Abstract
Background:
In 1998 we estimated that 34 per million infectious window period (WP) donations were entering the blood supply at the South African National Blood Service (SANBS). Selective use of donations based on donor race-ethnicity, reduced this risk to 26 per million donations, but was deemed unethical. Consequently, in 2005 SANBS eliminated race-ethnicity-based collection policies and implemented Individual Donation Nucleic Acid Testing (ID-NAT). We describe the change in donor base demographics, resulting HIV detection rates and residual transfusion transmissible (TT)-HIV risk.
Study design and Methods:
From 2005 to 2015 ~7.7 million donations were tested for anti-HIV and HIV-RNA. Number of donations, HIV prevalence, ID-NAT yield (HIV RNA-positive, antibody-negative) rate, serology yield rate (RNA-negative, antibody-positive) and residual TT-HIV risk were analysed by donor type, race-ethnicity, age and gender. Multiple regression analysis was performed to investigate the determinants of HIV-positive and NAT yield donations.
Results:
The combined strategy of increasing donations from black donors and implementing ID-NAT increased the proportion of donations from black donors from 6% in 2005 to 30% in 2015 (p<0.00001), and reduced the TT risk from 24 to 13 per million transfusions. ID-NAT interdicted 481 (1:16,100) seronegative WP donations, while one TT case (0.13/million) was documented. Race-ethnicity and donor type were highly significant predictors of HIV positivity, with adjusted odds ratio for first-time donors of 12.5 (95% CI: 11.9,13.1) and for black race-ethnicity of 31.1 (28.9,33.4). There was no statistically significant difference in WP-NAT yield rate between first-time and repeat donors when controlled for race. The proportion of serology yields among HIV infected donors increased from 0.27% to 2.4%.
Conclusion:
ID-NAT enabled SANBS to increase the number of donations from black donors 5-fold while enhancing the safety of the blood supply.
Introduction:
According to a “between-census” community survey done in 2016 that sampled 1.3 million households of the 56 million people living in South Africa, approximately 7.06 million people are HIV positive, providing an estimated adult HIV prevalence and incidence of 18% and 0.91 per 100 person-years, respectively.1 In this environment, the South African National Blood Service (SANBS) collects approximately 800,000 blood donations annually from voluntary non-remunerated blood donors which are processed into blood components that are provided to approximately 400,000 patients each year.
In 1998, despite screening donations with a sensitive HIV p24 antigen (p24Ag) test, it was estimated that a significant rate (34 per million) of infectious window period (WP) donations could be entering the blood supply, a projection supported by confirmed reports of transfusion-transmitted (TT) HIV infections each year2,3. A structured risk management program, which included stringent education, product triage and donor race-ethnicity as risk markers, was implemented in 1999, and these measures were successful at reducing the estimated residual risk of TT-HIV infection from 34 per million to 26 per million red blood cell (RBC) transfusions3. However, the selective use of donations based on donor race-ethnicity, where donations from black African donors were discarded, was unethical and unsustainable. Consequently, it was decided, after estimating that individual donation nucleic acid amplification technology (ID-NAT) would reduce the TT-HIV risk to 17 per million RBC transfusions,4 to implement ID-NAT and change the risk management program by eliminating race-ethnicity based collection and utilization policies. It was anticipated, that the implementation of ID-NAT would provide a safety buffer as donor eligibility policies were progressively modified to increase donations by the black African majority population4.
South Africa was the first country globally to implement ID-NAT nationwide. On October 3rd 2005: 1) all blood donations were screened for HIV RNA, HBV DNA and HCV RNA, 2) p24Ag testing was discontinued, 3) donor race-ethnicity was removed as a marker of risk categorization, and 4) a donor education and motivation campaign was launched to increase the black African donor base. With these measures SANBS aimed to maintain a sufficient and safe blood supply for the patients of South Africa. In this paper, we describe how the changes in donor base demographics over ten years and the implementation of ID-NAT has affected HIV detection rates and residual TT-HIV risk estimates.
Material and Methods:
Screening and confirmation testing algorithm
Prospective blood donors complete a donor questionnaire (DQ) that examines donor health (to protect the donor) and risk behaviour (to protect the patient) prior to donating blood. Positive responses to risk behaviour questions such as “Are you HIV positive?”, “In the past 6 months have you had sexual contact with more than one person” and “In the past six months have you started having sexual contact with a new sexual partner” will prohibit blood donation at this time eliciting a temporary deferral from blood donation until the “risk behaviour” has ceased.
SANBS screens all blood donations using the Prism (Abbott, Delkenheim, Germany) 3rd generation anti-HIV, HBsAg and anti-HCV chemiluminescent immunoassays (ChLIA) in parallel with the Procleix Ultrio (Plus) NAT assay (Grifols, Barcelona, Spain) for HIV RNA, HCV RNA, and HBV DNA. An extensive confirmation algorithm (previously described)5 is in place for HIV, which classifies donations based on blood collected at the time of donation into four categories: 1) HIV negative, 2) HIV concordant positive (HIV RNA by NAT and anti-HIV by ChIA), 3) HIV “NAT yield” (RNA positive [NAT repeat reactive], anti-HIV negative) and 4) HIV “serology yield” (RNA negative, anti-HIV ChIA repeat reactive and Western blot positive). To confirm the viral status of donors classified as NAT or serology yield cases, SANBS recalls these donors (approximately seventy percent return). NAT yield cases are confirmed when donors seroconvert at follow-up, whereas serology yield donors must remain Western blot positive but RNA negative (these reflect cases of spontaneous [elite] control or undisclosed antiretroviral therapy (ART)). In addition, for further confirmation of HIV-RNA only positive donors that do not return for follow up, samples from stored fresh frozen plasma (FFP) units are tested by replicate Procleix Ultrio (Plus) discriminatory HIV (dHIV) NAT (Grifols, Barcelona, Spain) and quantitative PCR (Cobas Ampliprep/Cobas Taqman HIV-1 vs 2, Roche, Basel, Switzerland or Real time HIV-1 m2000rt, Abbott, Delkenheim, Germany). NAT yield plasma samples are also tested using a p24Ag assay (Innotest MAb, Immunogenetics, Ghent, Belgium) to ascertain whether the donation would have been detected as HIV infected using the screening strategy in place before Oct 2005. The South African National institute of Communicable Diseases (NICD) sequences the POL region of HIV using plasma from the NAT yield donations to determine drug resistance and genotypes.
Characterization of donor demographics
Demographics including gender, race-ethnicity, date of birth and donation site are routinely collected from donors from the self-reported pre-donation questionnaire. The type of blood donation, i.e., from a first time (FT), repeat (<1 year from previous donation) or a lapsed (>1 year from previous donation) donor, is recorded in the blood establishment computer system.
