Background:
The effectiveness of nonabsorbable and biodegradable nasal packing is still controversial, and the choice of nasal packing type can alter the outcome of endoscopic sinus surgery. This study compared the effectiveness of Posisep and Merocel as nasal packing materials with regard to hemostasis, adhesion, wound healing, patient’s satisfaction and health-related quality of life after endoscopic sinus surgery (ESS).
Methods:
A prospective, randomized, double-blinded, controlled trial was conducted in patients with chronic rhinosinusitis refractory undergoing symmetrical bilateral ESS. At the completion of surgery, a nasal packing (either Merocel or Posisep) was randomly chosen and placed into the middle meatus of each nasal cavity. All patients were scheduled for follow-up visits at 24 hours, 5 days, 3 weeks, and 5 weeks days after surgery. Health-related quality of life was measured using the Sino-Nasal Outcome Test (SNOT-22). The overall inflammatory burden of chronic rhinosinusitis was measured by the Lund-Mackay postoperative endoscopic score (LMES).
Results:
Among 62 patients included in data analysis (n = 31 for each group), the mean age was 42.4 years and 54.8% were females. Patients with Posisep after ESS had more improvement and better symptoms measured through SNOT-22 and LMES at 24 hours, 5 days, and 3 weeks than those with Merocel after ESS. While some aspects measured by LMES such as discharge and scarring were still better until 5 weeks after surgery, all symptoms measured by SNOT-22 were similar between the 2 groups 5 weeks after surgery.
Conclusion:
Posisep containing chitosan provided patients with a better quality of life throughout the early recovery period compared with Merocel. Although more studies are needed, our findings support the use of Posisep after ESS.
Keywords: endoscopic sinus surgery, Merocel, nasal packing, Posisep, quality of life
Key Points.
Patients with Posisep after endoscopic sinus surgery had better outcomes than Merocel.
Sino-Nasal Outcome Test was better in Posisep at 24 hours, 5 days and 3 weeks.
Lund-Mackay postoperative endoscopic score was better in the Posisep group at 5 days, 3 weeks, and 5 weeks.
1. Introduction
In recent years, endoscopic sino-nasal surgery (ESS) has become a standard treatment for medically refractory chronic rhinosinusitis, especially in the presence of nasal polyps or suspected fungal in CT-scan.[1–3] The ESS, also known as mucosal sparing technique, has been reported to have minimal damage and complications. Bleeding and adhesion formation are the 2 most common complications after surgery, and the latter with a rate of 11% to 54% is the main cause of revision surgery.[4]
Nasal stenting is the most common procedure to prevent postoperative bleeding, formation of scar tissue and synechiae in the middle meatus. Many nonabsorbable products have been used after ESS, such as gauze strip or polyvinyl acetate (Merocel), Merogel/Meropack (Medtronic Xomed); Nasopore (Polyganics, Groningen, The Netherlands) or Floseal (Baxter International Inc., Deerfield, IL).[5] However, nonabsorbable products often cause many discomforts, such as facial pain, nasal blockage, sleep apnea, which decrease patient’s quality of life (QoL). However, there is no consensus on whether nasal packing is needed after ESS. Materials packing should be used in such a way that the type of middle meatus packing was chosen by surgeons.[6]
The effectiveness of nonabsorbable and biodegradable nasal packing is still controversial and the choice of nasal packing type can alter the outcome of endoscopic sinus surgery.[7] Wang et al[8] found that the prevalence of synechiae in nonabsorbable material groups ranged from 4.6% to 8.0% and in absorbable material groups from 8.0% to 35.7%. In contrast, Petros et al showed that absorbable packings produce less synechia and adhesion than nonabsorbable packings and are more effective than not putting anything in the middle meatus.[9] Merocel is one of the most common nasal packs which is compressed, dehydrated nonabsorbable sponge and can expand its size inside the nasal cavity through the rehydrated mechanism and thus create hemostasis by compressing blood vessels. However, many studies have shown that Merocel has some adverse effects on patient’s QoL, including nasal pain, obstructive airway and epistaxis after removal.[5,8]
Chitosan has many useful properties including hemostasis, anti-adhesion, antimicrobial activity, hypoallergenicity, biodegradability, and non-toxicity.[10] Thanh Ngoc Ha et al[11] conducted a study to evaluate the effectiveness of Chitosan-Dextran gel on the neoostia and found that Chitosan-Dextran produced significantly less stenosis and reduced the need for revision surgery. Recently, a new form of chitosan, carboxymethylated chitosan-based intranasal splint has been introduced as Posisep (Hemostasis, LLC, 5000 Township Parkway St. Paul, MN 55110). Posisep has been approved by the United States Food and Drug Administration for use in intranasal packing in the middle meatus after ESS.
The aim of this study was to compare the effectiveness of Posisep and Merocel as nasal packing materials with regard to hemostasis, adhesion, wound healing, patient’s satisfaction and health-related QoL after ESS.
