Abstract
Pomegranate peel powder (PPP) is a rich source of many bioactive components particularly polyphenols that are interlinked to various technological and functional properties. In the present study, chicken tender pops were developed with incorporation of PPP, and its effect on quality attributes and storage stability of the product were evaluated. The treatments were formulated using 0%, 3%, 6%, and 9% PPP in replacement of chicken. The physicochemical properties, texture profile, instrumental color, sensory attributes, and storage stability were assessed for 21 days at refrigeration temperature, at a regular interval of 7 days. The results indicated that the inclusion of PPP significantly (p < .05) increased the dietary fiber from 0.25% in T0 to 1.45% in T3 at Day 0 and WHC 43.60% ± 0.02 in T0 to 49.36% ± 0.02 in T3 at Day 0, whereas the moisture content significantly reduced from 60.05% ± 0.03 in T0 to 55.08% ± 0.01 in T3 at the start of the study. In addition, the values of TBARS were significantly (p < .05) reduced for treated samples 0.72 mg MDA/Kg in T3 as compared to control 1.17 mg MDA/Kg on the 21st day of storage, whereas a significant increase (p < .05) in TPC from 0.90 mg GAE/g to 3.87 mg GAE/g in T0 to T3 was observed at the start of the study. For TPA, a significant (p < .05) increase was noticed in hardness, chewiness, and gumminess, whereas cohesiveness and springiness showed a non‐significant (p > .05) change in treated samples in relation to control, and the instrumental color (L* and a*) decreased significantly. However, pH, crude fiber, fat, ash, and protein content showed non‐significant (p > .05) variations over time. The sensory evaluation suggested that chicken tender pops supplemented with 6% PPP (T2) presented high overall acceptability and balanced organoleptic properties. Hence, it can be concluded that PPP can be effectively utilized as a natural fiber source, antioxidant, and antimicrobial agent in novel functional foods.
Keywords: chicken tender pops, functional food, microbiological evaluation, oxidative potential, pomegranate Peel powder, sensory profile, storage stability
The study evaluated the effects of incorporation of pomegranate peel powder on physiochemical, biochemical, and sensory attributes in development of chicken tender pops in refrigeration storage. Results show that use of pomegranate peel power enhanced antioxidant concentration, antimicrobial properties, sensory characteristics, and improved nutritional profiles while exhibiting potential of natural preservatives.
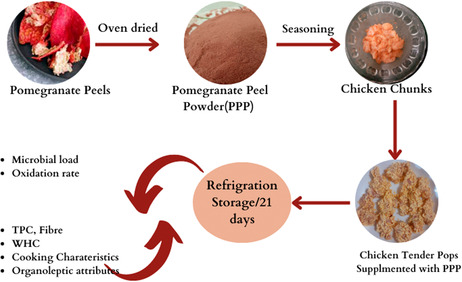
1. INTRODUCTION
Pomegranate (Punica granatum) is a spherical fruit that is a member of the Punicaceae family which was first cultivated in Iran and India and then spread throughout the Mediterranean basin (El Barnossi et al., 2021). Global pomegranate production is around 8.1 million tons, with a planting area of 835,950 hectares (Pienaar & Barends‐Jones, 2021). The global pomegranate market is anticipated to grow from 208.9 million USD in 2020 to 322.9 million USD by 2026 (River Country, 2021). Pomegranate fruit comprises two parts, an edible part that is 50% of the fruit, and the other 50% is the peel (Rafraf et al., 2017). Pomegranate is a valuable fruit because of its nutritional components such as minerals, proteins, crude fibers, vitamins, alkaloids, organic acids, fatty acids, flavonoids, polyphenols, isoflavones, and pectin that are associated with various therapeutic and technological benefits (Pirzadeh et al., 2021; Rahmani et al., 2017; Viuda‐Martos et al., 2010). It is usually consumed fresh or processed into different products such as juice, jam, oil, wine, or dietary supplements (Kahramanoglu & Usanmaz, 2016). However, the industrial processing of pomegranate produces enormous amounts of by‐products, mainly peels (40%–50%) which are disposed of as waste without any valorization that jeopardizes the environment (Ali et al., 2019). On the other hand, Pomegranate peel can be valorized to produce pomegranate peel powder and peel extracts containing many functional biomolecules (Jalal et al., 2018; Singh et al., 2019). These valorized products could be incorporated into the food chain to promote bio‐economy and satisfy sustainable development principles (Ben‐Othman et al., 2020; Sharayei et al., 2019).
Poultry consumption, particularly chicken meat, is associated with many positive health benefits and is considered more valuable than other meats because of its low energy value with high nutritional density (Bordoni & Danesi, 2017; Millen et al., 2014). In addition, it contains considerable amounts of long‐chain n‐3 polyunsaturated fatty acids, trace minerals (Fe and Zn), and B group vitamins, along with minute amounts of biotin, folic acid, pantothenic acid, and vitamin E (Barroeta, 2007). However, chicken meat lacks the dietary fiber essential to maintain human health by avoiding various ailments (Verma & Banerjee, 2010). Furthermore, using artificial preservatives to preserve the nutritional and quality attributes of meat products is associated with negative health effects such as allergy, asthma, cancer, hyperactivity, hypersensitivity, and neurological damage (Anand & Sati, 2013; Smaoui et al., 2019). Therefore, meat industries and researchers are focused on discovering natural substitutes to replace these synthetic additives with renewable biomass that is a natural safe source of many functional biomolecules (Pateiro et al., 2018; Žugčić et al., 2019).
Pomegranate peels are an excellent source of minerals (calcium, magnesium, potassium, phosphorus, and sodium) (Jalal et al., 2018), bioactive peptides (Hernández‐Corroto et al., 2020), and polysaccharides (Zhu et al., 2015). Furthermore, it contains high volumes of phytochemicals, mainly flavonoids (anthocyanins, catechin, epicatechin, and gallocatechin), hydrolyzable tannins (ellagitannins and gallotannins), and phenolic acids (caffeic acid, ellagic acid, and gallic acid) (Kaderides et al., 2020). The presence of these bioactive compounds is associated with a diverse range of biological activities (antibacterial, antifungal, and antimicrobial), therapeutic properties (anticarcinogenic, antihypertensive, anti‐inflammatory, antimutagenic, and antioxidant), and technological functions in foods (antioxidant, antimicrobial, emulsifying agent, oil‐holding and water‐holding agent colorant, flavoring, and nutraceuticals). Furthermore, secondary metabolites promote the avoidance and treatment of many chronic conditions, such as Alzheimer's disease, cardiovascular diseases, diabetes, and obesity (Jalal et al., 2018; Kandylis & Kokkinomagoulos, 2020; Ko et al., 2021).
The current study is aimed to develop a functional meat product (chicken tender pops) by effectively utilizing pomegranate peel powder and exploring its efficacy as a natural dietary fiber source, antioxidant, and antimicrobial agent. Furthermore, the effect of PPP incorporation on physiochemical characteristics, proximate composition, cooking characteristics, oxidative stability, instrumental color, texture profile, and sensory attributes of chicken tender pops was also assessed to predict the product's storage quality at refrigeration for 21 days.
2. MATERIALS AND METHODS
2.1. Procurement of raw materials
The pomegranate (Punica granatum) was procured from the local market of Lahore, Pakistan. The fresh boneless broiler chicken meat was purchased from the local Superstore in Lahore, Pakistan. The meat was packed in small bags of LDPE and held in a refrigerator at 4 ± 2°C for 6 h and later used to develop chicken tender pops.
2.2. Preparation of pomegranate peel powder (PPP)
The PPP was prepared using the method of Jalal et al. (2018), with few modifications. Briefly, fresh pomegranates were washed thoroughly with distilled water to remove surface dust. The arils were separated from rinds and then cut into medium‐sized pieces. Pomegranate peels (rind) were placed in a tray and dried using a hot‐air oven DOF‐230E (Bievopeak, Japan) at 50 ± 2°C for 48 hrs. Dried peels were cooled and ground enough to pass through a 20‐mesh sieve to obtain a fine powder with uniform particle size. Pomegranate peel powder was transferred in zipped‐lock high‐density polyethylene bags and stored at room temperature 20 ± 3°C for physicochemical analysis and later used in product development.
2.3. Physicochemical analysis of pomegranate peel powder (PPP)
Proximate analysis (moisture, fat, protein, ash, and crude fiber) was performed using standard protocols of AOAC (2005). The pH of PPP was determined using a pH meter described by Jalal et al. (2018). Two gram of the sample was combined with 20 mL of methanol and left for 2 days to allow for maximum leaching to analyze the TPC of PPP. Folin–Ciocalteu reagent 1.5 mL was added into extract 0.5 mL and incubated at 25°C for 5 min. After incubation, 6% sodium carbonate 1.5 mL was added and incubated again for 90 min in a dark room. The absorbance of the resulting blue color mixture was measured at 725 nm, and the total phenolic content was articulated as mg GAE per 100 mL of a sample (Mahmoud et al., 2017).
