Abstract
Thoracic endovascular aortic repair (TEVAR) is a new alternative surgical treatment for aortic pathologies, which is more minimally invasive. The aim of current study was to summarize the single-center experience of general anesthesia for patients undergoing TEVAR. In adult patients undergoing surgery for congenital heart disease, the strategy of “fast-track” anesthesia with early extubation in theater is associated with a shorter intensive care unit (ICU) stay, and lower health-care-related costs. Fast-track anesthesia has not been assessed in patients under TEVAR. Adult patients who received general anesthesia for TEVAR in our center from January 2020 to December 2020 were included. Baseline characteristics, airway management, anesthetic techniques and major complications were collected. A total of 204 (171 male, mean age 58.1 ± 11.5 years) patients met inclusion criteria for this study. The distribution of pathologies included 29 descending thoracic aneurysms, 87 type B dissections, and 88 intramural hematoma/perforating aortic ulcer. Etomidate was the induction agent in 190 (93.1%) patients, compared with propofol in 16 (7.8%). Cisatracurium was the muscle relaxant in 201 (98.5%), compared with rocuronium in 3 (1.5%). Midazolam (benzodiazepines) was given to 124 (60.8%) patients during anesthesia induction. General anesthesia was maintained with sevoflurane in 85.3% (174) patients, dexmedetomidine in 201 (98.5%) and propofol in 204 (100%). Postoperative length of stay (LOS) in the hospital was 6.0 (5.0–7.8) days. LOS in the ICU was 23.0 (20.0–27.8) hours. Overall neurologic event rate was 2.0% (n = 4) (spinal cord ischemia 1.5% [n = 3]; stroke 0.5% [n = 1]). After matching, patients who received “fast-track” anesthesia had a shorter LOS in ICUs (21.0 [18.0–24.0] vs 24.0 [20.0–44.0] hours; P = .005), and a shorter postoperative LOS in hospital (5.0 [4.0–7.0] vs 6.0 [5.0–8.0] days; P = .001). There were no in-hospital deaths. Fast-track anesthesia is feasible and safe in patients underwent TEVAR. This management strategy is associated with shorter LOS of ICU and total postoperative hospital stays. An early extubation strategy should be implemented for hemodynamically stable patients.
Keywords: fast-track anesthesia, management, TEVAR
1. Introduction
Acute aortic syndrome (AAS) is an acutely presenting, potentially fatal pathology within the wall of the aorta. AAS consists of aortic dissection, intramural hematoma, and penetrating atherosclerotic ulcer.[1] Aortic diseases can be broadly classified as thoracic aortic aneurysm, abdominal aortic aneurysm, and AAS.[2] Despite important advances in diagnostic and therapeutic interventions, data derived from registries and population-based studies highlight that the burden of aortic diseases remains high.
In the past, the traditional treatment was conservative treatment and surgical repair. Since 1994, thoracic endovascular aortic repair (TEVAR) was introduced as a less invasive alternative to open surgical repair for treatment of descending thoracic aortic aneurysms.[3] Although endovascular techniques were initially reserved for patients not suitable for conventional surgery, its availability and relative ease of application led to rapid expansion of indications. Much studies had reported the safety and feasibility of this therapy in various patient groups.[4–6] Providing anesthesia for patients undergoing TEVAR is complex and requires profound knowledge and clinical experience. Studies on best anesthetic management are scarce. The aim of this study is to summarize the general anesthetic management of TEVAR in 2020 and the short-term prognosis of patients (during hospitalization).
2. Methods
2.1. Study design and population
In this retrospective study, we analyzed TEVAR cases performed between January 2020 and December 2020. The authors’ institutional ethics committee approved the investigational protocol (2019–1301). Because of the retrospective nature of this study, patient consent was waived.
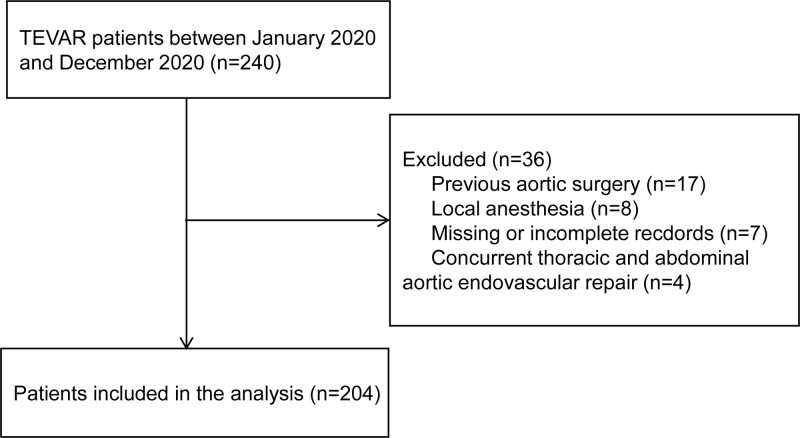
Data on all patients > 18 years of age who underwent TEVAR at authors’ hospital between January 2020 and December 2020 were obtained and studied. The inclusion criteria were as follows: adult patients (>18 years old); patients undergoing TEVAR. Exclusion criteria included: patients who received previous aortic surgery; patients whose anesthesia records were missing or incomplete; patients undergoing TEVAR with local anesthesia; and concurrent thoracic and abdominal aortic endovascular repair. The remaining 204 patients were included for a retrospective analysis.
2.2. Data collection
Data including demographic characteristics, medical history, procedural data, anesthesia management, and outcomes were collected for analysis. Patients’ demographics included age, sex, body mass index (BMI), comorbidities, and history of smoking. Data were recorded by 2 researchers.
2.3. Definitions
Fast-track anesthesia was defined as on-table extubation of patients in the operation theater.
Post-operative delirium had been defined as an acute change in cognitive status characterized by fluctuating attention and consciousness at any time after surgery until discharge.[7] Descriptive words documenting the presence of delirium include: mental status change, confusion, disorientation, agitation, delirium, inappropriate behavior, inattention, hallucinations, combative (e.g., pulling out lines or tubes), etc.
