Abstract
In the context of a large and growing burden of diabetes-related morbidity and missed opportunities to employ evidence-based care and prevention, the World Health Organization (WHO) has initiated the Global Diabetes Compact to prioritize evidence-based interventions, resources, and objective-setting to reduce the global burden of diabetes. In this report we describe the scientific basis that informed the recommendations of the key health objectives and target levels for the Compact. We considered metrics across 4 domains (structural, system- or policy-level factors; processes of care; biomarkers and behaviours; and health events and outcomes) and risk tiers (diagnosed diabetes, high risk, or whole population). An expert group reviewed and prioritized metrics according to their health importance, modifiability, data availability, and the degree to which they represent gaps and areas of global inequality. We reviewed global distributions of levels for each metric of interest to set target levels for future attainment. This process led to 5 country-level core metrics and target levels for UN member states: 1) at least 80% of the persons with diabetes should be diagnosed; 2) 80% of those with diagnosed diabetes having HbA1c levels below 8.0%; 3) 80% with diagnosed diabetes having blood pressure levels below 140/90 mmHg; 4) 60% with diagnosed diabetes using statins, and, 5) 100% of persons with type 1 diabetes having continuous access to insulin, blood glucose meters andtest strips. In addition, we propose several complementary metrics that currently have limited global coverage but warrant surveillance scale-up. These include, among persons with diabetes, documentation and routine collection of all-cause and cause-specific mortality estimates, and incidence of end-stage kidney disease, lower-extremity amputations, and incidence of diabetes. We also identified important areas for which the development of metrics is still required, including primary prevention of diabetes and integrated care. Achieving the overarching goals of the Global Diabetes Compact will require multi-sectoral efforts applied to individuals, health systems, policies, and country-level actions.
BACKGROUND AND RATIONALE
Diabetes mellitus is one of the world’s most challenging public health problems due to its high and growing prevalence and the diverse and extensive morbidity it causes, impacting individuals, health systems, and national economies1,2. Recent global estimates indicate that 537 million adults have the condition, of whom 80% live in low- and middle-income countries (LMICs)1,3. Further, the global impact and costs of diabetes are expected to continue to grow considerably, disproportionately affecting LMICs and the most disadvantaged people of high-income countries (HICs)4–6.
Despite the relentless growth of diabetes, the pathways to its adverse outcomes are highly modifiable across a broad continuum of its pathogenesis and many of interventions are cost-effective and feasible to implement. For people with diagnosed diabetes, delivery of essential medications, management of glycaemia and other cardiometabolic risk factors, alongside early screening for complications via well-organized care reduce acute and chronic complications and extend life 2,7–10. Further, type 2 diabetes can be delayed or prevented through intensive lifestyle interventions and medications directed at high-risk individuals or through population-wide changes to dietary quality, physical activity levels, and levels of obesity11–15.
Unfortunately, population-based studies have shown that the delivery of evidence-based care for people with diabetes is sub-optimal even in well-resourced health systems. Many countries have high proportions of their diabetes16 populations undiagnosed and without timely care for extended periods17–19. In HICs, the achievement of recommended targets of risk factor for complications such HbA1c and blood pressure control ranges from 50-70% and only about 20% meet all recommended targets 20–22. Levels are worse in LMICs, where only about half have good glycaemic control and about one in four have good blood pressure control6,18,23,24. Multicomponent quality-improvement initiatives have shown sustained benefits in achievement of diabetes care goals and vascular complications, even in low resource settings, but have had limited global reach25,26. Similarly, the implementation of primary prevention programmes has been variable and non-systematic at best2,27.
In the context of a large and growing burden of diabetes-related morbidity and missed opportunities to employ evidence-based care and prevention, the World Health Organization (WHO) recently announced the Compact18,27,28. Building on the Global Action Plan for the Prevention and Control of NCDs and on resolution 74.4 of World Health Assembly (Reducing the burden of non-communicable diseases through strengthening the prevention and control of diabetes), the Global Diabetes Compact sets priority metrics and target levels to serve as diabetes-related health objectives for all countries of the world to achieve by 2030. Based on prior successes in HIV29 and the premise that measurement drives action, the Compact targets are intended to drive prioritisation of interventions and resources for diabetes at the national, regional, and global levels30,31.
Setting targets for diabetes is challenging because of the breadth and diversity of the problem, as there are opportunities to affect risk for diabetes, progression, and outcomes across the disease course. The various targets include risk factors for diabetes in the whole population, progression to diabetes in high-risk populations, and preventing complications and mortality among those with diabetes. At the same time, it is important that health objectives be focused, parsimonious, simple, and relatively easy to measure.
In this report we describe the scientific basis that informed the selection of key health objectives and target levels for the Global Diabetes Compact. To do this, we review and describe the range of options for target metrics, including their strengths, weaknesses, and feasibility to measure and implement. Based on this literature synthesis and systematic prioritization process, we propose core and complementary metrics, their definitions, and target levels for the Compact to stimulate global action.
SUMMARY OF METHODS AND APPROACH:
To prioritize metrics and target levels, we used the following process (Figure 1). First, we organized potential metrics across 4 domains (policy and system-level factors, processes of care, biomarkers and behaviours, and long-term health outcomes) and risk tiers (diagnosed diabetes, high risk for diabetes, whole population)32. Second, the authors reviewed, scored and filtered, and then prioritized metrics through a consensus-based process according to their health importance, modifiability, data availability, and the degree to which they represent areas of global inequality. This led to a set of “core”, “complementary”, and “base” metrics. The core metrics are intended for priority implementation by UN member states and monitoring by the Global Diabetes Compact. The complementary metrics currently lack adequate global data availability or consensus-based definitions, and thus are currently unsuitable for recommendation as core Compact metrics but should be considered for scale-up in population health data and surveillance systems. Base metrics are additional processes or health indicators that are essential for the calculation of core and complementary metrics. Third, we reviewed published and unpublished data on the current levels of attainment of the chosen metrics, by global region and country and evidence from modelling-based studies to estimate the expected health impact of meeting different target levels. Fourth, we used the information and evidence from these steps to propose a set of target levels for core metrics. Finally, the proposed metrics and target levels were presented to a WHO-convened, international review panel and reviewed by member states’ ministries of health and WHO regional offices. Our recommendations incorporate input from all steps in this process.
Figure 1.
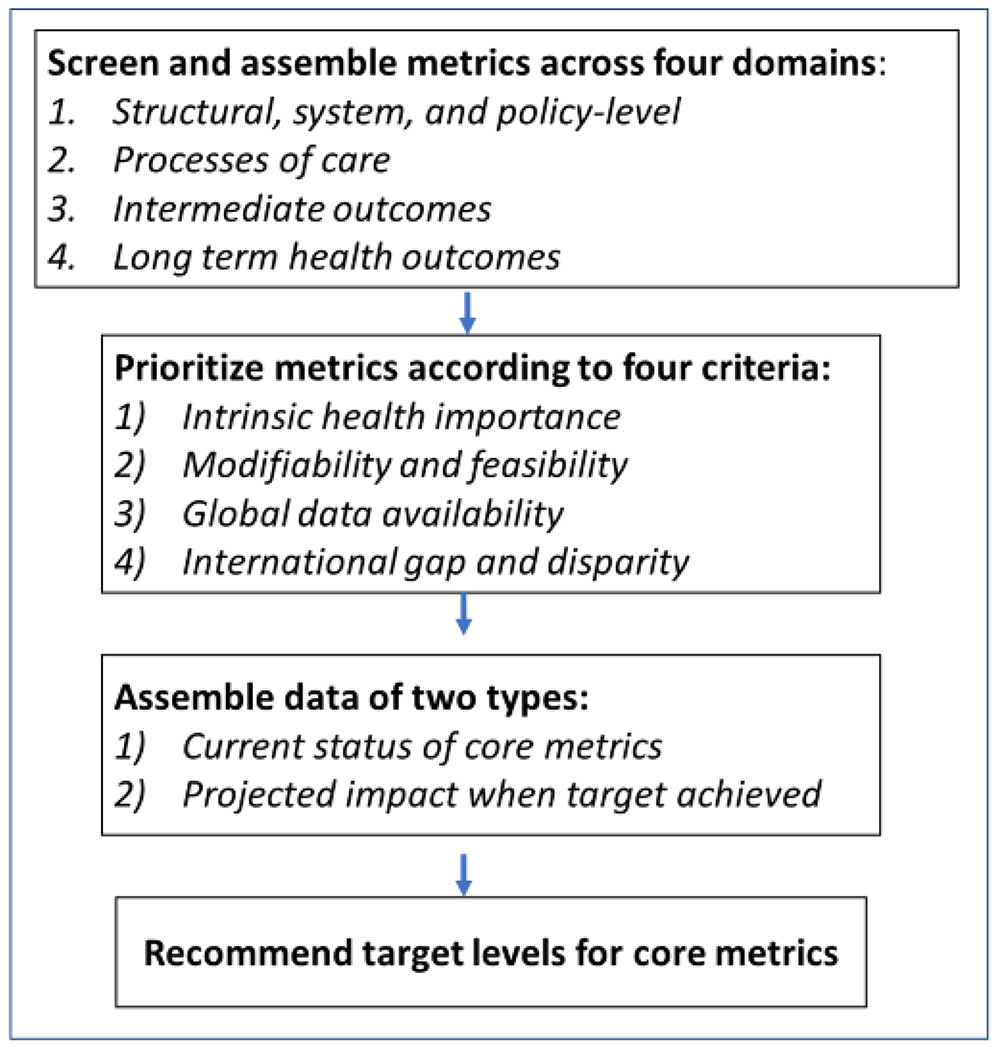
Methods and steps to recommend Global Diabetes Compact metrics and targets.
