Abstract
The Oncology Grand Rounds series is designed to place original reports published in the Journal into clinical context. A case presentation is followed by a description of diagnostic and management challenges, a review of the relevant literature, and a summary of the authors' suggested management approaches. The goal of this series is to help readers better understand how to apply the results of key studies, including those published in Journal of Clinical Oncology, to patients seen in their own clinical practice.
Accurate staging of the mediastinal lymph nodes in resectable non–small-cell lung cancer (NSCLC) is critically important to determine the overall stage of the tumor and guide subsequent management. The staging process typically begins with positron emission tomography (PET) or computed tomography imaging; however, imaging alone is inadequate, and tissue acquisition is required for confirmation of nodal disease. Mediastinoscopy was long considered the gold standard for staging of mediastinal lymph nodes, but, recently, endobronchial ultrasound–guided (EBUS) fine-needle aspiration (FNA) has become the standard of care. EBUS-FNA, in combination with supplementary technologies, such as intranodal forceps biopsy and esophageal ultrasonography, has a high sensitivity and specificity for the diagnosis of nodal metastases. EBUS-FNA is also capable of assessing N1 disease and obtaining adequate tissue for tumor genomic analysis to help guide treatment. In the case of negative findings on EBUS, a confirmatory video mediastinoscopy is still recommended by the European Society of Thoracic Surgeons guidelines. However, whether confirmatory mediastinoscopy is necessary is a matter of debate, and it is not commonly performed in North America. To address this question, Bousema and colleagues performed a randomized noninferiority trial to determine rates of unforeseen nodal metastases after EBUS alone versus EBUS with confirmatory mediastinoscopy in patients with resectable NSCLC. The authors concluded that EBUS alone is noninferior to EBUS with confirmatory mediastinoscopy. These findings affirm our current practice to forgo confirmatory mediastinoscopy after negative findings on EBUS.
Confirmatory mediastinoscopy is not required after a negative EBUS to stage non–small-cell lung cancer.
CASE PRESENTATION
A 66-year-old woman with a 10-pack-year smoking history and a medical history of hypertension presented with a new cough and intermittent right chest wall discomfort. A chest x-ray revealed a right upper lobe mass. Computed tomography (CT) imaging showed a 4.5-cm right upper lobe mass and mediastinal adenopathy (Fig 1). Subsequent positron emission tomography (PET) imaging demonstrated hypermetabolic activity at the upper lobe mass, with a maximum standardized uptake value (SUVmax) of 15.6, and PET-avid ipsilateral hilar, 4R, and 2R nodes. There was no evidence of extrathoracic disease on PET imaging, and findings on magnetic resonance imaging of the brain were normal. The patient was otherwise healthy, with a Zubrod performance status of 1. Thoracic surgical assessment determined that the patient was a candidate for surgery and that all disease could be resected.
FIG 1.
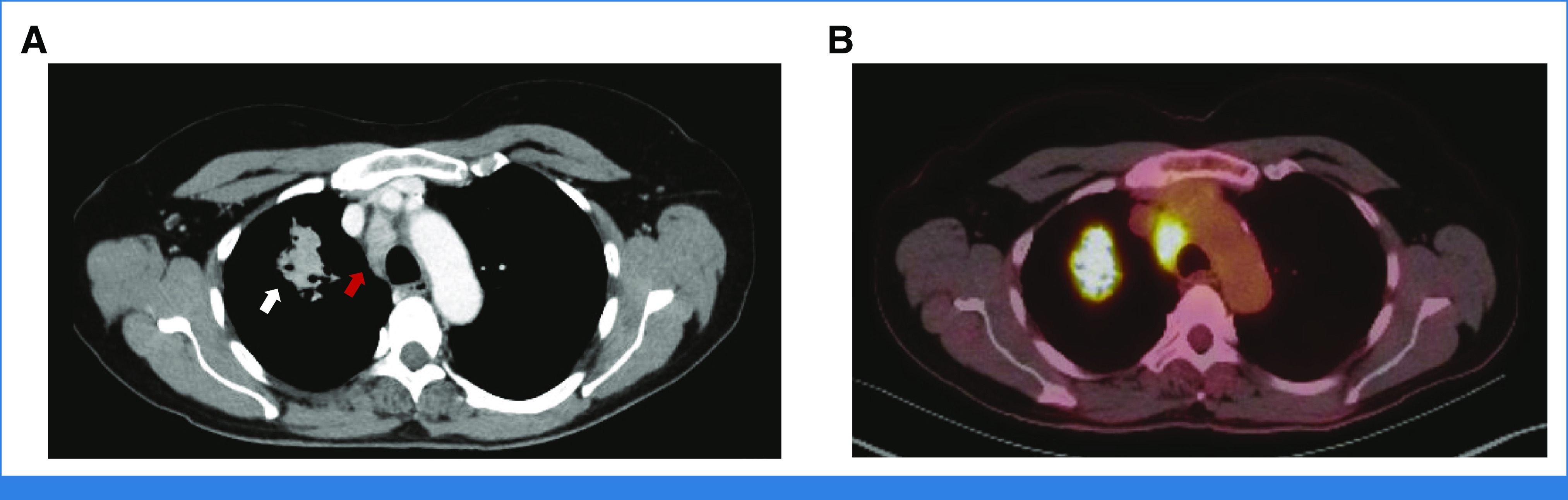
(A) Preoperative CT scan demonstrating a spiculated 4.5-cm right upper lobe mass (white arrow) with ipsilateral mediastinal lymphadenopathy (red arrow). (B) Corresponding positron emission tomography-CT scan showing hypermetabolic activity of the primary tumor and mediastinal lymph nodes. CT, computed tomography.
