PURPOSE
Given the increasing incidence of early-onset colorectal cancer (CRC; diagnosed before age 50 years) worldwide, it is important to identify modifiable risk factors. We investigated whether alcohol consumption in the young population correlated with an increased early-onset CRC risk that differed by tumor location and sex.
PATIENTS AND METHODS
We investigated the association between average daily alcohol consumption and the risk of early-onset CRC among 5,666,576 individuals age 20-49 years using data from the Korean National Health Insurance Service (2009-2019). Alcohol consumption levels of nondrinker, light (reference), moderate, and heavy drinker were defined as 0, <10, 10 to <30, and ≥30 g/d for men and 0, <10, 10 to <20, and ≥20 g/d for women, respectively. Multivariate Cox proportional hazards models were used to estimate adjusted hazard ratios (aHRs) with 95% CIs.
RESULTS
We identified 8,314 incident early-onset CRC cases during the follow-up period. Moderate and heavy drinkers showed an increased risk of early-onset CRC compared with light drinkers (aHR, 1.09 [95% CI, 1.02 to 1.16] and aHR, 1.20 [95% CI, 1.11 to 1.29], respectively). Subgroup analysis by tumor location showed positive dose-response significance for early-onset distal colon and rectal cancers, but not for proximal colon cancer. The dose-response association between drinking frequency and risk of early-onset CRC was significant, with a 7%, 14%, and 27% increased risk for 1-2, 3-4, and ≥5 d/wk compared with nondrinkers, respectively.
CONCLUSION
Excessive alcohol consumption increases the risk of CRC onset before age 50 years. Thus, effective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second most common cause of cancer-related deaths worldwide,1 and the incidence of early-onset CRC (diagnosed before age 50 years, approximately 10% of incident CRC diagnoses) continues to increase.1,2 By 2030, 11% and 23% of colon and rectal cancers, respectively, will occur in younger adults (age <50 years).2 Compared with late-onset CRC (diagnosed after age 50 years), which mostly occurs in the proximal colon, early-onset CRC is commonly found in the rectum and distal colon,3-5 especially in men.3 Although 30% of early-onset CRC occurs in those with a family history or hereditary syndrome, most cases are sporadic and possibly exacerbated by environmental or lifestyle factors.3,4 Sedentary behavior,6 consumption of sugar-sweetened beverages,7 obesity,8 metabolic syndrome,9,10 total vitamin D intake,11 and alcohol intake12,13 may contribute to the risk of early-onset CRC, but studies of the risk factors of early-onset CRC by tumor location, despite a predominance in the left colon, are limited.
CONTEXT
Key Objective
Could an increase in alcohol consumption in the young population be associated with an increased incidence of early-onset colorectal cancer (CRC) diagnosed before age 50 years?
Knowledge Generated
In this nationwide population-based study, moderate or heavy alcohol consumption was associated with an increased risk of early-onset CRC, particularly distal colon and rectal cancers. The dose-response association between increased amount and frequency of alcohol consumption and increased risk of CRC was significant.
Relevance (A.H. Ko)
-
This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC, offering potential risk reduction/mitigation strategies for this growing phenomenon.*
*Relevance section written by JCO Associate Editor Andrew H. Ko, MD, FASCO.
Alcohol consumption is a leading risk factor for carcinogenesis and cancer mortality worldwide.14 The International Agency for Research on Cancer has classified ethanol in alcoholic beverages as carcinogenic to humans (group 1),14 with epidemiologic evidence of cancer causation.15 Despite the carcinogenic effect, global per-capita alcohol consumption increased from 5.9 to 6.5 L between 1990 and 2017 and is estimated to reach 7.6 L by 2030,16 potentially increasing the overall global burden of alcohol-attributable cancers. Of the 1.34 billion individuals who consumed alcohol in 2020, 59.1% were 15 to 39 years old, with harmful alcohol consumption mainly concentrated in this age group.17 Moreover, alcohol use confers a threefold higher burden of alcohol-attributed diseases in men than in women.18
Excessive alcohol consumption in the young population may be a risk factor for early-onset CRC,12,13 although large-scale study data are lacking regarding whether dose-response association between alcohol consumption and early-onset CRC differs according to tumor location. We investigated the association between alcohol consumption and the early-onset CRC risk by tumor location and sex, while focusing on graded increasing amounts of alcohol consumption and drinking frequency.