SCIENTIFIC RATIONALE AND OPTIONS FOR METRICS AND TARGETS
Taxonomy and Range of Options for Health Metrics:
Target-setting for public health efforts is credited with influencing major successes in public health, ranging from vaccine delivery to the reductions in HIV and CVD-related mortality29 Numerous criteria have been used to establish health metrics and their targets. Metrics can be applied to individuals (e.g. clinical health conditions, biomarkers, or behaviours), or to health care providers and health systems (e.g. indicators of the delivery of interventions, or the presence of policies, or processes)33. Metrics may also represent actions or policies taken by broader institutions or governments. For the Compact, we have organized metrics into four domains: structural, system- or policy-level factors; processes of care, intermediate outcomes, health events and outcomes. (Table 1).
Table 1.
Potential metrics for the for the Global Diabetes Compact, stratified by domain and risk tiers.
| Structural, system, or policy factors | Processes of care | Intermediate outcomes | Health events and outcomes | |
|---|---|---|---|---|
| Diagnosed diabetes | National or regional health system diabetes registry Guidelines and dissemination efforts Presence of Decision support tools Facilities with essential medicines |
Diagnosis of diabetes Receiving treatment among diagnosed Availability of essential medicines Team-based care Statin use Diabetes education Vaccinations Foot exam Eye exam Renal testing |
Glycaemic control Controlled blood pressure Controlled lipids Microalbuminuria |
Diabetes prevalence Diabetes incidence Hyperglycaemic emergencies DM-related death hospitalisation CKD prevalence Incidence of lower extremity amputation Retinopathy prevalence Incidence of end stage kidney disease Incidence of CVD events Incidence of CVD death |
| High risk | Programmes or support for nutritional counselling Programmes/support for structured lifestyle interventions Guidelines for testing and referral |
Structured lifestyle programme Counselling for diet/exercise Testing for diabetes Metformin prescriptions Glycaemic assessments for gestational DM |
Intermediate hyperglycaemia Controlled blood pressure Controlled Lipids Body mass index |
Diabetes prevalence Diabetes incidence |
| Whole population | Facilities with essential medicines Population-based survey with blood glucose Presence of a policy to increase physical activity Presence of incentives for healthy diet programmes Food policy taxation (sugar sweetened beverages) Policies for smoking prevention |
Smoking cessation services Proportion of population with healthcare coverage for DM and CVD risk factors |
Physical activity levels Body mass index Fruit and vegetable consumption |
Diabetes prevalence Diabetes incidence |
In this framework, structural, system- or policy-level factors address multiple aspects of health services delivery or can target the entire population. For example, systematic reviews have shown that the assembly of multi-disciplinary teams for care management and decision-support via patient registries improves risk factors and management that should improve health outcomes 34–38. Processes of care are procedures conducted by health care providers or individuals or steps that are considered essential on the pathway to affecting biomarkers, behaviours, and long-term health outcomes39. For example, dilated eye exams, foot exams, and regular monitoring of cardiovascular, renal, and metabolic indices are considered crucial to the prevention of diabetes complications. Similarly, monitoring the uptake of lifestyle interventions is important, as such interventions reduce the incidence of diabetes 13,14. Intermediate outcomes are biomarkers and behaviours that were selected if they have been shown to be independently associated with long-term diabetes-related health outcomes, ideally established through randomized controlled trials. For example, reducing HbA1c, blood pressure, and lipids are associated with reduced microvascular and macrovascular health outcomes and related mortality2,7,8,40,41Finally, diabetes-related health events and outcomes are defined as those that have a direct impact on individual-level quality of life or health system burden and differentiate health outcomes in the diabetes population from those without diabetes (Table 1). They may include indicators of disease burden like diabetes prevalence and incidence, as well as the incidence of diabetes-related complications like lower extremity amputations (LEAs), end-stage kidney disease (ESKD), or CVD mortality42..
The metrics can also be organized according to the risk tier or stage of the disease that they primarily affect, including persons with diagnosed diabetes, persons at high risk (such as intermediate hyperglycaemia), or the whole population (Table 1). For example, managing blood glucose is likely most important in persons with diagnosed diabetes and improving overall dietary quality and physical activity, or applying policies such as taxes or incentives to promote healthful behaviours may be particularly important in the general population43.
Criteria for Prioritizing Metrics for Diabetes
The selection of any given metric has advantages and disadvantages. For example, reducing health events and outcomes are closest to the ultimate goals of clinical and public health practices, but can be difficult to measure, difficult to modify in the short term, and are uninformative about what factors are driving change44. Processes of care may be immediately measurable and responsive to interventions in the short term but may not predict health changes well1,45,46. Biomarkers and behaviours are both modifiable and predictive of long-term outcomes, have generally standardized measurement approaches with reasonable global reach41; however, there is a lack of consensus on the appropriate target thresholds, and obtaining reliable and comparable measures across different settings can be difficult. System and policy-level metrics have wide variation in adoption, can be difficult to implement in the short-term, have modest effect sizes, or inconsistently predict health outcomes at the individual level when achieved10,45,47. However, they have the potential to efficiently affect multiple risk factors and large segments of the population.
The selection of different population risk tiers also has trade-offs. Focusing on people with established disease or high risk may meet immediate health system demands and have more evidence for short-term effectiveness but not achieve the long-term goal of preventing the condition itself. Interventions aimed at the whole population depend upon policy-level interventions that can be difficult to measure and have unclear magnitudes of effect but may have important benefits over longer time horizons45. Focusing on prevention among at-risk adults with individualized prevention approaches has established efficacy but few examples of successful population-wide scale-up exist.
To prioritize metrics for the Compact, we considered their performance against four main criteria (Table 2)16,33. First, priority metrics should be of intrinsic health importance or else be a factor or intervention that strongly predicts major health events or outcomes. Second, a good metric should be modifiable via scalable interventions across diverse settings. Third, priority metrics should have good global data availability and acceptable measurement properties, be reasonably consistent across settings and be measurable through practical surveillance approaches. Fourth, priority metrics should ideally represent a gap and area of global inequality that is modifiable. The best core metrics should score well on all criteria, but data availability is a particular limiting factor. For the Compact to proceed with 2030 targets and determine recent or current levels as a baseline it is essential to use metrics that do not require new infrastructure development to collect.
Table 2.
Criterion and rating scale for potential metrics of the Global Diabetes Compact.
| Criterion | Excellent | Good | Fair |
|---|---|---|---|
| Intrinsic health importance or strong evidence for prediction or benefit on major health outcomes. | Major health outcome affecting QOL (e.g., MI, LEA). | Biomarker or intervention with clear causal linkage to health outcome. | Process, intervention, or factor with potential linkage. |
| Modifiable with scalable interventions targeting the metric. | Clearly efficacious and scalable via evidence-based means. | Moderately efficacious and scalable. | Lacking clear scalability – or – clear health effect if scalable. |
| Strong global data availability with acceptable measurement properties. | Currently available for 75% of countries. | Currently available for 25 - 75% of countries. | Available for fewer than 25% of countries. |
| International gap and disparity | Large proportion of population affected and large international variation | Large proportion of population affected – OR - large international variation | Modest international gap or limited variation |
Once metrics are identified, the selection of appropriate target levels presents an additional challenge. Health targets should ideally be specific, measurable, achievable, realistic, and time bound (SMART)48, 16,33 They should also be ambitious enough to affect health outcomes, which can present a difficult balance to achieve alongside the need to be realistic and achievable. Many approaches have been used to set targets in public health efforts33. Some approaches start with a static baseline level and then assign a relative or absolute percentage improvement, or calculate a target based on the minimally statistically significant change. Other methods evaluate the baseline trend and set the target to either maintain or add a percentage improvement to the slope. Others assign targets to be consistent with clinical guidelines. Finally, other approaches set fixed targets to be applied universally across settings, using the best current level across the subgroups, or else by setting an optimal level based on consensus and multiple criteria. If biomarkers are to be expressed as dichotomous targets, they also require a decision about the threshold to be used. This is typically based on clinical guidelines but sometimes aims to identify a level of risk that represents poor care or high risk for which virtually all settings should aim to reach. We considered each of these methods and data summarized below to arrive at consensus-based recommendations.
