PURPOSE
We determined the safety and efficacy of coadministration of CD19- and CD22-chimeric antigen receptor (CAR) T cells in patients with refractory disease or high-risk hematologic or isolated extramedullary relapse of B-acute lymphoblastic leukemia.
PATIENTS AND METHODS
This phase II trial enrolled 225 evaluable patients age ≤ 20 years between September 17, 2019, and December 31, 2021. We first conducted a safety run-in stage to determine the recommended dose. After interim analysis of the first 30 patients treated (27 at the recommended dose) showing that the treatment was safe and effective, the study enrolled additional patients according to the study design.
RESULTS
Complete remission was achieved in 99.0% of the 194 patients with refractory leukemia or hematologic relapse, all negative for minimal residual disease. Their overall 12-month event-free survival (EFS) was 73.5% (95% CI, 67.3 to 80.3). Relapse occurred in 43 patients (24 with CD19+/CD22+ relapse, 16 CD19–/CD22+, one CD19–/CD22–, and two unknown). Consolidative transplantation and persistent B-cell aplasia at 6 months were associated with favorable outcomes. The 12-month EFS was 85.0% (95% CI, 77.2 to 93.6) for the 78 patients treated with transplantation and 69.2% (95% CI, 60.8 to 78.8) for the 116 nontransplanted patients (P = .03, time-dependent covariate Cox model). All 25 patients with persistent B-cell aplasia at 6 months remained in remission at 12 months. The 12-month EFS for the 20 patients with isolated testicular relapse was 95.0% (95% CI, 85.9 to 100), and for the 10 patients with isolated CNS relapse, it was 68.6% (95% CI, 44.5 to 100). Cytokine release syndrome developed in 198 (88.0%) patients, and CAR T-cell neurotoxicity in 47 (20.9%), resulting in three deaths.
CONCLUSION
CD19-/CD22-CAR T-cell therapy achieved relatively durable remission in children with relapsed or refractory B-acute lymphoblastic leukemia, including those with isolated or combined extramedullary relapse.
INTRODUCTION
Autologous CD19-directed chimeric antigen receptor (CAR) T-cell therapy has revolutionized the management of relapsed or refractory pediatric acute lymphoblastic leukemia (ALL).1-6 Registry data show that tisagenlecleucel induces complete remission in 85.5% of cases and results in a 12-month event-free survival (EFS) of 52.4% in children treated for relapsed or refractory B-ALL.7 Approximately 50% of patients experienced relapse within 1 year,3-5,7 owing to loss of CAR T-cell persistence or loss of CD19 antigen because of splice variants, acquired genetic mutations, or lineage switch.8,9 Although CD22-targeted CAR T-cell therapy induces complete remission in 70%-80% of the patients in whom CD19-targeted CAR T-cell therapy failed, most experience relapse again.10-12 These observations led some investigators to use CAR T-cell therapy as a bridge to allogeneic transplantation,13 whereas others developed dual CD19-/CD22-targeted treatment to overcome antigen escape relapse.14-19
CONTEXT
Key Objective
Does coadministration of CD19- and CD22-chimeric antigen receptor T-cell therapy result in durable event-free survival in children with refractory disease or high-risk hematologic or extramedullary relapse of B-acute lymphoblastic leukemia?
Knowledge Generated
In this clinical trial that included 225 children, the 12-month event-free survival was 69.2% in patients treated for hematologic relapse without consolidative allogeneic hematopoietic cell transplantation, 95% for isolated testicular relapse and 68.6% for isolated CNS relapse.
Relevance (S. Bhatia)
-
Coadministration of CD19- and CD22-chimeric antigen receptor T-cell therapy may be a promising therapeutic strategy for patients with relapsed or refractory B-acute lymphoblastic leukemia. However, longer follow-up is needed to determine the durability of the response.*
*Relevance section written by JCO Associate Editor Smita Bhatia, MD, MPH.
Three recent studies showed the safety and feasibility of dual CD19-/CD22-targeted CAR T-cell therapy, but the results were not superior to those of the CD19-CAR T-cell therapy.14-16 Three other studies tested sequential administration of CD19-CAR T cells and CD22-CAR T cells, which yielded complete remission rates of 96%, 100%, and 85% and a 1-year leukemia-free survival of 52.9%, 79.5%, and 67.5%, respectively.17-19 Although this approach was associated with low rates of antigen-escape relapse, the limited CAR T-cell persistence raised concern of impending antigen-positive relapse.17-19 Preclinical studies have shown that CD19-targeting CAR T cells can downregulate CD22 expression in a subset of tumor cell line models.20 Therefore, we hypothesized that coadministration of CD19- and CD22-targeted CAR T cells would improve efficacy on the basis of the fundamental treatment principle for ALL that combination therapy forestalls the development of drug resistance and a preclinical model showing that simultaneous targeting may reduce the risk of antigen loss.21 Moreover, coadministration would avoid repeated lymphodepleting chemotherapy that eradicates CD19-CAR T cells. Here, we report the results of our clinical trial using this treatment approach.
PATIENTS AND METHODS
Study Design and Patient Population
This study (Chinese Clinical Trial Registry: ChiCTR2000032211), an open-label phase II, multicenter clinical trial, enrolled patients between September 17, 2019, and December 31, 2021. The study protocol and detailed eligibility criteria for three study cohorts are provided in the Protocol (online only). The first cohort for the safety run-in stage enrolled patients with refractory leukemia and hematologic relapse who did not achieve remission after ≥ 2 courses of remission induction or were ineligible for allogeneic transplantation. The second cohort for the phase II trial enrolled patients with refractory disease or hematologic relapse with unfavorable genotype, persistent disease after ≥ 2 treatment regimens for relapse, prior CD19-CAR T therapy, or allogeneic transplantation. The third cohort consisted of patients with isolated extramedullary relapse and negative minimal residual disease (MRD) defined as <0.01% of leukemia cells in bone marrow by flow cytometry. The study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review boards. Written informed consent was obtained from the parents, guardians, or patients, as appropriate.
