Abstract
Continuous and reliable monitoring of blood pressure and cardiac function is of great importance for diagnosing and preventing cardiovascular diseases. However, existing cardiovascular monitoring approaches are bulky and costly, limiting their wide applications for early diagnosis. Here, we developed an intelligent blood pressure and cardiac function monitoring system based on a conformal and flexible strain sensor array and deep learning neural networks. The sensor has a variety of advantages, including high sensitivity, high linearity, fast response and recovery, and high isotropy. Experiments and simulation synergistically verified that the sensor array can acquire high-precise and feature-rich pulse waves from the wrist without precise positioning. By combining high-quality pulse waves with a well-trained deep learning model, we can monitor blood pressure and cardiac function parameters. As a proof of concept, we further constructed an intelligent wearable system for real-time and long-term monitoring of blood pressure and cardiac function, which may contribute to personalized health management, precise and early diagnosis, and remote treatment.
A wearable and intelligent cardiovascular monitoring system based on a deep-learning-assisted strain sensor array is developed.
INTRODUCTION
Cardiovascular diseases cause tens of millions of deaths yearly and are one of the major diseases worldwide that threaten peoples’ lives (1–6). The early monitoring, diagnosis, and timely intervention of cardiovascular diseases by measuring blood pressure and cardiac function parameters are essential to assess cardiovascular status, which can effectively reduce the risk of cardiovascular-related death and improve quality of life (7–13). Pulse wave is the physiological signal of the human body that directly reflects the cardiovascular status, which has been widely used as an indispensable technical means in diagnosing and treating diseases (14–19). The arterial pulse wave has multiple peaks and notches, including advancing wave, reflected wave, dicrotic notch, and dicrotic wave, which correspond to heart beating and blood reflection in the vessels (20–22). The traditional ways to monitor detailed heart and cardiac status rely on bulky and professional equipment such as cardiac ultrasound, electrocardiograph, and intravenous cannula (23–26) and require patients to go to the hospital, hindering timely diagnosis and precise treatment. With the development of modern society and the growth in personalized medical demand, portable and intelligent systems for real-time, precise, and long-term cardiovascular monitoring are highly desired (27–33).
In recent years, flexible electronics are developing rapidly and showing great potential in health management, medical care, and human-machine interaction (34–44). In particular, the vigorous development of artificial intelligence and machine learning has further promoted the capabilities and prospects of flexible and wearable electronics (45–52). With the assistance of artificial intelligence, in-depth analysis and data mining of physiological signals collected by wearable electronics can be realized, promoting the capability of flexible electronics in disease diagnosis and precise treatment. Various wearable sensors such as those based on photoplethysmography (PPG) (53, 54), resistive (55, 56), piezoelectric (57, 58), triboelectric (59–61), ultrasonic (62, 63), and bioimpedance (64) working mechanisms have been developed to continuously monitor pulse. Among them, resistive-type strain sensors have features of high sensitivity, high accuracy, easy data acquisition, no need for pre-pressure, and unperceivable attachment on skin surfaces (29, 65, 66), promising their practical applications. However, for most people without professional knowledge, especially patients with weak pulse waves, it is not easy to locate the precise position of the pulse to get accurate and detailed signals (62). Therefore, positioning difficulties and unavoidable slippage of the sensors on skin surfaces are substantial challenges for their practical applications. It is valuable to develop highly sensitive, easy to be operated, and intelligent wearable systems that can realize the precise and long-term monitoring of pulse and the real-time further analysis and evaluation of heart status and cardiac function.
Here, we developed a wearable, user-friendly, and intelligent health monitoring system by combining a highly sensitive strain sensor array and deep learning neural networks, which can monitor blood pressure and cardiac function without requiring precise positioning and professional knowledge. The sensor array contains six high-performance strain sensors, which have a collection of compelling parameters, including high linearity (coefficient of determination of 0.9996), high sensitivity [gauge factor (GF) of 9.81], fast response (40 ms) and recovery (80 ms), and high isotropy. Experiments and simulation synergistically verified that at least one sensor out of the six can acquire high-precise and feature-rich pulse waves as long as the array was placed near the artery on the wrist. On the basis of the wearable sensor array, we further developed a well-trained deep learning model and constructed an intelligent system for continuous blood pressure and cardiac status monitoring. The combination of the feature-rich pulse data and deep learning neural networks enables the prompt evaluation of cardiovascular function. The mean differences and standard deviations of the predicted systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean blood pressure (MBP) compared with the values collected with a medical continuous noninvasive arterial pressure (CNAP) system were 0.045 ± 3.235, 0.221 ± 3.101, and 0.162 ± 2.652 mmHg, respectively, indicating that this portable and wearable system has high precision and reliability. In addition, the obtained cardiac function parameters are also consistent with those measured with professional medical equipment. We constructed an integrated wearable system and demonstrated its applications in precise and real-time monitoring of blood pressure and cardiac status. We propose that the wearable and user-friendly integrated system may present a convenient, continuous, and insusceptible monitoring technique for blood pressure and cardiovascular status, thus contributing to the further development of wearable and intelligent devices for personal health management, precise diagnosis, and remote medical treatment.
