PURPOSE
There is an unmet need for therapeutic options that prolong survival for patients with heavily pretreated, metastatic castration-resistant prostate cancer (mCRPC). The phase III, open-label KEYLYNK-010 study evaluated pembrolizumab plus olaparib versus a next-generation hormonal agent (NHA) for biomarker-unselected, previously treated mCRPC.
METHODS
Eligible participants had mCRPC that progressed on or after abiraterone or enzalutamide (but not both) and docetaxel. Participants were randomly assigned (2:1) to pembrolizumab plus olaparib or NHA (abiraterone or enzalutamide). The dual primary end points were radiographic progression-free survival (rPFS) by blinded independent central review per Prostate Cancer Working Group–modified RECIST 1.1 and overall survival (OS). Time to first subsequent therapy (TFST) was a key secondary end point. Safety and objective response rate (ORR) were secondary end points.
RESULTS
Between May 30, 2019, and July 16, 2021, 529 participants were randomly assigned to pembrolizumab plus olaparib and 264 to NHA. At final rPFS analysis, median rPFS was 4.4 months (95% CI, 4.2 to 6.0) with pembrolizumab plus olaparib and 4.2 months (95% CI, 4.0 to 6.1) with NHA (hazard ratio [HR], 1.02 [95% CI, 0.82 to 1.25]; P = .55). At final OS analysis, median OS was 15.8 months (95% CI, 14.6 to 17.0) and 14.6 months (95% CI, 12.6 to 17.3), respectively (HR, 0.94 [95% CI, 0.77 to 1.14]; P = .26). At final TFST analysis, median TFST was 7.2 months (95% CI, 6.7 to 8.1) versus 5.7 months (95% CI, 5.0 to 7.1), respectively (HR, 0.86 [95% CI, 0.71 to 1.03]). ORR was higher with pembrolizumab plus olaparib versus NHA (16.8% v 5.9%). Grade ≥3 treatment-related adverse events occurred in 34.6% and 9.0% of participants, respectively.
CONCLUSION
Pembrolizumab plus olaparib did not significantly improve rPFS or OS versus NHA in participants with biomarker-unselected, heavily pretreated mCRPC. The study was stopped for futility. No new safety signals occurred.
INTRODUCTION
Next-generation hormonal agents (NHAs) and docetaxel are mainstays of first-line metastatic castration-resistant prostate cancer (mCRPC) setting and in combination with androgen deprivation therapy (ADT) in the metastatic hormone-sensitive prostate cancer (mHSPC) setting.1,2 Therapeutic approaches for biomarker-unselected mCRPC after NHA and docetaxel include NHA switch, cabazitaxel, and recently the radioligand lutetium-177-PSMA-617 (177Lu-prostate-specific membrane antigen [PSMA]-617),1-5 but additional active regimens supported by high levels of evidence are needed.
CONTEXT
Key Objective
Does the combination of pembrolizumab plus olaparib yield better outcomes than a next-generation hormonal agent (NHA) switch for patients with metastatic castration-resistant prostate cancer after receipt of one prior NHA and docetaxel?
Knowledge Generated
Pembrolizumab plus olaparib did not significantly improve radiographic progression-free survival or overall survival versus NHA switch in the randomized, double-blind, phase III KEYLYNK-010 study. Pembrolizumab plus olaparib was associated with more adverse events compared with NHA treatment, but the safety profile of this combination did not suggest additive toxicity.
Relevance (M.A. Carducci)
-
This internationally conducted study is one a recent string of studies reinforcing the challenges associated with the immunosuppressive tumor microenvironment found in prostate cancer. Although discouraging, continued exploration of immune checkpoint inhibitors with other novel agents based on preclinical and clinical studies such as this is needed.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
Immune checkpoint inhibitors demonstrated antitumor activity in certain patients with prostate cancer, although none are recommended by guidelines for biomarker-unselected mCRPC.1,2 The anti-PD-1 antibody pembrolizumab may be used for a subset of patients on the basis of its approvals for the treatment of unresectable or metastatic microsatellite instability-high (MSI-high), mismatch repair deficient, or tumor mutational burden-high (TMB ≥10 mutations/megabase) solid tumors, as determined by a Food and Drug Administration–approved test, that progressed after prior treatment and have no satisfactory alternative options in the United States.
Preclinical models and human studies suggest that prostate cancer has an immunosuppressive tumor microenvironment (TME) with low effector T-cell infiltration.6-8 Aggressive, heavily pretreated, and advanced prostate cancers are associated with increased PD-L1 expression,9-11 and may be susceptible to immunotherapy plus combination partners targeting immunosuppression.12 The poly(ADP-ribose) polymerase (PARP) inhibitor olaparib is approved for the treatment of patients with mCRPC with deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene mutations (in the United States) or BRCA1 or BRCA2 mutations (in the European Union and Japan) that progressed after NHA treatment. The phase III PROpel study reported that the addition of olaparib to first-line abiraterone for mCRPC with or without HRR mutations significantly improved imaging-based progression-free survival (PFS; hazard ratio [HR], 0.66 [95% CI, 0.54 to 0.81]; P < .001); median overall survival (OS) was prolonged by >7 months (maturity, 47.9%; HR, 0.81 [95% CI, 0.67 to 1.00]; P = .054).13,14 Preclinical evidence suggests PARP inhibitors may sensitize tumors to immune checkpoint inhibitor therapy by upregulating tumor cell PD-L1 expression and activating STING-dependent pathways independent of BRCA mutational status.15,16 Pembrolizumab plus olaparib showed antitumor activity and a safety profile consistent with the individual agents in participants with biomarker-unselected, docetaxel-pretreated mCRPC in Cohort A of the phase Ib/II KEYNOTE-365 study.17,18
The randomized, open-label, phase III KEYLYNK-010 (ClinicalTrials.gov identifier: NCT03834519) study evaluated efficacy and safety of pembrolizumab plus olaparib versus NHA switch in participants with biomarker-unselected mCRPC that progressed on abiraterone or enzalutamide (but not both) and docetaxel.
METHODS
Study Design
The KEYLYNK-010 study was conducted at 193 study sites across six regions. The Protocol (online only) and all amendments were approved by the appropriate ethics committees at each center. The study was conducted in accordance with Good Clinical Practice guidelines. An external data monitoring committee (eDMC) oversaw the study and assessed interim results. All participants provided written informed consent.
Eligible participants were male, age 18 years and older, and had histologically or cytologically confirmed mCRPC not preselected for HRR gene alterations that was progressing during continued ADT (serum testosterone <50 ng/dL) as determined by prostate-specific antigen (PSA) levels, radiographically by RECIST 1.1, or radiographically in bone by Prostate Cancer Working Group (PCWG). Participants had an Eastern Cooperative Oncology Group performance status of ≤1, adequate organ function, prior abiraterone acetate (either for mHSPC or mCRPC) or enzalutamide (for mCRPC), but not both, and disease progression during or after prior docetaxel. Tissue and blood samples for exploratory biomarker analysis and genetic testing were collected prospectively at screening. Testing occurred after study initiation (Data Supplement [Methods], online only).
Participants were randomly assigned (2:1), stratified by prior NHA (abiraterone v enzalutamide) and presence of measurable disease at baseline (yes v no), to receive 200 mg pembrolizumab intravenously once every 3 weeks (for ≤35 cycles) plus 300 mg olaparib orally twice daily, or 1,000 mg abiraterone acetate orally once daily plus 5 mg prednisone/prednisolone orally twice daily (if prior enzalutamide) or 160 mg enzalutamide orally once daily (if prior abiraterone). Neither participants nor investigators were blinded to treatment assignment. Participants discontinued study treatment upon request at any time for any reason, or because of verified radiographic disease progression, intercurrent illness, prolonged treatment interruption, unacceptable toxicity, investigator's decision to discontinue therapy, protocol noncompliance, or completion of 35 cycles of pembrolizumab. Pembrolizumab interruptions or discontinuations were permitted. Twice-daily olaparib could be dose-reduced to 250 mg, and then to 200 mg, followed by discontinuation. Re-escalation of the olaparib dose was not permitted.