CLINICAL CHALLENGES IN EVALUATION AND MANAGEMENT
Lung cancer remains the leading cause of cancer-related death worldwide, accounting for 1.8 million deaths in 2020.1 In suspected cases of non–small-cell lung cancer (NSCLC), a confirmatory tissue diagnosis is routinely performed, and the correct clinical stage of the cancer is required to formulate a treatment plan for the patient. Radiographic staging studies include CT, PET, and/or magnetic resonance imaging. Although abnormal findings on radiographic imaging are concerning for advanced-stage disease, they are not definitive, and tissue acquisition is commonly required to accurately stage the disease. Given the proclivity of lung cancer to spread to intrapulmonary (N1) and mediastinal lymph nodes (N2 and N3), invasive staging of the hilar and mediastinal lymph nodes is commonly performed. Confirmation of positive nodal tissue indicates advanced-stage disease (stage II or III) and is an indication to consider neoadjuvant therapy before surgical resection of the primary tumor.2
Staging of the mediastinum begins with CT and/or PET-CT scan. PET has a sensitivity of 77% and a specificity of 86% for the identification of disease in mediastinal lymph nodes.3 However, PET has a false-negative rate of 7% to 16% and a false-positive rate of 45%-48%.4,5 A recent review of more than 1,400 patients with clinical stage I NSCLC on PET imaging found occult pN1 and pN2 disease in 8% and 10% of patients, respectively.6
Current guidelines recommend invasive mediastinal staging for all cases of operable NSCLC, with the exception of small (≤3 cm) peripheral tumors with no clinical evidence of nodal disease.2,7 Although invasive mediastinal evaluation is a well-accepted intervention for patients with cN2 disease, select patients with cN0/N1 disease also have a high risk of occult N2 disease. A systematic review and meta-analysis by Leong et al8 reported an incidence of pN2 disease of 14%-21% in patients with cN0/N1 disease. Unsurprisingly, in this study, the prevalence of mediastinal disease in CT-negative patients was 21%, compared with 14% in PET-CT–negative patients. In patients with PET-negative mediastinal nodes, tumor features suggestive of occult N2 disease included nonsquamous cell histologic type, central location of the tumor, presence of cN1 disease, and high mediastinal node-to-tumor SUVmax ratio.9 Therefore, invasive mediastinal staging should be strongly considered for these patients.
The first use of mediastinoscopy was reported by Carlens10 in 1959, after which mediastinoscopy was long considered the gold standard for mediastinal staging in patients with potentially operable NSCLC. This procedure allows sampling of the 2R, 2L, 4R, 4L, and 7 mediastinal nodal stations. Although there are reports of increasing mediastinal nodal assessment using a more invasive transcervical extended mediastinal lymphadenectomy, this approach has not been widely accepted.11 In a meta-analysis of mediastinal staging with mediastinoscopy that included more than 9,000 patients, Silvestri et al3 found a median sensitivity of 78% and a median negative predictive value of 91%. As a result, mediastinoscopy has been the approach of comparison in recent studies investigating imaging modalities such as PET and more recently adopted staging techniques such as endobronchial ultrasound–guided (EBUS)-transbronchial needle aspiration (TBNA) and endoscopic ultrasound-fine-needle aspiration (EUS-FNA).
