Abstract
Introduction:
Several interventions have been found to be effective for reversing prediabetes in adults. This systematic review and meta-analysis aims to compare the effectiveness of such interventions.
Methods:
MEDLINE, Embase, and Cochrane Library databases were searched for articles published between January 1, 2000 and June 27, 2018. RCTs in adults with prediabetes, testing non-surgical interventions lasting ≥3 months, and reporting the number of participants achieving normal glucose levels at intervention end were eligible. The pooled risk difference (RD) and number needed to treat (NNT) for achieving normoglycemia were estimated using a random-effects, arm-based network meta-analysis. The strength of the evidence was assessed using Grading of Recommendations Assessment, Development, and Evaluation. Data were obtained in 2018 and analyzed in 2019 and 2021.
Results:
Of 54 studies included in the systematic review, 47 were meta-analyzed (n=26,460, mean age=53 years, 46% male, 31% White). Studies included 27 arms testing lifestyle modification interventions, 25 medications, 5 dietary supplements, and 10 Chinese medicine. There were 35 control/placebo arms. At a median follow-up of 1.6 years, more participants in the lifestyle modification groups achieved normoglycemia than control (RD=0.18, NNT=6). The strength of the evidence was strong for lifestyle modification. Over a median follow-up of 2.7 years, more participants receiving glucagon-like peptide-1 receptor agonists (RD=0.47, NNT=2), alpha-glucosidase inhibitors (RD=0.29, NNT=4), and insulin sensitizers (RD=0.23, NNT=4) achieved normoglycemia than control. The strength of evidence was moderate for these medications.
Discussion:
Though several pharmacological approaches can reverse prediabetes, lifestyle modification provides the strongest evidence of effectiveness and should remain the recommended approach to address this condition.
INTRODUCTION
Prediabetes is a state of impaired glucose regulation where blood glucose levels are elevated but do not reach type 2 diabetes thresholds.1 Though the true global burden of prediabetes is likely unknown, current estimates suggest the global prevalence ranges from 8% to 58%, depending on which diagnostic cut offs are employed.2,3 People with prediabetes have increased risk for mortality, cardiovascular disease, and renal disease,4–9 even if they do not develop diabetes. Overall, prediabetes represents a substantial economic burden for current healthcare expenditure10 and a potential future increase in diabetes healthcare costs.
There is evidence that lifestyle modification, some medications, and alternative medicine can reverse prediabetes in adults. Specifically, alpha-glucosidase inhibitors (AGIs), thiazolidinediones, Chinese medicine, vitamin D, and improvements in physical activity and dietary behaviors have been found to be effective for reversing prediabetes.11–15 This has significant health benefits. In China, the 20-year follow up of the Daqing diabetes prevention study showed participants who returned to normal glucose levels during the intervention had lower diabetes incidence rates than those who remained in the prediabetes state.16 Another study from China showed adults with prediabetes who returned to normal glucose levels had lower risk for cardiovascular events than those who progressed to diabetes.17 In the U.S., the Diabetes Prevention Program Outcomes Study showed reversion to normal glucose regulation was associated with lower diabetes incidence and a lower prevalence of microvascular complications.18–20
It remains unknown which treatments or interventions are most effective for reversing prediabetes. To answer this question, this study aims to synthetize and compare the effectiveness of diverse interventions for reverting prediabetes to normal glucose regulation that were tested via RCTs. This study could inform policy and practice directed at managing prediabetes. This is particularly important given current debates around the use of pharmaceutical drugs to treat prediabetes and about whether prediabetes should be diagnosed and treated.
EVIDENCE ACQUISITION
Data Sources and Searches
MEDLINE, Embase, and Cochrane Library databases were searched systematically for articles published from January 1, 2000 to June 27, 2018. Investigators used combinations of Medical Subject Headings and search terms such as prediabetes, prevention, regression, and risk reduction (an example of a full search string is presented in Appendix Table 1). Relevant published meta-analyses were also reviewed to identify additional studies to include. The review protocol was registered in PROSPERO (CRD42017067750) and the present report adheres to PRISMA guidelines for reporting systematic reviews incorporating network meta-analyses of healthcare interventions.21
Study Selection
Eligible studies were those conducted among adults with prediabetes, testing any non-surgical intervention aimed to prevent diabetes or improve glucose regulation, lasting ≥3 months, using a randomized controlled design with any comparison group (i.e., control or lower intensity/alternative intervention), and reporting achievement of normoglycemia as an outcome. Prediabetes was defined according to the American Diabetes Association or World Health Organization criteria as follows22,23: fasting blood glucose level of 5.6–7.0 mmol/L or 6.1–7.0 mmol/L, a 2-hour post 75-mg oral glucose tolerance test (OGTT) plasma blood glucose level of 7.8–11.0 mmol/L, or hemoglobin A1c measure of 5.7%–6.4%. Studies testing surgeries; studies conducted in individuals with gestational diabetes, type 1 diabetes, children, or with other chronic diseases (e.g., women with polycystic ovary syndrome, men with HIV); and animal studies were excluded. Two reviewers independently screened study abstracts and full texts; discrepancies on inclusion decisions were resolved through discussions. Reviewers were not masked to author or journal names during this process.
Data Extraction and Risk of Bias Assessment
A standardized electronic fillable data form was designed to extract the data of interest. Two reviewers conducted the data extraction for all studies. A third reviewer conducted a second data extraction among 20% of the studies to detect and correct any data entry errors. For the primary outcome, the number of participants with prediabetes who achieved normal blood glucose levels by the end of the active intervention period was extracted. Reversion to normal glucose levels was defined as achieving fasting blood glucose levels of <5.6 or <6.1 mmol/L, OGTT plasma blood glucose levels of <7.8 mmol/L, or hemoglobin A1c <5.7% at the end of the intervention. Additional information extracted included treatment tested (e.g., medication type, dose), study characteristics (e.g., country, duration), and participant characteristics (e.g., age, sex, baseline BMI, race). For studies that did not report the data of interest, corresponding authors were contacted to request the information.
Risk of bias in individual studies was assessed by 3 independent reviewers, with discrepancies resolved through discussions. The Cochrane Collaboration tool was used,24 which assesses the risk of bias in a study based on 7 domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. For each domain, studies were categorized as having low, high, or unclear risk of bias based on established criteria outlined in the tool. High risk of bias in a domain was interpreted as plausible bias that seriously weakens confidence in the results; low risk of bias was plausible bias unlikely to seriously weaken confidence in the results; and unclear risk of bias indicated there was insufficient information to issue a judgment. For this review, overall study risk of bias was categorized as follows: low when ≥5 domains were deemed as low risk and high when ≥2 domains were deemed as high or unclear.
Data Synthesis and Analyses
For analyses, duplicate reports from the same study were excluded, and only the report with the most relevant and complete data was used. In studies testing different doses or combinations of the same treatments, the arm with the most intensive intervention/dose was included (e.g., longer intervention duration, higher medication dose). Data from published articles were obtained in 2018. The initial data analysis was conducted in 2019 and updated in 2021.
To obtain pooled effects for the various interventions in 1 parsimonious model, an arm-based network meta-analysis was conducted.25 For this, all study arms were first divided into intervention and control arms: Intervention arms were those receiving any intervention or treatment, whereas control arms received no intervention or placebo. Placebo or control arms that received a lifestyle modification intervention were categorized as lifestyle intervention arms. After this, arms from all studies were grouped according to the specific intervention/medication tested and the geometry of the network was summarized graphically in a network plot.
The pooled relative risk (RR) and the risk difference (RD) between intervention and control, as well as between treatments, were estimated using a random-effects frequentist model weighted by the inverse variance. A multivariate mixed model approach was used to account for the correlations in multi-arm studies.26 The number needed to treat was computed as 1 ÷ RD for each intervention/treatment. Between-study heterogeneity for treatment effects was assessed using the I2 statistic and the p-value for heterogeneity (via the Cochrane’s Q test). An I2 value ≥75% or a p-value <0.05 was considered high between-study heterogeneity.27 Publication bias was assessed via Egger’s test and considered present if the test was significant (p<0.05).
Subgroup analyses according to participant sex, age, race, BMI, weight loss percentage, and normoglycemia ascertainment (e.g., based on fasting glucose vs. or glucose tolerance) were conducted to explore sources of heterogeneity in treatment effects. Consistency between direct and indirect estimates was assessed via node splitting, where network estimates were split into the contribution of direct and indirect evidence to identify inconsistency in specific treatment comparisons in the network. Finally, a sensitivity analysis was conducted to isolate the treatment effects among studies with low risk of bias. The analyses described were conducted using the Netmeta package28 in R, version 1.2–1. An independent statistician ran the analyses described here separately to identify and address potential errors and inconsistencies in the data or analyses.
Strength of the Evidence Assessment
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach for network meta-analysis29 was used to determine the strength of the evidence summarized in the meta-analysis. For this, the strength of the evidence for each treatment was categorized as strong, moderate, low, or very low based on the presence of risk of bias, inconsistency of results, imprecision, publication bias, and magnitude of treatment effects. In this study, the evidence for all treatments was considered strong to start: The evidence was downgraded by 1 level (e.g., from strong to moderate) if 20%–39% of studies testing a specific intervention/treatment had high risk of bias, or if publication bias was present (Egger’s test p<0.05), or if the observed effect heterogeneity was moderate (I2=50%–74% according to existing recommendations27). The strength of the evidence was downgraded by 2 levels (e.g., from strong to low) if ≥40% of studies testing a treatment had high risk of bias, if the observed effect heterogeneity was high (I2 ≥75%27), or if the 95% confidence intervals for the effect estimate were wide.
EVIDENCE SYNTHESIS
Systematic Review
Of 3,547 identified titles, 54 articles met the inclusion criteria and were included in the systematic review (Appendix Figure 1). Participant and intervention characteristics across the 54 studies are presented in Appendix Table 2. The 54 studies enrolled 35,289 participants with a mean age of 53 years, 39% of participants were male, and 30% of participants were White/European. Prediabetes was identified using fasting blood glucose in 2 studies, OGTT in 19 studies, and a combination of fasting blood glucose, OGTT, or hemoglobin Alc in 33 studies. Seventeen studies used the American Diabetes Association diagnostic criteria, 24 used the World Health Organization diagnostic criteria, and 6 did not report the criteria used. Most of the studies (41%) were conducted in Asia, 31% in North America, 15% in Europe, 11% across multiple countries, and 2% in Oceania.
Regarding risk of bias, the assessment showed most studies reported how the random sequence was generated (n=25), how blinding of participants was conducted (n=26), and how group allocation was concealed (n=18), granting them low risk of bias in these domains. Blinding of outcome assessors was explicitly reported in 8 studies and not reported in the rest of the studies. However, the authors concluded this did not affect glucose outcome assessment and assigned a low risk of bias for this domain to all 54 studies. No significant protocol deviations were found across 54 studies, and 49 had low percentage of missing data (Appendix Figure 2). Based on the criteria set for this study, 40 studies were considered to have low risk and 14 studies to have high risk of bias.
Meta-Analysis
Of the 54 studies identified in the systematic review, 6 were additional reports of trials already included and 1 study did not provide sufficient data for the meta-analysis. The remaining 47 studies were included in the meta-analysis (n=26,460, mean age=53 years, 46% male, 31% White, mean BMI=30 kg/m2). These studies contributed 67 intervention arms (including placebo or control arms that received a lifestyle modification intervention) and 35 control/placebo arms. Intervention and control/placebo arms were balanced in demographic and clinical characteristics at baseline (Table 1).
Table 1.
