PURPOSE
Resectable non–small-cell lung cancer (NSCLC) with a high probability of mediastinal nodal involvement requires mediastinal staging by endosonography and, in the absence of nodal metastases, confirmatory mediastinoscopy according to current guidelines. However, randomized data regarding immediate lung tumor resection after systematic endosonography versus additional confirmatory mediastinoscopy before resection are lacking.
METHODS
Patients with (suspected) resectable NSCLC and an indication for mediastinal staging after negative systematic endosonography were randomly assigned to immediate lung tumor resection or confirmatory mediastinoscopy followed by tumor resection. The primary outcome in this noninferiority trial (noninferiority margin of 8% that previously showed to not compromise survival, Pnoninferior < .0250) was the presence of unforeseen N2 disease after tumor resection with lymph node dissection. Secondary outcomes were 30-day major morbidity and mortality.
RESULTS
Between July 17, 2017, and October 5, 2020, 360 patients were randomly assigned, 178 to immediate lung tumor resection (seven dropouts) and 182 to confirmatory mediastinoscopy first (seven dropouts before and six after mediastinoscopy). Mediastinoscopy detected metastases in 8.0% (14/175; 95% CI, 4.8 to 13.0) of patients. Unforeseen N2 rate after immediate resection (8.8%) was noninferior compared with mediastinoscopy first (7.7%) in both intention-to-treat (Δ, 1.03%; UL 95% CIΔ, 7.2%; Pnoninferior = .0144) and per-protocol analyses (Δ, 0.83%; UL 95% CIΔ, 7.3%; Pnoninferior = .0157). Major morbidity and 30-day mortality was 12.9% after immediate resection versus 15.4% after mediastinoscopy first (P = .4940).
CONCLUSION
On the basis of our chosen noninferiority margin in the rate of unforeseen N2, confirmatory mediastinoscopy after negative systematic endosonography can be omitted in patients with resectable NSCLC and an indication for mediastinal staging.
INTRODUCTION
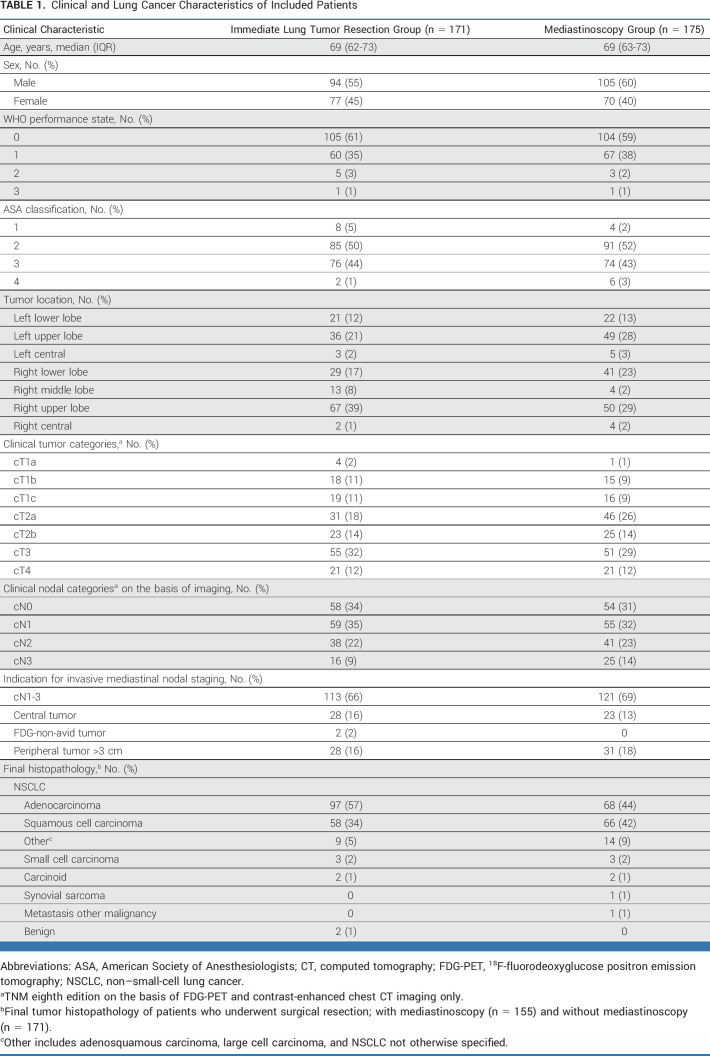
Lung cancer is one of the most frequently diagnosed cancers and accounts for 19% of cancer deaths worldwide.1 Primary clinical staging includes computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). Potential surgical candidates with suspicious hilar and/or mediastinal lymph nodes on imaging (cN1-3), or a centrally located, fluorodeoxyglucose (FDG)-non-avid or large (>3 cm) peripherally located tumor are recommended to undergo invasive mediastinal nodal staging before surgical resection.2 Of all surgically treated patients, 68% have a preoperative indication for invasive mediastinal staging.3
CONTEXT
Key Objective
Despite guideline recommendations, the value of confirmatory mediastinoscopy after tumor-negative endosonography as part of mediastinal staging is under debate in patients with resectable non–small-cell lung cancer and a high probability of mediastinal nodal involvement. The effect of omitting confirmatory mediastinoscopy on relevant clinical outcomes has never been evaluated in a randomized setting. To our knowledge, this study is the first to report randomized data on omitting mediastinoscopy after negative systematic endosonography.
Knowledge Generated
The omission of confirmatory mediastinoscopy and proceeding to immediate lung tumor resection demonstrated an unforeseen N2 rate after definite surgical lung tumor resection of 8.8%. Despite a mediastinal lymph node metastasis detection rate of 8.0% by mediastinoscopy in the control group, the unforeseen N2 rate after immediate resection did not exceed the predefined noninferiority boundary, thereby providing evidence of the redundancy of confirmatory mediastinoscopy.
Relevance
Implementation of the current findings prevents patients from morbidity of confirmatory mediastinoscopy, it reduces the lung cancer staging period, and it probably saves health care costs.
The ASTER trial demonstrated a 79% sensitivity for videomediastinoscopy to detect nodal metastases compared with 85% for endosonography. Confirmatory mediastinoscopy after negative endosonography increased the sensitivity to 94%.4 Guidelines therefore recommend confirmatory mediastinoscopy after cN0-1 endosonography in patients with cN1-3, while it should be considered in patients with centrally located, FDG-non-avid or peripheral tumors >3 cm2,5,6
After publication of the ASTER trial, the use of endosonography (either alone or combined with confirmatory mediastinoscopy) increased, whereas the use of mediastinoscopy alone decreased.3,7,8 The role of confirmatory mediastinoscopy is under debate owing to its limited nodal metastasis detection rate, associated morbidity, and delay in start of lung cancer treatment.3,9,10 Randomized data regarding immediate lung tumor resection after endosonography versus additional confirmatory mediastinoscopy are lacking.8,9
Omitting confirmatory mediastinoscopy after negative endosonography will probably lower the diagnostic sensitivity and increase undesirable unforeseen N2 (uN2) after surgery. The MEDIASTrial (Netherlands Trial Register NL6344) assesses whether omitting mediastinoscopy leads to an unacceptable increase in uN2 rate, on the basis of a clinically determined noninferiority limit, to allow potential improvements in morbidity, quality of life, and health economics.
METHODS
Trial Design
The study Protocol of the MEDIASTrial has previously been published and was conducted as a randomized controlled noninferiority trial at 23 hospitals in the Netherlands and Belgium.11 Our hypothesis was that omitting mediastinoscopy leads to a higher uN2 rate at final surgical resection (ie, our primary research question to test for noninferiority), but inversely reduces morbidity, improves quality of life, and reduces costs (ie, our secondary research question).
