Abstract
We have previously reported that the accessory protein Vpr from human immunodeficiency virus type 1 forms cation-selective ion channels in planar lipid bilayers and is able to depolarize intact cultured neurons by causing an inward sodium current, resulting in cell death. In this study, we used site-directed mutagenesis and synthetic peptides to identify the structural regions responsible for the above functions. Mutations in the N-terminal region of Vpr were found to affect channel activity, whereas this activity was not affected by mutations in the hydrophobic region of Vpr (amino acids 53 to 71). Analysis of mutants containing changes in the basic C terminus confirmed previous results that this region, although not necessary for ion channel function, was responsible for the observed rectification of wild-type Vpr currents. A peptide comprising the first 40 N-terminal amino acids of Vpr (N40) was found to be sufficient to form ion channels similar to those caused by wild-type Vpr in planar lipid bilayers. Furthermore, N40 was able to cause depolarization of the plasmalemma and cell death in cultured hippocampal neurons with a time course similar to that seen with wild-type Vpr, supporting the idea that this region is responsible for Vpr ion channel function and cytotoxic effects. Since Vpr is found in the serum and cerebrospinal fluids of AIDS patients, these results may have significance for AIDS pathology.
Although the accessory protein Vpr from human immunodeficiency virus type 1 (HIV-1) is not essential for viral replication in CD4+ T cells, it has been suggested that it plays important roles either in the early or in the late stages of the life cycle (for a review, see reference 18). It accelerates viral replication in primary macrophages/monocytes (3), possibly through its role in the transport of the preintegration complex to the nucleus (11, 13). Other demonstrated effects of Vpr include arresting cells in the G2 phase of the cell cycle (4, 9, 12, 16, 30, 32) and causing apoptosis and/or cell death (2, 21, 34), as well as playing a role in transactivation (7). Vpr is predominantly located in the nucleus of infected cells (19, 23) and is also present in virus particles. Recently, Levy et al. (17, 18) demonstrated that Vpr can also be found in the sera of AIDS patients as well as in the cerebrospinal fluid of patients with neurological disorders (AIDS dementia).
Vpr appears to have several structural regions including an N-terminal region predicted by the Chou-Fasman algorithm to be α-helical (amino acids 16 to 35); a hydrophobic, possibly α-helical region (amino acids 53 to 71); and a basic C terminus with two repeats of a conserved H(F/S)RIG motif. Mutational analysis has suggested that the N-terminal region of Vpr is important for nuclear localization (8, 38), virion incorporation (8, 24, 25, 38), and oligomerization (39) while the C terminus is mainly responsible for causing cell cycle arrest (6, 8, 16, 40) and mitochondrial dysfunction (2, 22).
The mechanism of action for any of the functions of Vpr is as yet unknown. We reported previously that Vpr can form cation-selective ion channels in planar lipid bilayers (27). In addition, extracellular Vpr is able to associate with the cell membrane and cause a large inward sodium current resulting in depolarization and eventually cell death in cultured rat hippocampal neurons (28). Recently, several other small viral proteins including M2 from influenza A virus (14, 29), NB from influenza B virus (35), and Vpu from HIV-1 (10, 33), all of which possess a single hydrophobic region, have also been reported to form ion channels in planar lipid bilayers, suggesting a wider role for ion channels in the replication of enveloped viruses.
In this study, we investigated, with site-directed mutagenesis and synthetic peptides, which structural regions of Vpr are responsible for the formation of ion channels in planar lipid bilayers as well as for plasmalemma depolarization and cytotoxic effects on intact hippocampal neurons. Our results indicate that the first 40 N-terminal amino acids of Vpr are sufficient to form ion channels in planar lipid bilayers and to produce cytotoxic effects in hippocampal neurons which are probably due to depolarization of the plasmalemma. Mutational analysis indicates that the positively charged C terminus is not necessary for ion channel formation but is responsible for the rectification of currents at positive potentials observed with wild-type (WT) Vpr. The C-terminal amino acids 76 to 96 can cause cell death of cultured hippocampal neurons but without plasmalemma depolarization comparable to that caused by Vpr. For the first time, these results directly link the region of Vpr responsible for ion channel function with its cytotoxic effects on cultured neurons. The results may be useful for the development of therapies to treat AIDS symptoms.
MATERIALS AND METHODS
Site-directed mutagenesis.
With the exception of VprΔ1, which corresponds to a form of Vpr with the C terminus truncated (27), and VprΔ2, which was generated by PCR, mutations in Vpr were generated by double-stranded mutagenesis of the plasmid p2GEX-Vpr (27) by the unique site elimination technique (unique site elimination kit; Pharmacia). The selection primer (5′ GCTGTTAGCAGGCCTATTAAGTTCTG 3′) changed the unique ApaI site in the p2GEX plasmid to StuI and was used in conjunction with the target primers GAACCCGTCCACATGTTCGGTATTATT, GAATGGACACTGCAGCTTTTAGAGGAG, GGACACTAGAGCTTCTGCAGCAGCTTAAGAAT, and GAAATGGAGCTAGCCAATCCTAGACTGAATTCC to generate the mutations VprE58Q, VprE21Q, VprE24Q, and VprR95Q, respectively. Mutations were confirmed by DNA sequencing performed in the Biomolecular Resource Facility (John Curtin School of Medical Research, Australian National University, Canberra, Australia).
Protein expression and purification.
Recombinant WT Vpr was purified as described previously (27) and appeared homogeneous on Coomassie brilliant blue R250-stained polyacrylamide gels (see Fig. 1B, lane 1). We estimate this preparation to be >99% pure. Mutant Vpr proteins were expressed and purified by the same method, and while the degree of purity as estimated by polyacrylamide gel electrophoresis varied depending on the particular mutation, we estimate all mutant preparations to be >90% pure (data not shown). This level of purity was considered sufficient for experiments aimed at detecting changes in the mutant proteins’ ion channel activity compared with that of the WT. Briefly, the proteins were expressed as glutathione S-transferase fusion proteins in Escherichia coli and purified by affinity chromatography with glutathione-agarose resin (Sigma). The mutant Vpr proteins were then cleaved from glutathione S-transferase with thrombin and further purified by cation-exchange high-pressure liquid chromatography (HPLC). Purified mutant Vpr was identified by sodium dodecyl sulfate gels and Western blotting with polyclonal anti-Vpr antibodies raised in rabbits (Fig. 1C) (27) and stored in 20 mM Tris (pH 7.0) containing the zwitterionic detergent 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS; 0.5%), glycerol (20%), and 500 mM NaCl. Vpr with the C terminus truncated (VprΔ1) was purified as described elsewhere (27).