HIV infection detection rates
We calculated HIV prevalence for FT donors (by year and demographic subcategories) by dividing the number of HIV confirmed positive donations (concordant positive, confirmed NAT yield and serology yield donations) by the total number of blood donations from FT donors, with results expressed as percentages. The rates of HIV infected donations from repeat and lapsed donors were calculated similarly and expressed per 100,000 donations, as were the NAT yield and serology yield rates in the different donation categories.
TT risk analysis.
To calculate the TT-HIV risk for the ten year period we used the window period NAT yield ratio model6. The proportional increase in the black African donor base and the residual TT-HIV risk was estimated annually for donations from all donors, FT donors, and repeat donors. The annual TT-HIV residual risk was estimated for ID-NAT screened blood transfusions, as well as on the assumption that blood had been screened by p24Ag testing instead of ID-NAT. For the ratio modelling we used detection periods (DP) of 10.1 days for the NAT-positive/p24Ag-negative/anti-HIV negative detection period7,8 and 5.3 days for the p24Ag-positive/anti-HIV-negative detection period7. To determine the infectious pre-ID-NAT window period in risk day equivalents, formulas published by Weusten et al. were used, with 50% and 95% lower limits of detection (LODs) by ID-NAT of 2.7 and 18.4 copies/mL9, a transfusion plasma volume of 20 mL for RBC components, a 50% minimum infectious dose (MID50) of 3.1 (range between 1 and 10) HIV virions10 and a doubling time of the virus during the ramp-up phase of infection of 0.85 days7; this yielded a residual infectious WP for ID-NAT screened blood of 2.9 days. To determine the pre-p24Ag infectious WP in risk day equivalents we used a 50% and 95% LOD for p24Ag EIA of 10,000 and 64,000 copies/mL8, which yielded an infectious WP of 13.0 days for p24Ag and anti-HIV screened RBC donations. We also investigated the impact of increasing the MID50 to less conservative values for stored RBCs of between 10 and 1000 virions10 which reduced the infectious WP for ID-NAT/anti-HIV screened blood from 2.9 to between 1.7 and 0.04 days, respectively, and for p24Ag/anti-HIV screened blood from 13.0 days to between 11.6 and 5.9 days, respectively. The rates of detection of donations confirmed as NAT yields were used to estimate the ID-NAT residual TT-HIV risks, and the rates of NAT yield donations that tested p24Ag positive were used to estimate the p24Ag residual TT-HIV risks.
Lookback and Traceback
Lookback was performed on the recipient of the previous donation when a subsequent donation from the donor confirmed HIV positive (donor triggered), whereas Traceback was performed when a recipient alleges potential acquisition of HIV from a transfusion (recipient triggered). If the recipient and donor are both HIV positive, phylogenetic sequencing was performed by NICD to confirm transmission. Due to inconsistent lookback documentation until 2010, we limited reporting from 2010 to 2015.
Regression analysis
We fit logistic regression models for total HIV-positive donations (concordant, NAT yield and serology yield) and for NAT yield donations. Crude and adjusted odds ratios were computed (using single and multiple predictors, respectively) and 95% confidence intervals (CI) estimated using the profile likelihood method. Owing to potential bias resulting from the small number of events, we applied the Firth correction to NAT yield models11. The predictors investigated were donor type, donor race-ethnicity, donor age, gender and geographic region of collection. Estimation was performed using the logistf R package12.
Results
Donor demographics