2. Methods
2.1. Settings and patients
A prospective, randomized, double-blinded, controlled trial was conducted from October 2018 to June 2019 at the University Medical Center, Ho Chi Minh City. All patients older than 18 years with chronic rhinosinusitis refractory to medical therapy and undergoing symmetrical bilateral ESS were included in this study. Patients who were pregnant or breast-feeding, had sino-nasal involvement with a systemic disease, or had seafood allergy were excluded. A minimum sample size of 26 for each group was needed to have a statistical power of at least 80% to detect an effect size of 0.8, assuming an average of 2-point difference in each item of the Sino-Nasal Outcome Test (22 item) (SNOT-22) and a standard deviation of difference of 2.5.
2.2. Procedures
Ethics approval was granted by the ethics committee for biomedical research at the University of Medicine and Pharmacy at Ho Chi Minh City (431/DHYD-HDDD). All patients provided written informed consent. All surgeries were performed under general anesthesia for all patients by an experienced surgeon who was also an expert in ESS. At the completion of surgery, a nasal packing (either Merocel or Posisep) was randomly chosen and placed into the middle meatus of each nasal cavity by the ESS resident (Fig. 1). For randomization, the resident randomly chose 1 envelope from a block of 2 envelopes that contained Merocel or Posisep. Patients did not know which nasal packing they received.
Figure 1.
Intraoperative application of Posisep (A) and Merocel (B) in the middle meatus. IF = inferior turbinate, MT = middle turbinate, NS = nasal septum, P = Posisep, M = Merocel.
Based on findings from a previous study that Merocel nasal packing removal 24 hours after ESS was less painful than removal 48 hours after ESS,[12] in our study, Merocel packings were removed 24 hours after surgery. Patients were discharged and prescribed oral antibiotics for 7 days, saline nasal irrigation 2 times daily, and topical steroid therapy for 2 weeks. All patients were scheduled for follow-up visits at 24 hours, 5 days, 3 weeks, and 5 weeks after the surgery.
2.3. Measurements
Patient’s characteristics were collected including gender, age, comorbidity, and type of sinusitis. To compare the effectiveness of treatment between the 2 groups, postoperative bleeding, health-related QoL and endoscopic appearances were recorded.
Health-related QoL was measured using the SNOT-22, which provides a validated means to objectively quantify patient’s perception of chronic rhinosinusitis burden both before and after ESS.[13] The SNOT - 22 includes 2 categories: questions about physical symptoms (rhinology, ear, and facial symptoms) and questions about health and QoL (sleep function and psychological symptoms). All questionnaires included a transition rating that compared preoperative and postoperative health-related QoL problems on a 5-point scale (0= “No problem,” 1 = “Very mild problem,” 2 = “Moderate problem,” 4 = “Severe problem,” and 5 = “Problem as bad as it can be”). The total score ranges from 0 to 110, higher scores represent worse symptoms. Patients were excluded if both preoperative and postoperative evaluations were not completed. In this study, SNOT-22 was used at baseline (pre-operation), 24 hours, 5 days, 3 weeks, and 5 weeks after the surgery.
The overall inflammatory burden of chronic rhinosinusitis was measured by the Lund-Mackay postoperative endoscopic score (LMES). LMES is a staging system for the left and right sides of nose to assess polyps (0 = absence of polyps, 1 = polyps in middle meatus, 2 = polyps beyond middle meatus), edema (0 = absent, 1 = mild, 2 = severe), discharge (0 = no discharge, 1 = thin discharge, 2 = purulent discharge), crusting and scarring (0 = absent, 1 = mild, 2 = severe).[14] LMES were reviewed at each follow-up visit (5 days, 3 weeks, and 5 weeks). Postoperative complications such as excessive epistaxis were also noted. All measurements during follow-up visits were conducted through clinical interviews by study staff who did not know whether Merocel or Posisep was used for the patients.
3. Statistical analysis
Statistical analysis was performed using SPSS version 26.0 (SPSS Inc, Chicago, IL). Student’s t tests were used to compare the scores of SNOT-22, LMES, Lund-Mackay and Lund-Kenedy between the 2 groups. Chi-square tests or Fisher exact tests were used when appropriate to examine the differences of qualitative characteristics of patients between the 2 groups. The level of statistical significance was set at 0.05.
