Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
The combined analysis of SOFT-TEXT compared outcomes in 4,690 premenopausal women with estrogen/progesterone receptor–positive (ER/PgR+) early breast cancer randomly assigned to 5 years of exemestane + ovarian function suppression (OFS) versus tamoxifen + OFS. After a median follow-up of 9 years, exemestane + OFS significantly improved disease-free survival (DFS) and distant recurrence-free interval (DRFI), but not overall survival, compared with tamoxifen + OFS. We now report DFS, DRFI, and overall survival after a median follow-up of 13 years. In the intention-to-treat (ITT) population, the 12-year DFS (4.6% absolute improvement, hazard ratio [HR], 0.79; 95% CI, 0.70 to 0.90; P < .001) and DRFI (1.8% absolute improvement, HR, 0.83; 95% CI, 0.70 to 0.98; P = .03), but not overall survival (90.1% v 89.1%, HR, 0.93; 95% CI, 0.78 to 1.11), continued to be significantly improved for patients assigned exemestane + OFS over tamoxifen + OFS. Among patients with human epidermal growth factor receptor 2-negative tumors (86.0% of the ITT population), the absolute improvement in 12-year overall survival with exemestane + OFS was 2.0% (HR, 0.85; 95% CI, 0.70 to 1.04) and 3.3% in those who received chemotherapy (45.9% of the ITT population). Overall survival benefit was clinically significant in high-risk patients, eg, women age < 35 years (4.0%) and those with > 2 cm (4.5%) or grade 3 tumors (5.5%). These sustained reductions of the risk of recurrence with adjuvant exemestane + OFS, compared with tamoxifen + OFS, provide guidance for selecting patients for whom exemestane should be preferred over tamoxifen in the setting of OFS.
INTRODUCTION
The SOFT-TEXT combined analysis assessed the role of the aromatase inhibitor (AI) exemestane versus tamoxifen in premenopausal women with ER/PgR+ early breast cancer receiving ovarian function suppression (OFS). The most recent analysis after a 9-year median follow-up (MFU)1 showed sustained improvements with exemestane + OFS versus tamoxifen + OFS in disease-free survival (DFS; hazard ratio [HR], 0.77; 95% CI, 0.67 to 0.90) and distant recurrence-free interval (DRFI) but not overall survival (HR, 0.98; 95% CI, 0.79 to 1.22). Given the potential late recurrences of ER/PgR+ breast cancer2,3 and late-emergent survival benefit of adjuvant AIs versus tamoxifen in postmenopausal women,4 we report the 12-year SOFT-TEXT late treatment effects on DRFI and overall survival and benefits in women with human epidermal growth factor receptor 2 (HER2)-negative tumors and in those at high risk of disease relapse.
METHODS
The SOFT-TEXT designs and conduct have been described previously.5,6 Patients were randomly assigned 1:1 to receive 5 years of tamoxifen + OFS or exemestane + OFS, stratified by the use of adjuvant chemotherapy and lymph node status.
The present report focused on DRFI and time from random assignment until first appearance of invasive breast cancer recurrence at a distant site and overall survival and time from random assignment until death from any cause. Statistical analyses followed previous reports5; HRs were also estimated in time intervals 0 to < 5, 5 to < 10, and ≥ 10 years (or 0 to < 5 and ≥ 5 years for the cohorts; Data Supplement, online only).
RESULTS
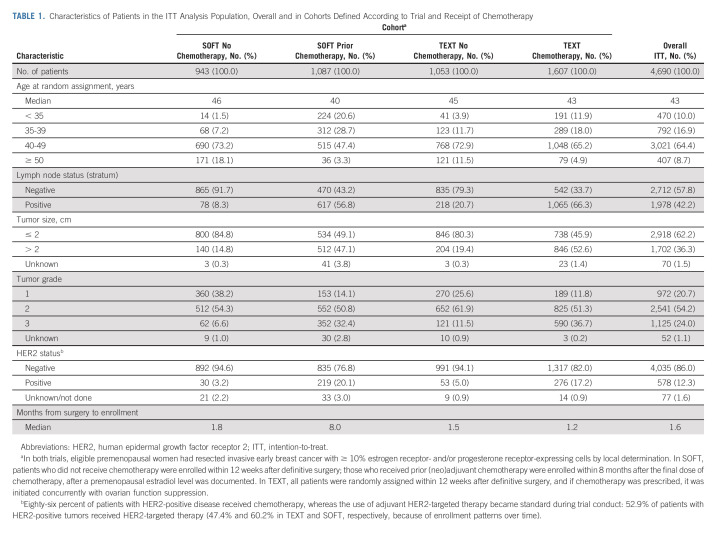
The ITT population included 4,690 premenopausal women randomly assigned from November 2003-April 2011 to exemestane + OFS or tamoxifen + OFS (Table 1 and Data Supplement). Most patients (86.0%) had HER2-negative tumors. At database lock (May 2021), the MFU was 13 years.
TABLE 1.
Characteristics of Patients in the ITT Analysis Population, Overall and in Cohorts Defined According to Trial and Receipt of Chemotherapy
Deaths were reported for 473 patients, 85.6% after a breast cancer event, 6.8% after second (nonbreast) malignancy, and few in the absence of any cancer event (0.4% of all patients, including four cardiovascular deaths in the chemotherapy cohorts; Data Supplement). Patients assigned exemestane + OFS did not have a significantly different hazard of death compared with tamoxifen + OFS (90.1% v 89.1% 12-year overall survival, HR, 0.93; 95% CI, 0.78 to 1.11; P = .43; Fig 1C). The hazard of death was higher for exemestane + OFS versus tamoxifen + OFS during years 0-5 (HR, 1.34; 95% CI, 0.98 to 1.84) and then lowered with longer follow-up (HR, 0.72; 95% CI, 0.54 to 0.95 years 5-10 and HR, 0.88; 95% CI, 0.61 to 1.28 ≥ 10 years).
FIG 1.
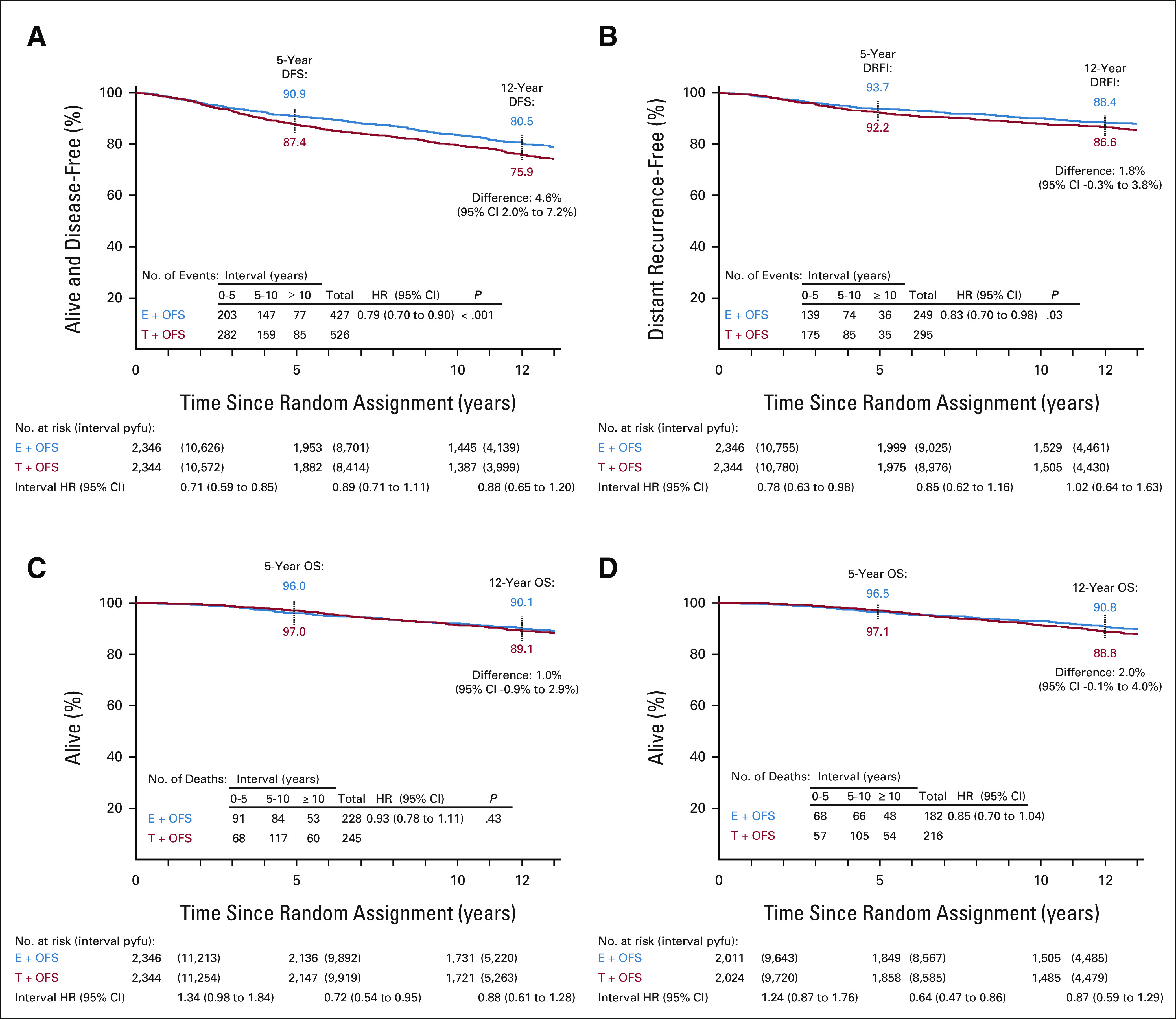
Outcomes after a 13-year median follow-up. Kaplan-Meier estimates of (A) DFS, (B) DRFI, (C) OS distributions in the ITT population, and (D) OS in the predominant subgroup with HER2-negative cancers. Reported are 5- and 12-year event-free percentages and 12-year difference (E + OFS minus T + OFS; with 95% CI). Stratified HRs with 95% CIs are reported, with log-rank P values in the ITT population only. In addition, numbers of events, pyfu, and HRs are provided for time intervals of 0 to < 5 years, ≥ 5 to < 10 years, and ≥ 10 years. DFS, disease-free survival; DRFI, distant recurrence-free interval; E, exemestane; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; ITT, intention-to-treat; OFS, ovarian function suppression; OS, overall survival; pyfu, patient-years of follow-up; T, tamoxifen.