PRIORITIZATION OF METRICS AND TARGETS
Using the categories of metrics described in Table 1 and the criteria described in Table 2, these led to 5 core, 10 complementary, and two base metrics that have the best combination of health importance, modifiability, global data availability, and equity (Figure 2). These metrics can also be organized along a continuum, from the base metrics that are essential to understand the general status of the epidemic (e.g., prevalence) and to assemble essential data to calculate metrics, to metrics of prevention, processes of care, and then to the outcomes essential to estimate health impact. The primary distinction between core and complementary metrics are that core metrics can currently be assessed in many countries using health surveys or registries, whereas complementary metrics require more surveillance infrastructure, scale-up, or international consensus on operational definitions and measurement specification.
Figure 2. Proposed core, complementary, and base metrics for the Global Diabetes Compact.
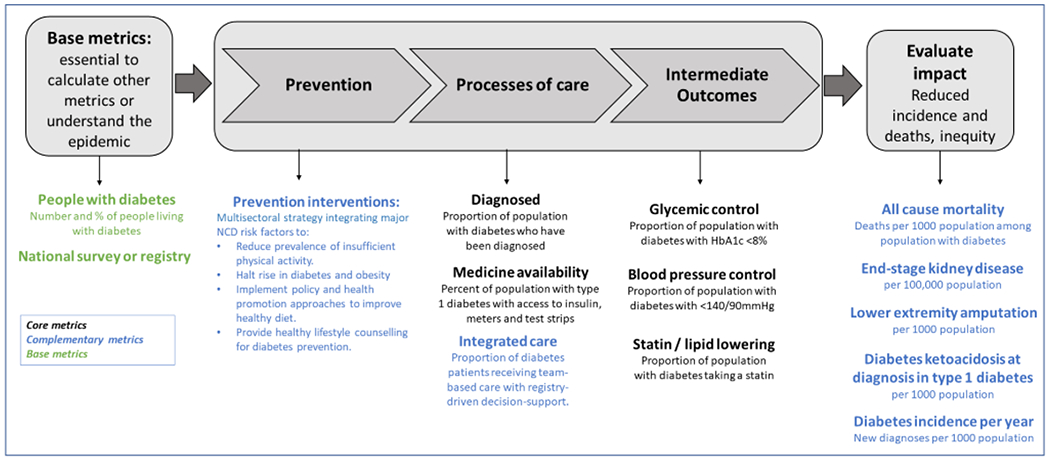
Recommended core metrics shown in black, complementary metrics in blue, and base metrics in green. The core metrics are intended for priority implementation by UN member states and monitoring by the Global Diabetes Compact. The complementary metrics currently lack adequate global data availability or consensus-based definitions but should be considered for scale-up in population health data and surveillance systems. Base metrics are additional processes or health indicators that are essential for the calculation of core and complementary metrics.
Core Metrics
The proposed core metrics include the following:
The proportion of cases that are diagnosed out of the total number with diabetes. The total number with diabetes is defined by either self-reported prior diagnosis, taking diabetes medications, or having elevated HbA1c or fasting glucose.
The proportion of adults with diagnosed diabetes with controlled HbA1c, defined as less than 8%.
The proportion of adults with diagnosed diabetes who have controlled blood pressure, defined as less than 140/90 mmHg.
The proportion of adults with diagnosed diabetes aged ≥ 40 years taking a statin.
The proportion of the population with type 1 diabetes having continuous access to insulin, blood glucose meters, and test strips..
Diagnosis of diabetes was selected as a core metric because it is an essential step in linking those affected with treatments and preventive screenings for diabetes complications. Although the effectiveness of community-based testing and population-wide screening remains unclear and not established by randomized controlled trials (RCTs)49,50, opportunistic testing in clinical practice is recommended if the health care system is considered sufficient to handle increasing case loads. It has also been shown to be cost-effective in some HICs if paired with identification of high-risk individuals for lifestyle change9,51,52. Further, the levels of diagnosis have been shown to be starkly low in many LMICs18,24. Attaining good HbA1c control reduces risk of acute, microvascular, and to a lesser extent, macrovascular outcomes 2,7,8. Improving blood pressure levels and taking statins reduce risk for CVD events in persons with diagnosed diabetes2,53. Ensuring insulin access was prioritized because of its recognized lack of availability in some settings, with the result of deaths and high complication rates, often among children and young adults,54,55.
Three of the metrics (glycaemic control, blood pressure, statin use) are highly modifiable using affordable medications available in primary care, particularly if supported by team-based care. Diabetes diagnosis and insulin availability can each be improved through concerted health system or policy-level interventions. The metrics can be quantified with STEPs surveys or other nationally representative surveys56 or via other WHO surveillance systems such as the WHO biennial Country Capacity survey57–59.
These metrics may also be specified in different ways. If data are collected from respondents with both diagnosed diabetes and undiagnosed diabetes, countries will have the option of considering levels of control among the total population with diabetes as the denominator instead of those with diagnosed diabetes60. A potentially more precise formulation of diagnosis over time is the number of undiagnosed cases divided by the number without diagnosed diabetes (i.e, undiagnosed cases plus non-cases)61, but this alternative metric is less intuitive to policy makers and has not been applied internationally.
Complementary and Base Metrics:
Several additional complementary and base metrics are important to monitor delivery of evidence-based interventions or are long-term health outcomes of diabetes. All-cause mortality in people with diabetes, end-stage kidney disease, and lower-extremity amputations among the population with diagnosed diabetes were each prioritized because they are intrinsically important health outcomes, highly modifiable via established evidence-based practices, and lend themselves to standardized, objective, population-based monitoring. They also represent good sentinel indicators of secondary prevention because they are affected by multiple aspects of recommended care. Incidence of diagnosed diabetes is an important metric because it reflects a change in the direction of the diabetes epidemic with more sensitivity and is less affected by mortality than is prevalence. However, its assessment requires either very large panel surveys or population-based registries that are available only in a few countries62,63. A fifth metric, the percent of cases of type 1 diabetes who have diabetic ketoacidosis (DKA) at diagnosis, is a recognized proxy for timely diagnosis of type 1 diabetes64. In addition to being a cause of morbidity, subsequent DKA, and mortality, timely diagnosis of type 1 diabetes is considered modifiable through improved community awareness about signs and symptoms of type 1 diabetes64.
Improving the delivery and effectiveness of both primary prevention and integrated care are also essential to reduce incidence of diabetes, and its complications, respectively. However, both areas lack consensus in how to quantify and measure success. The WHO has recommended goals of reducing by 10% the prevalence of insufficient physical activity and halting the rise in diabetes and obesity, along with recommending numerous policy and health promotion approaches to improve healthy diet to reduce diabetes risk30. In addition, the WHO Package of Essential Noncommunicable (PEN) disease interventions includes recommendations for healthy lifestyle counselling for diabetes prevention, as well as for organization of care to improve risk factor management65. In some settings, the proportion of high-risk adults with access to diabetes prevention interventions may be considered14,66,67. Similarly, the proportion of patients receiving team-based care with registry-driven decision support are important to facilitate attainment of core targets2. However, to operationalize both of these metrics, there would need to be investments in adequate data systems and agreement about the standardized definitions of these interventions as well about measurement approaches68,69. In addition to the core and complementary metrics, having a national or sub-national population survey in place and measuring diabetes prevalence (Figure 1) with both self-report and some type of glycaemic measures are essential base metrics for the calculation of core metrics, as well as for ongoing monitoring.
Additional potential metrics:
As diabetes is affected by multiple aetiologies and evidence-based options across stages of disease, there are many other potential metrics. Gestational diabetes is an important contributor to the diabetes burden and a key target for prevention of morbidity, but there remains little global consensus on definition and diagnostic criteria, and there is uncertainty over benefits of screening and long-term benefits of treatment70. Treatment with guideline-directed medical therapy, such as taking blood pressure- and glucose-lowering medications, are often used as metrics in cascades of care, and available data suggests that the primary gap in treatment is due to people who have not been diagnosed. Further, the accuracy of treatment status using self-report is unclear and is complicated by the increasing number of medications and drug classes available. In addition, some individuals may be appropriate for management using lifestyle interventions only, which is generally not captured in questions on treatment. Processes of care, including receipt of HbA1c tests, foot, and eye exams are considered essential elements of high-quality diabetes care. However, they were not prioritized because they are inconsistently associated with later health outcomes 10,45,46. Additional policy or system-level factors such as policies to increase physical activity were not prioritized because of difficulties in measurement and lack of agreement about intervention effectiveness45. Upstream risk factors such as body mass index, physical activity levels, and dietary behaviours were also considered but not prioritized because of either difficulty in measurement or lack of agreement about the feasibility of altering them, or inadequate specificity in predicting diabetes incidence or complications.