Baseline Participant Characteristics Across Treatment Arms
| Arms | Study participant characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Male sex | Race | Weight (kg) Mean (SD) |
BMI (kg/m2) Mean (SD) |
FBG (mmol/l) Mean (SD) |
PLG (mmol/l) Mean (SD) |
A1c (%) Mean (SD) |
Normoglycemiaa% | |
| Control (N=35; n=10,164) |
52.9 (6.0) |
44% | White 20% | 84.9 (11.3) |
29.7 (3.7) |
5.9 (0.4) |
8.5 (1.9) |
6.0 (0.4) |
31% |
| Intervention (N=67; n=15,948) |
53.1 (6.0) |
40% | White 30% | 84.5 (12.6) |
29.4 (4.3) |
5.8 (0.4) |
8.6 (1.4) |
6.0 (0.4) |
45% |
| AGIs (N=5; n=1,703) |
57.3 (5.8) |
55% | Asian 60% | 79.9 (10.9) |
26.3 (3.1) |
6.1 (0.3) |
8.9 (0.5) |
5.5 (0.6) |
53% |
| DPP-4 (N=1; n=8) |
49.3 (5.7) |
38% | Latino 100% | 84.3 (18.1) |
31.3 (3.6) |
5.5 (0.5) |
8.9 (0.9) |
6.1 (0.3) |
63% |
| Fenofibrate (N=1, n=20) |
NR | 0% | Asian 100% | NR | NR | NR | NR | NR | 55% |
| GLP-1 (N=2; n=1,488) |
50.5 (4.2) |
24% | White 83% | 107.0 (0.8) |
37.3 (2.1) |
5.7 (0.3) |
7.7 (0.4) |
5.7 (0.1) |
66% |
| Chinese medicine (N=10; n=1,111) |
51.6 (3.3) |
46% | Asian 100% | 66.7 (3.4) |
25.6 (1.6) |
6.0 (0.4) |
9.0 (0.5) |
6.4 (0.5) |
44% |
| Insulin secretagogues (N=1; n=16) |
58.0 (2.0) |
11% | White 100% | NR | 27.9 (1.5) |
5.3 (0.1) |
7.7 (0.2) |
NR | 56% |
| Insulin sensitizers (N=13, n=4,342) |
52.3 (7.3) |
38% | White 19% | 85.3 (7.7) |
30.5 (2.6) |
5.6 (0.4) |
8.9 (0.5) |
5.9 (0.2) |
43% |
| L-arginine (N=1; n=66) |
57.2 (11.7) |
51% | White 100% | NR | 30.4 (5.3) |
5.8 (0.7) |
9.1 (1.0) |
NR | 42% |
| Lifestyle modification (N=27; n=4,065) |
53.6 (6.1) |
39% | Asian 44% | 83.9 (12.5) |
29.6 (5.1) |
5.9 (0.4) |
8.6 (2.2) |
6.0 (0.4) |
41% |
| Lipase inhibitor (N=1; n=67) |
43.9 (0.6) |
19% | White 88% | 99.0 (0.6) |
35.6 (0.1) |
5.8 (0.1) |
NR | NR | 72% |
| Magnesium (N=1; n=59) |
53.6 (6.1) |
NR | Latino 100% | NR | 30.6 (6.4) |
6.3 (0.4) |
7.7 (1.9) |
NR | 51% |
| RAS blockade (N=1; n=2,623) |
54.7 (10.9) |
40% | Asian 44% | 84.8 (18.8) |
30.9 (5.6) |
NR | NR | NR | 43% |
| Vitamin D (N=3; n=380) |
56.3 (7.1) |
66% | White 67% | 102.6 (10.2) |
29.6 (3.1) |
6.0 (0.4) |
7.7 (0.7) |
6.1 (0.1) |
23% |
Crude total percentage of participants that achieved normoglycemia at the end of the intervention.
AGIs, alpha-glucosidase inhibitors; DPP-4, dipeptidyl peptidase 4 inhibitors; FBG, fasting blood glucose; GLP-1, glucagon-like peptide 1 receptor agonists; N, total number of arms; n, total number of participants; NR, not reported; PLG, 2-hour post-load glucose; RAS, renin-angiotensin system.
Of the intervention arms, 27 tested lifestyle modification strategies (through dietary or physical activity/exercise improvements), 25 medications, 5 dietary supplements (vitamin D, magnesium, and L-arginine), and 10 Chinese medicine. Medications tested included insulin sensitizers (metformin, rosiglitazone, and pioglitazone), insulin secretagogues (glipizide), AGIs (acarbose and voglibose), dipeptidyl peptidase 4 inhibitors (linagliptin), glucagon-like peptide 1 receptor agonists ([GLP-1]; liraglutide, dapagliflozin, and exenatide), fenofibrate, renin–angiotensin system blockers (rampiril), and a lipase inhibitor (orlistat). Figure 1 shows the asymmetrical network plot where lifestyle modification (27 arms, n=4,065) and insulin sensitizers (13 arms, n=4,342) were the most tested approaches against control/placebo arms.
Figure 1.
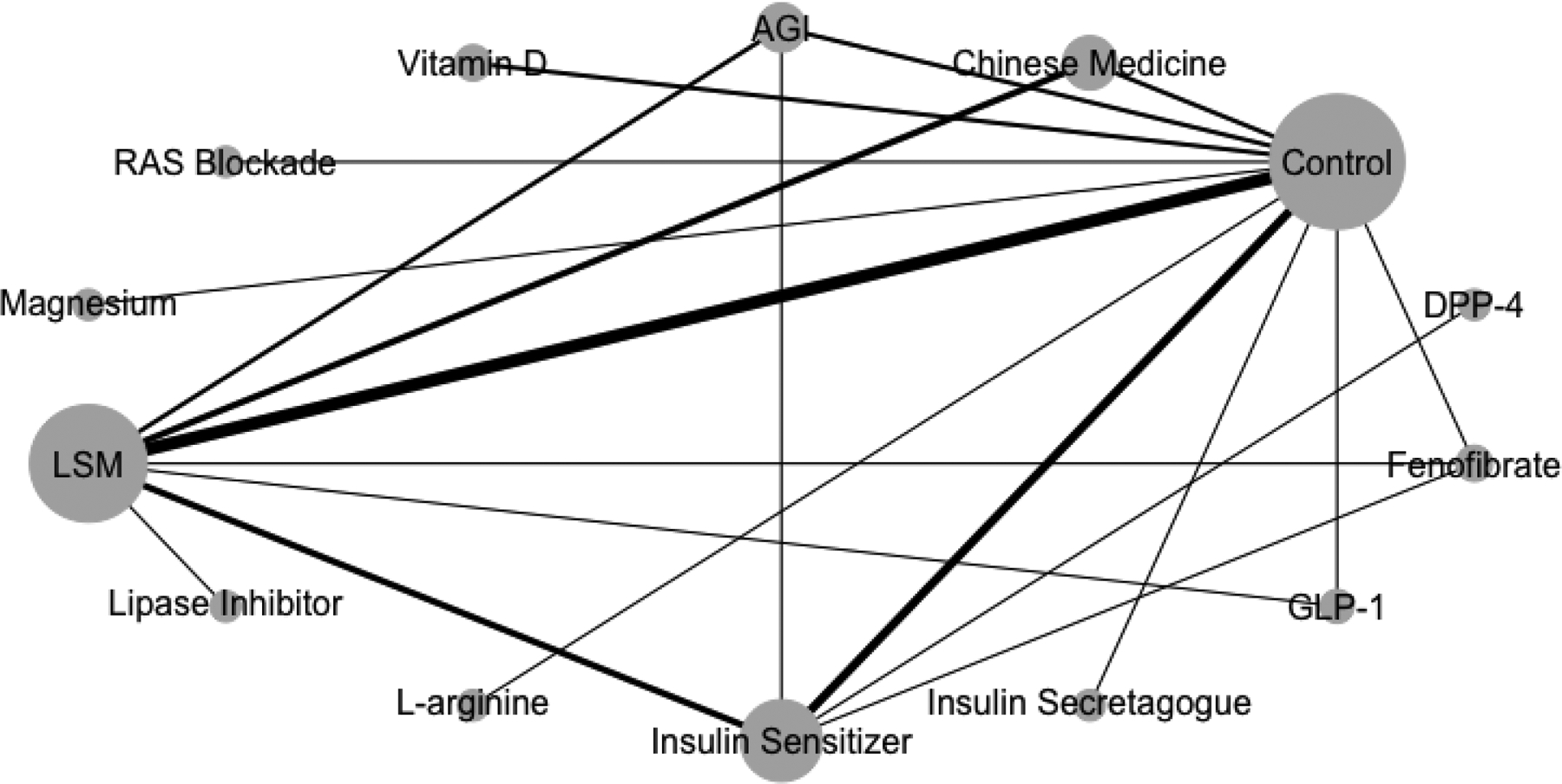
Network structure showing an asymmetrical network.
Notes: The size of the bubble represents the number of studies testing a treatment and the thickness of the connecting lines the number of arms testing that comparison.
AGI, alpha-glucosidase inhibitors; DPP-4, dipeptidyl peptidase 4 inhibitors; GLP-1, glucagon-like peptide 1 receptor agonists; LSM, lifestyle modification; RAS, renin-angiotensin system.
Meta-analytic estimates for the different treatments are presented in Table 2 and Figure 2. Groups receiving GLP-1 were more likely to achieve normoglycemia than control groups after a median follow-up of 1.7 years (68% vs 21%; RD=0.47, 95% CI=0.22, 0.72; RR=3.53, 95% CI=1.69, 7.37; NTT=2). Individuals in the lipase inhibitor group were more likely to achieve normoglycemia than control after a median follow-up of 1.6 years (61% vs 21%; RD=0.40, 95% CI=0.04, 0.76; RR=2.57, 95% CI=1.14, 5.81; NNT=3). Individuals in the fenofibrate group were more likely to achieve normoglycemia than control after a median follow-up of 0.5 years (58% vs 21%; RD=0.37, 95% CI=0.02, 0.71; RR=1.85, 95% CI=0.82, 4.19; NNT=3). Groups receiving AGIs were more likely to achieve normoglycemia than control after a median follow-up of 2.7 years (50% vs 21%; RD=0.29, 95% CI=0.14, 0.43; RR=2.01, 95% CI=1.40, 2.91; NTT=4). Those receiving insulin sensitizer agents were also more likely to achieve normoglycemia than control after a median follow-up of 2.0 years (44% vs 21%; RD=0.23, 95% CI=0.14, 0.33; RR=1.62, 95% CI=1.23, 2.14; NNT=4).
Table 2.
Risk Difference (95% CI) Relative to Control (First Column) and to Treatment
| CON | RAS | VIT-D | LSM | SEC | L-ARG | SEN | AGIs | DPP-4 | CHIN | FEN | LIP | MAG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAS | 0.04 (−0.27; 0.35) |
||||||||||||
| VIT-D | 0.09 (−0.09; 0.28) |
−0.05 (−0.41; 0.31) |
|||||||||||
| LSM |
0.18
(0.10; 0.25) |
−0.13 (−0.45; 0.18) |
−0.08 (−0.29; 0.12) |
||||||||||
| SEC. | 0.15 (−0.31; 0.61) |
−0.11 (−0.66; 0.44) |
−0.06 (−0.55; 0.44) |
0.03 (−0.44; 0.49) |
|||||||||
| L-ARG | 0.20 (−0.14; 0.55) |
−0.16 (−0.62; 0.30) |
−0.11 (−0.50; 0.28) |
−0.03 (−0.38; 0.32) |
−0.05 (−0.62; 0.52) |
||||||||
| SEN |
0.23
(0.14; 0.33) |
−0.19 (−0.52; 0.13) |
−0.14 (−0.35; 0.07) |
−0.06 (−0.16; 0.05) |
−0.08 (−0.55; 0.38) |
−0.03 (−0.39; 0.33) |
|||||||
| AGIs |
0.29
(0.14; 0.43) |
−0.24 (−0.58; 0.10) |
−0.19 (−0.43; 0.05) |
−0.11 (−0.26; 0.04) |
−0.13 (−0.61; 0.34) |
−0.08 (−0.46; 0.29) |
−0.05 (−0.21; 0.11) |
||||||
| DPP-4 | 0.36 (−0.22; 0.94) |
−0.32 (−0.97; 0.34) |
−0.26 (−0.87; 0.35) |
−0.18 (−0.76; 0.40) |
−0.21 (−0.95; 0.53) |
−0.16 (−0.83; 0.52) |
−0.13 (−0.70; 0.45) |
−0.07 (−0.67; 0.52) |
|||||
| CHIN |
0.31
(0.19; 0.42) |
−0.26 (−0.59; 0.07) |
−0.21 (−0.43; 0.01) |
−0.13 (−0.24; −0.02) |
−0.16 (−0.63; 0.32) |
−0.10 (−0.47; 0.26) |
−0.07 (−0.22; 0.07) |
−0.02 (−0.20; 0.16) |
0.05 (−0.54; 0.64) |
||||
| FEN |
0.37
(0.02; 0.71) |
−0.33 (−0.79; 0.14) |
−0.27 (−0.67; 0.12) |
−0.19 (−0.54; 0.15) |
−0.22 (−0.79; 0.35) |
−0.17 (−0.65; 0.32) |
−0.14 (−0.48; 0.21) |
−0.08 (−0.45; 0.29) |
−0.01 (−0.68; 0.66) |
−0.06 (−0.42; 0.30) |
|||
| LIP |
0.40
(0.04; 0.76) |
−0.36 (−0.83; 0.12) |
−0.31 (−0.71; 0.10) |
−0.23 (−0.58; 0.13) |
−0.25 (−0.83; 0.33) |
−0.20 (−0.70; 0.30) |
−0.17 (−0.54; 0.20) |
−0.12 (−0.50; 0.27) |
−0.04 (−0.72; 0.64) |
−0.10 (−0.47; 0.27) |
−0.03 (−0.53; 0.46) |
||
| MAG |
0.44
(0.10; 0.78) |
−0.40 (−0.85; 0.06) |
−0.34 (−0.73; 0.05) |
−0.26 (−0.61; 0.09) |
−0.29 (−0.86; 0.28) |
−0.23 (−0.72; 0.25) |
−0.20 (−0.56; 0.15) |
−0.15 (−0.52; 0.22) |
−0.08 (−0.75; 0.59) |
−0.13 (−0.49; 0.23) |
−0.07 (−0.55; 0.41) |
−0.04 (−0.53; 0.46) |
|
| GLP-1 |
0.47
(0.22; 0.72) |
−0.43 (−0.83; −0.03) |
−0.38 (−0.69; −0.06) |
−0.30 (−0.54; −0.05) |
−0.32 (−0.84; 0.20) |
−0.27 (−0.69; 0.16) |
−0.24 (−0.50; 0.03) |
−0.19 (−0.47; 0.10) |
−0.11 (−0.74; 0.52) |
−0.17 (−0.43; 0.10) |
−0.10 (−0.52; 0.32) |
−0.07 (−0.50; 0.36) |
−0.03 (−0.45; 0.39) |
Note: The table reads top to bottom, starting with the first column/row and moving down within the column to each row below. Estimates are ranked according to effect size from top to bottom, and by significance level from left to right. Bolded text indicates significant estimates.
