (2011). SPARC functions as an anti-stress factor by inactivating p53 through Akt-mediated MDM2 phosphorylation to promote melanoma cell survival. Oncogene 30 (49), 4887–4900. 10.1038/onc.2011.198 [DOI] [PubMed] [Google Scholar]
- Fenouille N., Tichet M., Dufies M., Pottier A., Mogha A., Soo J. K., et al. (2012). The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS One 7 (7), e40378. 10.1371/journal.pone.0040378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S. (2004). NF-kappaB: Holy Grail for rheumatoid arthritis? Arthritis Rheum. 50 (8), 2381–2386. 10.1002/art.20468 [DOI] [PubMed] [Google Scholar]
- Fu Y., Luo L., Fan Y., Tang M. (2021). Downregulation of secreted protein acidic and rich in cysteine in human trabecular meshwork cells. Exp. Ther. Med. 22 (4), 1126. 10.3892/etm.2021.10560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. Y., Han R. B., Wang X., Ge S. H., Li H. L., Deng T., et al. (2015). Change of SPARC expression after chemotherapy in gastric cancer. Cancer Biol. Med. 12 (1), 33–40. 10.7497/j.issn.2095-3941.2014.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Yin S. P., Xie X. S., Xu D. D., Du W. D. (2017). The relationship between stromal cell derived SPARC in human gastric cancer tissue and its clinicopathologic significance. Oncotarget 8 (49), 86240–86252. 10.18632/oncotarget.21133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z. W., Liu C., Yang L., He T., Wu X. N., Zhang H. Z., et al. (2021). SPARC overexpression promotes liver cancer cell proliferation and tumor growth. Front. Mol. Biosci. 8, 775743. 10.3389/fmolb.2021.775743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido M. C., Requena L., Kutzner H., Ortiz P., Pérez-Gómez B., Rodriguez-Peralto J. L. (2014). Desmoplastic melanoma: Expression of epithelial-mesenchymal transition-related proteins. Am. J. Dermatopathol. 36 (3), 238–242. 10.1097/DAD.0b013e3182987441 [DOI] [PubMed] [Google Scholar]
- Gaudet P., Livstone M. S., Lewis S. E., Thomas P. D. (2011). Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform 12 (5), 449–462. 10.1093/bib/bbr042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachi A., Cugno M., Gualtierotti R. (2022). Disease-modifying anti-rheumatic drugs improve the cardiovascular profile in patients with rheumatoid arthritis. Front. Cardiovasc Med. 9, 1012661. 10.3389/fcvm.2022.1012661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R. E., McNally P. G., Cox A., Dziadek M., Rumble J., Cooper M. E., et al. (1995). SPARC gene expression is reduced in early diabetes-related kidney growth. Kidney Int. 48 (4), 1216–1225. 10.1038/ki.1995.405 [DOI] [PubMed] [Google Scholar]
- Gilles C., Bassuk J. A., Pulyaeva H., Sage E. H., Foidart J. M., Thompson E. W. (1998). SPARC/osteonectin induces matrix metalloproteinase 2 activation in human breast cancer cell lines. Cancer Res. 58 (23), 5529–5536. [PubMed] [Google Scholar]
- Girotti M. R., Fernández M., López J. A., Camafeita E., Fernández E. A., Albar J. P., et al. (2011). SPARC promotes cathepsin B-mediated melanoma invasiveness through a collagen I/α2β1 integrin axis. J. Invest. Dermatol 131 (12), 2438–2447. 10.1038/jid.2011.239 [DOI] [PubMed] [Google Scholar]
- Goyal L., Zheng H., Abrams T. A., Miksad R., Bullock A. J., Allen J. N., et al. (2019). A phase II and biomarker study of sorafenib combined with modified FOLFOX in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 25 (1), 80–89. 10.1158/1078-0432.Ccr-18-0847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. L., Fishbein M. C., Hong L. S., Krysan K., Minna J. D., Shay J. W., et al. (2014). A novel molecular pathway for Snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis. Cancer Prev. Res. (Phila) 7 (1), 150–160. 10.1158/1940-6207.Capr-13-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Zhang M., Chen Y., Guo S. (2017). The clinical significance of secreted protein acidic and rich in cysteine expression in breast cancer tissue and its association with prognosis. J. Cancer Res. Ther. 13 (5), 833–836. 10.4103/jcrt.JCRT_424_17 [DOI] [PubMed] [Google Scholar]
- Güttlein L. N., Benedetti L. G., Fresno C., Spallanzani R. G., Mansilla S. F., Rotondaro C., et al. (2017). Predictive outcomes for HER2-enriched cancer using growth and metastasis signatures driven by SPARC. Mol. Cancer Res. 15 (3), 304–316. 10.1158/1541-7786.Mcr-16-0243-t [DOI] [PubMed] [Google Scholar]
- Haber C. L., Gottifredi V., Llera A. S., Salvatierra E., Prada F., Alonso L., et al. (2008). SPARC modulates the proliferation of stromal but not melanoma cells unless endogenous SPARC expression is downregulated. Int. J. Cancer 122 (7), 1465–1475. 10.1002/ijc.23216 [DOI] [PubMed] [Google Scholar]
- Hao S., Lv J., Yang Q., Wang A., Li Z., Guo Y., et al. (2019). Identification of key genes and circular RNAs in human gastric cancer. Med. Sci. Monit. 25, 2488–2504. 10.12659/msm.915382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries L. W., McCulloch L. J., Holley J. E., Rawling T. J., Welters H. J., Kos K. (2013). A role for SPARC in the moderation of human insulin secretion. PLoS One 8 (6), e68253. 10.1371/journal.pone.0068253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanin I., Elzoghby A. (2020). Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist 3 (4), 930–946. 10.20517/cdr.2020.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellinger J. W., Schömel F., Buse J. V., Lenz C., Bauerschmitz G., Emons G., et al. (2020). Identification of drivers of breast cancer invasion by secretome analysis: Insight into CTGF signaling. Sci. Rep. 10 (1), 17889. 10.1038/s41598-020-74838-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K., Tsuchihara M., Nakatsura T. (2010). Silencing of secreted protein acidic and rich in cysteine inhibits the growth of human melanoma cells with G arrest induction. Cancer Sci. 101 (4), 913–919. 10.1111/j.1349-7006.2009.01476.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y. H., Lien H. C., Hwa H. L., Kuo W. H., Chang K. J., Hsieh F. J. (2010). SPARC (osteonectin) in breast tumors of different histologic types and its role in the outcome of invasive ductal carcinoma. Breast J. 16 (3), 305–308. 10.1111/j.1524-4741.2009.00899.x [DOI] [PubMed] [Google Scholar]
- Hu L., He F., Huang M., Zhao Q., Cheng L., Said N., et al. (2020). SPARC promotes insulin secretion through down-regulation of RGS4 protein in pancreatic β cells. Sci. Rep. 10 (1), 17581. 10.1038/s41598-020-74593-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H. W., Jiang F., Huang Q., Liao Z. J., Ding G. (2015). Re-sensitization of 5-FU resistance by SPARC through negative regulation of glucose metabolism in hepatocellular carcinoma. Tumour Biol. 36 (1), 303–313. 10.1007/s13277-014-2633-2 [DOI] [PubMed] [Google Scholar]
- Hua H. W., Jiang H. S., Jia L., Jia Y. P., Yao Y. L., Chen Y. W., et al. (2021). SPARC regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma. Cancer Biomark. 32 (4), 425–433. 10.3233/cbm-200101 [DOI] [PubMed] [Google Scholar]