CURRENT GLOBAL STATUS OF METRICS; VARIATION, LEVELS, AND COVERAGE
To inform the selection of target levels for the metrics for the Compact, we synthesized three types of evidence: 1) Recent and current population-based national estimates to provide realistic baselines; 2) Estimates of trends in rates of metrics over time from various settings to identify a plausible and realistic magnitude of change over time; 3) Estimates of projected health benefit and costs associated with meeting versus not meeting targets.
Region and country-specific estimates.
We assembled data from systematic reviews, a subset of studies from 65 LMICs from the Global Health and Population Project on Access to Care for Cardiometabolic diseases (HPACC) collaborators, and from published estimates from 26 HIC estimates containing levels for the metrics specified and selected for the Compact18,71 24 60. The HPACC is a pooled dataset of WHO STEPS and other nationally representative population-based surveys. As the sample size of persons with diagnosed diabetes was small for many studies, we only used the subset of studied with at least 150 persons in the metric denominator18,24. For the complementary metrics, we also assembled data from previously published reviews of diabetes incidence, all-cause and CVD mortality, and incidence of diabetes-related complications62,72,73.
Tables 3–4 and appendix tables and figures present regional and country-specific estimates for core metrics. Levels of each of the core metrics varied considerably around the world. Among all countries, the median percent diagnosed was 64% (interquartile range 14%). Of diagnosed individuals, the median percent with HbA1c <8%, blood pressure <140/90mmHg, and using statins were 68%, 52%, and 12% respectively. Few studies exist on trends in the attainment of these targets over time. Where they exist, they tend to find large increases during the 1990s and 2000s but generally flat or marginally increasing trends since 2010. In the U.S., for example, the proportion meeting targets increased 12-13 percentage points (PPTs) from 1999-2009 but have been relatively stagnant since 20,22,74–76.
Table 3.
Median levels of percent of the population attaining target levels for core metrics for all regions of the world, and according to world region.
| Total diabetes prevalence | Diagnosed / total diabetes population | Glycaemic control (HbA1c <8%) / diagnosed diabetes (%) | Blood pressure control (<140/90mmHg) / diagnosed diabetes | Statin / diagnosed diabetes population | ||
|---|---|---|---|---|---|---|
| All regions | Mean | 9.5 | 61.1 | 67.1 | 51.9 | 25.9 |
| All regions | Median | 8.3 | 63.7 | 68.1 | 52.8 | 12.3 |
| All regions | IQR | 5.7 | 14.1 | 13.4 | 21.9 | 27.0 |
| East Asia & Pacific | 12.2 | 53.4 | 58 | 52.2 | 12.3 | |
| Europe & Central Asia | 8.3 | 63.7 | 80.1 | 43.7 | 23.6 | |
| Latin America & Caribbean | 9.8 | 69.9 | 68.2 | 65.4 | 10.0 | |
| Middle East & North Africa | 11.5 | 63.7 | 67.6 | 50.8 | 25.1 | |
| North America | 11.7 | 74.1 | 69.9 | 76.4 | 60 | |
| South Asia | 8.6 | 51.6 | 67.3 | 52.8 | 13.4 | |
| Sub-Saharan Africa | 6.6 | 56.7 | 61.6 | 44.8 | 11 | |
Estimates assembled from four primary types of sources: IDF Diabetes Atlas, Global Health and Population Project on Access to Care for Cardiometabolic diseases (HPAAC) collaborators, literature reviews, and web-sites containing estimates from national diabetes surveillance systems. References listed in Appendix.
Table 4.
Published estimates for complementary metrics among people with diabetes in WHO member states.
| Country | Income | DM IRⴕ | All-cause mortality rate § |
ESKD IRⴕ | LEA IRⴕ | DKA Prevalence | |
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| East Asia & Pacific | |||||||
| Australia | HIC | - | 790* | - | - | - | 24.9 |
| Japan | HIC | 88 | - | - | - | - | - |
| New Zealand | HIC | - | - | - | - | - | 26.3 |
| South Korea | HIC | - | 940* | - | - | - | - |
| Data unavailable for 25 countries | |||||||
|
| |||||||
| Europe & Central Asia | |||||||
| Austria | HIC | - | - | - | - | - | 38.0 |
| Czechia | HIC | - | - | - | - | - | 28.8 |
| Denmark | HIC | 6.2 | 4070 | 3680 | - | - | 20.7 |
| Finland | HIC | 35 | 4260* | - | - | 4.8 | - |
| France | HIC | 96 | - | - | - | 15.8 | - |
| Germany | HIC | 87 | - | - | 16.7 | 4.8 | 26.8 |
| Ireland | HIC | - | - | - | - | 17.6 | - |
| Italy | HIC | 40 | 3450* | - | 10.4 | 15.3 | 41.2 |
| Latvia | HIC | - | 5470 | 4380 | - | - | - |
| Luxembourg | HIC | - | - | - | - | - | 43.8 |
| Netherlands | HIC | 37.3 | 970 | 880 | - | 25.1 | - |
| Norway | HIC | 39.8 | 4500 | 4760 | - | - | 22.1 |
| Portugal | HIC | 97.2 | - | - | - | - | - |
| Slovenia | HIC | - | - | - | - | - | 40.3 |
| Spain | HIC | - | - | - | 5.9 | 34.4 | - |
| Sweden | HIC | - | 3380* | - | - | - | 19.5 |
| UK | HIC | 36.9 | 2100 | 2240 | - | 17.6 | 25.0 |
| Russia | UMIC | - | 2320 | - | - | - | - |
| Data unavailable for 31 countries | |||||||
|
| |||||||
| Latin America & Caribbean | |||||||
| Chile | HIC | - | 19.9* | - | - | - | - |
| Brazil | UMIC | 200 | - | - | - | - | - |
| Mexico | UMIC | 140 | - | - | - | - | - |
| Peru | UMIC | 194.9 | - | - | - | - | - |
| Data unavailable for 29 countries | |||||||
|
| |||||||
| Middle East & North Africa | |||||||
| Israel | HIC | 108 | 1070* | - | - | - | - |
| Data unavailable for 18 countries | |||||||
|
| |||||||
| North America | |||||||
| Canada | HIC | 61.6 | 1220* | - | 13.3 | - | - |
| USA | HIC | 71 | 6400* | - | 20 | 28.4 | 36.9 |
|
| |||||||
| South Asia: Data unavailable | |||||||
|
| |||||||
| Sub-Saharan Africa: Data unavailable | |||||||
Incidence Rates per 10,000 person-years
Mortality rate per 100,000 people
Total for both sexes
Published data for LEAs, CVD, and all-cause mortality among persons with diabetes, and incidence of diagnosed diabetes, is mostly limited to high-income countries63,72,73,77 (Table 4). Where data exist, absolute rates vary considerably due to variation in the sampling approach, the outcome definition, and in the true underlying rate. For example, rates of LEAs across most countries range from 5 to 34 per 10,000 per year with an average of about 18 per 10,000 per year. Annual rates of all-cause mortality vary from 10 to 60 per 1000, with an average of about 23. Estimates for diabetes related ESKD use the overall population as the denominator. Thus, the increase in ESKD incidence observed across most countries is affected by the increasing prevalence of diabetes. The annual incidence of diagnosed diabetes tends to range from 1 to 10 per 1000, with an average of roughly 7 per 1000. Although these metrics lend themselves to international standardization, existing published estimates are difficult to compare because of variations in sampling methods and denominators, outcome definitions, and population standardization approaches78. For these reasons, as well as the lack of availability in current surveillance systems, the Compact did not set global targets for these conditions.
Estimating health impact of meeting core metrics
Few studies have examined the health effects that could be achieved by changing target levels. Each of the core metrics has well-established cost-effectiveness or is cost saving with the exception of screening for undiagnosed diabetes, wherein some degree of targeting by age and risk is required to make it cost-effective51. Quality improvement programs have achieved reductions in HbA1c, blood pressure, and lipid levels that would be expected to reduce CVD incidence and all-cause mortality by 40%79. Similarly, model-based estimates from a recent Lancet Commission also suggest that the application of integrated care to improve diabetes care and prevention targets could reduce cardiovascular (CVD) complications of diabetes by half and for those with poor control, increase life expectancy by 5 years from age 40 2.
