Abstract
The murine gammaherpesvirus 68 (MHV-68) replicates in respiratory epithelial cells, where it establishes a persistent, latent infection limited predominantly to B lymphocytes. The virus-specific CD4+ T-cell response in C57BL/6 mice challenged intranasally with MHV-68 is detected first in the mediastinal lymph nodes and then in the cervical lymph nodes and the spleen. The numbers of MHV-68-specific CD4+ T cells generated in congenic mice homozygous for disruption of the β2-microglobulin gene tended to be higher, indicating that the absence of the CD8+ set in this group resulted in a compensatory response. The peak frequency within the splenic CD4+ T-cell population may reach 1:50 in the acute response; it then drops to 1:400 to 1:500 within 4 months and stays at that level in the very long term. Sorting for L-selectin (CD62L) expression established that all virus-specific CD4+ T cells were initially CD62Llow, with >80% maintaining that phenotype for the next 14 months. The overall conclusion is that MHV-68-specific CD4+ T cells remain activated (CD62Llow) and at a stable frequency in the face of persistent infection.
The murine gammaherpesvirus 68 (MHV-68) replicates in respiratory epithelium following intranasal (i.n.) challenge and then persists as a latent infection of B lymphocytes (13, 17) or non-B cells (16, 23, 25). The virus-specific CD8+ T-cell response has generally been thought to control the acute, lytic phase of this disease. The infectious process is fatal in BALB/c (H-2d) mice depleted of CD8+ T cells by treatment with monoclonal antibodies (MAbs), while CD4+ T-cell-deficient major histocompatibility complex (MHC) class II−/− (H-2b) mice survive and initially limit the extent of viral replication in the lung (2–4). However, the virus later reactivates in the MHC class II−/− mice and causes a chronic, wasting disease that is ultimately fatal. Also, though CD8+ T-cell-deficient (26) β2-microglobulin-deficient (β2M−/−) (H-2b) mice failed to clear MHV-68 from the spleen after high-dose intraperitoneal infection, they did not succumb and remained healthy in the long term (25). This, together with the late onset of disease in the MHC class II−/− mice, indicates that CD4+ T cells are important effectors of immunity in the MHV-68 model. The possible mechanisms of action are currently being analyzed in other experiments (unpublished data).
The present, longitudinal study focuses on the prevalence and activation phenotype of the MHV-68-specific CD4+ T cells. Some have argued that the maintenance of a substantial set of immune CD4+ T cells depends on the continued presence of antigen-antibody complexes on follicular dendritic cells (7, 8, 10). Previous experiments established that this is not the case for CD4+ T-cell memory to the influenza A viruses, which is well maintained in immunoglobulin-deficient (Ig−/−) μMT mice (21). Perhaps, however, viral protein continues to be present in some other form. It is virtually impossible to prove the absolute absence of antigen from any in vivo system, though a spectrum of experiments with virus-specific CD8+ T cells indicate that persistence of the inducing peptide is not essential for the maintenance of memory (8, 11).
The MHV-68 model allows us to turn the question around and to analyze the generation and maintenance of the CD4+ T-cell response to a virus that does genuinely persist. A previous, kinetic study of the immune response to Sendai virus, a negative-strand RNA virus that is thought to be readily eliminated, established that the numbers of memory CD4+ T cells declined with time, though there was a late enrichment in very old mice due to the loss of the naive T-cell compartment (20). Also, the Sendai virus-specific CD4+ T cells progressively reverted to the naive CD62Lhigh phenotype (5). The experiments described here establish that the CD4+ T-cell response to a persistent virus like MHV-68 is indeed different in character.
MATERIALS AND METHODS
Mice and virus.
Female C57BL/6J (B6) and congenic β2M−/− mice (26) were purchased from the Jackson Laboratory (Bar Harbor, Maine). They were held in specific-pathogen-free conditions, except for i.n. infection at 6 to 12 weeks of age with 600 PFU of MHV-68 under Avertin anesthesia. The virus stocks were grown on owl monkey kidney (OMK) cells from an isolate originally provided by A. A. Nash.
Lymphocyte sampling and flow cytometry.