AGI, alpha-glucosidase inhibitors; L-ARG, L-arginine; CHIN, Chinese medicine; DPP-4, dipeptidyl peptidase 4 inhibitors; FEN, fenofibrate; GLP-1, glucagon-like peptide 1 receptor agonists; LIP, lipase inhibitor; LSM, lifestyle modification; MAG, magnesium; SEN, insulin sensitizers; SEC, insulin secretagogue; RAS, renin-angiotensin system; VIT-D, vitamin D.
Figure 2.
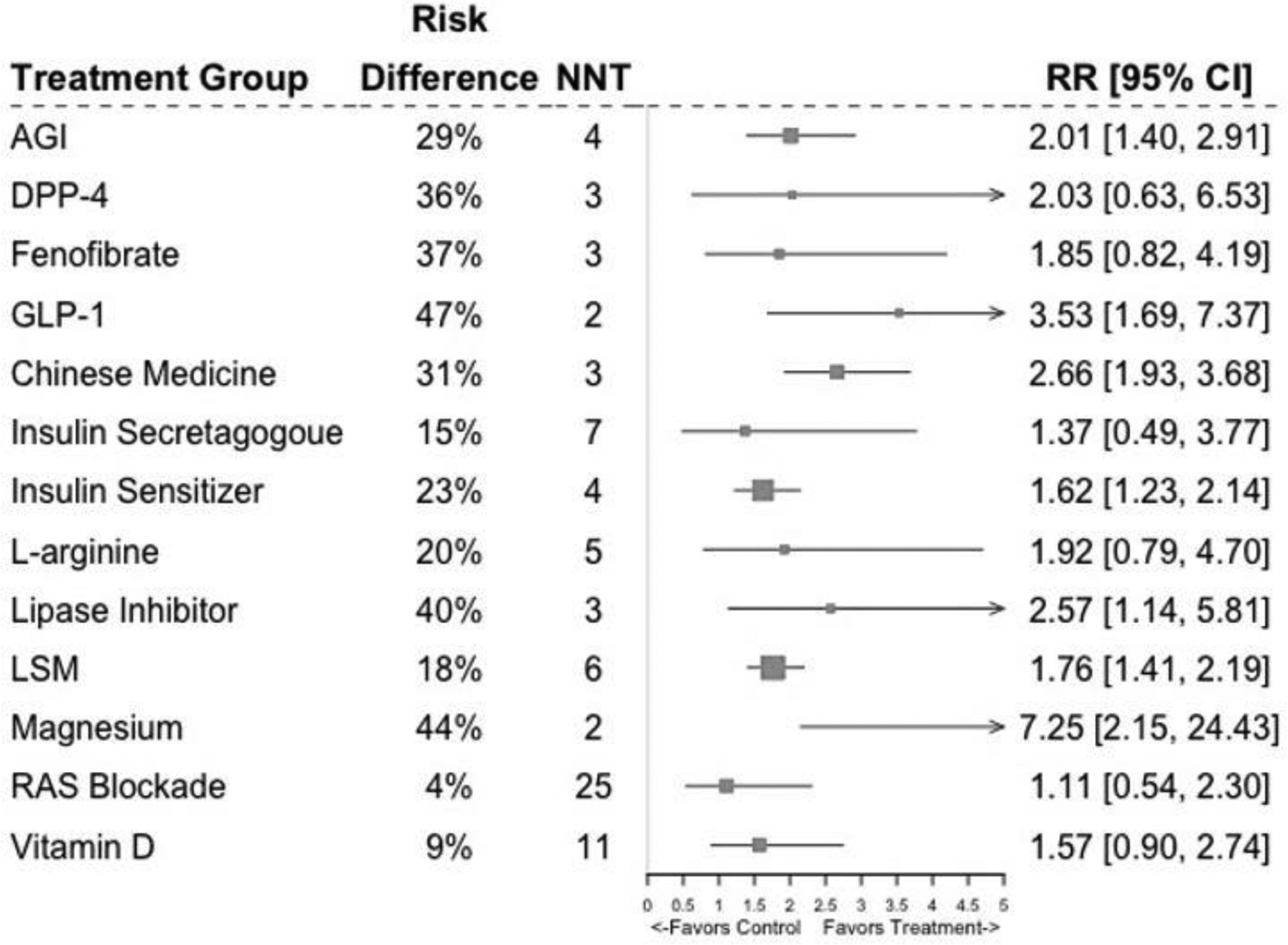
Forest plot showing pooled effects for each treatment against control/placebo.
AGI, alpha-glucosidase inhibitors; DPP-4, dipeptidyl peptidase 4 inhibitors; GLP-1, glucagon-like peptide 1 receptor agonists; LSM, lifestyle modification; RAS, renin-angiotensin system; NNT, number needed to treat; RD, risk difference; RR, relative risk.
Regarding non-pharmacological interventions, individuals receiving magnesium supplements were more likely to achieve normoglycemia than control (65% vs 21%; RD=0.44, 95% CI=0.10, 0.78; RR=7.25, 95% CI=2.15, 24.43; NNT=2) at a median follow-up of 0.3 years. Chinese medicine groups were also more likely to achieve normoglycemia than control at a median follow-up of 1 year (52% vs 21%; RD=0.31, 95% CI= 0.19, 0.42; RR=2.66, 95% CI=1.93, 3.68; NNT=3). Finally, lifestyle modification groups were more likely to achieve normoglycemia than control at a median follow-up of 1.6 years (39% vs 21%; RD=0.18, 95% CI=0.10, 0.25; RR=1.76, 95% CI=1.41, 2.19; NNT=6).
The full network meta-analysis showed some treatments achieved higher normoglycemia levels relative to other treatments (Table 2). Specifically, the GLP-1 group was more likely to achieve normoglycemia than renin–angiotensin system blockade medication (RD= −0.43, 95% CI= −0.83, −0.03), vitamin D (RD= −0.38, 95% CI= −0.69, −0.06), and lifestyle modification groups (RD= −0.30, 95% CI= −0.54, −0.05). Similarly, Chinese medicine groups were more likely to achieve normoglycemia than lifestyle modification groups (RD= −0.13, 95% CI= −0.24, −0.02). No other significant between-treatment differences were observed. Effect estimates in the full network model were heterogeneous for both RR (I2=82.7%, 95% CI=77.4%, 86.7%) and RD (I2=91%, 95% CI=88.8%, 92.8%).
Consistency and Strength of the Evidence Assessment
Subgroup analyses comparing intervention versus control arms showed effect estimates varied according to clinical and study characteristics (Appendix Table 3). Specifically, AGIs (RD=0.24, 95% CI=0.11, 0.38), Chinese medicine (RD=0.27, 95% CI=0.13, 0.41), and lifestyle modification approaches (RD=0.15, 95% CI=0.07, 0.24) had beneficial effects in studies where participants were aged ≥50 years, but null effects were observed in studies where participants were aged <50 years. AGIs were beneficial in studies where <80% of participants were White (RD=0.35, 95% CI=0.18, 0.53) and in studies where mean BMI was <30 kg/m2 (RD=0.35, 95% CI=0.19, 0.52), but no effects were observed in studies where ≥80% of participants were White (RD=0.04, 95% CI= −0.13, 0.21) and where mean BMI was ≥30 kg/m2 (RD=0.04, 95% CI= −0.25, 0.34).
Lifestyle modification approaches had beneficial effects in studies where impaired glucose tolerance or impaired fasting glucose were used to determine normoglycemia achievement (RD=0.21, 95% CI=0.08, 0.33), whereas no effects were observed in studies where only impaired glucose tolerance was used (RD=0.10, 95% CI= −0.04, 0.24). AGIs and insulin sensitizers had a beneficial effect among participants who lost ≥4% of their baseline weight (RD=0.55, 95% CCI=0.25, 0.86 and RD=0.43, 95% CI=0.21, 0.66, respectively), whereas null effects were observed among participants that lost <4% (RD=0.04, 95% CI= −0.22, 0.31 and RD=0.10, 95% CI= −0.02, 0.23, respectively). Finally, shorter duration studies (≤12 months) had larger effect sizes than longer duration studies (>12 months), reaching 2 times higher normoglycemia estimates for all treatments (Appendix Table 3).
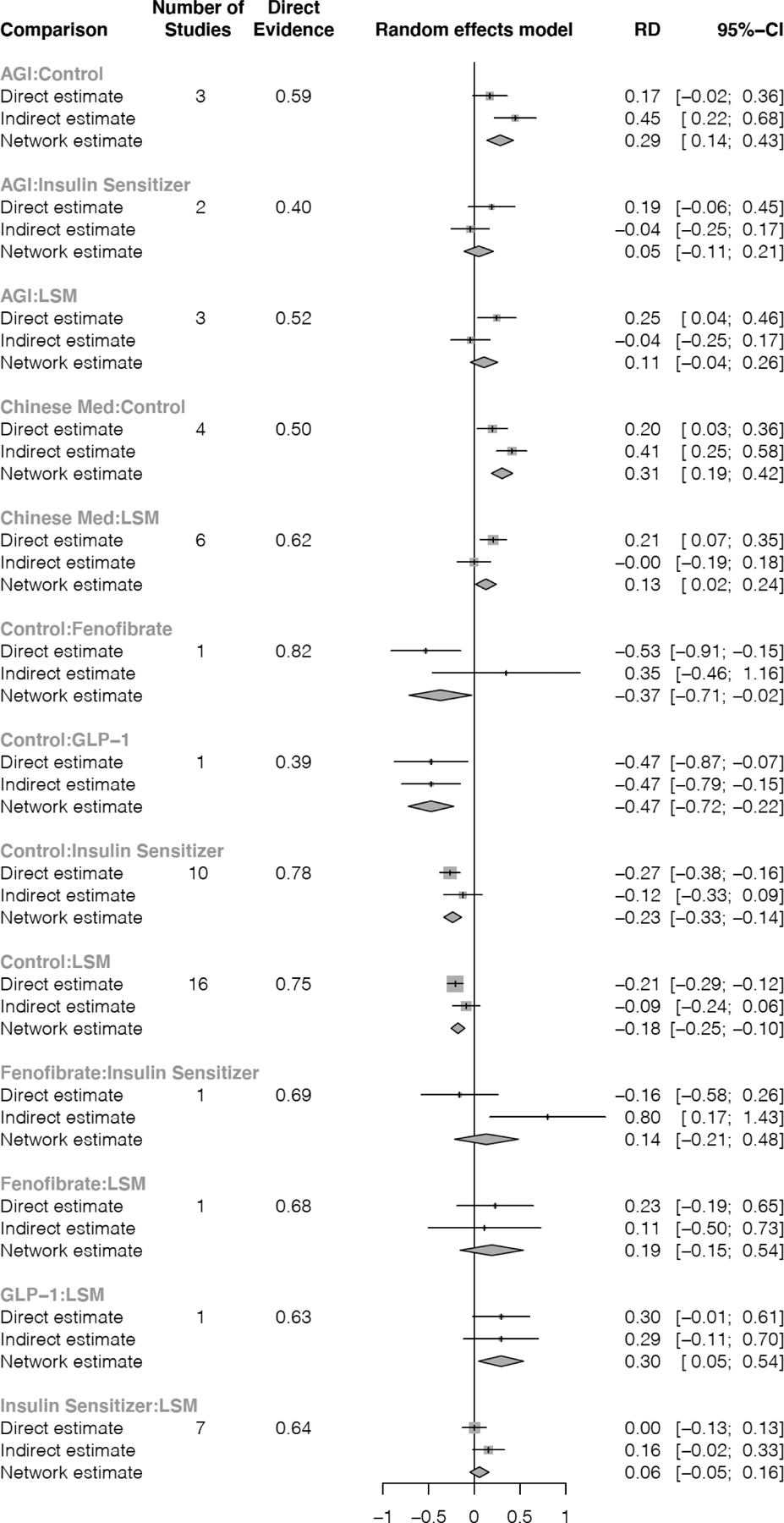
Sensitivity analyses including only 34 studies that were deemed to have low risk of bias yielded similar normoglycemia estimates to those obtained in analyses including all studies (Appendix Table 4). Regarding consistency, estimates from direct and indirect evidence were consentient (i.e., in the same direction) for most treatments (Appendix Figure 3). The main discrepancy was found in the AGI versus lifestyle modification comparison, with direct evidence from head-to-head studies showing AGIs were superior to lifestyle (RD=0.25, 95% CI=0.04, 0.46), and indirect evidence showing no difference (RD= −0.04, 95% CI= −0.25, 0.17).