DFS continued to be significantly improved with exemestane + OFS versus tamoxifen + OFS (80.5% v 75.9%, 4.6% [95% CI, 2.0% to 7.2%] absolute improvement, HR, 0.79; 95% CI, 0.70 to 0.90; Fig 1A). Of 953 DFS events (Data Supplement), 52.0% were distant recurrences (61.0% of events during 0-5 years and 42.7% > 5 years). Patients assigned exemestane + OFS experienced a 1.8% (95% CI, −0.3% to 3.8%) absolute improvement in 12-year DRFI compared with those assigned tamoxifen + OFS (88.4% v 86.6%; HR, 0.83; 95% CI, 0.70 to 0.98; P = .03; Fig 1B). The estimated DFS and DRFI benefits were strongest during years 0-5 and attenuated during years 5-10 and ≥ 10 years (Fig 1 and Data Supplement).
Clinicopathologic characteristics and patterns of recurrence differed between patients who received or did not receive chemotherapy and by trial; only 81 of 544 distant recurrences and 79 of 473 deaths were in the no-chemotherapy cohorts. Modest absolute 12-year DRFI benefits were evident in the no-chemotherapy cohorts (each HR, 0.67; 1.8% in SOFT, 1.4% in TEXT), ranging from 93.8% to 97.7% (Data Supplement). The 12-year overall survival was > 95% in both treatment groups in the no-chemotherapy cohorts, with no excess deaths reported among patients assigned exemestane + OFS compared with tamoxifen + OFS (Data Supplement).
In the chemotherapy cohorts, the DRFI benefit was homogeneous across trials (each HR, 0.86; 12-year DRFI absolute benefit 1.9% SOFT; 2.4% TEXT; Data Supplement). The differences in 12-year overall survival were −0.7% in SOFT (HR, 1.06; 95% CI, 0.79 to 1.43) and +2.6% in TEXT (HR, 0.85; 95% CI, 0.65 to 1.11; Data Supplement). For both cohorts, as compared with years 0-5 (each HR > 1), there was a consistent reduction in hazard of death for exemestane + OFS versus tamoxifen + OFS after ≥ 5 years (SOFT HR, 0.84; 95% CI, 0.57 to 1.22; TEXT HR, 0.74; 95% CI, 0.53 to 1.03).
Outcomes According to HER2 Status
In the predominant HER2-negative subgroup (4,035 patients, 53.3% received chemotherapy), a reduction in hazard of death with exemestane + OFS versus tamoxifen + OFS was apparent (HR, 0.85; 95% CI, 0.70 to 1.04; Fig 1D), with a 2.0% improvement in the 12-year overall survival (90.8% exemestane + OFS v 88.8% tamoxifen + OFS). The greatest absolute improvements in overall survival were achieved in patients at higher risk of relapse, ie, women age < 35 years (+4.0%) and those with tumors > 2 cm (+4.5%) and grade 3 tumors (+5.5%; Fig 2). In both chemotherapy cohorts, there was a 3.3% absolute overall survival improvement (84.4% v 81.1% in SOFT; 86.8% v 83.5% in TEXT; Fig 2 and Data Supplement). Overall, the observed hazards of death were low but higher for exemestane + OFS versus tamoxifen + OFS (HR, 1.24; 95% CI, 0.87 to 1.76) during years 0-5; after year 5, exemestane + OFS showed substantially lower hazard versus tamoxifen + OFS (years 5-10 HR, 0.64; 95% CI, 0.47 to 0.86; ≥ 10 years HR, 0.87; 95% CI, 0.59 to 1.29; Data Supplement). In patients with HER2-positive disease, outcomes continued to favor tamoxifen + OFS versus exemestane + OFS (Fig 2).
FIG 2.
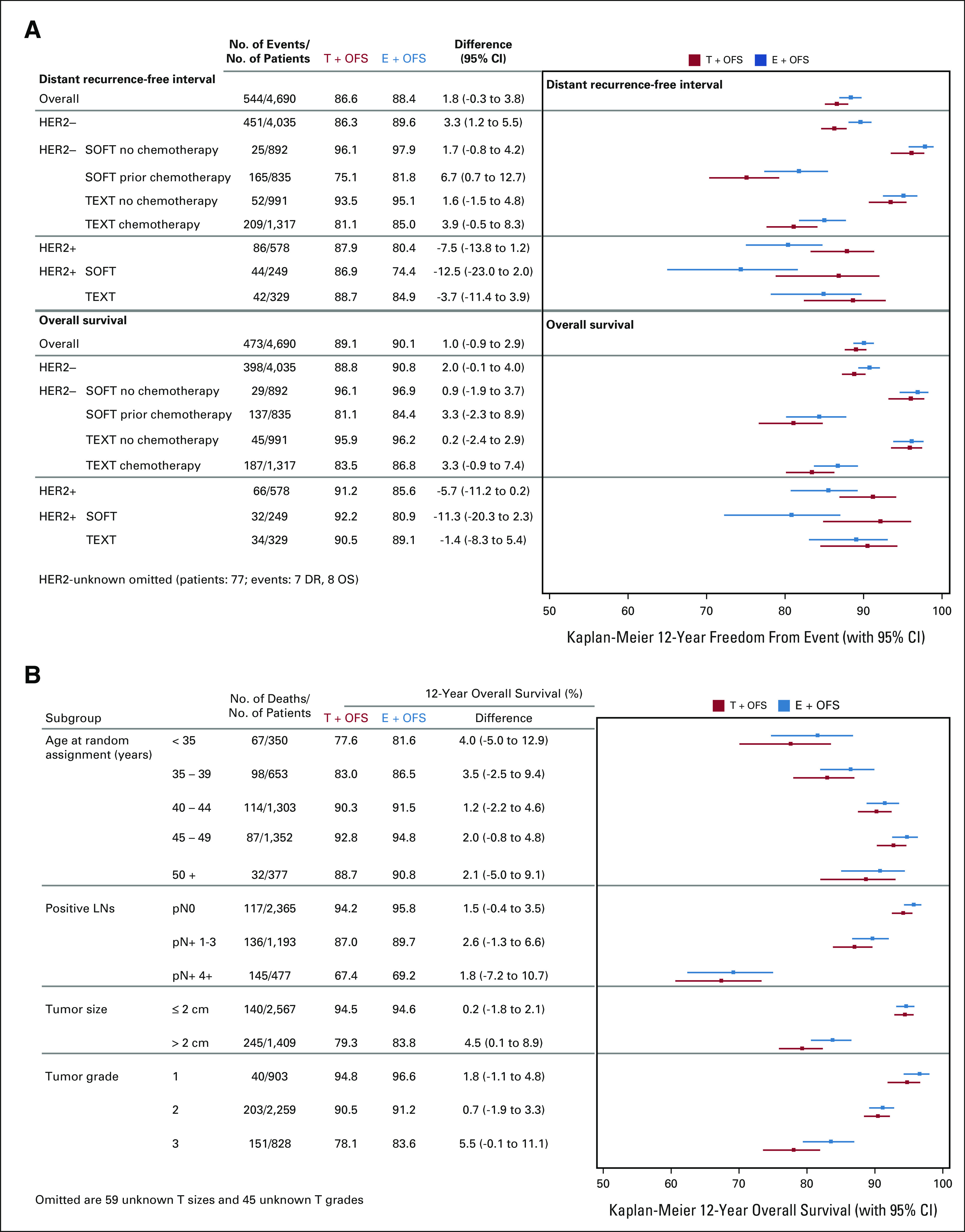
Kaplan-Meier estimates of 12-year outcomes (with 95% CIs) according to treatment assignment. The median follow-up is 13 years. Estimates and difference (exemestane + OFS minus tamoxifen + OFS) are presented for (A) DRFI and overall survival in the ITT population, in HER2 subgroups, and within HER2 status according to the cohort or trial and (B) for overall survival among 4,035 patients who had hormone receptor–positive/HER2-negative cancers in clinicopathologic subgroups. DRFI, distant recurrence-free interval; E, exemestane; HER2, human epidermal growth factor receptor 2; ITT, intention-to-treat; OFS, ovarian function suppression; T, tamoxifen.
DISCUSSION
The updated analysis of SOFT-TEXT after a 13-year MFU confirmed a sustained reduction in recurrence with adjuvant exemestane + OFS compared with tamoxifen + OFS in premenopausal women with ER/PgR+ breast cancer. In the ITT population, absolute improvements were retained in both 12-year DFS (+4.6%) and DRFI (+1.8%). Treatment effects on recurrence tended to attenuate over time, being strongest in years 0-5 with no further improvement after ≥ 10 years. Overall survival was excellent with both treatments, not improved by exemestane + OFS (90.1% v 89.1% in patients assigned tamoxifen + OFS); the lack of survival benefit from exemestane + OFS is at least in part attributable to early emergent, persistent favorable outcomes with tamoxifen + OFS in the HER2-positive subgroup. Deaths without breast cancer or second (nonbreast) cancer (Data Supplement) were rare and not higher with exemestane. Similar findings, reported in the postmenopausal meta-analysis of adjuvant AIs versus tamoxifen,4 contrast data of increased cardiovascular deaths in premenopausal women undergoing oophorectomy7,8 and are reassuring for the safety of 5-year AI + OFS in premenopausal patients. The EBCTCG meta-analysis9 showed that in premenopausal women receiving OFS, AIs versus tamoxifen reduced the relative risk of recurrence by 21% and of distant recurrence by 17%; no significant difference in breast cancer mortality or overall survival was found, but follow-up beyond 10 years was extremely limited.
Distinct outcomes persisted long term according to the baseline risk of recurrence and the choice to administer chemotherapy or not. The 12-year overall survival > 95% in women selected not to receive adjuvant chemotherapy confirmed that premenopausal patients at lower risk of relapse have excellent long-term outcomes with risk-adapted endocrine therapy even in the presence of node-positive disease (8.3% in SOFT and 20.7% in TEXT).