4. Results
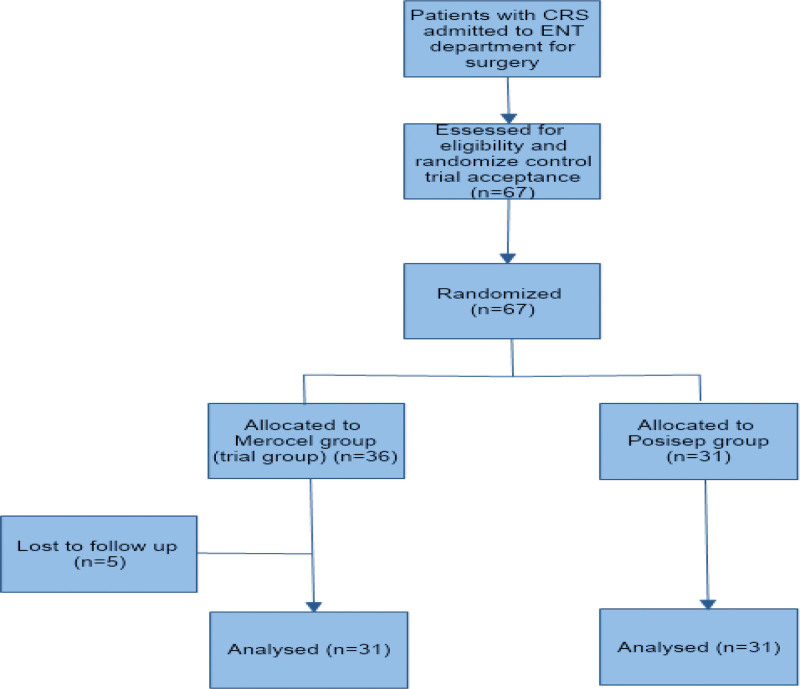
Among 67 patients who participated in this study (n = 36 in Merocel group and n = 31 in Posisep group), 5 patients (all in the Merocel group) did not return for any follow-up visits and were excluded from the analysis (Fig. 2) and no patient underwent revision sinus surgery. Among 62 patients included in the data analysis (n = 31 for each group), the mean age was 42.4 years and 54.8% were females. There was no statistically significant difference between the 2 groups in age, gender, chronic diseases, type of sinusitis, and Lund-Mackay computed tomography score. However, additional procedures were significantly different between the 2 groups (Table 1). No difference was found in the extent of ESS between the 2 groups (Table 2).
Figure 2.
Study flow diagram. CRS = Chronic rhinosinusitis.
Table 1.
Patient’s baseline characteristics between the Posisep packing group and the Merocel packing group.
| MEROCEL (N = 31) | POSISEP (N = 31) | P value | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Gender | Male | 14 | 45.2 | 13 | 41.9 | .798 |
| Female | 17 | 54.8 | 18 | 58.1 | ||
| Age (yr) | Mean (Standard deviation) | 42.4 | (13.4) | 47.8 | (14.3) | .131 |
| Comorbidity | Diabetes mellitus | 0 | 0.0 | 2 | 6.4 | .619 |
| Heart disease | 3 | 9.7 | 4 | 12.9 | ||
| Heart disease & diabetes mellitus | 2 | 6.4 | 2 | 6.5 | ||
| No | 26 | 83.9 | 23 | 74.2 | ||
| Type of sinusitis | Allergic fungal Sinusitis | 4 | 12.9 | 10 | 32.3 | .186 |
| Nonpolypoid rhinosinusitis | 20 | 64.5 | 15 | 48.4 | ||
| Polypoid rhinosinusitis | 7 | 22.6 | 6 | 19.3 | ||
| Lund-Mackay computed tomography score | Mean (Standard deviation) | 7.9 | (4.5) | 6.87 | (5.01) | .397 |
| Additional procedure | Septoplasty | 13 | 41.9 | 4 | 12.9 | .030 |
| Turbinoplasty | 3 | 9.7 | 3 | 9.7 | ||
| No additional procedure | 15 | 48.4 | 24 | 77.4 | ||
Table 2.
Characteristics of endoscopic sinus surgery between the Posisep packing group and the Merocel packing group.
| MEROCEL | POSISEP | P value | |||
|---|---|---|---|---|---|
| n | %% | n | % | ||
| MMA + BAE + FRS | 1 | 3.2 | 1 | 3.2 | .698 |
| MMA + BAE + SS | 1 | 3.2 | 1 | 3.2 | |
| MMA + BAPE + SS | 1 | 3.2 | 0 | 0 | |
| MMA + BAPE + SS + FRS | 3 | 9.7 | 3 | 9.7 | |
| MMA + FRS | 1 | 3.2 | 0 | 0 | |
| MMA | 11 | 35.5 | 6 | 19.4 | |
| MMA + SS | 1 | 3.2 | 1 | 3.2 | |
| MMA + BAE | 0 | 0 | 3 | 9.7 | |
| MMA + BAPE | 1 | 3.2 | 1 | 3.2 | |
| MMA + BAPE + FRS | 10 | 32.3 | 12 | 38.7 | |
| SS | 1 | 3.2 | 3 | 9.7 | |
BAE = bilateral anterior ethmoidectomy, BAPE = anterior posterior ethmoidectomy, FRS = frontal recess surgery, MMA = Middle meatal antrostomy, SS = sphenoid sinusotomy.
