Abstract
INTRODUCTION:
Transradial approach (TRA) is a new technique proposed for performing cerebral angiography and neuro-interventional procedures. In this article, we sought to provide a step-by-step guide for carrying out a diagnostic cerebral angiography using this approach and summarize our center’s experience.
MATERIAL AND METHODS:
Records of patients since January 2020 were investigated, and data on demographic indices, reports of the procedures, outcomes, and complications were extracted. Then, these data were used to develop a step-by-step instruction for TRA cerebral angiography.
RESULTS:
Two hundred eighty-nine patients matched our eligibility criteria with a mean age of 50 years and a female-to-male ratio of 1.18. Overall, TRA was carried out successfully for 97.2% (281 patients). In case TRA failed, transfemoral approach was considered for the procedure. Three minor complications (two vasospasm and one small hematoma) and two major complications (one pseudoaneurysm of the radial artery and one radial artery avulsion) were observed.
CONCLUSION:
This article covers challenges a neurointerventionalist may face during a diagnostic cerebral angiography using TRA. Furthermore, our findings indicated that cerebral angiography with TRA might be performed safely and with a great success rate.
Keywords: Cerebral angiography, neuro-interventional, transradial approach (TRA)
Introduction
The transfemoral approach (TFA) is the most common access for cerebral angiography and neuroendovascular procedures.[1] In recent years, by adopting the radial-first approach from interventional cardiology, several centers have successfully transitioned from femoral to radial-first access in most neuroendovascular procedures.[2,3,4,5,6] For a transition from TFA to transradial approach (TRA), gaining new skill sets and experiences are needed since the radial artery is smaller than the femoral artery in diameter size, and advanced techniques in manipulating catheters are required for catheter formation in the aortic arch and for accessing to the major cervical arteries.[7,8] As such, the learning curve for TRA could be slower and more challenging than TFA.[4,7] Furthermore, TRA has its own challenges that require neurointerventionalists to be aware of and know how to deal with it.[9]
Although several neurointerventional centers have reportedly switched their practices from TFA to TRA, the majority of the neurointerventionalists have generally kept the traditional TFA. We have gradually shifted from TFA-first toward TRA-first in our center in Iran during the last 2 years. In this article, to share our experience, we provide a stepwise and practical approach to TRA for neuroendovascular procedures and discuss potential challenges dealt with during the procedures.
Methods
Since January 2020, patients in our center have had the choice to choose the access method for the cerebral angiogram and elective neuroendovascular procedures. Since then, 289 patients were undergone radial access for a diagnostic angiogram. In the majority of procedures, the 5-french Simmons-shaped catheter includes Cook Beacon® (Cook Medical [Bloomington], [Indiana, United States]), Performa®, (Merritt Medical [Utah, United States]) and Supertorque®, (Cordis [Florida, United States]) were utilized as the Terumo gliding Simmons catheter is not available in our market. For each patient, informed consent for the procedure was obtained. This study was approved by the Institutional Review Board of Mashhad University of Medical Sciences with the registration number 4001116.
Patient selection
TRA was proposed to all patients over 16-year-old and underwent cerebral angiography or neuroendovascular procedures except for mechanical thrombectomy. Allen test is no longer a predictor of ischemic hand events and was not used to select the patients.[10] The right radial artery is the first choice for access, and in case it is failed, the right ulnar artery was used. If ulnar access is not suitable or fails, the access is switched to the femoral artery. The left radial artery is used if the left vertebral artery (VA) is not accessible through the right radial approach.
Technique
Anesthesia and pain relief
After positioning the patient on the angiography table, the patient’s hand is placed on the designed pad in an anatomic position with the palm facing upward. For distal radial access, the patient’s hand is put in a neutral position with a snuff box in the best view. The anesthetic team includes anesthesiologists for general anesthesia and/or nurses for moderate sedation. For moderate sedation, generally, 1 mg of midazolam and 50–100 of fentanyl will be injected intravenously (IV). After preparing skin at the access site with iodine-based disinfectant and draping, 5 ml lidocaine 2% is locally injected around the radial access puncture site. If the patient still feels uncomfortable, the anesthetic team will administer propofol for more sedation. For the majority of interventional procedures, we prefer general anesthesia. Patients’ vital signs, including pulse rate, blood pressure, and pulse oximetry are continuously monitored during the operation.