We defined acute kidney injury (AKI) using the consensus Kidney Disease Improving Global Outcomes criteria as at least a 50% and/or a 0.3 mg/dL increase in serum creatinine level (to convert to micromoles per liter, multiply by 88.4) relative to the preoperative reference value.[8,9] The highest postoperative value is limited within 5 days postoperatively. Postoperative fever was defined as body temperature of >38ºC.[10,11]
2.4. Statistical analysis
Categorical variables are presented as numbers and percentages. For the descriptive analysis of continuous variables, mean value with standard deviation (for a normal distribution) and median value with interquartile range (IQR) (for non-normal distribution), were calculated. We used the Student’s t test, Mann–Whitney U test, χ2 test, or Fisher’s exact test to compare differences between 2 groups where appropriate. A 2-sided α of less than 0.05 was considered statistically significant. A 1:1 propensity match analysis was used to balance important clinical factors, such as age, gender, BMI, elective or emergency and operative procedure. Statistical analyses were done using the SPSS 25 (SPSS Inc., an IBM company, Chicago, USA).
2.5. Surgical protocol
The procedures were performed by the same operation team. All patients were administered general anesthesia in our hybrid operating theater. The femoral artery was exposed surgically or percutaneously using suture device.
All the 204 patients were treated by TEVAR, and the detailed procedures of TEVAR were reported in previous literature by the same surgeon team.[12] If the lesion involves the aortic arch, the technique of reconstruction of the arch vessels (chimney, fenestrated, periscopes or TEVAR concomitant with supra-arch bypass) was determined by the surgeon according to the characteristics of aortic pathologies.[13]
Antibiotic prophylaxis was administered intravenously before the procedure. Heparin was administered upon the access site was surgically exposed and activated clotting time (ACT) was maintained beyond 200s throughout the procedure; the ACT was checked every 60 minutes.
At the end of the procedure, after the removal of the large sheath, the arteriotomy was repaired. After surgery, the patient was returned to the intensive care unit (ICU). All patients underwent postoperative computed-tomographic angiography prior to discharge.
3. Results
3.1. Baseline characteristics
In total, 240 cases were identified between January 2020 and December 2020. After applying exclusion criteria and excluding cases with missing data, the final sample size was 204 cases (Fig. 1).
Figure 1.
Study flow chart. TEVAR = thoracic endovascular aortic repair.
Among 204 patients, mean age was 58.1 ± 11.5 years and 171 (83.8%) were male. The mean BMI was 26.3 ± 3.4 kg/m2, and the most common comorbidities were hypertension (168, 82.4%) and dyslipidemia (67, 32.8%). Demographic data and clinical features of the overall population are shown in Table 1.
Table 1.
Baseline clinical characteristics of patient population.
| Parameter | All (n = 204) | Non-fast (n = 90) | Fast (n = 114) | P value |
|---|---|---|---|---|
| Age, yr | 58.1 ± 11.5 | 56.6 ± 11.1 | 59.2 ± 11.7 | .109 |
| Male | 171 (83.8) | 79 (87.8) | 92 (80.7) | .173 |
| BMI, kg/m2 | 26.3 ± 3.4 | 27.1 ± 3.5 | 25.6 ± 3.2 | .001* |
| Comorbidity | ||||
| Hypertension | 168 (82.4) | 73 (81.1) | 95 (83.3) | .679 |
| Coronary heart disease | 37 (18.1) | 16 (17.8) | 21 (18.4) | .906 |
| Diabetes | 27 (13.2) | 10 (11.1) | 17 (14.9) | .426 |
| Dyslipidemia | 67 (32.8) | 35 (38.9) | 32 (28.1) | .102 |
| Ischemic stroke | 22 (10.8) | 6 (6.7) | 16 (14.0) | .092 |
| Current or previous smoke | 129 (63.2) | 66 (73.3) | 63 (55.3) | .008* |
| Previous PCI | 14 (6.8) | 7 (7.8) | 7 (6.1) | .646 |
| ASA class | ||||
| II | 18 (8.8) | 6 (6.7) | 12 (10.5) | .335 |
| III–IV | 186 (91.2) | 84 (93.3) | 102 (89.5) | |
| Preoperative medications | ||||
| Aspirin | 25 (12.2) | 11 (12.2) | 14 (12.3) | .99 |
| P2Y12 antagonist | 8 (3.9) | 1 (1.1) | 7 (6.1) | .14 |
| LMWH | 3 (1.42) | 2 (2.2) | 1 (0.9) | .836 |
| Statin | 57 (27.9) | 24 (26.7) | 33 (28.9) | .719 |
| ACEI/ARB | 84 (41.1) | 35 (38.9) | 49 (43.0) | .555 |
| Anticoagulant | 1 (0.4) | 0 (0) | 1 (0.9) | 1.000 |
| Beta blockers | 153 (75) | 70 (77.8) | 83 (72.8) | .416 |
| CCB | 165 (80.8) | 78 (86.7) | 87 (76.3) | .062 |
| Elective | 193 (94.6) | 80 (88.9) | 113 (99.1) | .004* |
| Pathology | ||||
| Aneurysm | 29 (14.2) | 9 (10.0) | 20 (17.5) | .001* |
| Dissection | 87 (42.6) | 51 (56.7) | 36 (31.6) | |
| IMH/PAU | 88 (43.1) | 30 (33.3) | 58 (50.9) | |
| Hemoglobin, g/L | 134 ± 16 | 133 ± 15 | 134 ± 17 | .781 |
| White blood cell count, ×109/L | 7.82 ± 2.64 | 8.47 ± 2.95 | 7.30 ± 2.25 | .002* |
| Platelet count, ×109/L | 236 ± 87.6 | 244 ± 90 | 230 ± 86 | .235 |
| D-dimer, μg/mL | 1.19 (0.48–2.55) | 1.25 (0.76–2.72) | 1.13 (0.35–2.44) | .145 |
| Albumin, g/L | 37.59 ± 3.46 | 37.89 ± 3.89 | 37.41 ± 3.10 | .32 |
Categorical data are given as numbers and percentages. Continuous data are given as mean value with standard deviation (for a normal distribution) and median value with interquartile range (25th–75th percentiles) (for a non-normal distribution). P values are calculated by Student’s t test, Mann–Whitney U test, χ2 test, or Fisher’s exact test, as appropriate.