Meaningful 12-year overall survival improvements in the predominant HER2-negative subgroup were now observed after a 13-year MFU, as high as 3.3% in both chemotherapy cohorts; ongoing follow-up will provide a better assessment of any additional survival benefit. Women with HER2-negative tumors with high-risk clinicopathologic characteristics experienced the greatest absolute improvements in 12-year overall survival when treated with exemestane + OFS compared with tamoxifen + OFS, ranging 4.0% to 5.5%.
The monarchE trial reported significant short-term iDFS and DRFS benefits from adding the CDK4/6 inhibitor abemaciclib to standard adjuvant endocrine therapy in patients at high risk of relapse.10 It is currently unknown if the impact of abemaciclib in premenopausal women (43% of patients) is independent of the endocrine backbone11 or limited to women treated with tamoxifen alone, 41% of enrolled premenopausal women despite their high-risk disease characteristics.12
In conclusion, with a 13-year MFU, a reduction not only in recurrences but also in mortality emerged for exemestane + OFS versus tamoxifen + OFS in women with HER2-negative disease, most clinically meaningful for those at higher risk of relapse. No overall survival benefit with exemestane + OFS was evident in women at lower risk of relapse not receiving chemotherapy. Given the burden of treatment intensification on quality of life,13,14 proper selection of women most likely to benefit is paramount.
ACKNOWLEDGMENT
We thank the patients, clinicians, trial staff, and pathologists who participated in the TEXT and SOFT clinical trials; the International Breast Cancer Study Group (IBCSG), the Breast International Group (BIG), BIG cooperative groups, and the US National Cancer Institute National Clinical Trials Network (NCI NCTN) for their collaboration; and Dr Larry Norton and Dr Jeffrey Abrams for supporting the international collaboration between the IBCSG, BIG and the US NCI NCTN through the breast cancer committee of Alliance for Clinical Trials in Oncology. SOFT and TEXT conduct were supported by Pfizer; Pfizer and Ipsen provided the study drugs. Lists of the investigators in SOFT, TEXT, and the International Breast Cancer Study Group, a division of ETOP IBCSG Partners Foundation, can be found in Appendix 1 (online only).
APPENDIX 1. SOFT and TEXT Investigators and the International Breast Cancer Study Group
Steering Committee: P. Francis (Chair, SOFT Co-Chair), G. Fleming (SOFT Co-Chair), O. Pagani (TEXT Co-Chair), B. Walley (TEXT Co-Chair), M. Regan (Trial Statistician), S. Loi, M. Colleoni, L. Blacher, H. Bonnefoi, L. De Meulemeester, E. Ciruelos, A. Coates, S. El-Abed, R. Gelber, A. Hiltbrunner, H. Roschitzki-Voser, R. Kammler, S. Loibl, B. Ruepp, H. Shaw, V. Stearns, R. Torrisi, G. Viale, K. DeMontille (Pfizer), J. Amauri Soares (Ipsen)
IBCSG Scientific Committee: M. Colleoni (Chair), S. Loi (Co-Chair)
IBCSG Scientific Executive Committee (until 30 June 2021): M. Colleoni, A. Di Leo, F. Boyle, G. Jerusalem, S. Loi, M.M. Regan, G. Viale
ETOP IBCSG Partners Foundation Board (effective from 01 July 2021): R Stahel (President), S. Aebi, P. Baas, M. Colleoni, R. Gelber, S. Loi, K. McGregor, S. Peters, S. Popat, R. Rosell
ETOP IBCSG Partners Foundation Coordinating Center, Bern, Switzerland: A. Hiltbrunner (Director), A. Gasca, R. Kammler, R. Maibach, M. Rabaglio-Poretti, H. Roschitzki, S. Roux, B. Ruepp, J. Schroeder
IBCSG Statistical Center, Dana-Farber Cancer Institute, Boston, MA, USA: M. Regan (Director), C. Bouzan, R. Gelber, A. Giobbie-Hurder, H. Huang, K. Price
IBCSG Data Management Center, Frontier Science & Technology Research Foundation, Amherst, NY, USA: L. Blacher (Director), H. Shaw (Lead Trial Coordinator), M. Blackwell, M. Greco, A. Mora de Karausch, D. Narayanan, K. Scott, R. Starkweather
IBCSG Central Pathology Office, European Institute of Oncology, Division of Pathology, Milan, Italy: G. Viale (Director), S. Andrighetto, O. Biasi, P. Dell’Orto, L. Russo
ETOP IBCSG Partners Foundation Quality of Life Office, Bern, Switzerland: J. Bernhard, K. Ribi
Breast International Group (BIG), Brussels, Belgium: M. Piccart-Gebhart, D. Cameron, S. El- Abed,
U.S. National Cancer Institute: J. Abrams, L. Korde, M. Mooney, J.A. Zujewski
Alliance (CALGB) Pathology Coordinating Office, Alliance Biorepository at Ohio State, Ohio State University, Columbus, OH, USA: W. Frankel, L. McCart, R. Jewell, D. Rohrer
Dana-Farber Cancer Institute, Boston, MA, USA (US FDA IND): E. Winer, J. Savoie
Pfizer Study Support: K. DeMontille, S. Salem, S. Simon
Ipsen Study Support: J. Amauri Soares, F. Baton
Participating Centers and Principal Investigators
Centers with accrual of more than 1 patient
SOFT
BREAST INTERNATIONAL GROUP (BIG)
INTERNATIONAL BREAST CANCER STUDY GROUP, A DIVISION OF ETOP IBCSG PARTNERS FOUNDATION
Breast Cancer Trials Australia and New Zealand (BCT-ANZ), Australia; P. Francis, I. Laycock
Austin Health, Heidelberg, Victoria; J. Stewart
Ballarat Oncology and Haematology Services, Wendouree, Victoria; G. Kannourakis
Border Medical Oncology, Wodonga, Victoria; C. Underhill
Box Hill Hospital, Box Hill, Victoria; J. Chirgwin
Calvary Mater Newcastle, Waratah, New South Wales; A. van der Westhuizen
Canberra Hospital, The, Garran, Australian Capital Territory; N. Gorddard
Chris O’Brien Lifehouse, The, Canperdown, New South Wales; J Beith
Coffs Harbour Health Campus, Coffs Harbour, New South Wales; K. Briscoe
Concord Repatriation General Hospital, Concord, New South Wales; P. Beale
Launceston General Hospital, Launceston, Tasmania; S. Gauden
Liverpool Hospital, Liverpool, New South Wales; E. Moylan
Macarthur Cancer Therapy Centre, Campbelltown, New South Wales; S. Della-Fiorentina
Manning Rural Referral Hospital, Taree, New South Wales; E. Livshin
Maroondah Hospital, Ringwood East, Victoria; J. Chirgwin
Mater Hospital, The, North Sydney, New South Wales; F. Boyle
Monash Medical Centre, East Bentleigh, Victoria; M. White
Nambour Hospital, Nambour, Queensland; G. Hawson
Peter MacCallum Cancer Center, East Melbourne, Victoria; P.A. Francis
Riverina Cancer Care Centre, Wagga Wagga, New South Wales; J. Hill
Royal Adelaide Hospital, Adelaide, South Australia; M. Bochner
Royal Brisbane and Women's Hospital, Herston, Queensland; M. Nottage
Royal Hobart Hospital, Hobart, Tasmania; I. Byard
Royal North Shore Hospital, St Leonards, New South Wales; S. Baron-Hay
St Andrews Toowoomba Hospital, Toowoomba, Queensland; P. Vasey
St George Hospital, Kogarah, New South Wales; J. Lynch
St John of God Hospital, Bunbury, Western Australia; A. Kiberu
St John of God Hospital, Subiaco, Western Australia; D. Tsoi
St Vincents Hospital, Fitzroy, Victoria; R. Snyder
St Vincent's Hospital, Darlinghurst, New South Wales; R. Dear
Tweed Hospital, The, Tweed Heads, New South Wales; E. Abdi
Victorian Breast and Oncology Care, Melbourne, Victoria; M. Chipman
Breast Cancer Trials Australia and New Zealand(BCT-ANZ), New Zealand
Auckland City Hospital, Auckland; S. Wilson
Christchurch Hospital, Christchurch; K. Gardner
Palmerston North Hospital, Palmerston North; R. Isaacs
Waikato Hospital, Hamilton; I. Campbell
Brazil
Hospital de Clinicas de Porto Alegre, Porto Alegre; J. Villanova Biazús
Grupo Oncológico Corporativo Chileno de Investigación (GOCCHI), Chile; B. Muller
Instituto Nacional del Cancer, Santiago; R. Torres
Hospital San Juan de Dios, Santiago; S. Torres
Hospital San Borja Arriaran, Santiago; J. Letzkus
Hospital Clinico de la Universidad de Chile, Santiago; O. Barajas
Hospital Dr Sotero Del Rio, Santiago; H. Rojas
Centro De Patologia Mamaria, Santiago; M.E. Bravo
Hospital Base de Valdivia, Valdivia; B. Cardemil
Instituto De Radiomedicina, Vitacura; S. Solé
Hungary
National Institute of Oncology, Budapest; I. Láng
India
Tata Memorial Hospital, Mumbai; V. Parmar
Italy
Centro di Riferimento Oncologico, Aviano; S. Spazzapan
Azienda Sanitaria di Bolzano, Bolzano; C. Graiff Ospedali Riuniti di Bergamo, Bergamo; C. Tondini
Ospedale degli Infermi, Biella; M. Clerico
Unita Operativa de Medicina Oncologica, Ospedale Ramazzini, Carpi; A. Fabrizio