PATIENTS AND METHODS
Study Design
Using the South Korean population database of the National Health Insurance Service (NHIS), this retrospective population-based cohort study analyzed nationally representative data of 5.7 million Korean young adults (age 20-49 years). The NHIS provides a single-insurer health insurance coverage to all resident Korean citizens (approximately 50 million) that provides a biannual free health checkup.19 The NHIS database comprises the health information, such as demographic, socioeconomic variables, and health checkup and claims data, of all insured individuals. The NHIS health checkup programs include anthropometric measurements (height, weight, waist circumference, and blood pressure), hearing and visual acuity checks, laboratory tests, health behavior surveys (smoking, alcohol consumption, and regular exercise), and medical and family history, as described previously.10 As the NHIS database represents the entire Korean population, NHIS data can be used for nationwide population-based studies.
In Korea, the Basic Act on Health Examination was enacted in April 2008, and detailed questionnaires on drinking, smoking, and physical activity were added in 2009.20 Thus, we included individuals who underwent NHIS health checkups between January 1, 2009, and December 31, 2009 (index year), and tracked them until the end of 2019 to identify incident early-onset CRC (age <50 years). The participation rate for NHIS health checkups was 67% in 2009. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB number: X-2007-627-905). All procedures involving human participants were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The requirement for informed consent was waived because the data were publicly available and deidentified before the analysis.
Study Population
Figure 1 depicts the enrollment process for this cohort. The legal drinking age in Korea is 19 years. We initially screened 6,114,839 individuals (age 20-49 years) who underwent health checkups in 2009. Exclusions included 407,414 individuals because of incomplete data (Data Supplement, online only), 36,157 individuals because of malignancy diagnosed before 2009, and 4,692 individuals diagnosed with CRC within 1 year of enrollment (excluded to rule out pre-existing CRC). We included 5,666,576 (3,362,414 men; 2,304,162 women) participants and they were censored on attaining age 50 years or until December 31, 2019 (mean follow-up 7.4 ± 2.9 years).
FIG 1.
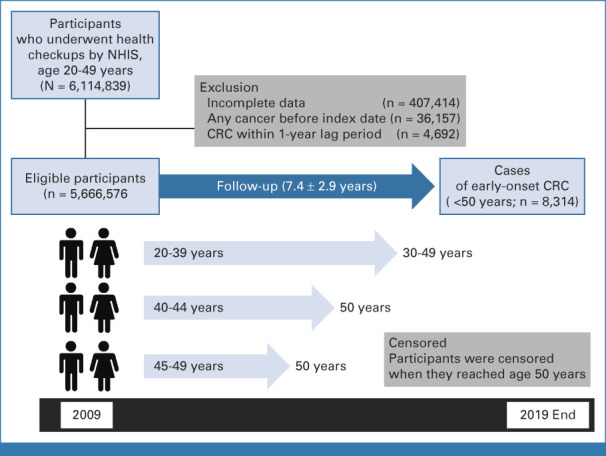
Flowchart showing the enrollment process for the study cohort. CRC, colorectal cancer; NHIS, National Health Insurance Service.
Exposure: Alcohol Consumption Calculation
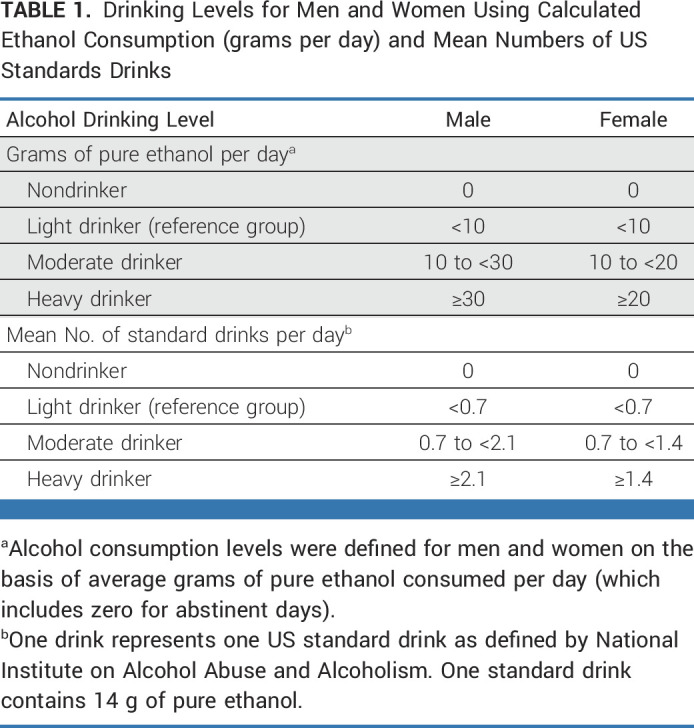
Using a standardized self-reported questionnaire at enrollment (Data Supplement), the average daily alcohol consumption was calculated using the drinking frequency (numbers of days per week) and the typical amount consumed on each occasion (the amount of standard cups). In Korea, one standard cup containing 8 g of ethanol is equivalent to one cup (50 mL) of Soju (a Korean distilled spirit) or one cup (220 mL) of beer.21 Given the regional differences in the definition of a standard drink containing ethanol (from 8 to 14 g),22,23 alcohol consumption was converted into grams of ethanol per day, instead of standard drinks per day (Data Supplement). The alcohol drinking levels were defined as four categories (nondrinkers, light [reference], moderate, and heavy drinkers) for men and women referring to previous studies (Table 1).24-26 Alcohol drinking consumption was also expressed in terms of US standard drinks (14 g of pure alcohol) as defined by the National Institute on Alcohol Abuse and Alcoholism.