RESULTS
Device design and working principle
Figure 1 shows the key concept of this work. The integrated wearable system for blood pressure and cardiac function monitoring consists of a data acquisition module, a data preprocessing module, and a deep learning analysis module. The data acquisition is realized with a sensor array, which contains six strain sensors named sensors A to F and placed on the wrist while working. Figure 1B shows the structure of the strain sensors. Carbonized silk georgette (CSG) with twisted warp and twisted weft yarns is used as the active layer. Laser-cutting nickel fabric with high conductivity serves as the electrodes, and ultrathin Ecoflex layers are used for encapsulation. The whole sensor array shows excellent flexibility, good biocompatibility, and high structural and chemical stability. Compared to carbonized silk with other structures, carbonized georgette has the advantages of thinness, isotropy, and high sensitivity to tensile strain, endowing it with the ability to detect tiny pulse (67). It can stably and conformally attach to the skin even when the wrist is bent or twisted with the assistance of a medical glue (fig. S1 and movie S1). The sensor array can be randomly placed near the radial artery on the wrist for acquiring high-quality pulse wave signals.
Fig. 1. Illustration showing the structure and working mechanism of the deep learning–assisted strain sensor array for monitoring blood pressure and cardiac function.
(A) Schematic and photograph of the monitoring system attached on the wrist. (B) Schematic showing the structure of the sensor array and images of the carbonized silk. (C) Schematic showing the deformation of the strain sensor induced by the radial artery pulse and its working mechanism. (D) Typical radial artery pulse of a healthy person, showing plenty of features, including heartbeat total time (TT), systolic upstroke time (UT), total time (TT), reflected wave transit time (RWTT), systolic-diastolic time (PPT), left ventricular ejection time (LVET), derivative-systolic upstroke time (DPT), amplitude of the advancing wave (HP), amplitude of the reflected wave (HT), and amplitude of the dicrotic wave (HD). (E) Architecture of the deep learning model. CNN, convolutional neural network.
As illustrated in Fig. 1C, the blood periodically flows under the heart’s drive, resulting in changes in the arterial diameter and deformation of the skin. The strain sensor can monitor pulse by converting skin deformation to resistance changes, which is different from PPG and ultrasonic technique in principle and has the advantages of high sensitivity, high accuracy, easy data acquisition, no need for pre-pressure, and simple device structure. Furthermore, the resistance changes collected by the strain sensors are converted into voltage changes through the resistance-to-voltage module based on Ohm’s law. Finally, the voltage changes are collected by the high-speed analog-to-digital converter (ADC) module and further transmitted to the data preprocessing module. Figure 1D shows a magnified typical arterial pulse waveform of a healthy person. Our sensor can acquire pulse waves with high precision and rich feature, from which plenty of features such as advancing wave, reflected notch, reflected wave, dicrotic notch, dicrotic wave, and their derivative features can be extracted. To efficiently analyze the obtained waveform, we further developed a deep learning algorithm based on a supervised convolution neural network, which consists of four convolutional layers and two fully connected layers, and thus realized automatic analysis on the blood pressure and cardiac function.