Single-cell suspensions of spleen, mediastinal lymph node (MLN) and cervical lymph node (CLN) were obtained by pressing the organs through a fine mesh. The lymphocytes that were used only for phenotyping (22) were stained by incubating 106 cells in individual wells of a 96-well round-bottom microtiter plate with MAbs diluted in a FACS (fluorescence-activated cell sorting) medium containing 1% bovine serum albumin and 0.1% NaN3 in phosphate-buffered saline (PBS). After a 30-min incubation on ice, cells were washed three times with FACS medium, fixed with 1% ice-cold paraformaldehyde in PBS, and analyzed on a FACScan using CellQuest version 3.1 software (Becton Dickinson, San Jose, Calif.). Lymphocyte populations stained in 1% bovine serum albumin–PBS and sorted into CD4+, CD4+ CD62Llow, CD4+ CD62Lhigh, CD4+ CD44low, CD4+ CD44high, CD4+ CD69low, and CD4+ CD69high populations on a FACStar Plus (Becton Dickinson) were used for functional studies. The purity of the sorted cell populations was always >95%. The conjugated anti-CD4-phycoerythrin anti-CD62L-fluorescein isothiocyanate (FITC), anti-CD44-FITC, and anti-CD69-FITC MAbs were purchased from Pharmingen (San Diego, Calif.).
Assay for T helper cell precursor (Thp) cytokine production.
The CTLL (interleukin-2 [IL-2] and IL-4-dependent) cell line was grown in RPMI 1640 medium supplemented with IL-2. The cells were washed free of IL-2, and 5 × 103 cells were added to 50 μl of cell culture supernatants. Plates were incubated for 24 h at 37°C in 10% CO2, the last 6 h with [3H]thymidine (1 μCi/well). Thymidine incorporation was assayed in a Wallac Betaplate scintillation counter.
LDA.
The limiting-dilution analysis (LDA) assay was based on that described previously for Sendai virus (20). In brief, dilutions of sorted populations from pooled samples of three to four mice were incubated in replicates of 24 with 5 × 104 T-cell-depleted, uninfected, or MHV-68-infected, and irradiated (3,000 rads), syngenic spleen cells (antigen-presenting cells [APCs]). The cultures were incubated for 4 days at 37°C in 10% CO2; then 50-μl aliquots of the supernatant were transferred to new plates, and lymphokine activity was measured by the CTLL assay. Cultures giving counts in the CTLL assay greater than 3 standard deviations (SD) above the mean values obtained with medium alone were recorded as positive. Estimations of frequencies were calculated by applying the Poisson formula.
IFN-γ enzyme-linked immunospot (ELISpot) assay.
Nitrocellulose-bottomed 96-well plates (Millipore, Bedford, Mass.) were coated overnight at 4°C with rat anti-mouse gamma interferon (IFN-γ) (10 μg/ml; Pharmingen), washed five times with PBS, and blocked for 1 h at 37°C with RPMI 1640 medium supplemented with 10% fetal calf serum, l-glutamine (2 mM), 2-mercaptoethanol (55 μM), and penicillin (60 μg/ml) (complete medium). The APCs were naive splenocytes infected with MHV-68 (1 PFU/cell) for 1 h at 37°C. The control APCs were left untreated. All APCs were irradiated (3,000 rads), washed twice, and added (5 × 105/well) to duplicate threefold dilutions (104 to 105/well) of the responder lymphocytes. The samples were then incubated for 48 h in complete medium with recombinant IL-2 (10 U/ml) at 37°C in 5% CO2. Secreted cytokine was detected with biotinylated rat anti-mouse IFN-γ (2 μg/ml; Pharmingen) and streptavidin-alkaline phosphatase (DAKO, Carpinteria, Calif.). The plates were washed five times with PBS–0.05% Tween 20 after each incubation, and spots were visualized with 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium substrate (Sigma, St. Louis, Mo.) and counted microscopically. The mean number of spots with untreated APCs (1 to 2 spots/50,000 cells) was subtracted from the mean number of spots with MHV-68-infected APCs to give the number of virus-specific T cells. The results are expressed as reciprocal CD4+ T-cell frequencies.