Efficacy and safety assessment methods are provided in the Data Supplement.
End Points
Dual primary end points were radiographic PFS (rPFS) per PCWG-modified RECIST 1.1 by blinded independent central review (BICR) and OS (definitions provided in the Data Supplement). Time to first subsequent therapy (TFST) was a key secondary end point. Secondary end points included objective response rate (ORR) and duration of response (DOR) per PCWG-modified RECIST 1.1 by BICR, time to PSA progression, and safety.
Statistical Analysis
Efficacy was assessed in all randomly assigned participants (intention-to-treat [ITT] population). Safety was assessed in all randomly assigned participants who received ≥1 dose of study treatment (as-treated population). Event rates over time for rPFS, OS, TFST, and time to PSA progression were estimated by the nonparametric Kaplan-Meier method. HRs and 95% CI were estimated with a Cox regression model (stratified by prior NHA therapy [abiraterone v enzalutamide] and measurable disease at baseline [yes v no]) with Efron's method of tie handling and treatment group as the single covariate. Between-treatment differences were evaluated using a log-rank test with the same stratification factors. Planned study sample size was approximately 780 participants (approximately 520 to pembrolizumab plus olaparib and approximately 260 to NHA). The study had approximately 90% power to detect superior OS with pembrolizumab plus olaparib over NHA with HR = 0.725 with approximately 482 OS events at an initial overall α = .02 (one-sided), and approximately 90% power to detect superior rPFS (HR, 0.65) with approximately 360 rPFS events at an initial overall α = .005 (one-sided). The overall type-I error rate was strongly controlled at 2.5% (one-sided) using the Maurer and Bretz graphical method,19 with 2.0% and 0.5% allocated to test OS and rPFS, respectively. No initial α was allocated to TFST, planned to be tested only if the rPFS null hypothesis was rejected. Statistical testing for rPFS was conducted at the first protocol-specified interim analysis (IA1), planned to occur after ≥360 rPFS events and ≥241 deaths (Data Supplement [Table S1]). This study was stopped for futility after the second interim analysis (IA2) per the eDMC and final testing for OS occurred at IA2 after ≥386 OS events. The P value boundary for significance was .005 (one-sided) for rPFS and .0093 (one-sided) for OS.
SAS software, version 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses. The full statistical analysis plan is provided in the protocol (Data Supplement).
RESULTS
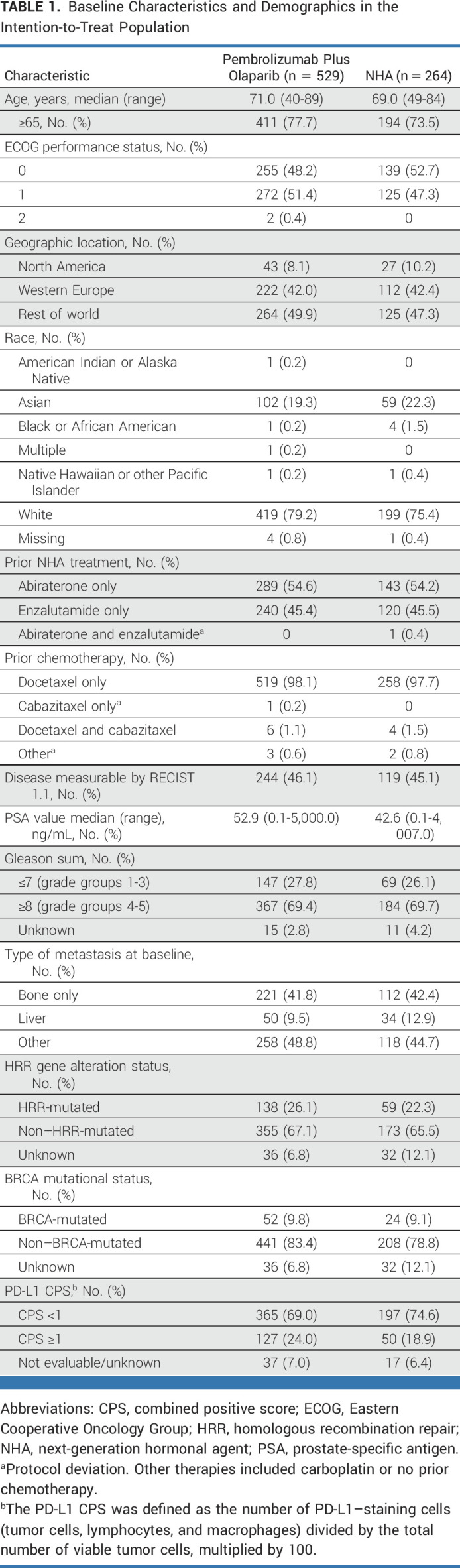
Between May 30, 2019, and July 16, 2021, 793 participants were randomly assigned to receive pembrolizumab plus olaparib (n = 529) or NHA (n = 264; Fig 1). Baseline characteristics were balanced between arms (Table 1). HRR mutations and BRCA mutations were detected in 138 (26.1%) and 52 (9.8%) participants in the pembrolizumab plus olaparib arm and in 59 (22.3%) and 24 (9.1%) participants in the NHA arm, respectively. One hundred twenty-seven (24.0%) and 50 (18.9%) participants in each arm, respectively, had mCRPC with PD-L1 combined positive score (CPS) ≥1. Among 316 participants with evaluable samples, 6 (1.9%) had MSI-high status; no further analysis was performed on this subgroup. The median time from random assignment to the data cutoff date (July 19, 2021) was 12.7 months (range, 0.1-25.7) at IA1, and 18.7 months (range, 6.1-31.7) at IA2 (data cutoff date, January 18, 2022). The median number of doses of pembrolizumab was 7.0 (range, 1-35). Median average daily doses of olaparib, abiraterone, or enzalutamide were 595.7 mg (range, 212-600), 1,000.0 mg (range, 643-1,200), and 160.0 mg (range, 96-160), respectively. Among the ITT population at IA2, 272 (51.4%) participants in the pembrolizumab plus olaparib arm and 146 (55.3%) participants in the NHA arm had received subsequent anticancer therapy (Data Supplement [Tables S2A and S2B]), most commonly cabazitaxel (n = 197 [37.2%] and n = 108 [40.9%], respectively).
FIG 1.
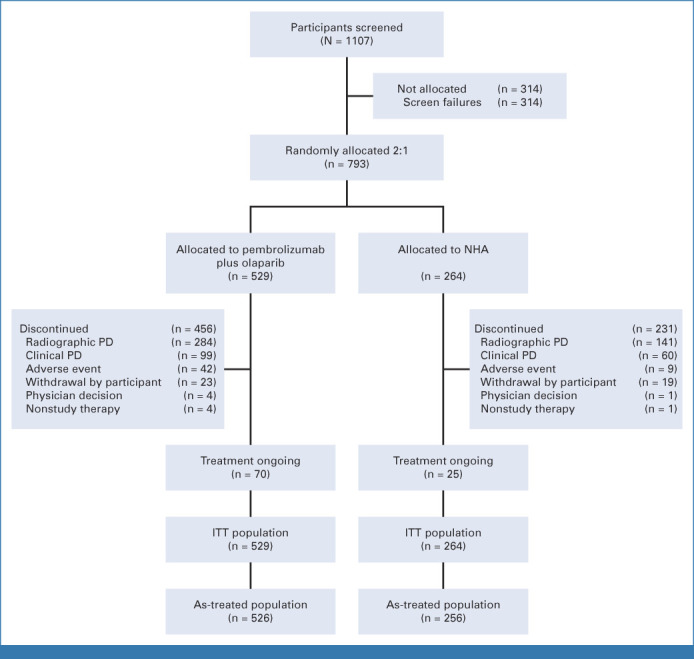
CONSORT diagram. ITT, intention-to-treat; NHA, next-generation hormonal agent; PD, progressive disease.