Performance of the strain sensor array
The strain sensor based on CSG has high sensitivity and good linearity in the strain range of 0 to 200%. Figure 2A shows a typical plot of the relative change in resistance versus strain of the CSG strain sensor, where R0 and ΔR represent the initial resistance and the resistance change, respectively. The sensor shows a GF of 8.81 with a high determination fitting coefficient of determination of 0.9996. The I-V curves show that the resistance of the strain sensor increases about 8.8 times monotonically as the sensor is stretched from the pristine state to 100% strain (fig. S2A). It is noted that the linearity of this sensor is superior to most reported strain sensors, endowing it with the ability to monitor pulse with high conformity in various usage scenarios with different pre-stretching states (65, 66). In addition to high sensitivity and high linearity, the sensor also has attractive features including good reliability, fast response (40 ms) and recovery time (80 ms), and high durability. When the strain sensor is loaded with cyclic tensile strain in the range of 1 to 100%, the sensor shows similar electrical responses, indicating its high reliability (fig. S2B). To evaluate the response time and the recovery time of the CSG strain sensor, we loaded and unloaded a small strain of 0.5% on the sensor. According to the resistance-time curve, the response time and recovery time were calculated to be 40 and 80 ms, respectively, which satisfies the need for pulse monitoring applications (fig. S2C). At the same time, the sensor also shows high stability and durability in long-term strain loading-unloading cycles (fig. S2D). It is noted that the elastic fabric electrode has a relatively stable resistance to mechanical deformation and its resistance increases by less than 1% of the whole system resistance even under 35% tensile strain (fig. S3), alleviating motion interference during practical applications. In addition, the sensors prepared in different patches using the same process showed consistent performance (fig. S4), facilitating its practical applications.
Fig. 2. Experimental electromechanical response and simulation results of the strain sensor array.
(A) Relative change in resistance of the strain sensor versus the applied strain, showing high linearity. The experiment was repeated three times independently with similar results. (B and C) Relative change in resistance of the strain sensor array while being pressed at different sites. The experiment was repeated three times independently with similar results. (D) Schematic illustration of the sensor array collecting pulse. (E) Deformation distribution in the active layer from the bottom view while the center of the array is pressed. (F) Deformation of different sensors while the center of the array is pressed.
On the basis of the CSG strain sensor, we constructed a strain sensor array with six strain sensors and evaluated its electromechanical performance. To ensure effective coverage of the radial artery, we selected sensors with effective working size of 0.4 cm × 0.4 cm, which is slightly wider than the diameter of human radial artery (fig. S5) (68). Six sensors (named A to F in counterclockwise order) were arranged into a rectangular array to strike a balance between high accuracy and low integration complexity. To simulate various situations in which the user randomly placed the sensor array near the radial artery on the wrist in practical applications, we pressed different sites of the sensor array with a finger and collected the electrical response of each sensor. The results show that the sensor closest to the pressed site produces the most pronounced electrical response (Fig. 2B). For example, when pressing site C, sensor C shows a relative change in resistance of 56%, while sensors A, B, D, E, and F show changes of 11%, 21%, 8%, 2%, and 11%, respectively (Fig. 2C). We obtained similar results when pressing other sites. All the experiments proved that at least one sensor in the array can show a satisfying response regardless of the pressed site (fig. S6).
The user placed the sensor array on the wrist to collect pulse signals, and at least one sensor in the array deforms with the sensor and heartbeat (Fig. 2D). To understand and validate the experimental observations, we simulated the deformation of the sensor array under a specific force. The model is simplified as a thin plate. The pulse is modeled as a constant upward force. When the radial artery was in the center of the sensor array, the sensors closest to the artery (sensors B and E) generated the most substantial deformation (Fig. 2, E and F). Other sensors distributed symmetrically from the artery show similar deformations to each other. We obtained similar results when the radial artery was located elsewhere relative to the sensor array (fig. S7). All the results indicate that no matter where the pressure is applied on the array, at least one sensor can produce a pronounced electrical response for obtaining high-quality pulse waves.