Although the development of video-assisted mediastinoscopy has markedly improved visualization for the procedure, mediastinoscopy is associated with uncommon but significant complications, including recurrent nerve injury, hemorrhage, and injury to the trachea or esophagus.7,12 In 1978, Wang et al13 first described the use of a bronchoscopy needle aspiration technique for the biopsy of paratracheal lesions, a technique they later applied to the diagnosis and staging of NSCLC. EBUS can sample the same nodal stations as mediastinoscopy, as well as hilar and interlobar (stations 10 and 11) N1 nodes.7 EBUS has a sensitivity of 69% to 99% and a specificity of 99% for the detection of pN2 disease.14 It can distinguish between N0 and N1 disease with a sensitivity, specificity, and negative predictive value of 76.2%, 100%, and 96.2%, respectively.15 Even in patients without cN2 disease on imaging, systematic sampling via EBUS has a pooled sensitivity of 49%, a pooled specificity of 100%, and a mean negative predictive value of 91%.8 Because of its high sensitivity and specificity and its less-invasive nature, EBUS is now the recommended modality, over mediastinoscopy, for evaluating the mediastinum in North America and Europe.3,7
Additional techniques have been developed to further increase the diagnostic yield of EBUS-FNA. EBUS-intranodal forceps biopsy (EBUS-IFB) uses forceps through the bronchoscope, under ultrasound guidance, to sample lung lesions and lymph nodes.16 A recent meta-analysis showed that EBUS-IFB increased the diagnostic yield of EBUS-FNA from 67% for EBUS-FNA alone to 92% for EBUS-FNA with EBUS-IFB.16 Esophageal endosonography has also been used to supplement EBUS-TBNA; sensitivity increases from 72% for EBUS alone to 86% for EBUS and endosonography used together.17 Given the importance of tumor genomic analysis to guide the treatment of patients with NSCLC, modalities used to biopsy the primary tumor and stage the mediastinum must obtain sufficient tissue for these analyses. Multiple studies have shown that EBUS-FNA yields enough cancer cells for polymerase chain reaction (PCR) detection of common driver mutations in NSCLC. In a prospective study, EGFR and ALK genotyping with EBUS samples was successful in 99% of cases.18 Additionally, a recent study found that a tissue surface area of ≥1 mm is required for next-generation sequencing (NGS) and that DNA sequencing with EBUS-FNA samples was successful in 84% of cases.19
An analysis of recent trends demonstrated that EBUS is increasingly used for the evaluation of mediastinal lymph nodes, compared with mediastinoscopy.20 This is a direct result of the ASTER trial, an international randomized controlled trial that compared (1) endosonography followed by surgical staging in patients with negative findings on endosonography and (2) surgical staging alone in patients with resectable NSCLC and an indication for mediastinal staging.21 The outcome of interest was the sensitivity of these modalities to detect metastatic disease in mediastinal nodes. Endosonography had a sensitivity of 85% to detect nodal metastases, compared with 79% for mediastinoscopy. When endosonography findings were negative, the addition of subsequent mediastinoscopy increased sensitivity to 94%. Furthermore, the combination of endosonography and mediastinoscopy prevented unnecessary thoracotomy because of undetected mediastinal nodal metastases in one of seven patients, compared with mediastinoscopy alone. The authors concluded that (1) endosonography should be the first-line approach for mediastinal nodal staging and that (2) although the additional use of mediastinoscopy for patients with negative findings on endosonography increases the sensitivity for the detection of nodal metastases from 85% to 94%, 11 patients would need to undergo mediastinoscopy to identify one patient with mediastinal nodal metastasis. Therefore, as noted by the authors in their concluding remarks, negative findings on endosonography should not routinely prompt mediastinoscopy. Finally, recently published data from the ASTER trial demonstrated equivalent 5-year survival between EBUS with mediastinoscopy and mediastinoscopy alone for mediastinal staging.22
A frequent clinical conundrum for the thoracic surgeon is the approach for patients with clinically suspicious mediastinal lymph nodes on imaging who have negative findings on adequate EBUS. As noted, the European Society of Thoracic Surgeons guidelines recommend mediastinoscopy after negative findings on adequate EBUS.7 However, because of the risks posed by an additional procedure and the potential delay to curative resection, many have questioned whether confirmatory mediastinoscopy is necessary. Two meta-analyses have attempted to answer this question by looking at rates of unforeseen N2 (uN2) disease found at the time of surgical resection after EBUS with or without mediastinoscopy. Bousema et al23 found that the rate of uN2 disease was 10% for both EBUS alone and EBUS with mediastinoscopy. Furthermore, the complication rate associated with confirmatory mediastinoscopy was 6%, which includes grade 3 or 4 complications in 1.9% of patients and procedure-related death in 0.5% of patients. In addition, Sanz-Santos et al24 found that the negative predictive value of EBUS-TBNA was 79%, which increased to 97% with the addition of confirmatory mediastinoscopy. However, the number of patients needed to treat in this analysis was substantial, at 23.8.