Using the GRADE assessment, 33% of studies testing lifestyle modification approaches had high risk of bias due to lack of blinding to intervention. However, this was judged as not placing a major influence on the primary outcome. Given that no other GRADE criteria were of concern, the strength of the evidence for lifestyle modification was deemed strong. For studies testing insulin sensitizers, AGIs, and GLP-1, the strength of the evidence was deemed as moderate. This was due to the high heterogeneity in treatment effects observed in studies testing these treatments (I2=60%–85%), the fact that 33% of AGI studies and 40% of insulin sensitizer studies were at high risk of bias, and due to the imprecision in the GLP-1 effect estimate (there were only 2 studies). The strength of the evidence for Chinese medicine was deemed as low because 30% of studies had high risk of bias, the effect estimate was imprecise, and publication bias was present (Egger’s test p<0.001). Finally, the strength of the evidence for fenofibrate, the lipase inhibitor, and the magnesium supplement was deemed very low because the effect estimates were imprecise and there was a single study testing each treatment that had high risk of bias. The full GRADE assessment for all treatments is presented in Appendix Table 5.
DISCUSSION
This systematic review and meta-analysis of RCTs compared the effectiveness of diverse interventions for reversing prediabetes. It found that non-pharmacological approaches (lifestyle modification, Chinese medicine, and magnesium supplementation) as well as pharmacological approaches (GLP-1, AGIs, insulin sensitizers, lipase inhibitors, and fenofibrate) can reverse prediabetes. The achieved normoglycemia levels were larger for pharmacological interventions (23%–47%) than for non-pharmacological approaches (18%–44%). However, considering the risk of bias, precision, heterogeneity, and publication bias present in the included studies,29 the strength of the evidence is strongest for lifestyle modification, moderate for pharmacological agents, and low or very low for the remaining approaches. Based on these findings, and in line with current expert recommendations,30 lifestyle modification should be the first-line approach for treating prediabetes.
Groups receiving lifestyle modification interventions were 18% more likely to achieve normal glucose levels than control groups, with 6 people needing the intervention for one to reverse to normal. A previous meta-analysis of 6 studies showed that improvements in dietary and physical activity behaviors were associated with 53% higher RR for reversing to normal glucose levels among people with prediabetes,14 which aligns with the RR observed in this study. In this meta-analysis, lifestyle modification was most beneficial in studies where participants were aged >50 years, suggesting this age group may experience the greatest benefits. Studies where only OGTT was used to determine prediabetes reversion did not achieve significant reversion levels; however, this may be explained by the fact that most lifestyle modification approaches using OGTT were compared against other treatments (e.g., a drug or a Chinese medicine) as opposed to a control condition. As the strength of the evidence for lifestyle modification studies is the strongest,29 these findings support current expert statements30 recommending lifestyle modification as the first-line approach for treating prediabetes.
Regarding pharmacological interventions, GLP-1 receptor agonists achieved 47%, AGIs achieved 29%, and insulin sensitizers (metformin, rosiglitazone, and pioglitazone) achieved 23% higher reversion estimates than control groups. To get 1 person with prediabetes to reverse to normal glucose levels, 2, 4, and 4 people would need treatment, respectively. These findings align with previous estimates showing AGIs and insulin sensitizers are associated with 2 times higher normoglycemia odds than control groups.13 Subgroup analyses suggest AGIs may be more beneficial in older age groups (≥50 years), people without obesity (BMI <30 kg/m2), and among more racially diverse populations (<80% White). Additionally, the effect of AGIs and insulin sensitizers seems to be mediated by weight loss given only those who lost ≥4% of their baseline body weight experienced benefits. As the strength of the evidence was moderate for these medications and regulatory agencies have not approved medications for treating prediabetes, these drugs should not be used for this purpose.
Although this study found Chinese medicine, a lipase inhibitor (orlistat), fenofibrate, and a magnesium supplement were associated with higher normoglycemia levels than control groups, the strength of the evidence was low or very low for these approaches. Studies focused on rigorously examining the effects of these approaches on prediabetes reversion are lacking; aside from Chinese medicine, only 1 study per treatment was included in this meta-analysis. Evidence is more prominent for Chinese medicine, which has led to meta-analyses examining the effects of this approach on prediabetes reversal11 and potential mechanisms of action.31 Evidence on magnesium supplementation for reversing prediabetes may emerge in the near future.32 Overall, there is no sufficient evidence, neither support from expert recommendations, or approval from regulatory agencies to use these approaches for treating prediabetes.
Because the literature search was completed on June 27 of 2018, potentially eligible studies published after this date were not included. To determine whether the present analysis needed updating, the authors conducted a new search from this date up to August 20, 2021. They found 194 new study titles, of which 47 abstracts were screened, and 8 studies met the eligibility criteria. Of these, 4 studies tested lifestyle modification approaches (diet and physical activity strategies): 3 studies found 8%–25% higher normoglycemia levels in intervention compared with control groups,33–35 and 1 found no difference.36 Two studies tested medications: lorcaserin (serotonin receptor agonist) which was associated with a non-significant increase in normoglycemia among 185 patients with prediabetes,37 and saxagliptin (dipeptidyl peptidase 4 inhibitor), which promoted reversion to normal glucose tolerance in 11 of 12 patients receiving it.38 The remaining 2 studies tested vitamin D supplementation among 121 Asian Indian women,39 and Gymnema sylvestre (a plant from India) among 30 patients.40 Compared with control groups, more participants in the vitamin D (51% vs 44%) and Gymnema sylvestre group (47% vs 0%) returned to normal glucose levels. Because these studies are not likely to change the results, the authors decided not to update the present analyses.
One final point is the fact that 21% of control participants reversed to normal glucose levels in the meta-analysis; this falls within previous estimates indicating that 17%–42% people with prediabetes reverse to normal glucose levels within 11 years.41 Though some individuals with prediabetes reverse to normal without intervention, those who remain in the prediabetes state have increased risk for developing cardiovascular and renal diseases, even if they do not develop diabetes.4–6 Hence, working toward reversing prediabetes is a worthwhile approach for lowering cardiometabolic risk, as supported by long-term evidence from RCTs.16,18–20 Although there is a debate about whether a prediabetes diagnosis should be used,42 this meta-analysis supports expert statements recommending identification and treatment of this condition.2,30 However, the authors recognize that, unlike with diabetes remission, there is no consensus definition on what prediabetes remission, reversal, or normal glucose regulation restoration might constitute, which warrants further attention.43
Limitations
These findings should be interpreted considering some limitations. There was heterogeneity in study characteristics and effect estimates, which was partially explained in subgroup analyses. Twelve percent of the studies had high risk of bias; however, in sensitivity analyses excluding these studies, neither effect estimates nor study conclusions changed. There was inconsistency between direct and indirect evidence in the AGI versus lifestyle modification comparisons; hence, indirect comparisons between these treatments should be interpreted with caution. Few medication studies reported results after a washout period: this prevented the investigators from assessing whether the effect of these medication persisted after withdrawal. Finally, the search terms employed for the systematic review were in English and it is possible that studies published in other languages (e.g., Spanish) may have been missed.
CONCLUSIONS
This systematic review and meta-analysis of RCTs identified a range of pharmacological and non-pharmacological strategies that can reverse prediabetes. However, only lifestyle modification interventions provide strong evidence of effectiveness, which supports current expert statements recommending this as the first-line approach for treating prediabetes. To date, neither medications nor alternative approaches are recommended in expert statements or have been approved by regulatory authorities for treating prediabetes—the present findings support these conclusions. Findings from this study can motivate preventive medicine professionals to introduce lifestyle modification approaches in their clinical practice to treat prediabetes. Treating prediabetes could potentially offset, or at least delay, future medication needs and economic costs to individuals and the payer systems where they live. With close to 400 million people living with prediabetes worldwide, the potential of reversing this condition is significant.
ACKNOWLEDGMENTS
KIG was supported by the National Heart, Lung, and Blood Institute of NIH (K01HL149479). MKA, KMVN, and MBW were partially supported by the Georgia Center for Diabetes Translation Research (P30DK111024). KMVN, UPG, and MBW were supported by the National Heart, Lung, and Blood Institute of the NIH (R01HL125442). All authors had full access to study data and take responsibility for the integrity and accuracy of the data analyses. The study sponsor/funder was not involved in the design of the study; the collection, analysis, or interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Appendix Table 1.
Search String Used for PubMed Search
| 1. Prediabetes terms (results=48,541) | “prediabetic state”[MeSH] OR prediabetic OR prediabetes OR pre-diabetic OR pre-diabetes OR “glucose intolerance”[MeSH] OR impaired fasting glucose OR impaired glucose tolerance |
| 2. Normoglycemia terms (results=242,248) | normoglycemic OR normoglycemia OR normoglycaemic OR normoglycemia OR normal glucose tolerance OR normal fasting glucose OR “hypoglycemic agents”[MeSH] OR “hypoglycemic agents” |
| 3. Outcomes (results=6,498,412) | Treatment outcome OR remission OR reverse OR reduction OR reversal OR risk reduction OR risk OR regulation OR prevention |
| 4. 1 AND 2 AND 3 (results 9,373 humans and animals) | ((((“prediabetic state”[MeSH] OR prediabetic OR prediabetes OR pre-diabetic OR pre-diabetes OR “glucose intolerance”[MeSH] OR impaired fasting glucose OR impaired glucose tolerance))) AND (((normoglycemic OR normoglycemia OR normoglycaemic OR normoglycemia OR normal glucose tolerance OR normal fasting glucose OR “hypoglycemic agents”[MeSH] OR “hypoglycemic agent*”)))) AND (Treatment outcome OR remission OR reverse OR reduction OR reversal OR risk reduction OR risk OR regulation OR prevention) |
| 5. Limit 4 to animals (results 2,362) | ((((“prediabetic state”[MeSH] OR prediabetic OR prediabetes OR pre-diabetic OR pre-diabetes OR “glucose intolerance”[MeSH] OR impaired fasting glucose OR impaired glucose tolerance))) AND (((normoglycemic OR normoglycemia OR normoglycaemic OR normoglycemia OR normal glucose tolerance OR normal fasting glucose OR “hypoglycemic agents”[MeSH] OR “hypoglycemic agent*”)))) AND (Treatment outcome OR remission OR reverse OR reduction OR reversal OR risk reduction OR risk OR regulation OR prevention) Filters: Other Animals |
| 6. #4 NOT #5 AND date from 2001+ (Results=5,503) | (((((“prediabetic state”[MeSH] OR prediabetic OR prediabetes OR pre-diabetic OR pre-diabetes OR “glucose intolerance”[MeSH] OR impaired fasting glucose OR impaired glucose tolerance)) AND (normoglycemic OR normoglycemia OR normoglycaemic OR normoglycemia OR normal glucose tolerance OR normal fasting glucose OR “hypoglycemic agents”[MeSH] OR “hypoglycemic agents”)) AND (Treatment outcome OR remission OR reverse OR reduction OR reversal OR risk reduction OR risk OR regulation OR prevention))) NOT (((((“prediabetic state”[MeSH] OR prediabetic OR prediabetes OR pre-diabetic OR pre-diabetes OR “glucose intolerance”[MeSH] OR impaired fasting glucose OR impaired glucose tolerance)) AND (normoglycemic OR normoglycemia OR normoglycaemic OR normoglycemia OR normal glucose tolerance OR normal fasting glucose OR “hypoglycemic agents”[MeSH] OR “hypoglycemic agents”)) AND (Treatment outcome OR remission OR reverse OR reduction OR reversal OR risk reduction OR risk OR regulation OR prevention)) AND Animals[Mesh:noexp]) AND ((“2001/01/01”[PDat] : “2030/12/31”[PDat])) |
Appendix Table 2.