During the safety run-in stage, one of the three patients treated at the initial dose of 1 × 107 CAR T cells/kg developed a grade 4 neurotoxicity. None of the subsequent three patients experienced grade ≥ 3 toxicity at a de-escalated dose of 5.0 × 106 CAR T cells/kg, which was determined as the recommended dose for patients with hematologic relapse. A dose between 5 × 106 and 1 × 107 CAR T cells/kg was used to treat isolated extramedullary relapse to enhance CAR T-cell proliferation in the setting of low antigen stimulation. After an interim analysis of the first 30 patients showed that the treatment was safe (Data Supplement, online only) and their EFS was superior to that of 46 historical patients treated with CD19-CAR T cells (Data Supplement), the study continued as planned. Consolidative transplantation was planned only for patients with KMT2A- or ZNF384-rearranged B-ALL to avoid myeloid lineage switch.22,23
Treatment
Generally, within 3 days of eligibility confirmation, CD3+ T lymphocytes were collected from peripheral blood (1-2 mL/kg) and CAR T cells were manufactured at the Shanghai Children's Medical Center. Briefly, after Ficoll-Hypaque gradient centrifugation and anti-CD3 Microbeads sorting, T cells were stimulated by anti-CD3/CD28 beads for 24-48 hours and were transduced with CD19-specific or CD22-specific CAR lentiviral vectors with 4-1BB costimulatory and CD3 zeta signaling domains. CD19- and CD22-specific CAR T cells were cultured separately. After 5-7 days in culture, CD19- and CD22-CAR T cells were pooled together at a ratio of 1:1, washed, resuspended in saline solution with 2.5% human serum albumin, and transported to the participating medical center (Data Supplement) where the patient received the infusion on day 0. The coordination of the timing of CAR T-cell production and lymphodepleting chemotherapy with fludarabine and cyclophosphamide are shown in the Data Supplement.
Outcomes
The primary end points included the recommended phase II dose of combined CD19- and CD22-CAR T cells, CAR T-cell infusion–related adverse effects, complete remission rate at day 28 postinfusion, and EFS and overall survival (OS) at 12 months with or without consolidative transplantation. Exploratory analyses were performed on the effect of sustained B-cell aplasia (as defined by the detection of < 1% CD19+ lymphocytes in peripheral blood or bone marrow) on treatment outcomes and the safety and outcomes of patients treated for isolated extramedullary relapse. Quantification of CAR T-cell persistence in peripheral blood and cytokine profiling are provided. Cytokine release syndrome and neurotoxicity related to CAR T-cell therapy were graded per the American Society of Transplant and Cellular Therapy criteria24; other adverse events were captured using the Common Terminology Criteria for Adverse Events (version 4.03). Complications were managed per the consensus statement of Mahadeo et al25 with minor modifications.
Statistical Analysis
EFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazards regression model was used for univariate and multivariate analyses of prognostic factors. Transplant was regarded as a time-dependent covariate in the Cox regression model for comparisons between patients who did or did not receive transplant, and display of survival curves was generated according to the method by Bernasconi et al.26 All analyses were preplanned as described in the protocol. Additional details are provided in the Data Supplement. Outcome data were updated on May 31, 2022.
RESULTS
Trial Population and Treatment
We enrolled 232 patients in the study, of whom 225 were evaluable, including 194 with refractory disease or hematologic relapse and 31 with isolated extramedullary relapse (Fig 1). The baseline characteristics are summarized in the Data Supplement. The median age at the time of enrollment for patients treated for refractory disease or hematologic relapse was 7.6 years (interquartile range [IQR], 4.8-10.8; range, 0.8-19.6 years). The median time from enrollment to infusion was 7 days (range, 6-12 days). The median dose of combined CD19- and CD22-CAR T cells was 5.6 × 106/kg (IQR, 4.1-7.6 × 106; range, 1.3-13.0 × 106). The median dose of CD19-CAR T cells was 2.7 × 106/kg (IQR, 1.9-3.7 × 106), and that of CD22-CAR T cells was 2.8 × 106/kg (IQR, 2.1-4.0 × 106). The median ratio of CD19-CAR T-cell dose to that of CD22-CAR T-cell dose was 0.94 (IQR, 0.78-1.19).
FIG 1.
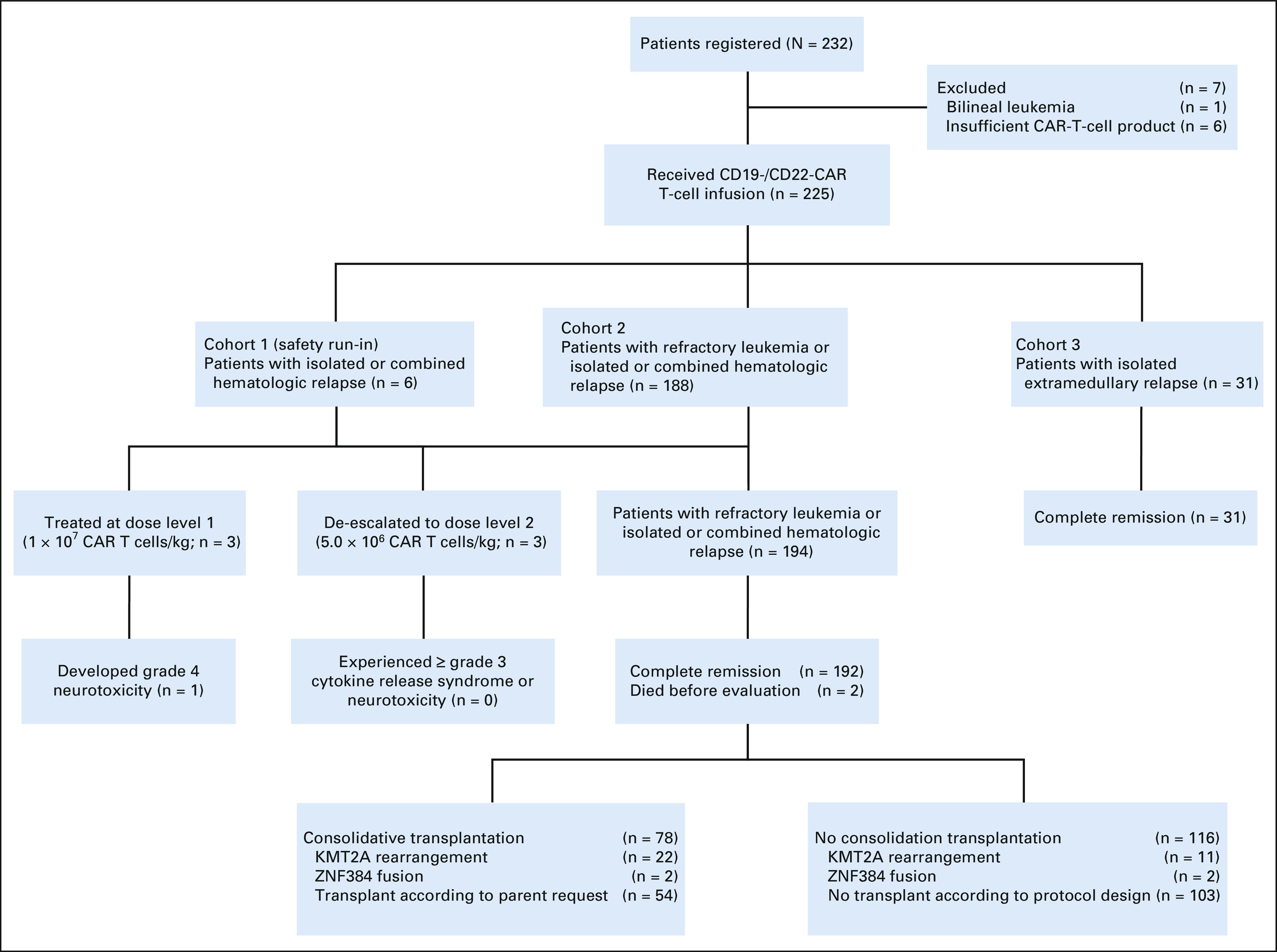
CONSORT diagram. Seven patients were excluded from the study because of the diagnosis of acute bilineal leukemia or insufficient CAR T-cell production (< 1 × 106 CAR T cells/kg). The first six patients with hematologic relapse (cohort 1) were treated in the safety run-in stage. Subsequent 188 patients with refractory leukemia or hematologic relapse (cohort 2) were treated with recommended phase II dose. Among the total 194 patients in cohort 1 and cohort 2, 192 achieved complete remission, of whom 78 received consolidative transplantation. All 31 patients with isolated extramedullary disease (cohort 3) achieved complete remission. CAR, chimeric antigen receptor.