TABLE 1.
Drinking Levels for Men and Women Using Calculated Ethanol Consumption (grams per day) and Mean Numbers of US Standards Drinks
Assessment of Covariates
Participant smoking status during the study (nonsmoker, past smoker, and current smoker) and pack-years were recorded. Regular exercise was defined as ≥20 minutes of vigorous-intensity (≥3 times/wk) or ≥30 minutes of moderate-intensity (≥5 times/wk) exercise.27 Low income was defined as the lowest quantile of income-based insurance contributions. Comorbidities (hypertension, diabetes mellitus [DM], and dyslipidemia) were identified from the NHIS health checkup and claims data. Hypertension was defined on the basis of the International Classification of Disease, 10th Revision, Clinical Modification (ICD-10-CM) codes I10-I13 or I15 with any claim for prescription of antihypertensive medications, or a recorded blood pressure ≥140/90 mmHg in the index year. DM was defined as a fasting glucose level ≥126 mg/dL, or at least one annual claim for prescription of antidiabetic medications under the ICD-10 codes E11-E14. Dyslipidemia was defined as a serum total cholesterol level ≥240 mg/dL, or at least one annual claim for prescription of lipid-lowering medications under the ICD-10 code E78.
Study Outcomes
The primary outcome was the incidence of early-onset CRC diagnosed before age 50 years. CRC was defined as C18-20 on the basis of the ICD-10-CM codes (C18.0-18.4 for proximal colon cancer; C18.5-18.7 and C19.0 for distal colon cancer; C18.9 for unspecified colon cancer; C20.0 for rectal cancer) and the registration code for cancer (V193). In the subgroup analysis by tumor subsites, malignant neoplasms in overlapping sites in the colon (C18.8) were excluded because they were not specific to one location. The proportion of overlapping sites in the colon was 0.5% (46/8,314) in early-onset CRC cases. Carcinoid tumors were excluded.
Statistical Analysis
We evaluated the dose-response association between average daily alcohol consumption and the risk of early-onset CRC in subgroup analyses by anatomic site (proximal colon, distal colon, rectum, and unspecified colon). Baseline participant characteristics are presented as descriptive statistics and compared using the independent t test or chi-square test. The outcome incidence was calculated by dividing the number of events by total person-years of follow-up. Multivariable Cox proportional hazard regression analyses were performed to estimate the hazard ratios (HRs) and 95% CIs. Model 1 was unadjusted; model 2 was adjusted for age (years); model 3 was adjusted for age (years), sex (male v female), smoking status (never, former, or current), regular exercise (yes v no), and low income (on the basis of the lowest quintile); and model 4 was further adjusted for the variables in model 3 and comorbidities (hypertension, DM, and dyslipidemia). Sex variable was not adjusted in the sex-specific models 3 and 4. Linear trend tests were performed with the variables of interest defined on a continuous scale. To examine whether the association between alcohol consumption patterns and early-onset CRC risk differed by anatomic site, HR and 95% CI were estimated in a multivariable Cox proportional hazard model. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and results with two-sided P values <.05 were considered significant.