Oncologia Medica Fano Italy, Fano; R. Mattioli
Ospedale Civile di Lecco, Lecco; M. Visini
Fondazione Salvatore Maugeri, Pavia; A. Bernardo
Ospedale degli Infermi, Rimini; L. Gianni
Ospedale di Circolo e Fondazione Macchi, Varese; G. Pinotti
Dipartimento di Oncologia, Azienda Ospedaliero-Universitaria di Udine, Udine; F. Puglisi
Peru
Instituto de Enfermedades Neoplásicas, Lima; H.L. Gomez
South Africa
Sandton Oncology Centre, Johannesburg; D. Vorobiof
Sweden
Sahlgrenska University Hospital, Gothenburg; P. Karlsson
Central Hospital Karlstad, Karlstad; B. Loden
Karolinska University Hospital, Stockholm; J. Bergh
Lund University Hospital, Lund; P. Malmström
Skaraborg Hospital Skovde, Skovde; A. Nissborg
Southern Elfsborg Hospital Boras, Boras; P. Karlsson
Swiss Association for Clinical Cancer Research (SAKK), Switzerland Centre Hospitalier Universitaire Vaudois, Lausanne; K. Zaman
Inselspital, Berne; M. Rabaglio
Kantonsspital St Gallen, St Gallen; T. Ruhstaller
Rätisches Kantonos-/Regionalspital, Chur; R. von Moos
Kantonsspital Basel, Basel; C. Rochlitz
Onkologiezentrum Thun-Berner Oberland, Thun; D. Rauch
Oncocare Engeried, Bern; K. Buser
Zürich Frauenklinik, Zürich; N. Gabriel
Brust-Zentrum Zurich, Zurich; C. Rageth
Kantonsspital Aarau (AG), Aarau; A. Schoenenberger
Tumor Zentrum Hirslanden Klinik, Aarau; R. Popescu
Kantonsspital Baden, Baden; C. Caspar
Tumor und Brustzentrum Zetup St Gallen, St Gallen; H.J. Senn
SOLTI, SPAIN; E. CIRUELOS
Hospital Clínic i Provincial de Barcelona, Barcelona; M. Muñoz
Hospital Universitari Vall D' Hebron, Barcelona; M. Bellet
Hospital Universitario 12 de Octubre, Madrid; E. Ciruelos
Centro Oncológico MD Anderson, Madrid; R. Márquez
Hospital Son Llàtzer, Palma de Mallorca; J. G. Catalán
Clinica Univ. De Navarra, Pamplona; J. M. Aramendia
Instituto Valenciano de Oncología, Valencia; M.A. Climent
Hospital Son Espases (Palma de Mallorca), Palma de Mallorca; A. Perelló
Complejo Hospitalario Universitario de Santiago De Compostela, Santiago de Compostela; R. López
H.U. Arnau de Vilanova, Lleida; S. Morales
Hospital Universitario Virgen Macarena, Sevilla; J.A. Virizuela
Hospital Clínico Universitario de Valencia, Valencia; B. Bermejo
Hospital Ramón Y Cajal, Madrid; N. Martínez Jáñez
Hospital Sant Joan de Reus, Reus; M. Melé
Hospital Reina Sofía De Córdoba, Córdoba; J.R. de la Haba
Complejo Hospitalario Universitario De Gran Canaria Dr Negrín, Las Palmas de Gran Canaria; Negrín; S. Saura
Hospital Sant Pau i Santa Tecla, Tecla; C. Pérez Segura
Central and East European Oncology Group (CEEOG); J. Jassem
Poland
Medical University of Gdansk, Gdansk, Poland; J. Jassem
Serbia
Institute of Oncology & Radiology of Serbia, Belgrade, Serbia; Z. Neskovic-Konstantinovic
European Organisation for Research and Treatment of Cancer (EORTC); S. Marreaud, J. Bogaerts
Belgium
ZNA Middelheim, Antwerpen; A. Vandebroek
Cliniques Universitaires St-Luc UCL, Brussels; M. Berliere
U.Z. Gasthuisberg, Leuven; P. Neven
Centre Hospitalier Universitarie Sart Tilman, Liège; G. Jerusalem
Hopital De Jolimont, Haine St Paul; C. Mitine
Clinique Sainte Elisabeth, Namur; S. Henry
Algemeen Ziekenhuis Sint-Augustinus, Wilrijk; L. Dirix
Centre Hospitalier Etterbeek Ixelles, Brussels; El Ali Ziad
France
Centre Henri Becquerel, Rouen; C. Moldovan
Institut Claudius Regaud, Toulouse; F. Dalenc
Institut Jean Godinot, Reims; C. Jouannaud
Centre Leon Berard, Lyon; T. Bachelot
Institut Bergonie, Bordeaux; H. Bonnefoi
Centre Georges Francois-Leclerc, Dijon; I. Desmoulins
Centre Rene Huguenin, Saint-Cloud; E. Brain
Institut Curie, Paris; J.Y. Pierga
Centre Eugene Marquis, Rennes; T. de la Motte Rouge
C.H.R.U. de Limoges, Limoges; L. Venat-Bouvet
Clinique Mutualiste de l’Estuaire, Saint-Nazaire; V. Delecroix
Clinique De L'alliance, Tours; A. Fignon
Institut Gustave Roussy, Villejuif; M. Saghatchian
Israel
Rambam Medical Center, Haifa; G. Fried
Netherlands
The Netherlands Cancer Institute, Amsterdam; S. Sonke
Onze Lieve Vrouwe Gasthuis, Amsterdam; J. Meerum Terwogt Leids
Universitair Medisch Centrum, Leiden; J. Kroep
Portugal
Centro de Lisboa, Lisboa; A. Moreira
GERMAN BREAST GROUP (GBG); O. ORTMANN, K. REIßMÜLLER, S. LOIBL
DRK Kliniken Berlin Köpenick, Berlin; A. Kleine-Tebbe
Praxis Dr Tessen, Goslar; H.W. Tessen
Martin-Luther-Universität Halle-Wittenberg, Halle an der Saale; C. Thomssen
Universitätsfrauenklinik Erlangen, Erlangen; M.W. Beckmann
Klinikum Mittelbaden/Stadtklinik Baden-Baden, Baden-Baden; A. Hahn
Dr Horst Schmidt Kliniken, Wiesbaden; F. Lorenz-Salehi
St Vincentius Kliniken, Karlsruhe; O. Tomé
Klinikum Landshut GmbH, Landshut; I. Bauerfeind
Universitäts Frauenklinik, Frankfurt/Main; C. Solbach
Caritas-Krankenhaus St Josef, Regensburg; O. Ortmann
Krankenhaus der Barmherzigen Brüder, Regensburg; H. Stauder
Cancer Trials Ireland (formerly All Ireland Cooperative Oncology Research Group; ICORG)
Beaumont Hospital, Dublin; L. Grogan
Mater Misericordiae Hospital, Dublin; J. McCaffrey
Mater Private Hospital, Dublin; J. McCaffrey
Univiversity College Hospital Galway, Galway; M. Keane
South Infirmary-Victoria University Hospital, Cork; S. O’Reilly
Adelaide, Meath & National Children’s Hospital, Dublin; J. Walshe
ICR-CTSU on behalf of the National Cancer Research Institute (NCRI) Breast Clinical Studies Group, United Kingdom; R. Coleman, J. Bliss, S. Kernaghan, N. Atkins
South Tyneside District Hospital, South Shields, Tyne & Wear; G. Mazdai
Weston Park Hospital, Sheffield, South Yorkshire; R. Coleman
Mount Vernon Hospital, Northwood, Middlesex; A. Makris
Luton & Dunstable Hospital, Luton; A. Makris
Clatterbridge Centre for Oncology, Wirral; S. O’Reilly
Great Western Hospital, Swindon; D. Cole
New Cross Hospital, Wolverhampton; M Churn
Whiston Hospital, Prescot; H. Ines
Aberdeen Royal Infirmary, Aberdeen; R. Todd
Royal Marsden Hospital - Fulham, London; I.E. Smith
Royal Marsden Hospital - Sutton, Surrey; I.E. Smith
York Hospital, York; J. Joji
St James Univ Hospital, Leeds; T. Perren
Harrogate District Hospital, Harrogate; J. Joji
Stepping Hill Hospital, Stockport; A. Chittalia
Russells Hall Hospital, Dudley; P. Ramachanara
US NCI NATIONAL CLINICAL TRIALS NETWORK (NCTN)
Alliance for Clinical Trials in Oncology; E. Winer, L. Carey, A. Partridge, J. Ingle
ECOG-ACRIN Cancer Research Group; N. Davidson, V. Stearns, R. O’Regan, S. Gluck
Canadian Cancer Trials Group; K. Pritchard, T. Whelan, K. Gelmon, M. Webster
NRG Oncology; C. Geyer Jr, N. Wolmark, T Mamounas, J. White, S. Swain
SWOG; G. Hortobagyi, S. Martino, J. Gralow, A. Scott
North American Participating Centers
Canada
Doctor H. Bliss Murphy Cancer Center, St John's, Newfoundland; J. McCarthy
BCCA-Vancouver Cancer Center, Vancouver, British Columbia; H. Kennecke
CHUM- Hotel Dieu du Montreal, Montreal, Quebec; A. Robidoux
Hopital Du Sacre-Coeur de Montreal, Montreal, Quebec; J. Roy
Hôpital Charles LeMoyne, Greenfield Park, Quebec; C. Prady
Cancer Centre of Southeastern Ontario at Kingston General Hospital, Kingston, Ontario; V. Kumar
Ottawa Hospital Research Institute, Ottawa, Ontario; S.F. Dent
Thunder Bay Regional Health Science Centre, Thunder Bay, Ontario; D. Vergidis
Health Sciences North, Sudbury, Ontario; P.G. Lopez
Juravinski Cancer Centre at Hamilton Health Sciences, Hamilton, Ontario; R. G. Tozer
Odette Cancer Centre, Toronto, Ontario; K.I. Pritchard
London Regional Cancer Center, London, Ontario; K.R. Potvin
Cancercare Manitoba, Winnipeg, Manitoba; D. Grenier
Cross Cancer Institute, Edmonton, Alberta; K.S. Tonkin
Tom Baker Cancer Center, Calgary, Alberta; B.A. Walley
BCCA Cancer Center for the Southern Interior, Kelowna, British Columbia; S. Ellard
BCCA-Fraser Valley Cancer Center, Surrey, British Columbia; G. K. Pansegrau
Allan Blair Cancer Centre, Regina, Saskatchewan; M. Salim
United States of America
Providence Alaska Medical Center, Anchorage, AK; J.E. Anderson
University of Alabama, Birmingham, AL; R. Diasio
University of California at Los Angeles (UCLA), Los Angeles, CA; P.A. Ganz
University of Southern California, Los Angeles, CA; C.A. Russel