TABLE 1.
Baseline Characteristics and Demographics in the Intention-to-Treat Population
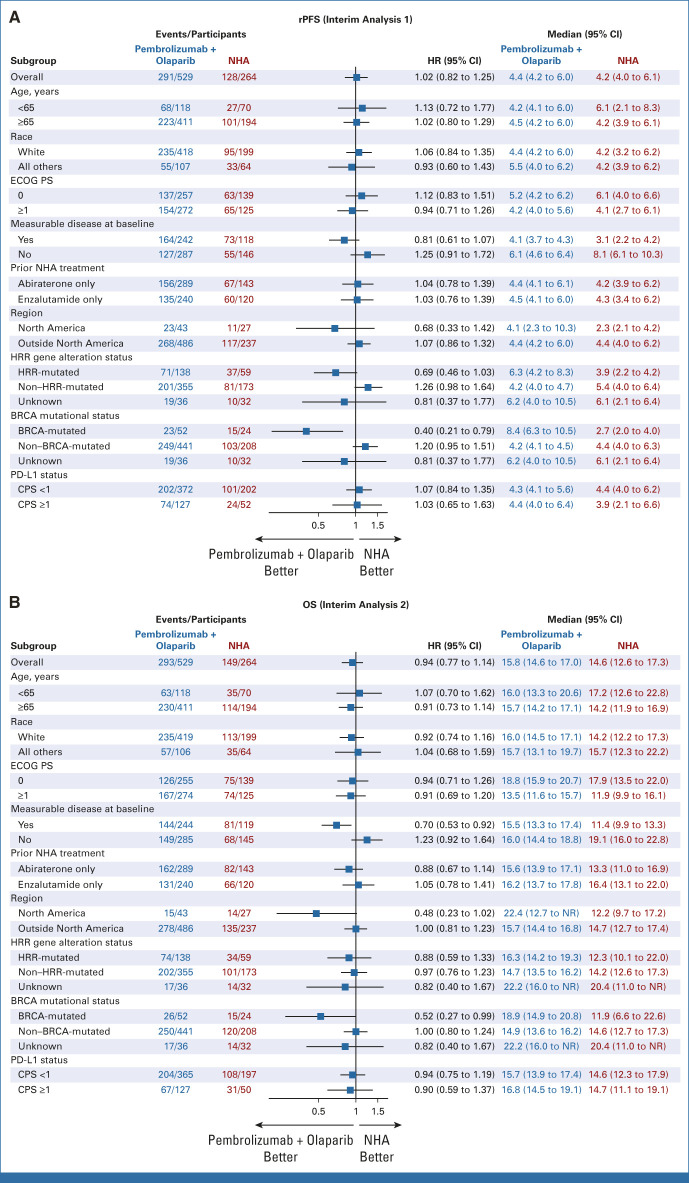
At IA1, 291 (55.0%) rPFS events had occurred in the pembrolizumab plus olaparib arm and 128 (48.5%) had occurred in the NHA arm. Median rPFS was 4.4 months (95% CI, 4.2 to 6.0) with pembrolizumab plus olaparib and 4.2 months (95% CI, 4.0 to 6.1) with NHA (HR, 1.02 [95% CI, 0.82 to 1.25]; P = .5544; Fig 2A). Estimated rPFS rate at 12 months was 16.3% (95% CI, 11.8 to 21.3) and 21.1% (95% CI, 14.1 to 29.1), respectively. Similar results were observed at IA2 (median rPFS, 4.6 months [95% CI, 4.2 to 6.0] v 4.2 months [95% CI, 4.0 to 6.1]; HR, 0.96, 95% CI, 0.79 to 1.16; Data Supplement [Fig S1A]). After 293 (55.4%) deaths in the pembrolizumab plus olaparib arm and 149 (56.4%) deaths in the NHA arm at IA2, the median OS was 15.8 months (95% CI, 14.6 to 17.0) versus 14.6 months (95% CI, 12.6 to 17.3; HR, 0.94 [95% CI, 0.77 to 1.14]; P = .2616; Fig 2B). Estimated OS rate at 12 months was 64.3% (95% CI, 59.9 to 68.5) versus 59.4% (95% CI, 52.9 to 65.4), respectively. Subgroup analyses of rPFS at IA1 and OS at IA2 are shown in Figures 3A and 3B. rPFS and OS results were generally consistent across subgroups, including PD-L1 status, with HRs for rPFS and OS of 1.07 (95% CI, 0.84 to 1.35) and 0.94 (95% CI, 0.75 to 1.19) in participants with CPS <1 and 1.03 (95% CI, 0.65 to 1.63) and 0.90 (95% CI, 0.59 to 1.37) in participants with CPS ≥1, respectively. Among participants with HRR mutations, the HRs for rPFS and OS were 0.69 (95% CI, 0.46 to 1.03) and 0.88 (95% CI, 0.59 to 1.33), respectively. Among participants with BRCA mutations, the HRs for rPFS and OS were 0.40 (95% CI, 0.21 to 0.79) and 0.52 (95% CI, 0.27 to 0.99), respectively.
FIG 2.
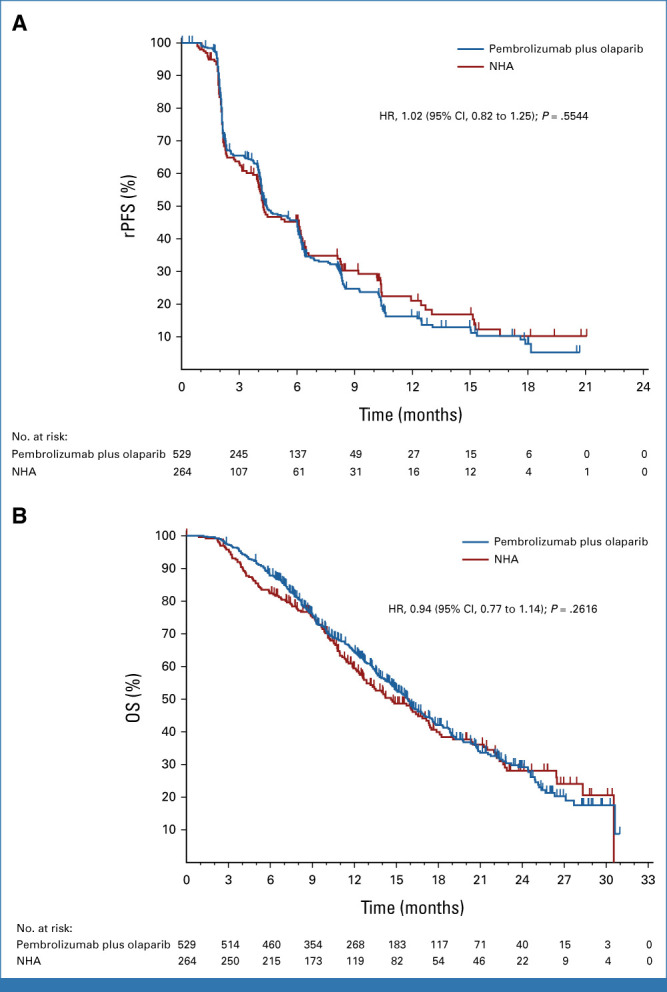
rPFS and OS in the intention-to-treat population. Kaplan-Meier estimates of (A) rPFS per PCWG-modified RECIST 1.1 by blinded independent central review at the first interim analysis and (B) OS at the second interim analysis in the trial groups. Tick marks indicate censored observations. HR, hazard ratio; NHA, next-generation hormonal agent; OS, overall survival; PCWG, Prostate Cancer Working Group; rPFS, radiographic progression-free survival.