RESULTS
Baseline Characteristics
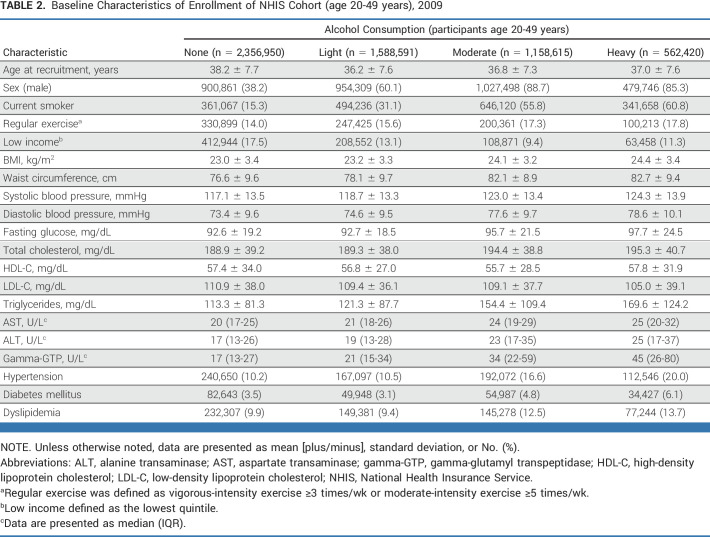
Table 2 presents baseline characteristics of the participants by drinking status. Among the 5,666,576 participants (age 20-49 years), nondrinkers, light, moderate, and heavy drinkers comprised 41.6%, 28.0%, 20.4%, and 9.9%, respectively. Compared with light drinkers (reference), moderate and heavy drinkers were more likely to be older, male, current smokers, and regular exercisers; have higher values of BMI, waist circumference, blood pressure, fasting glucose, total cholesterol, liver enzymes, and gamma-glutamyl transpeptidase; and have more comorbidities (hypertension, DM, and dyslipidemia).
TABLE 2.
Baseline Characteristics of Enrollment of NHIS Cohort (age 20-49 years), 2009
Early-Onset CRC Risk by Average Daily Alcohol Consumption
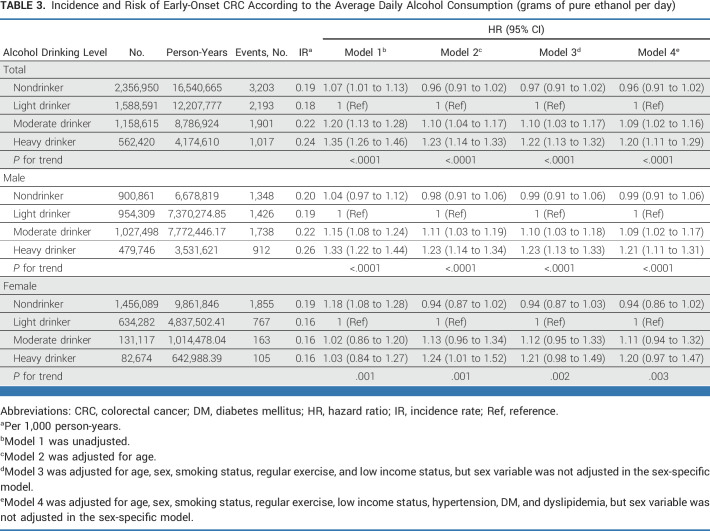
During a median 9.1 (IQR, 5.5-9.3) years, 8,314 early-onset CRC diagnoses were documented among the 5,666,576 participants. Compared with light drinking (reference), the risk of early-onset CRC was significantly associated with moderate and heavy drinking (adjusted HR [aHR], 1.09 [95% CI, 1.02 to 1.16] and aHR, 1.20 [95% CI, 1.11 to 1.29], respectively), after adjusting for confounding factors (Table 3). This dose-response relationship between alcohol drinking levels and the risk of early-onset CRC was observed in both men and women (P for trend <.0001 and =.003, respectively). However, the results were not statistically significant in women with moderate and heavy drinking levels (aHR, 1.11 [95% CI, 0.94 to 1.32] and aHR, 1.20 [95% CI, 0.97 to 1.47], respectively) compared with those with light drinking.
TABLE 3.
Incidence and Risk of Early-Onset CRC According to the Average Daily Alcohol Consumption (grams of pure ethanol per day)
Early-Onset CRC Risk by Alcohol Consumption According to Tumor Location
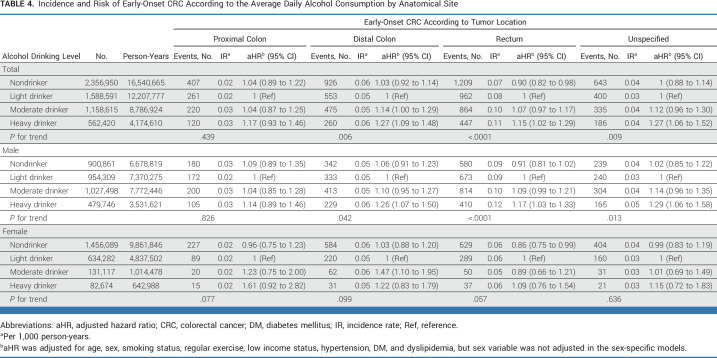
The aHRs of early-onset CRC for alcohol consumption levels by tumor location are shown in Table 4. Graded increasing amounts of alcohol consumption were significantly associated with a higher risk of early-onset CRC by the following anatomic sites: distal colon, rectum, and unspecified colon (P for trend = .006, <.0001, and =.009, respectively). However, no significant association was observed in the proximal colon (P for trend = .439). Compared with light drinkers, moderate and heavy drinkers had a 14% and 27% increased risk of distal colon cancer, respectively, and heavy drinkers had a 15% and 27% increased risk of rectal cancer and unspecified colon cancer, respectively. Interestingly, nondrinkers had a 10% reduced risk of rectal cancer compared with light drinkers (aHR, 0.90 [95% CI, 0.82 to 0.98]).