ACEI/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blockers, ASA = American Society of Anesthesiologists, BMI = body mass index, CCB = calcium channel blockers, IMH = intramural hematoma, LMWH = low-molecular-weight heparin, PAU = penetrating aortic ulcer, PCI = percutaneous coronary intervention.
P < .05.
3.2. Anesthesia management
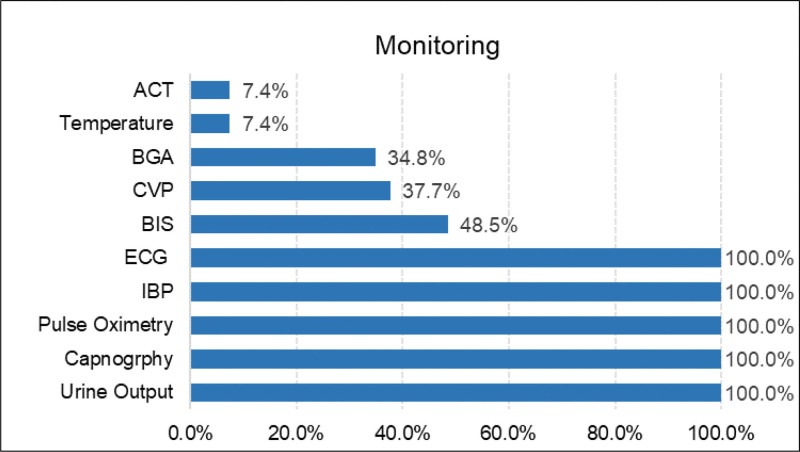
In the operating room, patients received routine monitoring, including ECG, invasive arterial blood pressure, pulse oximetry, capnography, and urine output, while central venous pressure, and the bispectral index (BIS) were not part of the routine monitoring. Invasive arterial blood pressure was instituted before anesthesia induction. Although invasive arterial line monitoring is required, the location of the arterial line should be discussed with the vascular surgeon. The left radial artery is usually chosen for arterial cannulation. If additional stent grafts may require left brachial access or if thoracic aortic stent grafts may cover the left subclavian artery take-off, arterial access should be obtained in the right arm. Details are shown in Figure 2.
Figure 2.
Monitoring during TEVAR. ACT = activated clotting time, BGA = blood gas analysis, CVP = central venous pressure, BIS = bispectral index, IBP = invasive blood pressure, TEVAR = thoracic endovascular aortic repair.
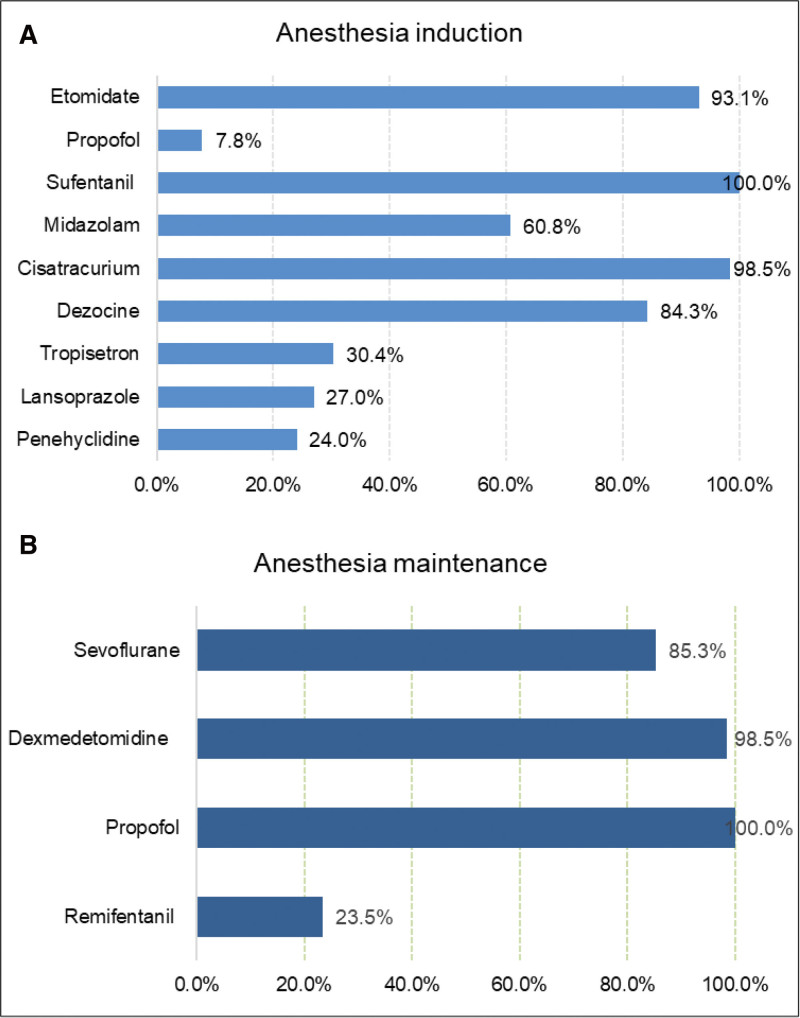
In our center, no anesthetic premedication was administered. Commonly used induction agents are etomidate (0.2–0.3 mg/kg) 93.1% (190), midazolam (1–2 mg) 60.8% (124), sufentanil (0.3–0.5 μg/kg) 100% (204), cisatracurium (0.15–0.2 mg/kg) 98.5% (201), and some adjuncts such as dezocine (5–10 mg), lansoprazole (30 mg), tropisetron (5 mg), and penehyclidine (0.5–1 mg) (Fig. 3A).
Figure 3.
(A) Agents used for anesthesia induction. (B) Agents used for anesthesia maintenance.
During maintenance of general anesthesia, propofol (2–4 mg/kg/h), dexmedetomidine (0.2–0.4 μg/kg/h) and sevoflurane (1%) were regularly used in most cases (Fig. 3B). In addition, we found that ultra-short-acting remifentanil (0.1 μg/kg/min) was used in 23.5% (48) of patients during the maintenance of anesthesia. Additional doses of sufentanil and muscle relaxant were given intraoperatively if necessary. Ninety-seven (47.5%) of patients were administered a continuous infusion of cisatracurium.