In the ten years following implementation of revised donor eligibility and recruitment policies and implementation of ID-NAT, SANBS collected 7,736,125 whole blood donations of which 947,594 (12.2%) were collected from FT donors, 877,385 (11.3%) from lapsed donors (>1 year inter-donation interval) and 5,910,737 (76.4%) from frequent repeat donors (<1 year inter-donation interval). The majority of blood donations came from white (67%), male (59%), and >30 years old donors (58%) (Supplementary Table 1). The only significant change in demographics in the donor base over the decade was the number and proportion of collections by different racial-ethnic groups (Figure 1). An additional 203,417 donations were collected from black African donors in 2015 compared to 2005, with the proportion of donations from black African donors increasing from 6% in 2005 to 30% in 2015(p<0.00001). The proportion of donations collected from black African FT donors increased from 19% in 2005 to 54% in 2015 by an additional 44,171 donations. Moreover, black African repeat donors contributed an additional 132,989 donations in 2015 compared with 2005, increasing the proportion of repeat donations from black African donors from 5% to 26% (p<0.00001).
Figure 1:
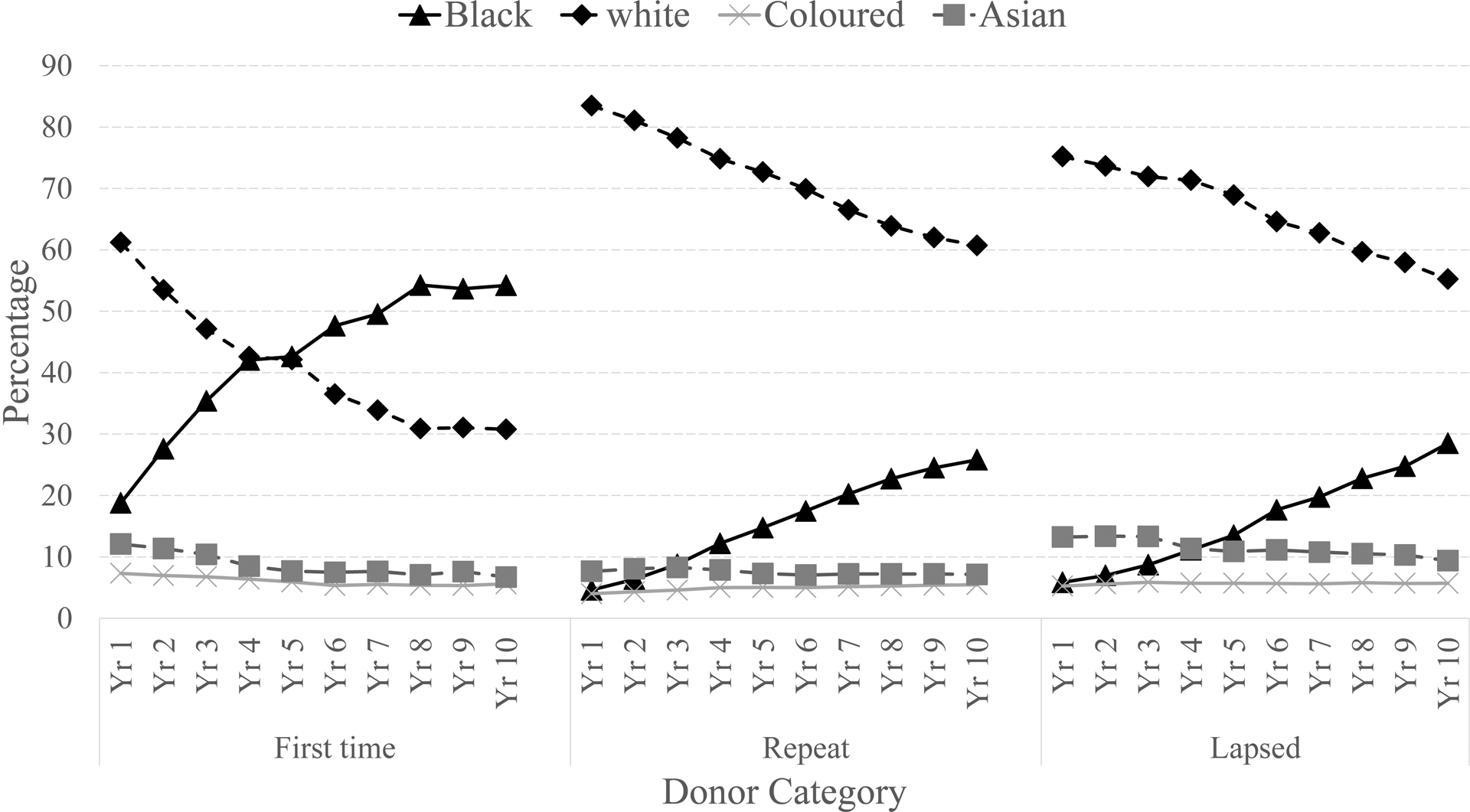
Proportion of collections over a ten year period by ethnic group and donor type
HIV infection rates by donation and donor demographic categories
There were a total of 15,702 (0.2%) HIV positive donations over the 10 year period of which 10,765 (69%) were from FT donors, 2,430 (15%) from lapsed donors and 2,504 (16%) from frequent repeat donors (Table 1). In the first 5 years there was a significant increase in HIV prevalence in FT donors, from 0.70% to 1.27% (p<0.00001), but that rate later decreased to 1.14% (supplementary Table 2, Figure 2). During the ten years the prevalence of HIV in black African FT donors decreased from 3.18% to 1.97% (p<0.00001) (Supplementary Table 2 & Figure 2).
Table 1:
Total HIV positive donations and NAT yield and serology yield rates by donation status, donor demographics and SANBS region.
| Collections | Total HIV Positive Cases | NAT Yield Cases | Serology Yield Cases | ||||
|---|---|---|---|---|---|---|---|
| Na | N | % | N | x:105 | N | x:105 | |
| Race by Donor type | |||||||
| All | 7 736 202 | 15 702 | 0.20 | 481 | 6.2 | 206 | 2.7 |
| Repeat | 5 910 780 | 2 504 | 0.04 | 300 | 5.1 | 3 | 0.1 |
| FT | 947 632 | 10 765 | 1.14 | 137 | 14.5 | 185 | 19.5 |
| Lapsed | 877 381 | 2 430 | 0.28 | 44 | 5.0 | 18 | 2.1 |
| Black-African | 1 506 435 | 13 810 | 0.92 | 411 | 27.3 | 189 | 12.5 |
| Repeat | 939 621 | 2 075 | 0.22 | 245 | 26.1 | 2 | 0.2 |
| FT | 418 418 | 9 919 | 2.37 | 129 | 30.8 | 171 | 40.9 |
| Lapsed | 148 363 | 1 813 | 1.22 | 37 | 24.9 | 16 | 10.8 |
| Non Black-African | 6 229 767 | 1 892 | 0.03 | 70 | 1.1 | 17 | 0.3 |
| Repeat | 4 971 159 | 429 | 0.01 | 55 | 1.1 | 1 | 0.0 |
| FT | 529 214 | 846 | 0.16 | 8 | 1.5 | 14 | 2.6 |
| Lapsed | 729 018 | 617 | 0.08 | 7 | 1.0 | 2 | 0.3 |
| Race | |||||||
| White | 5 155 955 | 833 | 0.02 | 36 | 0.7 | 5 | 0.1 |
| Black African | 1 506 435 | 13 810 | 0.92 | 411 | 27.3 | 189 | 12.5 |
| Asian | 621 231 | 239 | 0.04 | 9 | 1.4 | 3 | 0.5 |
| Coloured | 397 316 | 630 | 0.16 | 20 | 5.0 | 5 | 1.3 |
| Unknown | 55 265 | 190 | 0.34 | 5 | 9.0 | 4 | 7.2 |
| Gender | |||||||
| Male | 4 586 361 | 5 995 | 0.13 | 186 | 4.1 | 50 | 1.1 |
| Female | 3 149 832 | 9 706 | 0.31 | 295 | 9.4 | 156 | 5.0 |
| Unknown | 9 | 1 | 11.11 | ||||
| Age | |||||||
| <20 | 1 392 820 | 2 975 | 0 | 99 | 7.2 | 23 | 1.7 |
| 20 to 25 | 1 072 738 | 3 810 | 0.36 | 142 | 13.2 | 23 | 2.1 |
| 26 to 30 | 749 781 | 2 993 | 0.40 | 93 | 12.4 | 31 | 4.1 |
| 31 to 40 | 1 520 635 | 3 661 | 0.24 | 84 | 5.5 | 80 | 5.3 |
| 41 to 50 | 1 470 292 | 1 682 | 0.11 | 49 | 3.3 | 33 | 2.2 |
| >50 | 1 529 936 | 581 | 0 | 14 | 1.5 | 16 | 1.9 |
409 donors had unknown donor type and were not included in the table
Figure 2:
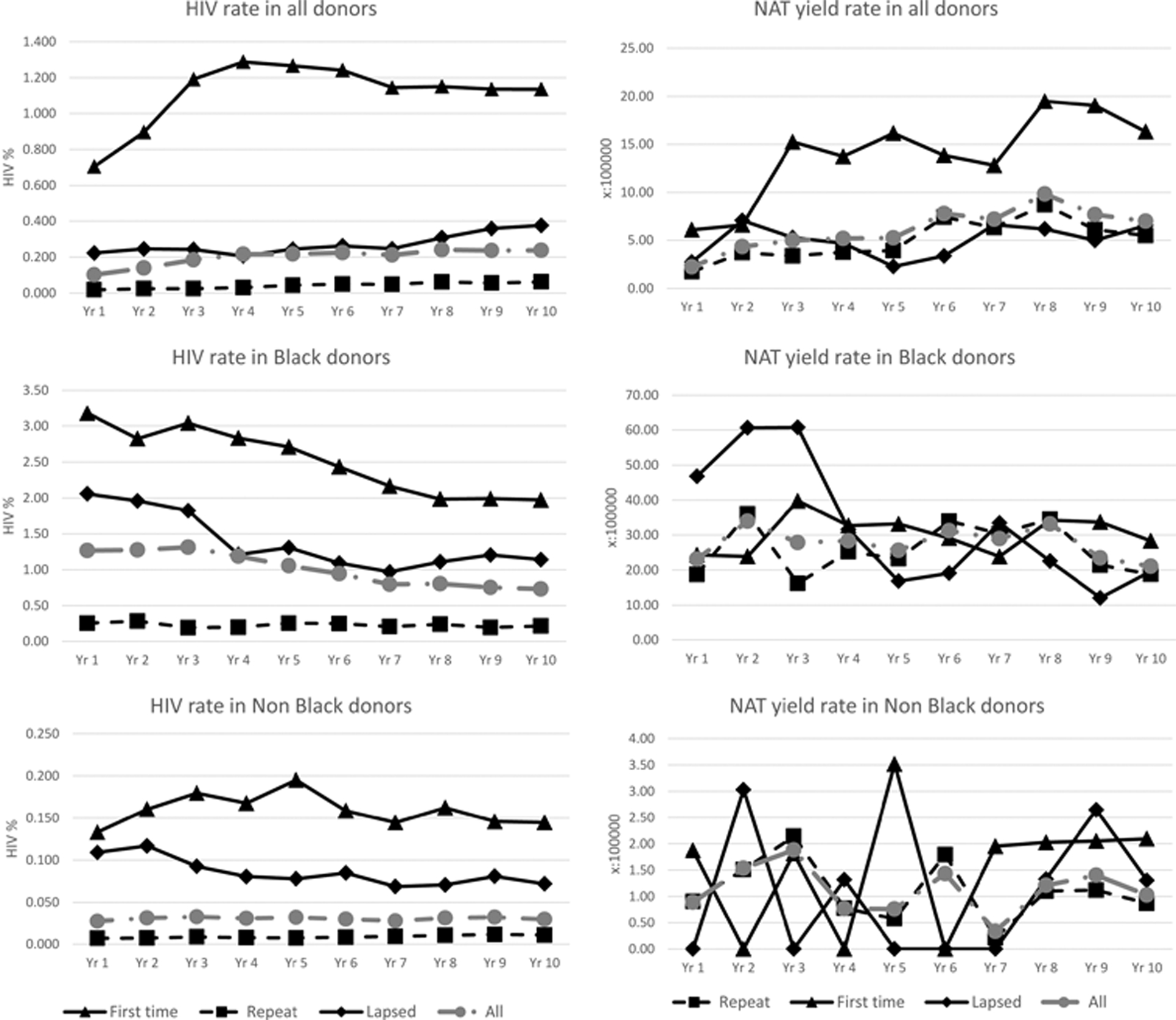
HIV rate and NAT yield rate trend by race and donor type