TABLE 4.
Incidence and Risk of Early-Onset CRC According to the Average Daily Alcohol Consumption by Anatomical Site
For men, heavy drinking had a 26%, 17%, and 29% increased risk of distal colon, rectal, and unspecified colon cancers, respectively, compared with light drinkers. For women, moderate drinking had a 47% increased risk of distal colon cancer compared with light drinking. By contrast, among women, nondrinkers had a 14% reduced risk of rectal cancer compared with light drinkers (aHR, 0.86 [95% CI, 0.75 to 0.99]).
Early-Onset CRC Risk by Drinking Frequency of Alcohol Consumption
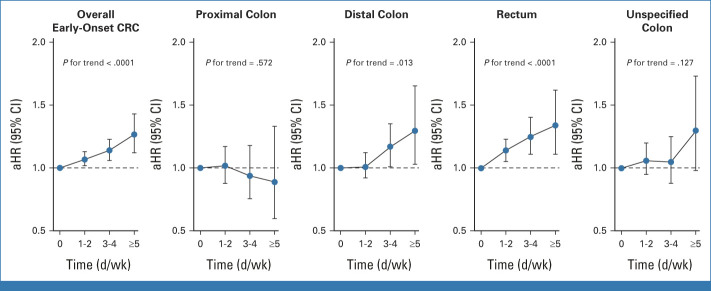
The dose-response association between drinking frequency and the risk of early-onset CRC was significant with a 7%, 14%, and 27% increased risk for 1-2, 3-4, and ≥5 drinking days per week compared with nondrinkers, respectively (P for trend <.0001; Fig 2, Data Supplement). According to tumor location, the risk of distal colon cancer was significantly associated with increasing frequency of alcohol consumption, with a 17% and 30% increased risk for 3-4 and ≥5 drinking days per week compared with nondrinkers, respectively (P for trend = .013; Data Supplement). The risk of rectal cancer was significantly associated with a 14%, 25%, and 34% increased risk for 1-2, 3-4, and ≥5 drinking days per week compared with nondrinkers, respectively (P for trend <.0001).
FIG 2.
Association between the early-onset CRC incidence and drinking frequency per week according to tumor location. aHR was adjusted for age, sex, smoking status, regular exercise, low-income status, hypertension, DM, and dyslipidemia. aHR, adjusted HR; CRC, colorectal cancer; DM, diabetes mellitus; HR, hazard ratio.
DISCUSSION
To our knowledge, this study is the first to investigate the sex and tumor-site differences in the association between graded increasing alcohol consumption and early-onset CRC risk. Our study showed that moderate and heavy drinking increased the risk of early-onset CRC by 9% and 20%, respectively, compared with light drinking. Subanalysis by tumor location showed that the positive dose-response association between alcohol consumption and early-onset CRC risk was mainly driven by left-sided colon, distal colon, and rectal cancers.