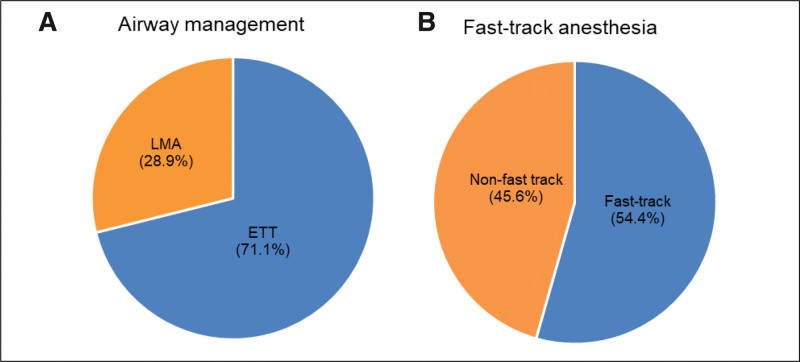
All patients were mechanically ventilated, 71.1% (145) by endotracheal intubation and 28.9% (59) by laryngeal mask airway (Fig. 4A). Overall, 54.4% (111) of the patients were under fast-track anesthesia (Fig. 4B). Mechanical ventilation was set volume-controlled ventilation, with a tidal volume of 6 to 8 mL/kg (employing ideal body weight). The fraction of inspired oxygen was set from 0.4 to 0.7 to maintain oxygen saturations measured by pulse oximetry greater than or equal to 95%.
Figure 4.
(A) Airway management during TEVAR. (B) Fast-track anesthesia. ETT = endotracheal intubation, LMA = laryngeal mask airway, TEVAR = thoracic endovascular aortic repair.
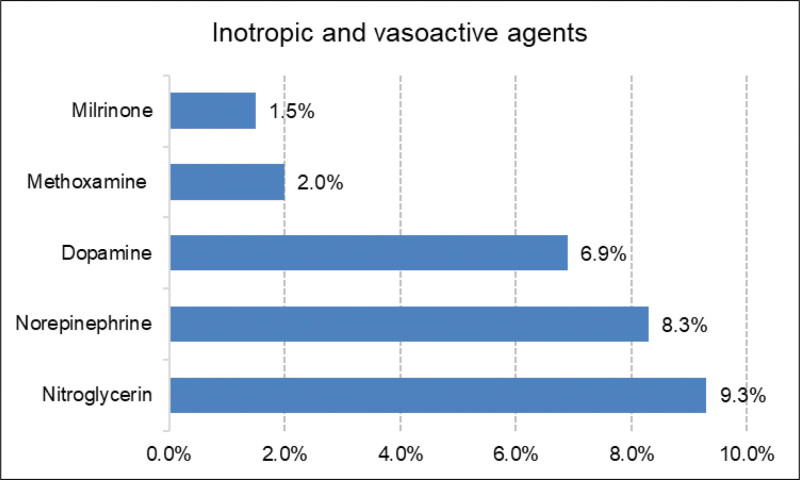
For the management of hypertension, nitroglycerin infusion was used in 19 (9.3%) patients. For persistent hypotension, norepinephrine infusion was used in 17 (8.3%) patients and dopamine infusion was used in 14 (6.9%) patients (Fig. 5). The patients received inotropic/vasoactive agent infusion intraoperatively. The surgeon and ICU physician will adjust the inotropic/vasoactive agents according to the patients’ condition, upon arrival at the ICU after surgery.
Figure 5.
Inotropic and vasoactive agents used during TEVAR. TEVAR = thoracic endovascular aortic repair.
3.3. Outcomes
The median operation time was 75 (IQR 60–95, min.–max. 30–250) minutes. Intraoperative variables are described in Table 2. At the end of the procedure only 1 patient received intravenous protamine to reverse the anticoagulation. ACT was checked in 80% of patients upon arrival ICU, and protamine was not administered in any patient.
Table 2.
Intraoperative and postoperative variables of included patients.
| Parameter | All (n = 204) | Non-fast (n = 90) | Fast (n = 114) | P value |
|---|---|---|---|---|
| Surgical data | ||||
| Contrast volume, mL | 81 (70–95) | 83 (75–100) | 80 (70–91) | .045* |
| First dose of heparin, U/kg | 58 (50–63) | 58 (51–65) | 57 (50–63) | .332 |
| Operative time, min | 75 (60–95) | 75 (60–108) | 70 (60–90) | .046* |
| Operative procedure | ||||
| TEVAR | 111 (54.4) | 47 (52.2) | 64 (56.1) | .155 |
| TEVAR+aortic arch bypass | 3 (1.5) | 3 (3.3) | 0 (0) | |
| Chimney/periscope/fenestration | 90 (44.1) | 40 (44.4) | 50 (43.9) | |
| Intraoperative volume | ||||
| Blood loss, mL | 20 (20–30) | 20 (20–30) | 20 (20–30) | .053 |
| Urine output, mL | 150 (60–300) | 150 (60–400) | 150 (74–300) | .872 |
| Fluid infusion, mL | 500 (500–800) | 500 (300–800) | 600 (500–913) | .084 |
| Blood product transfusion | 1 (0.4) | 1 (1.1) | 0 (0) | .441 |
| Postoperative outcomes | ||||
| LOS in the ICU, h | 23.0 (20.0–27.8) | 24.0 (20.0–70.5) | 21.5 (19.0–24.0) | .001* |
| LOS in the hospital, d | 6.0 (5.0–7.8) | 7.0 (5.0–8.3) | 5.0 (4.0–7.9) | <.001* |
| Complications | ||||
| Unplanned reintubation | 1 (0.5) | 1 (1.1) | 0 (0) | .441 |
| Pulmonary infection | 1 (0.5) | 1 (1.1) | 0 (0) | .441 |
| Delirium | 12 (5.8) | 7 (7.8) | 5 (4.4) | .375 |
| AKI | 7 (3.4) | 6 (6.7) | 1 (0.9) | .045* |
| Pulmonary embolism | 2 (1.0) | 1 (1.1) | 1 (0.9) | 1.000 |
| Minor stroke | 1 (0.5) | 0 (0) | 1 (0.9) | 1.000 |
| Spinal ischemia | 3 (1.5) | 2 (2.2) | 1 (0.9) | .584 |
| Arm ischemia | 1 (0.5) | 0 (0) | 1 (0.9) | 1.000 |
| Type I endoleak | 1 (0.5) | 0 (0) | 1 (0.9) | 1.000 |
| Unplanned operation | 3 (1.5) | 3 (3.3) | 0 (0) | .084 |
| Postoperative fever | 15 (7.4) | 10 (11.1) | 5 (4.4) | .103 |
| Death | 0 (0) | 0 (0) | 0 (0) | 1.000 |
Categorical data are given as numbers and percentages. Continuous data are given as mean value with standard deviation (for a normal distribution) and median value with interquartile range (25th–75th percentiles) (for a non-normal distribution). P values are calculated by Student’s t test, Mann–Whitney U test, χ2 test, or Fisher’s exact test, as appropriate.