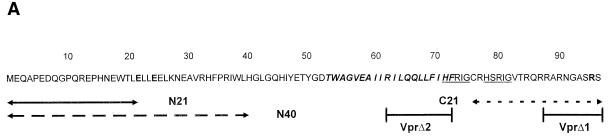
FIG. 1.
(A) Amino acid sequence of Vpr. The hydrophobic region is in boldface italics, the H(F/S)RIG motifs are underlined, and the sites of point mutations are indicated in boldface. The synthetic peptides are indicated underneath the sequence, as are the sites of the deletions in VprΔ1 and VprΔ2. (B) Purity of WT Vpr preparation. Lanes 1 and 2, Coomassie blue-stained gel of the Vpr preparation and molecular mass markers, respectively; lane 3, Western blot with antibody specific to the C terminus of Vpr. (C) Western blots of HPLC-purified fractions of Vpr mutant proteins probed with AbN. The arrows indicate the sizes of full-length Vpr and C-terminally truncated forms of Vpr (VprΔ1). Numbers at left show molecular masses in kilodaltons.
Synthetic peptides.
Vpr peptides comprising the first 21 (N21) or 40 (N40) N-terminal amino acids and the last 21 (C21) C-terminal amino acids (see Fig. 1) were synthesized in the Biomolecular Resource Facility (Australian National University) with an Applied Biosystems model 477a machine. The peptides were further purified by reverse-phase HPLC with a C18 column to yield a single clear peak in the elution profile. Mass spectroscopy (MALDITOF; matrix-assisted laser desorption-time of flight) of the purified N40 peptide revealed a single molecular ion of the correct molecular mass for the amino acid sequence indicated in Fig. 1. For bilayer experiments, peptides were dissolved in cis solution (500 mM NaCl buffered at pH 6.0 with 10 mM 2-[N-morpholino]ethanesulfonic acid [MES], 10 mM mercaptoethanol) and added to the cis chamber to obtain final peptide concentrations of 8 to 40 μM. For confocal laser scanning microscopy (CLSM), peptides were dissolved in CHAPS buffer (20 mM Tris, 0.25% CHAPS, 10% glycerol, pH 7.0) at 6 mM.
Recording of ion channel activity.
Purified WT and mutant Vpr and synthetic peptides were tested for their ability to induce channel activity in planar lipid bilayers as described elsewhere (27). Briefly, bilayers were formed from a mixture of 1-palmitoyl-2-oleoyl phosphatidylethanolamine and 1-palmitoyl-2-oleoyl phosphatidylcholine (8:2 weight ratio; Avanti Polar Lipids) dissolved in n-decane (50 mg/ml). For experiments with the C-terminal peptides, the negatively charged lipid 1-palmitoyl-2-oleoyl phosphatidylserine (PS) was added to the lipid mixture (5:3:2 ratio of phosphatidylethanolamine to PS to phosphatidylcholine, respectively). The lipid mixture was painted onto 150- to 200-μm-diameter apertures in the wall of a 2-ml Delrin cup separating cis and trans chambers containing 50 and 500 mM salt solutions, respectively, adjusted to pH 6 with 10 mM MES. Voltages were measured in the trans chamber with respect to the grounded cis chamber with an Axopatch 200 amplifier (Axon Instruments). An aliquot (10 to 100 μl) of HPLC fractions containing mutant Vpr (in 20 mM Tris-HCl–20% glycerol–0.5% CHAPS and up to 415 mM NaCl) or an aliquot of synthetic Vpr peptides (in cis solution) was added to the cis chamber, which was stirred until channel activity was seen. Currents were recorded and analyzed as described elsewhere (27). Buffer controls, including HPLC column fractions not containing Vpr, failed to produce any channel activity when tested in the bilayer assay. In addition, inhibition of channel activity by the Vpr C-terminal specific antibody (AbC) (27) was routinely checked as a means of confirming that the activity was indeed caused by the Vpr polypeptide and not due to some minor contaminant (see also Fig. 5).
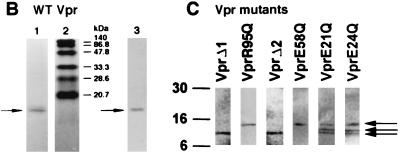
FIG. 5.
Inhibition of activity by an antibody recognizing the C terminus of Vpr. Shown are examples of currents generated in planar lipid bilayers by WT Vpr (A), VprE58Q (B), and VprE24Q (C) at 0-mV holding potential, before (“0mV”) and after (“+AbC”) addition of 50 μl of affinity-purified antibodies raised against a peptide (C21) comprising the C-terminal 21 amino acids of Vpr (see reference 27) to the trans chamber. The dashed lines indicate the zero current levels. All-points histograms of the data are plotted to the right of each trace.
Cell culture.
Rat hippocampal neurons were prepared from neonatal rats as previously reported (31). Briefly, after removal and trituration of the hippocampi from neonatal rats, dissociated cells were plated on glass coverslips pretreated with poly(l-lysine) and cultured for 5 to 15 days in a humidified incubator (5% CO2) in minimal essential medium supplemented with fetal bovine serum (10%), serum extender (0.1%), glucose (6%), penicillin (2%), and streptomycin (2%).
Plasmalemma depolarization.