Alcohol use has been declining in many Western European countries over the past 30 years but has increased in several Asian countries.16 Korea has high alcohol consumption (annual per-capita alcohol consumption 10.2 L, higher than the global average 6.4 L in 2016),28,29 and daily consumption increased from 8.4 g in 1988 to 15 g in 2016-2018.28 Alcohol quantities were notably higher in men than in women, and highest in men age 30-49 years.28 This heavy drinking tendency of men in their 30s and 40s may contribute to the increased incidence of early-onset CRC among young men in Korea. The multinational cancer registry data for 2008-2012 showed that the incidence of early-onset CRC was highest (12.9 per 100,000), and the most rapid increase occurred in South Korea (average annual percentage change, 4.2).30
Possible mechanisms for the association between alcohol consumption and colorectal carcinogenesis may include a genotoxic effect of acetaldehyde, a metabolite of ethanol,14,15 tissue injury by reactive oxygen species and nitrogen species,31 changes in folate metabolism,32 and alcohol-induced gut dysbiosis.33 Besides the toxic effects of alcohol and its carcinogenic metabolites, heavy drinkers are predisposed to a poor diet low in folate and fiber, which could further augment alcohol-induced colorectal carcinogenesis.34 The CRC risk increases with higher alcohol consumption in a dose-response manner.35 A meta-analysis of 57 studies showed a causal relationship between high alcohol intake and increased CRC risk (pooled relative ratio 1.21 for moderate [≥2 drinks/d] and 1.52 for heavy [≥4 drinks/d] drinking).36 A recent meta-analysis suggested that alcohol consumption is a risk factor for early-onset CRC.37 Syed et al12 reported that alcohol use (v nondrinker) was significantly associated with the risk of early-onset CRC (odds ratio, 1.71 [95% CI, 1.62 to 1.80]). Rosato et al13 reported that two or more drinks per day of alcohol increased the risk of CRC diagnosis before age 45 years by 1.6-fold. However, these studies focused on the current status of alcohol consumption (nondrinker v drinker) or the total amount of alcohol consumption, and data on frequency or gradually increasing amount of alcohol consumption with early-onset CRC risk are limited.
To investigate the association between the risk of early-onset CRC and alcohol consumption, our analysis focused on graded increasing alcohol consumption into four categories on the basis of calculated pure alcohol content (in grams, instead of standard drinks). Given the lack of international consensus on the amount of pure alcohol in a standard drink, regional definitions of standard drinks differ.23,38 The dose-response relationships between alcohol consumption and major health conditions are still controversial. Alcohol consumption at any level is associated with cancer risk (eg, breast cancer), but some studies have found that small amounts of alcohol consumption lowers the risk of cardiovascular diseases.39 As an ambivalent aspect of the small amount of alcohol consumption, a recent study has proposed the reference group as the nondrinker equivalence level, which means the minimal amount of alcohol consumption at which the risk of health loss for a drinker is equivalent to that of a nondrinker.17 In this study, our reference group was defined as the light drinker who consumes <10 g of alcohol per day.
The strength of our study is that, to our knowledge, for the first time, we show positive dose-response association between alcohol consumption and the risk of early-onset CRC according to tumor location. Tumor location–based analysis indicated that increased alcohol consumption showed a significant positive association for distal colon, rectal, and unspecified colon cancers, but not for proximal colon cancers. In addition to the drinking amount, increased drinking frequency was associated with a higher risk of early-onset CRC, and this association was also prominent for the distal colon and rectal cancers. Thus, excessive and frequent alcohol consumption may be associated with left-sided colon cancer risk in individuals younger than 50 years, which is important in the context of early-onset CRC.3 Epidemiologic studies have also shown that alcohol consumption is more strongly associated with rectal than colon cancer, as in our results.40-42
The effects of alcohol on colorectal carcinogenesis may differ by sex because of differences in alcohol metabolism and body composition.43,44 Lower alcohol dehydrogenase levels and higher body fat percentage increase blood alcohol concentrations; thus, women may be more susceptible to alcohol than men.43 Given the evidence that men and women differ in their susceptibility to alcohol-induced disease, a separate threshold was needed, with a lower cutoff value for women.39 Although we found that early-onset CRC risk tends to increase linearly in women by alcohol quantity, the analyses lacked statistical power, possibly because of the lower average alcohol consumption in women than in men. Interestingly, among women, nondrinkers had a 14% reduced risk of rectal cancer compared with light drinkers (aHR, 0.86 [95% CI, 0.75 to 0.99]). Therefore, even small amounts of alcohol consumption may be associated with an increased risk of rectal cancer in young women. Our study showed that heavy drinking was significantly associated with early-onset CRC mainly in men, although women with alcohol consumption also had an increased risk of early-onset CRC; therefore, the risk should not be underestimated by sex.