Participants
Consecutive patients with proven or suspected, resectable non–small-cell lung cancer (NSCLC) without distant metastasis, with centrally located, FDG-non-avid or large (>3 cm) peripherally located tumors or cN1-3 on imaging were enrolled. Imaging consisted of CT and FDG-PET in all patients. A systematic endosonographic assessment of nodal stations 4R-7-4L and additionally all CT-enlarged (>10 mm) and/or FDG-avid (standardized uptake value [SUV] >2.5) mediastinal nodal stations with tumor-negative cytology of N2-3 stations was mandatory for inclusion. In case of nodes with unsuspicious appearance on endosonography (<8 mm, oval shape, vague borders, and absence of hypoechoic texture), samples were not obligatory since node size <8 mm has shown to be a clinically feasible cutoff.12 Patients with suspected metastases to stations 5/6 were eligible for inclusion. Extended invasive staging of station 5/6 (through parasternal mediastinotomy or video-assisted thoracoscopic surgery) should have been performed if nodal spread to these stations would change treatment strategy according to the local multidisciplinary board. Exclusion criteria were neoadjuvant treatment, unresectable tumor (judged by a thoracic surgeon), contraindications for mediastinoscopy or lung resection (insufficient cardiopulmonary function), noncorrectable coagulopathy, age <18 years, inability to consent, or bulky cN2-3 disease. Also, patients with highly suspicious mediastinal lymph nodes (SUV >5 and at least three endosonographic malignant criteria [mentioned above]) but out of reach for conventional surgical resection (cervical or contralateral nodal stations) were not eligible for inclusion.11 Written informed consent was obtained from all patients.
Random Assignment
Patients were 1:1 assigned to undergo either immediate lung tumor resection and lymph node dissection (immediate lung tumor resection group) or confirmatory mediastinoscopy first followed by lung tumor resection in the absence of nodal metastases (mediastinoscopy group). Because of the invasive nature of mediastinoscopy, blinding was not possible. Stratification was performed per age group (≤66 years and >66 years) and type of center (academic or nonacademic) to minimize bias in a planned economic evaluation.
Mediastinoscopy
Mediastinoscopy consisted of a cervical videomediastinoscopy with sampling of nodal stations 4R-7-4L in accordance with the ESTS guideline, as well as station 2R for right-sided tumors according the Dutch guideline.13 Sampling station 2L in left-sided tumors was encouraged but not mandatory because of risks for recurrent laryngeal nerve palsy. Sampling consisted of at least four surgical biopsies (biopsy forceps ≥5 mm) or an entire lymph node per station. Frozen sections were not routinely performed on mediastinoscopy biopsies.
Lung Tumor Resection
Lung tumor resection consisted of an anatomic resection and dissection of at least three mediastinal stations (including the subcarinal station) according to international guidelines.14,15
Outcomes
The primary outcome was the presence of uN2 in the immediate resection group versus the mediastinoscopy group. The uN2 rate was calculated by dividing the number of patients with pathologically proven N2 resulting from lymph node dissection, not detected by endosonography or mediastinoscopy, by the total number of patients undergoing lymph node dissection. Histopathology was performed conform international guidelines and pathologists were unaware that patients participated in a trial.16 Exploratory subgroup analyzes were performed for the different indications for invasive staging. uN2 cases were categorized having single-level or multilevel nodal station uN2 and being detection errors (not detected by imaging, endosonography or mediastinoscopy) or sampling errors (benign lymphoid sampling results from endosonography and/or mediastinoscopy). Patients with radiologically suspect station 5/6 not undergoing extended staging in accordance with the multidisciplinary board advise, but with pathologically proven nodal spread to station 5/6 after final lymph node dissection were determined having foreseen N2. Major morbidity and 30-day mortality after mediastinoscopy and surgical resection were secondary outcomes and were scored during hospital stay and outpatient visits. Morbidity was scored according the Clavien-Dindo classification, considering grade I-II as minor and grade III-IV or laryngoscopic proven recurrent laryngeal nerve palsy as major morbidity.17
Trial Quality
This study was performed in accordance with the Declaration of Helsinki, 64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013. The medical ethical committee of Máxima MC approved the study, which was registered in the Netherlands Trial Register on July 6, 2017 (NL6344). The study protocol and statistical analysis plan were published open-access before knowledge of any results of this trial.11,18 On-site monitoring and clinical data collection were performed by independent professionals. Diagnostic and therapeutic procedures were performed by trained pulmonologists and thoracic surgeons, who received feedback on protocol violations that were exposed by study monitors to ensure continuous quality.
Noninferiority Margin and Sample Size
A systematic review being part of the research proposal of this study showed uN2 rates of 6.3% in the mediastinoscopy group versus 6.8% after immediate resection. From the ASTER trial, an uN2 rate as high as 14.3% was calculated in patients undergoing mediastinoscopy alone without compromising 5-year survival.19 On the basis of these numbers, we set the noninferiority margin at 8% (difference between 6.3% and 14.3%), resulting in a sample size of 171 patients in each group to achieve a power of 80% with an alpha error of 0.0250. With an assumed dropout rate of 5%, the aimed sample size was 360 patients.
Statistical Analysis
The complete statistical analysis plan was formerly published open-access.18 Intention-to-treat (ITT) analyses of uN2 were performed, in which patients with N2 disease detected by mediastinoscopy were excluded since they did not undergo lymph node dissection that was necessary for uN2 calculation. Unforeseen N2 is usually reported in this manner. All patients with complete mediastinoscopy and lymph node dissection procedures (conform study protocol) were included for the per protocol (PP) uN2 analysis (Fig 1). We calculated 95% CI of proportions using Wilson's approximation,20 while 95% CI for the difference in proportions (95% CIΔ) were calculated using the slightly more conservative Miettinen-Nurminen approximation.21 Noninferiority was concluded if the upper limits of the 95% CIΔ (UL 95% CIΔ) after ITT and PP were smaller than the absolute 8% margin from the observed uN2 rates for the mediastinoscopy group. For the secondary outcomes, we did include patients undergoing mediastinoscopy without subsequent lymph node dissection (because of proven N2 or dropout after mediastinoscopy) to include all morbidity associated with mediastinoscopy. The respective exclusion and inclusion of patients with positive mediastinoscopy in the primary and secondary analyses resulted in different denominators. To assess its effect, we additionally performed a modified uN2 analysis including patients with positive mediastinoscopy in the denominator. The analyses were performed using the Statistical Package for the Social Sciences version 24.0, NCSS Statistical Software 2007,22 and WinPepi version 11.22.23
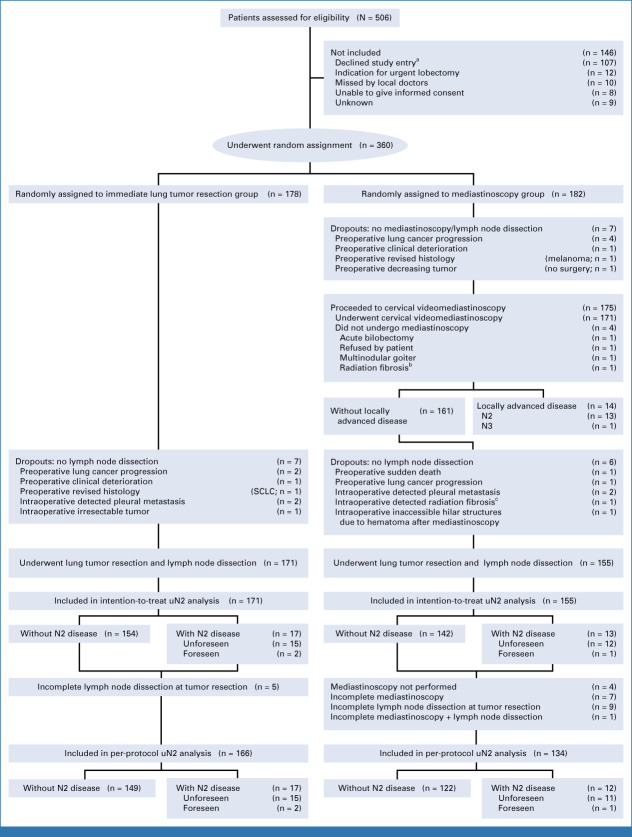
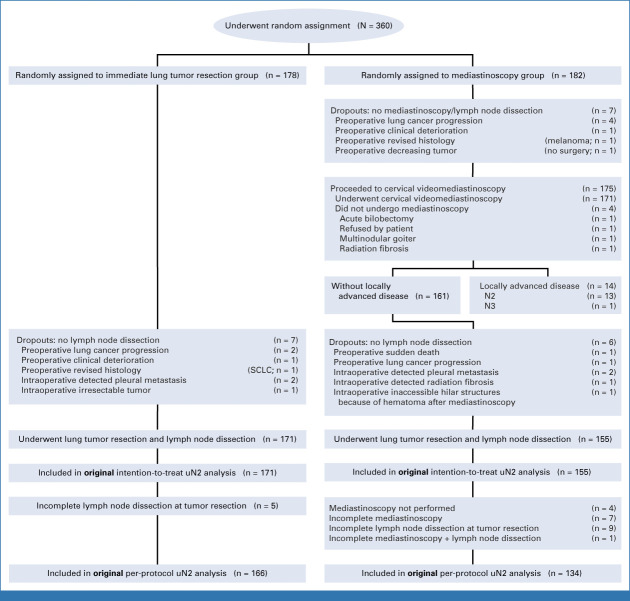
FIG 1.