Scripps Clinic - La Jolla, La Jolla, CA; J.F. Kroener
University of California San Diego Moores Cancer Center, San Diego, CA; B.A. Parker
John Muir Medical Center, Concord, CA; J.T. Ganey
Kaiser Permanente - Fremont, Fremont, CA; L. Fehrenbacher
Alta Bates Hospital, Berkeley, CA; D.H. Irwin
Kaiser Permanente Santa Teresa (San Jose), Vallejo, CA; L. Fehrenbacher
Mercy General Hospital, Carmichael, CA; M. Javeed
Kaiser Permanente-San Francisco, Vallejo, CA; L. Fehrenbacher
Santa Rosa Memorial Hospital, Santa Rosa, CA; I.C. Anderson
Stanford University Medical Center, Stanford, CA; I.L. Wapnir
Kaiser Permanente, San Diego, CA; J.A. Polikoff
Glendale Memorial Hospital and Health Center, Glendale, CA; G. Al-Jazayrly
Penrose-Saint Francis Healthcare, Colorado Springs, CO; E.R. Pajon
Front Range Cancer Specialists, Fort Collins, CO; D. Medgyesy
Longmont United Hospital, Longmont, CO; E.R. Pajon
The Shaw Regional Cancer Center, Aurora, CO; A.D. Elias
Greenwich Hospital, Greenwich, CT; B.J. Drucker
Norwalk Hospital, Norwalk, CT; R.C. Frank
Stamford Hospital, Stamford, CT; I. Tepler
Eastern Connecticut Hematology and Oncology Associates, Norwich, CT; K. Jagathambal
Northwest Connecticut Oncology - Hematology Associates, Torrington, CT; D.S. Brandt
Georgetown University Hospital, Washington, DC; C. Isaacs
Washington Hospital Center, Washington, DC; A. Aggarwal
Sibley Memorial Hospital, Washington, DC; F. Barr
Christiana Healthcare Services - Christiana Hospital, Newark, DE; D.D. Biggs
Memorial Cancer Institute, Hollywood, FL
Mount Sinai Medical Center CCOP, Miami Beach, FL; M.A. Schwartz
Holy Cross Hospital, Fort Lauderdale, FL; R.C. Lilenbaum
Sarasota Memorial Hospital, Sarasota, FL
Dekalb Medical Center, Atlanta, GA; T.E. Seay
Emory University, Altanta, GA; R.M. O’Regan
Memorial Health University Medical Center, Savannah, GA; H.C. Lebos
Atlanta Regional CCOP, Atlanta, GA; T.E. Seay
Augusta Oncology Associates, Inc., Augusta, GA; M.R. Keaton
St Joseph's/Candler Health System, Savannah, GA; M.A. Taylor
Mercy Medical Center - North Iowa, Mason City, IA; W.W. Bate
Medical Associates Clinic, Professional Corporation, Dubuque, IA; C. Holm
Loyola University Medical Center, Maywood, IL; K.S. Albain
Rush University Medical Center, Chicago, IL; M.A. Cobleigh
University of Chicago, Chicago, IL; H.L. Kindler
St Anthony Medical Center, Rockford, IL; R.E. Nora
Decatur Memorial Hospital, Decatur, IL; J.L. Wade
Memorial Medical Center, Springfield, IL; J.L. Wade
Ingalls Memorial Hospital, Harvey, IL; M.F. Kozloff
Carle Cancer Center CCOP, Urbana, IL; K.M. Rowland
Community Regional Cancer Care North, Indianapolis, IN; R. Walling
Indiana University Medical Center, Indianapolis, IN; K.D. Miller
Fort Wayne Medical Oncology/Hematology Incorporated, Fort Wayne, IN; S.R. Nattam
Northern Indiana Consortium, South Bend, IN; R.H. Ansari
Cancer Center of Kansas - Wichita, Wichita, KS; S.R. Dakhil
Via Christi Regional Medical Center, Wichita, KS; S.R. Dakhil
Louisiana State University, Shreveport, LA; G.M. Mills
Tufts Medical Center, Boston, MA; J.K. Erban
Massachusetts General Hospital, Boston, MA; H.J. Burstein
Dana-Farber Cancer Institute, Boston, MA; H.J. Burstein
Beth Israel Deaconess Medical Center, Boston, MA; H.J. Burstein
North Shore Cancer Center, Salem, MA; K.J. Krag
Suburban Hospital, Bethesda, MD; C.B. Hendricks
Johns Hopkins University, Baltimore, MD; A.C. Wolff
Anne Arundel Medical Center, Annapolis, MD; S.P. Watkins
Kaiser Permanente - Shady Grove Medical Center, Rockville, MD; L.C. Hwang
Eastern Maine Medical Center, Bangor, ME; H.M. Segal
Mercy Hospital, Portland, ME; R.C. Inhorn
William Beaumont Hospital, Royal Oak, MI; D. Zakalik
University of Michigan Medical Center, Ann Arbor, MI; A.F. Schott
Wayne State University, Detroit, MI; R.T. Morris
Mid-Michigan Medical Center, Midland, MI; M.R. Hurtubise
Regions Hospital, Minneapolis, MN; D.J. Schneider
United Hospital, St Paul, MN; P.J. Flynn
Duluth Clinic, Duluth, MN; R.J. Dalton
Mayo Clinic, Rochester, MN; J.N. Ingle
Saint Francis Regional Medical Center, Shakopee, MN; D.J. Schneider
Washington University School of Medicine, St Louis, MO; M.J. Naughton
Saint John's Regional Health Center, Springfield, MO; J.W. Goodwin Missouri
Baptist Medical Center, Saint Louis, MO; A.P. Lyss
Montana Cancer Consortium CCOP, Billings, MT; B.T. Marchello
University of North Carolina, Chapel Hill, NC; T.C. Shea
Mission Hospitals Inc, Asheville, NC; M.J. Messino
Forsyth Memorial Hospital, Winston-Salem, NC; J.O. Hopkins
Northeast Medical Center, Concord, NC; J.G. Wall
Hope, A Women's Cancer Center, Asheville, NC; D.J. Hertzel
Altru Hospital, Grand Forks, ND; T. Dentchev
Elliot Hospital, Manchester, NH; D. Weckstein
Dartmouth Hitchcock Medical Center, Lebanon, NH; P.A. Kaufman
New Hampshire Oncology-Hematology Associates, Concord, NH; C. Catcher
Saint Barnabas Medical Center, Livingston, NJ; R.A. Michaelson
Cooper Hospital University Medical Center, Newark, NJ; D.D. Biggs
Cancer Institute of New Jersey, New Brunswick, NJ; D.L. Toppmeyer
Cancer Institute of New Jersey At Hamilton, Trenton, NJ; D.L. Toppmeyer
University of Nevada At Reno Washoe Medical Center, Reno, NV
Saint Vincent's Hospital and Medical Center of New York, New York, NY; P. Klein
Memorial Sloan Kettering Cancer Center, New York, NY; C.A. Hudis
Weill Medical College of Cornell University, New York, NY; J. Leonard
Staten Island University Hospital, Staten Island, NY; M. Odaimi
Albert Einstein College/Medicine, Bronx, NY; C.M. Pellegrino
Montefiore Medical Center, Bronx, NY; C.M. Pellegrino
North Shore University Hospital, Manhasset, NY; D.R. Budman
Brookdale Hospital Medical Center, Brooklyn, NY; M.R. Kalavar
Roswell Park Cancer Institute, Buffalo, NY; E.G. Levine
Ohio State University Hospital, Columbus, OH; C.D. Bloomfield
Cleveland Clinic Foundation, Cleveland, OH; G.T. Budd
Case Western Reserve University, Cleveland, OH; P. Silverman
Fairview Hospital, Cleveland, OH; G.T. Budd
Aultman Hospital, Canton, OH; J.A. Schmotzer
Samaritan North Health Center, Dayton, OH; H.M. Gross
Lima Memorial Hospital, Toledo, OH; P.L. Schaefer
Cleveland Clinic Wooster Specialty Center, Wooster, OH; G.T. Budd
Kaiser Permanente, Portland, OR; N.R. Tirumali
Allegheny Cancer Center Network, Pittsburgh, PA; N. Wolmark
University of Pittsburgh, Pittsburgh, PA; A.M. Brufsky
Lancaster General Hospital, Lancaster, PA; R.J. Gottlieb
Abramson Cancer Center of the University of Pennsylvania, Philadelphia, PA; S.M. Domchek
Fox Chase Cancer Center, Philadelphia, PA; L.J. Goldstein
Chester County Hospital, West Chester, PA; W.E. Luginbuhl
St Mary Regional Cancer Center, Langhorne, PA; R.E. Reilly
Abington Memorial Hospital, Abington, PA; W.G. Andrews
Scranton Hematology Oncology, Scranton, PA; M. Hyzinski
Rhode Island Hospital, Providence, RI; W.M. Sikov
Women's and Infants Hospital, Providence, RI; D.S. Dizon
Sioux Valley Clinic - Oncology, Sioux Falls, SD; M.A. Mazurczak
Erlanger Medical Center, Chattanooga, TN; L.L. Schlabach
Jones Clinic, Germantown, TN; B.A. Mullins
Presbyterian Hospital of Dallas, Dallas, TX; J.F. Strauss
MD Anderson Cancer Center, Houston, TX; M.C. Green
Baylor College of Medicine, Houston, TX; R.M. Elledge
Doctor's Hospital of Laredo, Laredo, TX; G.W. Unzeitig
University of Vermont, Burlington, VT; S. Burdette-Radoux
Swedish Hospital Medical Center, Seattle, WA; S.E. Rivkin
University of Washington Medical Center, Seattle, WA; S.E. Rivkin
Southwest Washington Medical Center, Vancouver, WA; K.S. Lanier
University of Wisconsin, Madison, WI; J.A. Stewart
Saint Vincent Hospital, Green Bay, WI; T.J. Saphner
Midelfort Clinic, Eau Claire, WI; G.S. Nambudiri
Green Bay Oncology LTD at Saint Mary's Hospital, Green Bay, WI; T.J. Saphner
Marshall University Medical Center, Huntington, WV; M.R.B. Tria Tirona
TEXT
BREAST INTERNATIONAL GROUP (BIG)
INTERNATIONAL BREAST CANCER STUDY GROUP, A DIVISION OF ETOP IBCSG PARTNERS FOUNDATION
Breast Cancer Trials Australia and New Zealand (BCT-ANZ), Australia; P. Francis, I. Laycock
Austin Health, Heidelberg, Victoria; J. Stewart