- Huang Y., Zhang J., Zhao Y. Y., Jiang W., Xue C., Xu F., et al. (2012). SPARC expression and prognostic value in non-small cell lung cancer. Chin. J. Cancer 31 (11), 541–548. 10.5732/cjc.012.10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J. Y., Yen M. C., Jian S. F., Wu C. Y., Chang W. A., Liu K. T., et al. (2017). Secreted protein acidic and rich in cysteine (SPARC) induces cell migration and epithelial mesenchymal transition through WNK1/snail in non-small cell lung cancer. Oncotarget 8 (38), 63691–63702. 10.18632/oncotarget.19475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta Y., Nakatsura T., Kageshita T., Fukushima S., Ito S., Wakamatsu K., et al. (2005). Highly sensitive detection of melanoma at an early stage based on the increased serum secreted protein acidic and rich in cysteine and glypican-3 levels. Clin. Cancer Res. 11 (22), 8079–8088. 10.1158/1078-0432.Ccr-05-1074 [DOI] [PubMed] [Google Scholar]
- Ito M., Ito G., Kondo M., Uchiyama M., Fukui T., Mori S., et al. (2005). Frequent inactivation of RASSF1A, BLU, and SEMA3B on 3p21.3 by promoter hypermethylation and allele loss in non-small cell lung cancer. Cancer Lett. 225 (1), 131–139. 10.1016/j.canlet.2004.10.041 [DOI] [PubMed] [Google Scholar]
- Jandeleit-Dahm K., Rumble J., Cox A. J., Kelly D. J., Dziadek M., Cooper M. E., et al. (2000). SPARC gene expression is increased in diabetes-related mesenteric vascular hypertrophy. Microvasc. Res. 59 (1), 61–71. 10.1006/mvre.1999.2189 [DOI] [PubMed] [Google Scholar]
- Jiang X., Liu F., Wang Y., Gao J. (2019). Secreted protein acidic and rich in cysteine promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells and acquisition of cancerstem cell phenotypes. J. Gastroenterol. Hepatol. 34 (10), 1860–1868. 10.1111/jgh.14692 [DOI] [PubMed] [Google Scholar]
- John B., Naczki C., Patel C., Ghoneum A., Qasem S., Salih Z., et al. (2019). Regulation of the bi-directional cross-talk between ovarian cancer cells and adipocytes by SPARC. Oncogene 38 (22), 4366–4383. 10.1038/s41388-019-0728-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. E., Sharick J. T., Colbert S. E., Shukla V. C., Zent J. M., Ostrowski M. C., et al. (2021). Pten regulates collagen fibrillogenesis by fibroblasts through SPARC. PLoS One 16 (2), e0245653. 10.1371/journal.pone.0245653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. H., Oh D. J., Kang J. H., Rhee D. J. (2013). Regulation of SPARC by transforming growth factor β2 in human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 54 (4), 2523–2532. 10.1167/iovs.12-11474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. H., Oh D. J., Rhee D. J. (2011). Effect of hevin deletion in mice and characterization in trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 52 (5), 2187–2193. 10.1167/iovs.10-5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaochar S., Dong J., Torres M., Rajapakshe K., Nikolos F., Davis C. M., et al. (2018). ICG-001 exerts potent anticancer activity against uveal melanoma cells. Invest. Ophthalmol. Vis. Sci. 59 (1), 132–143. 10.1167/iovs.17-22454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Frankenne F., Noël A., Sakai N., Nagashima Y., Koshika S., et al. (2000). High production of SPARC/osteonectin/BM-40 in mouse metastatic B16 melanoma cell lines. Pathol. Oncol. Res. 6 (1), 24–26. 10.1007/bf03032654 [DOI] [PubMed] [Google Scholar]
- Kehlet S. N., Manon-Jensen T., Sun S., Brix S., Leeming D. J., Karsdal M. A., et al. (2018). A fragment of SPARC reflecting increased collagen affinity shows pathological relevance in lung cancer - implications of a new collagen chaperone function of SPARC. Cancer Biol. Ther. 19 (10), 904–912. 10.1080/15384047.2018.1480887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. I., Kim G. E., Lee J. S., Park M. H. (2017). In phyllodes tumors of the breast expression of SPARC (osteonectin/BM40) mRNA by in situ hybridization correlates with protein expression by immunohistochemistry and is associated with tumor progression. Virchows Arch. 470 (1), 91–98. 10.1007/s00428-016-2048-0 [DOI] [PubMed] [Google Scholar]
- Koblinski J. E., Kaplan-Singer B. R., VanOsdol S. J., Wu M., Engbring J. A., Wang S., et al. (2005). Endogenous osteonectin/SPARC/BM-40 expression inhibits MDA-MB-231 breast cancer cell metastasis. Cancer Res. 65 (16), 7370–7377. 10.1158/0008-5472.Can-05-0807 [DOI] [PubMed] [Google Scholar]
- Kos K., Wilding J. P. (2010). Sparc: A key player in the pathologies associated with obesity and diabetes. Nat. Rev. Endocrinol. 6 (4), 225–235. 10.1038/nrendo.2010.18 [DOI] [PubMed] [Google Scholar]
- Kos K., Wong S., Tan B., Gummesson A., Jernas M., Franck N., et al. (2009). Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose. Diabetes 58 (8), 1780–1788. 10.2337/db09-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis M. I., Giatromanolaki A., Brekken R. A., Sivridis E., Gatter K. C., Harris A. L., et al. (2003). Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 63 (17), 5376–5380. 10.1097/00130404-200309000-00013 [DOI] [PubMed] [Google Scholar]
- Kurtul N., Taşdemir E. A., Ünal D., İzmirli M., Eroglu C. (2017). Sparc: As a prognostic biomarker in rectal cancer patients treated with chemo-radiotherapy. Cancer Biomark. 18 (4), 459–466. 10.3233/cbm-161733 [DOI] [PubMed] [Google Scholar]
- Lane T. F., Iruela-Arispe M. L., Johnson R. S., Sage E. H. (1994). SPARC is a source of copper-binding peptides that stimulate angiogenesis. J. Cell Biol. 125 (4), 929–943. 10.1083/jcb.125.4.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J., Yasmin T., Sens D. A., Zhou X. D., Sens M. A., Garrett S. H., et al. (2010). SPARC gene expression is repressed in human urothelial cells (UROtsa) exposed to or malignantly transformed by cadmium or arsenite. Toxicol. Lett. 199 (2), 166–172. 10.1016/j.toxlet.2010.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C. P., Poon R. T., Cheung S. T., Yu W. C., Fan S. T. (2006). SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma. J. Pathol. 210 (4), 459–468. 10.1002/path.2068 [DOI] [PubMed] [Google Scholar]
- Le Bail B., Faouzi S., Boussarie L., Guirouilh J., Blanc J. F., Carles J., et al. (1999). Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J. Pathol. 189 (1), 46–52. [DOI] [PubMed] [Google Scholar]
- Ledda F., Bravo A. I., Adris S., Bover L., Mordoh J., Podhajcer O. L. (1997). The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J. Invest. Dermatol 108 (2), 210–214. 10.1111/1523-1747.ep12334263 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Lee J. A., Park H. S., Song Y. S., Jang Y. J., Kim J. H., et al. (2013). Associations among SPARC mRNA expression in adipose tissue, serum SPARC concentration and metabolic parameters in Korean women. Obes. (Silver Spring) 21 (11), 2296–2302. 10.1002/oby.20183 [DOI] [PubMed] [Google Scholar]