Bivariate (models 1–5) and multivariate (models 6–7) logistic regressions on HIV-positive donations are shown in Table 2. In bivariate models, very large crude odds ratios were observed for FT donation status (OR 27.10; 95% CI 25.95,28.31) and black race-ethnicity (OR 57.26; 53.43,61.45), while female sex was also highly significant (OR 2.36; 2.29,2.44). A multivariate model including these variables and also controlling for age and geographic region (model 7) resulted in lower adjusted odds ratios, but FT donation status (adjusted OR 12.49; 11.93,13.07), black African race-ethnicity (adj. OR 30.20; 28.13,32.47) and female sex (adj. OR 1.54; 1.49,1.59) remained highly significant.
Table 2:
Logistic regression models
| Models for HIV-positive donation | Models for NAT yield donation | |||||
|---|---|---|---|---|---|---|
| Models 1–5 | Model 6 | Model 7 | Models 8–12 | Model 13 | Model 14 | |
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) |
| Donor type | ||||||
| Repeat | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| FT | 27.10 (25.95,28.31) | 12.49 (11.93,13.07) | 12.51 (11.96,13.10) | 2.85 (2.33,3.48) | 1.03 (0.83,1.27) | 1.04 (0.84,1.28) |
| Lapsed | 6.55 (6.19,6.93) | 5.38 (5.09,5.70) | 5.44 (5.14,5.75) | 1.00 (0.72,1.35) | 0.76 (0.54,1.03) | 0.76 (0.54,1.03) |
| Race | ||||||
| White | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Black African | 57.26 (53.43,61.45) | 30.20 (28.13,32.47) | 31.08 (28.94,33.43) | 38.60 (27.93,55.08) | 31.76 (22.79,45.64) | 32.63 (23.36,46.99) |
| Asian | 2.38 (2.06,2.75) | 1.81 (1.57,2.09) | 1.65 (1.43,1.91) | 2.16 (1.00,4.23) | 2.24 (1.03,4.39) | 1.82 (0.83,3.63) |
| Coloured | 9.83 (8.86,10.90) | 6.91 (6.23,7.67) | 7.34 (6.60,8.15) | 7.29 (4.17,12.39) | 6.40 (3.66,10.89) | 6.44 (3.66,11.05) |
| Sex | ||||||
| Male | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Female | 2.36 (2.29,2.44) | 1.54 (1.49,1.59) | 1.61 (1.55,1.66) | 2.31 (1.92,2.78) | 2.07 (1.72,2.50) | 2.19 (1.82,2.65) |
| Age | ||||||
| 16 to 19 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| 20 to 25 | 1.67 (1.59,1.75) | 2.43 (2.32,2.55) | 2.57 (2.45,2.70) | 1.86 (1.44,2.41) | 2.15 (1.66,2.79) | 2.30 (1.78,2.99) |
| 26 to 30 | 1.87 (1.78,1.97) | 3.24 (3.08,3.42) | 3.50 (3.32,3.69) | 1.75 (1.32,2.32) | 2.12 (1.58,2.82) | 2.34 (1.75,3.12) |
| 31 to 40 | 1.13 (1.07,1.18) | 3.14 (2.99,3.30) | 3.43 (3.27,3.61) | 0.78 (0.58,1.04) | 1.34 (1.00,1.80) | 1.51 (1.12,2.03) |
| 41 to 50 | 0.54 (0.50,0.57) | 2.50 (2.35,2.66) | 2.72 (2.55,2.89) | 0.47 (0.33,0.66) | 1.15 (0.81,1.62) | 1.29 (0.90,1.82) |
| >50 | 0.18 (0.16,0.19) | 1.69 (1.54,1.85) | 1.85 (1.69,2.03) | 0.13 (0.07,0.22) | 0.55 (0.30,0.94) | 0.62 (0.34,1.06) |
| Region | ||||||
| Egoli | (ref) | (ref) | (ref) | (ref) | ||
| Eastern Cape | 1.04 (0.97,1.11) | 1.33 (1.25,1.43) | 1.33 (0.90,1.95) | 1.67 (1.13,2.46) | ||
| Free State/Northern Cape | 0.79 (0.73,0.84) | 1.52 (1.41,1.63) | 1.32 (0.90,1.92) | 2.30 (1.56,3.36) | ||
| Kwazulu-Natal | 1.43 (1.36,1.51) | 1.81 (1.72,1.90) | 2.24 (1.68,3.01) | 2.66 (1.98,3.61) | ||
| Mpumalanga | 1.78 (1.68,1.87) | 2.62 (2.48,2.76) | 1.73 (1.22,2.44) | 2.55 (1.80,3.61) | ||
| Northern | 0.94 (0.89,0.99) | 1.04 (0.98,1.10) | 1.20 (0.86,1.66) | 1.37 (0.98,1.92) | ||
| Vaal | 1.02 (0.96,1.08) | 1.40 (1.32,1.48) | 1.14 (0.80,1.63) | 1.57 (1.10,2.25) | ||
Bold indicates statistical significance (95% CI does not encompass 1)
HIV NAT yield rates by donation and donor demographic categories
ID-NAT interdicted 481 (1:16,100) HIV confirmed positive donations that were anti-HIV negative, of which 137 (1:7,000) were in FT donors, 44 (1:20,000) in lapsed donors and 300 (1:19,700) in frequent repeat donors. Of the 462 confirmed HIV NAT yield donations that were tested for p24Ag, 285 (61.7%) tested p24Ag negative and 177 (38.3%) tested p24Ag positive. One hundred and eighty three of the p24Ag negative NAT yield donations (1:32,300) were collected from frequent repeat donors, 80 from FT donors (1:11,800) and 22 from lapsed donors(1:39,900).