Enrollment, random assignment, and flow of study patients. N2 = ipsilateral mediastinal lymph node metastasis; N3 = contralateral lymph node metastasis; unforeseen N2 disease/uN2 = pathologically proven N2 disease at lymph node dissection at the time of tumor resection when previous mediastinal staging showed N0 or N1. aMain reasons for declining study entry were objection to clinical trials/randomization and preference for additional staging certainty with mediastinoscopy. bCervical radiation fibrosis from a previous nonpulmonary malignancy. cMediastinal radiation fibrosis from a previous nonpulmonary malignancy. SCLC, small-cell lung cancer.
Role of the Funding Source
The funding sources had no involvement in the study design, data analysis, data interpretation and the decision to submit the article for publication.
RESULTS
Patients
Between July 17, 2017, and October 5, 2020 (mean inclusion period 26 months per center), a total of 360 patients were enrolled; 178 were assigned to immediate lung tumor resection and 182 to mediastinoscopy. The study flowchart including 14 dropouts is presented in Figure 1 and baseline characteristics are presented Table 1.
TABLE 1.
Clinical and Lung Cancer Characteristics of Included Patients
Endosonography
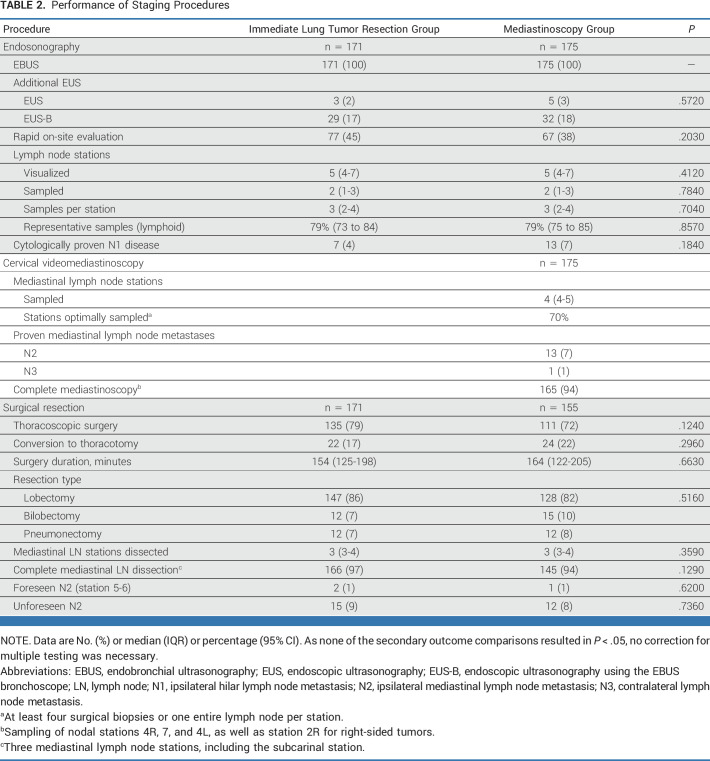
All patients underwent endobronchial ultrasonography (EBUS) conform protocol, added by endoscopic ultrasonography (EUS(B)) in 69 patients (20%). Moderate sedation was used in 186 patients (54%), propofol in 154 (44%), and no sedation in six patients (2%). Per patient, a median of five (IQR, 4-7) nodal stations were visualized, and two (IQR, 1-3) stations were sampled, taking a median of three (IQR, 2-4) samples per station. N1 metastases were cytologically proven in 20 of 346 patients (6%). Endosonography results were similar among groups (Table 2).
TABLE 2.
Performance of Staging Procedures
Mediastinoscopy
Cervical videomediastinoscopy was performed in 171 of 175 patients (98%). After random assignment, one patient refused mediastinoscopy and subsequently underwent lung tumor resection, one patient developed a thoracic empyema before undergoing mediastinoscopy and subsequently underwent emergency bilobectomy, and in two patients mediastinoscopy was prematurely aborted, one because of severe previous radiation effects for a cervical tumor and one because of a multinodular goiter (Fig 1). Mediastinoscopy encompassed a median of four (IQR, 4-5) stations per patient. All designated stations were assessed in 161 of 175 patients (92%; n = 9 missing one station, n = 1 missing two stations, and n = 4 no mediastinoscopy performed). Four surgical biopsies or one entire lymph node was harvested in 70% of stations (Table 2).
Lung Tumor Resection
The mean interval between endosonography and lung tumor resection was 28 days (95% CI, 26 to 30) in the immediate resection group versus 38 days (95% CI, 36 to 41) in the mediastinoscopy group. Six patients without mediastinal metastases at mediastinoscopy did not undergo resection; one suffered a sudden death 10 days after mediastinoscopy (no autopsy), one had progressive lung cancer, two had intraoperatively detected pleural metastases, one had severe mediastinal radiation fibrosis from a previous nonpulmonary malignancy, and in one patient, the hilar structures were inaccessible withholding lobectomy and lymph node dissection because of a severe hematoma after mediastinoscopy. This resulted in 171 operated patients with immediate resection and 155 patients after mediastinoscopy (Fig 1). Mediastinal lymph node dissection harvested a median of three (IQR, 3-4) stations, resulting in complete mediastinal lymph node dissection in 311 of 326 patients (95%). In 14 incomplete procedures, one station was missing, and in one incomplete procedure, three stations were dissected, except the subcarinal station. Lung tumor resection results were similar among groups (Table 2).
Mediastinal Nodal Metastases
The overall prevalence of mediastinal nodal metastases in the entire study population was 12.9% (44/340; 95% CI, 9.8 to 16.9). In the immediate resection group, N2 was postoperatively established in 9.9% (17/171; 95% CI, 6.3 to 15.3) including foreseen N2 in station 5/6 in 1.2% (2/171; 95% CI, 0.3 to 4.2). In the mediastinoscopy group, the rate of N2-3 detected by mediastinoscopy was 8.0% (14/175; 95% CI, 4.8 to 13.0; N2 n = 13; single-level n = 9) corresponding with a number needed to test (NNT) of 12.5 (100/8.0). After mediastinoscopy, the N2 rate among patients undergoing final resection was 8.4% (13/155; 95% CI, 5.0 to 13.8) including foreseen N2 in station 5/6 in 0.7% (1/155; 95% CI, 0.1 to 3.6). Herewith, the overall prevalence of N2-3 in the mediastinoscopy group was 16.0% (27/169; 95% CI, 11.2 to 22.3), higher but not significantly different from the immediate resection group (P = .0970; Table 3).