- Li A., Qu X., Li Z., Qu J., Song N., Ma Y., et al. (2015a). Secreted protein acidic and rich in cysteine antagonizes bufalin-induced apoptosis in gastric cancer cells. Mol. Med. Rep. 12 (2), 2926–2932. 10.3892/mmr.2015.3676 [DOI] [PubMed] [Google Scholar]
- Li J., Jiang H., Lv Z., Sun Z., Cheng C., Tan G., et al. (2022a). Articular fibrocartilage-targeted therapy by microtubule stabilization. Sci. Adv. 8 (46), eabn8420. 10.1126/sciadv.abn8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Song H. Y., Liu K., An M. M. (2015b). Correlation of secreted protein acidic and rich in cysteine with diabetic nephropathy. Int. J. Clin. Exp. Med. 8 (8), 12746–12755. [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhu Z., Zhao Y., Zhang Q., Wu X., Miao B., et al. (2019). FN1, SPARC, and SERPINE1 are highly expressed and significantly related to a poor prognosis of gastric adenocarcinoma revealed by microarray and bioinformatics. Sci. Rep. 9 (1), 7827. 10.1038/s41598-019-43924-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. (2022). Effect of knockdown of SPARC gene on proliferation and apoptosis of keloid fibroblasts. Anhui Medical University. [Google Scholar]
- Li X. L., Li J. L., Qiu D. J., Ma L. (2022b). Methylation-mediated expression of SPARC is correlated with tumor progression and poor prognosis of breast cancer. Neoplasma 69 (4), 794–806. 10.4149/neo_2022_211002N1401 [DOI] [PubMed] [Google Scholar]
- Li Y., Wang J., Ma X., Tan L., Yan Y., Xue C., et al. (2016a). A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int. J. Biol. Sci. 12 (8), 1022–1031. 10.7150/ijbs.15438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Li A. D., Xu L., Bai D. W., Hou K. Z., Zheng H. C., et al. (2016b). SPARC expression in gastric cancer predicts poor prognosis: Results from a clinical cohort, pooled analysis and GSEA assay. Oncotarget 7 (43), 70211–70222. 10.18632/oncotarget.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Y., Zhou A. F., Li G., Zhou L. Y., Pu P. M., Zhu K., et al. (2023). Chronic spinal cord compression associated with intervertebral disc degeneration in SPARC-null mice. Neural Regen. Res. 18 (3), 634–642. 10.4103/1673-5374.350210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S. M., Zhou X. J., Cai D., Zhou Q., Wang L. (2022). Overexpression of SPARC enhances the sensitivity of SKM-1 cells to Ara-C by regulating CPBP/MLKL. Chin. J. Exp. Hematol. 30 (05), 1508–1514. 10.19746/j.cnki.issn1009-2137.2022.05.031 [DOI] [PubMed] [Google Scholar]
- Liao P., Li W., Liu R., Teer J. K., Xu B., Zhang W., et al. (2018). Genome-scale analysis identifies SERPINE1 and SPARC as diagnostic and prognostic biomarkers in gastric cancer. Onco Targets Ther. 11, 6969–6980. 10.2147/ott.S173934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Zhao P., Jiang Y., Tang Y., Jin H., Pan Z., et al. (2016). Blood-brain-Barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano 10 (11), 9999–10012. 10.1021/acsnano.6b04268 [DOI] [PubMed] [Google Scholar]
- Lindner J. L., Loibl S., Denkert C., Ataseven B., Fasching P. A., Pfitzner B. M., et al. (2015). Expression of secreted protein acidic and rich in cysteine (SPARC) in breast cancer and response to neoadjuvant chemotherapy. Ann. Oncol. 26 (1), 95–100. 10.1093/annonc/mdu487 [DOI] [PubMed] [Google Scholar]
- Liu L., Hu F., Wang H., Wu X., Eltahan A. S., Stanford S., et al. (2019). Secreted protein acidic and rich in cysteine mediated biomimetic delivery of methotrexate by albumin-based nanomedicines for rheumatoid arthritis therapy. ACS Nano 13 (5), 5036–5048. 10.1021/acsnano.9b01710 [DOI] [PubMed] [Google Scholar]
- Liu Y., Feng Y., Wang X., Yang X., Hu Y., Li Y., et al. (2020). SPARC negatively correlates with prognosis after transarterial chemoembolization and facilitates proliferation and metastasis of hepatocellular carcinoma via ERK/MMP signaling pathways. Front. Oncol. 10, 813. 10.3389/fonc.2020.00813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M. V., Blanco P., Viale D. L., Cafferata E. G., Carbone C., Gould D., et al. (2006). Expression of a suicidal gene under control of the human secreted protein acidic and rich in cysteine (SPARC) promoter in tumor or stromal cells led to the inhibition of tumor cell growth. Mol. Cancer Ther. 5 (10), 2503–2511. 10.1158/1535-7163.Mct-06-0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukianova N., Zadvornyi T., Kashuba E., Borikun T., Mushii О., Chekhun V. (2022). Expression of markers of bone tissue remodeling in breast cancer and prostate cancer cells in vitro . Exp. Oncol. 44 (1), 39–46. 10.32471/exp-oncology.2312-8852.vol-44-no-1.17354 [DOI] [PubMed] [Google Scholar]
- Luo A., Kong J., Hu G., Liew C. C., Xiong M., Wang X., et al. (2004). Discovery of Ca2+-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene 23 (6), 1291–1299. 10.1038/sj.onc.1207218 [DOI] [PubMed] [Google Scholar]
- Luo L. Y., Wu J. H., Xiang X. X., Tang M., Fu Y. (2019). Research progress on the effect of cysteine-rich acidic secretory protein on glaucoma filtering bleb scarring. J. Shanghai Jiaot. Univ. Med. Ed. 39 (06), 685–688+684. 10.1021/jp512440z [DOI] [Google Scholar]
- Ma G. Y., Shi S., Zhang Y. R., Guo Z. B., Bai W. W., Zhang Z. G. (2022a). Prognostic significance of SPARC expression in non-small cell lung cancer: A meta-analysis and bioinformatics analysis. Oncol. Lett. 24 (5), 412. 10.3892/ol.2022.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Gao S., Xie X., Sun E., Zhang M., Zhou Q., et al. (2017a). SPARC inhibits breast cancer bone metastasis and may be a clinical therapeutic target. Oncol. Lett. 14 (5), 5876–5882. 10.3892/ol.2017.6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ma Y., Chen S., Guo S., Hu J., Yue T., et al. (2021). SPARC enhances 5-FU chemosensitivity in gastric cancer by modulating epithelial-mesenchymal transition and apoptosis. Biochem. Biophys. Res. Commun. 558, 134–140. 10.1016/j.bbrc.2021.04.009 [DOI] [PubMed] [Google Scholar]
- Ma W., Yan Y., Bai S., Zhou Y., Wang X., Feng Z., et al. (2022b). SPARC expression in tumor microenvironment induces partial epithelial-to-mesenchymal transition of esophageal adenocarcinoma cells via cooperating with TGF-β signaling. Cell Biol. Int. 47, 250–259. 10.1002/cbin.11927 [DOI] [PubMed] [Google Scholar]
- Ma Y. S., Chen G. W., Liu Y. C. (2017b). Research progress of SPARC in the mechanism of gastric cancer. Cancer Prev. Res. 44 (10), 694–697. 10.1007/s12253-012-9497-9 [DOI] [Google Scholar]
- Makridakis M., Roubelakis M. G., Bitsika V., Dimuccio V., Samiotaki M., Kossida S., et al. (2010). Analysis of secreted proteins for the study of bladder cancer cell aggressiveness. J. Proteome Res. 9 (6), 3243–3259. 10.1021/pr100189d [DOI] [PubMed] [Google Scholar]
- Maloney S. C., Marshall J. C., Antecka E., Orellana M. E., Fernandes B. F., Martins C., et al. (2009). SPARC is expressed in human uveal melanoma and its abrogation reduces tumor cell proliferation. Anticancer Res. 29 (8), 3059–3064. http://dx.doi.org/. [PubMed] [Google Scholar]