Baseline Participant and Intervention Characteristics for Each Study Included in the Systematic Review and Meta-Analysis
| Included only in Systematic Review (n=9) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Participant characteristics | Intervention characteristics | |||||||
| Author (year) Country |
Age (years) | N | Male | Race | BMI (kg/m2) | Mean Follow up (years) | Treatment tested | Dose |
| DeFronzo (2013)1 U.S. |
53 | 213 | 42% | 50% White | 33.0 | 2.4 (median) | Insulin Sensitizers (Pioglitazone) | 30‒45 mg per day for 2 years |
| Rosenstock (2010)2 U.S. |
4 | 73 | 18% | NR | 39.6 | NR | GLP-1 (Exenatide) plus diet | 10 ug 2 times per day with a 5 ug dose initiation period and caloric reduction in diet for all meals for 24 weeks |
| Perreault (2009a)3 U.S. |
50 | 850 | 35% | 54% White | 33.0 | NR | Lifestyle modification | 16 individual, 1-hour counseling sessions focused on increasing physical activity up to 150 minutes of moderate to vigorous intensity physical activity per week, delivered over 1 year |
| Perreault (2009b)3 U.S. |
51 | 832 | 36% | 57% White | 33.0 | NR | Insulin sensitizers (Metformin) | 850 mg 2 times per day delivered over 1 year |
| Perreault (2012a)4 U.S. |
NR | 736 | 35% | 53% White | 33.0 | 5.4 | Lifestyle modification | 16‒20 individual counseling sessions per year focused on increasing physical activity up to 150 minutes of moderate to vigorous intensity physical activity per week (participants could choose to do a minimum of 16 sessions and maximum of 20 sessions) for 1 year |
| Perreault (2012b)4 U.S. |
NR | 647 | 31% | 60% White | 33.0 | 5.4 | Insulin sensitizer (Metformin) | 850 mg 2 times per day for 1 year |
| Snehalatha (2009)5 India |
NR | 321 | NR | 100% White | 25.5 | 3.0 | Insulin sensitizer (Metformin) plus lifestyle modification | 500 g per day and advice on physical activity and diet for 3 years |
| Tripathy (2014)6 U.S. |
54 | 207 | 45% | NR | 33.4 | 2.4 | Insulin sensitizer (Pioglitazone) | 30 mg per day for 1 month and then 45 mg per day for 23 months |
| Tripathy (2016)7 U.S. |
54 | 152 | NR | NR | 33.4 | 2.4 | Insulin sensitizer (Pioglitazone) | 30 mg per day for a year |
| Included in Systematic Review and Meta-Analysis (n=47) | ||||||||
| Author (year) Country |
Age (years) | N | Male | Race | BMI (kg/m2) | Mean follow up (years) | Treatment tested | Dose |
| Barengolts (2015)8 U.S. |
58.2 | 87 | 100% | 100% Black | 32.4 | 1.0 | Vitamin D | 50,000 IU per week |
| Bennett (2004)9 Multiple countries |
62.7 | 9 | 56% | NR | 30.2 | 0.23 | Insulin Sensitizer (Rosiglitazone) | 4 mg per day for 12 weeks |
| Bhopal (2014)10 Scotland |
52.8 | 85 | 46% | 100% Asian | 30.6 | 3.0 | Lifestyle Modification | 15 visits from a dietician focused on weight loss over 3 years |
| Block (2015)11 U.S. |
55.0 | 163 | 68% | 67% White | 31.1 | 0.5 | Lifestyle Modification | Weekly emails with tailored behavior change support for 6 months |
| Chiasson (2002)12 Multiple countries |
54.3 | 682 | 48% | 97% White | 31.0 | 3.3 | AGI (Acarbose) plus Lifestyle Modification | 100 mg at 3 times per day for 3 years |
| DeFonzo (2011)13 U.S. |
53.0 | 303 | 42% | 51% White | 33.0 | 2.4 | Insulin Sensitizer (Pioglitazone) | 30 mg per day for first month. Then, 45 mg per day for remaining 23 months |
| DPP Research Group (2002a)14 U.S. |
50.9 | 1,073 | 34% | 56% White | 33.9 | 2.8 | Insulin Sensitizer (Metformin) | 850 mg at 2 times per day for 24 weeks |
| DPP Research Group (2002b)14 U.S. |
50.6 | 1,079 | 32% | 54% White | 33.9 | 2.8 | Lifestyle Modification | One-year lifestyle modification program including 2 phases: (1) an intensive 4-month phase of 16 individual counselling sessions focused on improving physical activity and dietary behaviors and (2) a maintenance phase including monthly individual counseling sessions delivered over 24 weeks |
| DREAM (2006)15 Multiple countries |
54.6 | 2,635 | 42% | NR | 30.8 | 3 (median) |
Insulin Sensitizer (Rosiglitazone) |
8 mg per day for 3 years |
| DREAM (2006)16 Multiple countries |
54.7 | 2,623 | 40% | NR | 30.9 | 3 (median) |
Insulin Sensitizer (Rampiril) | 5 mg per day for first 2 months 10 mg per day for next 10 months 15 mg per day until end of study (up to 3 years) |
| Dutta (2014a)17 India |
48.4 | 68 | 43% | NR | 26.3 | 2.4 | Vitamin D plus Lifestyle Modification | 60, 000 IU per week, 1,250 mg Calcium per month, and diet and physical activity |
| Dutta (2014b)17 India |
47.4 | 57 | 31% | NR | 26.8 | 2.4 | Lifestyle Modification | 1,250 mg Calcium per month and diet and physical activity |
| Eriksson (2006)18 Finland |
53.0 | 17 | 11% | NR | 27.9 | 3.2 | Insulin Secretagogue (Glipizide) | 2.5 mg per day for 6 months |
| Fan (2004a)19 China |
54.6 | 23 | 43% | 100% Asian | 25.8 | NR | Chinese Medicine | Jiangtang Bushen Recipe at 2‒3 administrations per week for 1 year |
| Fan (2004b)19 China |
57.0 | 22 | 50% | 100% Asian | 26.0 | NR | Lifestyle Modification | Guided aerobic exercise at 1 time per day for 30‒60 minutes each session for 1 year |
| Fang (2004a)20 China |
55.0 | 36 | 53% | 100% Asian | 25.3 | NR | Lifestyle Modification | NR |
| Fang (2004b)20 China |
50.0 | 45 | 56% | 100% Asian | 24.9 | NR | AGI (Acarbose) | NR |
| Fang (2004c)20 China |
50.0 | 44 | 54% | 100% Asian | 25.2 | NR | Insulin sensitizer (Flumamine) | NR |
| Fang (2014a)21 China |
55.0 | 223 | 53% | 100% Asian | 25.3 | 1.0 | Chinese medicine plus Lifestyle Modification | 8.8 g ShenZhu TiaoPi granules at 2 times per day and dietary advice for 1 year |
| Fang (2014b)21 China |
54.6 | 216 | 55% | 100% Asian | 25.3 | 1.0 | Lifestyle Modification | Dietary advice for 1 year |
| Gaddam (2015)22 India |
NR | 52 | NR | 100% Asian | 26.2 | 3.0 | Chinese medicine | Fenugreek 5 g powder 2 times per day for 3 years |
| Gagnon (2011)23 Canada |
54.8 | 22 | 55% | 100% White | 34.1 | 1.0 | Lifestyle Modification | 25 seminars about diet, exercise, and behavior change over 1 year |
| Gao (2013)24 China |
49.3 | 230 | 43% | 100% Asian | 25.1 | 3.0 | Chinese Medicine | Tangzhiping granules 5g at 2 times per day |
| Gonzalez-Heredia (2017a)25 Mexico |
51.9 | 8 | 37% | 100% Latin | 31.2 | 0.23 | Insulin Sensitizer (Metformin) | 500 mg per day for 3 months |
| Gonzalez-Heredia (2017b)25 Mexico |
49.3 | 8 | 37% | 100% Latin | 31.3 | 0.23 | DPP-4 (Linagliptin) | 5 mg per day for 3 months |
| Guerrero-Romero (2015)26 Mexico |
42.5 | 59 | 42% | 100% Latin | 30.6 | 0.23 | Magnesium | 30 mL bolus per day for 4 months |
| Heymsfield (2000)27 Multiple countries |
43.9 | 67 | 19% | 88% White | 35.6 | 1.6 | Lipase inhibitor (Orlistat) | 120 mg 3 times per day for 108 weeks |
| Heymsfield (2000)27 Multiple countries |
44.3 | 53 | 18% | 88% White | 36.0 | 1.6 | Lifestyle Modification | Individualized diet plan for 108 weeks |
| Huang (2016a)28 China |
52.0 | 60 | 52% | 100% Asian | NR | 2.1 | Chinese Medicine plus Lifestyle Modification | Tangyiping Granules 10 g at w times per day |
| Huang (2016b)28 China |
51.1 | 60 | 58% | 100% Asian | NR | 2.1 | Lifestyle Modification | Diet and physical activity modification for 12 weeks |
| Jorde (2016)29 Norway |
62.3 | 256 | 63% | 100% White | 30.1 | NR | Vitamin D | 20,000 IU per week for 5 years |
| Kawamori (2009)30 Japan |
55.7 | 897 | 60% | 100% Asian | 25.8 | 0.9 | AGI (Voglibose) | Dose not reported |
| Ke (2012a)31 China |
46.5 | 43 | 51% | 100% Asian | 28.7 | 0.5 | Chinese Medicine | Modified Linggui Zhugan decoction 2 times per day for 6 months |
| Ke (2012b)31 China |
45.7 | 38 | 67% | 100% Asian | 28.5 | 0.5 | Lifestyle Modification | Daily, low-calorie diet for 6 months |
| Kosaka (2012)32 Japan |
NR | 356 | 100% | 100% Asian | 24.0 | 4.0 | Lifestyle Modification | Tailored, individualized lifestyle change program over 4 years |
| Le Roux (2017)33 Multiple countries |
47.5 | 1,505 | 24% | 83% White | 38.8 | 2.8 | GLP-1 (liraglutide) | 3 mg per day for up to 160 weeks |
| Lehtovirta (2001)34 Finland |
57.3 | 20 | 70% | NR | 29.8 | 0.5 | Insulin Sensitizer (Metformin) | 500 mg 2 times per day for 6 months |
| Lian (2014a)35 China |
53.0 | 198 | 47% | 100% Asian | 25.2 | 1.0 | Chinese Medicine plus Lifestyle Modification | 1.6 g of Tianqi at 3 times per day and individualized dietary counseling for 12 months |
| Lian (2014b)35 China |
51.9 | 191 | 50% | 100% Asian | 25.5 | 1.0 | Lifestyle Modification | Individualized dietary counseling for 12 months |
| Lu (2011a)36 China |
62.4 | 46 | 53% | 100% Asian | 27.1 | 2.0 | Insulin Sensitizer (Metformin) | 0.25 g 3 times per day for 2 years |
| Lu (2011b)36 China |
62.4 | 49 | 53% | 100% Asian | 27.1 | 2.0 | AGI (Acarbose) | 50 mg 3 times per day for 2 years |
| Lu (2011c)36 China |
64.7 | 86 | 53% | 100% Asian | 26.9 | 2.0 | Lifestyle Modification | Educational sessions on diet and physical activity every month for 2 years |
| Lundkvist (2016)37 Sweden |
53.5 | 16 | 40% | 100% White | 35.8 | 0.5 | GLP-1 (Dapagliflozin and Exenatide) | 10 mg per day dapagliflozin and 2 mg per week of exenatide for up to 24 weeks |
| Monti (2012)38 Italy |
57.2 | 66 | 58% | 100% White | 30.4 | 2.5 | L-arginine | 3.2 g at 2 times per day for 1.5 years |
| Moore (2011)39 Australia |
61.3 | 183 | 41% | 100% White | 29.7 | 0.5 | Lifestyle Modification | Group educational sessions about diet and physical activity once a month for 6 months |
| O’Brien (2017a)40 U.S. |
45.5 | 30 | 0 | 100% Latin | 34.4 | 1.0 | Lifestyle Modification | 24 sessions on diet and physical activity behavior change over 1 year |
| O’Brien (2017b)40 U.S. |
45.8 | 27 | 0 | 100% Latin | 33.2 | 1.0 | Insulin Sensitizer (Metformin) | 850 mg at 2 times per day for 1 year |
| Oldroyd (2006)41 UK |
58.2 | 91 | 46% | 100% White | NR | 2.0 | Lifestyle Modification (Diet and physical activity) | Regular motivational counseling for diet and physical activity over 2 years |
| Penn (2009)42 UK |
56.8 | 41 | 41% | 100% White | 34.1 | 3.1 | Lifestyle Modification (Diet and physical activity) | 30-minute session addressing behavioral interventions to change diet and physical activity over 3 years |
| Ramachandran (2010a)43 India |
NR | 125 | NR | 100% Asian | NR | 3.0 | Insulin Sensitizer (Metformin) | 500 mg per day for 3 years |
| Ramachandran (2010b)43 India |
NR | 297 | NR | 100% Asian | NR | 3.0 | Lifestyle Modification (Diet and physical activity) | Physical activity and diet recommendations for 3 years |
| Roman-Ramos (2000)44 Mexico |
36.8 | 15 | 40% | 100% Latin | 29.6 | 0.23 | Insulin Sensitizer (Rosiglitazone) | 4 mg per day for 12 weeks |
| Shi (2016)45 China |
47.1 | 32 | 47% | 100% Asian | 22.9 | 0.23 | Chinese Medicine | One packet Jinlida granules per day for 12 weeks |
| Stentz (2016)46 U.S. |
43.1 | 12 | 25% | 83% Black | 40.5 | 0.5 | Lifestyle Modification (Diet) | Daily high-protein diets for 6 months |
| Sun (2015)47 China |
55.5 | 82 | 51% | 100% Asian | NR | 2.0 | Chinese Medicine plus Lifestyle Modification | Seven JinQi Jiangtang tablets 2 times per day and tailored exercise and diet program for 1 year |
| Wan (2010a)48 China |
NR | 20 | NR | 100% Asian | NR | 0.5 | Fenofibrate | 200 mg per day for 6 months |
| Wan (2010b)48 China |
NR | 24 | NR | 100% Asian | NR | 0.5 | Insulin Sensitizer (Metformin) | 500 mg 3 times per day for 6 months |
| Wan (2010c)48 China |
NR | 25 | NR | 100% Asian | NR | 0.5 | Lifestyle Modification | Diet plans that included recommendations to limit daily caloric, fat, and energy intake for 6 months |
| Wang (2000a)49 China |
64.0 | 30 | 52% | 100% Asian | 22.7 | 1.0 | AGI (Acarbose) | 50 mg at 3 times per day for 12 months |
| Wang (2000b)49 China |
63.0 | 30 | 53% | 100% Asian | 21.0 | 1.0 | Lifestyle Modification | Diet and physical activity recommendations for 12 months |
| Wei (2008)50 China |
51.3 | 68 | 44% | 100% Asian | NR | 0.5 | Chinese Medicine | Two packets of Tang No.1 granule at 2 times per day for 6 months |
| Xu (2013)51 China |
60.4 | 41 | 37% | 100% Asian | 26.8 | 1.0 | Lifestyle Modification | Daily meal replacement and physical activity coaching for 1 year |
| Zhou (2011)52 China |
57.3 | 59 | 19% | 100% Asian | NR | 0.5 | Lifestyle Modification | Diet adjustment and individualized physical activity interventions that involved sports for 6 months |
| Zinman (2010a)53 Canada |
55.0 | 103 | 35% | 75% White | 31.3 | 3.9 | Insulin Sensitizer (Rosiglitazone and Metformin) | 500 mg metformin and 2 mg rosiglitazone at 2 times per day for 1 year |
| Zinman (2010b)53 Canada |
57.3 | 104 | 32% | 75% White | 32.0 | 3.9 | Lifestyle Modification | Five 1-on-1 diet and physical activity modification sessions for 1 year |
| Zong (2015)54 China |
NR | 84 | 54% | 100% Asian | 26.1 | NR | Lifestyle Modification | Intensive nutrition and physical activity guidance |
AGI, alpha glucosidase inhibitors; DPP-4, dipeptidyl peptidase 4 inhibitors; GLP-1, glucagon-like peptide 1 receptor agonists; RAS, renin-angiotensin system; NR, not reported.