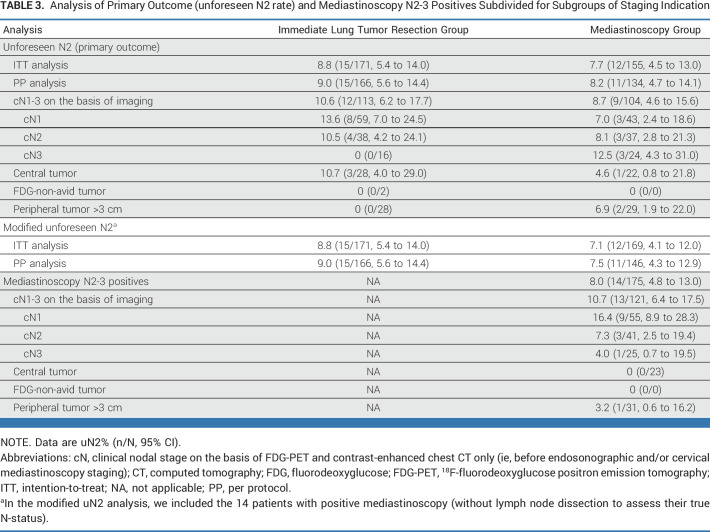
TABLE 3.
Analysis of Primary Outcome (unforeseen N2 rate) and Mediastinoscopy N2-3 Positives Subdivided for Subgroups of Staging Indication
Of the 14 patients with N2-3 detected at mediastinoscopy, nine had radiologic cN1 as indication for staging, corresponding with 16.4% (9/55; 95% CI, 8.9 to 28.3) positive mediastinoscopy results within this cN1 subgroup. After detection of N2-3 at mediastinoscopy, nine patients underwent definite chemoradiation, one received radiotherapy, one best supportive care, and two underwent neoadjuvant chemotherapy followed by lung tumor resection. The last patient had microscopic single-level N2 detected by mediastinoscopy and underwent subsequent lung tumor resection demonstrating no further nodal metastasis.
Unforeseen N2
In the ITT analysis, uN2 was found in 8.8% (15/171; 95% CI, 5.4 to 14.0) in the immediate resection group versus 7.7% (12/155; 95% CI, 4.5 to 13.0) in the mediastinoscopy group (Δ, 1.03%; UL 95% CIΔ, 7.2%; Pnoninferior = .0144). In the PP analysis, uN2 was found in 9.0% (15/166; 95% CI, 5.6 to 14.4) after immediate resection versus 8.2% (11/134; 95% CI, 4.7 to 14.1) with mediastinoscopy (Δ, 0.83%; UL 95% CIΔ, 7.3%; Pnoninferior = .0157). uN2 rates in patients with different indications for mediastinal staging were presented in Table 3. The most remarkable difference in uN2 rate was found among patients with cN1; 13.6% in the immediate resection group versus 7.0% in the mediastinoscopy group. The modified analyses also demonstrated that the upper margin of the difference in uN2 rate felt within the chosen acceptable upper limit favoring the immediate resection strategy (Table 3).
Details of uN2
After immediate resection, uN2 was multilevel in three patients (20%; one intranodal and two extranodal) and single-level in 12 patients (80%; seven intranodal and five extranodal). Eight uN2 cases (53%) were sampling errors (all benign lymphoid), all within reach of cervical mediastinoscopy. Seven uN2s (47%) were detection errors; two were located in the lower mediastinum (station 8 and 9, both no EUS(B)) and one was located in station 5/6.
In the mediastinoscopy group, uN2 was multilevel in one patient (8%; one extranodal) and single-level in 11 patients (92%; seven intranodal and four extranodal). Six (50%) uN2 cases were sampling errors (all benign lymphoid) and six (50%) were detection errors, one in station 9 (no EUS(B)), four in station 5/6, and one in station 3.
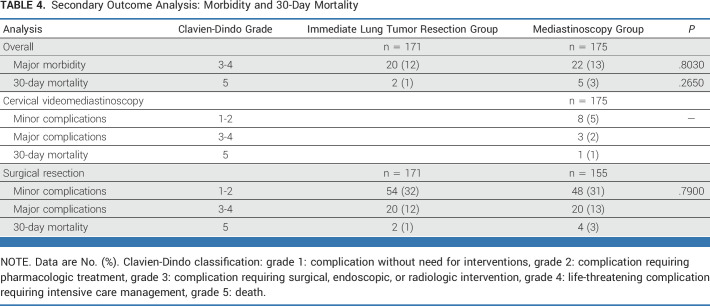
Major Morbidity and 30-Day Mortality
Overall, major morbidity and 30-day mortality was found in 12.9% (22/171; 95% CI, 8.7 to 18.7) after immediate resection versus 15.4% (27/175; 95% CI, 10.8 to 21.5) in the mediastinoscopy group (P = .4940; Table 4). Confirmatory mediastinoscopy resulted in minor complications in eight patients (4.6%) and major complications in three patients (1.7%): one had a surgical site infection requiring surgical drainage, one had a persistent laryngeal recurrent nerve palsy, and one had a postoperative bleeding requiring remediastinoscopy, which resulted in inaccessible hilar structures making lung tumor resection impossible. One patient (0.6%) suffered from a sudden death 10 days after mediastinoscopy, and no autopsy was performed.
TABLE 4.
Secondary Outcome Analysis: Morbidity and 30-Day Mortality
DISCUSSION
This multicenter randomized trial including patients with resectable NSCLC and a negative endosonography demonstrated noninferiority in uN2 for the immediate resection strategy. Confirmatory mediastinoscopy reduced the uN2 rate by only 1.03%, at the expense of 10-day delay for lung tumor resection, morbidity in 6.3% (potentially impeding curative treatment), mortality in 0.6%, and repeat general anesthesia in all patients involved.
A meta-analysis by Sanz-Santos showed an increase in negative predictive value from 79% to 92% by confirmatory mediastinoscopy after negative EBUS, with a NNT of 24.24 The underlying primary research question in our trial therefore was not to assess the inevitable loss in sensitivity by omitting mediastinoscopy, but to determine whether the expected increase in uN2 was within predefined limits. Our premise hereby was that the increase in uN2 will be counterbalanced by a reduction in the drawbacks of confirmatory mediastinoscopy (secondary outcome). When designing this trial, no consensus was available to determine an acceptable loss in sensitivity nor consensus on a combined outcome measure including loss in sensitivity and gain in morbidity. Since uN2 after final lung tumor resection represents the undesirable outcome of mediastinal staging and includes both benefits (nodal spread detection among patients with N2 disease) and potential harms (demonstrating absence of nodal spread among patients without N2 at the cost of morbidity) of confirmatory mediastinoscopy, we decided uN2 to be the most clinically relevant primary outcome measure. Importantly, we were able to determine an acceptable upper noninferiority limit for uN2 rate on the basis of the survival data of the ASTER trial.4,19
Our study demonstrates that confirmatory mediastinoscopy can be omitted in cN2-3 patients, whereas the subgroup of cN1 may deserve special consideration. Most patients with positive mediastinoscopy and uN2 after immediate resection were from the cN1 subgroup. Previous research suggested cN1 patients to be at high risk of uN2 because of a potential lower diagnostic accuracy of endosonography alone.25,26 To overcome this potential lower diagnostic accuracy, Leong demonstrated that with the addition of EUS(B) to EBUS, the sensitivity increased from 49% to 71% in cN0-1 patients.27 Although we demonstrated noninferiority including those cN1 patients in our study, further research and tailored mediastinal management of cN1 patients may still be considered.