- Maltzman J. S. (2022). A SPARC-ling link to inflammaging. Sci. Immunol. 7 (75), eade5698. 10.1126/sciimmunol.ade5698 [DOI] [PubMed] [Google Scholar]
- Maroni P., Bendinelli P., Morelli D., Drago L., Luzzati A., Perrucchini G., et al. (2015). High SPARC expression starting from dysplasia, associated with breast carcinoma, is predictive for bone metastasis without enhancement of plasma levels. Int. J. Mol. Sci. 16 (12), 28108–28122. 10.3390/ijms161225997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanism of SPARC and SPARCL-1 regulating autophagy in glutamate-induced hippocampal neuron injury (). Directed by chen, S. Haikou, China: Hainan Medical College. [Google Scholar]
- Meling M. T., Kiniwa Y., Ogawa E., Sato Y., Okuyama R. (2021). Increased expression of secreted protein acidic and rich in cysteine and tissue inhibitor of metalloproteinase-3 in epidermotropic melanoma metastasis. J. Dermatol 48 (11), 1772–1779. 10.1111/1346-8138.16125 [DOI] [PubMed] [Google Scholar]
- Meng S., Jing L., Zhang W., Wang F., Dong Y., Dong D. (2022). Research progress on serological indices and their clinical application in rheumatoid arthritis. J. Clin. Lab. Anal. 36 (9), e24576. 10.1002/jcla.24576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhadi S., Tam S., Li Q., Moghal N., Pham N. A., Tong J., et al. (2022). Integrative analysis of non-small cell lung cancer patient-derived xenografts identifies distinct proteotypes associated with patient outcomes. Nat. Commun. 13 (1), 1811. 10.1038/s41467-022-29444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo A., Yang S. W., Jiang Y. X., Zhao Y. L., Shi Y., Qian F., et al. (2017). Role of secreted protein acidic in hematogenous metastasis of gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 21 (19), 4311–4321. 10.1051/medsci/2007232221 [DOI] [PubMed] [Google Scholar]
- Moazzeni H., Mirrahimi M., Moghadam A., Banaei-Esfahani A., Yazdani S., Elahi E. (2019). Identification of genes involved in glaucoma pathogenesis using combined network analysis and empirical studies. Hum. Mol. Genet. 28 (21), 3637–3663. 10.1093/hmg/ddz222 [DOI] [PubMed] [Google Scholar]
- Naczki C., John B., Patel C., Lafferty A., Ghoneum A., Afify H., et al. (2018). SPARC inhibits metabolic plasticity in ovarian cancer. Cancers (Basel) 10 (10), 385. 10.3390/cancers10100385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M. A., Gerhard R., Fregnani J. H., Nonogaki S., Rierger R. B., Netto M. M., et al. (2011). Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res. Treat. 126 (1), 1–14. 10.1007/s10549-010-0867-2 [DOI] [PubMed] [Google Scholar]
- Nakajima M., Yoshino S., Kanekiyo S., Maeda N., Sakamoto K., Tsunedomi R., et al. (2018). High secreted protein acidic and rich in cysteine expression in peritumoral fibroblasts predicts better prognosis in patients with resectable gastric cancer. Oncol. Lett. 15 (1), 803–812. 10.3892/ol.2017.7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kamihagi K., Satakeda H., Katayama M., Pan H., Okamoto H., et al. (1996). Enhancement of SPARC (osteonectin) synthesis in arthritic cartilage. Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum. 39 (4), 539–551. 10.1002/art.1780390402 [DOI] [PubMed] [Google Scholar]
- Nakatani K., Seki S., Kawada N., Kitada T., Yamada T., Sakaguchi H., et al. (2002). Expression of SPARC by activated hepatic stellate cells and its correlation with the stages of fibrogenesis in human chronic hepatitis. Virchows Arch. 441 (5), 466–474. 10.1007/s00428-002-0631-z [DOI] [PubMed] [Google Scholar]
- Ng Y.-L., Klopcic B., Lloyd F., Forrest C., Greene W., Lawrance I. C. (2013). Secreted protein acidic and rich in cysteine (SPARC) exacerbates colonic inflammatory symptoms in dextran sodium sulphate-induced murine colitis. PLOS ONE 8, e77575. 10.1371/journal.pone.0077575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NikhalaShree S., Karthikkeyan G., George R., Shantha B., Vijaya L., Ratra V., et al. (2019). Lowered decorin with aberrant extracellular matrix remodeling in aqueous humor and tenon's tissue from primary glaucoma patients. Invest. Ophthalmol. Vis. Sci. 60 (14), 4661–4669. 10.1167/iovs.19-27091 [DOI] [PubMed] [Google Scholar]
- Nygaard G., Firestein G. S. (2020). Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 16 (6), 316–333. 10.1038/s41584-020-0413-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez J. L., Paraoan L., Hiscott P., Gray D., García-Fiñana M., Grierson I., et al. (2005). Differential expression of angioregulatory matricellular proteins in posterior uveal melanoma. Melanoma Res. 15 (6), 495–502. 10.1097/00008390-200512000-00003 [DOI] [PubMed] [Google Scholar]
- Ouboussad L., Burska A. N., Melville A., Buch M. H. (2019). Synovial tissue heterogeneity in rheumatoid arthritis and changes with biologic and targeted synthetic therapies to inform stratified therapy. Front. Med. (Lausanne) 6, 45. 10.3389/fmed.2019.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley P. J., Goff B. A., Gown A. M., Greer B. E., Sage E. H. (2000). Alterations in SPARC and VEGF immunoreactivity in epithelial ovarian cancer. Gynecol. Oncol. 78 (3 Pt 1), 336–341. 10.1006/gyno.2000.5894 [DOI] [PubMed] [Google Scholar]
- Pan M. R., Chang H. C., Chuang L. Y., Hung W. C. (2008). The nonsteroidal anti-inflammatory drug NS398 reactivates SPARC expression via promoter demethylation to attenuate invasiveness of lung cancer cells. Exp. Biol. Med. (Maywood) 233 (4), 456–462. 10.3181/0709-rm-257 [DOI] [PubMed] [Google Scholar]
- Peng F., Zhong Y., Liu Y., Zhang Y., Xie Y., Lu Y., et al. (2017). SPARC suppresses lymph node metastasis by regulating the expression of VEGFs in ovarian carcinoma. Int. J. Oncol. 51 (6), 1920–1928. 10.3892/ijo.2017.4168 [DOI] [PubMed] [Google Scholar]
- Penny S. M. (2020). Ovarian cancer: An overview. Radiol. Technol. 91 (6), 561–575. [PubMed] [Google Scholar]
- Phan E., Ahluwalia A., Tarnawski A. S. (2007). Role of SPARC--matricellular protein in pathophysiology and tissue injury healing. Implications for gastritis and gastric ulcers. Med. Sci. Monit. 13 (2), Ra25–30. 10.1051/medsci/2007232221 [DOI] [PubMed] [Google Scholar]
- Pieniazek M., Donizy P., Halon A., Leskiewicz M., Matkowski R. (2016). Prognostic significance of immunohistochemical epithelial-mesenchymal transition markers in skin melanoma patients. Biomark. Med. 10 (9), 975–985. 10.2217/bmm-2016-0133 [DOI] [PubMed] [Google Scholar]
- Piñero F., Dirchwolf M., Pessôa M. G. (2020). Biomarkers in hepatocellular carcinoma: Diagnosis, prognosis and treatment response assessment. Cells 9 (6), 1370. 10.3390/cells9061370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhajcer O. L., Benedetti L., Girotti M. R., Prada F., Salvatierra E., Llera A. S. (2008). The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 27 (3), 523–537. 10.1007/s10555-008-9135-x [DOI] [PubMed] [Google Scholar]