2.4. Manufacturing of chicken tender pops
Treatments of chicken tender pops were prepared using various concentrations of PPP replacing chicken meat. 0%, 3%, 6%, and 9% PPP were added to 80%, 77%, 74%, and 71% boneless meat, respectively, while other ingredients were added according to weight ratio (w/w) mentioned in Table 1. Boneless chicken breasts were washed with tap water, dried using a paper towel, and sliced into chunks of 8 g to 10 g. Chicken chunks were uniformly mixed with PPP, onion powder, garlic powder, black pepper, paprika, and iodized salt with the addition of chilled water according to the formulation mentioned in Table 1. After a marinating stay of 30 min, chunks were coated with flour and coarsely ground cornflakes. The functional chicken tender pops were packaged aerobically in low‐density polyethylene (LDPE) boxes and stored in the refrigerator for 21 days. Physiochemical properties, proximate composition, cooking characteristics, oxidative stability, microbiological studies, texture profile, instrumental color, and sensory attributes of chicken tender pops were evaluated every 7 days up to 21 days. To determine sensory characteristics and cooking characteristics, the developed product was air fried using air fryer DWAF, 3013 (Dawlance, Pakistan) for 10 min at 200 ± 2°C.
TABLE 1.
Formulation of chicken tender pops.
| Ingredients | Treatments | |||
|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |
| Chicken breast (g) | 80 | 77 | 74 | 71 |
| Pomegranate peel powder (g) | 0 | 3 | 6 | 9 |
| Paprika (g) | 2 | 2 | 2 | 2 |
| Garlic powder (g) | 2 | 2 | 2 | 2 |
| Onion powder (g) | 2 | 2 | 2 | 2 |
| Black pepper (g) | 2 | 2 | 2 | 2 |
| Salt (g) | 2 | 2 | 2 | 2 |
| Chilled water (mL) | 10 | 10 | 10 | 10 |
2.5. Determination of physiochemical parameters of chicken tender pops
The pH of chicken tender pops was estimated by the dipping probe of a digital pH meter (HANNA‐instrument, USA) in a homogenized sample by following the method of Santhi et al. (2020). The water‐holding capacity of samples was assessed as described by Rupasinghe et al. (2022), samples were placed between layers of filter paper and subjected to 10 kg weight for 5 min, and weight difference was expressed as WHC of samples.
2.6. Determination of proximate composition
Moisture, fat, protein, ash, and crude fiber content of chicken tender pops were determined by following the standard protocols of AOAC, 2005. Chopped samples of chicken tender pops were oven dried at 100°C for 2 h, cooled in a desiccator, and moisture content was measured as weight loss. The solvent extraction method was used to determine fat content; methanol was used as a solvent. Protein was assessed through the Kjeldahl method of digestion. Samples were subjected to a muffle furnace at a temperature of 550°C for ashing. For crude fiber determination, defatted samples were digested using acid and base. After digestion, the leftover material was weighed and ashed. The crude fiber was calculated as the difference in sample weight.
2.7. Determination of TPC and antioxidant activity (TBARS)
Folin–Ciocalteu method, as described by Firuzi et al. (2019), was used to determine the total phenolic content of the product with few modifications. 0.5 mL methanolic extract of the sample was mixed with 1.5 mL of Folin–Ciocalteu reagent and incubated at 25°C for 5 min. Afterward, 1.5 mL of sodium carbonate (6%) was added, and the sample was incubated in a dark room for about 90 min. The absorbance of the blue color mixture was taken at 725 nm, and total phenolic content was expressed as milligram gallic acid equivalents (GAE) per 100 mL of a sample. The assessment of TBARS was done to predict the lipid oxidation of the product. TBARS were measured using the method of Mashau et al. (2021) with few modifications, methanolic extracts of chicken tender pop samples were mixed with thiobarbituric acid, and centrifuged at 3000g for 15 min. Samples were heated at 95°C for 60 min in a water bath, followed by cooling at 25°C. The absorbance of samples was measured at 532 nm through the Tecan Sunrise spectrophotometer (Austria).
2.8. Texture profile analysis
The instrumental texture profile (hardness, chewiness, springiness, gumminess, cohesiveness, and resilience) of chicken tender pops was evaluated using a Universal TA‐XT plus texture analyzer (Stable Micro Systems, UK) and its propriety Exponent software, version V.5.1.1.0, as described by de Paiva et al. (2021). A slice of 1 × 1 cm was ligated from the treated samples of chicken tender pops. A double compression cycle test was implemented up to 50% strain compression by means of an aluminum cylinder probe of 3.6 cm diameter at a speed of 1 mm/s to acquire force–time deformation curves. Force versus time plots were used to estimate TPA values that were recorded at 0, 7, 14, and 21 days of storage at room temperature 25°C.
2.9. Instrumental color analysis
The instrumental color of chicken tender pops was determined using a Hunter lab calorimeter at 0, 7, 14, and 21 days of refrigerated storage. CIE L* (lightness), a* (redness), and b* (yellowness) of samples were measured (Shahamirian et al., 2019).
2.10. Determination of cooking characteristics
Cooking yield and cooking loss expressed the overall cooking characteristics of samples. Samples were air fried for 10 min at 200°C and then cooled at room temperature 25 ± 5°C. The cooking yield and loss were measured as weight differences before and after frying chicken tender pop, following Sunantha and Saroat (2011) and El‐Nashi et al. (2015).
2.11. Microbiological evaluation
The microbiological analysis for each treatment was performed according to International Standards (APHA, 2001). Maximum recovery solution was prepared by adding 10 g of sample in 90 mL of peptone water in a sterile stomacher bag for 2 min of blending in the stomacher (IUL Instruments, Mod. 1986/470, Spain). After that, serial dilutions up to 10−3 were prepared using 1 mL sample in 9 mL of peptone water. The samples were inoculated in particular culture media for enumeration of studied microorganisms: total viable count and psychotropic count (plate count agar; at 37°C for 48 h and 10°C for 5 days, respectively), coliform count (violet red bile agar; at 30°C for 48 h), Staphylococcus (Baird–Parker agar with egg yolk and 1% potassium tellurite, Hi‐media, Mumbai, India), Salmonella (XLD agar; at 35°C for 24 h), and Listeria monocytogenes (Meuller–Hinton agar, HiMedia, Mumbai, India). All microbial counts were calculated as logarithms of colony‐forming units per gram (log cfu/g). Staphylococcus, Salmonella, and Listeria analyses were performed at 0 and 21 days of storage, whereas all other investigations were made for the whole storage period at an interval of 7 days (Honrado et al., 2022).
2.12. Sensory evaluation
Sensory evaluation of chicken tender pops was carried out by an untrained consumer panel of 20 individuals using a 9‐point hedonic scale (score of 9 as excellent and 1 as extremely poor) at the Department of Food Sciences, University of the Punjab, Lahore, Pakistan. The items were air fried and served warm for a few minutes before sensory analysis. The samples were evaluated based on their general acceptability, juiciness, flavor, appearance, color, texture, and juiciness. Around 4.30 p.m., sensory evaluation was conducted in a setting with adequate lighting. The panelists offered potable water to rinse their mouths after each sample (Santhi et al., 2020).
2.13. Statistical analysis
Acquired data were articulated as the mean values of three replicates, and standard deviations were analyzed statistically by evaluating variance using SPSS version 25.0. Two‐way ANOVA and LSD's post hoc analysis were used for multiple comparisons. For all tests, p‐values of (p < .05) were considered statistically significant.
3. RESULTS AND DISCUSSIONS
3.1. Physiochemical analysis of pomegranate peel powder
The physicochemical composition of PPP is illustrated in Table 2. The percentage of moisture, crude fiber, fat, protein, and ash were 7.28 ± 1.50, 13.91 ± 0.02, 1.61 ± 0.08, 3.78 ± 0.14, and 3.63 ± 0.05, respectively. The results of our studies are in line with those of Kushwaha et al. (2013) and Rowayshed et al. (2013). According to our studies' results, pomegranate peel powder's pH was 4.86 ± 0.02, which was in near accordance with Jalal, Pal, Ahmad, et al. (2018). TPC in the methanolic extract of PPP was 23.59 ± 0.02 mg GAE/g, which is responsible for its excellent radical scavenging properties. Our findings are parallel to those of Jalal, Pal, Hamdani, et al. (2018), who reported the TPC of methanol extract of PPP to be 24.00 mg GAE/g.
TABLE 2.
Physicochemical chemical composition of PPP.
| Moisture | 7.28 ± 1.50% |
| Fat | 1.61 ± 0.08% |
| Protein | 3.78 ± 0.14% |
| Crude fiber | 13.91 ± 0.02% |
| Ash | 3.63 ± 0.05% |
| pH | 4.8 |
| TPC | 23.59 ± 0.02 mg GAE/g of dry peel |
Abbreviation: PPP, Pomegranate Peel Powder.
3.2. Physiochemical parameters of chicken tender pops
The incorporation of pomegranate peel powder did not significantly change the pH of chicken tender pop as depicted in Table 3. However, a non‐significant (p > .05) decrease was observed in treated samples compared to the control, which could be linked to higher acidity of pomegranate peel powder. During refrigerated storage, pH of all treatments decreased non‐significantly (p > .05), possibly due to growth of lactic acid bacteria or the conversion of glycogen into lactic acid. A similar effect was reported by Rupasinghe et al. (2022) for frozen storage of chicken wings marinated with several fruit juices. The water‐holding capacity (WHC) represents the amount of water retained by a product on the application of external force. WHC of treated samples was significantly (p < .05) improved by the addition of pomegranate peel powder, which is recorded as 43.60%, 46.36%, 47.87%, and 49.36% for T0 (0% PPP), T1 (3% PPP), T2 (6% PPP), and T3 (9% PPP) respectively. Similar results were reported by Akhtar et al. (2015), who described that the incorporation of 3% of PPP in beef sausages enhances the WHC of the products. On the other hand, results indicated a decline in WHC with the passage of storage. However, the decline rate was less in the treated sample, which complies with the results of Rupasinghe et al. (2022), who concluded that WHC of chicken wings marinated with different fruit juices reduced with the progression of storage period.