Appendix Figure 1.
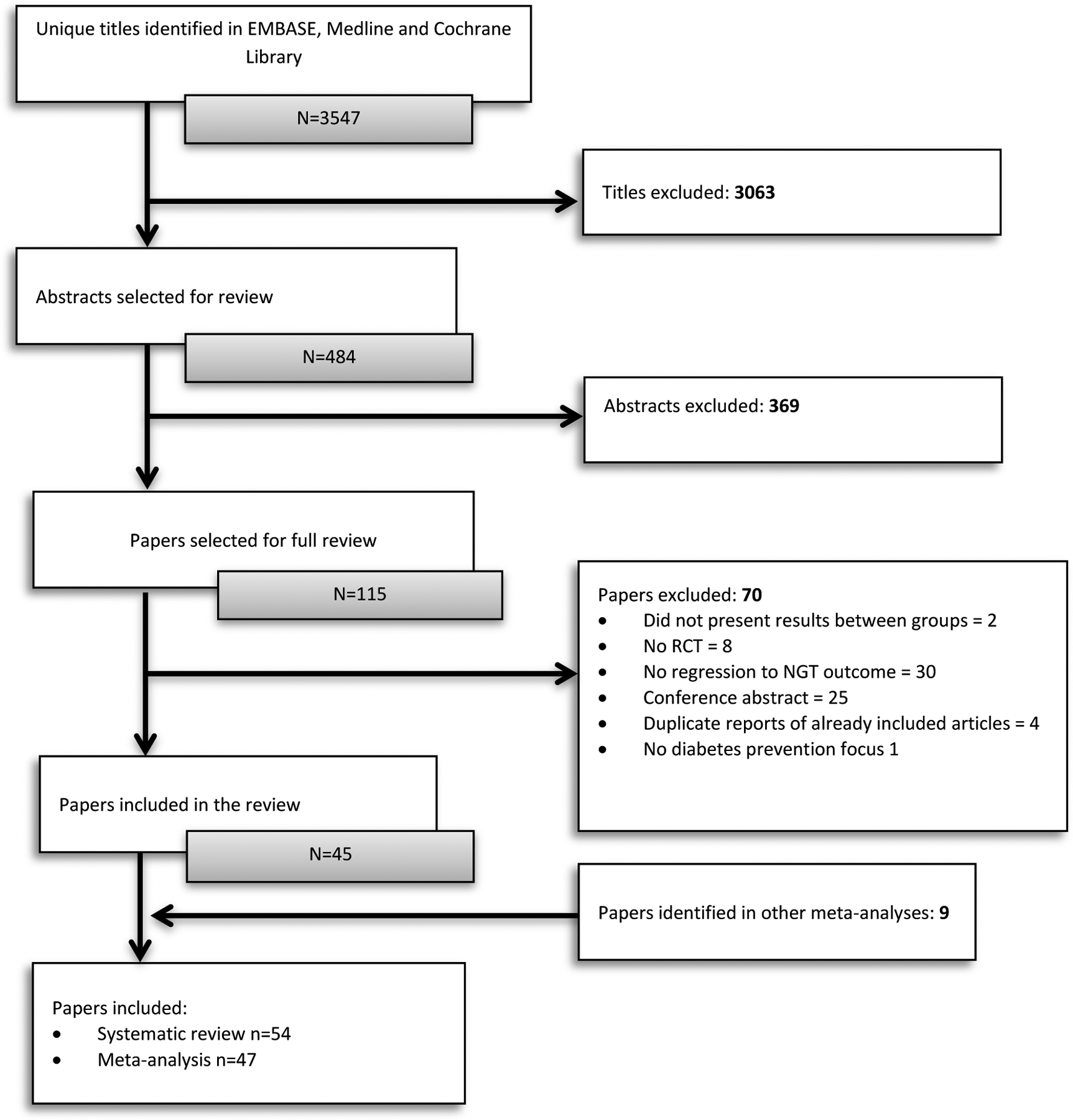
PRISMA study selection flow chart.
Appendix Figure 2.
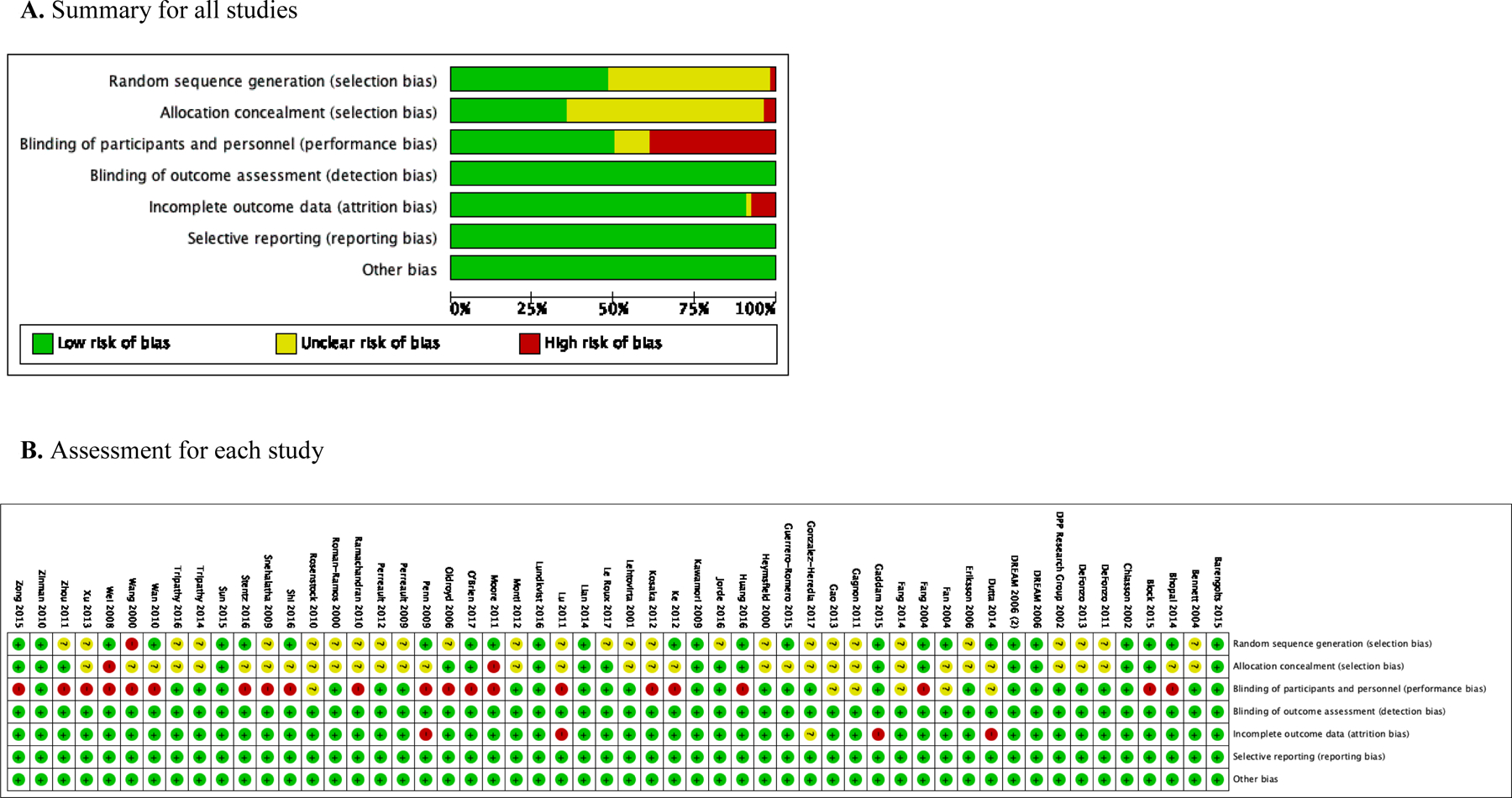
Risk of bias assessment results.
Appendix Table 3.