- Porte H., Triboulet J. P., Kotelevets L., Carrat F., Prévot S., Nordlinger B., et al. (1998). Overexpression of stromelysin-3, BM-40/SPARC, and MET genes in human esophageal carcinoma: Implications for prognosis. Clin. Cancer Res. 4 (6), 1375–1382. [PubMed] [Google Scholar]
- Prada F., Benedetti L. G., Bravo A. I., Alvarez M. J., Carbone C., Podhajcer O. L. (2007). SPARC endogenous level, rather than fibroblast-produced SPARC or stroma reorganization induced by SPARC, is responsible for melanoma cell growth. J. Invest. Dermatol 127 (11), 2618–2628. 10.1038/sj.jid.5700962 [DOI] [PubMed] [Google Scholar]
- Puolakkainen P., Bradshaw A. D., Kyriakides T. R., Reed M., Brekken R., Wight T., et al. (2003). Compromised production of extracellular matrix in mice lacking secreted protein, acidic and rich in cysteine (SPARC) leads to a reduced foreign body reaction to implanted biomaterials. Am. J. Pathol. 162 (2), 627–635. 10.1016/s0002-9440(10)63856-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Lane T. F., Iruela-Arispe M. L., Ross R., Sage E. H. (1992). The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc. Natl. Acad. Sci. U. S. A. 89 (4), 1281–1285. 10.1073/pnas.89.4.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinella L., Orlando G., Ferrante C., Chiavaroli A., Brunetti L., Leone S. (2020). Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front. Physiol. 11, 578966. 10.3389/fphys.2020.578966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee D. J., Haddadin R. I., Kang M. H., Oh D. J. (2009). Matricellular proteins in the trabecular meshwork. Exp. Eye Res. 88 (4), 694–703. 10.1016/j.exer.2008.11.032 [DOI] [PubMed] [Google Scholar]
- Rocco M., Malorni L., Cozzolino R., Palmieri G., Rozzo C., Manca A., et al. (2011). Proteomic profiling of human melanoma metastatic cell line secretomes. J. Proteome Res. 10 (10), 4703–4714. 10.1021/pr200511f [DOI] [PubMed] [Google Scholar]
- Ryall C. L., Viloria K., Lhaf F., Walker A. J., King A., Jones P., et al. (2014). Novel role for matricellular proteins in the regulation of islet β cell survival: The effect of SPARC on survival, proliferation, and signaling. J. Biol. Chem. 289 (44), 30614–30624. 10.1074/jbc.M114.573980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S., Sidorov S., Ravussin E., Artyomov M., Iwasaki A., Wang A., et al. (2022). The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging. Immunity 55 (9), 1609–1626.e7. 10.1016/j.immuni.2022.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N. A., Elmarakby A. A., Imig J. D., Fulton D. J., Motamed K. (2008). SPARC ameliorates ovarian cancer-associated inflammation. Neoplasia 10 (10), 1092–1104. 10.1593/neo.08672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N. A., Najwer I., Socha M. J., Fulton D. J., Mok S. C., Motamed K. (2007c). SPARC inhibits LPA-mediated mesothelial-ovarian cancer cell crosstalk. Neoplasia 9 (1), 23–35. 10.1593/neo.06658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N., Frierson H. F., Sanchez-Carbayo M., Brekken R. A., Theodorescu D. (2013). Loss of SPARC in bladder cancer enhances carcinogenesis and progression. J. Clin. Invest. 123 (2), 751–766. 10.1172/jci64782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N., Najwer I., Motamed K. (2007a). Secreted protein acidic and rich in cysteine (SPARC) inhibits integrin-mediated adhesion and growth factor-dependent survival signaling in ovarian cancer. Am. J. Pathol. 170 (3), 1054–1063. 10.2353/ajpath.2007.060903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N. (2016). Roles of SPARC in urothelial carcinogenesis, progression and metastasis. Oncotarget 7 (41), 67574–67585. 10.18632/oncotarget.11590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N., Socha M. J., Olearczyk J. J., Elmarakby A. A., Imig J. D., Motamed K. (2007b). Normalization of the ovarian cancer microenvironment by SPARC. Mol. Cancer Res. 5 (10), 1015–1030. 10.1158/1541-7786.Mcr-07-0001 [DOI] [PubMed] [Google Scholar]
- Salvatierra E., Alvarez M. J., Leishman C. C., Rivas Baquero E., Lutzky V. P., Chuluyan H. E., et al. (2015). SPARC controls melanoma cell plasticity through Rac1. PLoS One 10 (8), e0134714. 10.1371/journal.pone.0134714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaletti S., Tripodo C., Cappetti B., Casalini P., Chiodoni C., Piconese S., et al. (2011). SPARC oppositely regulates inflammation and fibrosis in bleomycin-induced lung damage. Am. J. Pathol. 179 (6), 3000–3010. 10.1016/j.ajpath.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavelli K., Chatterjee A., Rhee D. J. (2015). Secreted protein acidic and rich in cysteine in ocular tissue. J. Ocul. Pharmacol. Ther. 31 (7), 396–405. 10.1089/jop.2015.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet L. F., Su R., Barathi V. A., Lee W. S., Poh R., Heng Y. M., et al. (2010). SPARC deficiency results in improved surgical survival in a novel mouse model of glaucoma filtration surgery. PLoS One 5 (2), e9415. 10.1371/journal.pone.0009415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet L. F., Su R., Toh L. Z., Wong T. T. (2012). In vitro analyses of the anti-fibrotic effect of SPARC silencing in human tenon's fibroblasts: Comparisons with mitomycin C. J. Cell Mol. Med. 16 (6), 1245–1259. 10.1111/j.1582-4934.2011.01400.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segat L., Milanese M., Pirulli D., Trevisiol C., Lupo F., Salizzoni M., et al. (2009). Secreted protein acidic and rich in cysteine (SPARC) gene polymorphism association with hepatocellular carcinoma in Italian patients. J. Gastroenterol. Hepatol. 24 (12), 1840–1846. 10.1111/j.1440-1746.2009.06009.x [DOI] [PubMed] [Google Scholar]
- Shan Z., Wang W., Tong Y., Zhang J. (2021). Genome-scale analysis identified NID2, SPARC, and MFAP2 as prognosis markers of overall survival in gastric cancer. Med. Sci. Monit. 27, e929558. 10.12659/msm.929558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S. (2018). Abnormal methylation of SPARC gene in gastric cancer and its significance. Shenyang, China: Chinese Medical University. [Google Scholar]
- Shi S., Ma H. Y., Han X. Y., Sang Y. Z., Yang M. Y., Zhang Z. G. (2022). Prognostic significance of SPARC expression in breast cancer: A meta-analysis and bioinformatics analysis. Biomed. Res. Int. 2022, 8600419. 10.1155/2022/8600419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2017). Cancer statistics, 2017. CA Cancer J. Clin. 67 (1), 7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- Smyth E. C., Nilsson M., Grabsch H. I., van Grieken N. C., Lordick F. (2020). Gastric cancer. Lancet 396 (10251), 635–648. 10.1016/s0140-6736(20)31288-5 [DOI] [PubMed] [Google Scholar]