Monitoring of pulse and extraction of key features with the sensor array
Figure 3A shows the typical pulse waveform of the radial artery obtained with our strain sensor. Compared to pulse waves collected with traditional PPG (fig. S8), our obtained pulse waves show much higher precision and obviously richer features. For example, we can only distinguish advanced wave (P) and dicrotic wave (D) from the pulse wave collected by the PPG method. In contrast, from the pulse waves collected with our strain sensor, the P, reflected notch (W), reflected wave (T), dicrotic notch (V), and dicrotic wave (D) can be extracted. The strain sensor shows the ability to detect high-quality pulse signals with a radial artery depth of up to 5 mm (fig. S9), fulfilling requirement for most users (69). In addition, for users with thick subcutaneous fat layers and radial artery depth up to 7 mm, satisfactory pulse signals can be acquired by applying an additional pressure on the skin (fig. S10). The high-precise and feature-rich pulse wave provides the basis for the subsequent analysis and prediction of blood pressure and cardiovascular function. Further, plenty of derivative features, including systolic upstroke time (UT), total time (TT), reflected wave transit time (RWTT), systolic-diastolic time (PPT), left ventricular ejection time (LVET), derivative-systolic upstroke time (DPT), amplitude ratio of the reflected wave to advancing wave (HT/HP), amplitude ratio of the dicrotic wave to advancing wave (HD/HP), systolic time @ 25% of the pulse amplitude (Up_25), diastolic time @ 25% of the pulse amplitude, width @ 50% of the pulse amplitude (Width_50), and width @ 70% of the pulse amplitude (Width_70) can be calculated based on the above feature points (as shown in fig. S11 and table S1). Besides, we investigated the long-term reliability of the sensor array. Figure 3 (B and C) shows radial artery pulse waves collected before and after 30 days of service. After working for a long time, the sensor still shows good pulse tracking ability, endowing it with potential for reliable and practical applications. Because of its good resistance to motion interference, the sensor can effectively monitor pulse during light physical activities such as walking (fig. S12). This feature facilitates continuous monitoring of pulse in everyday scenarios.
Fig. 3. Performance of the strain sensor array in acquiring pulse waves and the extracted key features.
(A) Typical pulse waveform of radial artery collected by one strain sensor, showing high precision and rich features. (B and C) Radial artery pulse collected before (B) and after (C) 30 days of service, showing long-term stability. The experiment was repeated three times independently with similar results. (D) Pulse waves collected with the sensor array from different locations, indicating its pulse acquisition ability without precise positioning. (E) UT, TT, RWTT, and PPT from the pulse signal. (F) LVET, DPT, HT/HP (amplitude ratio of the reflected wave to advancing wave), and HD/HP (amplitude ratio of the dicrotic wave to advancing wave). (G) Extracted systolic time @ 25% of the pulse amplitude (Up_25), diastolic time @ 25% of the pulse amplitude (Down_25), width @ 50% of the pulse amplitude (Width_50), and width @ 70% of the pulse amplitude (Width_70).
Figure 3D shows the pulse waves collected at different locations near the radial artery on the wrist without precise positioning. Sensors B and C can acquire pulse signals when the sensor is placed at site I. When the sensor is placed at site II, sensors A and B collect high-quality pulse waves. Sensors D, E, and F achieve high-quality pulse monitoring when the sensor array is moved to sites III and IV. The results show that, wherever the sensor array is placed, high-quality pulse waves can be captured at least by one of the sensors, eliminating the requirement for precise positioning. By comparison, the intensity and quality of the acquired pulse signals substantially decrease when the sensor deviates from the ideal location of the radial artery (as shown in fig. S13). Figure 3 (E to G) shows key features extracted from the pulse waves with our self-developed algorithm. The successful extraction of these features further proves the high quality of the captured pulse waves with the sensor array. These features fluctuate with the pulse, which correspond to the subtle changes in cardiac status, enabling the real-time monitoring of cardiovascular status.
Monitoring of blood pressure and cardiac function with deep learning
Combining the high-precise and feature-rich pulse waves with a well-trained convolutional neural network, blood pressure and cardiac function can be monitored. We developed an intelligent blood pressure and cardiac function monitoring system consisting of pulse acquisition, preprocessing, and deep learning modules (Fig. 4A). The pulse acquisition module contains a strain sensor array, a signal converter, and a multichannel ADC, which play the roles of converting pulse-to-resistance, resistance-to-voltage, and voltage acquisition, respectively. The self-developed algorithms have various preprocessing functions, including denoise, debase, segmentation, and classification. High-quality without motion artifacts pulse signals for data extraction are screened by the preprocessing module for subsequent deep learning. The impact of variability caused by sensor performance deviations and interference in pulse acquisition can be effectively reduced by normalization. The deep learning module, which contains four convolutional layers, three max-pooling layers, and two fully connected layers, processes pulse data and calculates blood pressure and cardiac function.
Fig. 4. The mechanism to monitor blood pressure and cardiac function based on the pulse waves obtained by the sensor array and deep learning neural networks.