The NAT yield rate in all race-ethnicities combined was significantly higher in FT (14.5/100,000) compared with repeat donors (5.1/100,000) (OR 2.85; 95% CI 2.33,3.48). However, as shown in Table 2, when controlling for race, sex, age and geographic region in multivariate models, the coefficients for donor type were no longer statistically significant (adj. OR 1.04; 0.84,1.28). In the full model (model 14), black African race-ethnicity remained a substantial and significant predictor (adj. OR 32.63; 23.36,46.99), while coloured race-ethnicity (adj. OR 6.44; 3.66,11.05) and female sex (adj. OR 2.19; 1.82,2.65) also remained significant.
HIV serology yield rates by donation and donor demographic categories
Serologic testing detected 206 confirmed HIV antibody positive, NAT negative donations (2.6/100,000), of which the majority (186) were from FT donors (Table 1 and supplementary Table 2). There was a significant increase in the rate of serology yield donations from FT donors during the 10-year period, from 3/100,000 to 39/100,000 (p=0.0001) (supplementary Table 2). When expressed as a percent of all HIV positive donations there was a similar increase over time from 0.27% in 2006 to 2.43% in 2015 (Figure 3).
Figure 3:
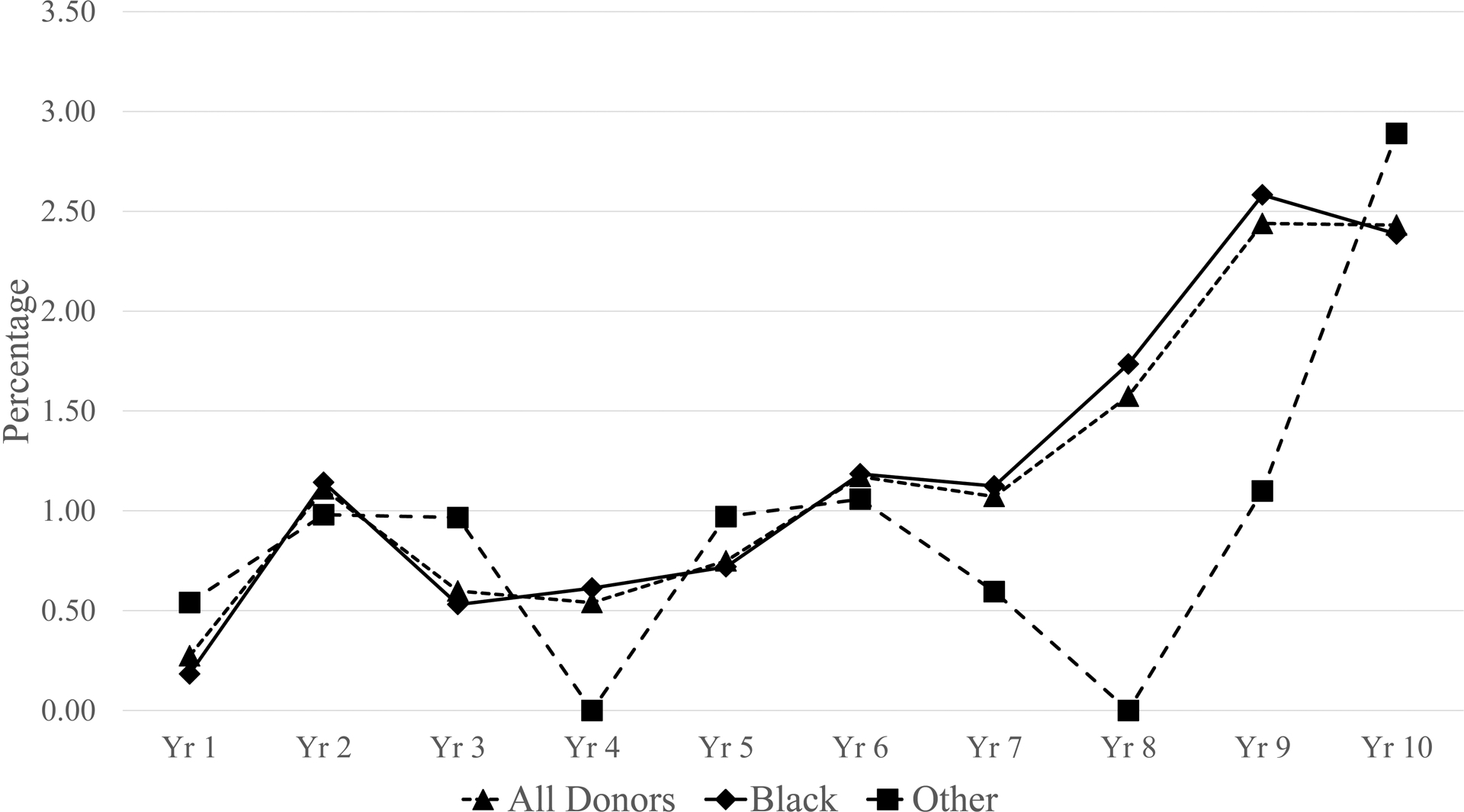
Proportion of HIV positives that are serology yields by year and race
Impact of screening strategy and changing donor demographics on WP transmission risk
Using a MID50 of 3.16 virions in the transfused RBC component plasma, the WP NAT yield ratio model estimated an overall residual risk of 11.9/million RBC transfusions for the ten year period. Figure 4a compares the TT risk by year when ID-NAT testing is performed compared to p24Ag screening (both in combination with a 3rd generation anti-HIV assay). Figure 4a also shows the TT risk estimated at 26/million RBC transfusions by Heyns and colleagues in the years immediately prior to the implementation of ID-NAT3. The TT risk in 2005 would have been 24/million RBC transfusions had ID-NAT not been implemented in place of p24Ag testing, and would have increased to 71.2/million RBC transfusions in 2015 given the increasing proportion of donations by black African donors. However, the WP TT risk was estimated to be much lower at 13.4/million RBC transfusions in year 10 following implementation of ID-NAT. Figure 4b shows the TT risk by donor type and year when NAT testing is performed. FT donors have a 3-fold higher TT risk than repeat and lapsed donors.