- Socha M. J., Said N., Dai Y., Kwong J., Ramalingam P., Trieu V., et al. (2009). Aberrant promoter methylation of SPARC in ovarian cancer. Neoplasia 11 (2), 126–135. 10.1593/neo.81146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Ding L., Zhang S., Wang W. (2018). MiR-29 family members interact with SPARC to regulate glucose metabolism. Biochem. Biophys. Res. Commun. 497 (2), 667–674. 10.1016/j.bbrc.2018.02.129 [DOI] [PubMed] [Google Scholar]
- Stanford S. M., Aleman Muench G. R., Bartok B., Sacchetti C., Kiosses W. B., Sharma J., et al. (2016). TGFβ responsive tyrosine phosphatase promotes rheumatoid synovial fibroblast invasiveness. Ann. Rheum. Dis. 75 (1), 295–302. 10.1136/annrheumdis-2014-205790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Feng J., Yi Q., Xu X., Chen Y., Tang L. (2018). SPARC acts as a mediator of TGF-β1 in promoting epithelial-to-mesenchymal transition in A549 and H1299 lung cancer cells. Biofactors 44 (5), 453–464. 10.1002/biof.1442 [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhang Y., Wu X., Chi P. (2020). A four gene-based risk score system associated with chemoradiotherapy response and tumor recurrence in rectal cancer by Co-expression network analysis. Onco Targets Ther. 13, 6721–6733. 10.2147/ott.S256696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Oshima T., Yoshihara K., Sakamaki K., Aoyama T., Cho H., et al. (2018). Clinical significance of secreted protein, acidic and cysteine-rich gene expression in patients with stage II/III gastric cancer following curative resection and adjuvant chemotherapy with S-1. Oncol. Lett. 15 (5), 7335–7343. 10.3892/ol.2018.8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S. S., Oh D. J., Kang M. H., Shepard A. R., Pang I. H., Rhee D. J. (2014). TGF-β2-mediated ocular hypertension is attenuated in SPARC-null mice. Invest. Ophthalmol. Vis. Sci. 55 (7), 4084–4097. 10.1167/iovs.13-12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szynglarewicz B., Kasprzak P., Donizy P., Biecek P., Halon A., Matkowski R. (2016). Ductal carcinoma in situ on stereotactic biopsy of suspicious breast microcalcifications: Expression of SPARC (Secreted Protein, Acidic and Rich in Cysteine) can predict postoperative invasion. J. Surg. Oncol. 114 (5), 548–556. 10.1002/jso.24373 [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. (1981). Osteonectin, a bone-specific protein linking mineral to collagen. Cell 26 (1 Pt 1), 99–105. 10.1016/0092-8674(81)90037-4 [DOI] [PubMed] [Google Scholar]
- Tian Y., Xing Y., Zhang Z., Peng R., Zhang L., Sun Y. (2020). Bioinformatics analysis of key genes and circRNA-miRNA-mRNA regulatory network in gastric cancer. Biomed. Res. Int. 2020, 2862701. 10.1155/2020/2862701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichet M., Prod'Homme V., Fenouille N., Ambrosetti D., Mallavialle A., Cerezo M., et al. (2015). Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat. Commun. 6, 6993. 10.1038/ncomms7993 [DOI] [PubMed] [Google Scholar]
- Tremble P. M., Lane T. F., Sage E. H., Werb Z. (1993). SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J. Cell Biol. 121 (6), 1433–1444. 10.1083/jcb.121.6.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodo C., Sangaletti S., Guarnotta C., Piccaluga P. P., Cacciatore M., Giuliano M., et al. (2012). Stromal SPARC contributes to the detrimental fibrotic changes associated with myeloproliferation whereas its deficiency favors myeloid cell expansion. Blood 120 (17), 3541–3554. 10.1182/blood-2011-12-398537 [DOI] [PubMed] [Google Scholar]
- Tu J., Chen W., Fang Y., Han D., Chen Y., Jiang H., et al. (2022). PU.1 promotes development of rheumatoid arthritis via repressing FLT3 in macrophages and fibroblast-like synoviocytes. Ann. Rheum. Dis. 82, 198–211. 10.1136/ard-2022-222708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello D. A., Andrews M. R., Brenton J. D. (2016). SPARC regulates transforming growth factor beta induced (TGFBI) extracellular matrix deposition and paclitaxel response in ovarian cancer cells. PLoS One 11 (9), e0162698. 10.1371/journal.pone.0162698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. M., Murphy-Ullrich J. E., Downs J. C., O'Brien C. J. (2014). The role of matricellular proteins in glaucoma. Matrix Biol. 37, 174–182. 10.1016/j.matbio.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Wallace D. M., Pokrovskaya O., O'Brien C. J. (2015). The function of matricellular proteins in the lamina cribrosa and trabecular meshwork in glaucoma. J. Ocul. Pharmacol. Ther. 31 (7), 386–395. 10.1089/jop.2014.0163 [DOI] [PubMed] [Google Scholar]
- Wan J., Wen D., Dong L., Tang J., Liu D., Liu Y., et al. (2015). Establishment of monoclonal HCC cell lines with organ site-specific tropisms. BMC Cancer 15, 678. 10.1186/s12885-015-1692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhang Z., Tang J., Tao H., Zhang Z. (2018a). Correlation between SPARC, TGFβ1, Endoglin and angiogenesis mechanism in lung cancer. J. Biol. Regul. Homeost. Agents 32 (6), 1525–1531. [PubMed] [Google Scholar]
- Wang J.-C., Lai S., Guo X., Zhang X., Crombrugghe B. d., Sonnylal S., et al. (2010). Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res. Ther. 12, R60. 10.1186/ar2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Fu M., Liu K., Wang N., Zhang Z., Zhou M., et al. (2018b). Matricellular proteins play a potential role in acute primary angle closure. Curr. Eye Res. 43 (6), 771–777. 10.1080/02713683.2018.1449222 [DOI] [PubMed] [Google Scholar]
- Wang L., Yang M., Shan L., Qi L., Chai C., Zhou Q., et al. (2012). The role of SPARC protein expression in the progress of gastric cancer. Pathol. Oncol. Res. 18 (3), 697–702. 10.1007/s12253-012-9497-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang l. Y., Yang Q. R. (2018). Changes and significance of peripheral blood mononuclear cells and serum SPARC expression in patients with ankylosing spondylitis. Shandong Med. 58 (16), 79–81. Jinan, China. [Google Scholar]
- Wang S., Li X. C., Liu Z. C., Rang J. T. (2019). Expression and prognosis correlation analysis of SPARC in gastric cancer. China Tumor Markers Acad. Conf. 13th Tumor Markers Young Sci. Forum), 233–234. [Google Scholar]
- Wang T., Srivastava S., Hartman M., Buhari S. A., Chan C. W., Iau P., et al. (2016). High expression of intratumoral stromal proteins is associated with chemotherapy resistance in breast cancer. Oncotarget 7 (34), 55155–55168. 10.18632/oncotarget.10894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang X., Chen Y., Zhu B., Xing Q. (2022a). Identification of hub biomarkers and exploring the roles of immunity, M6A, ferroptosis, or cuproptosis in rats with diabetic erectile dysfunction. Andrology 11, 316–331. 10.1111/andr.13265 [DOI] [PubMed] [Google Scholar]