Radial artery puncture and sheath selection
For arterial puncture, we use the technique described by Dev et al.[11] We extend the patient’s wrist to bring the radial artery to a more superficial position. The radial artery is located between the styloid process of the radius and the flexor carpi radialis. For diagnostic purposes, we usually use Prelude® 5F (Merit Medical TM ((Utah, United States]) and for interventions Preclude® 6F (Merit Medical TM). We use a modified Seldinger sheath insertion technique with a micropuncture kit.[12] Then, we inject a radial cocktail (made up of 100 μg of nitroglycerin) through the radial sheath and we administered 5000 units of heparin IV.
Navigating to the aortic arch
For catheterization of large vessels of the neck, 5 French Simmons 2 (Sim2) catheters are usually utilized.[5] Although catheterization with glide catheters is generally our first choice, we have used 5F nonglide catheters such as Torcon NB® Sim2 catheters Cook Medical (Bloomington, Indiana, United States) or Performa® which are available in our center. After sheath insertion, Sim2 catheter is navigated to the subclavian artery over a 0.035 hydrophilic wire. If there is any resistance to advancing the hydrophilic wire, we navigate under the road mapping technique. Using a road map, we engage the catheter into the right VA origin in case we plan to perform a posterior circulation angiogram from the right VA.
Forming Simmons
Sim2 catheter could be formed to its original shape in 4 different ways using the right common carotid artery (CCA), ascending aorta over the aortic valve, descending aorta, and left CCA. Hadley et al. describe seven ways to form a Sim2 catheter and three of them used ascending aorta over the aortic valve.[8]
While advancing the catheter over the hydrophilic wire from the right subclavian artery into the brachiocephalic artery, the right CCA could sometimes be selected by the catheter/hydrophilic wire without the need for forming the catheter. At this time, the glide wire is pushed as distal as possible in the right CCA or external carotid artery (ECA), and the catheter is advanced over the wire into the right CCA.
If the right CCA could not be selected directly, Sim2 may be formed in the aortic arch. In our practice, we generally advance the glide wire to the descending aorta then slide Sim2 over it, passing the catheter curved portion to the very proximal descending aorta from the brachiocephalic artery origin. The hydrophilic wire is then retracted to the straight portion of the catheter proximal to the curve. Holding both wire and catheter as a unit, they are then pushed into the ascending aorta with or without rotation to form the loop [Figure 1].
Figure 1.
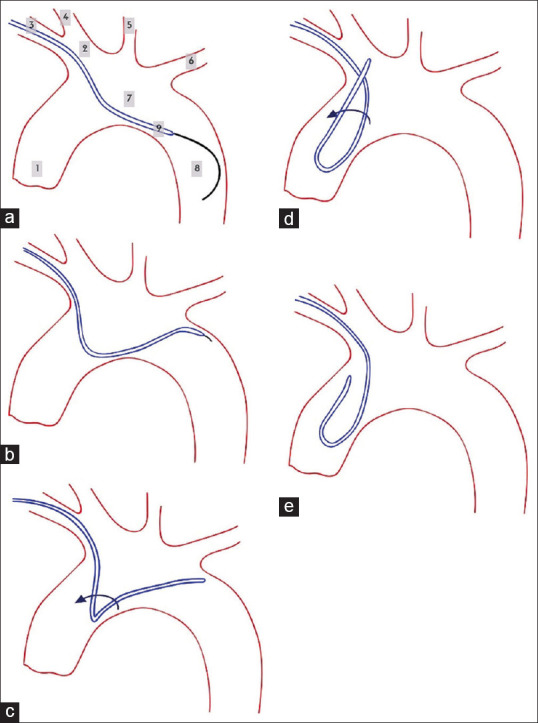
Forming Simmons in descending aorta. (a) Advancing wire and Simmons to descending aorta, (b) pulling back the wire and placing the catheter on the edge of the aorta, (c) pushing and rotating Simmons, (d) rotating Simmons to navigating great vessels, (e) final form of Simmons. The labels indicate 1: Ascending aorta, 2: Brachiocephalic artery, 3: Right subclavian artery, 4: Right CCA, 5: Left CCA, 6: Left subclavian artery, 7: Aortic arc, 8: Descending aorta, 9: The Catheter. CCA: Common carotid artery
In another technique, the ascending aorta is used to form Sim2 over the aortic valve. The hydrophilic wire is advanced into the ascending aorta over the aortic valve [Figure 2]. This maneuver should be done cautiously to avoid entering the into left ventricle inducing paroxysmal ventricular contraction or to enter the coronary arteries leading to dissection or perforation. These events could be avoided by carefully navigating the tip of the wire under fluoroscopy. The wire makes a loop over the aortic valve and is advanced into right CCA, left CCA, or descending aorta. In the next step, Sim2 will be advanced over the wire. When the catheter curve is passed the aortic valve, we pull back the wire, and Sim2 would be formed. In a bovine arch configuration (with a prevalence of 13%), the formation of the Simmons catheter directly in the left CCA would be easier.[13]
Figure 2.