A recent comprehensive study using STEPs data from 67 LMICs and microsimulation modelling found that increasing the percentage of the diabetes population that is diagnosed or who achieve glycaemic control by 10 PPTs reduced 10-year risk of microvascular disease complications (neuropathy, ESKD, retinopathy) but has a negligible effect on CVD60. An equivalent improvement in the proportion having controlled blood pressure control decreases 10-year risk of CVD events, ESRD and retinopathy by 8-18% while increasing the proportion meeting lipid goals by the same magnitude decreases 10-year CVD risk by 10%. Achieving 60% on diagnosis, treatment, and all three control metrics (glycaemia, blood pressure, and statin use) reduces CVD deaths by >40%, consistent with findings from a recent Lancet Commission68. The study also found that achieving targets was cost-effective below WHO thresholds for cost-effectiveness 71.
RECOMMENDED TARGET LEVELS FOR CORE METRICS
Selection of target levels ultimately requires a difficult balance between being ambitious, realistic, and obtainable. Table 5 presents recommended target levels for the core metrics. Our review suggests that target levels of 80% for the proportion of persons with diabetes who are diagnosed, and among those with diagnosed diabetes, 80%, 80%, and 60% meeting targets for HbA1c (<8%), blood pressure (<140/90mmHg), and statin use, respectively, are ambitious but achievable and would have large health benefits in many countries of the world. The gaps between current levels of attainment and the proposed targets vary considerably by region and country of the world. These target levels are generally consistent with the top 85 to the 100th percentile of countries of the world that currently have data.
Table 5.
Summary of global medians, 90th percentiles, and proposed targets for core metrics of the Global Diabetes Compact.
| Core Metric | Definition | Global median (%) | Global 90th percentile (%) | Proposed Global Target (%) |
|---|---|---|---|---|
| Percent diagnosed | Number diagnosed divided by number with clinical diabetes | 64 | 75 | 80 |
| Glycaemic control | Number controlled (HbA1c < 8%) divided by total diagnosed diabetes | 68 | 84 | 80 |
| Blood pressure control | Number controlled (BP < 140/90) divided by total diagnosed diabetes | 53 | 70 | 80 |
| Lipid treated | Number treated with statin divided by total with diagnosed diabetes | 12 | 47 | 60 |
| Medicine availability | Availability of insulin, meters, and glucose test-strips for persons with type 1 diabetes | N/A | N/A | 100 |
Estimates assembled from four primary types of sources: IDF Diabetes Atlas, Global Health and Population Project on Access to Care for Cardiometabolic diseases (HPAAC) collaborators, literature reviews, and web-sites containing estimates from national diabetes surveillance systems. References listed in Appendix.
Our review suggests that for the percent diagnosed metric, meeting the 80% target will require increases of 10 to 28 PPT improvements across regions. Meeting the target of 80% of persons with diagnosed diabetes having HbA1c levels <8% will require an average 12 PPT increase, ranging from 0 to 22 PPT across countries. Current levels of attainment of 80% of patients with diagnosed diabetes having blood pressure <140/80mmHg are highly variable and will require a 27 PPT increase globally; current gaps range from 10 PPT in North America to ~25-35 PPT in most regions. Current levels of attainment of the statin target are considerably below 60%, ranging from 10% to 25% across all regions outside of North America, where it is 60%. Thus, meeting the statin target will likely require substantial country-level policy actions, and country-specific target setting may again be appropriate. For the insulin availability metric, we propose an ambitious target of 100% because of insulin’s essential role in survival of persons with type 1 diabetes.
Setting targets for the complementary targets of incidence of diagnosed diabetes, and among persons with diagnosed diabetes, LEAs, ESKD, and mortality rates is difficult because of the high degree of baseline variability and the further needs in standardization of metrics. However, preliminary data suggests that country-level relative reductions of 50% over 10 years may be appropriate.
Monitoring and Achieving Global Targets
Long-term success of the Global Diabetes Compact will also depend upon consistent and accurate monitoring of the Compact targets as well as continued support and strengthening of comprehensive NCD surveillance systems. The assessment of core targets can be conducted via population-based surveys such as STEPs with inclusion of HbA1c measurement. However, few STEPs surveys have adequate sample sizes to assess metrics with high precision. Typical cross-sectional surveys with 2000 to 4000 participants have between 100 and 500 participants with diagnosed diabetes, which yields confidence intervals around core metrics of 10 to 20 percentage points. This may be adequate to assess whether a country is meeting a target at a single point in time, but generally insufficient to monitor trends over time. Thus, it will be important for member states to evaluate sample sizes and consider additional strategies (e.g., aggregating successive surveys; over-sampling) or monitoring systems in their evaluation plans. Additionally, to assess progress to achieving these targets will require repeated surveys, which many countries have yet to conduct. Alternatively, a sampling of sentinel facility or health systems may be considered.
The Global Diabetes Compact is intended to drive country-level efforts to strengthen national capacity, leadership, and multi-sectoral action, with a particular focus on achieving universal health coverage, strengthening and orienting health systems around NCDs through primary care, reducing modifiable risk factors and underlying social determinants of health, and strengthening surveillance and monitoring. Adoption of the complementary targets related to long-term health outcomes (i.e., diabetes complications) will generally require new surveillance systems, such as condition-specific or population registries based on linkage of primary and secondary care, medications and laboratory values, as well as additional consensus-based agreement on specific epidemiologic definitions. The proposed metrics for prevention interventions and integrated care are conceivably attainable through modification of current surveys and surveillance systems but require further consensus-based development of definitions, methods of assessment, and target levels.
Although the Global Diabetes Compact focuses on diagnosis and reducing complications through risk factor control and access to essential medications for persons with diabetes, the breadth of the diabetes challenge calls for efforts to reduce diabetes incidence through a combination of individual-targeted and population-wide approaches. Effective lifestyle-based prevention will relieve the burden on health systems while improving metabolic and cardiovascular risk factor profiles. Thus, the Compact should be viewed in the context of broader approaches to reduce the burden of diabetes through prevention as well as through efforts to ensure health care access and strengthening of health systems. As such, it is related to and builds on UN roadmaps for NCD prevention and is supported by recent Lancet Commissions addressing the global challenges of using data to transform diabetes care globally and in Sub-Saharan Africa2,6,30,31 . The priority actions range from scaling up diagnosis and medication availability to improving skills and competencies, to building clinical decision supports and population monitoring systems (Table 6). The metrics and targets are not intended to cover the full range of health objectives and actions to address the needs of the diabetes epidemic. Rather, they are intended to capture areas of missed opportunity and be an important first step. The goals are clearly measurable and, if attained, will have significant impact on health outcomes.
Table 6.
Diabetes-relevant priorities of the Global Action Plan for the Prevention of Non-Communicable Diseases.
| • Scaling up diagnosis of diabetes to initiate cost-effective medical and behavioral risk factor management. |
| • Improving availability, affordability, and equitable access to essential medicines, including life-saving insulin, and technologies. |
| • Enhancing skills and capacity of health care providers to povide team-based comprehensive care for diabetes management. |
| • Establishing continuous quality improvement systems for disease management and prevention with an emphasis on evidence-based guidelines, treatment protocols, and decision tools. |
| • Improving information management and sharing across settings to optimize the ability of local data registries and electronic medical records to support clinical and health services decisions. |
| • Development of facility- or health-system level diabetes registries where feasible to assist in both patient care and population monitoring. |
Based on references 30 (World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. World Health Organization. 2013.) and 31 (World Health Organization. Reducing the burden of noncommunicable diseases through strengthengin prevention and control of diabetes. 2021)
Supplementary Material
Acknowledgements:
*Diabetes Targets Expert Consultation Group: Carlos A Aguilar-Salinas9, Glennis Andall-Brereton10, Felicia Anumah11, Pablo Aschner12, Abdul Basit13, Faraja Chiwanga14, Naomi Levitt15, Bolormaa Norov16, An Pan17, Joao Filipe Raposo18, Lela Sturua19, JS Thakur20, Thaksaphon Mek Thamarangsi21
9Instituto Nacional de Ciencias, Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico
10Public health consultant, Port of Spain, Trinidad and Tobago
11University of Abuja, Abuja Nigeria
12Javeriana University School of Medicine, Bogota, Colombia
13Diabetic Association of Pakistan, Karachi, Pakistan
14Muhimbili National Hospital, Dar es Salaam, Tanzania
15Department of Medicine, University of Cape Town, South Africa
16Nutrition department of the National Center for Public Health, Ulaanbaatar, Mongolia
17Huazhong University of Science and Technology, Wuhan, PR China
18APDP Diabetes Portugal and NOVA Medical School, NOVA University, Lisbon, Portugal
19National Center for Disease Control and Public Health, Tbilisi, Georgia
20Department of Community Medicine &School of Public Health, Chandigarh, India
21International Health Policy Program, Ministry of Public Health, Bangkok, Thailand
The Global Health and Population Project on Access to Care for Cardiometabolic diseases (HPAAC) collaborators contributed important data from population surveys that contributed to the recommendation of target levels and review by the Diabetes Targets Expert Consultation Group. Contributors and affiliations of the HPAAC are as follows: Kokou Agoudavi22; Krishna Aryal23 ; Rifat Atun24; Silver Bahendeka25; Brice Bicaba26; Pascal Bovet27; Garry Brian28; Albertino Damasceno29; Justine Davies30; Maria Dorobantu31; Farshad Farzadfar32; David Flood33; Gladwell Gathecha34; Pascal Geldsetzer35; Mongal Gurung36; David Guwatudde 37; Corine Houehanou38; Dismand Houinato39; Nahla Hwalla40; Lindsay Jaacks41; Khem Karki42; Demetre Labadarios43 ; Nuno Lunet 44; Jennifer Manne-Goehler45; Maja Marcus46; Joao Martins47; Mary Mayige48; Bolormaa Norov49; Moghaddam Sahar Saeedi50; Quesnel-Crooks Sarah51; Abla Sibai52; Lela Sturua53; Michaela Theilmann54 ; Lindiwe Tsabedze55 ; Sebastian Vollmer 56; Zhaxybay Zhumadilov57.