(A) Schematic illustration of the system, which contains pulse acquisition, preprocessing, and deep learning modules. ADC, analog-to-digital converter; BP, blood pressure; CFP, cardiac function parameters. (B) Violin plots of the predicted and measured diastolic blood pressure (DBP), systolic blood pressure (SBP), and mean blood pressure (MBP), indicating the high accuracy of the blood pressure monitoring system. The violin plots include box plots (defined as Q1 and Q3 quartiles, and median) with a kernel density estimation over the points. (C) Bland-Altman plot of the differences between the predicted systolic blood pressure using our system (SBP_Pre) and the measured systolic blood pressure using the CNAP system (SBP_Mea). (D) Bland-Altman plot of the differences between the predicted diastolic blood pressure using our system (DBP_Pre) and measured diastolic blood pressure using the CNAP system (DBP_Mea). (E to H) Violin plots of the predicted and measured cardiac function parameters, including PPV, CO, SV, and SVR. The violin plots include box plots (defined as Q1 and Q3 quartiles, and median) with a kernel density estimation over the points.
We collected pulse waves of three participants without precise positioning using our strain sensor array. At the same time, we used a medical CNAP system to collect the blood pressure and cardiac function parameters for comparison. After acquisition and preprocessing, we obtained about 6000 sets of pulse data and the corresponding blood pressure and cardiac function parameters. Eighty percent of the data were used to train the model, and the remaining 20% were used for performance evaluation.
The loss of the deep learning model for blood pressure monitoring decreases rapidly with training sessions and converges after tens of epochs (fig. S14). The violin plot (Fig. 4B) presents the DBP, SBP, MBP predicted with the trained neural network model, and the values collected by the CNAP system. The coefficient of determination (R2 score) of the results was calculated to be 0.87, which shows high agreement between the obtained blood pressure with our system and the CNAP system. In addition, to further investigate the agreement between the predicted blood pressure obtained from our neural network and the obtained blood pressure by the CNAP system, we performed a Bland-Altman analysis. The result reveals that the mean differences and standard deviations in SBP, DBP, and MBP between our results and the CNAP results were 0.045 ± 3.235, 0.221 ± 3.101, and 0.162 ± 2.652 mmHg (Fig. 4, C and D, and fig. S15), respectively, indicating that our deep learning–assisted strain sensor arrays can obtain high reliable blood pressures without precise positioning.
In addition, we trained and evaluated cardiac function parameters, including pulse pressure variation (PPV), cardiac output (CO), stroke volume (SV), and systemic vascular resistance (SVR), with the similar neural networks. The results are shown in Fig. 4 (E to H). The models used to evaluate cardiac function also converged rapidly with training sessions (fig. S16). The violin plots of the predicted cardiac function parameters are consistent with the results measured with the CNAP system. Besides, the prediction performance may be further improved through increasing the training data (70). These results prove the ability of our system for monitoring cardiac function.
Applications of the system in health monitoring
On the basis of strain sensor arrays and well-trained deep learning algorithms, we constructed an intelligent and user-friendly blood pressure and cardiac function parameter monitoring system (Fig. 5A). A user can wear the conformal flexible sensor array on the wrist and acquire high-quality pulse signals without precise positioning. Wireless multichannel ADC (Xiao ESP32S3) can be used to avoid the need for wired connections (figs. S17 and S18). The collected pulse signals can be transmitted to the intelligent terminal through Wi-Fi. Then, blood pressure and cardiac function parameters can be calculated through the deep learning model and the results can be displayed to the user. Until now, the data preprocessing and deep learning algorithms are only able to deeply analyze the pulse signals offline. In future endeavors, a further improved wearable system can be developed by constructing custom-designed flexible printed circuits. In addition, real-time analysis of pulse signals can be achieved through optimized algorithms and hardware platforms. Furthermore, in future scenarios, the intelligent terminal can upload these data to doctors through 5G or Wi-Fi and then the doctor may provide on-time analysis or instruction on the user’s cardiac state.