In vitro depletion.
Lymphocte subsets were removed (8) by incubation with MAbs (Pharmingen) to CD8α (53.6.72), CD4 (GK1.5), or NK1.1-FITC (5E6) for 30 min on ice, followed by washing and depletion with sheep anti-rat and sheep anti-mouse Ig Dynabeads (Dynal) for 40 min at 4°C. The depleted cells were then incubated in the ELISpot assay, without adjusting the numbers for cell loss. Depleted cell populations were tested for purity by staining with anti-CD8β, anti-CD4 (RM4-4), or anti-NK1.1 (5E6). In general, <1% of the depleted subset remained.
RESULTS
Characterization by LDA.
The kinetics of the MHV-68-specific CD4+ T-cell response were analyzed by LDA prior to the development of the more sensitive and reproducible ELISpot assay (see below). Most of this information is not presented, but activation phenotypes and Thp frequencies are shown in Table 1 for several time points where the LDA worked well in the technical sense. The MHV-68-specific CD4+ T cells present from 4 to 19 months after infection were generally CD44high and CD69low, while the CD62L phenotype was more variable (Table 1). This pattern is comparable to the profiles determined previously by LDA for CD4+ memory T cells reactive to viruses that do not persist (5, 20).
TABLE 1.
Determination of Thp activation status by LDAa
| Organ | Phenotype | Reciprocal of Thp frequency
|
||
|---|---|---|---|---|
| 4 mo | 11 mo | 19 mo | ||
| Spleen | CD4+ | 745 | 70 | 581 |
| CD4+ CD44low | 6,348 | 2,875 | 10,703 | |
| CD4+ CD44Lhigh | 177 | 62 | 212 | |
| CD4+ CD62Llow | ND | 80 | 544 | |
| CD4+ CD62Lhigh | 562 | 70 | 2,975 | |
| CD4+ CD69low | 703 | 102 | 217 | |
| CD4+ CD69high | 1,192 | 344 | 2,306 | |
| CLN | CD4+ | 11,617 | 9,768 | 10,178 |
| MLN | CD4+ | 52,262 | 15,795 | 9,288 |
B6 mice were infected i.n. with 600 PFU of MHV-68, and sorted T-cell populations from pool of three to four animals were stimulated with virus-infected, irradiated syngeneic spleen cells for 4 days. ND, none done.
ELISpot assay.
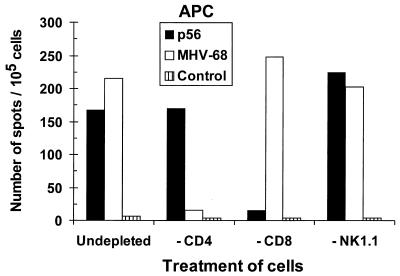
The IFN-γ ELISpot assay used here, in which exposes the lymphocytes are exposed for 48 h to APCs pulsed with whole MHV-68, has not been published previously as a method for determining Thp frequencies. Like the peptide stimulation ELISpot assay used to quantify CD8+ T cells, it is clearly more sensitive (data not shown) than previously developed LDA protocols (11, 20) that read out by cytotoxicity (CD8 after 6 days) or IL-2 production (CD4 after 4 days) (Table 1). However, stimulation with virus-infected cells also has the potential to activate the CD8+ set. Analyzing the response profiles for enriched CD4+ and CD8+ T-cell populations established that this was not a problem. The CD8+ T cells produce significant amounts of IFN-γ only following exposure to a high dose of the cognate peptide (Fig. 1, −CD4), while the CD4+ set responds to the virus-infected APCs (Fig. 1, −CD8). The response profiles to APCs pulsed with live or heat-inactivated MHV-68 were also compared. No significant differences were detected, and there was no evidence of any response when the immune cells were incubated with control lysates of uninfected OMK cells (see the legend to Fig. 1).
FIG. 1.