AKI = acute kidney injury, TEVAR = thoracic endovascular aortic repair.
P < .05.
The median postoperative length of stay (LOS) in the ICU was 23.0 (IQR 20.0–27.8, min.–max. 2–188) hours and the hospital 6.0 (IQR 5.0–7.8, min.–max. 2–29) days. The complication rate in the overall group was low, with a reintubation of 1 (0.5%), due to arrhythmia resuscitation intubation. In the postoperative period, 1 (0.5%) developed pulmonary infection due to preoperative bronchiectasis.
Twelve (5.8%) of 204 patients developed delirium after surgery. Postoperative AKI was observed in 3.4% of patients, and pulmonary embolism occurred in 2 patients (1.0%). Two patients had been found to have an asymptomatic pulmonary embolism on the postoperatively CT scan. One minor stroke was recorded in the short term, and gradually recovered at discharge. There were 3 (1.5%) spinal cord ischemia (SCI) after TEVAR in our series of which one was a permanent paraplegia. None of the patients had type II endoleak, but a small type I endoleak occurred in 1 patient, without reintervention. Three (1.5%) patients returned to operating room for unplanned operation, 2 (1.0%) of these due to punctured femoral artery injury and one developing retrograde type A dissection after TEVAR. In total, 15 of 204 (7.4%) patients presented fever within 72h in our study. There were no in-hospital deaths.
3.4. Matched cohorts
Before propensity-score matching, there were some differences between the fast and non-fast group. With the use of propensity-score matching (1:1), 69 patients who received fast-track anesthesia were matched 69 who received non-fast-track anesthesia. After matching, baseline characteristics between the 2 groups were well balanced (Table 3).
Table 3.
Baseline clinical characteristics in PSM-matched cohorts.
| Parameter | Non-fast (n = 69) | Fast (n = 69) | P value |
|---|---|---|---|
| Age, yr | 58.1 ± 10.3 | 57.5 ± 12.1 | .752 |
| Male | 59 (85.5) | 60 (87.0) | .805 |
| BMI, kg/m2 | 26.4 ± 3.4 | 26.3 ± 3.0 | .871 |
| Comorbidity | |||
| Hypertension | 55 (79.7) | 58 (84.1) | .507 |
| Coronary heart disease | 12 (17.4) | 13 (18.8) | .825 |
| Diabetes | 8 (11.6) | 11 (15.9) | .459 |
| Dyslipidemia | 26 (37.7) | 22 (31.9) | .475 |
| Ischemic stroke | 4 (5.8) | 11 (15.9) | .056 |
| Current or previous smoke | 50 (72.5) | 41 (59.4) | .106 |
| Previous PCI | 7 (10.1) | 6 (8.7) | .854 |
| ASA class | |||
| II | 5 (7.2) | 5 (7.2) | 1.000 |
| III–IV | 64 (92.8) | 64 (92.8) | |
| Preoperative medications | |||
| Aspirin | 9 (13.0) | 7 (10.1) | .595 |
| P2Y12 antagonist | 1 (1.4) | 5 (7.2) | .208 |
| LMWH | 2 (2.9) | 1 (1.4) | 1.000 |
| Statin | 22 (31.9) | 21 (30.4) | .854 |
| ACEI/ARB | 29 (42.0) | 37 (53.6) | .173 |
| Anticoagulant | 0 (0) | 1 (1.4) | 1.000 |
| Beta blockers | 55 (79.7) | 51 (73.9) | .771 |
| CCB | 62 (89.9) | 542 (78.3) | .063 |
| Elective | 69 (100) | 69 (100) | 1.000 |
| Pathology | |||
| Aneurysm | 7 (10.1) | 10 (14.5) | .057 |
| Dissection | 36 (52.2) | 22 (31.9) | |
| IMH/PAU | 26 (37.7) | 37 (53.6) | |
| Hemoglobin, g/L | 132 ± 15 | 135 ± 17 | .492 |
| White blood cell count, ×109/L | 8.08 ± 4.67 | 7.52 ± 2.22 | .180 |
| Platelet count, ×109/L | 246 ± 92 | 244 ± 84 | .918 |
| D-dimer, μg/mL | 1.21 (0.73–2.30) | 1.14 (0.34–2.45) | .356 |
| Albumin, g/L | 37.99 ± 4.08 | 37.50 ± 3.13 | .426 |
Categorical data are given as numbers and percentages. Continuous data are given as mean value with standard deviation (for a normal distribution) and median value with interquartile range (25th–75th percentiles) (for a non-normal distribution). P values are calculated by Student’s t test, Mann–Whitney U test, χ2 test, or Fisher’s exact test, as appropriate.
ACEI/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blockers, ASA = American Society of Anesthesiologists, BMI = body mass index, CCB = calcium channel blockers, IMH = intramural hematoma, LMWH = low-molecular-weight heparin, PAU = penetrating aortic ulcer, PCI = percutaneous coronary intervention, PSM = propensity-score matching.
In the matched cohort, fast-track anesthesia associated with a significantly shorter postoperative LOS in ICU (21.0 [18.0–24.0] vs 24.0 [20.0–44.0] hours; P = .005). The total postoperative hospital LOS was also significantly shorter in patients with fast-track anesthesia (5.0 [4.0–7.0] vs 6.0 [5.0–8.0] days; P = .001) (Table 4).