Depolarization of the plasmalemma after extracellular addition of purified Vpr mutant proteins or synthetic Vpr peptides was assessed qualitatively by CLSM (15) in conjunction with the anionic potential-sensitive dye bis(1,3-dibutylbarbituric acid)trimethine oxonol [DiBa-C4(3)] (Molecular Probes) as described previously (28). Hippocampal neurons grown on coverslips were exposed to 3 μl of 55% bath solution (140 mM NaCl, 5 mM KCl, 3 mM CaCl2, 2 mM MgCl2, 10 mM glucose, 10 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid], pH 7.3); 20% DiBa-C4(3) (final concentration of 1 μM); 83 mM NaCl; and 16.7% either CHAPS buffer (negative control), WT Vpr in CHAPS buffer (final concentration of 1 μM), or synthetic peptides in CHAPS buffer (final concentration, 1 mM). Confocal images were recorded at several time points up to 15 min at 37°C. As a positive control, cultured hippocampal neurons were exposed to the above solutions, substituting 1 M KCl for the 16.7% CHAPS buffer (final concentration of 200 mM KCl), which depolarizes the transmembrane potential (26).
Cytotoxic effects.
The cytotoxic effect of purified Vpr mutant protein (1 μM) or synthetic Vpr peptides (1 mM) on cultured rat hippocampal neurons was assessed with CLSM with the membrane-impermeable, nucleic acid-staining fluorescent dye propidium iodide (PI; Molecular Probes) as described previously (28). Cells were prepared for CLSM as described above for plasmalemma depolarization experiments, except that PI (400 mg/ml in H2O) was added instead of DiBa-C4(3). For analysis, cells showing intense nuclear staining, indicative of cell death, were counted at various time points and expressed as a percentage of the total number of cells as previously described (28).
RESULTS
Mutations in the N-terminal region change ion selectivity.
Inspection of the Vpr amino acid sequence by predictive algorithms indicates three clear structural regions: a basic C-terminal region, a hydrophobic region (amino acids 53 to 71), and an N-terminal region containing a predicted amphipathic α-helix (amino acids 16 to 35). The Vpr mutations generated in this study (Table 1 and Fig. 1A) were designed to investigate the importance of these three regions for ion channel formation by Vpr in planar lipid bilayers. The mutant forms of Vpr were expressed in E. coli and purified as described in Materials and Methods (Fig. 1C) before reconstitution into planar lipid bilayers (27).
TABLE 1.
Vpr mutant proteins and synthetic peptides used in this study
| Mutant or peptide (location and designation) | Description | Effect on channel activity |
|---|---|---|
| Mutants | ||
| C-terminal region | ||
| VprΔ1a | Deletion of 8 to 9 amino acids from the C terminus | No rectification |
| VprR95Q | Point mutation disrupting the proposed salt bridge between R95 and N terminus | Channel activity as WT Vpr |
| Hydrophobic region | ||
| VprΔ2 | Deletion of 11 amino acids (63 to 73) and point mutation G75R | Channel activity as WT Vpr |
| VprE58Q | Point mutation disrupting the proposed salt bridge between E58 and R62 | Channel activity as WT Vpr |
| N-terminal region | ||
| VprE21Q | Point mutation in the proposed N-terminal α-helix (amino acids 16 to 34) | Altered Na+ permeability |
| VprE24Q | Point mutation in the proposed N-terminal α-helix (amino acids 16 to 34) | Altered Na+ permeability |
| Peptides | ||
| C-terminal region, C21 | Peptide containing the last 21 C-terminal amino acids | Channels only under special conditions |
| N-terminal region | ||
| N21 | Peptide containing the first 21 N-terminal amino acids | No channel activity |
| N40 | Peptide containing the first 40 N-terminal amino acids | Channel activity as WT |
VprΔ1 represents C-terminally truncated Vpr obtained by purification as described elsewhere (27).
C-terminal mutations.
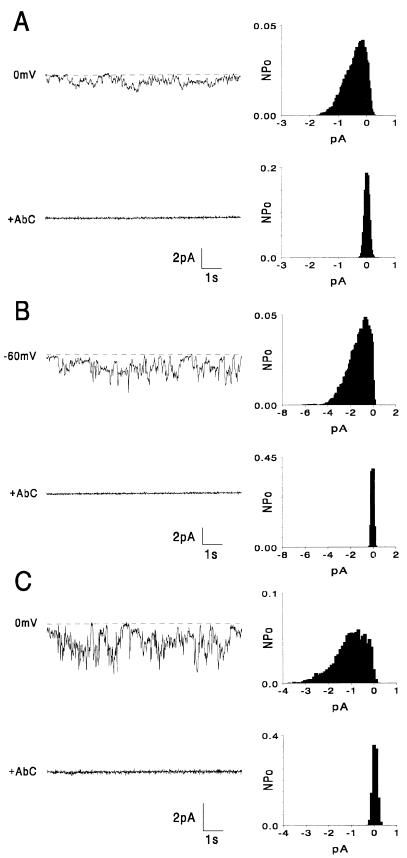
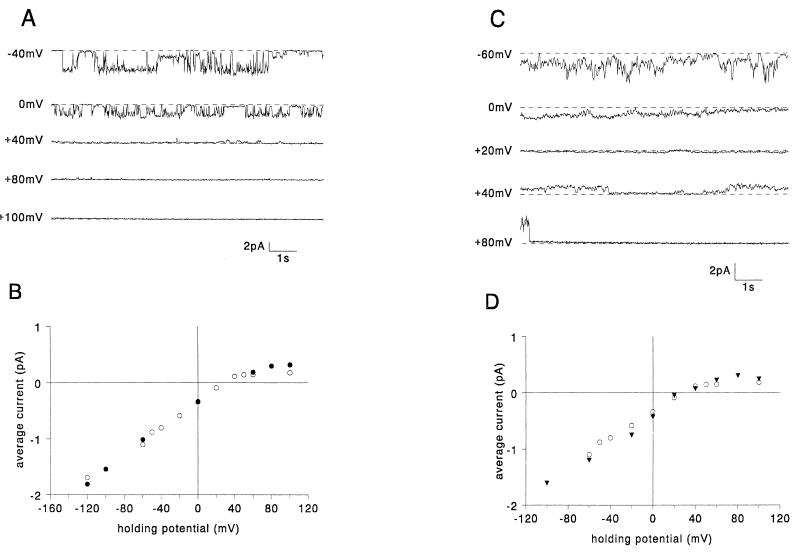
We have previously reported that the C-terminal 8 or 9 amino acids of Vpr are not necessary for channel activity (27). C-terminally truncated Vpr (VprΔ1 [Table 1]) was found to form channels with an ion selectivity similar to that of WT Vpr, but they do not rectify at positive potentials (Fig. 2B). Originally, we proposed, based on a computer-generated structural model, a number of specific electrostatic interactions between positively charged amino acids in the C-terminal region and negatively charged residues in the N-terminal region (27). In particular, arginine 95 appeared to be involved in three salt-bridge interactions with N-terminal glutamates (E6, E13, and E17). To test the role of these proposed interactions in channel function, the mutant VprR95Q was constructed. Rather than altering channel properties, this mutant Vpr protein exhibited ion channel activity indistinguishable from that of WT Vpr in planar lipid bilayers (Fig. 2C and D). While further mutations are required in this region to identify the key residues of the Vpr C terminus that affect rectification, the results support the idea that the C terminus is not necessary for ion channel formation.