The results of the MEDIASTrial, published in the companion to this article,25 represent the findings of the first multicenter randomized controlled trial to investigate the added value of performing a mediastinoscopy after negative findings on EBUS. This trial is an extension of the ASTER study and involved many of the same groups of patients from the Netherlands and Belgium.21 All patients included in the study had resectable NSCLC and an indication for mediastinal nodal staging. The primary end point of this noninferiority trial was the rate of uN2 disease on surgical pathology. After negative findings on EBUS, patients were randomly assigned to undergo immediate resection and lymph node dissection or confirmatory mediastinoscopy followed by resection. The rate of uN2 disease in the immediate resection cohort was 8.8%, which was noninferior to the rate of 7.7% in the mediastinoscopy cohort. Mediastinoscopy detected N2/3 disease in 8.0% of patients; however, the additional procedure delayed definitive surgical resection by an average of 10 days. Furthermore, although overall morbidity was not significantly different between the approaches (12.9% for immediate resection v 15.4% for mediastinoscopy first), 6.3% of patients undergoing mediastinoscopy had complications as a direct result of the procedure. Three of these patients had major complications, including 1 with a postmediastinoscopy bleed requiring remediastinoscopy, which eventually precluded the patient from curative resection. Overall, the results of the MEDIASTrial show that patients with an indication for mediastinal staging for NSCLC and negative findings on EBUS can forgo confirmatory mediastinoscopy and proceed directly to surgical resection.
OUR APPROACH TO MANAGEMENT
Our patient presented to the clinic with a 4.5-cm lung mass concerning for malignancy. Given the tumor size and the hypermetabolic mediastinal lymph nodes on PET-CT, the patient underwent EBUS-FNA. Pathology from the 2R lymph node revealed thyroid transcription factor 1–negative adenocarcinoma. PCR analysis identified no EGFR or ALK alterations, but the tumor was PD-L1–positive at 20%. The patient had clinical stage IIIA disease (cT2bN2M0) according to the eighth edition of the American Joint Committee on Cancer Staging Manual.26 After a multidisciplinary discussion and in accordance with National Comprehensive Cancer Network guidelines, the patient received two of a planned three cycles of neoadjuvant pemetrexed, carboplatin, and nivolumab.2 The patient developed a rash and hypersensitivity to the nivolumab; thus, for the third cycle, she received chemotherapy only. A restaging PET-CT showed no PET avidity in the mediastinal nodes and a reduction in the size of the mass, with a mild decrease in SUVmax, from 15.6 before treatment to 11.4 after treatment (Fig 2). The patient underwent an R0 right upper lobe lobectomy and complete mediastinal lymph node dissection. Her final pathology was ypT1aN0M0, with viable tumor present in 24% of the specimen. Broad-panel NGS confirmed a KRAS G12D mutation, mutations in KEAP1 and STK11, and a deletion in SMARCA4. She received no adjuvant therapy, and her follow-up CT scan at 6 months after surgery showed no evidence of disease.
FIG 2.