22Togo Ministry of Health, Lome, Togo
23Public Health Promotion and Development Organization, Kathmandu, Nepal
24Department of Global Health and Population, Harvard T.H. Chan School of Public Health, Harvard University, Boston, MA, USA
25Department of Internal Medicine, MKPGMS Uganda Martyrs University, Kampala
26Institut National de Santé Publique, Burkina Faso
27Ministry of Health, Victoria, Seychelles
28The Fred Hollows Foundation New Zealand
29Faculty of Medicine, Eduardo Mondlane University, Maputo, Mozambique
30Institute for Applied Health Research, University of Birmingham, UK
31University of Medicine and Pharmacy Carol Davila, Bucharest, Romania
32Non-Communicable Diseases Research Center, Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
33Division of Hospital Medicine, Department of Medicine, University of Michigan, Ann Arbor, Michigan, USA
34Department of Non-Communicable Diseases, Ministry of Health, Nairobi, Kenya
35Division of Primary Care and Population Health, Stanford University
36Health Research and Epidemiology Unit, Ministry of Health, Thimphu, Bhutan
37Department of Epidemiology and Biostatistics, School of Public Health, Makerere University, Kampala, Uganda
38Laboratory of Epidemiology of Chronic and Neurological Diseases, Faculty of Health Sciences, University of Abomey-Calavi, Cotonou, Benin
39Laboratory of Epidemiology of Chronic and Neurological Diseases, Faculty of Health Sciences, University of Abomey-Calavi, Cotonou, Benin
40Faculty of Agricultural and Food Sciences, American University of Beirut, Beirut, Lebanon
41Global Academy of Agriculture and Food Security, The University of Edinburgh, Midlothian, United Kindom
42Department of Community Medicine and Public Health, Institute of Medicine, Tribhuvan University, Kathmandu, Nepal
43Faculty of Medicine and Health Sciences, Stellenbosch University, Stellenbosch, South Africa
44Department of Public Health and Forensic Health Sciences and Medical Education, Faculty of Medicine, University of Porto, Porto, Portugal
45Division of Infectious Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
46Department of Economics and Centre for Modern Indian Studies, University of Goettingen, Göttingen, Germany
47Faculty of Medicine and Health Sciences, National University of East Timor, Dili, Timor-Leste
48National Institute for Medical Research, Dar es Salaam, Tanzania
49National Center for Public Health, Ulaanbaatar, Mongolia
50Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
51Non-Communicable Diseases, Caribbean Public Health Agency, Port of Spain, Trinidad and Tobago
52Epidemiology and Population Health Department, Faculty of Health Sciences American University of Beirut, Beirut, Lebanon
53Non-Communicable Disease Department, National Center for Disease Control and Public Health, Tbilisi, Georgia
54Heidelberg Institute of Global Health, Faculty of Medicine and University Hospital, Heidelberg University, Heidelberg, Germany
55Ministry of Health, Mbabane, Eswatini
56Department of Economics and Centre for Modern Indian Studies, University of Goettingen, Göttingen, Germany
57Nazarbayev University School of Medicine, Nur-Sultan, Kazakhstan
REFERENCES
- 1.Federation ID. IDF Diabetes Atlas. 2021. www.diabetesatlas.org.
- 2.Chan JCN, Lim LL, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2021; 396(10267): 2019–82. [DOI] [PubMed] [Google Scholar]
- 3.Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2020; 162: 108072. [DOI] [PubMed] [Google Scholar]
- 5.Bommer C, Heesemann E, Sagalova V, et al. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol 2017; 5(6): 423–30. [DOI] [PubMed] [Google Scholar]
- 6.Atun R, Davies JI, Gale EAM, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol 2017; 5(8): 622–67. [DOI] [PubMed] [Google Scholar]
- 7.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317(7160): 703–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131): 837–53. [PubMed] [Google Scholar]
- 9.Jonas DE, Crotty K, Yun JDY et al. Screening for prediabetes and type 2 diabetes: Updatesd evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021. [DOI] [PubMed] [Google Scholar]
- 10.Holman N, Knighton P, O’Keefe J, et al. Completion of annual diabetes care processes and mortality: A cohort study using the National Diabetes Audit for England and Wales. Diabetes Obes Metab 2021; 23(12): 2728–40. [DOI] [PubMed] [Google Scholar]
- 11..GtCPS. Diabetes prevention and control: combined diet and physical activity promotion programs to prevent type 2 diabetes among people at increased risk (abbreviated). 05/15/2014 2014. www.thecommunityguide.org/diabetes/combineddietandpa.html. (accessed 07/09/2014.
- 12.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012; 31(1): 67–75. [DOI] [PubMed] [Google Scholar]
- 13.Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. British Medical Journal 2007; 334(7588): 299–302B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haw JS, Galaviz KI, Straus AN, et al. Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern Med 2017; 177(12): 1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global Diabetes Prevention Interventions: A Systematic Review and Network Meta-analysis of the Real-World Impact on Incidence, Weight, and Glucose. Diabetes Care 2018; 41(7): 1526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali M, Siegel K, Chandrasekar E, Tandon N, Montoya P, et al. , editor. Diabetes: An Update on the Pandemic and Potential Solutions: Washington, DC: World Bank; 2021. [PubMed] [Google Scholar]
- 17.Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults for 2013 for the IDF Diabetes Atlas. Diabetes Res Clin Pract 2013. [DOI] [PubMed] [Google Scholar]
- 18.Manne-Goehler J, Geldsetzer P, Agoudavi K, et al. Health system performance for people with diabetes in 28 low- and middle-income countries: A cross-sectional study of nationally representative surveys. PLoS Med 2019; 16(3): e1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009; 32(2): 227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999-2018. N Engl J Med 2021; 384(23): 2219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med 2014; 161(10): 681–9. [DOI] [PubMed] [Google Scholar]
- 22.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. NEnglJMed 2013; 368(17): 1613–24. [DOI] [PubMed] [Google Scholar]
- 23.Manne-Goehler J, Atun R, Stokes A, et al. Diabetes diagnosis and care in sub-Saharan Africa: pooled analysis of individual data from 12 countries. Lancet Diabetes Endocrinol 2016; 4(11): 903–12. [DOI] [PubMed] [Google Scholar]
- 24.Flood D. Diabetes indicators reqeusted for the WHO Global Diabetes Compact. 2021. [Google Scholar]
- 25.Ali MK, Singh K, Kondal D, et al. Effectiveness of a Multicomponent Quality Improvement Strategy to Improve Achievement of Diabetes Care Goals: A Randomized, Controlled Trial. Ann Intern Med 2016; 165(6): 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali MK, Chwastiak L, Poongothai S, et al. Effect of a Collaborative Care Model on Depressive Symptoms and Glycated Hemoglobin, Blood Pressure, and Serum Cholesterol Among Patients With Depression and Diabetes in India: The INDEPENDENT Randomized Clinical Trial. Jama 2020; 324(7): 651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flood D, Hane J, Dunn M, et al. Health system interventions for adults with type 2 diabetes in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med 2020; 17(11): e1003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt D, Hemmingsen B, Matzke A, et al. The WHO Global Diabetes Compact: a new initiative to support people living with diabetes. Lancet Diabetes Endocrinol 2021; 9(6): 325–7. [DOI] [PubMed] [Google Scholar]
- 29.Medlock J, Pandey A, Parpia AS, Tang A, Skrip LA, Galvani AP. Effectiveness of UNAIDS targets and HIV vaccination across 127 countries. Proc Natl Acad Sci U S A 2017; 114(15): 4017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Organization WH. Global action plan for the prevention and control of noncommunicable diseases 2013-2020. World Health Organization. . 2013. [Google Scholar]
- 31.Organziation WH. Reducing the burden of noncommunicable diseases through strengthengin prevention and control of diabetes. 2021. [Google Scholar]
- 32.Ayanian JZ, Markel H. Donabedian’s Lasting Framework for Health Care Quality. N Engl J Med 2016; 375(3): 205–7. [DOI] [PubMed] [Google Scholar]
- 33.Hubbard K, Talih M, Klein RJ, Huang TD. Target-setting methods in healthy people 2030. Healthy People Statistical Notes 2020; 28. [Google Scholar]