Consequences of depleting different responder cell types for the IFN-γ ELISpot analysis. B6 mice were primed i.n. with 600 PFU of MHV-68. Pooled spleen cells taken at day 12 after infection were depleted in vitro of the CD4, CD8, or NK1.1 subset, or left untreated, and incubated in the ELISpot assay with MHV-68-infected APCs or APCs pulsed with an H-2Db-restricted MHV-68 peptide (p56). The control APCs were uninfected. In a previous experiment, the numbers (per 105 cells) for APCs pulsed with different preparations were as follows: live MHV-68, 349; heat-inactivated MHV-68, 215; mock infected (OMK cell lysate), <4.0; and untreated, <2.0.
Acute phase of the CD4+ T-cell response.
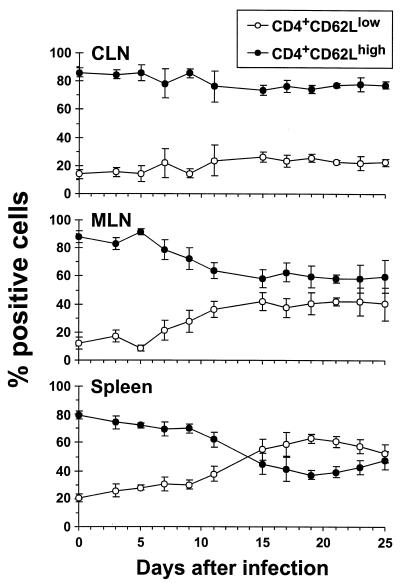
The numbers of activated (CD62Llow) CD4+ T cells increase progressively in the MLN and spleen following infection of B6 mice with MHV-68, reaching levels as high as 60% of the total CD4+ set in the spleen by day 19 after virus challenge (Fig. 2). In the past, it would have been considered highly unlikely that many of these CD62Llow CD4+ T cells could indeed be specific for the inducing virus. However, recent studies using tetrameric complexes of MHC class I glycoprotein plus peptide to quantitate the primary CD8+ T-cell response to lymphocytic choriomeningitis virus (LCMV) and the secondary response to influenza A viruses have shown that from 30 to 50% of the CD8+ T cells in the spleen can indeed be specific for the inducing pathogen (6, 12). Comparable numbers have been derived for the LCMV model by ELISpot analysis following in vitro stimulation with the appropriate MHC class I-restricted peptide (1).
FIG. 2.
Phenotypic analysis of the total CD4+ T-cell population. B6 mice were infected i.n. with 600 PFU of MHV-68, and CLN, MLN, and spleen cells from individual animals were stained with MAbs to CD4 and L-selectin (CD62L) for flow cytometric analysis. Data points represent means ± SD for four to five animals, expressed as percent CD4+ T cells.
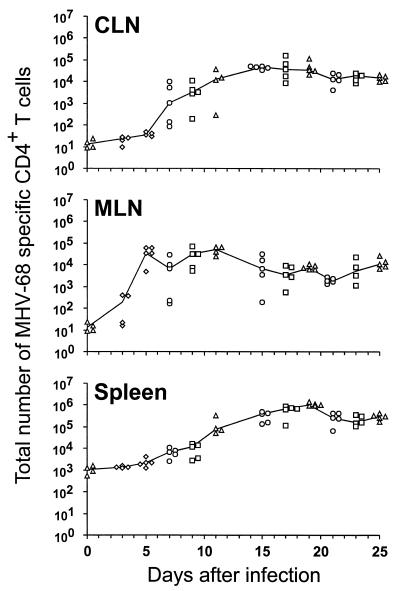
The spleen and lymph node CD4+ T-cell populations shown in Fig. 2 were analyzed for viral specificity by the ELISpot technique. Virus infected β2M−/− spleen cells were used as APCs to avoid any possibility that the assay was detecting CD8+ T cells, though the results in Fig. 1 indicate that this would be unlikely. Determining Thp frequencies for normal, uninfected mice established that the limit of detection of this assay is about 1:30,000 to 1:40,000 CD4+ T cells (Table 2). Calculating the MHV-68-specific Thp numbers (Fig. 3) from the frequencies determined by ELISpot (Table 2) and the CD4+ T-cell counts (Fig. 2) showed very clearly that the response could be detected first in the MLN (day 5) and then in the CLN (day 7). The Thp numbers remained fairly stable in the MLN from days 11 to 25 after infection (Fig. 3), with the frequencies being as high as 1:100 in the CD4+ set (Table 2). The counts in the CLN and spleen, however, tended to increase until days 17 to 19 (Fig. 3).