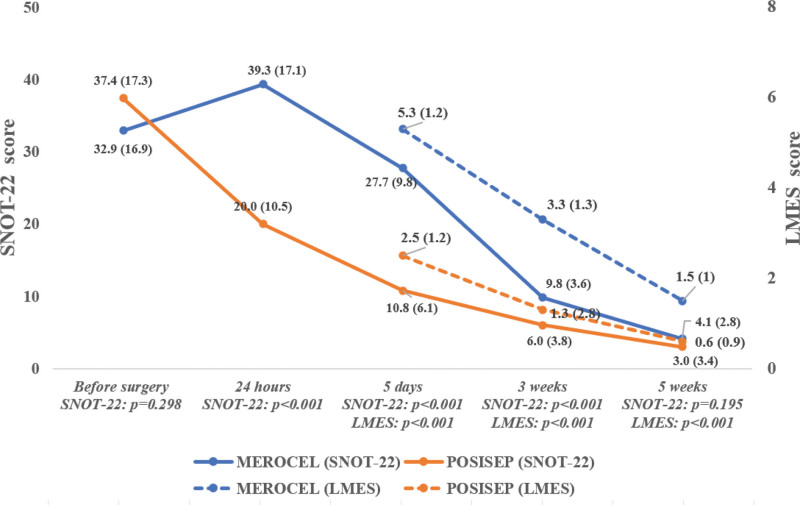
Figure 3 presents the changes of SNOT-22 score and LMES score in the 2 groups. There was no significant difference in SNOT-22 score between Merocel and Posisep before surgery. However, a significantly higher level of decrease in SNOT-22 scores and thus more improvement in symptoms was found in the Posisep group compared with the Merocel group 24 hours, 5 days, and 3 weeks after surgery. No significant difference in SNOT-22 score between the 2 groups was found 5 weeks after surgery. Moreover, a decreasing pattern in LMES score was found in both Merocel and Posisep groups. However, the level of decrease was significantly higher in the Posisep group at all follow-up visits (i.e., 5 days, 3 weeks, 5 weeks). The rate of postoperative bleeding was not different between the 2 groups (Merocel n = 1, 3.2% vs Posisep n = 2, 6.4%).
Figure 3.
SNOT-22 and LMES between the Posisep packing group and the Merocel packing group. LMES = The Lund-Mackay postoperative endoscopic score, SNOT-22 = Sino-Nasal Outcome Test (22 item).
In-depth analysis of the differences between the 2 groups with regard to SNOT-22 and LMES is presented in Table 3 and Table 4. In Table 3, the frequency of most of the symptoms measured in the SNOT-22 was significantly lower in the Posisep group than in the Merocel group at 24 hours, 5 days, and 3 weeks after surgery. However, all symptoms were similar in both groups 5 weeks after surgery, except runny nose. In Table 4, nasal polyps were rare in both groups and were not different between the 2 groups after surgery at all evaluation points. In terms of mucosal edema and crusting, the Posisep group had a significantly higher level of wound healing at 5 days and 3 weeks after surgery (P < .001). However, there was no statistically significant difference at 5 weeks postoperatively. Scarring (adhesion formation) according to LMES was not different between the 2 groups at day 5 but was significantly lower in the Posisep group at 3 weeks and 5 weeks. The rate of thin discharge and purulent discharge was significantly higher in the Merocel group at all evaluation points. Epistaxis in the Posisep group was not significantly different from that in the Merocel group (Merocel n = 1, 3.2% vs Posisep n = 2, 6.4%).
Table 3.
Multivariate regression analysis comparing pre- and postsurgical SNOT-22 questions between the Posisep packing group and the Merocel packing group.
| Item | SNOT-22 content | Before surgery | 24 hours | 5 days | 3 weeks | 5 weeks | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Merocel | Posisep | P value | Merocel | Posisep | P value | Merocel | Posisep | P value | Merocel | Posisep | P value | Merocel | Posisep | P value | ||
| 1 | Need to blow nose | 2.1 (1.3) | 1.9 (1.3) | .695 | 2.3 (1.4) | 1.4 (0.9) | .005 | 2.2 (1.0) | 0.9 (0.7) | <.001 | 0.8 (0.6) | 0.3 (0.5) | .001 | 0.3 (0.6) | 0.2 (0.5) | .352 |
| 2 | Nasal blockage | 2.5 (1.5) | 2.3 (1.5) | .607 | 3.7 (1.3) | 1.7 (1.1) | <.001 | 2.6 (0.9) | 1.0 (0.5) | <.001 | 1.0 (0.7) | 0.5 (0.7) | .008 | 0.5 (0.6) | 0.3 (0.5) | .258 |
| 3 | Sneezing | 1.8 (1.1) | 2.0 (1.3) | .465 | 2.0 (1.2) | 1.2 (0.8) | .008 | 1.9 (0.9) | 0.7 (0.8) | <.001 | 0.9 (0.6) | 0.5 (0.6) | .026 | 0.4 (0.5) | 0.4 (0.6) | 1.000 |