Table 4.
Intraoperative and postoperative variables in PSM-matched cohorts.
| Parameter | Non-fast (n = 69) | Fast (n = 69) | P value |
|---|---|---|---|
| Surgical data | |||
| Contrast volume, mL | 83 (75–100) | 80 (70–90) | .011* |
| First dose of heparin, U/kg | 58 (53–66) | 56 (48–59) | .008* |
| Operative time, min | 75 (60–95) | 65 (59–84) | .067 |
| Operative procedure | |||
| TEVAR | 36 (52.2) | 39 (56.5) | .733 |
| TEVAR+aortic arch bypass | 1 (1.4) | 0 (0) | |
| Chimney/periscope/fenestration | 32 (46.4) | 30 (43.5) | |
| Intraoperative volume | |||
| Blood loss, mL | 20 (20–30) | 20 (20–30) | .171 |
| Urine output, mL | 150 (60–350) | 150 (80–300) | .886 |
| Fluid infusion, mL | 500 (400–800) | 500 (500–850) | .263 |
| Postoperative outcomes | |||
| LOS in the ICU, h | 24.0 (20.0–44.0) | 21.0 (18.0–24.0) | .005* |
| LOS in the hospital, d | 6.0 (5.0–8.0) | 5.0 (4.0–7.0) | .001* |
| Complications | |||
| Unplanned reintubation | 1 (1.4) | 0 (0) | 1.000 |
| Pulmonary infection | 1 (1.4) | 0 (0) | 1.000 |
| Delirium | 5 (7.2) | 4 (5.8) | 1.000 |
| AKI | 2 (2.9) | 1 (1.4) | 1.000 |
| Pulmonary embolism | 1 (1.4) | 1 (1.4) | 1.000 |
| Minor stroke | 0 (0) | 0 (0) | 1.000 |
| Spinal ischemia | 2 (2.9) | 0 (0) | .496 |
| Arm ischemia | 0 (0) | 1 (1.4) | 1.000 |
| Type I endoleak | 0 (0) | 0 (0) | 1.000 |
| Unplanned operation | 2 (2.9) | 0 (0) | .496 |
| Postoperative fever | 7 (10.1) | 3 (4.3) | .325 |
Categorical data are given as numbers and percentages. Continuous data are given as mean value with standard deviation (for a normal distribution) and median value with interquartile range (25th–75th percentiles) (for a non-normal distribution). P values are calculated by Student’s t test, Mann–Whitney U test, χ2 test, or Fisher’s exact test, as appropriate.
AKI = acute kidney injury, PSM = propensity-score matching, TEVAR = thoracic endovascular aortic repair.
P < .05.
4. Discussion
Experience with endovascular treatment in a wide spectrum of aortic diseases demonstrated the feasibility of this approach with encouraging early and long-term results.[6,14]
General anesthesia is popular due to the patient’s stress and anxiety and the need for the patient to hold their breath for brief periods of time and remain still intraoperatively. The choice of general anesthesia often is based on the preference of the operating surgeon and the anesthesiologist.
Minimizing patient movement and respiratory motion was especially important when angiography was performed. For example, angiography was performed to clarify the location of the proximal entry tear and the major vessel takeoff proximity to the landing zone. The overall intraoperative anesthetic goal is to establish a stable hemodynamic environment that provides optimal security for safe stent deployment while maintaining good cardiac function and adequate blood flow to the spinal cord and vital organs.
During TEVAR less than half of the patients were monitored for BIS, central venous pressure, and blood gas analysis. These different monitoring choices may be related to the preference of anesthesiologist. Some of them thought the BIS monitor could measure the real depth of anesthesia and reduce the possibility of awareness with recall during general anesthesia; however, this view is controversial. Avidan et al[15] demonstrated that anesthesia awareness occurred even when BIS value was within the target ranges. Their findings do not support BIS monitoring as part of routine practice. Some anesthesiologists believe that central venous catheter should be inserted before operation in order to facilitate the rapid transfusion of intravenous fluids and blood products, to monitor central venous pressure, and to allow the use of vasopressors and inotropes. This preference may be related to experience and the patient’s pathology. Vascular surgeons frequently perform fluoroscopy after heparin administration, so anesthesiologists are not allowed to step into the working field of the surgeon freely due to the radiation hazards. Only 7.4% of patients had ACT records probably due to missed registration by the anesthesiologist.
Etomidate was administered to almost all patients at anesthesia induction. It has a limited effect on patient’s respiratory and circulatory systems, which is beneficial to maintaining hemodynamic stability. Dezocine was given as an adjuvant in more than 80% of patients. Dezocine is a partial μ-receptor agonist, a κ-receptor antagonist, and a norepinephrine and serotonin reuptake inhibitor.[16] Although dezocine is a μ-opioid receptor partial antagonist, theoretically, it could antagonize the antinociceptive effects of morphine. However, when used in combination with morphine, dezocine concentration-dependently enhances the analgesic effects of morphine.[17] The combination of dezocine and sufentanil produce an additive effect for relieving the acute nociception after gynecological laparoscopic surgery.[18] Dezocine significantly reduced the incidence of etomidate-induced myoclonus[19] and inhibited opioid-induced cough.[20] Although dezocine is not a well- known drug in Western countries, it is widely applied as perioperative pain analgesic agent in China for decades.[20]
Maintenance of general anesthesia is a combination of sevoflurane and intravenous anesthetics in almost all patients. Sufentanil was used for intraoperative analgesia in all patients. Forty-eight (23.5%) of patients were combined with remifentanil, which may be related to fast-track anesthesia.
Fast-track anesthesia strategy was associated with a reduction in LOS of ICU and total postoperative hospital stays. Fast-track anesthesia was not associated with an increase in postoperative complications in our cohort. A retrospective study of 711 procedures reported that early extubation was associated with a shorter ICU stay and not associated with an increase in postoperative complications in patients undergoing surgery for congenital heart disease.[21] Our results are consistent with that literature. In our center, 54.4% of patients are fast-track anesthesia, achieving early extubation in the operating theater. Fast-track anesthesia appears to be safe and feasible for the majority of patients under TEVAR in routine practice, when fast-track anesthesia is appropriate. After the procedure is complete, hemodynamically stable patients should be routinely extubated to facilitate neurologic examination and reduce postoperative respiratory support duration.