- Wang Z., Hao B., Yang Y., Wang R., Li Y., Wu Q. (2014). Prognostic role of SPARC expression in gastric cancer: A meta-analysis. Arch. Med. Sci. 10 (5), 863–869. 10.5114/aoms.2014.46207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Song H. Y., An M. M., Zhu L. L. (2015). Association of serum SPARC level with severity of coronary artery lesion in type 2 diabetic patients with coronary heart disease. Int. J. Clin. Exp. Med. 8 (10), 19290–19296. [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Xia Q., Su W., Zhang M., Gu Y., Xu J., et al. (2022b). The commonness in immune infiltration of rheumatoid arthritis and atherosclerosis: Screening for central targets via microarray data analysis. Front. Immunol. 13, 1013531. 10.3389/fimmu.2022.1013531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Okano T., Gon T., Yoshida N., Fukumoto K., Yamada S., et al. (2022). Difficult-to-treat rheumatoid arthritis: Current concept and unsolved problems. Front. Med. (Lausanne) 9, 1049875. 10.3389/fmed.2022.1049875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins G., Douglas-Jones A., Bryce R., Mansel R. E., Jiang W. G. (2005). Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostagl. Leukot. Essent. Fat. Acids 72 (4), 267–272. 10.1016/j.plefa.2004.12.003 [DOI] [PubMed] [Google Scholar]
- Wei Y., Wang Y., Xia D., Guo S., Wang F., Zhang X., et al. (2017). Thermosensitive liposomal codelivery of HSA-paclitaxel and HSA-ellagic acid complexes for enhanced drug perfusion and efficacy against pancreatic cancer. ACS Appl. Mater Interfaces 9 (30), 25138–25151. 10.1021/acsami.7b07132 [DOI] [PubMed] [Google Scholar]
- Wong S. L., Sukkar M. B. (2017). The SPARC protein: An overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease. Br. J. Pharmacol. 174 (1), 3–14. 10.1111/bph.13653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. Y., Crowley D., Bronson R. T., Hynes R. O. (2008). Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer. Clin. Exp. Metastasis 25 (2), 109–118. 10.1007/s10585-007-9126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Li L., Yang M., Liu H., Yang G. (2011). Elevated plasma levels of SPARC in patients with newly diagnosed type 2 diabetes mellitus. Eur. J. Endocrinol. 165 (4), 597–601. 10.1530/eje-11-0131 [DOI] [PubMed] [Google Scholar]
- Wu J., Zhang J. R., Jiang X. Q., Cao X. G. (2017). Correlation between secreted protein acidic and rich in cysteine protein expression and the prognosis of postoperative patients exhibiting esophageal squamous cell carcinoma. Mol. Med. Rep. 16 (3), 3401–3406. 10.3892/mmr.2017.6959 [DOI] [PubMed] [Google Scholar]
- Xia Y., Inoue K., Du Y., Baker S. J., Reddy E. P., Greenblatt M. B., et al. (2022). TGFβ reprograms TNF stimulation of macrophages towards a non-canonical pathway driving inflammatory osteoclastogenesis. Nat. Commun. 13 (1), 3920. 10.1038/s41467-022-31475-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X. X., Tang M. (2018). Mechanism of cysteine-rich acidic secretory protein in the field of primary open-angle glaucoma. Acta Ophthalmol. 33 (03), 195–199. 10.3978/j.issn.1000-4432.2018.04.06 [DOI] [Google Scholar]
- Xu B., Mcleod H. L., Teer J. K., Liao P., Liu R., Li W., et al. (2018). Genome-scale analysis identifies SERPINE1 and SPARC as diagnostic and prognostic biomarkers in gastric cancer. Archive "OncoTargets Ther. 11, 6969–6980. 10.2147/ott.S173934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yang S., Gu X., Shen H., Wang L., Xu W., et al. (2019). SPARC correlates with unfavorable outcome and promotes tumor growth in lung squamous cell carcinoma. Exp. Mol. Pathol. 110, 104276. 10.1016/j.yexmp.2019.104276 [DOI] [PubMed] [Google Scholar]
- Xu L., Ping F., Yin J., Xiao X., Xiang H., Ballantyne C. M., et al. (2013). Elevated plasma SPARC levels are associated with insulin resistance, dyslipidemia, and inflammation in gestational diabetes mellitus. PLoS One 8 (12), e81615. 10.1371/journal.pone.0081615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. J., Yao X. Q., Zheng X. l., Li D. P., Huo L. C. (2022b). Research progress on the mechanism of acupuncture-moxibustion intervention in rheumatoid arthritis based on NF-κB signaling pathway. Massage Rehabilitation Med. 13 (17), 50–54+60. 10.19787/j.issn.1008-1879.2022.17.012 [DOI] [Google Scholar]
- Xu Y., Zhang Z., He J., Chen Z. (2022a). Immune effects of macrophages in rheumatoid arthritis: A bibliometric analysis from 2000 to 2021. Front. Immunol. 13, 903771. 10.3389/fimmu.2022.903771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L. Y., Hu N., Song Y. M., Zou S. M., Shou J. Z., Qian L. X., et al. (2006). Tissue microarray analysis reveals a tight correlation between protein expression pattern and progression of esophageal squamous cell carcinoma. BMC Cancer 6, 296. 10.1186/1471-2407-6-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L. Y., Zou S. M., Zheng S., Liu X. Y., Wen P., Yuan Y. L., et al. (2011). Expressions of the γ2 chain of laminin-5 and secreted protein acidic and rich in cysteine in esophageal squamous cell carcinoma and their relation to prognosis. Chin. J. Cancer 30 (1), 69–78. 10.5732/cjc.010.10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M., Kanda K., Li N. C., Fukumori T., Oka N., Kanayama H. O., et al. (2001). Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J. Urol. 166 (6), 2495–2499. 10.1016/s0022-5347(05)65623-6 [DOI] [PubMed] [Google Scholar]
- Yamashita K., Upadhay S., Mimori K., Inoue H., Mori M. (2003). Clinical significance of secreted protein acidic and rich in cystein in esophageal carcinoma and its relation to carcinoma progression. Cancer 97 (10), 2412–2419. 10.1002/cncr.11368 [DOI] [PubMed] [Google Scholar]
- Yan P., He Y., Xie K., Kong S., Zhao W. (2018). In silico analyses for potential key genes associated with gastric cancer. PeerJ 6, e6092. 10.7717/peerj.6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Xia Y., Wang S., Sun C. (2022a). Prognostic value of SPARC in hepatocellular carcinoma: A systematic review and meta-analysis. PLoS One 17 (8), e0273317. 10.1371/journal.pone.0273317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. P., Jian Y. Q., Liu Y. B., Xie Q. L., Yu H. H., Wang B., et al. (2022b). Heilaohuacid G, a new triterpenoid from Kadsura coccinea inhibits proliferation, induces apoptosis, and ameliorates inflammation in RA-FLS and RAW 264.7 cells via suppressing NF-𝜅B pathway. Phytother. Res. 36 (10), 3900–3910. 10.1002/ptr.7527 [DOI] [PubMed] [Google Scholar]
- Yang Y., Tang X., Song X., Tang L., Cao Y., Liu X., et al. (2019). Evidence for an oncogenic role of HOXC6 in human non-small cell lung cancer. PeerJ 7, e6629. 10.7717/peerj.6629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D., Zhang P. B., Gong S., Liu L., Ren Z. Q. (2020). Effects of gastric bypass surgery on the expression of SPARC protein and GLUT-4 protein in adipose tissue of non-obese GK rats. Chin. J. Basic Clin. General Surg. 27 (06), 685–690. [Google Scholar]
- Yardley D. A. (2013). nab-Paclitaxel mechanisms of action and delivery. J. Control Release 170 (3), 365–372. 10.1016/j.jconrel.2013.05.041 [DOI] [PubMed] [Google Scholar]