TABLE 3.
Physicochemical parameters of chicken tender pops supplemented with different concentrations of pomegranate peel powder during refrigerated storage at 4 ± 2°C for 21 days.
| Variables | Storage (days) | Treatments | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| pH | 0 | 6.22 ± 0.01k | 6.22 ± 0.02j | 6.21 ± 0.02h | 6.20 ± 0.01g |
| 7 | 6.22 ± 0.02m | 6.21 ± 0.02j | 6.20 ± 0.02i | 6.19 ± 0.02g | |
| 14 | 6.20 ± 0.02L | 6.20 ± 0.01f | 6.20 ± 0.02e | 6.17 ± 0.03d | |
| 21 | 6.19 ± 0.01i | 6.20 ± 0.02c | 6.18 ± 0.01b | 6.16 ± 0.02a | |
| WHC (%) | 0 | 43.60 ± 0.02f | 46.36 ± 0.03d | 47.87 ± 0.02b | 49.36 ± 0.02a |
| 7 | 41.61 ± 0.01i | 44.85 ± 0.025e | 46.36 ± 0.03d | 47.84 ± 0.02c | |
| 14 | 38.60 ± 0.05k | 42.36 ± 0.02h | 44.86 ± 0.01e | 46.35 ± 0.01d | |
| 21 | 35.12 ± 0.01L | 39.86 ± 0.02j | 43.35 ± 0.02g | 44.84 ± 0.02e | |
Note: Values are expressed as mean ± S.D.; means with different letters superscripts are significant (p < .05).
Abbreviations: T0: Chicken tender pops with PPP; T1: Chicken tender pops with 3% PPP; T2: Chicken tender pops with 6% PPP; and T3: Chicken tender pops with 9% PPP.
3.3. Proximate composition of chicken tender pops
The moisture content of chicken tender pops was significantly (p < .05) decreased by the addition of pomegranate peel powder ranging from 60.05 ± 0.03% to 55.08 ± 0.005%, attributed to the replacement of meat with dried peel powder. A considerable drop in moisture content was observed during storage associated with evaporation into surroundings, but this decrease was lower in treated samples, as elucidated in Table 4. Sharma and Yadav (2020) also reported reduced moisture content in chicken meat patties prepared with pomegranate peel powder. The fat content of chicken tender pops increased in a non‐significant manner with the progression of the storage period due to the breakdown of lipoprotein into lipids and protein (El‐Nashi et al., 2015). A minor increase in the protein content of treated samples is associated with a low amount of protein in pomegranate peel powder. In contrast, a decrease in protein content with the progression of storage is associated with protein breakdown and removal of water‐soluble amino acids along with moisture removal. Similar results were observed by El‐Nashi et al. (2015) for beef sausages enriched with pomegranate peel powder. The addition of pomegranate peel powder did not affect the ash content of chicken tender pops remarkably; however, a slight increase in ash content was observed for all treatments as the progression of the storage period. The crude fiber content of chicken tender pops considerably increased from 0.256 ± 0.005% to 1.45 ± 0.010%. The enhancement of crude fiber is associated with a higher concentration of dietary fiber in pomegranate by‐products (Rowayshed et al., 2013). Bhol and John Don Bosco (2013) also claimed an increase in the dietary fiber of bread fortified with powder of pomegranate by‐products.
TABLE 4.
Proximate composition of chicken tender pops supplemented with different concentrations of pomegranate peel powder during refrigerated storage at 4 ± 2°C for 21 days.
| Variables | Storage (days) | Treatments | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| Moisture (%) | 0 | 60.05 ± 0.03a | 58.08 ± 0.01b | 57.04 ± 0.02c | 55.08 ± 0.01e |
| 7 | 56.05 ± 0.01d | 54.04 ± 0.05f | 53.10 ± 0.01g | 52.03 ± 0.02i | |
| 14 | 52.07 ± 0.01h | 50.06 ± 0.01j | 49.07 ± 0.02k | 48.11 ± 0.02L | |
| 21 | 48.07 ± 0.02m | 47.08 ± 0.01n | 46.09 ± 0.01° | 45.08 ± 0.01p | |
| Fat (%) | 0 | 16.56 ± 0.02j | 16.57 ± 0.02ij | 16.58 ± 0.01ij | 16.58 ± 0.02i |
| 7 | 16.77 ± 0.02f | 16.65 ± 0.01h | 16.64 ± 0.01h | 16.64 ± 0.02h | |
| 14 | 17.36 ± 0.01b | 16.85 ± 0.01e | 16.74 ± 0.01g | 16.76 ± 0.02fg | |
| 21 | 17.57 ± 0.02a | 16.92 ± 0.01c | 16.88 ± 0.02d | 16.88 ± 0.01d | |
| Protein (%) | 0 | 16.85 ± 0.01h | 17.74 ± 0.01c | 17.75 ± 0.01abc | 17.76 ± 0.02a |
| 7 | 16.84 ± 0.02i | 17.73 ± 0.01f | 17.73 ± 0.02cd | 17.76 ± 0.01ab | |
| 14 | 16.82 ± 0.01j | 17.73 ± 0.02f | 17.72 ± 0.01de | 17.74 ± 0.02bc | |
| 21 | 16.81 ± 0.02j | 17.71 ± 0.02g | 17.70 ± 0.02e | 17.71 ± 0.01e | |
| Ash (%) | 0 | 1.98 ± 0.052f | 2.37 ± 0.01e | 2.76 ± 0.02c | 3.11 ± 0.01a |
| 7 | 1.98 ± 0.01f | 2.38 ± 0.02de | 2.76 ± 0.01bc | 3.11 ± 0.01a | |
| 14 | 1.98 ± 0.01f | 2.38 ± 0.01de | 2.76 ± 0.01bc | 3.11 ± 0.02a | |
| 21 | 1.20 ± 0.01g | 2.38 ± 0.02d | 2.78 ± 0.02b | 3.12 ± 0.01a | |
| Crude fiber (%) | 0 | 0.256 ± 0.01i | 0.663 ± 0.01f | 1.04 ± 0.02d | 1.45 ± 0.01a |
| 7 | 0.256 ± 0.02j | 0.662 ± 0.01g | 1.04 ± 0.01d | 1.44 ± 0.01ab | |
| 14 | 0.253 ± 0.02j | 0.660 ± 0.02g | 1.03 ± 0.01e | 1.43 ± 0.02bc | |
| 21 | 0.252 ± 0.01k | 0.657 ± 0.01h | 1.02 ± 0.02e | 1.42 ± 0.01c | |
Note: Values are expressed as mean ± S.D.; means with different letters superscripts are significant (p < .05).
Abbreviations: T0: Chicken tender pops with PPP; T1: Chicken tender pops with 3% PPP; T2: Chicken tender pops with 6% PPP; and T3: Chicken tender pops with 9% PPP.
3.4. TPC and antioxidant activity (TBARS) of chicken tender pops
Antioxidants are compounds that exert a free radical scavenging effect and bind them, preventing degenerative diseases (Villalobos‐Delgado et al., 2019). Pomegranate peel powder is an excellent source of polyphenols that are interlinked to its technological and therapeutic potential (Jalal et al., 2018). Including pomegranate peel powder in chicken tender pops considerably increased the TPC of treated samples ranging from 3.87 mg GAE/g to 0.90 mg GAE/g, as depicted in Figure 1. Devatkal and Naveena (2010) also reported that a higher concentration of phenolic compounds in pomegranate peel contributes to the enhancement of the total phenolic content of the products supplemented with powder. However, with the advancement of the storage period, TPC of chicken tender pops reduced, as reported by Awan et al. (2017), who evaluated the storage stability of garlic‐fortified chicken bites. The presence of little phenolic content (0.90 ± 0.055 mg GAE/g) in the control sample is attributed to spices used for seasoning. TBARS were significantly (p < .05) increased for both control and treated samples throughout refrigerated storage. However, the increase in treated samples was significantly lower than the control sample, as presented in Figure 2. Similarly, Vaithiyanathan et al. (2011) witnessed that dipping meat in a phenolic solution significantly (p < .05) reduced the TBARS of meat. However, the TBARS increased during the storage period, a consequence of lipid oxidation in muscle foods.
FIGURE 1.
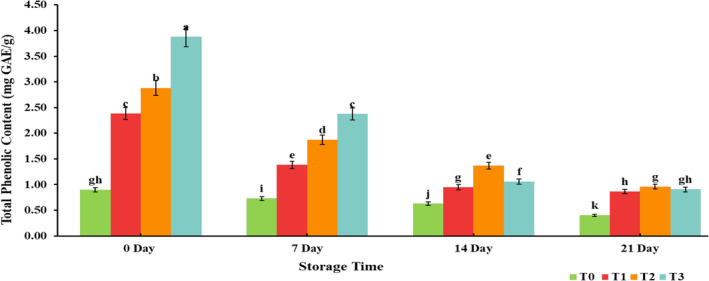
Effect of interaction between treatments and storage days for total phenolic content (mg of GAE/g) of chicken tender pops supplemented with different concentrations of pomegranate peel powder (PPP) during refrigerated storage at 4 ± 2°C for 21 days. Columns labeled with different letters are significantly different, p < .05 (n = 3). T0: control group; T1: 3% PPP; T2: 6% PPP; and T3: 9% PPP.