TABLE 2.
Thp frequency during acute MHV-68 infectiona
| Organ | Reciprocal of Thp frequency (mean ± SD for 4–5 animals)
|
|||
|---|---|---|---|---|
| Day 0 | Day 5 | Day 11 | Day 19 | |
| CLN | 34,498 ± 2,462 | 36,274 ± 2,543 | 1,193 ± 1,860 | 230 ± 113 |
| MLN | 29,345 ± 1,347 | 117 ± 105 | 107 ± 52 | 204 ± 111 |
| SPL | 21,530 ± 2,836 | 8,110 ± 3,144 | 327 ± 167 | 47 ± 7 |
B6 mice were infected i.n. with 600 PFU of MHV-68, and single-cell suspensions were stimulated in the ELISpot assay with virus-infected, irradiated β2M−/− spleen cells for 2 days.
FIG. 3.
Numbers of MHV-68-specific CD4+ T cells generated in the acute and persistent phases of the host response. B6 mice were infected i.n. with 600 PFU of MHV-68, and CLN, MLN, and spleen cells from individual animals were incubated for 48 h in the ELISpot assay. The numbers of MHV-68-specific CD4+ T cells in each organ were determined from the frequency, the percentage of CD4+ T cells, and total cell count. Each data point represents an individual mouse. The results were compiled from four experiments, indicated by the different symbols.
The highest numbers were found in the spleen in the long term (Fig. 3). This presumably reflects that the CD62Llow set does not traffic through the high endothelial venules to the lymph nodes, though there is no such CD62L-mediated gating requirement for T-cell entry to the spleen, leading to the concentration effect that is apparent in Fig. 2. The CD62Llow CD4+ T cells should, however, continue to reach the MLN and the CLN via afferent lymph. Comparison of the Thp frequencies and numbers (Table 2; Fig. 3) with the prevalence of CD62Llow CD4+ T cells (Fig. 2) indicated that most of this activated CD4+ set is not obviously specific for viral determinants that are expressed on MHV-68-infected APC populations. The very high prevalence of CD62Llow CD4+ T cells in the spleen from days 15 to 25 after infection (Fig. 2) may thus be a consequence of cytokine-induced bystander activation.
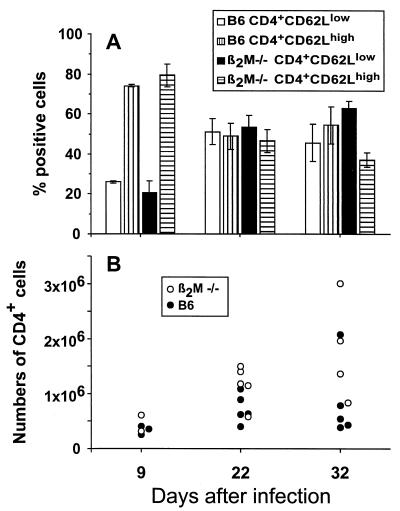
Mice with very low CD8+ T-cell numbers as a consequence of homozygous disruption of the β2M gene survive infection with MHV-68 (24). These β2M−/− mice were found to develop a CD4+ T-cell response that was generally comparable in character to that found for the normal β2M+/+ B6 controls (Fig. 4). The MHV-68-specific Thp numbers tended to be higher, although the difference was not statistically significant when tested with the Mann-Whitney test, in the β2M−/− group (Fig. 4), an observation in accord with other studies (15) showing that both the lytic (in the lung) and latent (in lymphoid tissue) virus loads tend to be greater in these CD8+ T-cell-deficient mice.
FIG. 4.
Comparison of CD4+ T-cell responses in β2M−/− and B6 (β2M+/+) mice. (A) The mice were infected i.n. with 600 PFU of MHV-68, and spleen cells from individual animals were analyzed by flow cytometry at different time points as described for Fig. 2. The histograms represent the means ± SD for two to five animals, expressed as percent CD4+ T cells. (B) The total number of MHV-68-specific CD4+ T cells was determined as described for Fig. 3. The data points represent individual animals.