Primary Outcome
Complete remission was achieved in 192 of the 194 patients (99.0% [95% CI, 97.5 to 100]); one patient died of neurotoxicity, and the other of cytokine release syndrome after treatment at the recommended dose. All 192 patients attained negative MRD status. With a median follow-up of 11.0 months after the infusion (IQR, 6.2-18.0 months; range, 0.1-32.4 months), relapse occurred in 43 patients (24 with CD19+/CD22+ relapse, 16 CD19–/CD22+, 1 CD19–/CD22–, and 2 unknown) with a cumulative risk of 22.2% (95% CI, 16.0 to 28.4). The 12-month EFS was 73.5% (95% CI, 67.3 to 80.3) and 69.2% (95% CI, 60.8 to 78.8) after censoring 78 patients for consolidative transplantation (Fig 2A), and the 12-month OS was 87.7% (95% CI, 82.9 to 92.9; Fig 2B).
FIG 2.
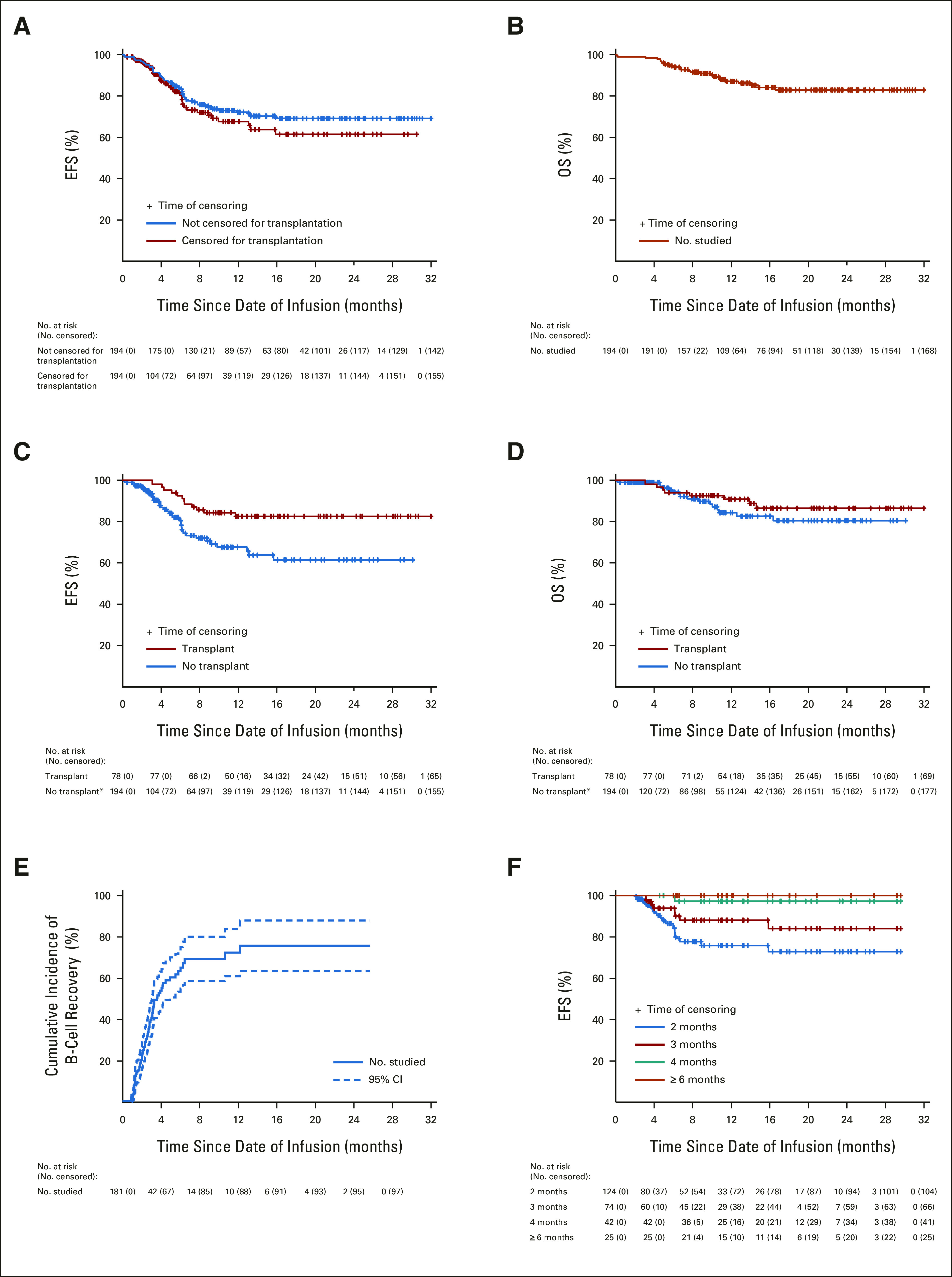
(A) The EFS for patients treated for hematologic relapse or refractory leukemia with or without censoring consolidative allogeneic hematopoietic cell transplantation. (B) The OS for patients treated for hematologic relapse or refractory leukemia with or without transplantation. (C) Comparisons of EFS and (D) OS between patients who did or did not receive consolidative allogeneic transplantation after CAR T-cell therapy. Transplant was regarded as a time-dependent factor; consequently, the initial total number at risk in the no-transplant group equaled the full sample size (indicated by an asterisk). (E) Cumulative incidence of B-cell recovery as defined by the detection of ≥ 1% CD19+ lymphocytes in bone marrow and/or peripheral blood samples by flow cytometry. Dashed lines denote 95% CI. (F) Landmark EFS analyses for patients with persistent B-cell aplasia reaching 2 months, 3 months, 4 months, and ≥ 6 months. Tick marks indicate the time of censoring. CAR, chimeric antigen receptor; EFS, event-free survival; OS, overall survival.