Subgroup Analyses Exploring Transitivity (i.e., Treatment Effect Heterogeneity) by Participant Characteristics and Study Follow Up
| Variable | AGI | Chinese medicine | Insulin sensitizer | Lifestyle modification | Vitamin D |
|---|---|---|---|---|---|
| Sex | |||||
| <50% Male | 0.20 (0.02, 0.39) | 0.24 (0.09, 0.38) | 0.23 (0.10, 0.36) | 0.21 (−0.17, 0.60) | |
| ≥50% Male | ‒ | 0.36 (0.22, 0.50) | 0.17 (0.01, 0.33) | 0.15 (0.07, 0.24) | 0.05 (−0.10, 0.20) |
| Age, years | |||||
| <50 | 0.51 (−0.10, 1.13) | 0.39 (−0.03, 0.81) | 0.41 (0.02, 0.80) | 0.27 (−0.09, 0.64) | 0.21 (−0.49, 0.92) |
| ≥50 | 0.24 (0.11, 0.38) | 0.27 (0.13, 0.41) | 0.14 (0.03, 0.25) | 0.15 (0.07, 0.24) | 0.04 (−0.26, 0.33) |
| Race | |||||
| <80% White | 0.35 (0.18, 0.53) | 0.24 (0.13, 0.35) | 0.18 (0.09, 0.27) | 0.12 (−0.13, 0.37) | |
| ≥80% White | 0.04 (−0.13, 0.21) | ‒ | 0.12 (0.11, 0.14) | 0.20 (0.08, 0.31) | 0.05 (−0.12, 0.23) |
| Reversion indicator | |||||
| IGT | 0.28 (0.07, 0.50) | 0.28 (0.12, 0.45) | 0.42 (0.19, 0.64) | 0.10 (−0.04, 0.24) | ‒ |
| IFG/IGT | 0.51 (0.18, 0.85) | 0.25 (0.01, 0.49) | 0.41 (0.16, 0.65) | 0.21 (0.08, 0.33) | 0.05 (−0.25, 0.36) |
| IFG + IGT | ‒ | 0.44 (0.09, 0.78) | 0.18 (0.05, 0.32) | 0.27 (0.12, 0.42) | 0.21 (−0.13, 0.56) |
| Baseline BMI (kg/m2) | |||||
| <30 | 0.35 (0.19, 0.52) | 0.34 (0.17, 0.51) | 0.14 (0.03, 0.25) | 0.21 (−0.13, 0.56) | |
| ≥30 | 0.04 (−0.25, 0.34) | ‒ | 0.17 (0.04, 0.30) | 0.25 (0.13, 0.37) | 0.05 (−0.17, 0.26) |
| Weight loss | |||||
| <4% | 0.04 (−0.22, 0.31) | 0.10 (−0.02, 0.23) | 0.16 (0.04, 0.27) | ||
| ≥4% | 0.55 (0.25, 0.86) | ‒ | 0.43 (0.21, 0.66) | 0.26 (0.15, 0.38) | ‒ |
| Follow up length | |||||
| ≤12 months | 0.49 (0.05, 0.93) | 0.41 (0.22, 0.60) | 0.44 (0.25, 0.63) | 0.26 (0.13, 0.39) | 0.04 (−0.35, 0.43) |
| >12 months | 0.22 (0.07, 0.36) | 0.21 (0.05, 0.37) | 0.11 (0.01, 0.22) | 0.11 (0.01, 0.20) | 0.12 (−0.09, 0.34) |
Notes: Bolded text indicates risk difference is significant between treatment and control. Estimates are intervention versus control risk difference (95% CI). Higher RD indicates greater normoglycemia difference between treatment and control arms.
IFG, impaired fasting glucose; IGT, impaired glucose tolerance. These represent the indicator used to determine achievement of normoglicemia at the end of the intervention.
Appendix Table 4.
Sensitivity Analysis Including Low Risk of Bias Studies (N=34). Treatment Arms Are Compared Against Control/Placebo Arms
| Treatment | RR (95% CI) | RD (95% CI) |
|---|---|---|
| AGI | 1.61 (1.04, 2.48) | 0.22 (0.03, 0.41) |
| Chinese medicine | 2.72 (1.79, 4.11) | 0.36 (0.20, 0.52) |
| Fenofibrate | 1.90 (0.81, 4.45) | 0.37 (0.01, 0.74) |
| GLP-1 | 3.48 (1.58, 7.63) | 0.47 (0.20, 0.74) |
| Insulin secretagogue | 1.37 (0.48, 3.87) | 0.15 (‒0.33, 0.63) |
| Insulin sensitizer | 1.68 (1.22, 2.31) | 0.27 (0.15, 0.39) |
| Lifestyle modification | 1.71 (1.29, 2.27) | 0.17 (0.07, 0.27) |
| L-arginine | 1.92 (0.76, 4.85) | 0.20 (‒0.17, 0.57) |
| Lipase inhibitor | 2.50 (1.05, 5.96) | 0.40 (0.01, 0.79) |
| Magnesium | 7.25 (2.10, 25.00) | 0.44 (0.07, 0.80) |
| RAS blockade | 1.11 (0.52, 2.39) | 0.04 (‒0.29, 0.38) |
| Vitamin D | 1.38 (0.69, 2.78) | 0.05 (‒0.20, 0.29) |
AGI, alpha glucosidase inhibitors; GLP-1, glucagon-like peptide 1 receptor agonists; RAS, renin-angiotensin system.
Appendix Figure 3.
Node-split showing risk difference obtained from direct treatment comparisons (i.e., in same study) and from indirect comparisons (i.e., obtained in this meta-analysis).
Appendix Table 5.
Results from the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)
| Treatment | GRADE | Rationale for grade |
|---|---|---|
| Lifestyle modification | ⊕ ⊕ ⊕ ⊕ Strong |
33% of studies have high risk of bias but this was mainly due to lack of blinding to intervention. Based on this, we concluded this should not downgrade the strength of the evidence. |
| Insulin Sensitizer | ⊕ ⊕ ⊕ ⊝ Moderate |
Downgraded by one level because 33% of studies had high risk of bias and given that observed heterogeneity was high (I2 = 85%). |
| AGI | ⊕ ⊕ ⊕ ⊝ Moderate |
Downgraded by one level because 40% of studies have high risk of bias and given that the observed heterogeneity was moderate (I2 = 60%). |
| GLP-1 | ⊕ ⊕ ⊕ ⊝ Moderate |
Downgraded by one level due to an imprecise effect estimate and given that the observed heterogeneity was moderate (I2 = 60%). |
| Chinese medicine | ⊕ ⊕ ⊝ ⊝ Low |
Downgraded by two levels because 30% of studies have high risk of bias, the effect estimate was imprecise, and given publication bias was present (Egger test p < 0.001). |
| RAS Blockade | ⊕ ⊕ ⊝ ⊝ Low |
Downgraded by two levels given that the confidence interval includes evidence of no effect and given there is a single study testing this treatment. |
| Insulin Secretagogue | ⊕ ⊕ ⊝ ⊝ Low |
Downgraded by two levels given that the confidence intervals include evidence of no effect and given there is a single study testing this treatment. |
| Vitamin D | ⊕ ⊕ ⊝ ⊝ Low |
Downgraded by two levels because 33% of studies have high risk of bias and given that the confidence intervals include evidence of no effect. |
| Magnesium | ⊕ ⊝ ⊝ ⊝ Very Low |
Downgraded by three levels because the effect estimate was imprecise and given that there is a single study at high risk of bias testing this treatment. |
| Lipase inhibitor | ⊕ ⊝ ⊝ ⊝ Very Low |
Downgraded by three levels due to the imprecise effect estimate and given that there is a single study at high risk of bias testing this treatment. |
| DPP-4 | ⊕ ⊝ ⊝ ⊝ Very Low |
Downgraded by three levels given that the confidence intervals include evidence of no effect and given that there is a single study at high risk of bias testing this treatment. |
| Fenofibrate | ⊕ ⊝ ⊝ ⊝ Very Low |
Downgraded by three levels because the effect estimate was imprecise and given that there is a single study at high risk of bias testing this treatment. |
| L-Arginine | ⊕ ⊝ ⊝ ⊝ Very Low |
Downgraded by three levels given that the confidence intervals include evidence of no effect and given that there is a single study at high risk of bias testing this treatment. |
AGI, alpha glucosidase inhibitors; DPP-4, dipeptidyl peptidase 4 inhibitors; GLP-1, glucagon-like peptide 1 receptor agonists; RAS, renin-angiotensin system.
Footnotes
The authors have no conflicts of interest to declare during the course of this work. No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Hostalek U Global epidemiology of prediabetes - present and future perspectives. Clin Res Diabetes Endocrinol. 2019;5:5. 10.1186/s40842-019-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas, 9th Edition. https://www.diabetesatlas.org/en/. Published 2019. Accessed on March 19, 2020.
- 3.Yip WCY, Sequeira IR, Plank LD, Poppitt SD. Prevalence of pre-diabetes across ethnicities: a review of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) for classification of dysglycaemia. Nutrients. 2017;9(11):1273. 10.3390/nu9111273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Prac. 2007;78(3):305–312. 10.1016/j.diabres.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–2155. 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 6.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988‒2014. Lancet Diabetes Endocrinol. 2018;6(5):392–403. 10.1016/s2213-8587(18)30027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016;33(12):1615–1624. 10.1111/dme.13113. [DOI] [PubMed] [Google Scholar]
- 8.Cai X, Zhang Y, Li M, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gujral UP, Jagannathan R, He S, et al. Association between varying cut-points of intermediate hyperglycemia and risk of mortality, cardiovascular events and chronic kidney disease: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2021;9(1):e001776. 10.1136/bmjdrc-2020-001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cefalu WT, Petersen MP, Ratner RE. The alarming and rising costs of diabetes and prediabetes: a call for action! Diabetes Care. 2014;37(12):3137–3138. 10.2337/dc14-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang B, Lian FM, Zhao XY, et al. Prevention of type 2 diabetes with the traditional Chinese patent medicine: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2017;131:242–259. 10.1016/j.diabres.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Pang B, Zhang Y, Liu J, et al. Prevention of type 2 diabetes with the Chinese herbal medicine Tianqi capsule: a systematic review and meta-analysis. Diabetes Ther. 2017;8(6):1227–1242. 10.1007/s13300-017-0316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phung OJ, Baker WL, Tongbram V, Bhardwaj A, Coleman CI. Oral antidiabetic drugs and regression from prediabetes to normoglycemia: a meta-analysis. Ann Pharmacother. 2012;46(4):469–476. 10.1345/aph.1q554. [DOI] [PubMed] [Google Scholar]
- 14.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):437–451. 10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Tan H, Tang J, et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: a systematic review and meta-analysis. Diabetes Care. 2020;43(7):1650–1658. 10.2337/dc19-1708. [DOI] [PubMed] [Google Scholar]
- 16.Shen XX, Wang JP, Chen YY, et al. Subjects with impaired glucose tolerance returned to normal glucose status for six years had lower long-term risk of diabetes: 20 years follow up of Daqing diabetes prevention study. Zhonghua Nei Ke Za Zhi. 2019;58(5):372–376. 10.3760/cma.j.issn.0578-1426.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Wu S, Song Q, Wang X. Reversion from pre-diabetes mellitus to normoglycemia and risk of cardiovascular disease and all-cause mortality in a Chinese population: a prospective cohort study. J Am Heart Assoc. 2021;10(3):e019045. 10.1161/jaha.120.019045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perreault L, Temprosa M, Mather KJ, et al. Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2014;37(9):2622–2631. 10.2337/dc14-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perreault L, Pan Q, Schroeder EB, et al. Regression from prediabetes to normal glucose regulation and prevalence of microvascular disease in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetes Care. 2019;42(9):1809–1815. 10.2337/dc19-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perreault L, Pan Q, Mather KJ, et al. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243–2251. 10.1016/s0140-6736(12)60525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. 10.7326/m14-2385. [DOI] [PubMed] [Google Scholar]
- 22.Association AD. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care. 2020;43(suppl 1):S14–S31. 10.2337/dc20-s002. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Classification of diabetes mellitus. https://www.who.int/publications/i/item/classification-of-diabetes-mellitus. Published 2019. Accessed September 8, 2020.