The prevalence of mediastinal nodal metastases after negative endosonography in our population was 12.9%, which is in line with literature, although it was nonsignificantly lower in the immediate resection group (9.9%) despite random assignment. This might be explained by left-sided paratracheal metastases that are not accessible by lymph node dissection without mediastinoscopy and a random imbalance of left-sided tumors that have an increased a priori chance of missed metastases in station 5/6 contributing to a higher N2 prevalence after negative endosonography. To test for such possible confounding factors, we performed an unplanned post hoc analysis with a correction for significant randomization imbalances (Appendix 2, online only). The higher rate of mediastinal nodal spread among patients receiving more diagnostic tests was also demonstrated by Sanz-Santos, demonstrating a 19.5% higher N2-3 prevalence in studies performing confirmatory mediastinoscopy.24 Although this meta-analysis showed large heterogeneity, the randomized ASTER trial found a difference of 10% in N2-3 prevalence as well without any effect on survival.4,19
Although management in patients with positive mediastinoscopy changed in 13 of 14 patients, in 92% of patients, confirmatory mediastinoscopy was negative and caused morbidity and treatment delay. In our opinion, the benefits of omitting mediastinoscopy for the entire group outweigh the potential for unnecessary surgical resection in a few, especially since the majority of false-negative endosonographies includes only minimal N2 disease. Single station and microscopic metastases have better survival compared with multiple station and macroscopic uN2.28,29 Moreover, lacking randomized data on this topic, retrospective studies found no survival benefit of neoadjuvant treatment compared with upfront surgery in patients with minimal N2.30,31 We observed that most uN2 cases in our study were single-level intranodal metastases, also after immediate resection. One of the strengths of the MEDIASTrial was the employment of independent data and monitoring specialists as well as upfront publication of protocol and statistical analysis plan. By clear instructions and quality control, we achieved high-quality performance of nearly all procedures. A limitation of our study is that only 20% of patients underwent additional EUS(B). This originates from our protocol prescribing that EUS(B) should preferably be added to EBUS. Combined systematic EBUS and EUS(B) with routine sampling of specified as well as imaging suspect lymph nodes has demonstrated to have additional diagnostic value over only a targeted approach.32,33 Although we already demonstrated noninferiority, addition of EUS(B) may further prevent patients from uN2. Moreover, despite our effort to optimize staging procedures, 13 uN2 metastases still were detection errors. Three were located in station 8/9 and may have been prevented by performing EUS(B), while six were out of reach for both endosonography and mediastinoscopy (station 3/5/6). Finally, as only two patients with FDG-non-avid tumors were included, conclusive statements on this subgroup were forgone.
Our population appears to be representative as two thirds of included patients had imaging suspected lymph nodes, having the highest risk for occult nodal metastases.2 In contrast to the ASTER trial, we performed this multicenter trial in both tertiary and secondary centers in the Netherlands and Belgium. Therefore, our results are widely applicable and expected to be easily implemented.
In conclusion, on the basis of our chosen noninferiority margin in the rate of unforeseen N2, confirmatory mediastinoscopy after negative systematic endosonography can be omitted in patients with resectable NSCLC and an indication for mediastinal staging.
ACKNOWLEDGMENT
The authors thank all patients who participated in the MEDIASTrial and all surgeons, pulmonary physicians, and research nurses who participated in patient recruitment and data collection in the MEDIASTrial. The authors also thank Prof. Dr. A. H. Zwinderman for his contribution to the post hoc statistical analysis. The members of MEDIASTrial study group are listed in Appendix 1 (online only).
APPENDIX 1. Members of MEDIASTrial study group
Nicole E. Papen-Botterhuis: Academy, Máxima MC, Veldhoven, the Netherlands; Maggy Youssef-El Soud: Department of Pulmonary Medicine, Máxima MC, Veldhoven, the Netherlands; Wim J. van Boven: Cardiothoracic Surgery, Amsterdam UMC location University of Amsterdam, the Netherlands; Johannes M.A. Daniels: Pulmonary Medicine, Amsterdam UMC location VU University Medical Center, the Netherlands; David J. Heineman: Cardiothoracic Surgery, Amsterdam UMC location VU University Medical Center, the Netherlands; Harmen R. Zandbergen: Cardiothoracic Surgery, Amsterdam UMC location VU University Medical Center, the Netherlands; Pepijn Brocken: Department of Pulmonary Medicine, HagaZiekenhuis, Den Haag, the Netherlands; Thirza Horn: Department of Surgery, HagaZiekenhuis, Den Haag, the Netherlands; Willem H. Steup: Department of Surgery, HagaZiekenhuis, Den Haag, the Netherlands; Jerry Braun: Department of Cardiothoracic Surgery, Leiden University Medical Center, Leiden, the Netherlands; Rajen S.R.S. Ramai: Department of Pulmonary Medicine, Leiden University Medical Center, Leiden, the Netherlands; Naomi Beck: Dutch Institute for Clinical Auditing, Leiden, the Netherlands; Fieke Hoeijmakers: Dutch Institute for Clinical Auditing, Leiden, the Netherlands; Nicole P. Barlo: Department of Pulmonary Medicine, Northwest Clinics, Alkmaar, the Netherlands; Martijn van Dorp: Department of Surgery, Northwest Clinics, Alkmaar, the Netherlands; W. Hermien Schreurs: Department of Surgery, Northwest Clinics, Alkmaar, the Netherlands; Anne-Marie C. Dingemans: Department of Pulmonary Medicine, Erasmus Medical Center, Rotterdam, the Netherlands; Roy T.M. Sprooten: Department of Pulmonary Medicine, Maastricht University Medical Center, Maastricht, NL; Jos G. Maessen: Department of Cardiothoracic Surgery, Maastricht University Medical Center, Maastricht, NL; Niels J.M. Claessens: Department of Pulmonary Medicine, Rijnstate Hospital, Arnhem, the Netherlands; Jan-Willem H.P. Lardenoije: Department of Surgery, Rijnstate Hospital, Arnhem, the Netherlands; Birgitta I. Hiddinga: Department of Pulmonary Medicine, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; Caroline Van De Wauwer: Department of Cardiothoracic Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; Anthonie J. van der Wekken: Department of Pulmonary Medicine, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands; Wessel E. Hanselaar: Department of Pulmonary Medicine, Franciscus Gasthuis en Vlietland, Rotterdam, the Netherlands; Robert ThJ Kortekaas: Department of Surgery, Franciscus Gasthuis en Vlietland, Rotterdam, the Netherlands; Martin P. Bard: Department of Pulmonary Medicine, Spaarne Gasthuis, Hoofddorp, the Netherlands; Herman Rijna: Department of Surgery, Spaarne Gasthuis, Hoofddorp, the Netherlands; Gerben P. Bootsma: Department of Pulmonary Medicine, Zuyderland Medical Center, Heerlen, the Netherlands; Yvonne L.J. Vissers: Department of Surgery, Zuyderland Medical Center, Heerlen, the Netherlands; Eelco J. Veen: Department of Surgery, Amphia Hospital, Breda, the Netherlands; Cor H. van der Leest: Department of Pulmonary Medicine, Amphia Hospital, Breda, the Netherlands; Emanuel Citgez: Department of Pulmonary Medicine, Medisch Spectrum Twente, Enschede, the Netherlands; Eino B. van Duyn: Department of Surgery, Medisch Spectrum Twente, Enschede, the Netherlands; Geertruid M.H. Marres: Department of Surgery, Albert Schweitzer Hospital, Dordrecht, the Netherlands; Eric R. van Thiel: Department of Pulmonary Medicine, Albert Schweitzer Hospital, Dordrecht, the Netherlands; Paul E. van Schil: Department of Thoracic and Vascular Surgery, Antwerp University Hospital, Antwerp, Belgium; Jan P. van Meerbeeck: MOCA/Thoraxoncology, Antwerp University Hospital, Antwerp, Belgium; Reinier Wener: Department of Pulmonary Medicine, Antwerp University Hospital, Antwerp, Belgium; Niels Smakman: Department of Surgery, Diakonessenhuis, Utrecht, the Netherlands; Femke van der Meer: Department of Pulmonary Medicine, Diakonessenhuis, Utrecht, the Netherlands; Mohammed D. Saboerali: Department of Pulmonary Medicine, Beatrixziekenhuis, Gorinchem, the Netherlands; Anne Marie Bosch: Department of Surgery, Hospital Gelderse Vallei, Ede, the Netherlands; Wouter K. de Jong: Department of Pulmonary Medicine, Hospital Gelderse Vallei, Ede, the Netherlands; Charles C. van Rossem: Department of Surgery, Maasstad Hospital, Rotterdam, the Netherlands; W. Johan Lie: Department of Pulmonary Medicine, Maasstad Hospital, Rotterdam, the Netherlands; Ewout A. Kouwenhoven: Department of Surgery, Hospital Group Twente (ZGT), Almelo, the Netherlands; A. Jeske Staal-van den Brekel: Department of Pulmonary Medicine, Hospital Group Twente (ZGT), Almelo, the Netherlands; Nike M. Hanneman: Department of Surgery, Ikazia Hospital, Rotterdam, the Netherlands; Roxane Heller-Baan: Department of Pulmonary Medicine, Ikazia Hospital, Rotterdam, the Netherlands; Valentin J.J.M. Noyez: Department of Vascular and Thoracic Surgery, Sint-Maarten General Hospital, Mechelen.