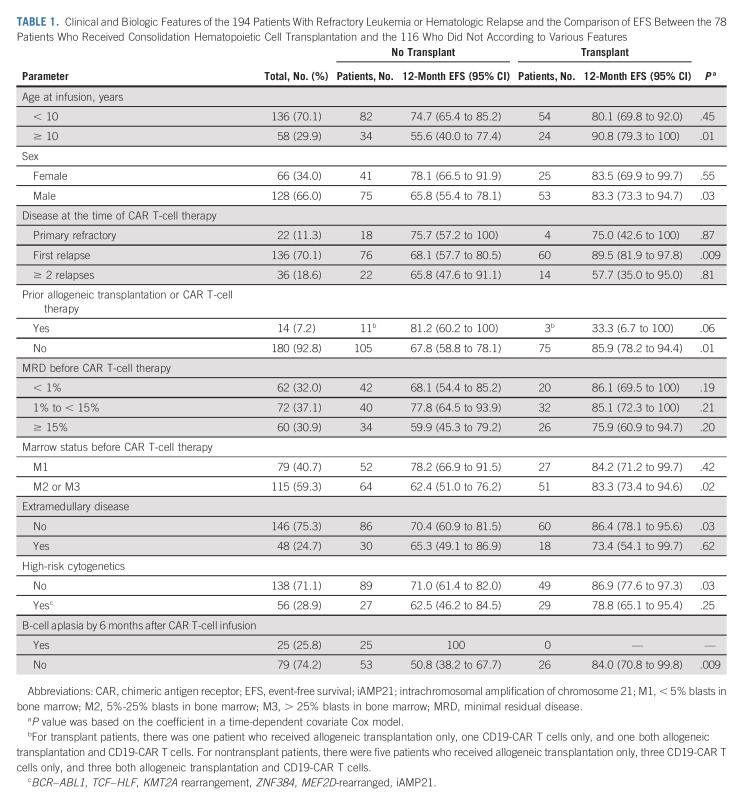
Consolidative transplantation was performed in 24 of the 37 patients with KMT2A-rearranged or ZNF384-rearranged ALL and in 54 patients because of parental request. Clinical and biologic characteristics of patients who did or did not undergo consolidative transplantation did not differ significantly, except that none who received transplantation had B-cell aplasia for ≥ 6 months after infusion (P < .001, Table 1). Patients who received transplantation had better 12-month EFS than did those who did not (P = .03, time-dependent covariate Cox model): 85.0% (95% CI, 77.2 to 93.6) versus 69.2% (95% CI, 60.8 to 78.8; Fig 2C). There was no significant difference in 12-month OS between patients who did or did not receive transplantation (P = .40, time-dependent covariate Cox model): 91.3% (95% CI, 84.8 to 98.3) versus 85.0% (95% CI, 78.1 to 92.6; Fig 2D). Transplantation was associated with better EFS for many categories of patients (Table 1).
TABLE 1.
Clinical and Biologic Features of the 194 Patients With Refractory Leukemia or Hematologic Relapse and the Comparison of EFS Between the 78 Patients Who Received Consolidation Hematopoietic Cell Transplantation and the 116 Who Did Not According to Various Features
Secondary Outcomes
B-cell aplasia occurred in the peripheral blood or bone marrow of all 181 patients analyzed by day 28 postinfusion. The median time to normal B-cell recovery (≥ 1%) in blood and/or bone marrow was 74.0 days (IQR, 47.8-97.8 days; range, 27-371 days). The cumulative incidence of loss of B-cell aplasia by 6 months postinfusion was 59.8% (95% CI, 50.4 to 69.2; Fig 2E). There was a steady improvement in EFS for patients who had persistent B-cell aplasia at 2 months after infusion and beyond: 77.0% (95% CI, 68.2 to 87.0), 88.7% (95% CI, 81.1 to 97.1), 97.4% (95% CI, 92.6 to 100), and 100% at 2, 3, 4, and ≥6 months, respectively (Fig 2F). Among the 116 patients who received only coadministration of CD19- and CD22-CAR T cells and did not undergo consolidative transplantation, MRD before CAR T-cell treatment < 15% (70.7% [95% CI, 60.6 to 82.5] v 54.6% [95% CI, 39.9 to 74.7], P = .04), M1 bone marrow status (76.3% [95% CI, 64.5 to 90.1] v 58.3% [95% CI, 46.8 to 72.5], P = .05), and persistent B-cell aplasia for ≥ 6 months were significantly associated with favorable 12-month EFS (100% v 47.2% [95% CI, 34.8 to 64.0], P < .001; Data Supplement).
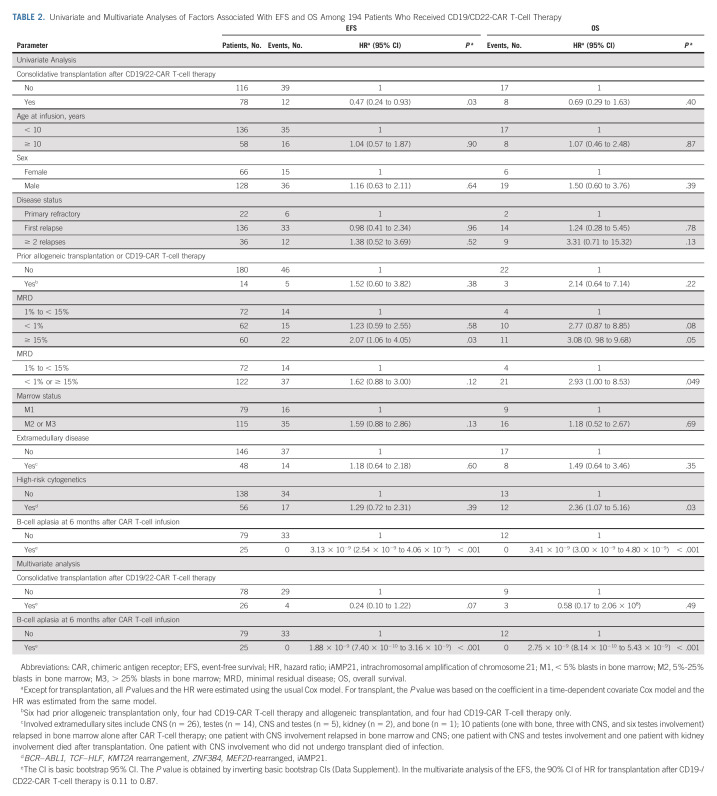
In the multivariate analysis, factors associated with better EFS included consolidative transplantation (hazard ratio, 0.24 [95% CI, 0.10 to 1.22], P = .07) and persistence of B-cell aplasia for ≥ 6 months postinfusion (100% event-free; hazard ratio, 1.88 × 10−9 [95% CI, 7.40 × 10−10 to 3.16 × 10−9], P < .001; Table 2).