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins N, Scott DA, Woods B. ‘Arm-based’ parameterization for network meta-analysis. Res Synth Methods. 2015;7(3):306–313. 10.1002/jrsm.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Carlin BP, Neaton JD, et al. Network meta-analysis of randomized clinical trials: reporting the proper summaries. Clin Trials. 2014;11(2):246–262. 10.1177/1740774513498322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. 2014;9(12):e115065. 10.1371/journal.pone.0115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brignardello-Petersen R, Murad MH, Walter SD, et al. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60–67. 10.1016/j.jclinepi.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2021;44(1). https://care.diabetesjournals.org/content/44/Supplement_1 [Google Scholar]
- 31.Tian J, Jin D, Bao Q, et al. Evidence and potential mechanisms of traditional Chinese medicine for the treatment of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(8):1801–1816. 10.1111/dom.13760. [DOI] [PubMed] [Google Scholar]
- 32.Liu D, Wen Q, Liu M, et al. Dietary supplements for prediabetes: a protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(20):e20347. 10.1097/md.0000000000020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nah EH, Chu J, Kim S, Cho S, Kwon E. Efficacy of lifestyle interventions in the reversion to normoglycemia in Korean prediabetics: one-year results from a randomised controlled trial. Prim Care Diabetes. 2019;13(3):212–220. 10.1016/j.pcd.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Röhling M, Kempf K, Banzer W, et al. Prediabetes conversion to normoglycemia is superior adding a low-carbohydrate and energy deficit formula diet to lifestyle intervention-a 12-month subanalysis of the ACOORH Trial. Nutrients. 2020;12(7):2022. 10.3390/nu12072022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wani K, Alfawaz H, Alnaami AM, et al. Effects of a 12-month intensive lifestyle monitoring program in predominantly overweight/obese Arab adults with prediabetes. Nutrients. 2020;12(2):464. 10.3390/nu12020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barengo NC, Acosta T, Arrieta A, et al. Early lifestyle interventions in people with impaired glucose tolerance in Northern Colombia: the DEMOJUAN Project. Int J Environ Res Public Health. 2019;16(8):1403. 10.3390/ijerph16081403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohula EA, Scirica BM, Inzucchi SE, et al. Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial. Lancet. 2018;392(10161):2269–2279. 10.1016/S0140-6736(18)32328-6. [DOI] [PubMed] [Google Scholar]
- 38.Rezki A, Fysekidis M, Chiheb S, Vicaut E, Cosson E, Valensi P. Acute and long-term effects of saxagliptin on post-prandial glycemic response in obese patients with impaired glucose tolerance. Nutr Metab Cardiovasc Dis. 2021;31(4):1257–1266. 10.1016/j.numecd.2020.12.025. [DOI] [PubMed] [Google Scholar]
- 39.Bhatt SP, Misra A, Pandey RM, Upadhyay AD, Gulati S, Singh N. Vitamin D supplementation in overweight/obese Asian Indian Women with prediabetes reduces glycemic measures and truncal subcutaneous fat: a 78 weeks randomized placebo-controlled trial (PREVENT-WIN Trial). Sci Rep. 2020;10:9844. 10.1038/s41598-020-67064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaytán Martínez LA, Sánchez-Ruiz LA, Zuñiga LY, González-Ortiz M, Martínez-Abundis E. Effect of gymnema sylvestre administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J Med Food. 2021;24(1):28–32. 10.1089/jmf.2020.0024. [DOI] [PubMed] [Google Scholar]
- 41.Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:Cd012661. 10.1002/14651858.cd012661.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ. 2014;349:g4485. 10.1136/bmj.g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalra S, Singal A, Lathia T. What’s in a name? Redefining type 2 diabetes remission. Diabetes Ther. 2021;12(3):647–654. 10.1007/s13300-020-00990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES FOR THE 54 STUDIES IDENTIFIED IN THE SYSTEMATIC REVIEW
- 1.DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104–1115. 10.1056/nejmoa1010949. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33(6):1173–1175. 10.2337/dc09-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF. Regression from pre-diabetes to normal glucose regulation in the Diabetes Prevention Program. Diabetes Care. 2009;32(9):1583–1588. 10.2337/dc09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243–2251. 10.1016/s0140-6736(12)60525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snehalatha C, Mary S, Selvam S, et al. Changes in insulin secretion and insulin sensitivity in relation to the glycemic outcomes in subjects with impaired glucose tolerance in the Indian Diabetes Prevention Programme-1 (IDPP-1). Diabetes Care. 2009;32(10):1796–1801. 10.2337/dc09-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tripathy D, Clement SC, Schwenke DC, et al. Baseline adiponectin levels do not influence the response to pioglitazone in ACT NOW. Diabetes Care. 2014;37(6):1706–1711. 10.2337/dc13-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripathy D, Schwenke DC, Banerji M, et al. Diabetes incidence and glucose tolerance after termination of pioglitazone therapy: results from ACT NOW. J Clin Endocrinol Metab. 2016;101(5):2056–2062. 10.1210/jc.2015-4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barengolts E, Manickam B, Eisenberg Y, Akbar A, Kukreja S, Ciubotaru I. Effect of high-dose vitamin D repletion on glycemic control in African-American males with prediabetes and hypovitaminosis D. Endocr Pract. 2015;21(6):604–612. 10.4158/ep14548.or. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett SM, Agrawal A, Elasha H, et al. Rosiglitazone improves insulin sensitivity, glucose tolerance and ambulatory blood pressure in subjects with impaired glucose tolerance. Diabet Med. 2004;21(5):415–422. 10.1111/j.1464-5491.2004.01155.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhopal RS, Douglas A, Wallia S, et al. Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: a family-cluster randomised controlled trial. Lancet Diabetes Endocrinol. 2(3):218–227. 10.1016/s2213-8587(13)70204-3. [DOI] [PubMed] [Google Scholar]
- 11.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240. 10.2196/jmir.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–2077. 10.1016/s0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Tripathy D, Schwenke DC, et al. Prevention of diabetes with pioglitazone in ACT NOW physiologic correlates. Diabetes. 2013;62:3920–3926. 10.2337/db13-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. 10.1056/nejmoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DREAM Trial Investigators. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551–1562. 10.1056/nejmoa065061. [DOI] [PubMed] [Google Scholar]
- 16.DREAM Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096–1105. 10.1016/s0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 17.Dutta D, Mondal SA, Choudhuri S, et al. Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: an open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103(3):e18–e23. 10.1016/j.diabres.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson JG, Lehtovirta M, Ehrnstrom B, Salmela S, Groop L. Long-term beneficial effects of glipizide treatment on glucose tolerance in subjects with impaired glucose tolerance. J Intern Med. 2006;259(6):553–560. 10.1111/j.1365-2796.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- 19.Fan GJ, Luo GB, Qin ML. [Effect of jiangtang bushen recipe in intervention treatment of patients with impaired glucose tolerance]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(4):317–320. [PubMed] [Google Scholar]
- 20.Fang YS, Li TY, Chen SY. Effect of medicine and non-medicine intervention on the outcomes of patients with impaired glucose tolerance: 5-year follow-up. Chinese Journal of Clinical Rehabilitation. 2004;8(30):6562–6563. [Google Scholar]
- 21.Fang Z, Zhao J, Shi G, et al. Shenzhu Tiaopi granule combined with lifestyle intervention therapy for impaired glucose tolerance: a randomized controlled trial. Complement Ther Med. 2014;22(5):842–850. 10.1016/j.ctim.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Gaddam A, Galla C, Thummisetti S, Marikanty RK, Palanisamy UD, Rao PV. Role of Fenugreek in the prevention of type 2 diabetes mellitus in prediabetes. J Diabetes Metab Disord. 2015;14(1):74. 10.1186/s40200-015-0208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagnon C, Brown C, Couture C, et al. A cost-effective moderate-intensity interdisciplinary weight-management programme for individuals with prediabetes. Diabetes Metab. 2011;37(5):410–418. 10.1016/j.diabet.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Zhou H, Zhao H, et al. Clinical research of traditional Chinese medical intervention on impaired glucose tolerance. Am J Chin Med. 2013;41(1):21–32. . [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Heredia T, Hernandez-Corona DM, Gonzalez-Ortiz M, Martinez-Abundis E. Effect of linagliptin versus metformin on glycemic variability in patients with impaired glucose tolerance. Diabetes Technol Ther. 2017;19(8):471–475. 10.1089/dia.2017.0020. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero-Romero F, Simental-Mendia LE, Hernandez-Ronquillo G, Rodriguez-Moran M. Oral magnesium supplementation improves glycaemic status in subjects with prediabetes and hypomagnesaemia: a double-blind placebo-controlled randomized trial. Diabetes Metab. 2015;41(3):202–207. 10.1016/j.diabet.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Heymsfield SB, Segal KR, Hauptman J, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Intern Med. 2000;160(9):1321–1326. 10.1001/archinte.160.9.1321. [DOI] [PubMed] [Google Scholar]
- 28.Huang YQ, Yang QF, Wang H, Xu YS, Peng W, Jiang YH. Long-term clinical effect of Tangyiping Granules () on patients with impaired glucose tolerance. Chin J Integr Med. 2016;22(9):653–659. 10.1007/s11655-016-2512-3. [DOI] [PubMed] [Google Scholar]
- 29.Jorde R, Sollid ST, Svartberg J, et al. Vitamin D 20 000 IU per week for five years does not prevent progression from prediabetes to diabetes. J Clin Endocrinol Metab. 2016;101(4):1647–1655. 10.1210/jc.2015-4013. [DOI] [PubMed] [Google Scholar]
- 30.Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373(9675):1607–1614. 10.1016/s0140-6736(09)60222-1. [DOI] [PubMed] [Google Scholar]
- 31.Ke B, Shi L, Jun-jie Z, Chen DS, Meng J, Qin J. Protective effects of modified linggui zhugan decoction combined with short-term very low calorie diets on cardiovascular risk factors in obese patients with impaired glucose tolerance. J Tradit Chin Med. 2012;32(2):193–198. 10.1016/s0254-6272(13)60010-2. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67(2):152–162. 10.1016/j.diabres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 33.le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–1409. 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 34.Lehtovirta M, Forsen B, Gullstrom M, et al. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabet Med. 2001;18(7):578–583. 10.1046/j.1464-5491.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 35.Lian F, Li G, Chen X, et al. Chinese herbal medicine Tianqi reduces progression from impaired glucose tolerance to diabetes: a double-blind, randomized, placebo-controlled, multicenter trial. J Clin Endocrinol Metab. 2014;99(2):648–655. 10.1210/jc.2013-3276. [DOI] [PubMed] [Google Scholar]
- 36.Lu YH, Lu JM, Wang SY, et al. Outcome of intensive integrated intervention in participants with impaired glucose regulation in China. Adv Ther. 2011;28(6):511–519. 10.1007/s12325-011-0022-4. [DOI] [PubMed] [Google Scholar]
- 37.Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: A 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab. 2017;19(1):49–60. 10.1111/dom.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monti LD, Setola E, Lucotti PC, et al. Effect of a long-term oral l-arginine supplementation on glucose metabolism: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14(10):893–900. 10.1111/j.1463-1326.2012.01615.x. [DOI] [PubMed] [Google Scholar]
- 39.Moore SM, Hardie EA, Hackworth NJ, et al. Can the onset of type 2 diabetes be delayed by a group-based lifestyle intervention? A randomised control trial. Psychol Health. 2011;26(4):485–499. 10.1080/08870440903548749. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien MJ, Perez A, Scanlan AB, et al. PREVENT-DM comparative effectiveness trial of lifestyle Intervention and metformin. Am J Prev Med. 2017;52(6):788–797. 10.1016/j.amepre.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldroyd JC, Unwin NC, White M, Mathers JC, Alberti KG. Randomised controlled trial evaluating lifestyle interventions in people with impaired glucose tolerance. Diabetes Res Clin Pract. 2006;72(2):117–127. 10.1016/j.diabres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009;9:342. 10.1186/1471-2458-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramachandran A, Arun N, Shetty AS, Snehalatha C. Efficacy of primary prevention interventions when fasting and postglucose dysglycemia coexist: analysis of the Indian Diabetes Prevention Programmes (IDPP-1 and IDPP-2). Diabetes Care. 2010;33(10):2164–2168. 10.2337/dc09-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Román Ramos R, Flores Sáenz JLE, Alarcón Aguilar FJ, Contreras Weber CC, Rivas Vilchis JF, Trujillo Arriaga HM. Normalization of impaired glucose tolerance with rosiglitazone. Invest Med Int. 2000;27(1):9–13. [Google Scholar]
- 45.Shi YL, Liu WJ, Zhang XF, et al. Effect of Chinese herbal medicine Jinlida granule in treatment of patients with impaired glucose tolerance. Chin Med J (Engl). 2016;129(19):2281–2286. 10.4103/0366-6999.190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stentz FB, Brewer A, Wan J, et al. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: randomized control trial. BMJ Open Diabetes Res Care. 2016;4(1):e000258. 10.1136/bmjdrc-2016-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X, Guo L, Shang H, et al. The cost-effectiveness analysis of JinQi Jiangtang tablets for the treatment on prediabetes: a randomized, double-blind, placebo-controlled, multicenter design. Trials. 2015;16:496. 10.1186/s13063-015-0990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan Q, Wang F, Wang F, et al. Regression to normoglycaemia by fenofibrate in pre-diabetic subjects complicated with hypertriglyceridaemia: a prospective randomized controlled trial. Diabet Med. 2010;27(11):1312–1317. 10.1111/j.1464-5491.2010.03107.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Xu WH, Wang GH. An evaluation on efficacy of acarbose interfering treatment on IGT. Shanxi Clin Med J. 2000;9(2):116–117. [Google Scholar]
- 50.Wei Y, Hong YZ, Ye X. Effect of Tang No.1 granule (1) in treating patients with impaired glucose tolerance. Chin J Integr Med. 2008;14(4):298–302. 10.1007/s11655-008-0298-7. [DOI] [PubMed] [Google Scholar]
- 51.Xu DF, Sun JQ, Chen M, et al. Effects of lifestyle intervention and meal replacement on glycaemic and body-weight control in Chinese subjects with impaired glucose regulation: a 1-year randomised controlled trial. Br J Nutr. 2013;109(3):487–492. 10.1017/s0007114512001328. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J [Life style interventions study on the effects of impaired glucose regulations in Shanghai urban communities]. Wei Sheng Yan Jiu. 2011;40(3):331–333. [PubMed] [Google Scholar]
- 53.Zinman B, Harris SB, Neuman J, et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE Trial): a double-blind randomized controlled study. Obstet Gynecol Surv. 2010;65(12):771–772. 10.1097/ogx.0b013e3182134487. [DOI] [PubMed] [Google Scholar]
- 54.Zong Y, Duan P, Ding X, Si L, Liu J, Tu P. [Effects of lifestyle and quantitative nutrition interventions on individuals with prediabetes]. Zhonghua Yi Xue Za Zhi. 2015;95(40):3293–3296. [PubMed] [Google Scholar]