Figure 4a:
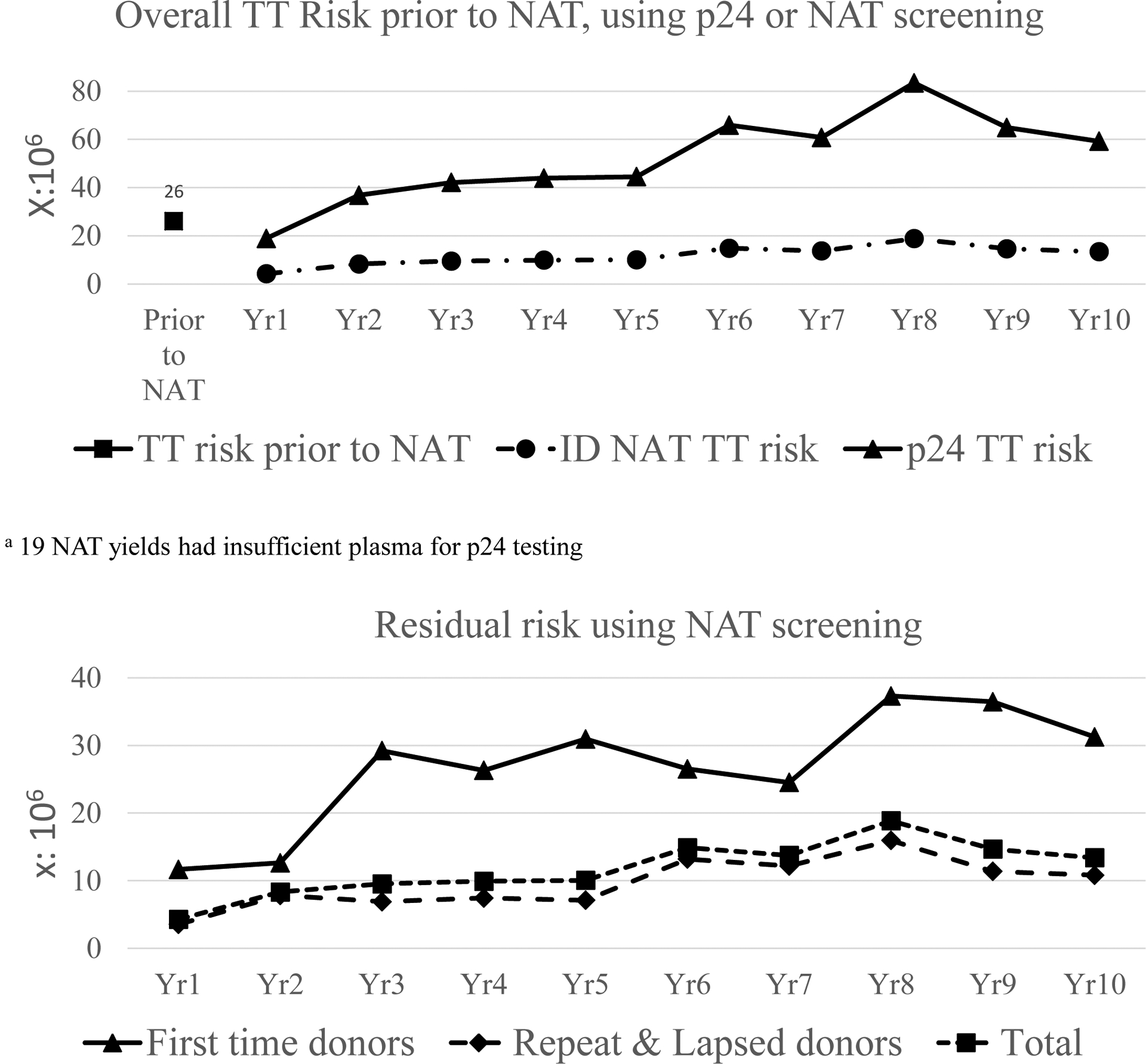
Annual TT risk per million donations when a p24 Ag and antibody or NAT and antibody screening strategy is used (compared to previously reported TT risk prior to the implementation of NAT). Annual Residual risk per million RBC transfusions for NAT screened FT, repeat and all donations
Supplementary Figure 1 shows the impact of alternative estimates of infectivity on the TT risk estimates. When the MID50 estimate is increased to between 31.6 and 316 virions in a RBC unit, the TT risk using ID-NAT and antibody screening is between 3.47 and 0.52 per million RBC transfusions compared to between 47.31 and 44.16 per million if the previous screening strategy of p24 Ag plus antibody testing had been maintained.
HIV genotypes and drug resistance profiles in NAT yield donors
We tested each NAT yield donation with sufficient volume and viral load for drug resistance and genotyping to establish these virological parameters for cases with recently acquired infections. Of the 481 NAT yields, 168 were unable to be tested due to viral loads <1000 copies/ml (minimal viral load for genetic testing at NICD) and 86 could not be amplified probably also due to low viral load or RNA stability. Of the 227 donations genotyped, 204 (90%) were subtype C without any drug resistance mutations and an additional 18 (8%) were subtype C with one or more drug resistance mutations. Five (2%) were non-subtype C (one A1, three B and one CRF02_AG) of which two (0.9%) of the subtype B donations had drug resistance mutations. These 20 donors who are HIV RNA only positive and have drug resistance mutations, suggest recent acquisition of HIV with drug resistance mutations.
Lookback and Traceback investigations
Between 2010 and 2015, 2,887 Lookback investigations (out of 5.8 million HIV-negative transfusions) were initiated based on lapsed or repeat donors who seroconverted to HIV antibody positivity. Of these 1,166 cases (40%) remained unresolved (i.e. no recipient outcome data were provided by hospitals), 396 (14%) patients had died after transfusion, 262 (9%) patients tested HIV positive prior to the transfusion, 236 (8%) patients tested HIV negative following investigation, 23 (0.85%) declined further testing and 15 (0.5%) patients tested HIV positive post-transfusion without documentation of their infection status prior to the implicated transfusion. Of these 15 HIV positive patients identified as possible TT cases, 8 showed no genetic linkage between the donor and recipient viruses, 2 were unlikely transfusion related due to a very short time interval to HIV positive results following transfusion (<6 days), 3 were unresolved but were unlikely to be TT cases (1 was already on ART 3 months after the transfusion and could not be amplified, and 2 never returned for testing), and 1 case was a confirmed HIV transmission following transfusion of a WP RBC unit. This case was confirmed by 100% sequence identity of HIV-RNA isolated from recipient and donor follow up samples. The Ultrio Plus S/CO ratio in the transfused donation was elevated at 0.65 but below the cut off of 1.00. Unfortunately, the low viral load could not be confirmed or quantified by replicate NAT testing because the frozen plasma component was not retrieved from the plasma fractionation manufacturer.