FIG. 2.
C-terminal mutants: examples of currents generated in planar lipid bilayers by VprΔ1 (A) and VprR95Q (C) at different holding potentials. The dashed lines indicate the zero current levels. The average currents are plotted versus holding potential for VprΔ1 (filled circles) (B), VprR95Q (filled squares) (D), and, for comparison, WT Vpr (open circles).
Hydrophobic region mutations.
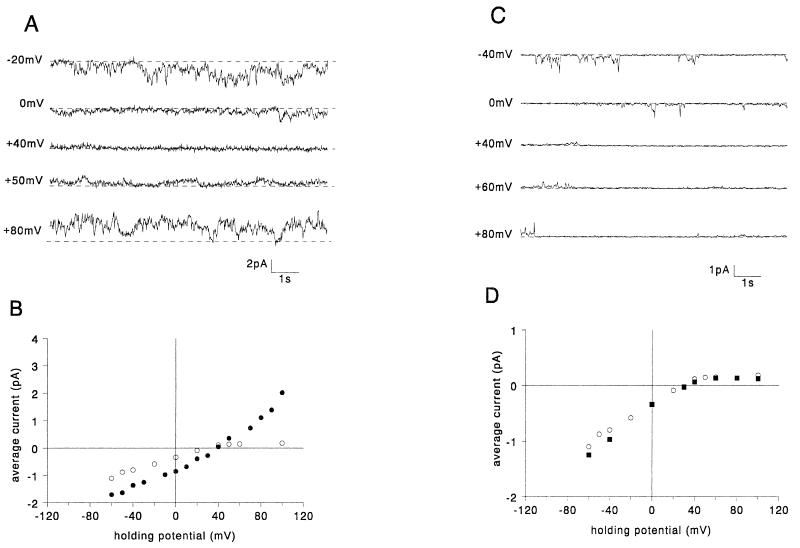
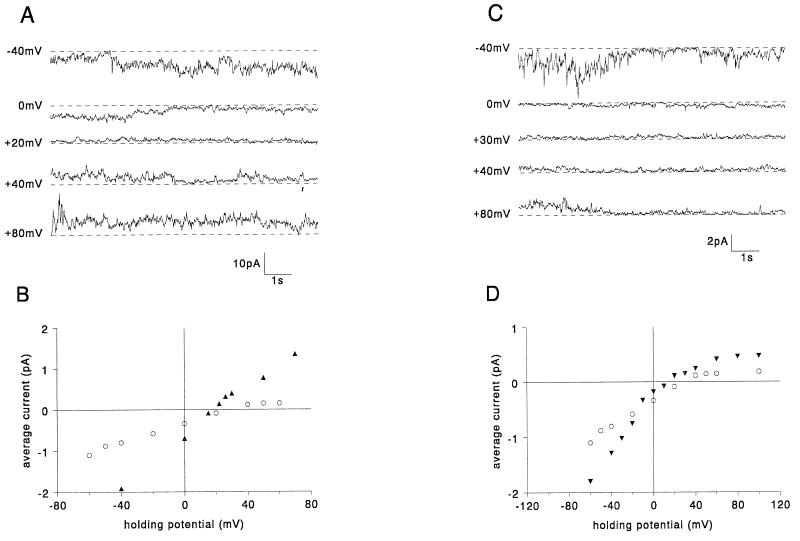
By analogy with other viral ion channels such as M2 from influenza A virus (14, 29), NB from influenza B virus (35), and Vpu from HIV-1 (10, 33), it seemed likely that the hydrophobic region of Vpr (amino acids 53 to 71) might form the transmembrane pore of the channel in the form of a bundle of α-helices. To test the role of this hydrophobic region in Vpr channel formation, the following two mutants were designed. The mutant VprΔ2 was designed to curtail the hydrophobic region so that the proposed hydrophobic α-helix would be too short to span the bilayer. VprE58Q was designed to disrupt the putative salt bridge in the proposed hydrophobic α-helix, leaving an unpaired positively charged residue to be buried in the bilayer. In all experiments in which either purified VprΔ2 (n = 38) or purified VprE58Q (n = 12) was tested in planar lipid bilayers, currents similar to those caused by WT Vpr were observed. Figure 3 shows typical channel recordings at different potentials and current-voltage relationships for VprΔ2 (A and B) and VprE58Q (C and D), respectively. It was concluded that the hydrophobic domain of Vpr was unlikely to play a role in ion channel function by forming a transmembrane α-helix.
FIG. 3.
Hydrophobic region mutants: examples of currents generated in planar lipid bilayers by VprΔ2 (A) and VprE58Q (C) at different holding potentials. The dashed lines indicate the zero current levels. The average currents are plotted versus holding potential for VprΔ2 (filled circles) (B), VprE58Q (filled inverted triangles) (D), and, for comparison, WT Vpr (open circles).