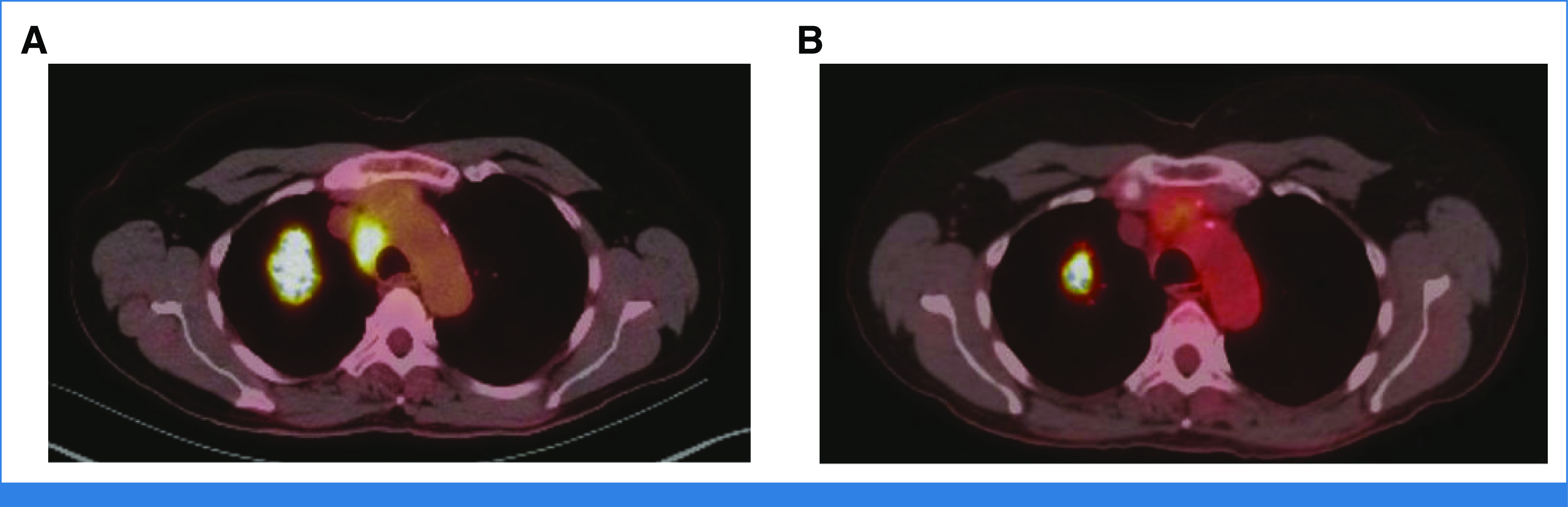
(A) Pretreatment PET-CT scan. (B) Restaging PET-CT scan after neoadjuvant chemotherapy and immunotherapy. PET-CT shows resolution of the avidity in the mediastinal lymph nodes and decreased size and lower maximum standardized uptake value of the primary tumor. PET-CT, positron emission tomography-computed tomography.
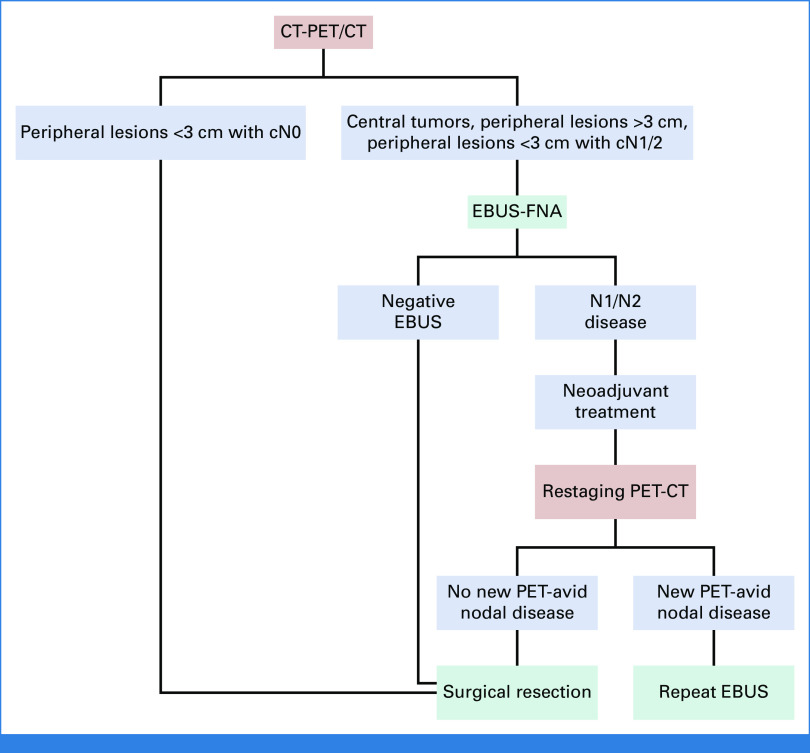
Our evaluation and treatment algorithm for resectable NSCLC is shown in Figure 3. In this case, our patient had positive findings on EBUS and therefore was referred for neoadjuvant treatment before surgical resection. However, if the result of her EBUS had been negative, we would not have performed a confirmatory mediastinoscopy, but we still would have considered neoadjuvant therapy because of the size of the tumor. Immediate surgical resection, likely with adjuvant therapy, would also have been a reasonable approach. Historically, mediastinoscopy was the primary invasive staging modality at our institution, but during the past 15 years, we have moved almost completely to EBUS-FNA for staging of suspicious N1/N2 nodes in patients with known or suspected NSCLC (Fig 4). Mediastinoscopy is reserved for the uncommon situations where EBUS-FNA results are inadequate for analysis of lesional tissue or lymphocytes and where there remains a strong clinical suspicion that N2 disease is present and that confirmation of such would affect treatment recommendations. More recently, we have transitioned to robotic bronchoscopy combined with EBUS-FNA to sample the primary tumor and stage the mediastinum, respectively.27 Our group has also shown that this approach secures enough tissue for PCR, NGS, and immunohistochemistry.28 This approach spares the patient an additional procedure, is cost-effective, and accelerates the implementation of a personalized care plan. Importantly, our thoracic surgeons perform the majority of EBUS-FNA and robotic bronchoscopy procedures on patients with NSCLC who are potential surgical candidates.
FIG 3.
Our institutional preoperative evaluation and treatment algorithm for resectable non–small-cell lung cancer. CT, computed tomography; EBUS, endobronchial ultrasound; FNA, fine-needle aspiration; PET, positron emission tomography.
FIG 4.
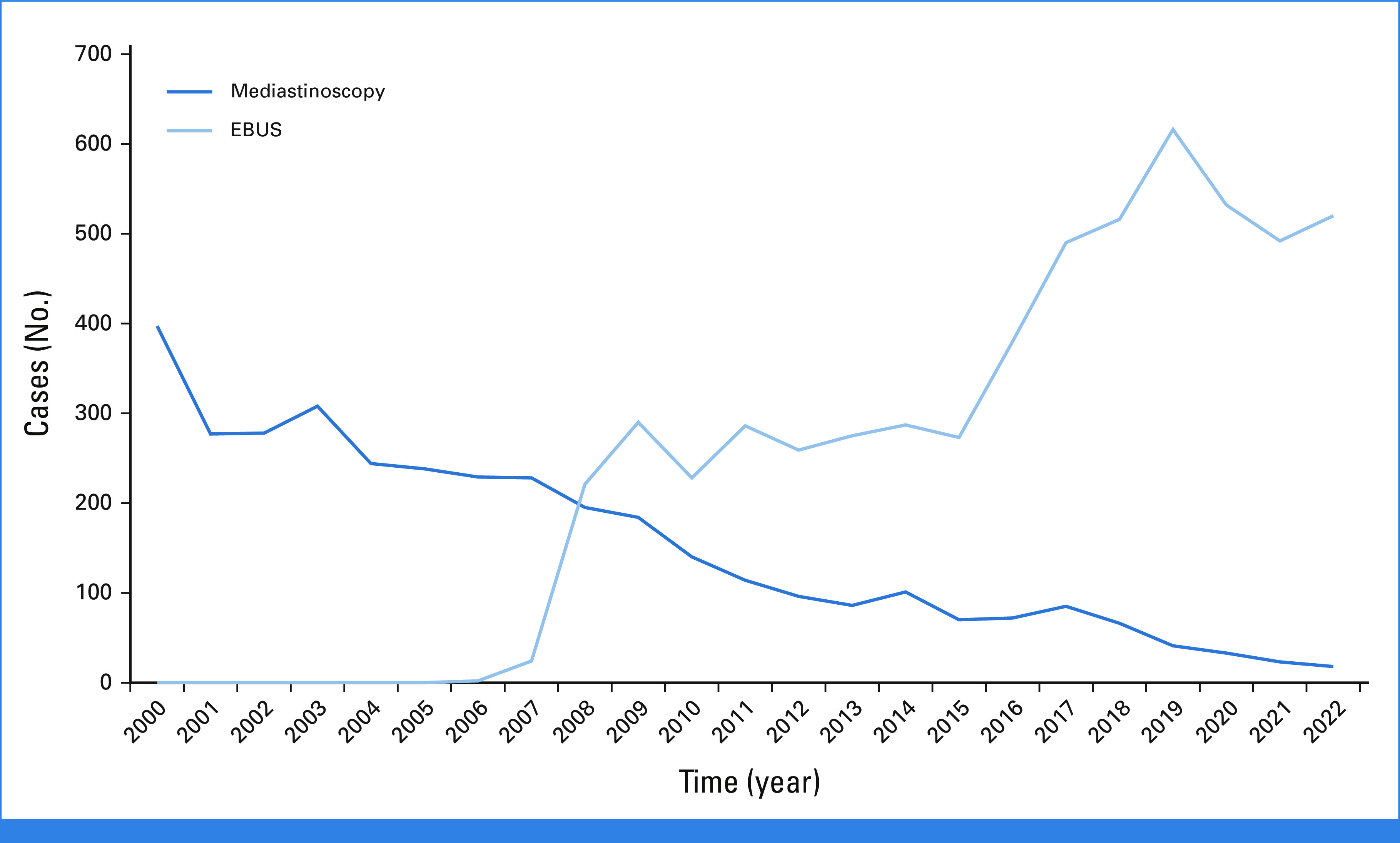
Trends in the use of mediastinoscopy and EBUS for mediastinal staging in patients with known or suspected lung cancer at Memorial Sloan Kettering Cancer Center (2000-2022). EBUS, endobronchial ultrasound.