Box Hill Hospital, Box Hill, Victoria; J. Chirgwin
Calvary Mater Newcastle, Waratah, New South Wales; A. van der Westhuizen
Coffs Harbour Health Campus, Coffs Harbour, New South Wales; K. Briscoe
Fiona Stanley Hospital, Murdoch, Western Australia; A. Redfern
Flinders Medical Centre, Bedford Park, South Australia; B. Koczwara
Launceston General Hospital, Launceston, Tasmania; S. Gauden
Lismore Base Hospital, A. Boyce
Liverpool Hospital, Liverpool, New South Wales; E. Moylan
Macarthur Cancer Therapy Centre, Campbelltown, New South Wales; S. Della-Fiorentina
Maroondah Hospital, Ringwood East, Victoria; J. Chirgwin
Peter MacCallum Cancer Centre, East Melbourne, Victoria; P. A. Francis
Riverina Cancer Care Centre, J. Hill
Royal Brisbane and Women’s Hospital, Herston, Queensland; M. Nottage
Royal Hobart Hospital, Hobart, Tasmania; I. Byard
St Vincent’s Hospital Melbourne, Fitzroy, Victoria; R. Snyder
Tamworth Rural Referral Hospital, Tamworth, New South Wales; F. Sardelic
Tweed Hospital, The, Tweed Heads, New South Wales; E. Abdi
Victorian Breast and Oncology Care, East Melbourne, Victoria; M. Chipman
Belgium
Institute Jules Bordet, Brussels; A. Gombos
Centre Hospitalier Peltzer-La Tourelle, Verviers; A. Barbeaux
Centre Hospitalier Regional de la Citadelle, Liège; J. P. Salmon
Centre Hospitalier Universitarie Sart Tilman, Liège; G. Jerusalem
U.Z. Gasthuisberg, Leuven; P. Neven
Egypt
Cairo Oncology Centre, Cairo; H. Azim
Hungary
National Institute of Oncology, Budapest; I. Láng
India
Tata Memorial Hospital, Mumbai; V. Parmar
Italy
Dipartimento di Oncologia, Azienda Ospedaliero-Universitaria di Udine, Udine; F. Puglisi
Centro di Riferimento Oncologico, Aviano; S. Spazzapan
Fondazione Salvatore Maugeri, Pavia; A. Bernardo
Istituto Europeo di Oncologia, Milano; M. Colleoni
Ospedale degli Infermi, Rimini; L. Gianni
Ospedale di Circolo e Fondazione Macchi, Varese; G. Pinotti
Ospedali Riuniti di Bergamo, Bergamo; C. Tondini
Sandro Pitigliani Medical Oncology Unit, Hospital of Prato, Prato; A. Di Leo
Spedali Civili, Brescia; E. Simoncini
Unita Operativa de Medicina Oncologica, Ospedale Ramazzini, Carpi; A. Fabrizio
Azienda Sanitaria di Bolzano, Bolzano; C. Graiff
Istituto Clinico Humanitas, Rozzano; A. Santoro
Peru
Instituto de Enfermedades Neoplásicas, Lima; H. Gomez
Slovenia
Institute of Oncology, Ljubljana; E. Skof
South Africa
Sandton Oncology Centre, Johannesburg; D. Vorobiof
Sweden
Sahlgrenska University Hospital, Gothenburg; P. Karlsson
Linkoping University Hospital, Linkoping; B. Linderholm
Swiss Association for Clinical Cancer Research (SAKK), Switzerland Centre Hospitalier Universitaire Vaudois, Lausanne; K. Zaman
Inselspital, Bern; M. Rabaglio
Oncocare Engeried, Bern; K. Buser
Institute of Oncology of Southern Switzerland (Ospedale San Giovanni, Bellinzona; Ospedale Regionale di Lugano, (Civico & Italiano), Lugano; Ospedale Regionale Beata Vergine, Mendrisio; Ospedale Regionale La Carità, Locarno; Istituto Cantonale di Patologia, Locarno); O. Pagani
Kantonsspital St Gallen, St Gallen; T. Ruhstaller
Rätisches Kantonos-/Regionalspital, Chur; R. von Moos
Kantonsspital Basel, Basel; C. Rochlitz
Onkologiezentrum Thun-Berner Oberland, Thun; D. Rauch
Zürich Frauenklinik, Zürich; N. Gabriel
GERMAN BREAST GROUP (GBG); O. ORTMANN, K. REIßMÜLLER, S. LOIBL
Caritas-Krankenhaus St Josef, Regensburg; O. Ortmann
Mammazentrum, Klinikum Deggendorf, Deggendorf; D. Augustin
St Vincentius Kliniken Karlsruhe, Karlsruhe; O. Tomé
Dr Horst Schmidt Kliniken, Wiesbaden; F. Lorenz-Salehi
Klinikum Mittelbaden, Baden-Baden; A. Hahn
Universitäts-Frauenklinik Lübeck, Lübeck; A. Rody
ICR-CTSU on behalf of the National Cancer Research Institute (NCRI) Breast Clinical Studies Group, United Kingdom; H. Earl, L. Hughes-Davies, J. Bliss, S. Kernaghan, N. Atkins
Addenbrookes Hospital, Cambridge; H. Earl
Peterborough District Hospital, Peterborough; K. McAdam
US NCI NATIONAL CLINICAL TRIALS NETWORK (NCTN)
Alliance for Clinical Trials in Oncology; E. Winer, L. Carey, A. Partridge, M. Goetz
ECOG-ACRIN Cancer Research Group; N. Davidson, V. Stearns, R. O’Regan, S. Gluck
Canadian Cancer Trials Group; K.I. Pritchard, T. Whelan, K. Gelmon, M. Webster
NRG Oncology; C.E. Geyer Jr, N. Wolmark, T Mamounas, J. White, S. Swain
SWOG; G. Hortobagyi, S. Martino, J. Gralow, A. Scott
North American Participating Centers
Canada
Cross Cancer Institute, Edmonton, Alberta; K.S. Tonkin
Tom Baker Cancer Center, Calgary, Alberta; B.A. Walley
London Regional Cancer Center, London, Ontario; K.R. Potvin
Juravinski Cancer Centre at Hamilton Health Sciences, Hamilton, Ontario; R.G. Tozer
Trillium Health Centre - W Toronto, Toronto, Ontario; J.A. Gapski
Hôpital Charles LeMoyne, Greenfield Park, Quebec; C. Prady
Allan Blair Cancer Center, Regina, Saskatchewan; M. Salim
Saskatoon Cancer Center, Saskatoon, Saskatchewan; A. Sami
The Vitalite Health Network - Dr Leon Richard Oncology Centre, Moncton, New Brunswick; P. Whitlock
Hopital du Sacre-Coeur de Montreal, Quebec; J. A. Roy
Windsor Regional Cancer Centre, Ontario; C. Hamm
United States of America
Presbyterian Hospital, Whittier, CA; J.H. Freimann
University of California at San Diego, San Diego, CA; J.E. Mortimer
San Francisco General, San Francisco, CA; H.S. Rugo
University of California at San Francisco, San Francisco, CA; C.J. Ryan
University of California San Diego Cancer Center, San Diego, CA; B.A. Parker
University of Colorado, Aurora, CO; A.D. Elias
The Shaw Regional Cancer Center, Aurora, CO; A.D. Elias
University of Connecticut, Farmington, CT; S. Tannenbaum
Walter Reed Army Medical Center, Washington, DC; D.C. Van Echo
University of Miami Sylvester Cancer Center, Miami, FL; S. Gluck
Mayo Clinic Jacksonville, Jacksonville, FL; E. Perez
Siouxland Hematology - Oncology Associates, Sioux City, IA; D.B. Wender
Saint Luke's Mountain States Tumor Institute, Boise, ID; T.A. Walters
Evanston Northwestern Healthcare, Evanston, IL; D.E. Merkel
John H. Stroger, Jr, Hospital of Cook County, Chicago, IL; H.A. Zaren
Resurrection Medical Center, Chicago, IL; C. G. Rose
University of Chicago, Chicago, IL; H.L. Kindler
Saint Joseph's Medical Center, South Bend, IN; R.H. Ansari
Memorial Hospital of South Bend, South Bend, IN; R.H. Ansari
Fort Wayne Medical Oncology/Hematology Incorporated, Fort Wayne, IN; S.R. Nattam
Northern Indiana Cancer Research Co, South Bend, IN; R.H. Ansari
Mount Carmel Regional Cancer Center, Pittsburg, KS
Stormont-Vail Regional Health Center, Topeka, KS; S.J. Vogel
Addison Gilbert, Gloucester, MA; A.P. McIntyre
Tufts Medical Center, Boston, MA; J.K. Erban
Massachusetts General Hospital, Boston, MA; H.J. Burstein
Dana-Farber Cancer Institute, Boston, MA; H.J. Burstein
Beth Israel Deaconess Medical Center, Boston, MA; H.J. Burstein
Faulkner Hospital, Boston, MA; H.J. Burstein
North Shore Cancer Center, Salem, MA; K.J. Krag
Emerson Hospital, Boston, MA; H.J. Burstein
Suburban Hospital, Bethesda, MD; C.B. Hendricks
University of Maryland Greenebaum Cancer Center, Baltimore, MD; K.H. Rak Tkaczuk
Mercy Medical Center, Baltimore, MD; D.A. Riseberg
William Beaumont Hospital, Royal Oak, MI; D. Zakalik
United Hospital, St Paul, MN; P.J. Flynn
Abbott-Northwestern Hospital, St Louis Park, MN; P.J. Flynn
Mercy Hospital, Coon Rapids, MN; P.J. Flynn
Mayo Clinic, Rochester, MN; J.N. Ingle
Saint John's Hospital - Healtheast, Minneapolis, MN; D.J. Schneider
Metro-Minnesota CCOP, Minneapolis, MN; P.J. Flynn
Washington School of Medicine, St Louis, MO; M.J. Naughto
Kansas City CCOP, Kansas City, MO; W.T. Stephenson
Montana Cancer Consortium CCOP, Billings, MT; B.T. Marchello
Moses H. Cone Memorial, Greensboro, NC; J.E. Feldmann
Mission Hospitals Inc, Asheville, NC; M.J. Messino
Hope, A Women's Cancer Center, Asheville, NC; D.J. Hetzel
Medcenter One Health Systems, Bismarck, ND; E.J. Wos
Dakota Clinic, Fargo, ND; K. Sen
University of Nebraska Medical Center, Omaha, NE; E.C. Reed
Portsmouth Regional Hospital, Portsmouth, NH; E.M. Bonnem
South Jersey Healthcare, Vineland, NJ; D.H. Blom
Virtua West Jersey Hospitals, Marlton, NJ; M.S. Entmacher