There are some unique features worthy of note, including: holding breath during the angiography to avoid position shifts caused by breathing; decreasing systolic blood pressure to below 90 mm Hg prior to deployment of the stent graft to prevent stent migration; and increasing mean arterial pressure (MAP) after aortic stent deployment to prevent SCI by ensuring adequate spinal cord perfusion. General guidelines for minimizing SCI include increasing MAP (i.e., ≥80 mm Hg) and draining cerebrospinal fluid (CSF) in order to maintain spinal cord perfusion.[22,23]
SCI can cause transient or permanent paraplegia. Anesthesiologists have a major role in spinal cord protection by careful management of the hemodynamics and institution of protocols for CSF drainage. The timing of CSF drainage insertion (preoperatively, intraoperatively or postoperatively) was different among institutions.[24] At our center, CSF drainage is not routinely placed preoperatively. Patients go to the ICU after surgery to be closely monitored by nurses. CSF drainage is placed immediately if SCI occurs. Acher et al[25] reviewed a number of studies concluding that, despite using widely vary neuroprotective measures, the incidence of SCI remained ranging from 3% to 10%. SCI remains a multifactorial problem with several etiologies, contributing factors and underlying aortic pathologies and may vary considerably among different patient cohorts. No single spinal cord protecting method is currently able to provide absolute safety. In addition to advanced modern surgical, anesthetic methods improved the safety of TEVAR include CSF drainage and MAP augmentation.[22]
Postoperative delirium was observed in 5.8% of patients. In the study by Liu et al,[26] the incidence of postoperative delirium in patients with type B aortic dissection who underwent TEVAR with/without concomitant procedures was 13.3%. Salata et al[27] reported that postoperative delirium was present in 14 (13%) patients in the endovascular aortic repair group (abdominal/thoraco-abdominal). This discordance may be due to different definitions of delirium and methods of detection, thus leading to different observed rates of delirium. In our study, delirium is clinically defined, rather than using a scoring system.
With a definition set at 50% or greater increase in baseline creatinine level, we observed an incidence of AKI after TEVAR of 3.4%. Drews and associates identified a 17% incidence of AKI, which markedly increased duration of hospitalization, and was independently associated with the risk for early mortality.[28] The experience of Piffaretti et al[29] detected 14% of patients with postoperative AKI. They observed that a preoperative depressed renal function, the thoracoabdominal extent of the aortic disease, and blood transfusion requirement were the most relevant predictive factors of AKI after TEVAR. The reasons for such discrepant results have been attributed to the differences among patient populations, heterogeneous etiology, extent of the aortic pathologies, and most importantly, the definitions of postoperative AKI are different.
Fever was observed in 7.4% of patients, which may be correlated to post-implantation syndrome (PIS) after TEVAR. PIS represents a systemic inflammatory response syndrome initially observed following endovascular aortic repair of infrarenal abdominal aortic aneurysm.[30] The major clinical features of PIS are postoperative fever despite negative blood cultures and leucocytosis. TEVAR series have shown postoperative fever rates ranging from 26.3% to 48.8%.[11,12,31,32]
5. Limitations
The present study has some limitations. First, it was a retrospective, observational single-center design, although we enrolled all patients undergoing TEVAR continually to minimize the selection bias. Second, it is a small study. This reduces the power of the study and increases the chances of type II statistical error. Third, although we used propensity score matched model to balance the variables, common confounders of retrospective may also be present. As such, our study should be confirmed using other clinical randomized controlled trials.
6. Conclusion
Fast-track anesthesia is feasible and safe in patients underwent TEVAR. This management strategy is associated with shorter LOS of ICU and total postoperative hospital stays. The intraoperative management should optimize end-organ perfusion, and adjust hemodynamic management as the procedure progresses. An early extubation strategy should be implemented for hemodynamically stable patients.
Author contributions
Data curation: Ying-chun Liu, Yan-ting Sun.
Formal analysis: Ying-chun Liu.
Investigation: Ying-chun Liu, Yan-ting Sun.
Methodology: Yun-tai Yao.
Project administration: Yan-ting Sun, Yun-tai Yao.
Resources: Yun-tai Yao.
Supervision: Yan-ting Sun, Yun-tai Yao.
Validation: Yun-tai Yao.
Writing – original draft: Ying-chun Liu.
Writing – review & editing: Ying-chun Liu.
Abbreviations:
- AAS
- acute aortic syndrome
- ACT
- activated clotting time
- AKI
- acute kidney injury
- BIS
- bispectral index
- BMI
- body mass index
- CSF
- cerebrospinal fluid
- ICU
- intensive care unit
- IQR
- interquartile range
- LOS
- length of stay
- MAP
- mean arterial pressure
- PIS
- post-implantation syndrome
- SCI
- spinal cord ischemia
- TEVAR
- thoracic endovascular aortic repair
This study has obtained IRB approval from (indicate the relevant board) and the need for informed consent was waived. This type of study consent for publication is not required.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Liu Y-c, Sun Y-t, Yao Y-t. Anesthesia management of patients undergoing thoracic endovascular aortic repair: A retrospective analysis of single center. Medicine 2023;102:32(e34508).
Contributor Information
Ying-chun Liu, Email: springshinyliu@163.com.
Yan-ting Sun, Email: 992188405@qq.com.