APPENDIX 2. Post Hoc Analysis
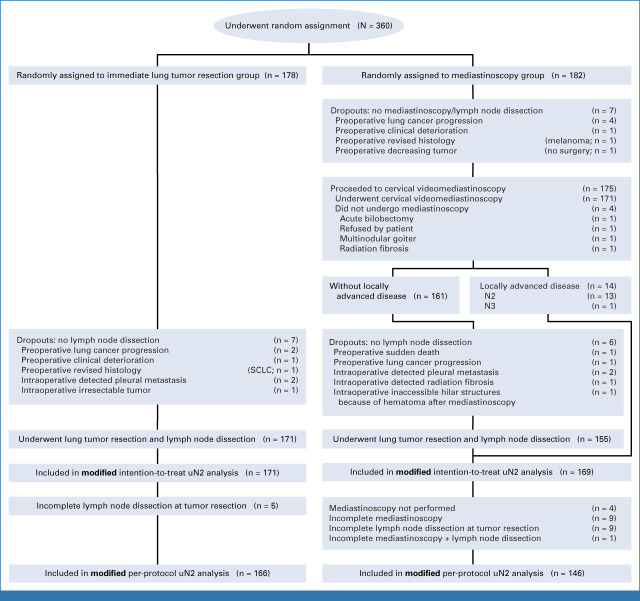
As important differences in baseline characteristics may exist despite random assignment, we performed a post hoc analysis to determine whether baseline characteristics were unevenly distributed (chi square test or Student T-test where appropriate, defined as P value <.05) among randomization groups for all primary analyses: the original intention-to-treat (ITT), the original per-protocol (PP), the modified ITT, and the modified PP. The formations of the different populations are presented in Appendix Figure A1 (original ITT and PP), and Appendix Figure A2 (modified ITT and PP).
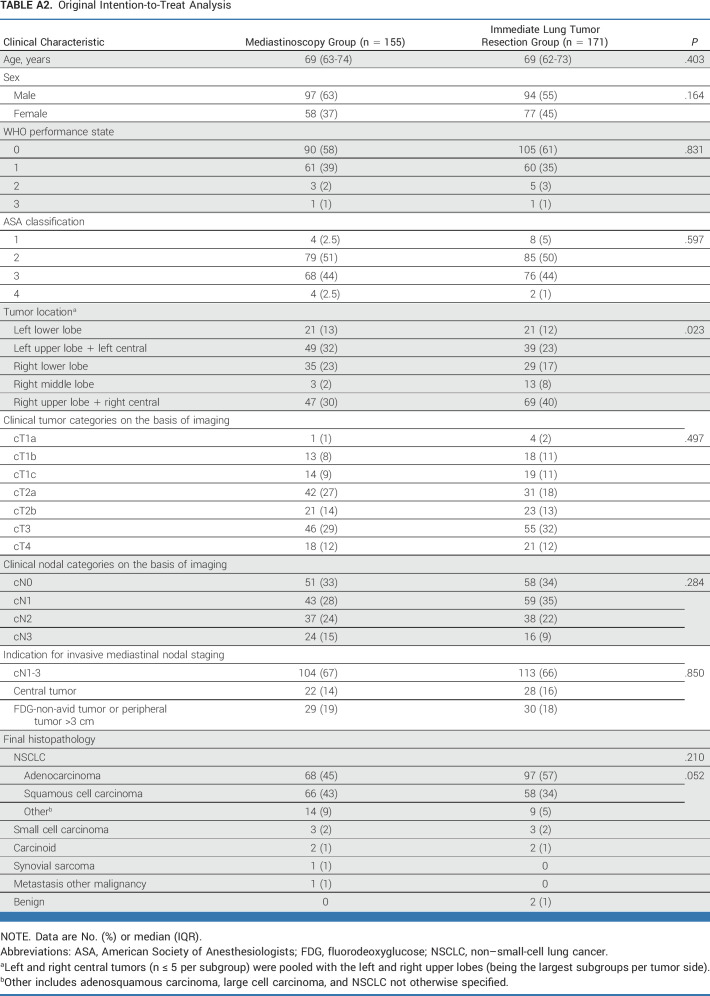
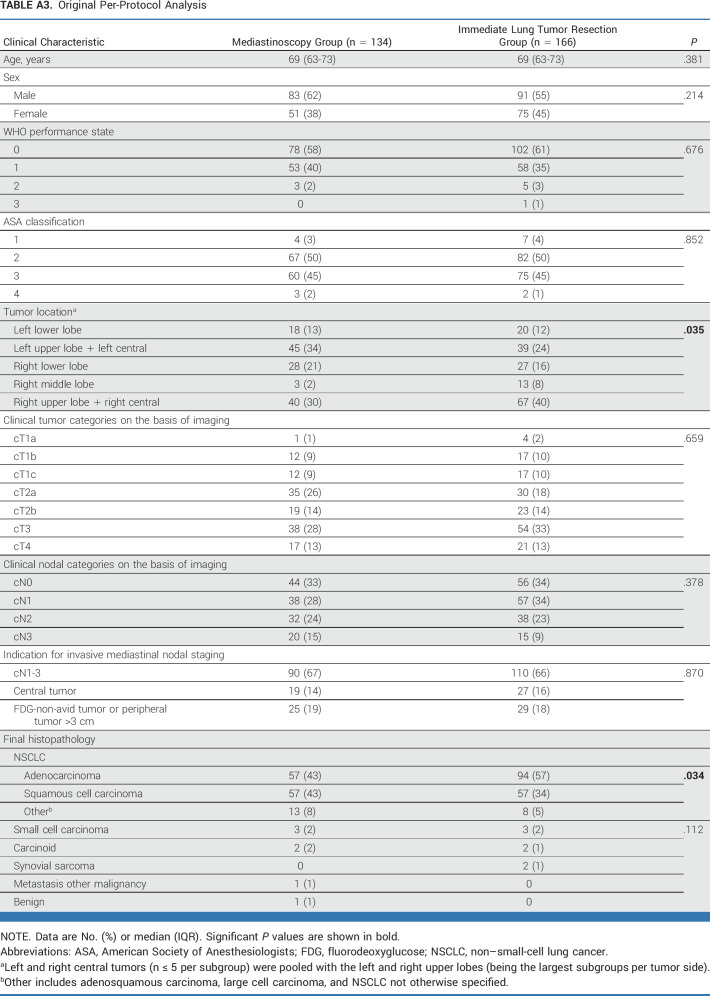
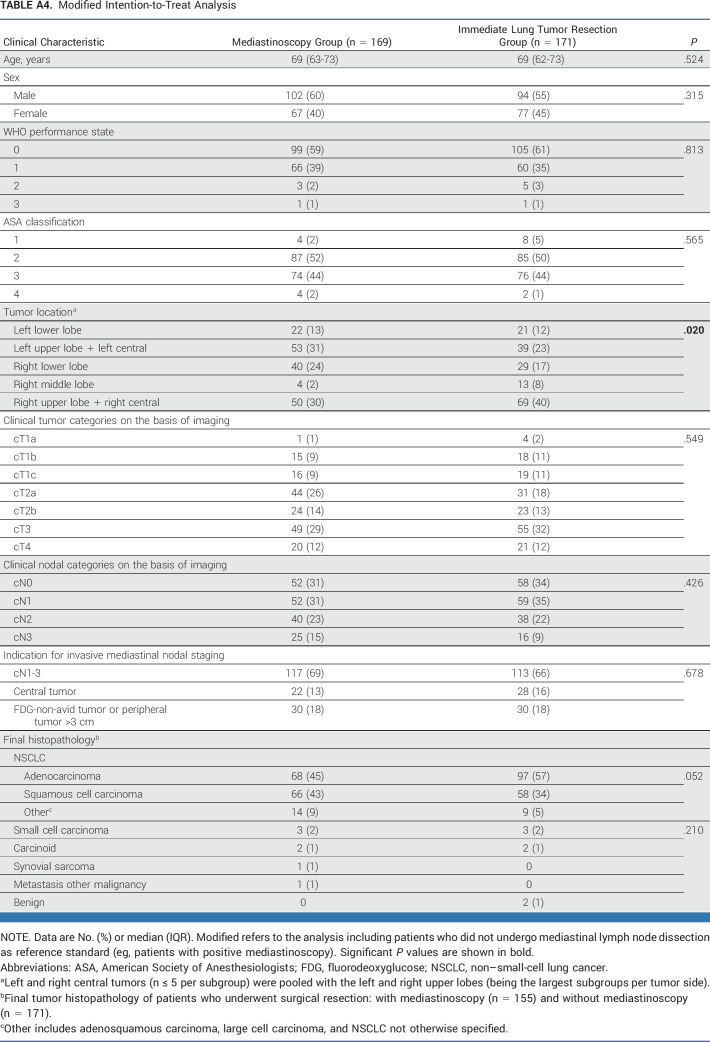
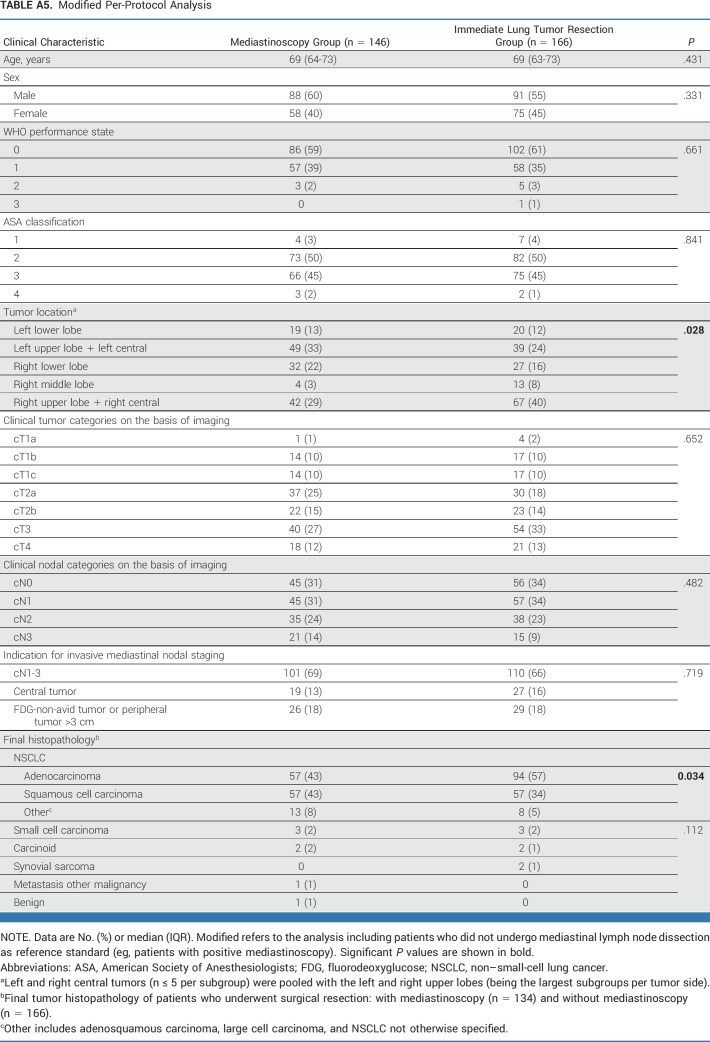
This post hoc analysis of baseline characteristics (Appendix Tables A2-A5) identified unbalanced random assignment in the ITT population (original analysis in Appendix Table A2, modified analysis in Appendix Table A3) regarding tumor location and in the PP population (original Appendix Table A4, modified Appendix Table A5) regarding tumor location and histology. For the adjusted post hoc analysis, left and right central tumors (n ≤ 5 per subgroup) were pooled with the left and right upper lobes (being the largest subgroups per tumor side).
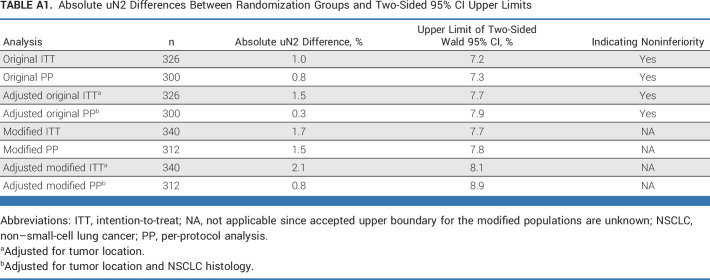
Subsequently, these variables were included in the adjusted post hoc generalized linear modeling. Unforeseen N2 was assessed as binomial response parameter with identity link to adjust for variables with significant baseline imbalances; the difference in proportions of unforeseen N2 and their upper limits of the Wald 95% two-sided confidence interval are presented in Appendix Table A1. Although the results lie within or very close to our chosen noninferiority limit, the accepted boundary for noninferiority cannot reliably be applied to the modified analyses with aberrant uN2 definition.