TABLE 2.
Univariate and Multivariate Analyses of Factors Associated With EFS and OS Among 194 Patients Who Received CD19/CD22-CAR T-Cell Therapy
Quantification of CAR T-Cell Persistence
By using quantitative polymerase chain reaction to detect the CAR transgene, we found that expansion occurred earlier for CD19-CAR T cells than for CD22-CAR T cells (peaked at mean ± SE: 7.3 ± 0.5 days v 10.9 ± 0.9 days, P = .0013) in 76 patients tested. CD19-CAR T cells had more robust expansion for longer duration than CD22-CAR T cells (Fig 3). Among the 21 relapsed patients tested, all 11 with CD19+/CD22+ relapse had lost CD19- and CD22-CAR T-cell persistence at relapse. Of the nine patients with CD19–/CD22+ relapse tested, four lost CD19-CAR T-cell persistence, but all nine lost CD22-CAR T cells at relapse. The patient with CD19–/CD22– relapse did not lose CD19- but lost CD22-CAR T-cell persistence at relapse.
FIG 3.
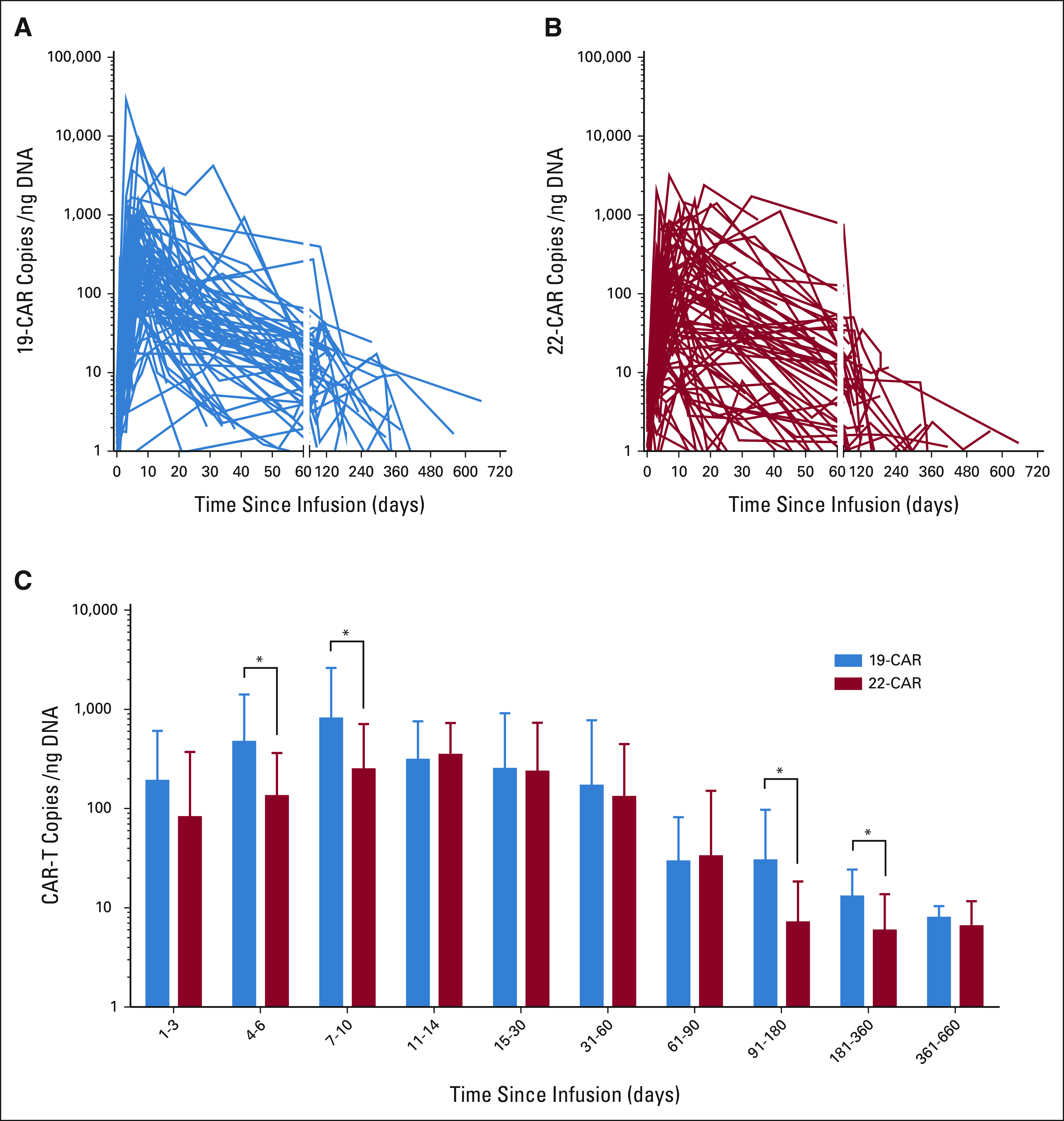
Expansion and persistence of (A) CD19, (B) CD22, and (C) both CD19 and CD22 CAR T cells in blood. The CAR-T copies/ng genome DNA of circulating CD19- and CD22-BB-3ζ CAR T cells as measured by quantitative real-time PCR showing significant differences at initial expansion and subsequent time points, ie, CAR-T copies/ng of genome DNA of CD19- versus CD22-BB-3ζ CAR T cells were 190.7 versus 81.9 at days 1-3 (P = .138), 468.8 versus 133.5 at days 4-6 (P = .028), 809.7 versus 248.4 at days 7-10 (P = .021), 310.1 versus 347.0 at days 11-14 (P = .721), 251.6 versus 236.0 at days 15-30 (P = .895), 170.4 versus 130.7 at days 31-60 (P = .679), 29.4 versus 33.0 at days 61-90 (P = .869), 30.1 versus 7.1 at days 91-180 (P = .039), 13.0 versus 5.9 at days 181-360 (P = .021), and 7.9 versus 6.5 at days 361-660 (P = .620). The asterisks in (C) denote significant differences in the CAR T-cell copies/ng DNA. CAR, chimeric antigen receptor.