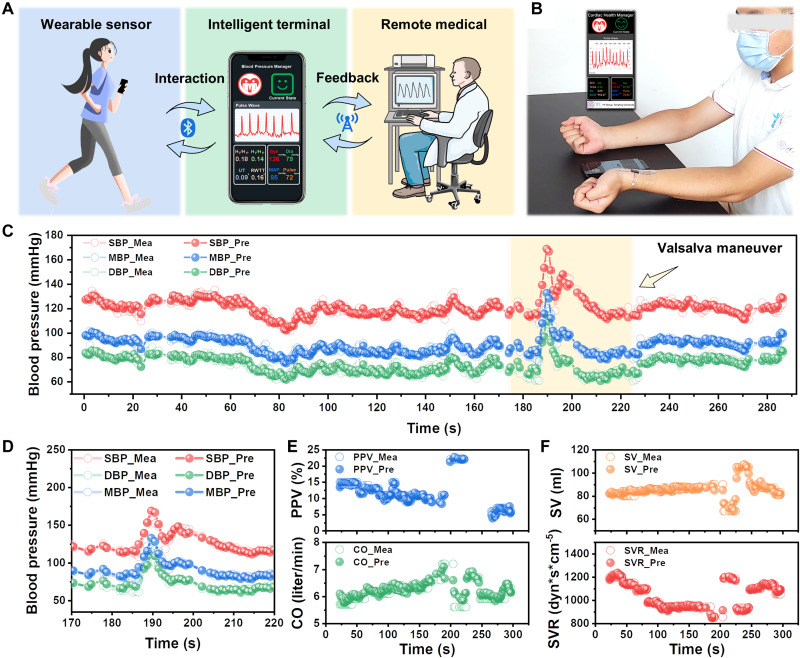
Fig. 5. Demonstration of the intelligent blood pressure and cardiac function monitoring system in practical application.
(A) Schematic diagram of the application scenario of the system. (B) Photograph of the health management system, which enabled real-time blood pressure and cardiac function monitoring using a mobile phone. (C) Predicted and measured blood pressure in a continuous 5-min test. (D) Predicted and measured blood pressure when the participant performed the Valsalva maneuver. (E and F) Predicted and measured cardiac function parameters in a continuous 5-min test, showing good consistency and indicating the reliable performance of the system.
We demonstrated the applications of the intelligent and user-friendly system in blood pressure monitoring and cardiac function evaluation (Fig. 5B). Participants were asked to perform the Valsalva maneuver during the test. The Valsalva maneuver is a method that can suddenly change the state of the heart and peripheral blood vessels within tens of seconds, which allows normal subjects to simulate the cardiac status of patients with hypertension and hypotension (71). The Valsalva maneuver happens in four phases, that is, the blood pumping of the heart and blood pressure increase, decrease, increase again, and finally return to normal.
Figure 5C shows the predicted blood pressure tracked measured blood pressure changes over a continuous 5-min test. Even when the subject’s cardiovascular state fluctuates dramatically over a short period, such as performing the Valsalva maneuver, the designed sensor array and deep learning model can effectively assess blood pressure (Fig. 5D). Besides, the predicted cardiac function parameters track the trend of measured values (Fig. 5, E and F), which is sufficient for most medical requirements. With the Valsalva maneuver, the PPV of the subjects increased notably and then returned to a lower level. CO and SV decreased first, then increased, and finally decreased, corresponding to the phases of the Valsalva maneuver, respectively. In contrast, SVR first increased, then decreased, and ultimately increased, which was consistent with the actual effect of the Valsalva maneuver. These results indicate that the health management system is a user-friendly, intelligent, reliable system for on-time and precise evolution of blood pressure and cardiovascular function, holding great potential for personal health management, early diagnosis, and remote medical treatment.
DISCUSSION
To conclude, we developed an intelligent and user-friendly blood pressure and cardiac function monitoring system based on a flexible highly sensitive strain sensor array and deep learning neural networks, which can be randomly placed near the artery on the wrist for data acquisition. The sensor array contains six high-performance strain sensors distributed in a rectangular area, and each of the sensor has high linearity (coefficient of determination of 0.9996), high sensitivity (GF of 9.81), fast response (40 ms) and recovery time (80 ms), and high isotropy. Both experimental and simulation results verified that at least one sensor in the array can obtain high-precise and feature-rich pulse waves wherever the sensor array is placed. From the high-quality pulse waves, we can extract plenty of features, including P, W, T, V, D, and their derivative features. In addition, we developed intelligent preprocessing algorithms and a deep learning model and realized the automatic extraction of critical features and output of blood pressure and cardiac function parameters. Remarkably, the mean differences and standard deviations in SBP, DBP, and MBP determinations between the predicted and measured were 0.045 ± 3.235, 0.221 ± 3.101, and 0.162 ± 2.652 mmHg, respectively. At the same time, cardiac function parameters can also be precisely tracked. As a proof of concept, we combined the sensor array with the deep learning model and constructed a user-friendly wearable system for on-time and precise monitoring of blood pressure and cardiac status. We believe that this work can contribute to the further development of technique for precise health monitoring, early diagnosis, and remote medical treatment.