TABLE A1.
Absolute uN2 Differences Between Randomization Groups and Two-Sided 95% CI Upper Limits
TABLE A2.
Original Intention-to-Treat Analysis
TABLE A3.
Original Per-Protocol Analysis
TABLE A4.
Modified Intention-to-Treat Analysis
TABLE A5.
Modified Per-Protocol Analysis
FIG A1.
Flowchart of original intention-to-treat and per-protocol populations. SCLC, small-cell lung cancer.
FIG A2.
Flowchart of modified intention-to-treat and per-protocol populations. SCLC, small-cell lung cancer.
Erik H.F.M. van der Heijden
Honoraria: Pentax Medical Devices (Inst), Siemens Healthineers (Inst), Janssen Oncology (Inst)
Consulting or Advisory Role: Johnson & Johnson/Janssen (Inst), Intuitive Surgical (Inst)
Research Funding: AstraZeneca (Inst), Philips Research (Inst), Pentax Medical Devices (Inst), Johnson & Johnson/Janssen (Inst)
Patents, Royalties, Other Intellectual Property: Patents issued, pending and planned in the field of advanced and navigation bronchoscopy (Inst)
Jouke T. Annema
Research Funding: Boston Scientific Foundation (Inst), Mauna Kea Technologies (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Respiratory Society International Congress, Barcelona, Spain, September 4-6, 2022.
SUPPORT
The MEDIASTrial was funded by The Netherlands Organisation for Health Research and Development (ZonMw; project number 843004109) and The Dutch Cancer Society (KWF; project number 11313). The funding sources had no involvement in the study design, data analysis, data interpretation and the decision to submit the article for publication.
CLINICAL TRIAL INFORMATION
NL6344 (MEDIASTrial)
J.T.A. and F.J.C.v.d.B. shared senior authorship.