| 4 | Runny nose | 2.2 (1.2) | 2.5 (1.5) | .418 | 2.9 (1.2) | 1.6 (1.0) | <.001 | 2.4 (0.8) | 1.3 (0.8) | <.001 | 1.2 (0.6) | 0.9 (0.8) | .098 | 0.7 (0.6) | 0.4 (0.6) | .040 |
| 5 | Cough | 1.8 (1.1) | 2.0 (1.3) | .474 | 2.3 (1.2) | 1.4 (0.9) | .002 | 2.0 (0.9) | 0.9 (0.7) | <.001 | 1.1 (0.7) | 0.6 (0.6) | .002 | 0.5 (0.6) | 0.3 (0.5) | .152 |
| 6 | Post-nasal discharge | 1.9 (1.1) | 2.5 (1.5) | .091 | 2.5 (1.2) | 1.7 (0.9) | .005 | 1.9 (1.0) | 1.1 (0.7) | <.001 | 0.7 (0.5) | 0.7 (0.6) | .828 | 0.6 (0.6) | 0.4 (0.6) | .087 |
| 7 | Thick nasal discharge | 1.8 (1.2) | 2.0 (1.5) | .469 | 2.2 (1.1) | 1.4 (1.2) | .004 | 1.8 (0.8) | 0.8 (1.0) | <.001 | 0.6 (0.5) | 0.4 (0.6) | .057 | 0.1 (0.3) | 0.2 (0.4) | .498 |
| 8 | Ear fullness | 1.5 (1.2) | 1.7 (1.5) | .645 | 2.1 (1.2) | 0.8 (0.8) | <.001 | 1.4 (0.9) | 0.2 (0.4) | <.001 | 0.3 (0.5) | 0.2 (0.4) | .143 | 0.1 (0.2) | 0.1 (0.2) | 1.000 |
| 9 | Dizziness | 1.3 (1.1) | 1.7 (1.4) | .194 | 1.6 (1.1) | 0.7 (0.8) | <.001 | 1.3 (0.8) | 0.2 (0.4) | <.001 | 0.3 (0.4) | 0.1 (0.2) | .040 | 0 (0) | 0 (0.2) | .321 |
| 10 | Ear pain | 1.1 (1.2) | 1.2 (1.2) | .759 | 1.5 (1.2) | 0.4 (0.6) | <.001 | 1.1 (0.8) | 0.1 (0.2) | <.001 | 0.2 (0.5) | 0.1 (0.2) | .112 | 0 (0) | 0 (0.2) | .313 |
| 11 | Facial pain/pressure | 2.3 (1.3) | 2.2 (1.6) | .932 | 2.1 (1.3) | 1.0 (1.0) | .001 | 1.3 (0.8) | 0.5 (0.7) | <.001 | 0.4 (0.5) | 0.2 (0.5) | .122 | 0.2 (0.4) | 0.1 (0.4) | .343 |
| 12 | Decreased sense of smell/taste | 1.1 (0.9) | 1.7 (1.3) | .030 | 1.6 (1.1) | 0.8 (0.9) | .002 | 1.3 (0.7) | 0.5 (0.7) | <.001 | 0.6 (0.6) | 0.2 (0.4) | <.001 | 0.2 (0.5) | 0.2 (0.4) | 1.000 |
| 13 | Difficulty falling asleep | 1.3 (1.3) | 1.7 (1.5) | .172 | 1.5 (1.2) | 0.9 (1.1) | .086 | 1.1 (0.8) | 0.5 (0.7) | .002 | 0.2 (0.4) | 0.1 (0.3) | .173 | 0.1 (0.3) | 0.3 (0.4) | .205 |
| 14 | Wake up at night | 1.1 (1.3) | 1.7 (1.2) | .052 | 1.5 (1.1) | 0.7 (1.0) | .006 | 0.9 (0.8) | 0.2 (0.5) | <.001 | 0.3 (0.5) | 0.1 (0.3) | .030 | 0.1 (0.2) | 0.1 (0.2) | 1.000 |
| 15 | Lack of a good night’s sleep | 1.5 (1.4) | 1.5 (1.1) | .844 | 1.8 (1.2) | 0.9 (0.9) | .001 | 1.0 (0.8) | 0.5 (0.7) | .011 | 0.4 (0.5) | 0.3 (0.5) | .594 | 0.1 (0.3) | 0.2 (0.4) | .498 |
| 16 | Wake up tired | 1.5 (1.4) | 1.5 (1.5) | .860 | 1.5 (1.2) | 0.8 (1.1) | .020 | 0.9 (0.7) | 0.4 (0.6) | .002 | 0.2 (0.4) | 0.3 (0.5) | .569 | 0 (0.2) | 0.1 (0.3) | .309 |
| 17 | Fatigue | 1.4 (1.4) | 1.5 (1.6) | .740 | 1.6 (1.2) | 0.7 (1.0) | .004 | 0.8 (0.7) | 0.3 (0.5) | .002 | 0.2 (0.4) | 0.4 (0.6) | .066 | 0 (0.2) | 0.2 (0.4) | .088 |
| 18 | Reduced productivity | 1.1 (1.4) | 1.4 (1.5) | .439 | 1.2 (1.2) | 0.4 (0.8) | .009 | 0.5 (0.6) | 0.2 (0.5) | .028 | 0.1 (0.3) | 0.4 (0.5) | .020 | 0.1 (0.2) | 0 (0.2) | .561 |
| 19 | Reduce concentration | 1.1 (1.2) | 1.2 (1.4) | .776 | 1.1 (1.1) | 0.6 (0.9) | .062 | 0.5 (0.6) | 0.1 (0.4) | .006 | 0 (0.2) | 0.2 (0.4) | .088 | 0 (0.2) | 0 (0) | .321 |
| 20 | Frustrated/ restless/irritable | 1.3 (1.4) | 1.4 (1.7) | .869 | 1.1 (1.2) | 0.5 (1.1) | .083 | 0.5 (0.6) | 0.1 (0.2) | .001 | 0.1 (0.3) | 0 (0) | .078 | 0 (0) | 0 (0.2) | .321 |
| 21 | Sad | 1.0 (1.1) | 1.2 (1.4) | .537 | 0.8 (1.1) | 0.2 (0.4) | .005 | 0.2 (0.5) | 0 (0.2) | .049 | 0.1 (0.2) | 0.2 (0.4) | .134 | 0 (0) | 0 (0.2) | .321 |
| 22 | Embarrassed | 0.5 (0.9) | 0.5 (1.1) | .796 | 0.5 (0.9) | 0 (0.2) | .007 | 0.1 (0.2) | 0 (0) | .156 | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - |
SNOT-22 = Sino-Nasal Outcome Test (22 item).