- Ye G. F. (2017). Cysteine-rich, acidic secreted glycoprotein regulates arterial homeostasis in human smooth muscle cells iin vitro . Jinan, China: Shan Dong University. [Google Scholar]
- Ye Z., Zeng Z., Shen Y., Chen Z. (2020). Identification of hub genes in peripheral blood mononuclear cells for the diagnosis of hepatocellular carcinoma using a weighted gene co-expression network analysis. Exp. Ther. Med. 20 (2), 890–900. 10.3892/etm.2020.8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Chen G., Liu Y., Liu S., Wang P., Wan Y., et al. (2010). Downregulation of SPARC expression decreases gastric cancer cellular invasion and survival. J. Exp. Clin. Cancer Res. 29 (1), 59. 10.1186/1756-9966-29-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G. K., Chan W. Y., Ng S. W., Chan P. S., Cheung K. K., Berkowitz R. S., et al. (2001). SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am. J. Pathol. 159 (2), 609–622. 10.1016/s0002-9440(10)61732-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng B., Sun Z., Zhao Q., Liu D., Chen H., Li X., et al. (2021). SEC23A inhibit melanoma metastatic through secretory PF4 cooperation with SPARC to inhibit MAPK signaling pathway. Int. J. Biol. Sci. 17 (12), 3000–3012. 10.7150/ijbs.60866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Zhang Y., Da J., Jia Z., Wu H., Gu K. (2020). Downregulation of SPARC expression decreases cell migration and invasion involving epithelial-mesenchymal transition through the p-FAK/p-ERK pathway in esophageal squamous cell carcinoma. J. Cancer 11 (2), 414–420. 10.7150/jca.31427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. L., Chen G. W., Liu Y. C., Wang P. Y., Wang X., Wan Y. L., et al. (2012a). Secreted protein acidic and rich in cysteine (SPARC) suppresses angiogenesis by down-regulating the expression of VEGF and MMP-7 in gastric cancer. PLoS One 7 (9), e44618. 10.1371/journal.pone.0044618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang P., Zhu J., Wang W., Yin J., Zhang C., et al. (2014). SPARC expression is negatively correlated with clinicopathological factors of gastric cancer and inhibits malignancy of gastric cancer cells. Oncol. Rep. 31 (5), 2312–2320. 10.3892/or.2014.3118 [DOI] [PubMed] [Google Scholar]
- Zhang K., Wu L., Lin K., Zhang M., Li W., Tong X., et al. (2021). Integrin-dependent microgliosis mediates ketamine-induced neuronal apoptosis during postnatal rat retinal development. Exp. Neurol. 340, 113659. 10.1016/j.expneurol.2021.113659 [DOI] [PubMed] [Google Scholar]
- Zhang X., Feng H., Du J., Sun J., Li D., Hasegawa T., et al. (2018a). Aspirin promotes apoptosis and inhibits proliferation by blocking G0/G1 into S phase in rheumatoid arthritis fibroblast-like synoviocytes via downregulation of JAK/STAT3 and NF-κB signaling pathway. Int. J. Mol. Med. 42 (6), 3135–3148. 10.3892/ijmm.2018.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Yang B., Du Z., Bai T., Gao Y. T., Wang Y. J., et al. (2012b). Aberrant methylation of SPARC in human hepatocellular carcinoma and its clinical implication. World J. Gastroenterol. 18 (17), 2043–2052. 10.3748/wjg.v18.i17.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Miao Y., Wang J., Zhou M., Fu M., Wang Y. (2018b). Matricellular protein levels in aqueous humor and surgical outcomes of trabeculectomy. Invest. Ophthalmol. Vis. Sci. 59 (10), 3906–3910. 10.1167/iovs.18-24534 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wang Z., Liu X., Shi M., Chen G., Zhang B., et al. (2012c). Correlation of KLF4 and SPARC expression with the clinical characteristics of non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 15 (12), 720–724. 10.3779/j.issn.1009-3419.2012.12.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao l., Shun J. B., Shi S. D., Shun Y. R., He W., et al. (2019). Genetic traceability practices in a large-size beef company in China. J. Xi'an Jiaot. Univ. Med. Ed. 40 (02), 222–228. 10.1016/j.foodchem.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Zhao S., Yu Q., Pan J., Zhou Y., Cao C., Ouyang J. M., et al. (2017). Redox-responsive mesoporous selenium delivery of doxorubicin targets MCF-7 cells and synergistically enhances its anti-tumor activity. Acta Biomater. 54, 294–306. 10.1016/j.actbio.2017.02.042 [DOI] [PubMed] [Google Scholar]
- Zhao X., Gabriëls R. Y., Hooghiemstra W. T. R., Koller M., Meersma G. J., Buist-Homan M., et al. (2022). Validation of novel molecular imaging targets identified by functional genomic mRNA profiling to detect dysplasia in barrett's esophagus. Cancers (Basel) 14 (10), 2462. 10.3390/cancers14102462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Wu S., Jing J. (2021). Identifying diagnostic and prognostic biomarkers and candidate therapeutic drugs of gastric cancer based on transcriptomics and single-cell sequencing. Pathol. Oncol. Res. 27, 1609955. 10.3389/pore.2021.1609955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C., Liang Y., Wang Q., Tan H. W., Liang Y. (2022). Construction and validation of a novel prediction system for detection of overall survival in lung cancer patients. World J. Clin. Cases 10 (18), 5984–6000. 10.12998/wjcc.v10.i18.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N. M. (2018). Mechanism of overexpression of SPARC gene in increasing chemosensitivity in gastric cancer peritoneal metastasis. Shenyang, China: Chinese Medical University. [Google Scholar]
- Zhou Y., Hofstetter W. L., He Y., Hu W., Pataer A., Wang L., et al. (2010). KLF4 inhibition of lung cancer cell invasion by suppression of SPARC expression. Cancer Biol. Ther. 9 (7), 507–513. 10.4161/cbt.9.7.11106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Zhang l. (2012). Study on the mechanism of NF-κB signaling pathway in the occurrence and development of osteoarthritis. Chin. J. Osteoporos. 18 (01), 78–82. 10.1016/j.avsg.2022.09.056 [DOI] [Google Scholar]
- Zhu A., Yuan P., Du F., Hong R., Ding X., Shi X., et al. (2016). SPARC overexpression in primary tumors correlates with disease recurrence and overall survival in patients with triple negative breast cancer. Oncotarget 7 (47), 76628–76634. 10.18632/oncotarget.10532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G. C., Gao L., He J., Long Y., Liao S., Wang H., et al. (2015). CD90 is upregulated in gastric cancer tissues and inhibits gastric cancer cell apoptosis by modulating the expression level of SPARC protein. Oncol. Rep. 34 (5), 2497–2506. 10.3892/or.2015.4243 [DOI] [PubMed] [Google Scholar]
- Zhu X. C., Dong Q. Z., Zhang X. F., Deng B., Jia H. L., Ye Q. H., et al. (2012). microRNA-29a suppresses cell proliferation by targeting SPARC in hepatocellular carcinoma. Int. J. Mol. Med. 30 (6), 1321–1326. 10.3892/ijmm.2012.1140 [DOI] [PubMed] [Google Scholar]
- Zhu Y. S. (2020). Osteonectin regulates osteoblast mineralization through p38MAPK pathway. Suzhou University. [Google Scholar]