We rarely restage the mediastinum with EBUS after neoadjuvant therapy if all disease is initially resectable and continues to be believed to be so. Indications for potential restaging of the mediastinum with EBUS-FNA are newly identified N3 disease or, more commonly, new PET-avid nodal disease. After neoadjuvant immunotherapy, the latter may represent true malignant progression, or it may be a variant of radiographic pseudoprogression, termed nodal immune flare, which mimics nodal disease progression but is nonmalignant and primarily consists of noncaseating granulomas on histology.29
In conclusion, the results of the MEDIASTrial reveal similar rates of uN2 disease on surgical pathology with or without confirmatory mediastinoscopy and support our practice during the past decade of forgoing mediastinoscopy after negative findings on EBUS.
David R. Jones
Consulting or Advisory Role: Merck, AstraZeneca
Speakers' Bureau: DAVA Oncology, Genentech/Roche
No other potential conflicts of interest were reported.
See accompanying Article, p. 3805
SUPPORT
Supported by the National Institutes of Health/National Cancer Institute (R01CA217169 and R01CA240472 to D.R.J. and Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: David R. Jones
Provision of study materials or patients: David R. Jones
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Mediastinal Staging in Non–Small-Cell Lung Cancer: Saying Goodbye to Mediastinoscopy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David R. Jones
Consulting or Advisory Role: Merck, AstraZeneca
Speakers' Bureau: DAVA Oncology, Genentech/Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. : Cancer statistics for the year 2020: An overview. Int J Cancer 149:778-789, 2021 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network : NCCN clinical practice guidelines in oncology (NCCN guidelines): Non-Small Cell Lung Cancer. Version 3.2023. April 10, 2023. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 3.Silvestri GA, Gonzalez AV, Jantz MA, et al. : Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e211S-e250S, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Li S, Zheng Q, Ma Y, et al. : Implications of false negative and false positive diagnosis in lymph node staging of NSCLC by means of 18F-FDG PET/CT. PLoS One 8:e78552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KY, Park HL, Kang HS, et al. : Clinical characteristics and outcome of pathologic N0 non-small cell lung cancer patients with false positive mediastinal lymph node metastasis on FDG PET-CT. In Vivo 35:1829-1836, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyas A, King RW, Ghanim AF, et al. : Clinical misstagings and risk factors of occult nodal disease in non-small cell lung cancer. Ann Thorac Surg 106:1492-1498, 2018 [DOI] [PubMed] [Google Scholar]