Table 4.
The Lund-Mackay postoperative endoscopic score (LMES) at postoperative visits.
| 5 days | 3 weeks | 5 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Merocel | Posisep | P value | Merocel | Posisep | P value | Merocel | Posisep | P value | |
| Polyp | |||||||||
| 0 | 29 (93.5) | 31 (100) | .492 | 30 (96.8) | 31 (100) | .999 | 30 (96.8) | 30 (96.8) | .999 |
| 1 | 2 (6.5) | 0 (0) | 1 (3.2) | 0 (0) | 1 (3.2) | 1 (3.2) | |||
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Edema | |||||||||
| 0 | 0 (0) | 17 (54.8) | <.001 | 7 (22.6) | 30 (96.8) | <.001 | 26 (83.9) | 30 (96.8) | .195 |
| 1 | 8 (25.8) | 14 (45.2) | 23 (74.2) | 1 (3.2) | 5 (16.1) | 1 (3.2) | |||
| 2 | 23 (74.2) | 0 (0) | 1 (3.2) | 0 (0) | 0 (0) | 0 (0) | |||
| Discharde | |||||||||
| 0 | 0 (0) | 5 (16.1) | <.001 | 0 (0) | 12 (38.7) | <.001 | 11 (35.5) | 22 (71.0) | .010 |
| 1 | 11 (35.5) | 24 (77.4) | 28 (90.3) | 18 (58.1) | 19 (61.3) | 9 (29.0) | |||
| 2 | 20 (64.5) | 2 (6.5) | 3 (9.7) | 1 (3.2) | 1 (3.2) | 0 (0) | |||
| Scarring | |||||||||
| 0 | 27 (87.1) | 31 (100) | .113 | 17 (54.8) | 29 (93.5) | .001 | 17 (54.8) | 25 (80.6) | .030 |
| 1 | 4 (12.9) | 0 (0) | 14 (45.2) | 2 (6.5) | 14 (45.2) | 6 (19.4) | |||
| 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Crust | |||||||||
| 0 | 0 (0) | 3 (9.7) | <.001 | 4 (12.9) | 27 (87.1) | <.001 | 27 (87.1) | 29 (93.5) | .671 |
| 1 | 9 (29.0) | 25 (80.6) | 26 (83.9) | 4 (12.9) | 4 (12.9) | 2 (6.5) | |||
| 2 | 22 (71.0) | 3 (9.7) | 1 (3.2) | 0 (0) | 0 (0) | 0 (0) | |||
Scoring: For polyps: 0 = absence of polyps; 1 = polyps in middle meatus only; 2 = polyps beyond middle meatus. For edema, scarring and crusting: 0 = absent; 1 = mild; 2 = severe. For discharge: 0 = no discharge; 1 = clear, thin discharge; 2 = thick, purulent discharge.
5. Discussion
This study is among the first to assess the effectiveness of a nonabsorbable pack (Posisep) for improving postoperative symptoms and mucosal healing after ESS as compared with a polyvinyl acetate sponge (Merocel). In this study, we found that patients with Posisep after ESS had more improvement and better symptoms measured through SNOT-22 and LMES at 24 hours, 5 days, and 3 weeks than those with Merocel after ESS. While some aspects measured by LMES such as discharge and scarring were still better until 5 weeks after surgery, all symptoms measured by SNOT-22 were similar between the 2 groups 5 weeks after surgery.