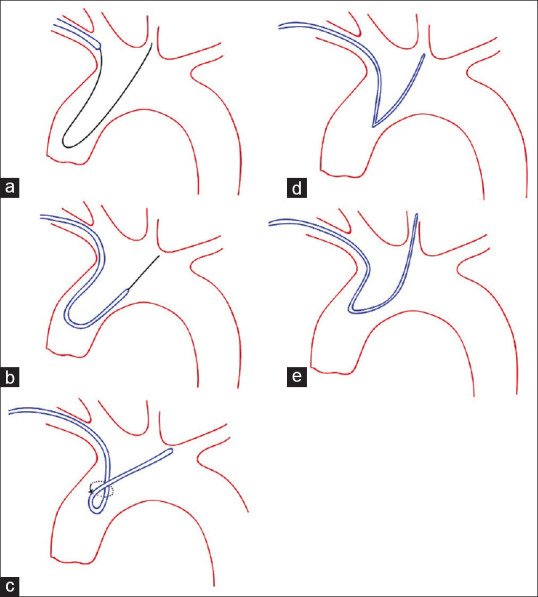
Shaping Simmons in ascending aorta, (a) advancing wire over aorta valve, (b) sliding Simmons over the wire, (c) pulling back the wire while rotating the Simmons, (d) Navigating Simmons to Great vessels, (e) pulling back the Simmons to engage the vessel
Navigation into great cervical arteries
To navigate into internal carotid arteries (ICAs) the hydrophilic wire is advanced into the distal cervical ICA under road mapping and the catheter is advanced over the wire. Occasionally, the catheter is pulled back into the ascending aorta when the catheter is pushed to slide over the wire, particularly with the nongliding catheters. In such cases, a stiffer hydrophilic 0.035 wire or a 260 cm stiff hydrophilic 0.035 wire is advanced into the distal ECA, preferably the internal maxillary artery (IMA), or sometimes into distal ICA and Sim2 is navigated over the wire to the distal part of the CCA or proximal part of the ICA until the curved potion of the Sim2 passes the aortic arch. At this time, the wire is pushed into the distal ICA and Sim2 is advanced over the wire. If Sim2 does not pass easily to the distal part of the CCA or proximal part of the ICA, it may be exchanged with Vertebral or Bern catheters over the 260 cm stiff hydrophilic wire while the distal tip of the wire is positioned in the distal part of the IMA.
Navigating the left VA could be difficult and challenging through right TRA. When left VA’s origin is very proximal and low in the left subclavian artery, left VA is easily catheterized with appropriate rotation and maneuvering of Sim2. If the origin of the left VA is high in the left subclavian artery and Sim2 could not easily navigate into the left VA, Simmons 3 can be used to address this challenge. Another alternative technique is to navigate the hydrophilic wire or sometimes stiff hydrophilic wire into the distal part of VA (V2) and then to slide the catheter over the wire. Of note, in the presence of an aberrant right subclavian artery, using intracranial support catheters would be helpful to pass the curvature added by this arterial variation.[14,15]
Closure
After completing the angiogram and withdrawing the catheter, the sheath is double flushed with saline and arterial blood pressure is checked. The puncture site is covered by TR Band® (Tayband TM, Lepu Medical Technology [Beijing, China]). The TR band is inflated with 15 ml of air. Then the air is gradually removed until blood return is noted. In the next step, the TR band is inflated with 3 ml of additional air. If the distal radial artery at the snuff box is punctured, we generally apply manual pressure for 10 min util hemostasis is achieved.
Postprocedure
We generally start deflating the TR Band® after 1.5–2 h at a rate of 2 ml every 10 min. If bleeding occurs during deflation of the TR band, it is re-inflated with 2 ml of air for 40 min. Generally, the patient is discharged 3–4 h after cerebral angiography is completed, and they are allowed to start their usual activities. The puncture site can be exposed to water after 48 h. Heavy activities are permitted 1 week after the procedure. Mild pain and discomfort are not unusual and can be managed by icepack or low-dose nonsteroidal anti-inflammatory drugs. There is no need to discontinue the patient’s medications, such as anticoagulants or antiplatelets, before, during, or after the cerebral angiogram.