FIG 3.
Subgroup analysis of rPFS and OS in the intention-to-treat population. Analysis of (A) rPFS per PCWG-modified RECIST 1.1 by blinded independent central review at the first interim analysis and (B) OS at the second interim analysis in key prespecified subgroups. CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; HRR, homologous recombination repair; NHA, next-generation hormonal agent; NR, not reached; OS, overall survival; PCWG, Prostate Cancer Working Group; PS, performance status; rPFS, radiographic progression-free survival.
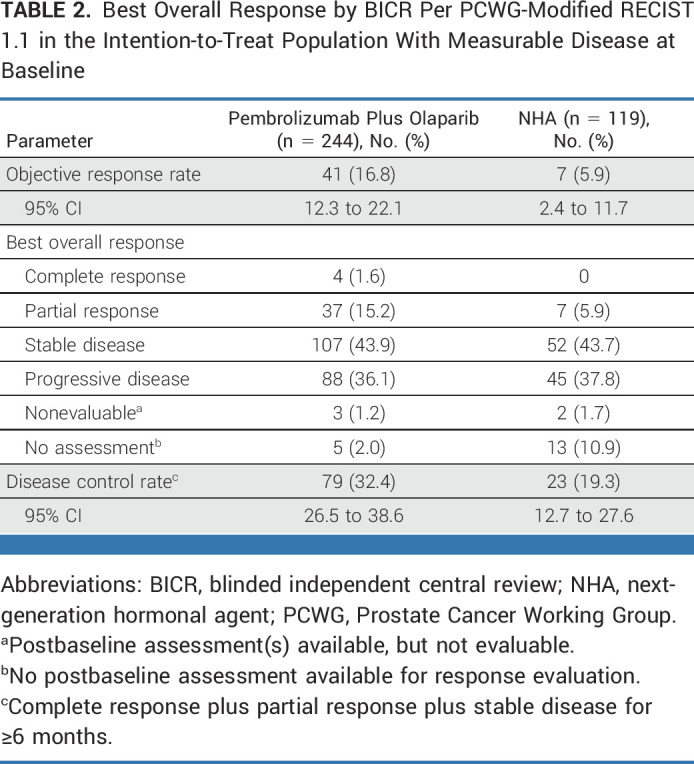
At IA1, 329 (62.2%) and 169 (64.0%) TFST events had occurred in the pembrolizumab plus olaparib and NHA arms, respectively. The median TFST was 7.2 months (95% CI, 6.7 to 8.1) versus 5.7 months (95% CI, 5.0 to 7.1), respectively (HR, 0.86 [95% CI, 0.71 to 1.03]; Fig 4). Since neither the rPFS nor OS dual primary end points were met, TFST was not formally statistically tested. Estimated TFST at 12 months was 28.8% (95% CI, 24.7 to 32.9) with pembrolizumab plus olaparib and 26.8% (95% CI, 21.4 to 32.6) with NHA at IA2. Two hundred forty-four (46.1%) participants in the pembrolizumab plus olaparib arm and 119 (45.1%) participants in the NHA arm had measurable disease and were evaluable for ORR at IA2. A complete or partial response occurred in 41 and in seven participants, respectively, with an ORR of 16.8% (95% CI, 12.3 to 22.1) and 5.9% (95% CI, 2.4 to 11.7; Table 2). Median DOR was 8.1 months (range, ≥1.9 to ≥24.2) with pembrolizumab plus olaparib and 8.5 months (range, ≥2.0 to 14.7) with NHA. At IA2, median time to PSA progression was 3.3 months (95% CI, 3.0 to 3.5) versus 3.5 months (95% CI, 3.2 to 4.3), respectively (HR, 1.11 [95% CI, 0.89 to 1.38]; Data Supplement [Fig S2]). PSA response (defined as ≥50% decrease in PSA levels from baseline among participants with available baseline PSA) was observed in 83/501 (16.6%; 95% CI, 13.4 to 20.1) and 47/247 (19.0%; 95% CI, 14.3 to 24.5) participants in the pembrolizumab plus olaparib versus NHA arms.
FIG 4.
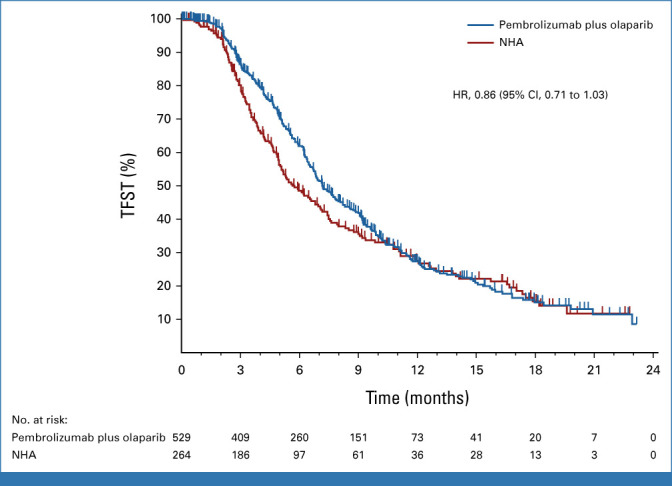
TFST in the intention-to-treat population. Kaplan-Meier estimates of TFST at the first interim analysis in the trial groups. Tick marks indicate censored observations. HR, hazard ratio; NHA, next-generation hormonal agent; TFST, time to first subsequent therapy.
TABLE 2.
Best Overall Response by BICR Per PCWG-Modified RECIST 1.1 in the Intention-to-Treat Population With Measurable Disease at Baseline
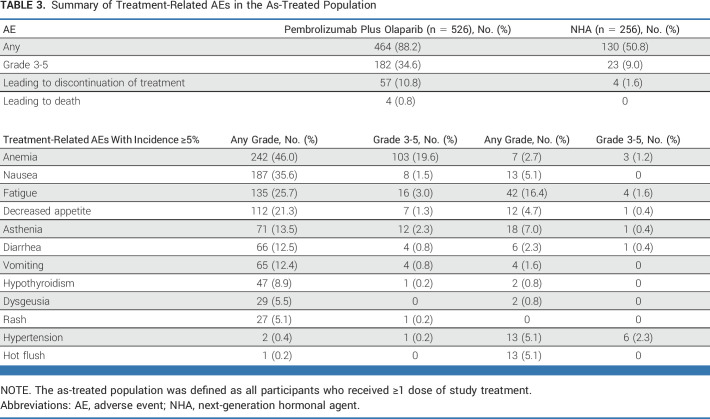
The as-treated population included 526 participants who received ≥1 dose of pembrolizumab plus olaparib and 256 participants who received ≥1 dose of NHA. The median duration (range) of therapy was 5.0 months (0.2-28.9) and 4.1 months (0.4-28.8), respectively. One hundred sixty-four (31.2%) participants had ≥1 olaparib dose reduction; 52 (9.9%) had two reductions. At least one any-grade, any-cause adverse event (AE) occurred in 516 (98.1%) and 237 (92.6%) participants in the pembrolizumab plus olaparib and NHA arms, and grade 3-5 any-cause AEs occurred in 301 (57.2%) and 101 (39.5%) participants, respectively (Table 3). In the pembrolizumab plus olaparib arm, 80 (15.2%) participants discontinued treatment and 21 (4.0%) participants died due to any-cause AEs. In the NHA arm, 9 (3.5%) participants discontinued treatment and 6 (2.3%) died due to any-cause AEs. Reasons for discontinuation for each drug and AEs resulting in death are provided in the Data Supplement ([Tables S3 and S4]). One hundred sixty-eight (31.9%) and 55 (21.5%) participants who received pembrolizumab plus olaparib versus NHA experienced ≥1 any-cause serious AE (Data Supplement [Table S5]), most commonly anemia (n = 18; 3.4%), pneumonia (n = 15; 2.9%), urinary tract infection (n = 10; 1.9%), and adrenal insufficiency (n = 9; 1.7%) with pembrolizumab plus olaparib and urinary tract infection (n = 4; 1.6%), hematuria (n = 4; 1.6%), anemia (n = 3; 1.2%), hyponatremia (n = 3; 1.2%), and pyelonephritis (n = 3; 1.2%) with NHA.