FIGURE 2.
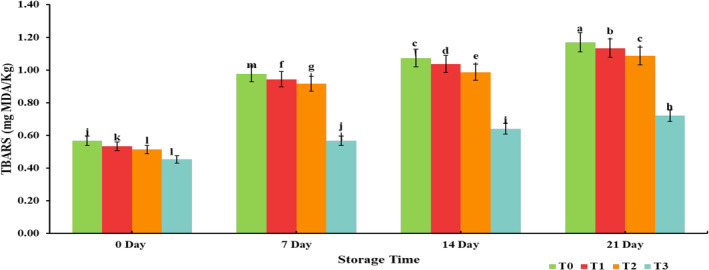
Effect of interaction between treatments and storage days for TBARS (mg of MDA/Kg) of chicken tender pops supplemented with different concentrations of pomegranate peel powder (PPP) during refrigerated storage at 4 ± 2°C for 21 days. Columns labeled with different letters are significantly different, p < .05 (n = 3). T0: control group; T1: 3% PPP; T2: 6% PPP; and T3: 9% PPP.
3.5. Texture profile analysis of chicken tender pops
The influence of PPP inclusion on the textural parameters of chicken tender pops was scrutinized while refrigerated storage and elucidated in Table 5. A significant (p < .05) increase was noticed in hardness, chewiness, and gumminess, whereas cohesiveness and springiness showed a non‐significant (p > .05) change in treated samples in relation to control. High concentrations of PPP followed by moisture loss contributed to the increased hardness in treated samples. Conversely, control samples showed low shear force values (Table 5) for chewiness when compared to treated samples (T1, T2, and T3). Our results are consistent with Sharma and Yadav (2020) and Yadav et al. (2016), who manifested a similar effect on the texture profile of processed chicken products by adding several fruits and their by‐products powders. Pietrasik et al. (2020) also reported increased hardness and chewiness in beef burgers supplemented with higher concentrations of pea fibers. In the same way, the gumminess of chicken tender pops was elevated ranging from 28.27 ± 0.05 N to 32.64 ± 0.01 N by the addition of PPP during storage because of increased hardness. Andrés et al. (2017) stated that the inclusion of pomegranate pomace increased the gumminess of lamb patties. However, grape and olive pomace showed considerably higher values than pomegranate pomace. For our studies, springiness and cohesiveness showed a non‐significant variation for control and treated samples, as illustrated in Table 5. López‐Vargas et al. (2014) found similar results for the springiness of beef burgers comprising various ratios of passion fruit powder. In contrast, the literature of Pereira et al. (2022) showed an increase in the springiness of beef burgers added with grape pomace powder. The reduced values of cohesiveness for treated samples may be assigned to the presence of low‐fat content and high crude fiber content in PPP reported by de Alencar et al. (2022). Contrary to our results, de Paiva et al. (2021) reported a significant increase in the cohesiveness of conventional meat nuggets prepared with acerola fruit powder, rosemary, and licorice extract.
TABLE 5.
Textural profile analysis of chicken tender pops supplemented with different concentrations of pomegranate peel powder during refrigerated storage at 4 ± 2°C for 21 days.
| Variables | Storage (days) | Treatments | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| Hardness (N) | 0 | 37.57 ± 0.03m | 47.85 ± 0.02L | 53.45 ± 0.02h | 58.40 ± 0.02d |
| 7 | 37.46 ± 0.02n | 48.13 ± 0.02k | 53.73 ± 0.02g | 58.65 ± 0.02c | |
| 14 | 37.32 ± 0.03° | 48.31 ± 0.01j | 53.91 ± 0.03f | 58.84 ± 0.02b | |
| 21 | 36.30 ± 0.03p | 48.49 ± 0.02i | 54.16 ± 0.03e | 59.03 ± 0.03a | |
| Chewiness (N) | 0 | 17.56 ± 0.02p | 24.69 ± 0.03L | 25.63 ± 0.02h | 26.61 ± 0.02d |
| 7 | 17.61 ± 0.02° | 24.78 ± 0.02k | 25.72 ± 0.01g | 26.71 ± 0.01c | |
| 14 | 17.66 ± 0.03n | 24.87 ± 0.03j | 25.81 ± 0.02f | 26.80 ± 0.01b | |
| 21 | 17.71 ± 0.03m | 24.96 ± 0.02i | 25.91 ± 0.02e | 26.89 ± 0.03a | |
| Gumminess (N) | 0 | 21.57 ± 0.01p | 28.27 ± 0.02L | 30.24 ± 0.02h | 32.25 ± 0.01d |
| 7 | 21.65 ± 0.01° | 28.40 ± 0.01k | 30.37 ± 0.01g | 32.38 ± 0.01c | |
| 14 | 21.73 ± 0.01n | 28.53 ± 0.02j | 30.50 ± 0.02f | 32.51 ± 0.02b | |
| 21 | 21.81 ± 0.02m | 28.66 ± 0.02i | 30.63 ± 0.01e | 32.64 ± 0.01a | |
| Springiness | 0 | 0.87 ± 0.02c | 0.85 ± 0.03cd | 0.83 ± 0.02de | 0.80 ± 0.02e |
| 7 | 0.88 ± 0.03b | 0.87 ± 0.04c | 0.85 ± 0.04cd | 0.82 ± 0.03de | |
| 14 | 0.88 ± 0.03b | 0.89 ± 0.02ab | 0.87 ± 0.03c | 0.84 ± 0.03d | |
| 21 | 0.91 ± 0.02a | 0.91 ± 0.03a | 0.89 ± 0.02ab | 0.86 ± 0.04cd | |
| Cohesiveness (%) | 0 | 0.52 ± 0.03a | 0.50 ± 0.02ab | 0.48 ± 0.01bc | 0.46 ± 0.02c |
| 7 | 0.51 ± 0.02ab | 0.49 ± 0.01b | 0.47 ± 0.02bc | 0.45 ± 0.03cd | |
| 14 | 0.51 ± 0.02ab | 0.47 ± 0.02bc | 0.46 ± 0.01c | 0.45 ± 0.03cd | |
| 21 | 0.49 ± 0.03b | 0.47 ± 0.01bc | 0.46 ± 0.02c | 0.43 ± 0.02d | |
Note: Values are expressed as mean ± S.D.; means with different letters superscripts are significant (p < .05).
Abbreviations: T0: Chicken tender pops with PPP; T1: Chicken tender pops with 3% PPP; T2: Chicken tender pops with 6% PPP; and T3: Chicken tender pops with 9% PPP.
3.6. Instrumental color analysis of chicken tender pops
Product color is regarded as the primary attribute that directly affects the consumer's purchasing intention and makes the product more eye appealing. The incorporation of PPP significantly (p < .05) affected the color of chicken tender pops during storage. A significant decrease was observed in lightness (L*) of treated samples as compared to control (as shown in Figure 3a); for the whole storage period, reduction in L* can be linked to the darker color of PPP and pigment dilution that leads to darker product color. Sáyago‐Ayerdi et al. (2009) reported a lower value of L* for chicken burgers enriched with grape dietary fibers. Similarly, the literature of Shahamirian et al. (2019) showed inhibition of brightness by the addition of pomegranate juice and rind powder extract in frozen burgers in relation to control. All treatments presented a drop in redness (a*), but it was more significant in the control sample than in treated samples (as illustrated in Figure 3b). The decline in a* is interlinked to the oxidation of myoglobin followed by accumulation of metmyoglobin that imparts a darker‐brown color to meat products. Similar results were observed by Devatkal et al. (2010), who added pomegranate seed extract to beef patties. However, more intense color was evident in control (3.14) than in treated samples 4.57, 4.69, and 4.78 at the end of storage, which can be attributed to the antioxidant potential of PPP. The addition of olive and grape pomace extracts to meat patties retained the intensity of red color in contrast to control sample (Andrés et al., 2017). The inclusion of PPP significantly (p < .05) reduced the yellowness (b*) in treated samples 12.15–12.37 than control 12.83. However, heterogeneous variation was observed for b* throughout the storage as depicted in Figure 3c. A significant drop was noticed for the treated sample on day 7, whereas on day 21 non‐significant (p > .05) increase was observed. Pereira et al. (2022) studied beef burgers' quality and sensory attributes, and correspondingly noticed a decrease in b* values as the proportion of grape pomace meal augmented. Firuzi et al. (2019) also reported a reduction in b* of frankfurter supplemented with various pomegranate fruit extracts. Contrarily, according to Estévez et al. (2005), increased b* was seen in frankfurter samples containing rosemary essential oil during chilled storage.
FIGURE 3.