Nasal packing with nonabsorbable or absorbable materials remains the most commonly used method for preventing wound adhesion and bleeding after ESS. However, nonabsorbable packings are criticized for their multiple defects, such as nasal airway obstruction, headache/pressure, painful mouth, pharynx dryness, and damage of mucosa. Absorbable nasal packings have advantages in preventing polyposis, lateralization of the middle turbinate and excellent wound healing, and acceptable patient comfort.[7,8,11,15] In the last 20 years, a wide range of absorbable middle meatus dressing has been developed and marketed, including carboxy-methyl-cellulose foam, collagen-based gel foam, hyaluronic acid-based films and foams, polyurethane sponges. Previous clinical trials have reported that synechia and granuloma formations, which were delayed in bioresorption, caused more crusting and made them less beneficial for additional debridement after ESS.[15,16]
Posisep, a resorbable chitosan-based nasal dressing recently approved by the Food and Drug Administration, has advantages through the hydrophilic characteristics of chitosan, antibacterial, hemostatic properties and has the ability to minimize postoperative adhesion, synechia and crusting when in contact with membranes of the mucosa.[10,17] A review by Kevin Hsu et al[17] has revealed the effectiveness of the chitosan-based product with respect to wound healing, degree of crusting and patient comfort. Young-Jun Chung et al[18] have shown that chitosan gel has rapid hemostasis ability after application to the surgical field, achieving complete hemostasis in 90.9% of the chitosan gel applied sides by 6 minutes, which is consistent with previous studies. Furthermore, there have been no adverse events reported in previous studies that evaluated the safety of chitosan gel in neurosurgical sheep model; according to postoperative magnetic resonance imaging scans and histological analysis, chitosan gel was safe and nontoxic to neural tissue and is comparable to Gelfoam.[19]
In this study, Posisep was favored by SNOT-22 and LME scores compared to Merocel. Moreover, this study demonstrated that the postoperative epistaxis was not significantly different between the 2 groups with a similar extent of surgery. There was still minimal bleeding after middle meatal antrostomy and middle meatal antrostomy plus sphenoid sinusotomy in 19 patients, and thus we applied anterior nasal packing. One patient in the Merocel group had epistaxis 7 hours after ESS and had to be self-limited. In the Posisep group, 1 patient was on anticoagulant daily due to cardiac valve disease; in another patient with epistaxis, the bleeding responded to acid tranxemic. Moreover, in our study, the first follow-up had the highest SNOT-22 scores in both groups, similar to that reported in Jordan clinical study.[20] It was because this was the time when retained material or crust in the nasal cavities could cause problem with pain, patient discomfort and the need for debridement. Patients who received Posisep had significant improvements in SNOT-22 at 5 days and 3 weeks. This is similar to the improvement in SNOT-22 reported with steroid-eluting stents at visit 2 in a recent study.[20] However, health-related QoL measured through SNOT-22 was similar in both groups at 5 weeks after surgery. Interestingly, our study reported improvements in SNOT-22 scores between preoperative and postoperative points for all patients. This finding is consistent with that reported by Zachary et al[13]
In our study, LMES score was significantly lower in the Posisep group than in the Merocel group through endoscopic evaluation of nasal cavities at 5 days, 3 weeks, and 5 weeks after surgery. However, the process of wound healing assessed with the LMES of mucosal edema and crusting in the Posisep group was not significantly different from that of the Merocel group at postoperative 5 days and 3 weeks. In a clinical setting, Young et al reported that in patients undergoing ESS, chitosan was known to have no adverse effect on the wound healing process at all evaluating time points (P > .05).[18] Moreover, in our study, there were significantly less scarring on the Posisep group follow-up visits at 5 days, 3 and 5 weeks after surgery (P < .05). Kevin et al revealed that chitosan is an effective agent to prevent scarring in patients who underwent ESS.[17] In general, scarring or adhesion occurs frequently after ESS and is the most common reason for revision surgery. Previous studies also reported that the severity of preoperative paranasal inflammation did not increase the incidence and degree of scarring formation.[17,18]
This study has several potential limitations that should be considered. Only short-term clinical impact of Posisep containing chitosan on SNOT-22 and LMES during the first 5 weeks after ESS was evaluated. It can be beneficial to follow-up patients to evaluate the long-term effect and other potential adverse events. Moreover, in this study we did not include a control group who did not receive packing, and thus the true effect of Posisep might not be fully quantified. However, as a standard procedure, it was not common to skip packing insertion following ESS. Thus, the inclusion of the control group in further studies should be with caution. Finally, in this study, we had a relatively small sample size, which can limit the generalization of our study findings. More studies with a larger sample size should be considered to confirm findings from our study.
6. Conclusion
In our study, we found that Posisep containing chitosan provided patients with a better health-related quality of life throughout the early recovery period compared with Merocel. More specifically, Posisep packing significantly improved postopeartive symptoms measured through SNOT-22 and LMES scores when compared with Merocel packing at 24 hours, 5 days, 3 weeks, and 5 weeks after surgery. Although more studies are needed, our findings support the use of Posisep after ESS.
Acknowledgments
The authors would like to thank the Director Board of University Medical Center Ho Chi Minh City for their support during the study and the patients who participated in this study.
Author contributions
Conceptualization: Huu Kien Pham, Trong Nguyen.
Data curation: Trong Nguyen, Tai Thanh Tran.
Formal analysis: Tai Thanh Tran, Truc Thanh Thai.
Methodology: Huu Kien Pham, Truc Thanh Thai.
Writing – original draft: Trong Nguyen, Tai Thanh Tran, Truc Thanh Thai.
Writing – review & editing: Huu Kien Pham, Truc Thanh Thai.
Abbreviations:
- ESS
- endoscopic sinus surgery
- LMES
- The Lund-Mackay postoperative endoscopic score
- QoL
- quality of life
- SNOT-22
- Sino-Nasal Outcome Test (22 item)
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Pham HK, Nguyen T, Tran TT, Thai TT. A comparison of effectiveness between Posisep and Merocel nasal packing after endoscopic sinus surgery: Findings from a randomized, double-blinded, controlled trial. Medicine 2023;102:32(e34782).