N-terminal mutations.
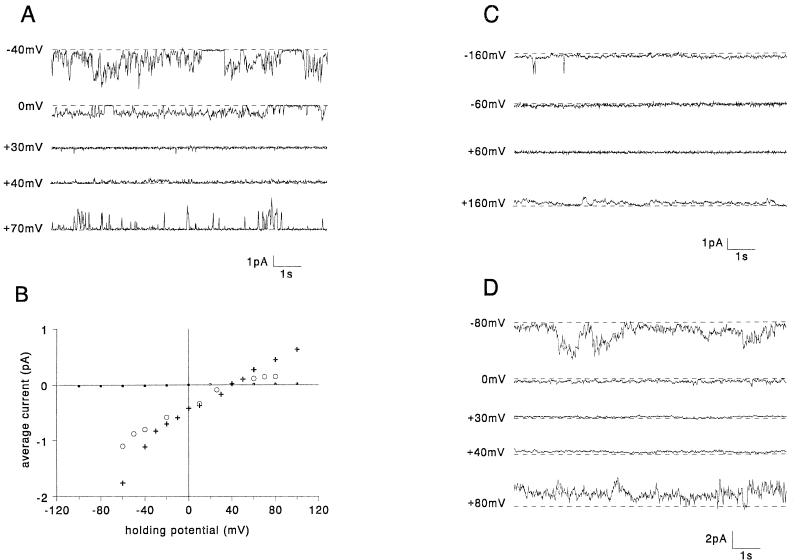
Since both the C terminus and hydrophobic region of Vpr appeared not to be necessary for ion channel formation by Vpr, the Vpr N-terminal region was examined. It has previously been noted (8, 25, 38) that the N-terminal region of Vpr has the propensity to form an α-helix based on structural predictive methods, and this is strongly supported by circular dichroism analysis of synthetic peptides corresponding to residues 17 to 34 of Vpr (20). Rather than attempting to completely disrupt the putative helical structure and hence severely impairing function without yielding any information as to which specific residues of the helix might be important, we chose to target residues that might be expected to be involved in lining the pore and/or interacting with the cations moving through the channel. To that end, point mutations in the N-terminal region, VprE21Q and VprE24Q, were designed to remove negative charges from the hydrophilic face of the proposed amphipathic α-helix. Both of these mutant proteins formed ion channels (22 and 20 experiments, respectively) that were less cation selective than those formed by WT Vpr. They both had reversal potentials more negative than +20 mV compared with +35 mV for WT Vpr channels (Fig. 4). The ratio of sodium to chloride ion permeability (calculated with the Goldman-Hodgkin-Katz equation based on ion activities) for VprE21Q and VprE24Q channels was at least four times lower than that for WT Vpr channels (PNa/PCl ratio of 2.5 for VprE21Q and VprE24Q and 11 for WT Vpr). This effect on sodium permeability of these mutations indicates that these residues are involved in the ion selectivity filter of the channel.
FIG. 4.
N-terminal mutants: examples of currents generated in planar lipid bilayers by VprE21Q (A) and VprE24Q (C) at different holding potentials. The dashed lines indicate the zero current levels. The average currents are plotted versus holding potential for VprE21Q (filled triangles) (B), VprE24Q (inverted filled triangles) (D), and, for comparison, WT Vpr (open circles).
Another striking feature of the channels formed by the VprE21Q mutant is that the currents do not rectify at positive potentials (Fig. 4A and B). It is possible that glutamate 21 may be involved in electrostatic interactions with residues in the C terminus (presumably not arginine 95) that lead to the rectification.
To confirm that the different channel properties observed in the preparation of these mutant proteins were directly attributable to Vpr, inhibition by an antibody specific to the Vpr C terminus (27) was tested (Fig. 5). In the case of both E21Q (data not shown) and E24Q, channel activity was abrogated by the presence of the antibody, in a fashion identical to that of either WT Vpr or the E58Q mutant (included as a control). It was concluded that the altered channel activity of the mutants was indeed due to Vpr, confirming also that the mutant derivatives were full length and not C-terminally truncated.
The N-terminal region of Vpr forms ion channels and kills intact neurons.
In an attempt to define a minimal region of Vpr that could still form an ion channel, synthetic peptides consisting of the first 21 (N21) and 40 (N40) amino acids of Vpr (Table 1) were tested in planar lipid bilayers. N21 did not cause any detectable currents in planar lipid bilayers at various holding potentials in eight experiments (Fig. 6B) in contrast to N40, which produced ion channel activity in 22 experiments. Peptide concentrations as low as 5 μM were observed to generate channel activity. However, at this concentration, inconveniently long periods of stirring were required before activity was observed. A 40 μM concentration was found to produce activity within about 5 min after addition to the cis chamber, and the size of the currents was similar to those generated by full-length recombinant WT Vpr (at approximately 2 to 20 nM). The channels had the same ion selectivity as WT Vpr channels. Channels formed by N40 did not show rectification at positive potentials, consistent with the absence of the C-terminal region. Figure 6A depicts a typical example of currents induced by N40 at different holding potentials and the current-voltage relationship (Fig. 6B). From these results, we conclude that the fundamental channel-forming structure in Vpr is located in the N-terminal region of the molecule and probably involves the predicted amphipathic α-helix that includes amino acids 16 to 35.
FIG. 6.
Synthetic peptides: examples of currents generated in planar lipid bilayers by N40 (A) and C21 (C and D) at different holding potentials. The dashed lines indicate the zero current levels. The average currents are plotted versus holding potential (B) for N40 (crosses), N21 (closed circles), and, for comparison, WT Vpr (open circles). Currents generated by C21 are shown under normal experimental conditions (C) or when phospholipids contained the negatively charged lipid PS (D).