TABLE 3.
Summary of Treatment-Related AEs in the As-Treated Population
A total of 464 (88.2%) participants with pembrolizumab plus olaparib and 130 (50.8%) participants with NHA had ≥1 any-grade treatment-related AE (Table 3). Grade 3-5 treatment-related AEs occurred in 182 (34.6%) participants with pembrolizumab plus olaparib, including four deaths (0.8%; one each due to immune-mediated hepatitis, pneumonia, craniocerebral injury, and renal failure). Grade 3-4 treatment-related AEs occurred in 23 (9.0%) participants with NHA, with no treatment-related deaths.
Any-grade and grade 3-5 immune-mediated AEs occurred, respectively, in 95 (18.1%) and 27 (5.1%) participants with pembrolizumab plus olaparib, including one grade 5 event (hepatitis), and in 14 (5.5%) and 3 (1.2%) participants with NHA (Data Supplement [Table S6]). Seventeen (3.2%) participants in the pembrolizumab plus olaparib arm and none in the NHA arm received high-dose (≥40 mg/d) systemic corticosteroids for immune-mediated AEs and infusion reactions.
DISCUSSION
The phase III KEYLYNK-010 study did not show a statistically significant improvement in rPFS or OS with pembrolizumab plus olaparib versus the active comparator NHA in participants with biomarker-unselected, previously treated mCRPC. The study was stopped for futility after IA2 on the basis of guidance from the eDMC. Although not formally tested per the prespecified multiplicity strategy, TFST analysis suggested that pembrolizumab plus olaparib may delay the need for next-line therapy versus NHA. In line with previous observations,17 pembrolizumab plus olaparib demonstrated antitumor activity, with a higher ORR than NHA in the ITT population with measurable disease. Subgroup analysis suggested a possible rPFS and OS benefit with pembrolizumab plus olaparib versus NHA in these participants. rPFS and OS outcomes were consistent with each regimen irrespective of tumor PD-L1 CPS.
The frequency of HRR gene alterations was 24.8% in the ITT population, consistent with prior observations using similar sequencing methods such as the PROpel (28.4% in the ITT population) and PROfound (28% among screened patients) studies.13,20 BRCA mutations were detected in 9.6% of the ITT population in KEYLYNK-010, similar to the frequency reported in PROpel (BRCA1 mutation rate, 1.5%; BRCA2 mutation rate, 9.2%).13 In KEYLYNK-010, rPFS and OS results favored pembrolizumab plus olaparib over NHA in the subset of participants with BRCA mutations, and similar (although less pronounced) trends were observed in the broader population with all HRR mutations. No formal statistical testing was prespecified or performed for these subgroups and no definitive conclusions could be drawn. This result was consistent with expectations on the basis of the known susceptibility of HRR-mutated and BRCA-mutated prostate cancers to PARP inhibition.13,20
More serious AEs, AEs leading to therapy discontinuation, and treatment-related AEs of any grade and grade ≥3 occurred with pembrolizumab plus olaparib versus NHA. The safety profile of pembrolizumab plus olaparib was consistent with prior observations and no new safety signals were observed.18,20,21
Efficacy findings for pembrolizumab plus olaparib in KEYLYNK-010 were consistent with reports from the biomarker-unselected population in Cohort A of KEYNOTE-365 (median rPFS, 4.5 months [95% CI, 4.0 to 6.5]; median OS, 14 months [95% CI, 10.4 to 18.2]).18 Outcomes for participants in the NHA arm were generally better than expected on the basis of historical observations. Although direct comparisons between trials cannot be made, the randomized, phase IV CARD study investigated cabazitaxel versus NHA switch in a population somewhat similar to KEYLYNK-010, that is, participants with mCRPC that progressed during 12 months of treatment with one prior NHA before or after ≥3 cycles of prior docetaxel. The median imaging-based PFS was 3.7 months (95% CI, 2.8 to 5.1) and median OS was 11.0 months (95% CI, 9.2 to 12.9) with NHA in CARD.5 Cabazitaxel demonstrated promising efficacy in CARD and was the most common subsequent anticancer therapy in KEYLYNK-010. Cabazitaxel's widespread use after NHA failure may partly account for the prolonged survival observed in our study. In the contemporaneous phase III VISION study, participants with PSMA-positive mCRPC with ≥1 prior NHA and 1-2 prior taxane regimens were randomly assigned to standard-of-care versus standard-of-care plus 177Lu-PSMA-617.3 More than 40% of participants had received two prior NHAs and more than 40% had received two prior taxanes. Therefore, the VISION population was more heavily pretreated than the KEYLYNK-010 and CARD populations but had a median imaging-based PFS of 8.7 months and median OS of 15.3 months in the 177Lu-PSMA-617 arm versus 3.4 months and 11.3 months with standard-of-care.3,5 Although few participants in the KEYLYNK-010 study received subsequent radiopharmaceutical therapy (approximately 7% in the pembrolizumab plus olaparib arm and approximately 10% in the NHA arm), the evolving later-line treatment landscape may result in better outcomes for control groups in future mCRPC studies compared with historical controls. These data should prove informative for future trial design assumptions, statistics, and sample size planning.
A potential limitation of the KEYLYNK-010 design was differing treatment practices for the broad population of patients with biomarker-unselected mCRPC across geographic regions. The study was not powered to formally test for ORR superiority in the ITT population or rPFS or OS superiority in subgroups, and some subgroup sizes were small. Biomarker analysis outside the scope of this report may help identify patients who could benefit from immunotherapy plus PARP inhibition.
Although immune checkpoint inhibitors have shown single-agent activity in select patients with prostate cancer, multiple phase III trials of anti-PD-(L)1 antibody monotherapies or combinations have reported negative results. KEYLYNK-010 underscores the challenges of an immunosuppressive prostate TME characterized by myeloid-derived suppressor cells, M2 macrophages, and suppressive cytokines such as transforming growth factor–beta.6-8,22 Combinations that potentially modulate the TME may be a future approach in mCRPC. Adding an anti–T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains antibody to anti-PD-1 therapy could enhance cluster of differentiation 8+ T- and natural killer–cell antitumor activity and alleviate the immunosuppressive effect of tumor-infiltrating regulatory T cells.23-25 Anti-PD-1 antibodies combined with multikinase inhibitors such as cabozantinib or lenvatinib that block myeloid-derived suppressor cells involved in tumor immune evasion are also being explored.26,27 Finally, ongoing efforts to better characterize immunologic parameters and tumoral molecular features may help refine patient selection for immunotherapy.28 With median OS estimates of only 11-15 months, a clear unmet need remains for additional effective treatment options for previously treated mCRPC,3,5 and studies are continually exploring how to best deploy immune checkpoint antibodies in the prostate cancer treatment continuum.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers, all primary investigators and their site personnel; Caryn Hampton of MSD for study support; Hai Yan Wu of MSD for statistical support; Cai Chen and Razvan Cristescu of MSD for genomic analysis support; Scot Ebbinghaus of MSD for study support and critical review; and Ina Nikolaeva of MSD for medical writing assistance.