- 7.De Leyn P, Dooms C, Kuzdzal J, et al. : Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 45:787-798, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Leong TL, Loveland PM, Gorelik A, et al. : Preoperative staging by EBUS in cN0/N1 lung cancer: A systematic review and meta-analysis. J Bronchology Interv Pulmonol 26:155-165, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Evison M, Morris J, Martin J, et al. : Nodal staging in lung cancer: A risk stratification model for lymph nodes classified as negative by EBUSTBNA. J Thorac Oncol 10:126-133, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Carlens E: Mediastinoscopy: A method for inspection and tissue biopsy in the superior mediastinum. Dis Chest 36:343-352, 1959 [DOI] [PubMed] [Google Scholar]
- 11.Zielinski M, Hauer L, Hauer J, et al. : Non-small cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg 37:776-780, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Czarnecka-Kujawa K, Yasufuku K: The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis 9:S83-S97, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang KP, Terry P, Marsh B: Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am Rev Respir Dis 118:17-21, 1978 [DOI] [PubMed] [Google Scholar]
- 14.Crombag LMM, Dooms C, Stigt JA, et al. : Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J 53:1800800, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Yasufuku K, Nakajima T, Waddell T, et al. : Endobronchial ultrasound-guided transbronchial aspiration for differentiating N0 versus N1 lung cancer. Ann Thorac Surg 96:1756-1760, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Agrawal A, Ghori U, Chaddha U, et al. : Combined EBUS-IFB and EBUS-TBNA vs EBUS-TBNA alone for intrathoracic adenopathy: A meta-analysis. Ann Thorac Surg 114:340-348, 2022 [DOI] [PubMed] [Google Scholar]
- 17.Korevaar DA, Crombag LM, Cohen JF, et al. : Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: A systematic review and meta-analysis. Lancet Respir Med 4:960-968, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Jeyabalan A, Bhatt N, Plummeridge MJ, et al. : Adequacy of endobronchial ultrasound-guided transbronchial needle aspiration samples processed as histopathological samples for genetic mutation analysis in lung adenocarcinoma. Mol Clin Oncol 4:119-125, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami S, Yokose