New York University Medical Center, New York, NY; A.D. Tiersten
Albert Einstein College/Medicine, Bronx, NY; C.M. Pellegrino
Roswell Park Cancer Institute, Buffalo, NY; E.G. Levine
Aultman Hospital, Canton, OH; J.A. Schmotzer
Geisinger Medical Center, Danville, PA; G.D.A. Padula
Sioux Valley Clinic - Oncology, Sioux Falls, SD; M.A. Mazurczak
University of Vermont, Burlington, VT; S. Burdette-Radoux
Mountainview Medical, Berlin, VT; S. Burdette-Radoux
Swedish Hospital Medical Center, Seattle, WA; S.E. Rivkin
University of Washington Medical Center, Seattle, WA; S.E. Rivkin
Aspirus Wausau Hospital Center, Wausau, WI; U. Gautam
Oncology Alliance-Glendale, Glendale, WI; R.D. Hart
West Virginia University, Morgantown, WV; J. Abraham
Olivia Pagani
Consulting or Advisory Role: Pfizer, Roche, Novartis, Debiopharm Group
Barbara A. Walley
Stock and Other Ownership Interests: Pfizer
Gini F. Fleming
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Curio Science, Physicians' Education Resource
Research Funding: Corcept Therapeutics (Inst), AbbVie (Inst), Iovance Biotherapeutics (Inst), Syros Pharmaceuticals (Inst), Sermonix Pharmaceuticals (Inst), Compugen (Inst), Plexxikon (Inst), Roche (Inst), GlaxoSmithKline (Inst), Celldex (Inst), AstraZeneca (Inst), Molecular Templates (Inst), CytomX Therapeutics (Inst), Astellas Pharma (Inst), K-Group Beta (Inst), Pfizer (Inst)
Other Relationship: DSI (Inst), Merck (Inst), Caris Life Sciences (Inst), Eisai (Inst), AstraZeneca (Inst)
Uncompensated Relationships: AbbVie
Marco Colleoni
Research Funding: Roche (Inst)
Henry L. Gomez
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Roche, Novartis, AstraZeneca, Bristol-Myers Squibb, Lilly
Research Funding: MSD Oncology
Carlo Tondini
Consulting or Advisory Role: Myriad Genetics, MSD Oncology, Amgen
Speakers' Bureau: Amgen
Travel, Accommodations, Expenses: Takeda, Amgen, MSD, Eli Lilly Italia SPA, Roche, Pfizer
Harold J. Burstein
This author is a Consultant Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Matthew P. Goetz
Consulting or Advisory Role: Lilly (Inst), AstraZeneca (Inst), Blueprint Medicines (Inst), Genzyme (Inst), ARC Therapeutics (Inst), Biotheranostics (Inst), Rna Diagnostics (Inst), Seattle Genetics (Inst)
Research Funding: Lilly (Inst), Pfizer (Inst), Sermonix Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Methods and Materials for Assessing Chemotherapy Responsiveness and Treating Cancer; Methods and Materials for using Butyrylcholinesterases to Treat Cancer; Development of Human Tumor Xenografts from Women with Breast Cancer Treated with Neoadjuvant Chemotherapy (Inst)
Travel, Accommodations, Expenses: Lilly
Eva M. Ciruelos
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, Novartis, Lilly, MSD Oncology, Daiichi Sankyo/Astra Zeneca, Gilead Sciences, Seattle Genetics
Speakers' Bureau: Lilly, Roche, Daiichi Sankyo/Astra Zeneca, Novartis
Travel, Accommodations, Expenses: Roche, Pfizer
Vered Stearns
Consulting or Advisory Role: Novartis
Research Funding: AbbVie (Inst), Pfizer (Inst), Novartis (Inst), Puma Biotechnology (Inst), Biocept (Inst)
Other Relationship: AstraZeneca
Hervé R. Bonnefoi
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca/Daiichi Sankyo
Silvana Martino
Consulting or Advisory Role: Merck, MorphoSys, Lilly, GlaxoSmithKline, Steba Biotech, Blue Print, Secura Bio, Pro Ed, TG Therapeutics, BeiGene, Secura Bio, 3D Communications
Charles E. Geyer Jr
Consulting or Advisory Role: Exact Sciences
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Daiichi Sankyo/Astra Zeneca (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: AbbVie, Genentech/Roche, Daiichi-Sankyo, AstraZeneca
Fabio Puglisi
Honoraria: Roche, MSD, AstraZeneca, Novartis, Pierre Fabre, Daiichi Sankyo, Eisai, Lilly, Pfizer
Consulting or Advisory Role: Roche, Amgen, Novartis, Pfizer, Eisai, Seattle Genetics, Pierre Fabre, AstraZeneca/Daiichi Sankyo, Viatris, Lilly, Gilead Sciences
Research Funding: Eisai, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, Celgene, GlaxoSmithKline, Amgen, AstraZeneca, MSD, Novartis, Lilly, Pfizer
Simon Spazzapan
Honoraria: Novartis, Daiichi-Sankyo, Eli Lilly Italia, AstraZeneca/MSD
Consulting or Advisory Role: Novartis, Seattle Genetics, AstraZeneca/Daiichi-Sankyo, MSD/AstraZeneca
Travel, Accommodations, Expenses: Roche, Celgene, Teva, Novartis
Thomas Ruhstaller
Honoraria: Lilly, Novartis (Inst), Daiichi Sankyo/Lilly
Consulting or Advisory Role: Novartis, Roche, AstraZeneca, Lilly
Expert Testimony: Novartis, Lilly
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Genentech/Roche
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Sherene Loi
Consulting or Advisory Role: Roche/Genentech (Inst), Aduro Biotech (Inst), Novartis (Inst), G1 Therapeutics (Inst), PUMA Biotechnology (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Seattle Genetics (Inst), BMS (Inst), Silverback Therapeutics (Inst), Pfizer (Inst), Gilead Sciences (Inst), Daiichi Sankyo/Lilly (Inst), Tallac Therapeutics (Inst)
Research Funding: Roche/Genentech (Inst), Novartis (Inst), Merck (Inst), Puma Biotechnology (Inst), Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), AstraZeneca (Inst), Nektar (Inst), Lilly (Inst)
Other Relationship: Roche Medical writing support
Alan S. Coates
Stock and Other Ownership Interests: Avita Medical Inc, CSL Limited, Ramsay Health Care, ResMed
Richard D. Gelber
Research Funding: AstraZeneca (Inst), Novartis (Inst), Roche (Inst), Merck (Inst)
Meredith M. Regan
Honoraria: Bristol-Myers Squibb, WebMD
Consulting or Advisory Role: Ipsen (Inst), Tolmar, Bristol-Myers Squibb, Debiopharm Group (Inst)
Research Funding: Pfizer (Inst), Ipsen (Inst), Novartis (Inst), Merck (Inst), AstraZeneca (Inst), Pierre Fabre (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Roche (Inst), TerSera (Inst), Debiopharm Group (Inst)
No other potential conflicts of interest were reported.
See accompanying editorial on page1339
DISCLAIMER
The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health and Social Care. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
PRIOR PRESENTATION
Presented at the 2021 San Antonio Breast Cancer Symposium, San Antonio, TX, December 7-11, 2021.
SUPPORT
SOFT and TEXT are sponsored by ETOP IBCSG Partners Foundation. Conduct is supported by the ETOP IBCSG Partners Foundation, which has included additional support for the IBCSG from the Frontier Science Foundation, Swiss Group for Clinical Cancer Research Switzerland, Oncosuisse, Cancer League Switzerland, Foundation for Clinical Cancer Research of Eastern Switzerland, grant U24 CA075362 from the US NCI. Longer-term follow-up of SOFT and TEXT has been supported also by grants to the IBCSG from Pfizer (WI223438), Ipsen, Debiopharm, TerSera, AstraZeneca (57735423), the Breast Cancer Research Foundation (16-185,17-187,18-003,19-011,20-011,21-011) and private donors. SOFT and TEXT conduct in the US and Canada have been supported by US NCI NCTN via the Alliance for Clinical Trials in Oncology (grant Nos. above). Supported by Breast Cancer Trials Australia and New Zealand (National Health and Medical Research Council grant Nos. 351161, 510788 and 1105058); Institute of Cancer Research Clinical Trials and Statistics Unit (ICR-CTSU) on behalf of the National Cancer Research Institute Breast Clinical Studies Group United Kingdom (NCRI-BCSG—ICR-CTSU Partnership), Cancer Research UK grant Nos. CRUKE/03/022, CRUKE/03/023, C1491/A15955; National Institute for Health Research Royal Marsden/Institute of Cancer Research Biomedical Research Centre (no grant No.); and National Institute for Health Research/Cambridge Biomedical Research Centre (no grant No.); Alliance for Clinical Trials in Oncology (US NIH grant No. U10CA180821); SWOG (US National Institutes of Health [NIH] grant Nos. U10CA180888, UG1CA233160, UG1CA233329); ECOG-ACRIN Cancer Research Group (US NIH grant Nos. U10CA180820, U10CA180794); NRG Oncology (US NIH grant Nos. U10CA180868, U10CA180822, UG1CA189867); Canadian Cancer Trials Group (US NIH grant No. U10CA180863, and Canadian Cancer Society grant No. 707213).