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon (Inst), Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology (Inst), Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene (Inst), Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, Ismar Health Care, Tempus, Orion, AIkido Pharma, ClinicalMind
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, Ismar Health Care, Medivation, Tempus, Orion, AIkido Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene, Clovis Oncology
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
Se Hoon Park
Honoraria: Merck, Pfizer, Ono Pharmaceutical
Consulting or Advisory Role: Janssen Oncology
Research Funding: Merck Sharp & Dohme LLC (Inst)
Jeffrey C. Goh
Stock and Other Ownership Interests: Immutep, ICON Cancer Care
Honoraria: Ipsen, MSD Oncology
Consulting or Advisory Role: GlaxoSmithKline, MSD, BMS, Janssen Oncology
Speakers' Bureau: Ipsen, Janssen, AstraZeneca/MedImmune, MSD Oncology, Pfizer/EMD Serono
Research Funding: BeiGene (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Travel, Accommodations, Expenses: Pfizer/EMD Serono, Bayer
Jae Lyun Lee
Stock and Other Ownership Interests: Johnson & Johnson/Janssen, Amgen, Merck, Innovent Biologics, Black Diamond Therapeutics, Karyopharm Therapeutics, Zymeworks
Honoraria: Bristol Myers Squibb, AstraZeneca, MSD
Consulting or Advisory Role: Merck, AstraZeneca, Sanofi, Oscotec, Astellas Pharma
Research Funding: Pfizer (Inst), Janssen (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), MSD (Inst), Bayer Schering Pharma (Inst), Seagen (Inst), GI Innovation (Inst), Amgen (Inst), Oscotec (Inst)
Niven Mehra
Consulting or Advisory Role: MSD Oncology (Inst), Janssen-Cilag, Bayer, Astellas Pharma, AstraZeneca
Research Funding: Pfizer (Inst), Janssen-Cilag (Inst), Astellas Pharma (Inst), Sanofi (Inst), Roche/Genentech (Inst), AstraZeneca/Merck (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Roche, Bristol Myers Squibb, MSD Oncology, Janssen-Cilag
Ray McDermott
Honoraria: Bayer, Sanofi, Janssen, Astellas Pharma, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Novartis, Clovis Oncology, Ipsen
Speakers' Bureau: MSD Oncology
Research Funding: Sanofi (Inst), Janssen (Inst), Bayer (Inst), Astellas Pharma (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Travel, Accommodations, Expenses: Pfizer, Janssen-Cilag, Roche, Ipsen
Núria Sala-Gonzalez
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb/Roche
Speakers' Bureau: Ipsen, Astellas Pharma
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Peter C. Fong
Consulting or Advisory Role: MSD
Travel, Accommodations, Expenses: Pfizer
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, MSD, Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo, Sanofi
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo, Sanofi
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Sandoz (Inst), Gilead Sciences (Inst), Roche (Inst), Daiichi Sankyo Europe GmbH (Inst), AbbVie
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Sciences, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Patricio Yanez
Honoraria: MSD (Inst), BMS Chile (Inst), AstraZeneca (Inst), Sanofi/Regeneron (Inst)
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb/Medarex, AstraZeneca
Speakers' Bureau: BMS Chile, AstraZeneca
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Yi-Hsiu Huang
Honoraria: Astellas Pharma, Janssen, Bayer, MSD, Ono Pharmaceutical, Pfizer, Ipsen, Viatris
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Stephen D. Begbie
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, MSD Oncology, Merck Serono, Pfizer
Research Funding: Astellas Medivation (Inst), Merck Serono (Inst), Janssen Oncology (Inst), Roche (Inst), Pfizer/EMD Serono (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Exelixis (Inst)
Rustem Airatovich Gafanov
Honoraria: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol Myers Squibb, Pfizer
Consulting or Advisory Role: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol Myers Squibb, Pfizer
Speakers' Bureau: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol Myers Squibb, Pfizer
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Maria De Santis
Honoraria: Roche/Genentech, Bayer, Novartis, Astellas Pharma, Immunomedics, Amgen, Janssen-Cilag, Ipsen, MSD Oncology, Merck Serono, Merck Sharp & Dohme, Pfizer, Sandoz-Novartis, Basilea, BioClin Therapeutics, Orion, Bristol Myers Squibb, Seagen, Ferring, Sanofi, AstraZeneca/MedImmune, Gilead Sciences, AAA HealthCare, Accord Healthcare, Exelixis/Ipsen
Consulting or Advisory Role: Pierre Fabre, Roche/Genentech, Ipsen, Astellas Pharma, Janssen, GlaxoSmithKline, Takeda, Bristol Myers Squibb, Merck Sharp & Dohme, Bayer, Sanofi, Ferring, Basilea, BioClin Therapeutics, AstraZeneca, BioSyn Healthy Pharma, Sandoz-Novartis, Amgen, Seagen, AAA HealthCare, Accord Healthcare, Novartis, Gilead Sciences, Orion Health
Travel, Accommodations, Expenses: Sanofi, Bayer, Janssen, Ipsen, Roche, Astellas Pharma, Bristol Myers Squibb, Merck Serono, Roche/Genentech
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Eli Rosenbaum
Stock and Other Ownership Interests: Brainsway, Conergent
Consulting or Advisory Role: MSD Oncology, Teva, Astellas Pharma, Bayer, Janssen
Speakers' Bureau: MSD Oncology
Research Funding: MSD Oncology (Inst)
Travel, Accommodations, Expenses: Bayer
Michael P. Kolinsky
Honoraria: Janssen, Bayer, EMD Serono
Consulting or Advisory Role: Janssen, Astellas Pharma, Ipsen, Bristol Myers Squibb, Merck, AstraZeneca, EMD Serono
Research Funding: Janssen (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Felipe Rey
Honoraria: AstraZeneca/MedImmune
Research Funding: AstraZeneca/MedImmune, MSD Oncology
Guilhem Roubaud
Honoraria: Astellas Pharma (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst)
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen-Cilag (Inst), AstraZeneca (Inst), Ipsen (Inst), AAA/Endocyte/Novartis (Inst), Merck/Pfizer (Inst), Bayer (Inst)
Research Funding: Bayer (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ for this study (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag, Bayer
Gero Kramer
Consulting or Advisory Role: Bayer, Janssen, AstraZeneca, MSD Life Science Foundation, Ferring, Astellas Pharma, Novartis, Sandoz-Novartis
Travel, Accommodations, Expenses: Janssen, Bayer
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Hiroyoshi Suzuki
Consulting or Advisory Role: Janssen Research & Development, AstraZeneca, Astellas Pharma, Bayer Yakuhin, Bayer Health, Roche, Sanofi, Nihon Medi-Physics, MSD K.K, Chugai Pharma, Daiichi Sankyo, Lilly, Janssen Oncology
Speakers' Bureau: Takeda, Astellas Pharma, AstraZeneca, Bayer Yakuhin, Sanofi, Janssen, Nippon Shinyaku, Daiichi-Sankyo
Research Funding: Takeda (Inst), Bayer Yakuhin (Inst), Kissei Pharmaceutical (Inst), Nihonkayaku (Inst), Chugai Pharma (Inst), Janssen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Ping Qiu
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Travel, Accommodations, Expenses: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Jinchun Zhang
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (Inst)
Jeri Kim
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Christian H. Poehlein
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Leadership: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Travel, Accommodations, Expenses: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Evan Y. Yu
Consulting or Advisory Role: Janssen, Bayer, Merck, Advanced Accelerator Applications, Oncternal Therapeutics, Aadi Bioscience
Research Funding: Dendreon (Inst), Merck (Inst), Seagen (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Blue Earth Diagnostics (Inst), Bayer (Inst), Lantheus Medical Imaging (Inst), Surface Oncology (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2022 European Society for Medical Oncology Congress, Paris, France, September 9-13, 2022.