T, Nemoto D, et al. : Suitability of bronchoscopic biopsy tissue samples for next-generation sequencing. Diagnostics (Basel) 11:391, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krantz SB, Howington JA, Wood DE, et al. : Invasive mediastinal staging for lung cancer by the Society of Thoracic Surgeons database participants. Ann Thorac Surg 106:1055-1062, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Annema JT, van Meerbeeck JP, Rintoul RC, et al. : Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: A randomized trial. JAMA 304:2245-2252, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Kuijvenhoven JC, Korevaar DA, Tournoy KG, et al. : Five-year survival after endosonography vs mediastinoscopy for mediastinal nodal staging of lung cancer. JAMA 316:1110-1112, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Bousema JE, van Dorp M, Noyez VJJM, et al. : Unforeseen N2 disease after negative endosonography findings with or without confirmatory mediastinoscopy in resectable non-small cell lung cancer: A systematic review and meta-analysis. J Thorac Oncol 14:979-992, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Sanz-Santos J, Almagro P, Malik K, et al. : Confirmatory mediastinoscopy after negative endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal staging of lung cancer: Systematic review and meta-analysis. Ann Am Thorac Soc 19:1581-1590, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Bousema JE, Dijkgraaf MGW, van der Heijden EHFM, et al. : Endosonography with or without confirmatory mediastinoscopy for resectable lung cancer: A randomized clinical trial. J Clin Oncol 41:3805-3815, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin MB, Greene FL, Edge SB, et al. : The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 67:93-99, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Kalchiem-Dekel O, Connolly JG, Lin IH, et al. : Shape-sensing robotic-assisted bronchoscopy in the diagnosis of pulmonary parenchymal lesions. Chest 161:572-582, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connolly JG, Kalchiem-Dekel O, Tan KS, et al. : Feasibility of shape-sensing robotic-assisted bronchoscopy for biomarker identification in patients with thoracic malignancies. J Thorac Cardiovasc Surg 166:231-240.E2, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cascone T, Weissferdt A, Godoy MCB, et al. : Nodal immune flare mimics nodal disease progression following neoadjuvant immune checkpoint inhibitors in non-small cell lung cancer. Nat Commun 12:5045, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]