Isolated Extramedullary Relapse
Thirty-one patients were treated for isolated extramedullary relapse (Data Supplement). Their median age was 7.6 years (IQR, 6.0-10.3; range, 1.4-15.5 years), the median time from enrollment to infusion was 7 days (range, 6-11 days), and the median dose of combined CD19- and CD22-CAR T cells was 7.0 × 106/kg (IQR, 5.3-8.9 × 106; range, 1.4-14.0 × 106). The median dose of CD19-CAR T cells was 3.0 × 106/kg (IQR, 2.2-4.1 × 106), and that of CD22-CAR T cells was 3.4 × 106/kg (IQR, 2.7-4.8 × 106). The median ratio of CD19-CAR T-cell dose to CD22-CAR T-cell dose was 0.87 (IQR, 0.77-1.01). Sixteen patients had one or more high-risk factors, including second or third relapse, prior allogeneic transplantation or CD19-CAR T-cell therapy, on-therapy relapse, or unfavorable genotypes. All patients experienced complete remission without local irradiation. With a median follow-up of 13.3 months, three of the 10 patients treated for CNS relapse had adverse events (two CNS relapses and one fatal neurotoxicity) and one of the 20 patients treated for testicular relapse developed hematologic relapse, resulting in a 12-month EFS of 68.6% (95% CI, 44.5 to 100) and 95.0% (95% CI, 85.9 to 100), respectively (Data Supplement). The patient with combined testicular and CNS relapse remained in complete remission for 14.4 months.
Adverse Events
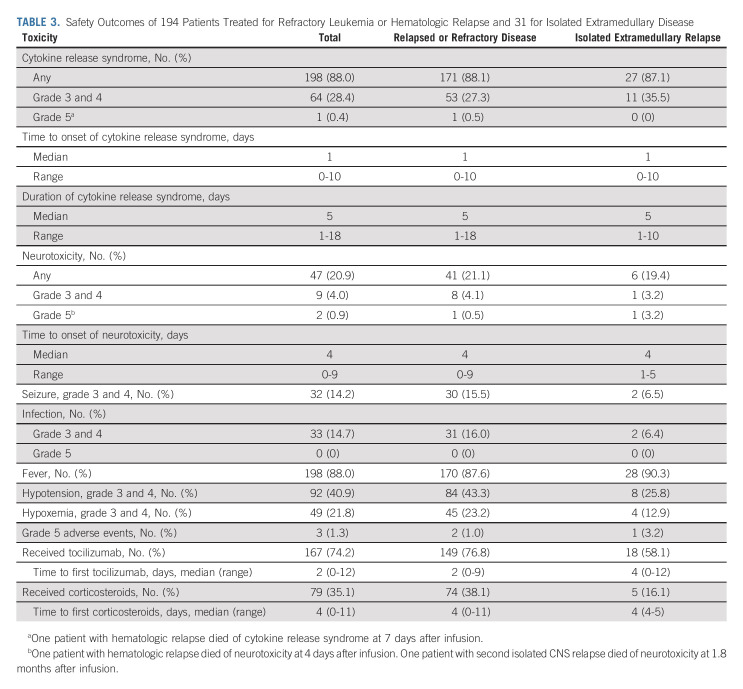
Toxicities that occurred within 4 weeks of infusion are shown in Table 3. Cytokine release syndrome developed in 198 (88.0%) patients, was grade ≥ 3 in 64 (28.4%) patients, and was fatal in one patient. Neurotoxicity occurred in 47 (20.9%) patients, was grade ≥ 3 in nine (4.0%) patients, and was fatal in two patients who received 12.0 × 106 and 5.6 × 106 CAR-T cells/kg, respectively. Grade 3 or 4 seizure developed in 14.2% of the patients and was more common in those presenting with isolated or combined CNS leukemia as compared with the other patients (10 of 42 v 22 of 183 patients). Grade 3 or 4 hypotension occurred in 40.9% of the patients. Tocilizumab was given to 167 (74.2%) patients, and corticosteroids to 79 (35.1%). The peak levels of interleukin-6 and interferon-gamma were significantly higher among patients with grade 3-4 cytokine release syndrome than in those with grade 0-2 (P < .001; Data Supplement).
TABLE 3.
Safety Outcomes of 194 Patients Treated for Refractory Leukemia or Hematologic Relapse and 31 for Isolated Extramedullary Disease
DISCUSSION
To our knowledge, in this largest prospective CAR T-cell trial for childhood ALL to date, CD19-/CD22-CAR T cells induced complete remission with negative MRD in 99.0% of the patients. Their 12-month EFS was 69.2% and 73.5% with or without censoring on consolidative transplantation, respectively, and their 12-month OS was 87.7%. These results appeared to be better than those of real-world experience with tisagenlecleucel.5,7,27 We attributed our favorable results partly to the simultaneous administration of two different CAR T cells to enhance early eradication of leukemia clones, thereby impeding the development of resistance. Compared with two large CD19 CAR-T studies,5,27 this trial yielded a higher complete remission rate (99% v 88% and 93.5%, respectively) and a lower relapse rate (22.2% v 36% and 31.5%, respectively), suggesting additional immune pressure via CD22 CAR-T cells. Our rapid manufacturing of the CAR T cells enabled infusion of fresh CAR T cells within approximately 1 week, which may also contribute to improved outcomes. Compared with cryopreserved CAR T cells, fresh CAR T cells are more functional and effective.28 The rapid and robust proliferation of our CAR T cells is suggested by the median onset of cytokine release syndrome of only 1 day and the median time to first tocilizumab treatment of 2 days.
The rapid production of CAR T cells in 7 days without leukapheresis also allowed us to optimize the timing of infusion on the basis of patient's clinical condition and total B-cell and blast counts, decreasing the need of bridging chemotherapy for patients with progressing disease during the waiting period. Disease burden, too low or too high, before CAR T-cell infusion was associated with disease recurrence.4,27,29-32 Decreased CAR T-cell persistence because of a lack of antigen stimulation has been associated with early loss of B-cell aplasia and CD19+ leukemia relapse in patients with low disease burden.4,30,31 High disease burden has been associated with CD19– relapse, a finding attributed to the development of resistance during leukemia proliferation or pre-existing minor population of CD19– disease, which was undetectable by standard flow cytometry but emerged after clearance of CD19+ disease.27,31,33 In this regard, our patients with MRD ≥ 15% have poorer EFS than those with levels < 15%.