SUPPORT
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
CLINICAL TRIAL INFORMATION
NCT03834519 (KEYLYNK-010)
DATA SHARING STATEMENT
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Emmanuel S. Antonarakis, Margitta Retz, Rustem Airatovich Gafanov, Felipe Rey, Ping Qiu, Jinchun Zhang, Jeri Kim, Christian H. Poehlein, Evan Y. Yu
Administrative support: Evan Y. Yu
Provision of study materials or patients: Emmanuel S. Antonarakis, Se Hoon Park, Jeffrey C. Goh, Sang Joon Shin, Jae Lyun Lee, Ray McDermott, Peter C. Fong, Margitta Retz, Yi-Hsiu Huang, Stephen D. Begbie, Rustem Airatovich Gafanov, Maria De Santis, Michael P. Kolinsky, Felipe Rey, Kun-Yuan Chiu, Makoto Sumitomo, Jinchun Zhang, Evan Y. Yu
Collection and assembly of data: Emmanuel S. Antonarakis, Se Hoon Park, Sang Joon Shin, Jae Lyun Lee, Ray McDermott, Peter C. Fong, Yi-Hsiu Huang, Stephen D. Begbie, Rustem Airatovich Gafanov, Maria De Santis, Eli Rosenbaum, Michael P. Kolinsky, Felipe Rey, Kun-Yuan Chiu, Gero Kramer, Makoto Sumitomo, Hiroyoshi Suzuki, Ping Qiu, Jinchun Zhang, Jeri Kim, Christian H. Poehlein, Evan Y. Yu
Data analysis and interpretation: Emmanuel S. Antonarakis, Se Hoon Park, Jeffrey C. Goh, Jae Lyun Lee, Niven Mehra, Ray McDermott, Núria Sala-Gonzalez, Peter C. Fong, Margitta Retz, Juan Pablo Sade, Stephen D. Begbie, Rustem Airatovich Gafanov, Maria De Santis, Michael P. Kolinsky, Felipe Rey, Guilhem Roubaud, Makoto Sumitomo, Francesco Massari, Hiroyoshi Suzuki, Ping Qiu, Jinchun Zhang, Jeri Kim, Christian H. Poehlein, Evan Y. Yu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab Plus Olaparib for Patients With Previously Treated and Biomarker-Unselected Metastatic Castration-Resistant Prostate Cancer: The Randomized, Open-Label, Phase III KEYLYNK-010 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Emmanuel S. Antonarakis
Honoraria: Sanofi, Dendreon (Inst), Medivation, Janssen Biotech, ESSA, Astellas Pharma, Merck, AstraZeneca, Clovis Oncology (Inst), Amgen, Bayer, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Celgene (Inst), Constellation Pharmaceuticals, Curium Pharma, Lilly, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, Ismar Health Care, Tempus, Orion, AIkido Pharma, ClinicalMind
Consulting or Advisory Role: Sanofi, Dendreon, Janssen Biotech, ESSA, Merck, AstraZeneca, Clovis Oncology, Lilly, Bayer, Amgen, Astellas Pharma, Blue Earth Diagnostics, Bristol Myers Squibb/Celgene, Constellation Pharmaceuticals, Curium Pharma, Exact Sciences, Foundation Medicine, GlaxoSmithKline, InVitae, Ismar Health Care, Medivation, Tempus, Orion, AIkido Pharma
Research Funding: Janssen Biotech (Inst), Johnson & Johnson (Inst), Sanofi (Inst), Dendreon (Inst), Aragon Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Genentech (Inst), Novartis (Inst), Astellas Pharma (Inst), Tokai Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Clovis Oncology (Inst), Constellation Pharmaceuticals (Inst), Celgene, Clovis Oncology
Patents, Royalties, Other Intellectual Property: Co-inventor of a biomarker technology that has been licensed to Qiagen
Travel, Accommodations, Expenses: Sanofi, Dendreon, Medivation
Se Hoon Park
Honoraria: Merck, Pfizer, Ono Pharmaceutical
Consulting or Advisory Role: Janssen Oncology
Research Funding: Merck Sharp & Dohme LLC (Inst)
Jeffrey C. Goh
Stock and Other Ownership Interests: Immutep, ICON Cancer Care
Honoraria: Ipsen, MSD Oncology
Consulting or Advisory Role: GlaxoSmithKline, MSD, BMS, Janssen Oncology
Speakers' Bureau: Ipsen, Janssen, AstraZeneca/MedImmune, MSD Oncology, Pfizer/EMD Serono
Research Funding: BeiGene (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Travel, Accommodations, Expenses: Pfizer/EMD Serono, Bayer
Jae Lyun Lee
Stock and Other Ownership Interests: Johnson & Johnson/Janssen, Amgen, Merck, Innovent Biologics, Black Diamond Therapeutics, Karyopharm Therapeutics, Zymeworks
Honoraria: Bristol Myers Squibb, AstraZeneca, MSD
Consulting or Advisory Role: Merck, AstraZeneca, Sanofi, Oscotec, Astellas Pharma
Research Funding: Pfizer (Inst), Janssen (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Roche/Genentech (Inst), AstraZeneca/MedImmune (Inst), MSD (Inst), Bayer Schering Pharma (Inst), Seagen (Inst), GI Innovation (Inst), Amgen (Inst), Oscotec (Inst)
Niven Mehra
Consulting or Advisory Role: MSD Oncology (Inst), Janssen-Cilag, Bayer, Astellas Pharma, AstraZeneca
Research Funding: Pfizer (Inst), Janssen-Cilag (Inst), Astellas Pharma (Inst), Sanofi (Inst), Roche/Genentech (Inst), AstraZeneca/Merck (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Roche, Bristol Myers Squibb, MSD Oncology, Janssen-Cilag
Ray McDermott
Honoraria: Bayer, Sanofi, Janssen, Astellas Pharma, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Novartis, Clovis Oncology, Ipsen
Speakers' Bureau: MSD Oncology
Research Funding: Sanofi (Inst), Janssen (Inst), Bayer (Inst), Astellas Pharma (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Travel, Accommodations, Expenses: Pfizer, Janssen-Cilag, Roche, Ipsen
Núria Sala-Gonzalez
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb/Roche
Speakers' Bureau: Ipsen, Astellas Pharma
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Peter C. Fong
Consulting or Advisory Role: MSD
Travel, Accommodations, Expenses: Pfizer
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Richard Greil
Honoraria: Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol Myers Squibb, MSD, Sandoz, AbbVie, Gilead Sciences, Daiichi Sankyo, Sanofi
Consulting or Advisory Role: Celgene, Novartis, Roche, Bristol Myers Squibb, Takeda, AbbVie, AstraZeneca, Janssen, MSD, Merck, Gilead Sciences, Daiichi Sankyo, Sanofi
Research Funding: Celgene (Inst), Merck (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Sandoz (Inst), Gilead Sciences (Inst), Roche (Inst), Daiichi Sankyo Europe GmbH (Inst), AbbVie
Travel, Accommodations, Expenses: Roche, Amgen, Janssen-Cilag, AstraZeneca, Novartis, MSD, Celgene, Gilead Sciences, Bristol Myers Squibb, AbbVie, Daiichi Sankyo
Patricio Yanez
Honoraria: MSD (Inst), BMS Chile (Inst), AstraZeneca (Inst), Sanofi/Regeneron (Inst)
Consulting or Advisory Role: MSD Oncology, Bristol Myers Squibb/Medarex, AstraZeneca
Speakers' Bureau: BMS Chile, AstraZeneca
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Yi-Hsiu Huang
Honoraria: Astellas Pharma, Janssen, Bayer, MSD, Ono Pharmaceutical, Pfizer, Ipsen, Viatris
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Stephen D. Begbie
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, MSD Oncology, Merck Serono, Pfizer
Research Funding: Astellas Medivation (Inst), Merck Serono (Inst), Janssen Oncology (Inst), Roche (Inst), Pfizer/EMD Serono (Inst), Bristol Myers Squibb (Inst), MSD (Inst), Exelixis (Inst)
Rustem Airatovich Gafanov
Honoraria: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol Myers Squibb, Pfizer
Consulting or Advisory Role: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol Myers Squibb, Pfizer
Speakers' Bureau: Janssen, Astellas Pharma, Bayer, Sanofi, MSD, Bristol Myers Squibb, Pfizer
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Maria De Santis
Honoraria: Roche/Genentech, Bayer, Novartis, Astellas Pharma, Immunomedics, Amgen, Janssen-Cilag, Ipsen, MSD Oncology, Merck Serono, Merck Sharp & Dohme, Pfizer, Sandoz-Novartis, Basilea, BioClin Therapeutics, Orion, Bristol Myers Squibb, Seagen, Ferring, Sanofi, AstraZeneca/MedImmune, Gilead Sciences, AAA HealthCare, Accord Healthcare, Exelixis/Ipsen
Consulting or Advisory Role: Pierre Fabre, Roche/Genentech, Ipsen, Astellas Pharma, Janssen, GlaxoSmithKline, Takeda, Bristol Myers Squibb, Merck Sharp & Dohme, Bayer, Sanofi, Ferring, Basilea, BioClin Therapeutics, AstraZeneca, BioSyn Healthy Pharma, Sandoz-Novartis, Amgen, Seagen, AAA HealthCare, Accord Healthcare, Novartis, Gilead Sciences, Orion Health