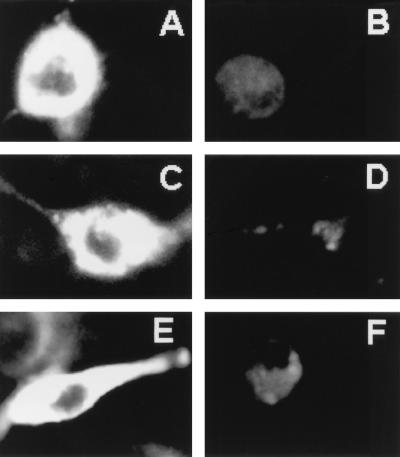
Qualitative assessments of transmembrane potential changes in intact neurons were performed as previously described with the potential-sensitive dye DiBa-C4(3) (28). Extracellular addition of N40 (at 1 mM) resulted in increased fluorescence of the dye as seen with WT Vpr (at approximately 1 μM [Fig. 7A]), indicating a similar level of depolarization. The approximately 1,000-fold relative potency difference between full-length WT Vpr and N40 thus agreed very well with the difference observed in the ability to form channels in planar lipid bilayers (see above).
FIG. 7.
Confocal images of hippocampal neurons in response to the external application of purified Vpr (A), C21 (B), N40 (C), N21 (D), 200 mM KCl (E), and CHAPS buffer (F) with the potential-sensitive dye DiBa-C4(3). Purified full-length Vpr or the synthetic peptides were added to a final concentration of 1 μM or 1 mM, respectively. Images were taken between 10 and 13 min after treatment at 37°C.
Exposure to N21 did not give an increased fluorescence (Fig. 7D), indicating that the plasmalemma was not significantly depolarized by this peptide. It was concluded that the N-terminal 40 amino acids of Vpr are mainly responsible for the ability of WT Vpr to depolarize the plasmalemma of neurons.
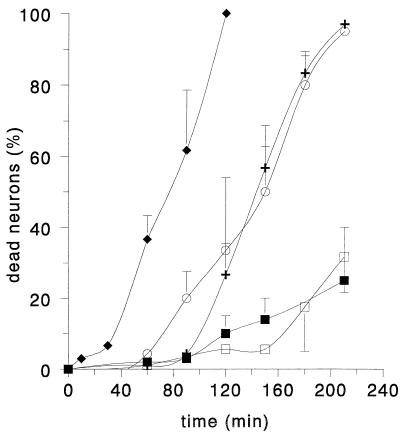
Death of cultured hippocampal neurons after exposure to N21 and N40 was determined with PI in combination with CLSM (see Materials and Methods). Exposure to N40 and WT Vpr resulted in similar rates of cell death, while exposure to N21 did not result in cell killing at rates higher than those in control experiments with CHAPS buffer (Fig. 8). The killing of neurons by the N40 peptide and by WT Vpr is consistent with their ability to form ion channels (Fig. 6) and to cause plasmalemma depolarization (Fig. 7).
FIG. 8.
Cytotoxic effects of extracellular addition of WT Vpr (1 μM) and Vpr peptides (1 mM) on hippocampal neurons expressed as percentages of dead neurons. The figure shows the effects of exposure to WT Vpr (open circles), N40 (crosses), C21 (filled diamonds), N21 (filled squares), and CHAPS buffer (open squares).
Unfortunately, it was not possible to inhibit depolarization or cell killing with the antibodies specific to the N (AbN) or C (AbC) terminus of Vpr. While AbN actually activates the channel activity of Vpr, AbC does not recognize the folded structure of Vpr in CHAPS buffer but rather recognizes the C terminus (which would be on the cytoplasmic side of the membrane) only after the conformational change that occurs upon membrane insertion (27). For that reason, AbC could also not be used for immunodepletion experiments. Our conclusion that the observed effects on neurons are a property of the Vpr N-terminal domain is thus based primarily on the high degree of purity of the full-length protein and N40 peptide (see Materials and Methods and also Fig. 1B). Because the WT Vpr and synthetic peptide were purified from completely independent sources which could not contain the same contaminating molecules, we can conclude that the effects are directly attributable to the predominant, common, Vpr-specific sequences in the preparations of both and not to two completely unrelated minor contaminating species.
A C-terminal peptide kills intact neurons.
A peptide corresponding to the last 21 amino acids at the C terminus of Vpr (C21) was also synthesized to assess its capacity to form ion channels and to affect intact neurons. Under our usual experimental conditions, the C21 peptide did not produce currents in planar lipid bilayers nor did exposure of intact neurons to extracellular C21 result in membrane depolarization detectable by DiBa-C4(3) fluorescence (Fig. 7B). However, this peptide consistently resulted in the highest rates of cell death as indicated by PI fluorescence (Fig. 8). This is consistent with the observations of Macreadie et al. (21), who reported that externally added peptides corresponding to the C-terminal 21 to 26 amino acids of Vpr kill yeast cells.
We decided to investigate further the effect of C21 on planar lipid bilayers. It was found that ionic currents could indeed be observed when the trans chamber was held at very high negative potentials (≥100 mV). These currents ceased upon returning to more positive potentials (Fig. 6C). It has been reported previously (1) that highly basic peptides more readily induce ionic currents in membranes containing phospholipids with negatively charged head groups. Accordingly, we tested C21 in planar lipid bilayers containing PS (Fig. 6D). Under these conditions, currents were observed at more moderate voltages. WT Vpr and the N40 peptide formed channels with identical characteristics in the presence or absence of PS (data not shown).
In summary, the C-terminal peptide of Vpr was found to be highly cytotoxic, but because the conditions needed to induce ion channel activity with C21 were not required for ion channel activity by full-length Vpr or N40, and because the C21 peptide did not cause membrane depolarization detectable with the fluorescent dye, we conclude that the cell killing by C21 may be caused by a mechanism different from that of WT Vpr. Further, while both regions of Vpr have cytotoxic effects on intact neurons, it appears that the N-terminal region of Vpr forms ion channels more readily than does the C-terminal region, which requires higher potentials or the presence of negatively charged phospholipids to form channels. In addition, the cytotoxicity of the C-terminal region is clearly reduced when it is incorporated into full-length Vpr. Therefore, the N-terminal region is more likely to be responsible for detrimental effects of Vpr on neurons (which may lead to AIDS dementia) during human HIV infection.