Contributor Information
Collaborators: Nicole E. Papen-Botterhuis, Maggy Youssef-El Soud, Wim J. van Boven, Johannes M.A. Daniels, David J. Heineman, Harmen R. Zandbergen, Pepijn Brocken, Thirza Horn, Willem H. Steup, Jerry Braun, Rajen S.R.S. Ramai, Naomi Beck, Fieke Hoeijmakers, Nicole P. Barlo, Martijn van Dorp, W. Hermien Schreurs, Anne-Marie C. Dingemans, Roy T.M. Sprooten, Jos G. Maessen, Niels J.M. Claessens, Jan-Willem H.P. Lardenoije, Birgitta I. Hiddinga, Caroline Van De Wauwer, Anthonie J. van der Wekken, Wessel E. Hanselaar, Robert ThJ Kortekaas, Martin P. Bard, Herman Rijna, Gerben P. Bootsma, Yvonne L.J. Vissers, Eelco J. Veen, Cor H. van der Leest, Emanuel Citgez, Eino B. van Duyn, Geertruid M.H. Marres, Eric R. van Thiel, Paul E. van Schil, Jan P. van Meerbeeck, Reinier Wener, Niels Smakman, Femke van der Meer, Mohammed D. Saboerali, Anne Marie Bosch, Wouter K. de Jong, Charles C. van Rossem, W. Johan Lie, Ewout A. Kouwenhoven, A. Jeske Staal-van den Brekel, Nike M. Hanneman, Roxane Heller-Baan, and Valentin J.J.M. Noyez
DATA SHARING STATEMENT
The data sets and/or analyzed data will be available from the principal investigator (F.v.d.B.) on reasonable request. The Data Management plan and Trial Master File are managed by the principal investigator (F.v.d.B.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: All authors
Collection and assembly of data: Jelle E. Bousema, Frank J.C. van den Broek
Data analysis and interpretation: Jelle E. Bousema, Marcel G.W. Dijkgraaf, Jouke T. Annema, Frank J.C. van den Broek
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Endosonography With or Without Confirmatory Mediastinoscopy for Resectable Lung Cancer: A Randomized Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Erik H.F.M. van der Heijden
Honoraria: Pentax Medical Devices (Inst), Siemens Healthineers (Inst), Janssen Oncology (Inst)
Consulting or Advisory Role: Johnson & Johnson/Janssen (Inst), Intuitive Surgical (Inst)
Research Funding: AstraZeneca (Inst), Philips Research (Inst), Pentax Medical Devices (Inst), Johnson & Johnson/Janssen (Inst)
Patents, Royalties, Other Intellectual Property: Patents issued, pending and planned in the field of advanced and navigation bronchoscopy (Inst)
Jouke T. Annema
Research Funding: Boston Scientific Foundation (Inst), Mauna Kea Technologies (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. : Global cancer statistics, 2012. CA Cancer J Clin 65:87-108, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Vilmann P, Clementsen PF, Colella S, et al. : Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 47:c1-c560, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Bousema JE, Heineman DJ, Dijkgraaf MGW, et al. : Adherence to the mediastinal staging guideline and unforeseen N2 disease in patients with resectable non-small cell lung cancer: Nationwide results from the Dutch Lung Cancer Audit—Surgery. Lung Cancer 142:51-58, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Annema JT, van Meerbeeck JP, Rintoul RC, et al. : Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: A randomized trial. JAMA 304:2245-2252, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Silvestri GA, Gonzalez AV, Jantz MA, et al. : Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e211S-e250S, 2013 [DOI] [PubMed] [Google Scholar]
- 6.NCCN Guidelines : NCCN clinical practice guidelines in oncology for non-small cell lung cancer V.3.2022. J Natl Compr Canc Netw 20:497-530, 2022 [DOI] [PubMed]
- 7.Bousema JE, Aarts MJ, Dijkgraaf MGW, et al. : Trends in mediastinal nodal staging and its impact on unforeseen N2 and survival in lung cancer. Eur Respir J 57:2001549, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Turner SR, Seyednejad N, Nasir BS: Patterns of practice in mediastinal lymph node staging for non-small cell lung cancer in Canada. Ann Thorac Surg 106:428-434, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Krantz SB, Howington JA, Wood DE, et al. : Invasive mediastinal staging for lung cancer by the society of thoracic surgeons database participants. Ann Thorac Surg 106:1055-1062, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Bousema JE, van Dorp M, Noyez VJJM, et al. : Unforeseen N2 disease after negative endosonography findings with or without confirmatory mediastinoscopy in resectable non–small cell lung cancer: A systematic review and meta-analysis. J Thorac Oncol 14:979-992, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Bousema JE, Dijkgraaf MGW, Papen-Botterhuis NE, et al. : MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): Study protocol of a multicenter randomised controlled trial. BMC Surg 18:27, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhoeven RLJ, Leoncini F, Slotman J, et al. : Accuracy and reproducibility of endoscopic ultrasound B-mode features for observer-based lymph nodal malignancy prediction. Respiration 100:1088-1096, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusch VW, Asamura H, Watanabe H, et al. : The IASLC lung cancer staging project A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 4:568-577, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Goldstraw P: Report on the international workshop on intrathoracic staging. London, October 1996. Lung Cancer 18:107-111, 1997 [Google Scholar]
- 15.Lardinois D, De Leyn P, Van Schil P, et al. : ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothoracic Surg 30:787-792, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Junker K: Histopathologic evaluation of mediastinal lymph nodes in lung cancer. Lung Cancer 45:S79-S83, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA: Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205-213, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousema JE, Annema JT, van der Heijden EHFM, et al. : MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): A statistical analysis plan. Trials 22:168, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuijvenhoven JC, Korevaar DA, Tournoy KG, et al. : Five-year survival after endosonography vs mediastinoscopy for mediastinal nodal staging of lung cancer. JAMA 316:1110-1112, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Newcombe RG: Interval estimation for the difference between independent proportions: Comparison of eleven methods. Stat Med 17:873-890, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Garner W: Constructing Confidence Intervals for the Differences of Binomial Proportions in SAS. Foster City, CA, Gilead Sciences, 2007 [Google Scholar]
- 22.Hintze J: NCSS, PASS and GESS. Kaysville, UT, NCSS, 2006 [Google Scholar]
- 23.Abramson JH: WINPEPI updated: Computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov 8:1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanz-Santos J, Almagro P, Malik K, et al. : Confirmatory mediastinoscopy after negative EBUS-TBNA for mediastinal staging of lung cancer: Systematic review and meta-analysis. Ann Am Thorac Soc 19:1581-1590, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Dooms C, Tournoy KG, Schuurbiers O, et al. : Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: A prospective multicenter study. Chest 147:209-215, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Beyaz F, Verhoeven RLJ, Schuurbiers OCJ, et al. : Occult lymph node metastases in clinical N0/N1 NSCLC; A single center in-depth analysis. Lung Cancer 150:186-194, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Leong TL, Loveland PM, Gorelik A, et al. : Preoperative staging by EBUS in cN0/N1 lung cancer: Systematic review and meta-analysis. J Bronchology Interv Pulmonol 26:155-165, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Garelli E, Renaud S, Falcoz PE, et al. : Microscopic N2 disease exhibits a better prognosis in resected non-small-cell lung cancer. Eur J Cardiothoracic Surg 50:322-328, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Su H, She Y, et al. : Which N descriptor is more predictive of prognosis in resected non-small cell lung cancer. Chest 159:2458-2469, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Thomas DC, Arnold BN, Rosen JE, et al. : The significance of upfront knowledge of N2 disease in non-small cell lung cancer. World J Surg 42:161-171, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Keshava HB, Tan KS, Dycoco J, et al. : How effective is neoadjuvant therapy followed by surgery for pathologic single-station N2 Non–Small cell lung cancer? Semin Thorac Cardiovasc Surg 33:206-216, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crombag LMM, Dooms C, Stigt JA, et al. : Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J 53:1800800, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Korevaar DA, Crombag LM, Cohen JF, et al. : Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: A systematic review and meta-analysis. Lancet Respir Med 4:960-968, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets and/or analyzed data will be available from the principal investigator (F.v.d.B.) on reasonable request. The Data Management plan and Trial Master File are managed by the principal investigator (F.v.d.B.).