In a bicistronic CD19-/CD22-targeted CAR T-cell study, five of 10 patients with progressive leukemia had negative or low CD19 expression but preserved CD22 expression.14 Similarly, three of the eight marrow relapses in another bicistronic CD19-/CD22-targeted study were CD19–, but only one was CD22–.15 A recent study of tandem CD19.22.BB.zeta CAR-T cells also demonstrated suboptimal CD22-targeting activity.16 Of our 43 relapsed patients, 17 lost CD19 expression, but only one lost CD22 expression in leukemic cells at relapse. Collectively, these data suggest relatively stronger CD19-specific immune pressure and inadequate CD22-CAR T activity, regardless of dual-targeting approach. Among our 21 relapsed patients tested, all lost CD22-CAR T cells, but six retained CD19-CAR T cells. By using quantitative polymerase chain reaction to detect the CAR transgene, we found that CD19-CAR T-cell expansion occurred earlier and for longer duration than CD22-CAR T-cell expansion, and CD19-CAR T cells had more robust expansion than CD22-CAR T cells. The lack of expansion and persistence of CD22-CAR T cells can be explained by lower CD22 versus CD19 antigen expression on leukemia blasts in general or by poor CD22-scFV signaling activity. Other explanations for more frequent loss of CD19 may include pre-existing CD19– leukemia cells being more frequent than CD22– leukemia cells before CAR T-cell therapy or acquired mutations and alternative splicing being more common with CD19.14,34-36 Studies are needed to determine whether enhancing CD22-CAR T-cell persistence and activity would improve outcomes, such as increasing the ratio of CD22- to CD19-CAR T-cell dose, repeated infusion of CD22-CAR T cells, and the use of alternative promotor-scFV-signaling domains or naive T cells.37
Hitherto, there were no reliable markers to predict relapse after CAR T-cell therapy. Hence, some investigators proposed to use CAR T-cell therapy as a bridge to consolidative transplantation for all patients. Consolidative transplantation provided long-term durable disease control in one CD19-CAR T-cell trial,13 but did not improve survival in another sequential CD19- and CD22-CAR T-cell study.17 In our trial, consolidative transplantation was associated with better EFS, a result not yet translated to better OS because some nontransplanted patients were salvageable, and others were still alive with disease. Persistent B-cell aplasia at 6 months and beyond was also an independent favorable prognostic factor in this study and was associated with an excellent 12-month EFS of 100%, suggesting that patients with this feature would not need transplantation. However, in a recent study of tisagenlecleucel, measuring B-cell aplasia was not as predictive of relapse as MRD detection by next-generation sequencing and also CD19– relapse could occur early and at higher frequency in patients with persistence of B-cell aplasia.38 Additional studies are needed to establish the clinical utility of measuring B-cell aplasia as a complimentary test.
Encouraged by the ability of CD19-CAR T cells to eradicate leukemic cells in cerebrospinal fluid of patients with relapsed CD19+ B-ALL,3,5 several studies tested this approach in the treatment of isolated extramedullary relapse.39-42 In a study of testicular relapse, six of seven patients were alive in remission for 5-23 months.40 In one study of CNS relapse, four of five patients remained alive in remission for 15-29 months.39 In an analysis of pooled data of 44 patients with CNS relapse from five studies, the 2-year relapse-free survival was 66%.41 In another recent consortium study, the 12-month relapse-free survival for the 22 patients with isolated CNS relapse was 66.1% and that for the 13 with combined CNS and hematologic relapse was 49.5%.42 In this study, all 31 patients with isolated testicular or CNS relapse attained complete remission. The 12-month EFS was 95.0% and 68.6% for patients treated for isolated testicular and CNS relapse, respectively. Notably, among our 48 patients treated for combined hematologic and extramedullary relapse, only one developed a subsequent extramedullary relapse (Table 2). These preliminary results are encouraging, and CAR T-cell therapy could become a therapeutic option for patients with extramedullary relapse.
We encountered relatively high frequencies of CAR T-cell–related grade 3 or 4 hypotension episodes (41.3%) and seizures (14.2%), which we attributed to rapid and robust CAR T-cell expansion. The seizure rate was especially high among patients with isolated or combined CNS leukemia (23.8% v 12.0% in the other patients) for whom anticonvulsant prophylaxis is now implemented.
This study had several limitations. We could not use our historical controls for the comparison of long-term outcomes because of a large proportion of patients in this trial undergoing consolidative transplantation. Another limitation is the lack of measurement of MRD with next-generation sequencing, which improved prediction of relapse beyond the assessment of B-cell aplasia.38 Longer follow-up is needed to determine if late CD19– relapse would occur as observed among those treated with tisagenlecleucel.38
See accompanying editorial on page 1646
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Supported in part by the National Natural Science Foundation of China (grant No. 81670174, B.L.; grant No. 81670136, J.T. And J.C.), the Shanghai Collaborative Innovation Center for Translational Medicine (grant No. TM201928, B.L.), the Research Programs of Shanghai Science and Technology Commission Foundation (grant No. 14411950600, J.C.), the US National Cancer Institute (grant No. CA21765, C.C. and C.-H.P.), the VIVA China Children's Cancer Foundation, and American Lebanese Syrian Associated Charities (C.C., J.J.Y., and C.-H.P.).
CLINICAL TRIAL INFORMATION
Chinese Clinical Trial Registry: ChiCTR2000032211
Jun J. Yang
Research Funding: Takeda (Inst)
Wing Leung
Employment: Miltenyi Biotec
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
No other potential conflicts of interest were reported.
T.W., Y.T., J.C., X.W., and S.H. contributed equally as cofirst authors; W.L., J.C., J.L., B.L., and C.H.P. contributed equally as colast authors.
DATA SHARING STATEMENT
Contact the corresponding author for additional information.