DISCUSSION
The results here identify the N-terminal region of Vpr, proposed to contain an amphipathic α-helix (amino acids 16 to 35), as the region responsible for ion channel formation by WT Vpr in planar lipid bilayers. The N-terminal peptide, N40, causes gross depolarization of the plasmalemma in neurons similar to that caused by WT Vpr and N40 kills cultured rat hippocampal neurons at a rate similar to that for WT Vpr, suggesting that ion channels formed by the N-terminal region of Vpr may be important for its depolarizing and cytotoxic effects. The ability of Vpr to form ion channels, depolarize the plasmalemma, and kill neurons could thus be mimicked by the first 40 N-terminal amino acids of Vpr. The N-terminal region of Vpr is highly conserved (36) and has been proposed to play important roles in other Vpr functions such as nuclear localization (8, 38), virion incorporation (8, 24, 25, 38), and oligomerization (39). A link between ion channel formation by Vpr and any of these roles requires further investigation.
The N40 peptide is approximately 1,000-fold less potent than full-length WT Vpr, both in its ability to form channels in planar lipid bilayers and in its ability to depolarize and kill neurons. Presumably, this reflects the fact that parts of Vpr additional to the N terminus play a role in either membrane insertion or maintenance of Vpr in the membrane. That both activities indicate the same difference in potency between full-length Vpr and N40 supports the notion that the activities are interrelated.
Our data showing that both E21Q and E24Q mutations in Vpr alter the ion selectivity of the channel, together with the circular dichroism studies (20) which show that the N-terminal region is α-helical, are consistent with the idea that both E21 and E24 are on the same hydrophilic face of an amphipathic helix that either lines the internal surface of the pore or contributes to a region of negative charge involved in the cation selection mechanism.
Results from this study demonstrate that the hydrophobic region of Vpr (amino acids 53 to 71), previously predicted to be the ion channel-forming region (27), is not essential for Vpr to form ion channels in planar lipid bilayers. Mutations disrupting this structural region of Vpr (VprΔ2 and VprE58Q) resulted in ion channel activity similar to that caused by WT Vpr (Fig. 2). Similarly, C-terminal truncation and a point mutation in the C terminus did not alter the ability of the mutants to form ion channels. The fact that a peptide comprising the C-terminal 21 residues of Vpr was able to conduct ions in planar lipid bilayers only under certain conditions—high negative potential or in the presence of negatively charged lipids, neither of which was needed for WT Vpr to form ion channels—supports our previous conclusion that the C terminus of Vpr does not play a major role in ion channel formation in lipid bilayers but is rather responsible for the rectification of currents in WT Vpr channel activity (27). The C-terminal peptide also failed to cause membrane depolarization detectable with the fluorescent dye in hippocampal neurons.
It has recently been reported (2, 21) that the extracellular application of synthetic peptides encompassing parts of the C-terminal region of Vpr including the conserved H(F/S)RIG domain can cause membrane permeabilization and cell death in yeast and mitochondrial dysfunction in CD4+ lymphocytes. Interestingly, the transmembrane potential in yeast and mitochondria is in the range of −150 to −200 mV (5, 37), which is similar to the potentials required to initiate ion channel activity in planar lipid bilayer experiments in this study. Neurons, in contrast, maintain transmembrane potentials of −50 to −75 mV, which are below the required potential for induction of ion channel activity observed with C21. We were unable to detect plasmalemma depolarization with the fluorescent dye in hippocampal neurons exposed to C21 (Fig. 7), although we did observe cytotoxic effects (Fig. 8). Thus, although we conclude that the Vpr N terminus is responsible for ion channel formation, plasmalemma depolarization, and neuronal death caused by full-length Vpr, we cannot exclude the possibility that the C terminus has additional roles under certain conditions in particular cell types. Further studies are needed to elucidate the cause of the cytotoxic activity of the C-terminal region of Vpr and its physiological relevance.
In conclusion, our results clearly indicate that the N-terminal domain of Vpr is the main region responsible for cation-selective channel activity in planar lipid bilayers and, more importantly, for the extracellular effects of Vpr on cultured hippocampal neurons including gross plasmalemma depolarization and cell death. Interestingly, preliminary results suggest that these effects are cell specific as they were not observed in cultured rat hepatoma cells exposed to WT Vpr (see also reference 28). Vpr has been reported to be present in the serum of AIDS patients (18), where because enzyme-linked immunosorbent assay quantification was not significantly affected by freezing and thawing or the presence of virus-disrupting agents, it was concluded that the majority of the Vpr was in a non-virus-associated form (1 to 100 pM). Although in this work we use a relatively high concentration of Vpr (1 μM) to facilitate our assays, in a previous study (28) we report that application of Vpr at 0.6 nM to hippocampal neurons caused a large cation current to be detected. It is feasible that, in vivo, long-term exposure to even lower concentrations of free Vpr may be able to impair neuron function. If the ion channel function of Vpr, which can cause plasmalemma depolarization and cell death, proves to be involved in causing neurological disorders observed in AIDS patients with high Vpr levels in the cerebrospinal fluid in vivo, new therapeutic approaches to block the Vpr ion channel may conceivably assist in treating these AIDS symptoms.