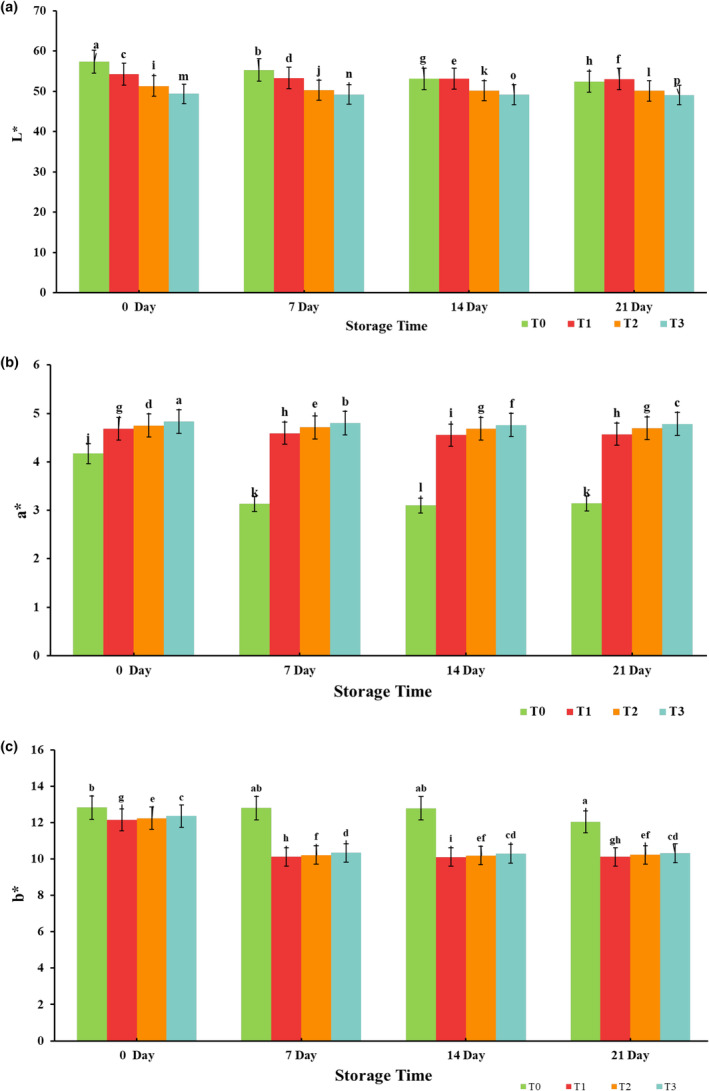
Effect of interaction between treatments and storage days for instrumental color (L*, a*, and b*) a, b, and c, respectively, of chicken tender pops supplemented with different concentrations of pomegranate peel powder (PPP) during refrigerated storage at 4 ± 2°C for 21 days. Columns labeled with different letters are significantly different, p < .05 (n = 3). T0: control group; T1: 3% PPP; T2: 6% PPP; and T3: 9% PPP.
3.7. Cooking characteristics of chicken tender pops
Cooking yield and cooking loss of tender chicken pop prepared with 0%, 3%, 6%, and 9% pomegranate peel powder were assessed, and the results are elucidated in Table 6. There was a considerable increase in the cooking yield of the treated sample compared to the control sample. The upsurge in cooking yield is attributed to the water‐retaining properties of pomegranate peel powder. Analogous effects were reported by Mashau et al. (2021) for the cooking yield of ground beef supplemented with moringa leaves powder. On the other hand, adding pomegranate peel powder reduced the cooking loss in developed products from 17.17% to 12.68%. According to El‐Nashi et al. (2015), the reduction in cooking loss is attributed to the WHC of pomegranate peel powder. However, the advancement of storage resulted in a significant decrease in cooking yield and a significant increase in cooking loss.
TABLE 6.
Cooking characteristics of chicken tender pops supplemented with different concentrations of pomegranate peel powder during refrigerated storage at 4 ± 2°C for 21 days.
| Variables | Storage (days) | Treatments | |||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| Cooking yield (%) | 0 | 82.58 ± 0.12ef | 84.67 ± 0.05d | 86.48 ± 0.15b | 88.40 ± 0.04a |
| 7 | 78.52 ± 0.051j | 83.19 ± 0.03e | 84.43 ± 0.03d | 85.43 ± 0.02c | |
| 14 | 74.52 ± 0.02k | 81.64 ± 0.04g | 82.93 ± 0.02ef | 82.36 ± 0.03fg | |
| 21 | 71.51 ± 0.02L | 78.64 ± 1.70j | 80.83 ± 0.02h | 79.85 ± 0.02i | |
| Cooking loss (%) | 0 | 17.17 ± 0.05L | 15.30 ± 0.05n | 14.88 ± 0.05° | 12.68 ± 0.50p |
| 7 | 20.64 ± 0.02g | 17.77 ± 0.02j | 17.35 ± 0.02k | 16.57 ± 0.03m | |
| 14 | 23.56 ± 0.03c | 20.75 ± 0.03f | 19.84 ± 0.03h | 19.57 ± 0.02i | |
| 21 | 28.46 ± 0.02a | 24.24 ± 0.02b | 23.32 ± 0.03d | 23.03 ± 0.02e | |
Note: Values are expressed as mean ± S.D.; means with different letters superscripts are significant (p < .05).
Abbreviations: T0: Chicken tender pops with PPP; T1: Chicken tender pops with 3% PPP; T2: Chicken tender pops with 6% PPP; and T3: Chicken tender pops with 9% PPP.
3.8. Microbiological studies of chicken tender pops
The effect of PPP supplementation on the microbiological safety of chicken tender pops during storage was investigated through the assessment of total viable count, psychotropic count, and coliform count along with Staphylococcus, Salmonella, and Listeria monocytogenes. The total viable count (TVC) for chicken tender pops significantly (p < .05) increased with the advancement of the storage period in all treatments as shown in Figure 4a. However, the increase in the TVC of treated samples was slightly less than that of the control sample. The TVC observed for the control sample was 2.54 ± 0.02 log cfu/g, whereas TVC for the treated sample (T3) was recorded as 1.33 ± 0.03 cfu/g. A similar trend was observed for the psychotropic count of chicken tender pops shown in Figure 4b, illustrating a significantly lower increase in the treated sample. The inhibitory effect is ascribed to the presence of different phenolic compounds in pomegranate peel that exerts an antimicrobial effect on the product. The results agree with Sharma and Yadav (2020), who observed the same inclination of microbial count for chicken patties supplemented with numerous by‐products of the pomegranate fruit. Chandralekha et al. (2012) also observed a significant decline in the microbial count of meatballs prepared by incorporating 5% pomegranate rind powder stored under refrigerated conditions. The coliform count decreased with the addition of PPP, as depicted in Figure 4c, which supports the potential of PPP as a natural preservative, although there was an increase in the number of coliforms with the progression of storage interval. The pragmatic results are in accordance with Kanatt et al. (2010) and Al‐Zoreky (2009), who reported that the antimicrobial action of PPP against different bacterial species is due to its interference with bacterial protein synthesis.
FIGURE 4.
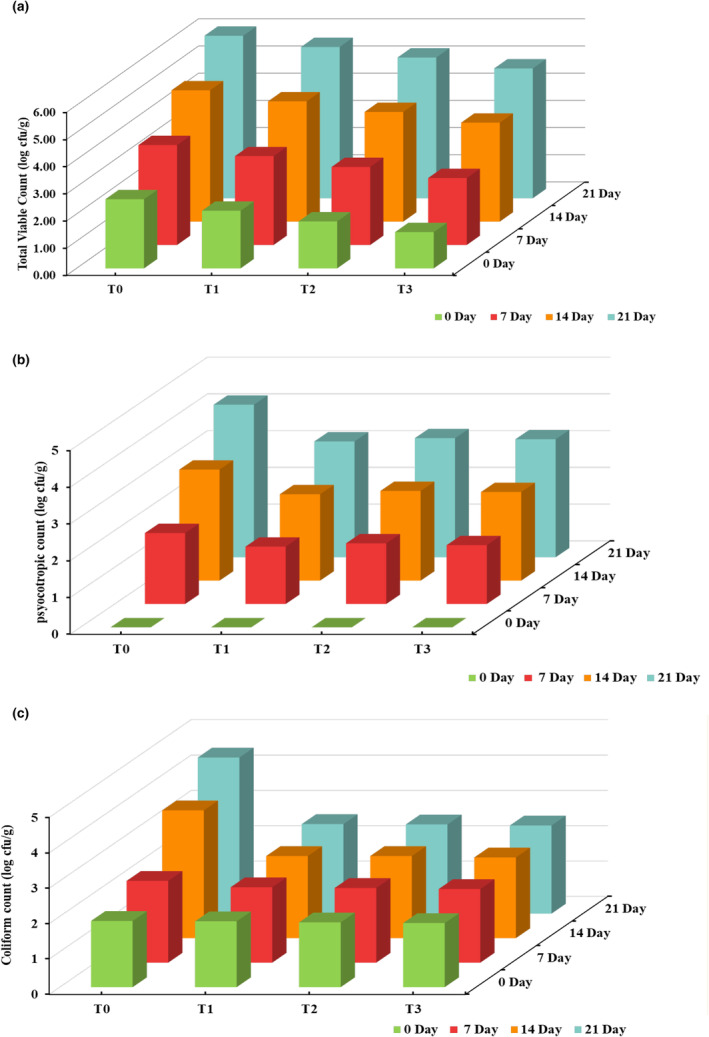
Effect of interaction between treatments and storage days for (a) total viable count (log cfu/g), (b) psychotropic count (log cfu/g); and c: coliform count (log cfu/g) of chicken tender pops supplemented with different concentrations of pomegranate peel powder (PPP) during refrigerated storage at 4 ± 2°C for 21 days. T0: control group; T1: 3% PPP; T2: 6% PPP; and T3: 9% PPP.
On the other hand, Staphylococcus, Salmonella, and Listeria were investigated as human pathogens at the beginning and the end of the study. The results showed negative values for all of these bacterial pathogens during the entire storage period hence conforming to safety legislation, Regulation EC No 2073/2005 (European Food Safety Authority, 2010). Furthermore, experimental results are supported by Honrado et al. (2022) and El‐Nashi et al. (2015), who witnessed similar results for low‐fat rabbit sausages and beef sausages developed using various concentrations of PPP.