Potential radial access complication and management
A thorough and complete review of complications and their management is beyond the scope of this article. However, briefly complications can be classified as intra versus postprocedural and further as hemorrhagic versus nonhemorrhagic.[16] Intraprocedural complications include radial artery spasm, arterial dissection, catheter kink, and radial artery perforation.[9,16,17,18]
Radial spasm is the most common complication reported in 4%–20% of cases.[16,19] In the majority of patients, radial spasm is mild and tolerable and can be managed by increasing sedation and analgesia or administration of more anti-spasmolytic medications (nitroglycerin and/or verapamil).[9,16] It is recommended to keep manipulating catheters and sheath as little as possible.[16] In moderate to severe cases, if radial artery spasm does not respond to sedation/analgesia and IV or intraarterial anti-spasmolytic medications, other measures may be required such as subcutaneous injection of lidocaine or anti-spasmolytic agents around the radial artery, flow-mediated dilatation by inflating blood pressure cuff, forearm warming, ulnar artery compression, and local lubricants (such as Viperslide or Rotaglide solution).[9,16] Deep sedation with propofol, general anesthesia, and axillary nerve block is reserved as the last option if other measures do not work.[9,16] To minimize this side effect, as recommended, we mildly sedate awake patients with midazolam and inject anti-spasmolytic agents such as nitroglycerin and/or verapamil into the radial artery after insertion of the sheath.[9,16,20]
A rare but severe complication is radial artery perforation, which could lead to forearm hematoma and even a compartment syndrome if it is not managed immediately.[9,16] It is usually due to the inadvertent advancement of wire into a small side branch.[16] Radial perforation accompanies severe forearm pain and/or vasovagal reactions following difficult navigation in tortuous or small vessels. If the wire is in place and could be passed across the injured site safely, the perforation may be sealed by advancing the sheath or glide catheter, allowing us to continue the procedure.[16] If the wire is not in place or the wire cannot advance across the perforation site safely, the procedure needs to be terminated, and the perforation site is compressed by inflating the blood pressure cuff to achieve hemostasis.[9,16] Occasionally, it is required to compress the perforation site for 15–20 min to achieve adequate hemostasis.[16] When the catheter is tightly entrapped in the radial artery, avulsion of the artery could occur during catheter removal despite following all the safety measures. In this situation, hemostatic compression could be sufficient to prevent hematoma in the forearm.
Simmons catheter’s shape is prone to kinking and knotting during manipulation, particularly in the very tortuous subclavian artery which is common in the elderly.[9,21] There are several techniques that can be used to come over this challenge: (1) advancing the hydrophilic wire or stiff hydrophilic wire in the catheter to pass and straighten the kink site, (2) untwisting the knot by clockwise or counter-clockwise rotation of the catheter, (3) external fixation of the distal part of the catheter by inflating the blood pressure cuff, (4) encasing the knot by using a long sheath, or (5) snaring the catheter through the femoral artery approach.[9,21]
Postprocedural complications are uncommon and include radial artery occlusion, pseudo-aneurysm, arteriovenous fistulae, nerve damage and regional pain syndrome, infection, and forearm hematoma with or without compartment syndrome.[9,16]
Our Center Experience
Two hundred and eighty-nine patients underwent radial access for cerebral angiography with a mean age of 50.42 (±17.6) years with a female-to-male ratio of 1.18 (157 vs. 132). The radial approach was successful in 97.2% (281) of patients and femoral access was obtained in the rest of the patients. In 70% (202) of patients, the radial artery was punctured and for others, if the radial approach was failed, the ulnar artery was used. Of 281 successful radial access, right VA was catheterized in 177 (63%) cases, right CCA or ICA or both in 271 (96%) cases (right CCA, 132 and ICA 148), left ICA or CCA or both in 264 (93.9%) cases (left CCA 140 and left ICA 131), right and left ECA in 29 (10.3%) and 23 (8.2%) cases respectively, and left VA in 134 cases. In eight patients, the left VA could not be catheterized directly, so the angiogram was performed through the left subclavian. In addition to 8 cases of unsuccessful radial access, 30 patients (10.7%) needed a cross-over to femoral access for completing the cerebral angiography due to failure of catheterization of desired cervical large arteries.