References
- [1].Mussa FF, Horton JD, Moridzadeh R, et al. Acute aortic dissection and intramural hematoma: a systematic review. JAMA. 2016;316:754–63. [DOI] [PubMed] [Google Scholar]
- [2].Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331–48. [DOI] [PubMed] [Google Scholar]
- [3].Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med. 1994;331:1729–34. [DOI] [PubMed] [Google Scholar]
- [4].Li S, Cai W, Li X, et al. Thoracic endovascular aortic repair for traumatic type B aortic dissection: a 5-year experience from a single center. Int Angiol. 2017;36:316–21. [DOI] [PubMed] [Google Scholar]
- [5].Massara M, Alberti A, Volpe P. Early and mid-term results of endovascular treatment of thoracic aorta diseases: a single-center experience. Semin Vasc Surg. 2020;32:111–6. [DOI] [PubMed] [Google Scholar]
- [6].Wiedemann D, Mahr S, Vadehra A, et al. Thoracic endovascular aortic repair in 300 patients: long-term results. Ann Thorac Surg. 2013;95:1577–83. [DOI] [PubMed] [Google Scholar]
- [7].Hussain M, Berger M, Eckenhoff RG, et al. General anesthetic and the risk of dementia in elderly patients: current insights. Clin Interv Aging. 2014;9:1619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kellum JA, Lameire N, Aspelin P, et al.; Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- [9].Huber M, Ozrazgat-Baslanti T, Thottakkara P, et al. Cardiovascular-specific mortality and kidney disease in patients undergoing vascular surgery. JAMA Surg. 2016;151:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gorla R, Erbel R, Kahlert P, et al. Clinical features and prognostic value of stent-graft-induced post-implantation syndrome after thoracic endovascular aortic repair in patients with type B acute aortic syndromes. Eur J Cardiothorac Surg. 2016;49:1239–47. [DOI] [PubMed] [Google Scholar]
- [11].Zhu Y, Luo S, Ding H, et al. Predictors associated with an increased prevalence of postimplantation syndrome after thoracic endovascular aortic repair for type B aortic dissection†. Eur J Cardiothorac Surg. 2019;55:998–1005. [DOI] [PubMed] [Google Scholar]
- [12].Shu C, Fang K, Luo M, et al. Emergency endovascular stent-grafting for acute type B aortic dissection with symptomatic malperfusion. Int Angiol. 2013;32:483–91. [PubMed] [Google Scholar]
- [13].Shu C, Fan B, Luo M, et al. Endovascular treatment for aortic arch pathologies: chimney, on-the-table fenestration, and in-situ fenestration techniques. J Thorac Dis. 2020;12:1437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fattori R, Nienaber CA, Rousseau H, et al.; Talent Thoracic Retrospective Registry. Results of endovascular repair of the thoracic aorta with the talent thoracic stent graft: the talent thoracic retrospective registry. J Thorac Cardiovasc Surg. 2006;132:332–9. [DOI] [PubMed] [Google Scholar]
- [15].Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–108. [DOI] [PubMed] [Google Scholar]
- [16].Liu R, Huang XP, Yeliseev A, Xi J, Roth BL. Novel molecular targets of dezocine and their clinical implications. Anesthesiology. 2014;120:714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morgan D, Cook CD, Smith MA, Picker MJ. An examination of the interactions between the antinociceptive effects of morphine and various mu-opioids: the role of intrinsic efficacy and stimulus intensity. Anesth Analg. 1999;88:407–13. [DOI] [PubMed] [Google Scholar]
- [18].Zhu H, Chen Y, Huang S, et al. Interaction of analgesic effects of dezocine and sufentanil for relief of postoperative pain: a pilot study. Drug Des Devel Ther. 2020;14:4717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhu Y, Yang Y, Zhou C, et al. Using dezocine to prevent etomidate-induced myoclonus: a meta-analysis of randomized trials. Drug Des Devel Ther. 2017;11:2163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].He LX, Yao YT, Shao K, et al. Efficacy of dezocine on preventing opioid-induced cough during general anaesthesia induction: a PRISMA-compliant systematic review and meta-analysis. BMJ Open. 2022;12:e052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bianchi P, Constantine A, Costola G, et al. Ultra-fast-track extubation in adult congenital heart surgery. J Am Heart Assoc. 2021;10:e020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Etz CD, Weigang E, Hartert M, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European association for cardio-thoracic surgery†. Eur J Cardiothorac Surg. 2015;47:943–57. [DOI] [PubMed] [Google Scholar]
- [23].Weigang E, Hartert M, Siegenthaler MP, et al. Perioperative management to improve neurologic outcome in thoracic or thoracoabdominal aortic stent-grafting. Ann Thorac Surg. 2006;82:1679–87. [DOI] [PubMed] [Google Scholar]
- [24].Awad H, Ramadan ME, El Sayed HF, et al. Spinal cord injury after thoracic endovascular aortic aneurysm repair. Can J Anaesth. 2017;64:1218–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Acher C, Acher CW, Marks E, et al. Intraoperative neuroprotective interventions prevent spinal cord ischemia and injury in thoracic endovascular aortic repair. J Vasc Surg. 2016;63:1458–65. [DOI] [PubMed] [Google Scholar]
- [26].Liu J, Yang F, Luo S, et al. Incidence, predictors and outcomes of delirium in complicated type B aortic dissection patients after thoracic endovascular aortic repair. Clin Interv Aging. 2021;16:1581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Salata K, Katznelson R, Beattie WS, et al. Endovascular versus open approach to aortic aneurysm repair surgery: rates of postoperative delirium. Can J Anaesth. 2012;59:556–61. [DOI] [PubMed] [Google Scholar]
- [28].Drews JD, Patel HJ, Williams DM, et al. The impact of acute renal failure on early and late outcomes after thoracic aortic endovascular repair. Ann Thorac Surg. 2014;97:2027–33; discussion 2033. [DOI] [PubMed] [Google Scholar]
- [29].Piffaretti G, Mariscalco G, Bonardelli S, et al. Predictors and outcomes of acute kidney injury after thoracic aortic endograft repair. J Vasc Surg. 2012;56:1527–34. [DOI] [PubMed] [Google Scholar]
- [30].Velázquez OC, Carpenter JP, Baum RA, et al. Perigraft air, fever, and leukocytosis after endovascular repair of abdominal aortic aneurysms. Am J Surg. 1999;178:185–9. [DOI] [PubMed] [Google Scholar]
- [31].Chang CK, Chuter TA, Niemann CU, et al. Systemic inflammation, coagulopathy, and acute renal insufficiency following endovascular thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009;49:1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Soejima T, Mizunoya K, Izumi Y, et al. Clinical features and significance of leukopenia occurring immediately after endovascular surgery. J Anesth. 2022;36:144–51. [DOI] [PubMed] [Google Scholar]