3.9. Sensory evaluation
Sensory attributes (appearance, color, texture, flavor, juiciness, tenderness, and overall acceptability) of prepared treatments (T0, T1, T2, and T3) containing different concentrations of pomegranate peel powder at different storage intervals are represented in Figure 5. Results indicated that the inclusion of pomegranate peel powder significantly improved the product's sensory attributes during storage with increased overall acceptability. However, in T3 (9% PPP), darker red color was observed with a slight dryness with the progression of storage. In general, the best sensory scores were received by T2 chicken tender pops containing 6% PPP. Hence, the panelists declared chicken tender pops with 6% pomegranate peel powder (T2) as the best treatment for all organoleptic properties during the study period. The results for sensory evaluation of color and textural properties (juiciness and tenderness) can be correlated with instrumental color analysis and texture profile analysis. Sensory scores for color were decreased as an increase in PPP concentration and results are analogous to L* and a* values which show increased darkness in product due to myoglobin oxidation and PPP color. Furthermore, instrumental texture analysis showed increased hardness and dryness because of moisture loss that will ultimately lower the juiciness and tenderness of the product.
FIGURE 5.
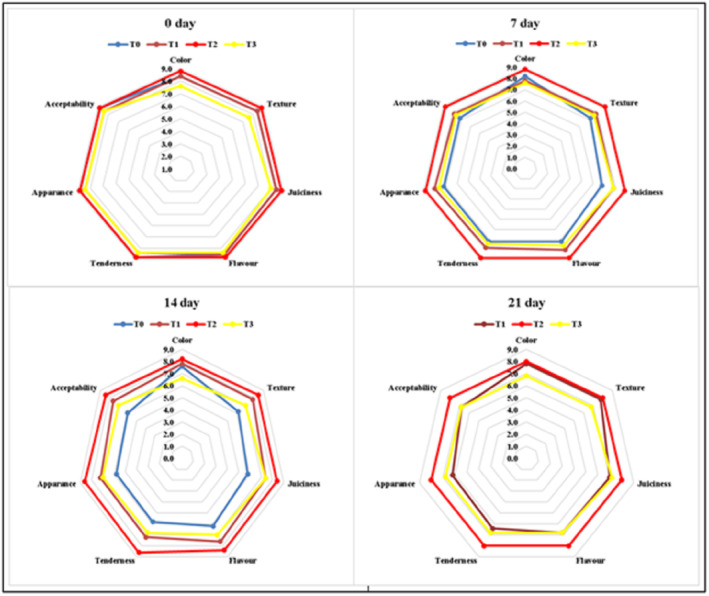
Effect of interaction between treatments and storage days for sensory scores of texture, juiciness, flavor, tenderness, appearance, and overall acceptability of chicken tender pops supplemented with different concentrations of pomegranate peel powder (PPP) during refrigerated storage at 4 ± 2°C for 21 days. T0: control group; T1: 3% PPP; T2: 6% PPP; and T3: 9% PPP.
3.10. Heatmap and hierarchical analysis
A heatmap was generated to analyze all variables pertaining to storage time and pomegranate peel powder concentration (Figure 6). In addition to data classification, a heatmap entails color comparison to make the findings more intrusive. Multiple separate clusters were observed for key variables, illustrating variances across them. The darkest red color, showing the highest concentration, was revealed for TPC in T3 (9% PPP), accompanied by coliform count and fat in the control sample at day 0. A slightly lighter color was depicted by T2 (6% PPP) and T3 at Day 0 for TPC and WHC and cooking yield, respectively.
FIGURE 6.
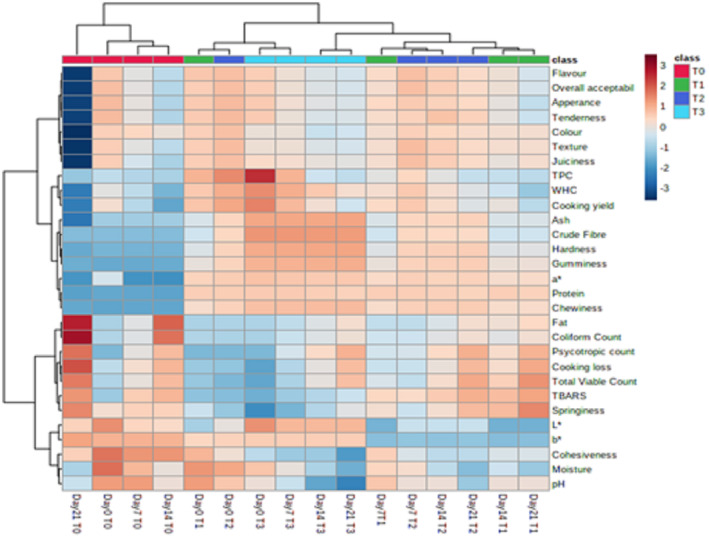
Heatmap analysis illustrating the correlation of all parameters in chicken tender pops supplemented with pomegranate peel powder during 21 days of storage.
Similarly, T0 indicated slight variation for coliform and fat on day 14, whereas T0 showed cooking loss on day 21. The moderate‐color cluster was noted in T3 for ash, crude fiber, gumminess, and hardness for the whole storage period. On the other hand, neutral color was displayed for moisture in T3 and T0 on days 7 and 14, respectively. Likewise, neutral color was noticed for organoleptic properties (flavor, tenderness, appearance, and overall acceptability) in T0 on day 7. The minimum values in a cluster were indicated by the dark blue color that was spotted in the control sample for all sensory attributes (appearance, color, flavor, tenderness, juiciness, texture, and overall acceptability) on the 21st day of storage.
3.11. Correlation and principal component analysis (PCA)
A regression analysis was performed to evaluate the correlation among the results of conducted assays on chicken tender pop samples supplemented with pomegranate peel powder shown in Figure 7. A significant positive correlation was observed between coliform count and fat. Similarly, the parameters hardness, chewiness, and gumminess were also observed to be positively correlated. Correlation analysis revealed that all the sensorial attributes (color, appearance, tenderness, flavor, texture, juiciness, and overall acceptability) were significantly correlated with each other in a positive correlation. Water‐holding capacity and cooking loss were also observed to be positively correlated. A comparatively neutral correlation was found between pH and organoleptic characters. Sensory properties were observed to be negatively correlated with coliform count and psychotropic count. Crude fiber, hardness, and gumminess depicted a negative correlation with cohesiveness indicating an inverse relation. Total plate count and cooking yield were also observed to be in a negative correlation with cooking yield.
FIGURE 7.
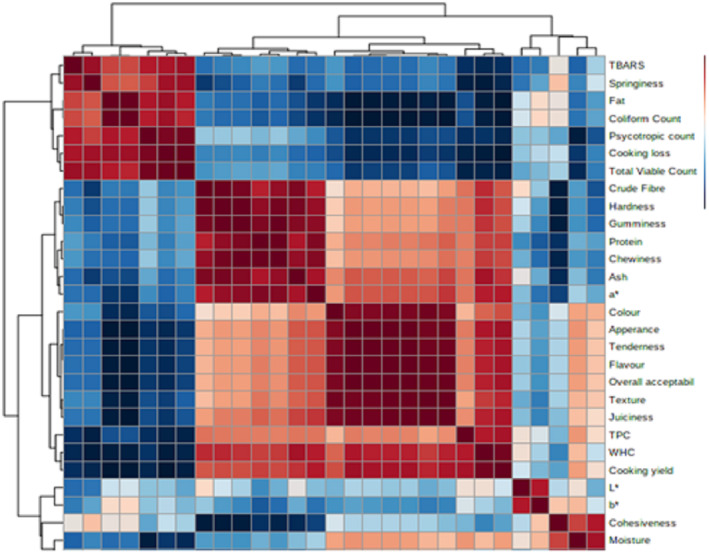
Correlation among various parameters of chicken tender pop samples supplemented with pomegranate peel powder during 21 days of storage.
Principal component analysis (PCA) was characterized by reducing a large number of variables to a small number of comprehensive variables, accurately expressing the total amount of data. Signal intensities are used in PCA to highlight the differences between the parameters that were taken into consideration. Figure 8 presents the results of the principal component analysis (PCA) of the pomegranate peel powder–enriched chicken tender pop samples. PCA illustrated maximum quantities of coliform count, b* (yellowness/blueness) value, and cooking loss of sample T3 (6% PPP) at day 7. Cooking yield and WHC revealed comparatively lower values for T1 on day 7. A similar trend was observed for chewiness and gumminess of T2 samples at day 14. Cooking loss and hardness were further reduced for samples T1, T2, and T3 at day 21. Minimum quantities were observed for control sample at day 0.
FIGURE 8.