Although this is the first large-scale study, to our knowledge, to investigate the association between early-onset CRC and alcohol consumption, the use of data from a claims database may affect the accuracy of the results. NHIS data have recently gained active utility in public health research19,45; however, the original purpose of this claims database was not research, but reimbursement and regulation of medical expenses. Owing to these characteristics of secondary data, operational definitions on the basis of disease codes could be overestimated or underestimated, which is a potential source of bias. However, a recent study showed that the operational definitions using NHIS claims data had high sensitivity (91.5%) and accuracy (84.9%) for CRC compared with the Korean Central Cancer Registration data.46
Our study has several limitations. First, lifestyle data, including alcohol-intake habits, were obtained from self-administered questionnaires; thus, respondents may have answered less or more than their actual drinking amount. Since alcohol consumption was ascertained at single point, it may differ from the actual chronic exposure of alcohol consumption. Second, analysis of the missing participants revealed that they were more likely to be male and younger than the final eligible participants (Data Supplement). The incidence rate of early-onset CRC in the missing population is slightly lower than that among final eligible participants (Data Supplement). Despite the relatively low exclusion rate of 6.7% (n = 407,414) among the 6,114,839 individuals initially screened, the characteristics of the missing population could potentially influence the study results. Third, we compared the risk of early-onset CRC in terms of drinking habits, sex, and tumor location, which leads to the issue of multiple comparisons. Fourth, this population-based study only covered Koreans, and the effect of alcohol on carcinogenesis (CRC risk) may vary by race.36,47 Larger studies involving various races are required to validate our findings. Fifth, it was difficult to identify participants who immigrated to other countries during the follow-up period; thus, they were not excluded. Sixth, some potential confounding factors, such as family history of CRC, inflammatory bowel disease, dietary intake, and colonoscopy history, could not be investigated. Finally, we could not determine the histologic and molecular subtypes that could affect CRC location.
In conclusion, this nationwide population-based study showed that graded increasing amount and higher frequency of alcohol consumption were associated with increased early-onset CRC risk in a dose-response manner that was statistically significant in left-sided colon cancer. These findings suggest the need for educational campaigns, alcohol control policies, and interventions to discourage alcohol consumption among young adults. Tailored screening approaches are needed even before age 50 years for individuals with excessive alcohol consumption. To develop effective CRC-preventive interventions at an early age, it is important to identify the modifiable risk factors for early-onset CRC.
SUPPORT
Supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C1140).
E.H.J. and K.H. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Kyungdo Han, Cheol Min Shin, Dong Ho Lee, Yoon Jin Choi
Collection and assembly of data: Kyungdo Han
Data analysis and interpretation: Eun Hyo Jin, Kyungdo Han, Cheol Min Shin, Seung Joo Kang, Joo Hyun Lim
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sex and Tumor-Site Differences in the Association of Alcohol Intake With the Risk of Early-Onset Colorectal Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Bailey CE, Hu CY, You YN, et al. : Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 150:17-22, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinicrope FA: Increasing incidence of early-onset colorectal cancer. N Engl J Med 386:1547-1558, 2022 [DOI] [PubMed] [Google Scholar]
- 4.Gausman V, Dornblaser D, Anand S, et al. : Risk factors associated with early-onset colorectal cancer. Clin Gastroenterol Hepatol 18:2752-2759.e2, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low EE, Demb J, Liu L, et al. : Risk factors for early-onset colorectal cancer. Gastroenterology 159:492-501.e7, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen LH, Liu PH, Zheng X, et al. : Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr 2:pky073, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hur J, Otegbeye E, Joh HK, et al. : Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 70:2330-2336, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu PH, Wu K, Ng K, et al. : Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol 5:37-44, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Zheng X, Zong X, et al. : Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 70:1147-1154, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin EH, Han K, Lee DH, et al. : Association between metabolic syndrome and the risk of colorectal cancer diagnosed before age 50 years according to tumor location. Gastroenterology 163:637-648.e2, 2022 [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Lipsyc-Sharf M, Zong X, et al. : Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology 161:1208-1217.e9, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syed AR, Thakkar P, Horne ZD, et al. : Old vs new: Risk factors predicting early onset colorectal cancer. World J Gastrointest Oncol 11:1011-1020, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosato V, Bosetti C, Levi F, et al. : Risk factors for young-onset colorectal cancer. Cancer Causes Control 24:335-341, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Rehm J, Shield KD, Weiderpass E: Alcohol consumption: A leading risk factor for cancer. Chem Biol Interact 331:109280, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Pflaum T, Hausler T, Baumung C, et al. : Carcinogenic compounds in alcoholic beverages: An update. Arch Toxicol 90:2349-2367, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Manthey