Long-term CD4+ T-cell response.
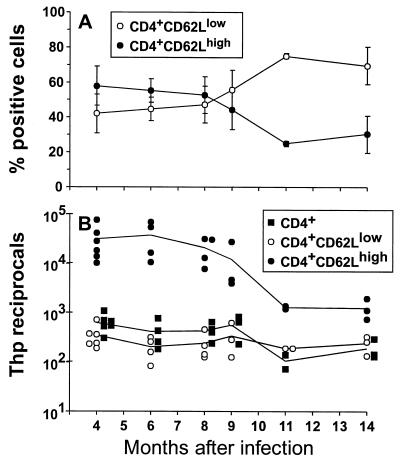
The long-term profiles for CD4+ Thp numbers determined by both ELISpot assay (Fig. 5) and LDA (Table 1) were remarkably constant from 4 to 14 months after infection. Furthermore, the great majority of the CD4+ T cells in MHV-68-infected animals retained the activated CD62Llow phenotype in the very long term (Fig. 5A), and the CD62Llow population was shown to contain the virus-specific CD4+ T cells (Fig. 5B). The frequency of the virus-specific component in the CD62Lhigh set did tend to increase with time (Fig. 5B). However, this could simply reflect enrichment, as the overall prevalence of all CD62Lhigh CD4+ T cells decreased from 8 months after infection (Fig. 5A) concomitant with thymic involution (14, 18, 19).
FIG. 5.
Characteristics of the long-term CD4+ T-cell response. (A) B6 mice were infected i.n. with 600 PFU of MHV-68, and the CD4+ T-cell phenotypes were determined for spleen cells taken at different times. Data points represent means ± SD for three to six animals, expressed as percent CD4+ cells. (B) The spleen cells were sorted and incubated for 72 h in the ELISpot assay. This incubation time is longer than that used in the acute experiments (Table 2; Fig. 3), as the time taken to develop substantial spots increases with age. Data points represent individual animals and are expressed as reciprocal frequencies.
DISCUSSION
The virus-specific CD4+ T-cell response has been analyzed previously in the long term by LDA following transient infection with LCMV (11) or by LDA and ELISpot assay following infection with Sendai virus (20). Neither virus was considered to persist, though there is some evidence that LCMV transcripts can be copied back into the genome (9). The numbers of Sendai virus-specific Thps tended to decrease until about 9 to 15 months after infection but then increased again progressively as the mice aged. This was thought to reflect the concentration of memory T cells as a consequence of the diminished output of naive precursors from the aging thymus. A similar effect was not, however, reported for the LCMV model (24). The Sendai virus-specific CD4+ T cells also tended to revert to the naive CD62Lhigh phenotype with time, an effect that has also been observed for the virus-specific CD8+ population (5, 20, 22).
The major difference between MHV-68 and Sendai virus is that the Thp frequencies remain consistently high and that the majority of the virus-specific CD4+ T cells do not progressively lose the activated CD62Llow phenotype. The profile for MHV-68 is thus what might be expected for a virus that persists in lymphoid tissue (13, 17) or non-B cells (16, 23, 25) and periodically reactivates to lytic phase, the situation that is thought to occur for this and other gammaherpesviruses. Even so, there is a minority component of the MHV-68-specific Thp population that does seem to be CD62Lhigh throughout the course of the response. Perhaps this naive CD62Lhigh set is comprised of low-affinity/avidity T cells.
The numbers of latently infected B lymphocytes that can be detected in spleen by the relatively insensitive infectious center assay does tend to decrease progressively with time in MHV-68-infected B6 mice (2). There is, however, always some evidence of persistence, especially in the bone marrow. What is intriguing is that the MHV-68-specific CD4+ Thp numbers remained stable and did not continue to increase in the long term. The argument that the general size of the memory CD8+ T-cell pool specific for lytic viruses (such as Sendai virus) that do not persist is determined by the magnitude of the clonal burst during the acute phase of the infection (8) also seems to be valid for the MHV-68-specific Thp response.