Travel, Accommodations, Expenses: Sanofi, Bayer, Janssen, Ipsen, Roche, Astellas Pharma, Bristol Myers Squibb, Merck Serono, Roche/Genentech
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Eli Rosenbaum
Stock and Other Ownership Interests: Brainsway, Conergent
Consulting or Advisory Role: MSD Oncology, Teva, Astellas Pharma, Bayer, Janssen
Speakers' Bureau: MSD Oncology
Research Funding: MSD Oncology (Inst)
Travel, Accommodations, Expenses: Bayer
Michael P. Kolinsky
Honoraria: Janssen, Bayer, EMD Serono
Consulting or Advisory Role: Janssen, Astellas Pharma, Ipsen, Bristol Myers Squibb, Merck, AstraZeneca, EMD Serono
Research Funding: Janssen (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Felipe Rey
Honoraria: AstraZeneca/MedImmune
Research Funding: AstraZeneca/MedImmune, MSD Oncology
Guilhem Roubaud
Honoraria: Astellas Pharma (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst)
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen-Cilag (Inst), AstraZeneca (Inst), Ipsen (Inst), AAA/Endocyte/Novartis (Inst), Merck/Pfizer (Inst), Bayer (Inst)
Research Funding: Bayer (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ for this study (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag, Bayer
Gero Kramer
Consulting or Advisory Role: Bayer, Janssen, AstraZeneca, MSD Life Science Foundation, Ferring, Astellas Pharma, Novartis, Sandoz-Novartis
Travel, Accommodations, Expenses: Janssen, Bayer
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Hiroyoshi Suzuki
Consulting or Advisory Role: Janssen Research & Development, AstraZeneca, Astellas Pharma, Bayer Yakuhin, Bayer Health, Roche, Sanofi, Nihon Medi-Physics, MSD K.K, Chugai Pharma, Daiichi Sankyo, Lilly, Janssen Oncology
Speakers' Bureau: Takeda, Astellas Pharma, AstraZeneca, Bayer Yakuhin, Sanofi, Janssen, Nippon Shinyaku, Daiichi-Sankyo
Research Funding: Takeda (Inst), Bayer Yakuhin (Inst), Kissei Pharmaceutical (Inst), Nihonkayaku (Inst), Chugai Pharma (Inst), Janssen (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
Ping Qiu
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Travel, Accommodations, Expenses: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Jinchun Zhang
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Research Funding: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (Inst)
Jeri Kim
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Christian H. Poehlein
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Leadership: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Stock and Other Ownership Interests: Merck & Co Inc
Travel, Accommodations, Expenses: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc
Evan Y. Yu
Consulting or Advisory Role: Janssen, Bayer, Merck, Advanced Accelerator Applications, Oncternal Therapeutics, Aadi Bioscience
Research Funding: Dendreon (Inst), Merck (Inst), Seagen (Inst), Daiichi Sankyo (Inst), Taiho Pharmaceutical (Inst), Blue Earth Diagnostics (Inst), Bayer (Inst), Lantheus Medical Imaging (Inst), Surface Oncology (Inst), Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Prostate cancer (version 1.2023). https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459
- 2.Parker C, Castro E, Fizazi K, et al. : Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1119-1134, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Sartor O, de Bono J, Chi KN, et al. : Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 385:1091-1103, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George DJ, Sartor O, Miller K, et al. : Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer 18:284-294, 2020 [DOI] [PubMed] [Google Scholar]
- 5.de Wit R, de Bono J, Sternberg CN, et al. : Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med 381:2506-2518, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Jayaprakash P, Ai M, Liu A, et al. : Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest 128:5137-5149, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sfanos KS, Bruno TC, Maris CH, et al. : Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 14:3254-3261, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasero C, Gravis G, Guerin M, et al. : Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res 76:2153-2165, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Gevensleben H, Dietrich D, Golletz C, et al. : The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin Cancer Res 22:1969-1977, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Bishop JL, Sio A, Angeles A, et al. : PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 6:234-242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haffner MC, Guner G, Taheri D, et al. : Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am J Pathol 188:1478-1485, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahmy O, Alhakamy NA, Khairul-Asri MG, et al. : Oncological response and predictive biomarkers for the checkpoint inhibitors in castration-resistant metastatic prostate cancer: A systematic review and meta-analysis. J Pers Med 12:8, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. : Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 10.1056/EVIDoa2200043 [DOI] [PubMed] [Google Scholar]
- 14.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. : Final overall survival (OS) in PROpel: Abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 41, 2023. (suppl 6; abstr LBA16) [Google Scholar]
- 15.Jiao S, Xia W, Yamaguchi H, et al. : PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 23:3711-3720, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen J, Zhao W, Ju Z, et al. : PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res 79:311-319, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu EY, Piulats JM, Gravis G, et al. : 612P Pembrolizumab (pembro) plus olaparib in patients with docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC): Update of KEYNOTE-365 cohort A with a minimum of 11 months of follow-up for all patients. Ann Oncol 32:S652-S653, 2021 [Google Scholar]
- 18.Yu EY, Piulats JM, Gravis G, et al. : Pembrolizumab plus olaparib in patients with metastatic castration-resistant prostate cancer: Long-term results from the phase 1b/2 KEYNOTE-365 cohort A study. Eur Urol 83:15-26, 2022 [DOI] [PubMed] [Google Scholar]
- 19.Maurer W, Glimm E, Bretz F: Multiple and repeated testing of primary, coprimary, and secondary hypotheses. Stat Biopharm Res 3:336-352, 2011 [Google Scholar]
- 20.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Antonarakis ES, Piulats JM, Gross-Goupil M, et al. : Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol 38:395-405, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majidpoor J, Mortezaee K: The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol 226:108707, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Banta KL, Xu X, Chitre AS, et al. : Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8(+) T cell responses. Immunity 55:512-526.e9, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu J, Maurice-Dror C, Lee DH, et al. : First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann Oncol 33:169-180, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Kurtulus S, Sakuishi K, Ngiow SF, et al. : TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest 125:4053-4062, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Lu X, Horner JW, Paul E, et al. : Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543:728-732, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu M, Zhang X, Gao X, et al. : Lenvatinib enhances T cell immunity and the efficacy of adoptive chimeric antigen receptor-modified T cells by decreasing myeloid-derived suppressor cells in cancer. Pharmacol Res 174:105829, 2021 [DOI] [PubMed] [Google Scholar]
- 28.Sena LA, Denmeade SR, Antonarakis ES: Targeting the spectrum of immune checkpoints in prostate cancer. Expert Rev Clin Pharmacol 14:1253-1266, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.