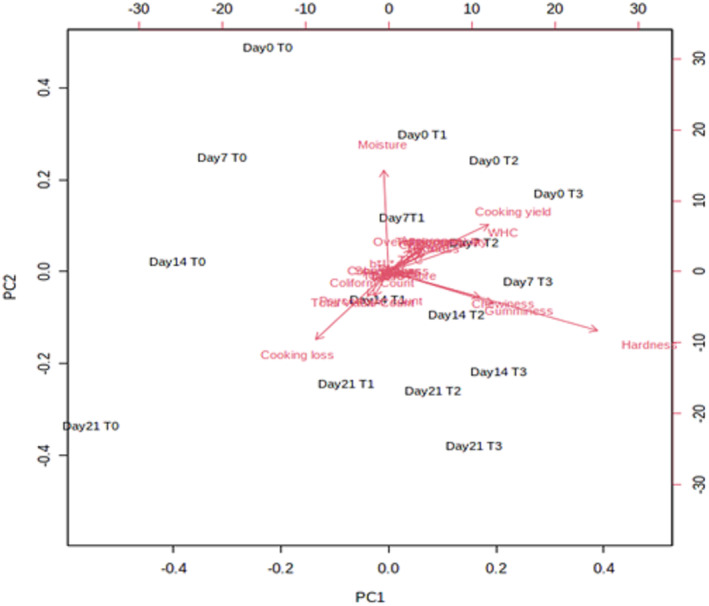
Principal component analysis (PCA) of different parameters of chicken tender pops supplemented with pomegranate peel powder during 21 days of storage.
4. CONCLUSION
Incorporating pomegranate peel powder reduced the moisture content and significantly improved the product's water‐holding capacity, ultimately enhancing the product's sensory attributes. Furthermore, the inclusion of PPP enhanced crude fiber content in treated samples compared to the control group. Significant elevation in total phenolic content indicated the potential of PPP to be used as a natural antioxidant. In contrast, reduced TBARS elucidated the positive impact of PPP on lipid auto‐oxidation. Hardness, chewiness, and gumminess were considerably affected, whereas springiness and cohesiveness showed minor variations. In addition, PPP retarded pigment oxidation was indicated by retained red color during storage. The reduced microbial load validates the antimicrobial potential of PPP ascribed to the presence of polyphenols and flavonoids. Pomegranate peel powder significantly improves the sensory attributes, but up to a specific limit, as for our study, it was at 6%. Based on our study, it can be concluded that chicken products supplemented with PPP have improved nutritional and sensory profiles and can be naturally preserved for up to 3 weeks at refrigeration temperature.
AUTHOR CONTRIBUTIONS
Zunaira Basharat: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal). Maryam Imran: Investigation (equal); visualization (equal); writing – review and editing (lead). Naeem Fatima: Investigation (supporting); visualization (supporting); writing – review and editing (supporting). Muhammad Wasim Sajid: Conceptualization (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (equal). Muhammad Rizwan Tariq: Conceptualization (equal); formal analysis (equal); methodology (equal); validation (equal). Shinawar Waseem Ali: Conceptualization (equal); data curation (equal); investigation (equal); writing – review and editing (equal). Zujaja Umer: Formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Humphrey Garti: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); writing – review and editing (equal).
FUNDING INFORMATION
We did not receive any funding for this work.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interest to declare.
ACKNOWLEDGEMENT
All the authors express deepest gratitude and appreciation to the Department of Food Sciences for their invaluable support during the research project. The resources, facilities, and expertise provided by the department were instrumental in the successful completion of this study.
Basharat, Z. , Imran, M. , Fatima, N. , Sajid, M. W. , Tariq, M. R. , Ali, S. W. , Umer, Z. , Safdar, W. , & Garti, H. (2023). Development of chicken tender pops by utilizing pomegranate peel powder. Food Science & Nutrition, 11, 4530–4546. 10.1002/fsn3.3412
Contributor Information
Muhammad Rizwan Tariq, Email: rizwan.foodsciences@pu.edu.pk.
Humphrey Garti, Email: hgarti@uds.edu.gh.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Akhtar, S. , Ismail, T. , Fraternale, D. , & Sestili, P. (2015). Pomegranate peel and peel extracts: Chemistry and food features. Food Chemistry, 174, 417–425. [DOI] [PubMed] [Google Scholar]
- Ali, A. , Chen, Y. , Liu, H. , Yu, L. , Baloch, Z. , Khalid, S. , & Chen, L. (2019). Starch‐based antimicrobial films functionalized by pomegranate peel. International Journal of Biological Macromolecules, 129, 1120–1126. [DOI] [PubMed] [Google Scholar]
- Al‐Zoreky, N. S. (2009). Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. International Journal of Food Microbiology, 134(3), 244–248. [DOI] [PubMed] [Google Scholar]
- Anand, S. P. , & Sati, N. (2013). Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. International Journal of Pharmaceutical Sciences and Research, 4(7), 2496. [Google Scholar]
- Andrés, A. I. , Petrón, M. J. , Adámez, J. D. , López, M. , & Timón, M. L. (2017). Food by‐products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Science, 129, 62–70. [DOI] [PubMed] [Google Scholar]
- AOAC . (2005). Official methods of analysis of AOAC international (18th ed.). Association of Official Analytical Chemists. [Google Scholar]
- APHA . (2001). Compendium of methods for the microbiological examination of foods (2001). American Public Health Association. [Google Scholar]
- Awan, K. A. , Butt, M. S. , Haq, I. , Ashfaq, F. , & Suleria, H. (2017). Storage stability of garlic fortified chicken bites. Journal of Food Chemistry and Nanotechnology, 3, 80–85. [Google Scholar]
- Barroeta, A. C. (2007). Nutritive value of poultry meat: Relationship between vitamin E and PUFA. World's Poultry Science Journal, 63, 277–284. [Google Scholar]
- Ben‐Othman, S. , Jõudu, I. , & Bhat, R. (2020). Bioactives from Agri‐food wastes: Present insights and future challenges. Molecules, 25, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhol, S. , & John Don Bosco, S. (2013). Enrichment of yeast leavened bread by pomegranate bagasse powder. Food Science, 2(5), 298–300. [Google Scholar]
- Bordoni, A. , & Danesi, F. (2017). Poultry meat nutritive value & human health. In Petracci M. Berri C. (Eds.), Poultry quality evaluation (pp. 279–290). Woodhead Publishing. [Google Scholar]
- Chandralekha, S. , Babu, A. J. , Moorthy, P. S. , & Karthikeyan, B. (2012). Studies on the effect of pomegranate rind powder extract as natural antioxidant in chicken meat balls during refrigerated storage. Journal of Advanced Veterinary Research, 2, 107–112. [Google Scholar]
- de Alencar, M. G. , de Quadros, C. P. , Luna, A. L. L. P. , Neto, A. F. , da Costa, M. M. , Queiroz, M. A. Á. , de Carvalho, F. A. L. , da Silva Araújo, D. H. , Gois, G. C. , Dos Anjos Santos, V. L. , da Silva Filho, J. R. V. , & de Souza Rodrigues, R. T. (2022). Grape skin flour obtained from wine processing as an antioxidant in beef burgers. Meat Science, 194, 108963. [DOI] [PubMed] [Google Scholar]
- de Paiva, G. B. , Trindade, M. A. , Romero, J. T. , & da Silva‐Barretto, A. C. (2021). Antioxidant effect of acerola fruit powder, rosemary and licorice extract in caiman meat nuggets containing mechanically separated caiman meat. Meat Science, 173, 108406. [DOI] [PubMed] [Google Scholar]
- Devatkal, S. K. , Narsaiah, K. , & Borah, A. (2010). Antioxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Science, 85(1), 155–159. [DOI] [PubMed] [Google Scholar]
- Devatkal, S. K. , & Naveena, B. M. (2010). Effect of salt, kinnow and pomegranate fruit by‐product powders on color and oxidative stability of raw ground goat meat during refrigerated storage. Meat Science, 85, 306–311. [DOI] [PubMed] [Google Scholar]
- El Barnossi, A. , Moussaid, F. , & Housseini, A. I. (2021). Tangerine, banana and pomegranate peels valorisation for sustainable environment: A review. Biotechnology Reports, 29, e00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Nashi, H. B. , Fattah, A. F. A. K. A. , Rahman, N. R. A. , & Abd El‐Razik, M. M. (2015). Quality characteristics of beef sausage containing pomegranate peels during refrigerated storage. Annals of Agricultural Sciences, 60, 403–412. [Google Scholar]
- Estévez, M. , Ventanas, S. , & Cava, R. (2005). Protein oxidation in frankfurters with increasing levels of added rosemary essential oil: Effect on color and texture deterioration. Journal of Food Science, 70(7), c427–c432. [Google Scholar]
- European Food Safety Authority . (2010). The assessment of the comparison of the Australian monitoring programme for carcasses to requirements in regulation (EC) No 2073/2005 on microbiological criteria on foodstuffs. EFSA Journal, 8(3), 1452. [Google Scholar]
- Firuzi, M. R. , Niakousari, M. , Eskandari, M. H. , Keramat, M. , Gahruie, H. H. , & Khaneghah, A. M. (2019). Incorporation of pomegranate juice concentrate and pomegranate rind powder extract to improve the oxidative stability of frankfurter during refrigerated storage. LWT, 102, 237–245. [Google Scholar]
- Hernández‐Corroto, E. , Plaza, M. , Marina, M. L. , & García, M. C. (2020). Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innovative Food Science and Emerging Technologies, 60, 102314. [Google Scholar]
- Honrado, A. , Aínsa, A. , Marquina, P. L. , Beltrán, J. A. , & Calanche, J. B. (2022). Low‐fat fresh sausage from rabbit meat: An alternative to traditional rabbit consumption. Meat Science, 194, 108973. [DOI] [PubMed] [Google Scholar]
- Jalal, H. , Pal, M. A. , Ahmad, S. R. , Rather, M. , Andrabi, M. , & Hamdani, S. (2018). Physico‐chemical and functional properties of pomegranate peel and seed powder. Journal of Pharmaceutical Innovation, 7, 1127–1131. [Google Scholar]