CLINICAL TRIAL INFORMATION
O.P. and B.A.W. contributed equally to this work.
M.M.R. and P.A.F. contributed equally to this work.
DATA SHARING STATEMENT
After publication, access to deidentified individual participant data may be requested by researchers by submitting a proposal (to stat_center@ibcsg.org), which will be reviewed for scientific merit and feasibility in accordance with IBCSG guidelines for collaborative research and data sharing policy.
AUTHOR CONTRIBUTIONS
Conception and design: Olivia Pagani, Barbara A. Walley, Gini F. Fleming, Harold J. Burstein, Matthew P. Goetz, Silvana Martino, Sherene Loi, Alan S. Coates, Richard D. Gelber, Aron Goldhirsch, Meredith M. Regan, Prudence A. Francis
Administrative support: István Láng, Silvana Martino, Barbara Ruepp
Provision of study materials or patients: Barbara A. Walley, István Láng, Henry L. Gomez, Carlo Tondini, Harold J. Burstein, Vered Stearns, Hervé R. Bonnefoi, Silvana Martino, Fabio Puglisi, Simon Spazzapan, Thomas Ruhstaller, Prudence A. Francis
Collection and assembly of data: Barbara A. Walley, Marco Colleoni, István Láng, Henry L. Gomez, Matthew P. Goetz, Eva M. Ciruelos, Vered Stearns, Hervé R. Bonnefoi, Silvana Martino, Thomas Ruhstaller, Barbara Ruepp, Sherene Loi, Richard D. Gelber, Meredith M. Regan, Prudence A. Francis
Data analysis and interpretation: Barbara A. Walley, Gini F. Fleming, István Láng, Henry L. Gomez, Carlo Tondini, Harold J. Burstein, Matthew P. Goetz, Vered Stearns, Silvana Martino, Claudio Chini, Fabio Puglisi, Simon Spazzapan, Thomas Ruhstaller, Eric P. Winer, Sherene Loi, Alan S. Coates, Meredith M. Regan, Prudence A. Francis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Exemestane With Ovarian Suppression in Premenopausal Breast Cancer: Long-Term Follow-Up of the Combined TEXT and SOFT Trials
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Olivia Pagani
Consulting or Advisory Role: Pfizer, Roche, Novartis, Debiopharm Group
Barbara A. Walley
Stock and Other Ownership Interests: Pfizer
Gini F. Fleming
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Curio Science, Physicians' Education Resource
Research Funding: Corcept Therapeutics (Inst), AbbVie (Inst), Iovance Biotherapeutics (Inst), Syros Pharmaceuticals (Inst), Sermonix Pharmaceuticals (Inst), Compugen (Inst), Plexxikon (Inst), Roche (Inst), GlaxoSmithKline (Inst), Celldex (Inst), AstraZeneca (Inst), Molecular Templates (Inst), CytomX Therapeutics (Inst), Astellas Pharma (Inst), K-Group Beta (Inst), Pfizer (Inst)
Other Relationship: DSI (Inst), Merck (Inst), Caris Life Sciences (Inst), Eisai (Inst), AstraZeneca (Inst)
Uncompensated Relationships: AbbVie
Marco Colleoni
Research Funding: Roche (Inst)
Henry L. Gomez
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Roche, Novartis, AstraZeneca, Bristol-Myers Squibb, Lilly
Research Funding: MSD Oncology
Carlo Tondini
Consulting or Advisory Role: Myriad Genetics, MSD Oncology, Amgen
Speakers' Bureau: Amgen
Travel, Accommodations, Expenses: Takeda, Amgen, MSD, Eli Lilly Italia SPA, Roche, Pfizer
Harold J. Burstein
This author is a Consultant Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Matthew P. Goetz
Consulting or Advisory Role: Lilly (Inst), AstraZeneca (Inst), Blueprint Medicines (Inst), Genzyme (Inst), ARC Therapeutics (Inst), Biotheranostics (Inst), Rna Diagnostics (Inst), Seattle Genetics (Inst)
Research Funding: Lilly (Inst), Pfizer (Inst), Sermonix Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Methods and Materials for Assessing Chemotherapy Responsiveness and Treating Cancer; Methods and Materials for using Butyrylcholinesterases to Treat Cancer; Development of Human Tumor Xenografts from Women with Breast Cancer Treated with Neoadjuvant Chemotherapy (Inst)
Travel, Accommodations, Expenses: Lilly
Eva M. Ciruelos
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, Novartis, Lilly, MSD Oncology, Daiichi Sankyo/Astra Zeneca, Gilead Sciences, Seattle Genetics
Speakers' Bureau: Lilly, Roche, Daiichi Sankyo/Astra Zeneca, Novartis
Travel, Accommodations, Expenses: Roche, Pfizer
Vered Stearns
Consulting or Advisory Role: Novartis
Research Funding: AbbVie (Inst), Pfizer (Inst), Novartis (Inst), Puma Biotechnology (Inst), Biocept (Inst)
Other Relationship: AstraZeneca
Hervé R. Bonnefoi
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca/Daiichi Sankyo
Silvana Martino
Consulting or Advisory Role: Merck, MorphoSys, Lilly, GlaxoSmithKline, Steba Biotech, Blue Print, Secura Bio, Pro Ed, TG Therapeutics, BeiGene, Secura Bio, 3D Communications
Charles E. Geyer Jr
Consulting or Advisory Role: Exact Sciences
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Daiichi Sankyo/Astra Zeneca (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: AbbVie, Genentech/Roche, Daiichi-Sankyo, AstraZeneca
Fabio Puglisi
Honoraria: Roche, MSD, AstraZeneca, Novartis, Pierre Fabre, Daiichi Sankyo, Eisai, Lilly, Pfizer
Consulting or Advisory Role: Roche, Amgen, Novartis, Pfizer, Eisai, Seattle Genetics, Pierre Fabre, AstraZeneca/Daiichi Sankyo, Viatris, Lilly, Gilead Sciences
Research Funding: Eisai, AstraZeneca, Roche
Travel, Accommodations, Expenses: Roche, Celgene, GlaxoSmithKline, Amgen, AstraZeneca, MSD, Novartis, Lilly, Pfizer
Simon Spazzapan
Honoraria: Novartis, Daiichi-Sankyo, Eli Lilly Italia, AstraZeneca/MSD
Consulting or Advisory Role: Novartis, Seattle Genetics, AstraZeneca/Daiichi-Sankyo, MSD/AstraZeneca
Travel, Accommodations, Expenses: Roche, Celgene, Teva, Novartis
Thomas Ruhstaller
Honoraria: Lilly, Novartis (Inst), Daiichi Sankyo/Lilly
Consulting or Advisory Role: Novartis, Roche, AstraZeneca, Lilly
Expert Testimony: Novartis, Lilly
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Genentech/Roche
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Sherene Loi
Consulting or Advisory Role: Roche/Genentech (Inst), Aduro Biotech (Inst), Novartis (Inst), G1 Therapeutics (Inst), PUMA Biotechnology (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst), Seattle Genetics (Inst), BMS (Inst), Silverback Therapeutics (Inst), Pfizer (Inst), Gilead Sciences (Inst), Daiichi Sankyo/Lilly (Inst), Tallac Therapeutics (Inst)
Research Funding: Roche/Genentech (Inst), Novartis (Inst), Merck (Inst), Puma Biotechnology (Inst), Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), AstraZeneca (Inst), Nektar (Inst), Lilly (Inst)
Other Relationship: Roche Medical writing support
Alan S. Coates
Stock and Other Ownership Interests: Avita Medical Inc, CSL Limited, Ramsay Health Care, ResMed
Richard D. Gelber
Research Funding: AstraZeneca (Inst), Novartis (Inst), Roche (Inst), Merck (Inst)
Meredith M. Regan
Honoraria: Bristol-Myers Squibb, WebMD
Consulting or Advisory Role: Ipsen (Inst), Tolmar, Bristol-Myers Squibb, Debiopharm Group (Inst)
Research Funding: Pfizer (Inst), Ipsen (Inst), Novartis (Inst), Merck (Inst), AstraZeneca (Inst), Pierre Fabre (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Roche (Inst), TerSera (Inst), Debiopharm Group (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Francis PA, Pagani O, Fleming GF, et al. : Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 379:122-137, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan H, Gray R, Braybrooke J, et al. : 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836-1846, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen RN, Esen B, Mellemkjær L, et al. : The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst 114:391-399, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) : Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 386:1341-1352, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Pagani O, Regan MM, Walley BA, et al. : Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 371:107-118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis PA, Regan MM, Fleming GF, et al. : Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372:436-446, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cusimano MC, Chiu M, Ferguson SE, et al. : Association of bilateral salpingo-oophorectomy with all cause and cause specific mortality: Population based cohort study. BMJ 375:e067528, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuesley KM, Protani MM, Webb PM, et al. : Hysterectomy with and without oophorectomy and all-cause and cause-specific mortality. Am J Obstet Gynecol 223:723.e1-723.e16, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Bradley R, Braybrooke J, Gray R, et al. : Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol 23:382-392, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbeck N, Rastogi P, Martin M, et al. : Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 32:1571-1581, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Paluch-Shimon S, Lueck H, Beith J, et al. : 153P Adjuvant endocrine therapy combined with abemaciclib in monarchE patients with high-risk early breast cancer: Disease characteristics and endocrine therapy choice by menopausal status. Ann Oncol 32:S427-S428, 2021 [Google Scholar]
- 12.Fleming GF, Pagani O, Regan MM, et al. : Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 33:658, 2022 [DOI] [PubMed] [Google Scholar]
- 13.Bernhard J, Luo W, Ribi K, et al. : Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): A combined analysis of two phase 3 randomised trials. Lancet Oncol 16:848-858, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribi K, Luo W, Walley BA, et al. : Treatment-induced symptoms, depression and age as predictors of sexual problems in premenopausal women with early breast cancer receiving adjuvant endocrine therapy. Breast Cancer Res Treat 181:347-359, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
After publication, access to deidentified individual participant data may be requested by researchers by submitting a proposal (to stat_center@ibcsg.org), which will be reviewed for scientific merit and feasibility in accordance with IBCSG guidelines for collaborative research and data sharing policy.