J, Shield KD, Rylett M, et al. : Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 393:2493-2502, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Bryazka D, Reitsma MB, Griswold MG, et al. : Population-level risks of alcohol consumption by amount, geography, age, sex, and year: A systematic analysis for the Global Burden of Disease Study 2020. Lancet 400:185-235, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griswold MG, Fullman N, Hawley C, et al. : Alcohol use and burden for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 392:1015-1035, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YH, Kang JG, Lee SJ, et al. : Underweight increases the risk of end-stage renal diseases for type 2 diabetes in Korean population: Data from the National Health Insurance Service health checkups 2009-2017. Diabetes Care 43:1118-1125, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Kang HT: Current status of the national health screening programs in South Korea. Korean J Fam Med 43:168-173, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo JE, Shin DW, Han K, et al. : Association of the frequency and quantity of alcohol consumption with gastrointestinal cancer. JAMA Netw Open 4:e2120382, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Sola J: Cardiovascular risks and benefits of moderate and heavy alcohol consumption. Nat Rev Cardiol 12:576-587, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Kalinowski A, Humphreys K: Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction 111:1293-1298, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Moriya A, Iwasaki Y, Ohguchi S, et al. : Roles of alcohol consumption in fatty liver: A longitudinal study. J Hepatol 62:921-927, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Chang Y, Ryu S, Kim Y, et al. : Low levels of alcohol consumption, obesity, and development of fatty liver with and without evidence of advanced fibrosis. Hepatology 71:861-873, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Bagnardi V, Rota M, Botteri E, et al. : Light alcohol drinking and cancer: A meta-analysis. Ann Oncol 24:301-308, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Yang HK, Han K, Kwon HS, et al. : Obesity, metabolic health, and mortality in adults: A nationwide population-based study in Korea. Sci Rep 6:30329, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SY, Kim HJ: Trends in alcohol consumption for Korean adults from 1998 to 2018: Korea national health and nutritional examination survey. Nutrients 13:609, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization : Global Status Report on Alcohol and Health 2018. Geneva, Switzerland, World Health Organization, 2019 [Google Scholar]
- 30.Siegel RL, Torre LA, Soerjomataram I, et al. : Global patterns and trends in colorectal cancer incidence in young adults. Gut 68:2179-2185, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Cederbaum AI: Alcohol metabolism. Clin Liver Dis 16:667-685, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varela-Rey M, Woodhoo A, Martinez-Chantar ML, et al. : Alcohol, DNA methylation, and cancer. Alcohol Res 35:25-35, 2013 [PMC free article] [PubMed] [Google Scholar]
- 33.Flemer B, Lynch DB, Brown JM, et al. : Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 66:633-643, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossi M, Keshavarzian A, Usman A, et al. : Colorectal cancer and alcohol consumption-populations to molecules. Cancers (Basel) 10:38, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boffetta P, Hashibe M: Alcohol and cancer. Lancet Oncol 7:149-156, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Fedirko V, Tramacere I, Bagnardi V, et al. : Alcohol drinking and colorectal cancer risk: An overall and dose-response meta-analysis of published studies. Ann Oncol 22:1958-1972, 2011 [DOI] [PubMed] [Google Scholar]
- 37.O'Sullivan DE, Sutherland RL, Town S, et al. : Risk factors for early-onset colorectal cancer: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 20:1229-1240.e5, 2022 [DOI] [PubMed] [Google Scholar]
- 38.Thomas B, John H-B: Brief Intervention for Hazardous and Harmful Drinking. Geneva, Switzerland, World Health Organization, 2001 [Google Scholar]
- 39.World Health Organization : International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland, World Health Organization, 2000 [Google Scholar]
- 40.Ferrari P, Jenab M, Norat T, et al. : Lifetime and baseline alcohol intake and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 121:2065-2072, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Bongaerts BW, van den Brandt PA, Goldbohm RA, et al. : Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int J Cancer 123:2411-2417, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Offermans NSM, Ketcham SM, van den Brandt PA, et al. : Alcohol intake, ADH1B and ADH1C genotypes, and the risk of colorectal cancer by sex and subsite in the Netherlands Cohort Study. Carcinogenesis 39:375-388, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Hur J, Smith-Warner SA, Rimm EB, et al. : Alcohol intake in early adulthood and risk of colorectal cancer: Three large prospective cohort studies of men and women in the United States. Eur J Epidemiol 36:325-333, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNabb S, Harrison TA, Albanes D, et al. : Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int J Cancer 146:861-873, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nam GE, Kim SM, Han K, et al. : Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med 15:e1002640, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang MS, Park M, Back JH, et al. : Validation of cancer diagnosis based on the National Health Insurance Service database versus the national cancer registry database in Korea. Cancer Res Treat 54:352-361, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizoue T, Inoue M, Wakai K, et al. : Alcohol drinking and colorectal cancer in Japanese: A pooled analysis of results from five cohort studies. Am J Epidemiol 167:1397-1406, 2008 [DOI] [PubMed] [Google Scholar]