Though it is possible to point to the initial antigen load as a key factor determining the magnitude of the subsequent memory T-cell pool, the present findings do not help us to understand the homeostatic mechanisms that control the size of that pool (3). This continues to be a major intellectual challenge for cellular immunologists. What keeps the system in balance?
ACKNOWLEDGMENTS
We thank Vicki Henderson for assistance with the manuscript, Richard Cross and Anne-Marie Hamilton-Easton for help with the FACS analysis, Kris Branum for assistance with the ELISpot and LDA assays, and Rhonda Cardin and Mehdi Mehrpooya for preparing the virus stocks.
This work was supported by Public Health Service grants AI38359 and CA21765 and by the American Lebanese-Syrian Associated Charities. J.P.C. is the recipient of a fellowship from the Alfred Benzon Foundation, Denmark.
REFERENCES
- 1.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 4.Ehtisham S, Sunil-Chandra N P, Nash A A. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewing C, Topham D J, Doherty P C. Prevalence and activation phenotype of Sendai virus-specific CD4+ T cells. Virology. 1995;210:179–185. doi: 10.1006/viro.1995.1329. [DOI] [PubMed] [Google Scholar]
- 6.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 7.Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- 8.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 9.Klenerman P, Hengartner H, Zinkernagel R M. A non-retroviral RNA virus persists in DNA form. Nature. 1997;390:298–301. doi: 10.1038/36876. [DOI] [PubMed] [Google Scholar]
- 10.Kosco-Vilbois M H, Gray D, Scheidegger D, Julius M. Follicular dendritic cells help resting B cells to become effective antigen-presenting cells: induction of B7/BB1 and upregulation of major histocompatibility complex class II molecules. J Exp Med. 1993;178:2055–2066. doi: 10.1084/jem.178.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 12.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 13.Nash A A, Sunil-Chandra N P. Interactions of the murine gammaherpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 14.Scollay R G, Butcher E C, Weissman I L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson, P. G., R. D. Cardin, J. P. Christensen, and P. C. Doherty. Immunological control of a murine γ-herpesvirus independent of CD8+ T cells. J. Gen. Virol., in press. [DOI] [PubMed]
- 16.Stewart J P, Usherwood E J, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunil-Chandra N P, Efstathiou S, Nash A A. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 18.Takeoka R G, Chen S-Y, Yago H, Boyd R L, Suehiro S, Shultz L D, Ansari A A, Greshwin M E. The murine thymus microenvironment: changes with age. Int Arch Allergy Immunol. 1996;111:5–12. doi: 10.1159/000237337. [DOI] [PubMed] [Google Scholar]
- 19.Thoman M L. The pattern of T lymphocyte differentiation is altered during thymic involution. In: Thoman M L, editor. Mechanisms of aging and development. Dublin, Ireland: Elsevier Science Ireland Ltd.; 1995. pp. 155–170. [DOI] [PubMed] [Google Scholar]
- 20.Topham D J, Doherty P C. Longitudinal analysis of the acute Sendai virus-specific CD4+ T cell response and memory. J Immunol. 1998;161:4530–4535. [PubMed] [Google Scholar]
- 21.Topham D J, Tripp R A, Hamilton-Easton A M, Sarawar S R, Doherty P C. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157:2947–2952. [PubMed] [Google Scholar]
- 22.Tripp R A, Hou S, Doherty P C. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 23.Usherwood E J, Stewart J P, Robertson K, Allen D J, Nash A A. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J Gen Virol. 1996;77:2819–2825. doi: 10.1099/0022-1317-77-11-2819. [DOI] [PubMed] [Google Scholar]
- 24.Varga S M, Welsh R M. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J Immunol. 1998;161:367–374. [PubMed] [Google Scholar]
- 25.Weck K E, Barkon M L, Yoo L I, Speck S H, Virgin H W. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol. 1996;70:6775–6780. doi: 10.1128/jvi.70.10.6775-6780.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zijlstra M, Bix M, Simister N E, Loring J M, Raulet D H, Jaenisch R. Beta 2-microglobulin deficient mice lack CD4−8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]