In terms of complications, three minor complications were recorded (1%). Two patients experienced severe vasospasm of the radial artery that required abandoning radial access and crossing over to the femoral approach. In one patient, a small hematoma in the right forearm was controlled by temporary cuff compression. Two major complications were reported in the records of patients (0.6%). One was a pseudoaneurysm in the proximal right radial artery that occurred in an 82-year-old female with a very tortuous radial artery. The pseudoaneurysm was treated with coil embolization through a TFA. Another one was radial artery avulsion in a 76-year-old female. It was treated by temporary compression of the forearm by the cuff. There was no report of any symptomatic stroke.
It may be beneficial to compare our results with other centers to gain a better understanding of the reliability and robustness of our findings since this report only reflects the experience of a single center. Stone et al. conducted a study in a single-center study and recruited 158 cases of radial approach angiography and compared the outcomes with 154 cases of femoral access. The diagnostic goal success rate for radial access was 97%, which is similar to our findings (97.2%). The femoral approach had a success rate of 99%. Stone et al. reported no significant difference in the complication rates of these two approaches, with a minor complication rate of 2.5% for the radial approach and 5.8% for the femoral (P value = 0.14). Also, no major complications were observed. Our study found major and minor complication rates of 0.6% and 1%, respectively.[22] Another study by Wang et al. investigated the data of 1,085 radial access and 1,229 femoral access. Their findings revealed that the TRA approach had a success rate of 98.6%, and the TFA had a success rate of 99.18%. This study noticed fewer complications such as local access complications and deep vein thrombosis in patients who underwent TRA compared to TFA.[23] Romano et al. have confirmed earlier findings by reporting a 97.6% success rate for the TRA and no major complications related to the radial artery.[24]
Conclusion
In this technical review, we aimed to cover all the main challenges that a neurointerventionalist may face during the TRA for diagnostic and neurointerventional procedures. Long-term outcomes and delayed complication rates should be investigated in larger-scale studies with extended follow-ups, but our study showed that the TRA for diagnostic neurovascular angiogram might be an effective and safe option.
Author Contribution
All authors contributed to developing the idea, writing the draft, and finalizing the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
Ashkan Mowla: Speakers Bureau/Consultant to Cerenovus, Stryker, Wallaby Medical, RapidAI, BALT, USA, LLC. Others have no disclosure.
References
- 1.Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): A randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 2.Zussman BM, Tonetti DA, Stone J, Brown M, Desai SM, Gross BA, et al. Aprospective study of the transradial approach for diagnostic cerebral arteriography. J Neurointerv Surg. 2019;11:1045–9. doi: 10.1136/neurintsurg-2018-014686. [DOI] [PubMed] [Google Scholar]
- 3.Osbun JW, Patel B, Levitt MR, Yahanda AT, Shah A, Dlouhy KM, et al. Transradial intraoperative cerebral angiography: A multicenter case series and technical report. J Neurointerv Surg. 2020;12:170–5. doi: 10.1136/neurintsurg-2019-015207. [DOI] [PubMed] [Google Scholar]
- 4.Snelling BM, Sur S, Shah SS, Khandelwal P, Caplan J, Haniff R, et al. Transradial cerebral angiography: Techniques and outcomes. J Neurointerv Surg. 2018;10:874–81. doi: 10.1136/neurintsurg-2017-013584. [DOI] [PubMed] [Google Scholar]
- 5.Joshi KC, Beer-Furlan A, Crowley RW, Chen M, Munich SA. Transradial approach for neurointerventions: A systematic review of the literature. J Neurointerv Surg. 2020;12:886–92. doi: 10.1136/neurintsurg-2019-015764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crockett MT, Selkirk GD, Chiu AH, Singh TP, McAuliffe W, Phillips TJ. Arterial access site complications in transradial neurointerventions: Single Center review of 750 consecutive cases. Clin Neuroradiol. 2020;30:639–42. doi: 10.1007/s00062-019-00866-1. [DOI] [PubMed] [Google Scholar]