During the same time there were 48 recipients of blood transfusions who reported contracting HIV from the transfusion (Traceback investigations). Of these 34 were established not to be TT HIV cases with all donors investigated and negative HIV test results subsequent to the implicated transfusion; however 14 Traceback cases remained unresolved as all implicated blood donors could not be contacted for further testing.
Discussion
Our 10-year analysis shows that screening blood donations by ID-NAT allowed for substantial increase in the number and proportion of donations from the majority black African population without reducing the safety of the blood supply in South Africa. Over ten years the proportion of donations from black African donors increased five-fold. The increase was seen in both FT and repeat donors, with the number (percent) of donations from black African FT donors increasing from 12,333 (19%) donations in 2005 to 56,504 (54%) donations in 2015 and collections from black African repeat donors increasing from 26,657 donations in 2005 (5%) to 159,646 donations (26%) in 2015. The TT-HIV risk was estimated at 26 per million RBC transfusions prior to the implementation of ID-NAT screening and expected to reduce to 17 per million RBC transfusions if screening by ID NAT is implemented.3 We estimated in this study that over 10 years following implementation of revised donor eligibility criteria, recruitment policies and ID-NAT, the TT HIV risk was reduced from 24 per million in 2005 (with p24 Ag and antibody but without ID-NAT testing) to 13.4 per million RBC transfusions in 2015 (with ID-NAT testing).
Black African race-ethnicity, female gender and donor age of <30 years were associated with higher prevalence of HIV infection, consistent with HIV infection demographic associations in the larger population in South Africa1. The HIV prevalence in black African FT donors was reduced by a third during the ten years, which we attribute to our improved pre-donation education programs.
When HIV NAT yield cases (representing recent infections within 1–4 weeks prior to donation) are analysed with all race ethnicities together, the rate is approximately 3-fold higher in FT donors compared to repeat donors (OR 2.85; 95% CI 2.33,3.48), while the rate in black African donors was 38-fold higher than in white donors. This higher rate of NAT yields in FT donors is therefore confounded by race. When controlled for race ethnicity, NAT yield donations were detected at the same rate in FT and repeat donors (Table 2), suggesting that non-compliance to the risk behaviour questions in the donor questionnaire is comparable, irrespective of prior donation education and experience. The fact that there was a significantly higher NAT yield rate in FT donors than in repeat donors overall, but no difference observed within ethnic groups, is thus explained by the higher proportion of black African donors in FT than in repeat donors. Similarly in a large international study of HIV infection rates in donors there was no difference in HIV NAT yield rates between FT and repeat donors in Asia and in Europe13. The higher rate of HIV NAT yield cases in black African female donors may well be due to gender inequality, which is often compounded by cultural, legal and political factors that impede a woman’s ability to protect herself from HIV14. In this case a prospective female donor may still be compliant to the risk behaviour question that asks about multiple sexual partners. In this study women were almost 3 fold more likely to be HIV positive and had double the NAT yield rate than men. Currently a study to determine risk factors that are not covered by the pre-donation questionnaire is underway to inform future enhancements of education and eligibility policies.
ID-NAT interdicted 481 HIV confirmed positive donations that tested negative for HIV antibodies, the majority of which would have been made into 2 or 3 blood components. Most transfused components from these interdicted donations would likely have caused TT-HIV in a recipient considering the viral loads ranged from low levels to >1 million copies/mL8. We identified only one confirmed case of TT-HIV during the ten-year period linked to a RBC component derived from an ID-NAT negative donation. This contrasts with 1–2 annual confirmed TT-HIV cases documented by SANBS’ Lookback programs during the 5 years prior to the implementation of ID-NAT.3 To our knowledge this is the first and only reported case of TT-HIV by ID-NAT tested blood in the world.
We acknowledge that the Lookback program in South Africa is not highly effective at detecting TT-HIV, since we could not determine whether TT-HIV occurred in 63% of the almost 3,000 cases investigated (40% unresolved, 14% deceased and 9% HIV positive at the time of transfusion). Therefore, it is possible that TT–HIV risk is higher than the 1 in 7.7 million transfusions issued over ten years that we documented. We modelled and reported a worst-case scenario of residual TT-HIV risk post ID-NAT of 12 per million transfusions, or 10 TT-HIV cases per year. However the infectivity of HIV in stored RBCs is likely 10–100 fold reduced relative to freshly collected blood10. When the MID50 estimate was changed from the very conservative 3.16 virions to a more likely 31.6–316 virions in the transfused component inoculum, a TT-HIV risk of 3.47 – 0.52 per million RBC transfusions was estimated, which is closer to the documented ID-NAT breakthrough TT rate of 0.13 per million (1/7.73 million). The true TT-HIV rate is probably somewhere between the 0.13/million donations (underestimated risk from lookback data) and 12/million donations (overestimated worst case modelling scenario), and likely in the range of the 0.5 to 3.5/million donations using MID50 estimates of between 31.6 and 316 virions.
Our breakthrough case highlights that ID-NAT screening does not remove all of the risk of TT-HIV. In our study, we did note that along with the increase in donations from black African donors, the TT-HIV risk increased annually over 8 years from 4/million to 19/million RBC transfusions, but then decreased in the last two years to 13/million which could be attributed to the national roll out of ART and resultant two fold decrease in HIV incidence from 1.86% to 0.91% during the last decade in the general population,1 which is reassuring for the future. Nevertheless, in the absence of pathogen inactivation for red blood cells, increasing donations from donors who are at a higher risk of an acute HIV infection must be implemented carefully, optimally with targeted donor education and extensive monitoring to balance blood safety with blood sufficiency.
Ten percent of the recently acquired HIV infections in donors had sequences consistent with transmitted drug resistance, similar to the finding of 9% ART drug resistant HIV in the first South African national survey of pre-treatment resistance.15 There were too few non-subtype C cases to investigate any differences in drug resistance prevalence between subtypes. This molecular surveillance aspect of our study demonstrates the important role of aligning national blood donor programs with public health reference laboratories to track changes in HIV genotypes and transmitted drug resistance in recently infected donors which would be similar to transmitted drug resistance in the general population.16,17
The increase in serology yields, using the same serological screening and confirmatory assays over the ten year period from 0.27 to 2.43% of HIV infected donors, is concerning. We are currently investigating the reasons for this increase, which is in part due to donations by donors who were aware of their HIV infections and taking ART which suppressed their viral loads to below the detection limit of even ID-NAT18 (Sykes W, Manuscript in preparation). This indicates that even more pre-donation education of donors is required.