REFERENCES
- 1.Agawa Y, Lee S, Ono S, Aoyagi H, Taniguchi T, Anzai K, Kirino Y. Interaction with phospholipid bilayers, ion channel formation, and antimicrobial activity of basic amphipathic alpha-helical model peptides of various chain lengths. J Biol Chem. 1991;266:20218–20222. [PubMed] [Google Scholar]
- 2.Arunagiri C, Macreadie I G, Hewish D, Azad A A. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2:69–76. doi: 10.1023/a:1026487609215. [DOI] [PubMed] [Google Scholar]
- 3.Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 4.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry E A, Hinkle P C. Measurement of the electrochemical proton gradient in submitochondrial particles. J Biol Chem. 1983;258:1474–1486. [PubMed] [Google Scholar]
- 6.Bodeus M, Margottin F, Dyrand H, Rouer E, Benarous R. Inhibition of prokaryotic cell growth by HIV1 Vpr. Res Virol. 1997;148:207–213. doi: 10.1016/s0923-2516(97)83990-8. [DOI] [PubMed] [Google Scholar]
- 7.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. Identification of HIV-1 vpr product and function. J Acquired Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 8.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau N R. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol. 1996;6:1096–1103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 10.Ewart G D, Sutherland T, Gage P W, Cox G B. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J Virol. 1996;70:7108–7115. doi: 10.1128/jvi.70.10.7108-7115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holsinger L J, Nichani D, Pinto L H, Lamb R A. Influenza A virus M2 ion channel protein: a structure-function analysis. J Virol. 1994;68:1551–1563. doi: 10.1128/jvi.68.3.1551-1563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jans D A, Jans P, Briggs L J, Sutton V, Trapani J A. Nuclear transport of granzyme B (fragmentin-2)-dependence on perforin in vivo and cytosolic factors in vitro. J Biol Chem. 1996;271:30781–30789. doi: 10.1074/jbc.271.48.30781. [DOI] [PubMed] [Google Scholar]
- 16.Jowett J B, Planelles V, Poon B, Shah N P, Chen M L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy D N, Refaeli Y, MacGregor R R, Weiner D B. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994;91:10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy D N, Refaeli Y, Weiner D B. The vpr regulatory gene of HIV. Curr Top Microbiol Immunol. 1995;193:209–236. doi: 10.1007/978-3-642-78929-8_11. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Z, Butcher D J, Murali R, Srinivasan A, Huang Z. Structural studies of synthetic peptide fragments derived from the HIV-1 Vpr protein. Biochem Biophys Res Commun. 1998;244:732–736. doi: 10.1006/bbrc.1998.8330. [DOI] [PubMed] [Google Scholar]
- 21.Macreadie I G, Arunagiri C K, Hewish D R, White J F, Azad A A. Extracellular addition of a domain of HIV-1 Vpr containing the amino acid sequence motif H(S/F)RIG causes cell membrane permeabilization and death. Mol Microbiol. 1996;19:1185–1192. doi: 10.1111/j.1365-2958.1996.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 22.Macreadie I G, Thorburn D R, Kirby D M, Castelli L A, Rozario N L, Azad A A. HIV-1 protein Vpr causes gross mitochondrial dysfunction in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410:145–149. doi: 10.1016/s0014-5793(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 23.Mahalingam S, Collman R G, Patel M, Monken C E, Srinivasan A. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virology. 1995;212:331–339. doi: 10.1006/viro.1995.1490. [DOI] [PubMed] [Google Scholar]
- 24.Mahalingam S, Khan S A, Jabbar M A, Monken C E, Collman R G, Srinivasan A. Identification of residues in the N-terminal acidic domain of HIV-1 Vpr essential for virion incorporation. Virology. 1995;207:297–302. doi: 10.1006/viro.1995.1081. [DOI] [PubMed] [Google Scholar]
- 25.Mahalingam S, Khan S A, Murali R, Jabbar M A, Monken C E, Collman R G, Srinivasan A. Mutagenesis of the putative alpha-helical domain of the Vpr protein of human immunodeficiency virus type 1: effect on stability and virion incorporation. Proc Natl Acad Sci USA. 1995;92:3794–3798. doi: 10.1073/pnas.92.9.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandler R N, Schaffner A E, Novotny E A, Lange G D, Barker J L. Flow cytometric analysis of membrane potential in embryonic rat spinal cord cells. J Neurosci Methods. 1988;22:203–213. doi: 10.1016/0165-0270(88)90041-6. [DOI] [PubMed] [Google Scholar]
- 27.Piller S C, Ewart G D, Premkumar A, Cox G B, Gage P W. Vpr protein of human immunodeficiency virus type 1 forms cation-selective channels in planar lipid bilayers. Proc Natl Acad Sci USA. 1996;93:111–115. doi: 10.1073/pnas.93.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piller S C, Jans P, Gage P W, Jans D A. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc Natl Acad Sci USA. 1998;95:4595–4600. doi: 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto L H, Dieckmann G R, Gandhi C S, Papworth C G, Braman J, Shaughnessy M A, Lear J D, Lamb R A, DeGrado W F. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc Natl Acad Sci USA. 1997;94:11301–11306. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planelles V, Jowett J B, Li Q X, Xie Y, Hahn B, Chen I S. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Premkumar L S, Gage P W, Chung S H. Coupled potassium channels induced by arachidonic acid in cultured neurons. Proc R Soc Lond B Biol Sci. 1990;242:17–22. doi: 10.1098/rspb.1990.0097. [DOI] [PubMed] [Google Scholar]
- 32.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schubert U, Ferrer Montiel A V, Oblatt Montal M, Henklein P, Strebel K, Montal M. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 1996;398:12–18. doi: 10.1016/s0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- 34.Stewart S A, Poon B, Jowett J B, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunstrom N A, Premkumar L S, Premkumar A, Ewart G, Cox G B, Gage P W. Ion channels formed by NB, an influenza B virus protein. J Membr Biol. 1996;150:127–132. doi: 10.1007/s002329900037. [DOI] [PubMed] [Google Scholar]
- 36.Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vacata V, Kotyk A, Sigler K. Membrane potentials in yeast cells measured by direct and indirect methods. Biochim Biophys Acta. 1981;643:265–268. doi: 10.1016/0005-2736(81)90241-8. [DOI] [PubMed] [Google Scholar]
- 38.Yao X J, Subbramanian R A, Rougeau N, Boisvert F, Bergeron D, Cohen E A. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao L J, Wang L, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 Vpr function. Oligomerization mediated by the N-terminal domain. J Biol Chem. 1994;269:32131–32137. [PubMed] [Google Scholar]
- 40.Zhou Y, Lu Y, Ratner L. Arginine residues in the C-terminus of HIV-1 Vpr are important for nuclear localization and cell cycle arrest. Virology. 1998;242:414–424. doi: 10.1006/viro.1998.9028. [DOI] [PubMed] [Google Scholar]