- Jalal, H. , Pal, M. A. , Hamdani, H. , Rovida, M. , & Khan, N. N. (2018). Antioxidant activity of pomegranate peel and seed powder extracts. Journal Pharmacognosy and Phytochemistry, 7(5), 992–997. [Google Scholar]
- Kaderides, K. , Mourtzinos, I. , & Goula, A. M. (2020). Stability of pomegranate peel polyphenols encapsulated in orange juice industry by‐product and their incorporation in cookies. Food Chemistry, 310, 125849. [DOI] [PubMed] [Google Scholar]
- Kahramanoglu, I. , & Usanmaz, S. (2016). Pomegranate production & marketing. CRC Press. [Google Scholar]
- Kanatt, S. R. , Chander, R. , & Sharma, A. (2010). Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. International Journal of Food Science and Technology, 45(2), 216–222. [Google Scholar]
- Kandylis, P. , & Kokkinomagoulos, E. (2020). Food applications and potential health benefits of pomegranate and its derivatives. Food, 9, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, K. , Dadmohammadi, Y. , & Abbaspourrad, A. (2021). Nutritional and bioactive components of pomegranate waste used in food and cosmetic applications: A review. Food, 10, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha, S. C. , Bera, M. B. , & Kumar, P. (2013). Nutritional composition of detanninated and fresh pomegranate peel powder. Journal of Environmental Science, Toxicology and Food Technology, 7, 38–42. [Google Scholar]
- López‐Vargas, J. H. , Fernández‐López, J. , Pérez‐Álvarez, J. Á. , & Viuda‐Martos, M. (2014). Quality characteristics of pork burger added with albedo‐fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co‐products. Meat Science, 97(2), 270–276. [DOI] [PubMed] [Google Scholar]
- Mahmoud, M. H. , Abou‐Arab, A. A. , & Abu‐Salem, F. M. (2017). Research article quality characteristics of beef burger as influenced by different levels of orange peel powder. American Journal of Food Technology, 12, 262–270. [Google Scholar]
- Mashau, M. E. , Ramatsetse, K. E. , & Ramashia, S. E. (2021). Effects of adding Moringa oleifera leaves powder on the nutritional properties, lipid oxidation and microbial growth in ground beef during cold storage. Applied Sciences, 11, 2944. [Google Scholar]
- Millen, B. E. , Wolongevicz, D. M. , de Jesus, J. M. , Nonas, C. A. , & Lichtenstein, A. H. (2014). 2013 American Heart Association/American College of Cardiology Guideline on lifestyle management to reduce cardiovascular risk: Practice opportunities for registered dietitian nutritionists. Journal of the Academy of Nutrition and Dietetics, 114, 1723–1729. [DOI] [PubMed] [Google Scholar]
- Pateiro, M. , Barba, F. J. , Domínguez, R. , Sant'Ana, A. S. , Khaneghah, A. M. , Gavahian, M. , & Lorenzo, J. M. (2018). Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Research International, 113, 156–166. [DOI] [PubMed] [Google Scholar]
- Pereira, A. , Lee, H. C. , Lammert, R., Jr. , Wolberg, C., Jr. , Ma, D. , Immoos, C. , Casassa, F. , & Kang, I. (2022). Effects of red‐wine grape pomace on the quality and sensory attributes of beef hamburger patty. International Journal of Food Science and Technology, 57(3), 1814–1823. [Google Scholar]
- Pienaar, L. , & Barends‐Jones, V. (2021). The economic contribution of South Africa's pomegranate industry. Aǧrı, 18(4), 57–64. [Google Scholar]
- Pietrasik, Z. , Sigvaldson, M. , Soladoye, O. P. , & Gaudette, N. J. (2020). Utilization of pea starch and fibre fractions for replacement of wheat crumb in beef burgers. Meat Science, 161, 107974. [DOI] [PubMed] [Google Scholar]
- Pirzadeh, M. , Caporaso, N. , Rauf, A. , Shariati, M. A. , Yessimbekov, Z. , Khan, M. U. , & Mubarak, M. S. (2021). Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Critical Reviews in Food Science and Nutrition, 61, 982–999. [DOI] [PubMed] [Google Scholar]
- Rafraf, M. , Hemmati, S. , Jafarabadi, M. A. , Moghaddam, A. , & Haghighian, M. K. (2017). Pomegranate (Punica Granatum L.) peel hydroalcoholic extract supplementation reduces pain & improves clinical symptoms of knee osteoarthritis: A randomized double‐blind placebo controlled study. Iranian Red Crescent Medical Journal, 19, e38577. [Google Scholar]
- Rahmani, A. H. , Alsahli, M. A. , & Almatroodi, S. A. (2017). Active constituents of pomegranates (Punica granatum) as potential candidates in the management of health through modulation of biological activities. Pharmacognosy Journal, 9, 689–695. [Google Scholar]
- Rowayshed, G. , Salama, A. , Abul‐Fadl, M. , Akila‐Hamza, S. , & Emad, A. M. (2013). Nutritional and chemical evaluation for pomegranate (Punica granatum L.) fruit peel and seeds powders by products. Middle East Journal of Applied Sciences, 3, 169–179. [Google Scholar]
- Rupasinghe, R. A. , Alahakoon, A. U. , Alakolanga, A. W. , Jayasena, D. D. , & Jo, C. (2022). Oxidative stability of vacuum‐packed chicken wings marinated with fruit juices during frozen storage. Food Science of Animal Resources, 42, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhi, D. , Kalaikannan, A. , & Natarajan, A. (2020). Characteristics and composition of emulsion‐based functional low‐fat chicken meat balls fortified with dietary fiber sources. Journal of Food Process Engineering, 43, e13333. [Google Scholar]
- Sáyago‐Ayerdi, S. G. , Brenes, A. , & Goñi, I. (2009). Effect of grape antioxidant dietary fiber on the lipid oxidation of raw & cooked chicken hamburgers. LWT‐Food Science and Technology, 42(5), 971–976. [Google Scholar]
- Shahamirian, M. , Eskandari, M. H. , Niakousari, M. , Esteghlal, S. , Hashemi Gahruie, H. , & Mousavi Khaneghah, A. (2019). Incorporation of pomegranate rind powder extract and pomegranate juice into frozen burgers: Oxidative stability, sensorial and microbiological characteristics. Journal of Food Science and Technology, 56(3), 1174–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharayei, P. , Azarpazhooh, E. , Zomorodi, S. , & Ramaswamy, H. S. (2019). Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT, 101, 342–350. [Google Scholar]
- Sharma, P. , & Yadav, S. (2020). Effect of incorporation of pomegranate peel and bagasse powder and their extracts on quality characteristics of chicken meat patties. Food Science of Animal Resources, 40, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, B. , Singh, J. P. , Kaur, A. , & Singh, N. (2019). Antimicrobial potential of pomegranate peel: A review. International Journal of Food Science and Technology, 54, 959–965. [Google Scholar]
- Smaoui, S. , Hlima, H. B. , Mtibaa, A. C. , Fourati, M. , Sellem, I. , Elhadef, K. , & Mellouli, L. (2019). Pomegranate peel as phenolic compounds source: Advanced analytical strategies and practical use in meat products. Meat Science, 158, 107914. [DOI] [PubMed] [Google Scholar]
- Sunantha, K. , & Saroat, R. (2011). Application of bromelain extract for muscle foods tenderization. Food and Nutrition Sciences, 2011, 393–401. [Google Scholar]
- Vaithiyanathan, S. , Naveena, B. M. , Muthukumar, M. , Girish, P. S. , & Kondaiah, N. (2011). Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4°C). Meat Science, 88, 409–414. [DOI] [PubMed] [Google Scholar]
- Verma, A. K. , & Banerjee, R. (2010). Dietary fibre as functional ingredient in meat products: A novel approach for healthy living—A review. Journal of Food Science and Technology, 47, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos‐Delgado, L. H. , Mateo, J. , Caro, I. , Ramos, M. Y. L. , Mendez, N. G. , Cansino, R. G. , & Mondragón, E. G. G. (2019). Natural antioxidants in fresh and processed meat. In Galanakis C. M. (Ed.), Sustainable meat production and processing (pp. 207–236). Academic Press. [Google Scholar]
- Viuda‐Martos, M. , Fernández‐López, J. , & Pérez‐Álvarez, J. A. (2010). Pomegranate and its many functional components as related to human health: A review. Comprehensive Reviews in Food Science and Food Safety, 9, 635–654. [DOI] [PubMed] [Google Scholar]
- Yadav, S. , Malik, A. , Pathera, A. , Islam, R. U. , & Sharma, D. (2016). Development of dietary fibre enriched chicken sausages by incorporating corn bran, dried apple pomace and dried tomato pomace. Nutrition and Food Science, 46, 16–29. [Google Scholar]
- Zhu, C. P. , Zhai, X. C. , Li, L. Q. , Wu, X. X. , & Li, B. (2015). Response surface optimization of ultrasound‐assisted polysaccharides extraction from pomegranate peel. Food Chemistry, 177, 139–146. [DOI] [PubMed] [Google Scholar]
- Žugčić, T. , Abdelkebir, R. , Alcantara, C. , Collado, M. C. , García‐Pérez, J. V. , Meléndez‐Martínez, A. J. , & Barba, F. J. (2019). From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends in Food Science and Technology, 83, 63–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