- 34.Lim LL, Lau ESH, Kong APS, et al. Aspects of Multicomponent Integrated Care Promote Sustained Improvement in Surrogate Clinical Outcomes: A Systematic Review and Meta-analysis. DIABETES CARE 2018; 41(6): 1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alharbi NS, Alsubki N, Jones S, Khunti K, Munro N, de Lusignan S. Impact of Information Technology-Based Interventions for Type 2 Diabetes Mellitus on Glycemic Control: A Systematic Review and Meta-Analysis. J Med Internet Res 2016; 18(11): e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levengood TW, Peng Y, Xiong KZ, et al. Team-Based Care to Improve Diabetes Management: A Community Guide Meta-analysis. Am J Prev Med 2019; 57(1): e17–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012; 379(9833): 2252–61. [DOI] [PubMed] [Google Scholar]
- 38.Adams J, Mytton O, White M, Monsivais P. Why Are Some Population Interventions for Diet and Obesity More Equitable and Effective Than Others? The Role of Individual Agency. PLoS Med 2016; 13(4): e1001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Health systems, patients factors, and quality of care for diabetes: a synthesis of findings from the TRIAD study. Diabetes Care 2010; 33(4): 940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rawshani A, Rawshani A, Franzén S, et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2018; 379(7): 633–44. [DOI] [PubMed] [Google Scholar]
- 41.Kontopantelis E, Springate DA, Reeves D, et al. Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia 2015; 58(3): 505–18. [DOI] [PubMed] [Google Scholar]
- 42.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014; 370(16): 1514–23. [DOI] [PubMed] [Google Scholar]
- 43.Essman M, Taillie LS, Frank T, Ng SW, Popkin BM, Swart EC. Taxed and untaxed beverage intake by South African young adults after a national sugar-sweetened beverage tax: A before-and-after study. PLoS Med 2021; 18(5): e1003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ali MK, Siegel KR, Laxy M, Gregg EW. Advancing Measurement of Diabetes at the Population Level. Curr Diab Rep 2018; 18(11): 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies JI, Reddiar SK, Hirschhorn LR, et al. Association between country preparedness indicators and quality clinical care for cardiovascular disease risk factors in 44 lower- and middle-income countries: A multicountry analysis of survey data. PLoS Med 2020; 17(11): e1003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Health systems, patients factors, and quality of care for diabetes: a synthesis of findings from the TRIAD study. diabetes care 2010; 33(4): 940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selby JV, Swain BE, Gerzoff RB, et al. Understanding the gap between good processes of diabetes care and poor intermediate outcomes: Translating Research into Action for Diabetes (TRIAD). MedCare 2007; 45(12): 1144–53. [DOI] [PubMed] [Google Scholar]
- 48.Doran GT. Management Review 1981; 70(11): 35–6. [Google Scholar]
- 49.Simmons RK, Echouffo-Tcheugui JB, Sharp SJ, et al. Screening for type 2 diabetes and population mortality over 10 years (ADDITION-Cambridge): a cluster-randomised controlled trial. Lancet 2012; 380(9855): 1741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffin SJ, Rutten G, Khunti K, et al. Long-term effects of intensive multifactorial therapy in individuals with screen-detected type 2 diabetes in primary care: 10-year follow-up of the ADDITION-Europe cluster-randomised trial. Lancet Diabetes Endocrinol 2019; 7(12): 925–37. [DOI] [PubMed] [Google Scholar]
- 51.Siegel KR, Ali MK, Zhou X, et al. Cost-effectiveness of Interventions to Manage Diabetes: Has the Evidence Changed Since 2008? Diabetes Care 2020; 43(7): 1557–92. [DOI] [PubMed] [Google Scholar]
- 52.Zhou X, Siegel KR, Ng BP, et al. Cost-effectiveness of Diabetes Prevention Interventions Targeting High-risk Individuals and Whole Populations: A Systematic Review. Diabetes Care 2020; 43(7): 1593–616. [DOI] [PubMed] [Google Scholar]
- 53.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008; 359(15): 1565–76. [DOI] [PubMed] [Google Scholar]
- 54.Beran D, Lazo-Porras M, Mba CM, Mbanya JC. A global perspective on the issue of access to insulin. Diabetologia 2021; 64(5): 954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Organization WH. WHO Model list of essential mediciens for children - 8th list, 2021. 2021. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.03 (accessed 12/08/2021.
- 56.Riley L, Guthold R, Cowan M, et al. The World Health Organization STEPwise Approach to Noncommunicable Disease Risk-Factor Surveillance: Methods, Challenges, and Opportunities. Am J Public Health 2016; 106(1): 74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang W, Winter R, Mallick L, Florey L, Burgert-Brucker C, Carter E. The relationship between the health service environment and service utilization: linking population data to health facilities data in Haiti and Malawi. Rockville, Maryland, USA: ICF International, 2015. [Google Scholar]
- 58.Burgert CR, Prosnitz D. Linking DHS household and SPA facility surveys: Data considerations and geospatial methods. Rockville, Maryland, USA: ICF International, 2014. [Google Scholar]
- 59.Assaf S, Kothari MT, Pullum T. An assessment of the quality of DHS anthropometric data, 2005-2014. Rockville, Maryland, USA: ICF International, 2015. [Google Scholar]
- 60.Basu S, Flood D, Geldsetzer P, et al. Estimated effect of increased diagnosis, treatment, and control of diabetes and its associated cardiovascular risk factors among low-income and middle-income countries: a microsimulation model. Lancet Glob Health 2021; 9(11): e1539–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brinks R, Hoyer A, Rolka DB, Kuss O, Gregg EW. Comparison of surveillance-based metrics for the assessment and monitoring of disease detection: simulation study about type 2 diabetes. BMC MEDICAL RESEARCH METHODOLOGY 2017; 17(ARTN 54). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magliano DJ, Chen L, Islam RM, et al. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol 2021; 9(4): 203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magliano DJ, Islam RM, Barr ELM, et al. Trends in incidence of total or type 2 diabetes: systematic review. Bmj 2019; 366: l5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cherubini V, Grimsmann JM, Åkesson K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia 2020; 63(8): 1530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Organization WH. WHO package of essential noncommunicable (PEN) disease interventions for primary health care. 2020. [Google Scholar]
- 66.Haw JS, Tantry S, Vellanki P, Pasquel FJ. National Strategies to Decrease the Burden of Diabetes and Its Complications. Current Diabetes Reports 2015; 15(9): 65. [DOI] [PubMed] [Google Scholar]
- 67.Ali MK, McKeever Bullard K, Imperatore G, et al. Reach and Use of Diabetes Prevention Services in the United States, 2016-2017. JAMA Netw Open 2019; 2(5): e193160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan JCN, Lim LL, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet 2020. [DOI] [PubMed] [Google Scholar]
- 69.Lim LL, Lau ES, Kong AP, et al. Aspects of multicomponent integrated care promote sustained improvement in surrogate clinical outcomes: a systematic review and meta-analysis. Diabetes Care 2018; 41(6): 1312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kapur A, McIntyre HD, Divakar H, et al. Towards a global consensus on GDM diagnosis: Light at the end of the tunnel? Int J Gynaecol Obstet 2020; 149(3): 257–61. [DOI] [PubMed] [Google Scholar]
- 71.Basu SFD, Geldsetzer P et al. Estimated impact of incrased diagnsois, treatm,ent, and control of diabetes mellitus among low- and middle-income countries: A microsimulatoin model. In Press 2021. [Google Scholar]
- 72.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia 2019; 62(1): 3–16. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, Islam RM, Wang J, et al. A systematic review of trends in all-cause mortality among people with diabetes. Diabetologia 2020; 63(9): 1718–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imperatore G, Cadwell BL, Geiss L, et al. Thirty-year trends in cardiovascular risk factor levels among US adults with diabetes: National Health and Nutrition Examination Surveys, 1971-2000. Am J Epidemiol 2004; 160(6): 531–9. [DOI] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention. National Diabetes Surveillance System. 2016. Available from http://www.cdc.gov/diabetes/statistics/index.htm. (accessed 2016/02/25.