Overall these analyses demonstrate that the change in donor eligibility and recruitment policies linked to introduction of ID-NAT were extremely successful, resulting in a five-fold increase in donations from black donors with a reduction in modelled TT-HIV risk of between 2- and 50-fold depending on infectivity of HIV in stored blood components. Moreover, in the ten year period the observed one TT-HIV case was approximately 20-fold lower as compared with the period before the introduction of ID-NAT when two cases per annum were observed2,3 However, going forward the trend in modelled residual risk must be continuously monitored to ensure the safety of the South African blood supply.
Supplementary Material
Figure 1 Supplementary: Impact of HIV-1 infectivity on residual WP transmission risk with ID-NAT (diamonds) and p24-Ag testing (squares) estimated over the 10 year screening period. Dotted lines represent 95% confidence intervals (CI) around 50% LODs i.e. 2.7 (2.0–3.5) and 10,000 (5000–20,000) copies/mL for ID-NAT and p24-Ag detection respectively, whereby variability in analytical sensitivity of NAT and p24-Ag reagent batches, RBC plasma transfusion volume and uncertainty in standardization in true copy or virion numbers is ignored.
Footnotes
Conflicts of interest: Nico Lelie may be paid by Grifols, the manufacturer of the NAT assay used, for review of the draft paper
There are no conflicts of interest for all other authors
References
- 1.Statistics South Africa -Mid Year population estimates. Statistical release 2017;http://www.statssa.gov.za/publications/P0302/P03022017.pdf. [Google Scholar]
- 2.Heyns Adu P Risk of transmitting HIV and other diseases with a blood transfusion in South Africa. . CME (South African med assoc) 1999: 854–61. [Google Scholar]
- 3.Heyns Adu P, Benjamin RJ, Swanevelder JP, Laycock ME, Pappalardo BL, Crookes RL, Wright DJ, Busch MP. Prevalence of HIV-1 in blood donations following implementation of a structured blood safety policy in South Africa. JAMA 2006;295: 519–26. [DOI] [PubMed] [Google Scholar]
- 4.Fang CT, Field SP, Busch MP, Heyns Adu P. Human immunodeficiency virus-1 and hepatitis C virus RNA among South African blood donors: estimation of residual transfusion risk and yield of nucleic acid testing. Vox Sang 2003;85: 9–19. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen M, Lelie N, Sykes W, Crookes R, Swanevelder J, Gaggia L, Le Roux M, Kuun E, Gulube S, Reddy R. Impact of individual-donation nucleic acid testing on risk of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission by blood transfusion in South Africa. Transfusion 2009;49: 1115–25. [DOI] [PubMed] [Google Scholar]
- 6.Busch MP, Glynn SA, Stramer SL, Strong DM, Caglioti S, Wright DJ, Pappalardo B, Kleinman SH, Group N-RNS. A new strategy for estimating risks of transfusion-transmitted viral infections based on rates of detection of recently infected donors. Transfusion 2005;45: 254–64. [DOI] [PubMed] [Google Scholar]
- 7.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003;17: 1871–9. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen M, Coleman C, Mitchel J, Reddy R, van Drimmelen H, Fickett T, Busch M, Lelie N. Comparison of human immunodeficiency virus assays in window phase and elite controller samples: viral load distribution and implications for transmission risk. Transfusion 2013;53: 2384–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weusten J, Vermeulen M, van Drimmelen H, Lelie N. Refinement of a viral transmission risk model for blood donations in seroconversion window phase screened by nucleic acid testing in different pool sizes and repeat test algorithms. Transfusion 2011;51: 203–15. [DOI] [PubMed] [Google Scholar]
- 10.Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion 2009;49: 2454–89. [DOI] [PubMed] [Google Scholar]
- 11.Firth D Bias Reduction of Maximum-Likelihood-Estimates. Biometrika 1993;80: 27–8. [Google Scholar]
- 12.Heinze G, Ploner M. Logistf: Firth’s Bias-Reduced Logistic Regression R Package version 1.22 2016. [Google Scholar]
- 13.Bruhn R, Lelie N, Custer B, Busch M, Kleinman S, International NATSG. Prevalence of human immunodeficiency virus RNA and antibody in first-time, lapsed, and repeat blood donations across five international regions and relative efficacy of alternative screening scenarios. Transfusion 2013;53: 2399–412. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Consolidated guideline on sexual and reproductive health and rights of women living with HIV. http://apps.who.int/iris/bitstream/handle/10665/254634/WHO-RHR-17.03-eng.pdf;jsessionid=63D4481D6B40CDC8EEA9A51E6140497E?sequence=1 2017. [PubMed]
- 15.Steegen K, Carmona S, Bronze M, Papathanasopoulos MA, van Zyl G, Goedhals D, MacLeod W, Sanne I, Stevens WS. Moderate Levels of Pre-Treatment HIV-1 Antiretroviral Drug Resistance Detected in the First South African National Survey. PLoS One 2016;11: e0166305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delwart E, Kuhns MC, Busch MP. Surveillance of the genetic variation in incident HIV, HCV, and HBV infections in blood and plasma donors: implications for blood safety, diagnostics, treatment, and molecular epidemiology. J Med Virol 2006;78 Suppl 1: S30–5. [DOI] [PubMed] [Google Scholar]
- 17.Delwart E, Slikas E, Stramer SL, Kamel H, Kessler D, Krysztof D, Tobler LH, Carrick DM, Steele W, Todd D, Wright DJ, Kleinman SH, Busch MP, Group N-R -IS. Genetic diversity of recently acquired and prevalent HIV, hepatitis B virus, and hepatitis C virus infections in US blood donors. J Infect Dis 2012;205: 875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeulen M, van den Berg K, Jacobs G, Custer B, Swanevelder R, Jentch U, Reddy R, Wiesner L, Maartens G, Murphy EL. Discovery of “False HIV Elite Controllers” among South African Blood donors ISBT. Copenhagen, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 Supplementary: Impact of HIV-1 infectivity on residual WP transmission risk with ID-NAT (diamonds) and p24-Ag testing (squares) estimated over the 10 year screening period. Dotted lines represent 95% confidence intervals (CI) around 50% LODs i.e. 2.7 (2.0–3.5) and 10,000 (5000–20,000) copies/mL for ID-NAT and p24-Ag detection respectively, whereby variability in analytical sensitivity of NAT and p24-Ag reagent batches, RBC plasma transfusion volume and uncertainty in standardization in true copy or virion numbers is ignored.