AUTHOR CONTRIBUTIONS
Conception and design: Jun J. Yang, Cheng Cheng, Wing Leung, Jing Chen, Jun Lu, Benshang Li, Ching-Hon Pui
Financial support: Benshang Li
Administrative support: Jing Chen, Benshang Li
Provision of study materials or patients: Tianyi Wang, Yanjing Tang, Fan Yang, Qing Cao, Juan Qian, Kang An, Chengjuan Luo, Xiang Wang, Wenhua Shi, Peifang Xiao, Xiaomin Yang, Jing Yang, Jingyan Tang, Wing Leung, Jing Chen, Jun Lu, Benshang Li
Collection and assembly of data: Tianyi Wang, Yanjing Tang, Jiaoyang Cai, Xinyu Wan, Shaoyan Hu, Xiaoxi Lu, Zhiwei Xie, Hui Jiang, Jingbo Shao, Fan Yang, Qing Cao, Jian Zhang, Jianmin Wang, Chengjuan Luo, Yan Miao, Xiang Wang, Lili Song, Hailong He, Wenhua Shi, Peifang Xiao, Xiaomin Yang, Jing Yang, Yiping Zhu, Ningling Wang, Longjun Gu, Qimin Chen, Jingyan Tang, Jun Lu, Ching-Hon Pui
Data analysis and interpretation: Yanjing Tang, Jiaoyang Cai, Xinyu Wan, Xiaohong Qiao, Hong Ren, Qing Cao, Juan Qian, Kang An, Chengjuan Luo, Huanhuan Liang, Yani Ma, Lixia Ding, Xiaomin Yang, Jing Yang, Wenjie Li, Yiping Zhu, Jingyan Tang, Cheng Cheng, Wing Leung, Jing Chen, Jun Lu, Ching-Hon Pui
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Coadministration of CD19- and CD22-Directed Chimeric Antigen Receptor T-Cell Therapy in Childhood B-Cell Acute Lymphoblastic Leukemia: A Single-Arm, Multicenter, Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jun J. Yang
Research Funding: Takeda (Inst)
Wing Leung
Employment: Miltenyi Biotec
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Novartis
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
No other potential conflicts of interest were reported.
REFERENCES
- 1.Grupp SA, Kalos M, Barrett D, et al. : Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368:1509-1518, 2013. [Erratum: N Engl J Med 374:998, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014. [Erratum: N Engl J Med 374:998, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385:517-528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner RA, Finney O, Annesley C, et al. : Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129:3322-3331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Laetsch TW, Buechner J, et al. : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439-448, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curran KJ, Margossian SP, Kernan NA, et al. : Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134:2361-2368, 2019. [Erratum: Blood 136:1374, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquini MC, Hu ZH, Curran K, et al. : Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4:5414-5424, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NN, Fry TJ: Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol 16:372-385, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlando EJ, Han X, Tribouley C, et al. : Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 24:1504-1506, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Fry TJ, Shah NN, Orentas RJ, et al. : CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24:20-28, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan J, Niu Q, Deng B, et al. : CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 33:2854-2866, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Shah NN, Highfill SL, Shalabi H, et al. : CD4/CD8 T-Cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: Updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol 38:1938-1950, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NN, Lee DW, Yates B, et al. : Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J Clin Oncol 39:1650-1659, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiegel JY, Patel S, Muffly L, et al. : CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Nat Med 27:1419-1431, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordoba S, Onuoha S, Thomas S, et al. : CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: A phase 1 trial. Nat Med 27:1797-1805, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shalabi H, Qin H, Su A, et al. : CD19/22 CAR T-cells in children and young adults with B-ALL: Phase I results and development of a novel bicistronic CAR. Blood 140:451-463, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Hu X, Cao W, et al. : Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood 135:17-27, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Pan J, Zuo S, Deng B, et al. : Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood 135:387-391, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Deng B, Yin Z, et al. : Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am J Hematol 96:671-679, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Schneider D, Xiong Y, Wu D, et al. : A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer 5:42, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin H, Ramakrishna S, Nguyen S, et al. : Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics 11:127-137, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner R, Wu D, Cherian S, et al. : Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 127:2406-2410, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberley MJ, Gaynon PS, Bhojwani D, et al. : Myeloid lineage switch following chimeric antigen receptor T-cell therapy in a patient with TCF3-ZNF384 fusion-positive B-lymphoblastic leukemia. Pediatr Blood Cancer 65:e27265, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DW, Santomasso BD, Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625-638, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. : Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol 16:45-63, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernasconi DP, Rebora P, Iacobelli S, et al. : Survival probabilities with time-dependent treatment indicator: Quantities and non-parametric estimators. Stat Med 35:1032-1048, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Myers RM, Taraseviciute A, Steinberg SM, et al. : Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol 40:932-944, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah NN, Johnson BD, Schneider D, et al. : Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nat Med 26:1569-1575, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Finney OC, Brakke HM, Rawlings-Rhea S, et al. : CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest 129:2123-2132, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillai V, Muralidharan K, Meng W, et al. : CAR T-cell therapy is effective for CD19-dim B-lymphoblastic leukemia but is impacted by prior blinatumomab therapy. Blood Adv 3:3539-3549, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dourthe ME, Rabian F, Yakouben K, et al. : Determinants of CD19-positive vs CD19-negative relapse after tisagenlecleucel for B-cell acute lymphoblastic leukemia. Leukemia 35:3383-3393, 2121 [DOI] [PubMed] [Google Scholar]
- 32.Kadauke S, Myers RM, Li Y, et al. : Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: A prospective clinical trial. J Clin Oncol 39:920-930, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabilloud T, Potier D, Pankaew S, et al. : Single-cell profiling identifies pre-existing CD19-negative subclones in a B-ALL patient with CD19-negative relapse after CAR-T therapy. Nat Commun 12:865, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotillo E, Barrett DM, Black KL, et al. : Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 5:1282-1295, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal J, Naqvi AS, Luo M, et al. : Heterogeneity of surface CD19 and CD22 expression in B lymphoblastic leukemia. Am J Hematol 93:E352-E355, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Bueno C, Barrera S, Bataller A, et al. : CD34+CD19-CD22+ B-cell progenitors may underlie phenotypic escape in patients treated with CD19-directed therapies. Blood 140:38-44, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz LM, Czerwinski DK, Levy R, Levy S: CD81 costimulation skews CAR transduction toward naive T cells. Proc Natl Acad Sci USA 119:e1910844119, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pulsipher MA, Han X, Maude SL, et al. : Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov 3:66-81, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubinstein JD, Krupski C, Nelson AS, et al. : Chimeric antigen receptor T cell therapy in patients with multiply relapsed or refractory extramedullary leukemia. Biol Blood Marrow Transplant 26:e280-e285, 2020 [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Wang Y, Ruan M, et al. : Treatment of testicular relapse of B-cell acute lymphoblastic leukemia with CD19-specific chimeric antigen receptor T cells. Clin Lymphoma Myeloma Leuk 20:366-370, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leahy AB, Newman H, Li Y, et al. : CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: A post-hoc analysis of pooled data from five clinical trials. Lancet Haematol 8:e711-e722, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabrizio VA, Phillips CL, Lane A, et al. : Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: A Pediatric Real World CAR Consortium report. Blood Adv 6:600-610, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Contact the corresponding author for additional information.