- 7.Zussman BM, Tonetti DA, Stone J, Brown M, Desai SM, Gross BA, et al. Maturing institutional experience with the transradial approach for diagnostic cerebral arteriography: Overcoming the learning curve. J Neurointerv Surg. 2019;11:1235–8. doi: 10.1136/neurintsurg-2019-014920. [DOI] [PubMed] [Google Scholar]
- 8.Hadley C, Srinivasan V, Burkhardt JK, Johnson J, Luther E, Strickland A, et al. Forming the simmons catheter for cerebral angiography and neurointerventions via the transradial approach-techniques and operative videos. World Neurosurg. 2021;147:e351–3. doi: 10.1016/j.wneu.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 9.Brunet MC, Chen SH, Peterson EC. Transradial access for neurointerventions: Management of access challenges and complications. J Neurointerv Surg. 2020;12:82–6. doi: 10.1136/neurintsurg-2019-015145. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand OF, Carey PC, Gilchrist IC. Allen or no Allen: That is the question! J Am Coll Cardiol. 2014;63:1842–4. doi: 10.1016/j.jacc.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Dev SP, Hillmer MD, Ferri M. Videos in clinical medicine. Arterial puncture for blood gas analysis. N Engl J Med. 2011;364:e7. doi: 10.1056/NEJMvcm0803851. [DOI] [PubMed] [Google Scholar]
- 12.Pancholy SB, Sanghvi KA, Patel TM. Radial artery access technique evaluation trial: Randomized comparison of Seldinger versus modified Seldinger technique for arterial access for transradial catheterization. Catheter Cardiovasc Interv. 2012;80:288–91. doi: 10.1002/ccd.23445. [DOI] [PubMed] [Google Scholar]
- 13.Zalocar LAD, Doroszuk G, Goland J. Transradial approach and its variations for neurointerventional procedures: Literature review. Surg Neurol Int. 2020;11:248. doi: 10.25259/SNI_366_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goland J, Doroszuk GF. Transradial approach to cerebral aneurysm occlusion in a patient with an aberrant right subclavian artery: A case report. Am J Case Rep. 2021;22:e931443. doi: 10.12659/AJCR.931443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majmundar N, Patel P, Gadhiya A, Patel NV, Gupta G, Agarwalla PK, et al. Left distal radial access in patients with arteria lusoria: Insights for cerebral angiography and interventions. J Neurointerv Surg. 2020;12:1231–4. doi: 10.1136/neurintsurg-2020-016199. [DOI] [PubMed] [Google Scholar]
- 16.Sandoval Y, Bell MR, Gulati R. Transradial artery access complications. Circ Cardiovasc Interv. 2019;12:e007386. doi: 10.1161/CIRCINTERVENTIONS.119.007386. [DOI] [PubMed] [Google Scholar]
- 17.Satti SR, Vance AZ. Radial access for neurovascular procedures. Semin Intervent Radiol. 2020;37:182–91. doi: 10.1055/s-0040-1709173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catapano JS, Fredrickson VL, Fujii T, Cole TS, Koester SW, Baranoski JF, et al. Complications of femoral versus radial access in neuroendovascular procedures with propensity adjustment. J Neurointerv Surg. 2020;12:611–5. doi: 10.1136/neurintsurg-2019-015569. [DOI] [PubMed] [Google Scholar]
- 19.Raelson C, Ahmed B. Prevention and Management of radial access complications. Curr Treat Options Cardiovasc Med. 2020;22:9. [Google Scholar]
- 20.Deftereos S, Giannopoulos G, Raisakis K, Hahalis G, Kaoukis A, Kossyvakis C, et al. Moderate procedural sedation and opioid analgesia during transradial coronary interventions to prevent spasm: A prospective randomized study. JACC Cardiovasc Interv. 2013;6:267–73. doi: 10.1016/j.jcin.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Dor I, Rogers T, Satler LF, Waksman R. Reduction of catheter kinks and knots via radial approach. Catheter Cardiovasc Interv. 2018;92:1141–6. doi: 10.1002/ccd.27623. [DOI] [PubMed] [Google Scholar]
- 22.Stone JG, Zussman BM, Tonetti DA, Brown M, Desai SM, Gross BA, et al. Transradial versus transfemoral approaches for diagnostic cerebral angiography: A prospective, Single-Center, non-inferiority comparative effectiveness study. J Neurointerv Surg. 2020;12:993–8. doi: 10.1136/neurintsurg-2019-015642. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Xia J, Wang W, Xu G, Gu J, Wang Y, et al. Transradial versus transfemoral approach for cerebral angiography: A prospective comparison. J Interv Med. 2019;2:31–4. doi: 10.1016/j.jimed.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano DG, Frauenfelder G, Tartaglione S, Diana F, Saponiero R. Trans-radial approach: Technical and clinical outcomes in neurovascular procedures. CVIR Endovasc. 2020;3:58. doi: 10.1186/s42155-020-00152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