- 76.Wang L, Li X, Wang Z, et al. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. Jama 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harding JL, Pavkov ME, Gregg EW, Burrows NR. Trends of Nontraumatic Lower Extremity Amputation in End-Stage Renal Disease and Diabetes, United States, 2000-2015. Diabetes Care 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia 2022; 65(1): 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao H, Shi L, Fonseca VA. Using the BRAVO Risk Engine to Predict Cardiovascular Outcomes in Clinical Trials With Sodium-Glucose Transporter 2 Inhibitors. Diabetes Care 2020; 43(7): 1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lind M, Garcia-Rodriguez LA, Booth GL, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia 2013; 56(12): 2601–8. [DOI] [PubMed] [Google Scholar]
- 81.Abouzeid M, Wikstrom K, Peltonen M, et al. Secular trends and educational differences in the incidence of type 2 diabetes in Finland, 1972-2007. European Journal of Epidemiology 2015; 30(8): 649–59. [DOI] [PubMed] [Google Scholar]
- 82.Ikonen TS, Sund R, Venermo M, Winell K. Fewer major amputations among individuals with diabetes in Finland in 1997-2007: a population-based study. diabetes care 2010; 33(12): 2598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boehme MW, Buechele G, Frankenhauser-Mannuss J, et al. Prevalence, incidence and concomitant co-morbidities of type 2 diabetes mellitus in South Western Germany - a retrospective cohort and case control study in claims data of a large statutory health insurance. BMC Public Health 2015; 15: 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karpati T, Cohen-Stavi CJ, Leibowitz M, Hoshen M, Feldman BS, Balicer RD. Towards a subsiding diabetes epidemic: trends from a large population-based study in Israel. Popul Health Metr 2014; 12(1): 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lombardo FL, Maggini M, De Bellis A, Seghieri G, Anichini R. Lower extremity amputations in persons with and without diabetes in Italy: 2001-2010. PLoS One 2014; 9(1): e86405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almaraz MC, Gonzalez-Romero S, Bravo M, et al. Incidence of lower limb amputations in individuals with and without diabetes mellitus in Andalusia (Spain) from 1998 to 2006. Diabetes Res Clin Pract 2012; 95(3): 399–405. [DOI] [PubMed] [Google Scholar]
- 87.Zghebi SS, Steinke DT, Carr MJ, Rutter MK, Emsley RA, Ashcroft DM. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes, Obesity & Metabolism; 19(11): 1537–45. [DOI] [PubMed] [Google Scholar]
- 88.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-Specific Trends From 2000-2011 in All-Cause and Cause-Specific Mortality in Type 1 and Type 2 Diabetes: A Cohort Study of More Than One Million People. Diabetes Care 2016; 39(6): 1018–26. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt MI, Bracco PA, Yudkin JS, et al. Intermediate hyperglycaemia to predict progression to type 2 diabetes (ELSA-Brasil): an occupational cohort study in Brazil. Lancet Diabetes Endocrinol 2019; 7(4): 267–77. [DOI] [PubMed] [Google Scholar]
- 90.Lok CE, Oliver MJ, Rothwell DM, Hux JE. The growing volume of diabetes-related dialysis: a population based study. Nephrol Dial Transplant 2004; 19(12): 3098–103. [DOI] [PubMed] [Google Scholar]
- 91.Guerrero-Núñez S, Valenzuela-Suazo S, Cid-Henríquez P. Effective Universal Coverage of Diabetes Mellitus Type 2 in Chile. Rev Lat Am Enfermagem 2017; 25: e2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monesi L, Baviera M, Marzona I, et al. Prevalence, incidence and mortality of diagnosed diabetes: evidence from an Italian population-based study. Diabet Med 2012; 29(3): 385–92. [DOI] [PubMed] [Google Scholar]
- 93.Forssas E, Keskimäki I, Reunanen A, Koskinen S. Widening socioeconomic mortality disparity among diabetic people in Finland. Eur J Public Health 2003; 13(1): 38–43. [DOI] [PubMed] [Google Scholar]
- 94.Fuentes S, Mandereau-Bruno L, Regnault N, et al. Is the type 2 diabetes epidemic plateauing in France? A nationwide population-based study. Diabetes Metab 2020; 46(6): 472–9. [DOI] [PubMed] [Google Scholar]
- 95.Fosse S, Hartemann-Heurtier A, Jacqueminet S, Ha Van G, Grimaldi A, Fagot-Campagna A. Incidence and characteristics of lower limb amputations in people with diabetes. Diabet Med 2009; 26(4): 391–6. [DOI] [PubMed] [Google Scholar]
- 96.Bruno G, Biggeri A, Merletti F, et al. Low incidence of end-stage renal disease and chronic renal failure in type 2 diabetes: a 10-year prospective study. Diabetes Care 2003; 26(8): 2353–8. [DOI] [PubMed] [Google Scholar]
- 97.Goto A, Goto M, Noda M, Tsugane S. Incidence of type 2 diabetes in Japan: a systematic review and meta-analysis. PLoS One 2013; 8(9): e74699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pildava S, Strēle I, Briģis G. The mortality of patients with diabetes mellitus in Latvia 2000-2012. Medicina (Kaunas) 2014; 50(2): 130–6. [DOI] [PubMed] [Google Scholar]
- 99.González-Villalpando C, Dávila-Cervantes CA, Zamora-Macorra M, Trejo-Valdivia B, González-Villalpando ME. Incidence of type 2 diabetes in Mexico: results of the Mexico City Diabetes Study after 18 years of follow-up. Salud Publica Mex 2014; 56(1): 11–7. [DOI] [PubMed] [Google Scholar]
- 100.Dale AC, Vatten LJ, Nilsen TI, Midthjell K, Wiseth R. Secular decline in mortality from coronary heart disease in adults with diabetes mellitus: cohort study. BMJ 2008; 337: a236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carrillo-Larco RM, Bernabé-Ortiz A. [Type 2 diabetes mellitus in peru: a systematic review of prevalence and incidence in the general population]. Rev Peru Med Exp Salud Publica 2019; 36(1): 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Comas J, Arcos E, Castell C, et al. Evolution of the incidence of chronic kidney disease Stage 5 requiring renal replacement therapy in the diabetic population of Catalonia. Nephrol Dial Transplant 2013; 28(5): 1191–8. [DOI] [PubMed] [Google Scholar]
- 103.Rawshani A, Rawshani A, Gudbjörnsdottir S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med 2017; 377(3): 300–1. [DOI] [PubMed] [Google Scholar]
- 104.Heintjes EM, Houben E, Beekman-Hendriks WL, et al. Trends in mortality, cardiovascular complications, and risk factors in type 2 diabetes. Neth J Med 2019; 77(9): 317–29. [PubMed] [Google Scholar]
- 105.van Houtum WH, Lavery LA. Regional variation in the incidence of diabetes-related amputations in The Netherlands. Diabetes Res Clin Pract 1996; 31(1-3): 125–32. [DOI] [PubMed] [Google Scholar]
- 106.Zghebi SS, Steinke DT, Carr MJ, Rutter MK, Emsley RA, Ashcroft DM. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes Obes Metab 2017; 19(11): 1537–45. [DOI] [PubMed] [Google Scholar]
- 107.Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes- and nondiabetes-related lower extremity amputation incidence before and after the introduction of better organized diabetes foot care: continuous longitudinal monitoring using a standard method. Diabetes Care 2008; 31(3): 459–63. [DOI] [PubMed] [Google Scholar]
- 108.Yashkin AP, Picone G, Sloan F. Causes of the change in the rates of mortality and severe complications of diabetes mellitus: 1992-2012. Med Care 2015; 53(3): 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weng W, Liang Y, Kimball ES, et al. Decreasing incidence of type 2 diabetes mellitus in the United States, 2007-2012: Epidemiologic findings from a large US claims database. Diabetes Res Clin Pract 2016; 117: 111–8. [DOI] [PubMed] [Google Scholar]
- 110.Icks A, Haastert B, Genz J, et al. Incidence of renal replacement therapy (RRT) in the diabetic compared with the non-diabetic population in a German region, 2002-08. Nephrol Dial Transplant 2011; 26(1): 264–9. [DOI] [PubMed] [Google Scholar]
- 111.de Sousa-Uva M, Antunes L, Nunes B, et al. Trends in diabetes incidence from 1992 to 2015 and projections for 2024: A Portuguese General Practitioner’s Network study. Prim Care Diabetes 2016; 10(5): 329–33. [DOI] [PubMed] [Google Scholar]
- 112.Buckley CM, O’Farrell A, Canavan RJ, et al. Trends in the incidence of lower extremity amputations in people with and without diabetes over a five-year period in the Republic of Ireland. PLoS One 2012; 7(7): e41492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johal S, Jamsen KM, Bell JS, et al. Do statin users adhere to a healthy diet and lifestyle? The Australian Diabetes, Obesity and Lifestyle Study. Eur J Prev Cardiol 2017; 24(6): 621–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