Contributor Information
Huu Kien Pham, Email: drphuchuu4@gmail.com.
Trong Nguyen, Email: drtrong265@gmail.com.
Tai Thanh Tran, Email: tai.tt3@umc.edu.vn.
References
- [1].Welch KC, Stankiewicz JA. A contemporary review of endoscopic sinus surgery: techniques, tools, and outcomes. Laryngoscope. 2009;119:2258–68. [DOI] [PubMed] [Google Scholar]
- [2].Ferreiro JA, Carlson BA, Thane Cody III D. Paranasal sinus fungus balls. Head Neck. 1997;19:481–6. [DOI] [PubMed] [Google Scholar]
- [3].Khalid AN, Quraishi SA, Kennedy DW. Long-term quality of life measures after functional endoscopic sinus surgery. Am J Rhinol. 2004;18:131–6. [PubMed] [Google Scholar]
- [4].May M, Levine HL, Mester SJ, et al. Complications of endoscopic sinus surgery: analysis of 2108 patients – incidence and prevention. Laryngoscope. 1994;104:1080–3. [DOI] [PubMed] [Google Scholar]
- [5].Weber R, Hochapfel F, Draf W. Packing and stents in endonasal surgery. Rhinology. 2000;38:49–62. [PubMed] [Google Scholar]
- [6].Orlandi RR, Lanza DC. Is nasal packing necessary following endoscopic sinus surgery? Laryngoscope. 2004;114:1541–4. [DOI] [PubMed] [Google Scholar]
- [7].Berlucchi M, Castelnuovo P, Vincenzi A, et al. Endoscopic outcomes of resorbable nasal packing after functional endoscopic sinus surgery: a multicenter prospective randomized controlled study. Eur Arch Otorhinolaryngol. 2009;266:839–45. [DOI] [PubMed] [Google Scholar]
- [8].Wang T-C, Tai C-J, Tsou Y-A, et al. Absorbable and nonabsorbable packing after functional endoscopic sinus surgery: systematic review and meta-analysis of outcomes. Eur Arch Otorhinolaryngol. 2015;272:1825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vlastarakos PV, Iacovou E, Fetta M, et al. How effective is postoperative packing in FESS patients? A critical analysis of published interventional studies. Eur Arch Otorhinolaryngol. 2016;273:4061–71. [DOI] [PubMed] [Google Scholar]
- [10].Raafat D, Sahl HG. Chitosan and its antimicrobial potential–a critical literature survey. Microb Biotechnol. 2009;2:186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ngoc Ha T, Valentine R, Moratti S, et al. A blinded randomized controlled trial evaluating the efficacy of chitosan gel on ostial stenosis following endoscopic sinus surgery. Int Forum Allergy Rhinol. 2013;3:573–80. [DOI] [PubMed] [Google Scholar]
- [12].Hajiioannou JK, Bizaki A, Fragiadakis G, et al. Optimal time for nasal packing removal after septoplasty. A comparative study. Rhinology. 2007;45:68–71. [PubMed] [Google Scholar]
- [13].Soler ZM, Jones R, Le P, et al. Sino-Nasal outcome test-22 outcomes after sinus surgery: a systematic review and meta-analysis. Laryngoscope. 2018;128:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Snidvongs K, Dalgorf D, Kalish L, et al. Modified Lund Mackay postoperative endoscopy score for defining inflammatory burden in chronic rhinosinusitis. Rhinology. 2014;52:53–9. [DOI] [PubMed] [Google Scholar]
- [15].Yan M, Zheng D, Li Y, et al. Biodegradable nasal packings for endoscopic sinonasal surgery: a systematic review and meta-analysis. PLoS One. 2014;9:e115458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chandra RK, Conley DB, Kern RC. The effect of FloSeal on mucosal healing after endoscopic sinus surgery: a comparison with thrombin-soaked gelatin foam. Am J Rhinol. 2003;17:51–5. [PubMed] [Google Scholar]
- [17].Hsu K, Ericksen M, Catalano P. Effect of a chitosan-based biodegradable middle meatal dressing after endoscopic sinus surgery: a prospective randomized comparative study. Sinusitis. 2016;1:3–12. [Google Scholar]
- [18].Chung Y-J, An S-Y, Yeon J-Y, et al. Effect of a chitosan gel on hemostasis and prevention of adhesion after endoscopic sinus surgery. Clin Exp Otorhinolaryngol. 2016;9:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rajiv S, Harding M, Bassiouni A, et al. The efficacy and safety of chitosan dextran gel in a burr hole neurosurgical sheep model. Acta Neurochir (Wien). 2013;155:1361–6. [DOI] [PubMed] [Google Scholar]
- [20].Rawl JW, McQuitty RA, Khan MH, et al. Comparison of steroid-releasing stents vs nonabsorbable packing as middle meatal spacers. Int Forum Allergy Rhinol. 2020;10:328–33. [DOI] [PubMed] [Google Scholar]