MATERIALS AND METHODS
Fabrication of the strain sensor and strain sensor array
The silk georgettes (Yunling Silk Co. Ltd.) were carbonized under a mixed atmosphere with argon (purity, 99.999%; gas flow, 200 sccm) and hydrogen (purity, 99.999%; gas flow, 20 sccm) in a tube furnace. The heat treatment schedule is divided into seven stages: (i) heat from ambient temperature to 150°C at the rate of 10°C min−1; (ii) keep at 150°C for 60 min; (iii) heat from 150° to 350°C at the rate of 5°C min−1; (iv) keep at 350°C for 180 min; (v) heat from 350° to 950°C at the rate of 3°C min−1; (vi) keep at 950°C for 120 min; (vii) naturally cool the system to ambient temperature.
The obtained CSG was cut into rectangular shapes and then connected to nickel fabric (Dongguan Xinshengyuan Nano Material Co.) electrodes with silver paste. The assembled sensors were encapsulated with Ecoflex (Ecoflex 00-50, Smooth-On Inc.). The thickness of the sensor is about 300 μm.
Characterization of the strain sensor and strain sensor array
The morphologies of silk and carbonized silk were characterized with a scanning electron microscope (Zeiss, GeminiSEM 500). The electromechanical performance of the strain sensor was collected using a digital meter (Keithley 2400) and a mechanical testing machine (Shimadzu, AGS-X). The electromechanical performance of the strain sensor array was collected using multichannel data acquisition system (Smacq, USB-3121). Each experiment was repeated for three times independently and produced similar results.
Finite element analysis
The induced deformation of the strain sensor array under the pulse stimulus was simulated based on Kirchhoff thin plate theory using MATLAB software. The pulse stimulus was simplified as a constant force. The strain sensor array was simplified as a thin plate model. Constant forces at different sites were loaded to the sensor array, and the corresponding induced deformations in the sensor array were calculated. The MATLAB program was uploaded to https://doi.org/10.5281/zenodo.8000626.
Pulse monitoring and collection of blood pressure and cardiac function parameters
A commercial medical glue (Liquid Band-aid, Zhende Medical Co. Ltd.) was brushed onto the skin, and then a strain sensor array was put around the radial artery before the glue was fully cured. The sensors were connected to a self-made resistance-to-voltage module via wires. To collect the pulse data in the laboratory, the sensors were connected to a self-made resistance-to-voltage module via wires, and then the data were collected and converted into voltage changes. A multichannel data acquisition system (Smacq, USB-3121) was used to collect the voltage changes, which were transmitted to a computer via cables. At the same time, the blood pressure and cardiac function parameters of the participant were collected through a continuous noninvasive blood pressure measurement system (CNSystems Medizintechnik GmbH).
Wireless pulse acquisition and transmission
A strain sensor array was put around the radial artery and attached to the skin without precise positioning. The sensors were connected to a self-made resistance-to-voltage module via wires. A wireless multichannel ADC (Xiao ESP32S3) was used to collect the voltage changes, which were transmitted to a cloud database (www.bemfa.com) via Wi-Fi. Subsequently, the pulse signals were transmitted to a computer via Wi-Fi for subsequent in-depth analysis.
Blood pressure and cardiac function parameter monitoring
The self-developed MATLAB program preprocessed the pulse signals collected by the strain sensor array. The pulse signal was denoised and debased by the wavelet filter (sym8), and then the pulse was segmented according to the pulse cycle. The pulse data were classified and filtered according to the standard, which can easily extract features. Further, the pulse data are normalized to reduce the influence of potential variability. The preprocessed data were fed into a deep learning model for blood pressure and cardiac function monitoring. The deep learning model contains four convolutional layers, three max-pooling layers, and two fully connected layers. Eighty percent of the data were used to train the deep learning model, and the remaining 20% were used for performance evaluation. Then, the evaluated blood pressure and cardiac function parameters were transmitted to a custom-designed app via Wi-Fi for health management.
Ethics declarations
Consent for the publication of identifiable images of research participants to publish was obtained. The data were obtained with the informed consent of all participants. The Institutional Review Board of Tsinghua University approved this study (no. 20220118).
Acknowledgments
We thank T. Yi, Z. Wang, and H. Weng from Peking University First Hospital for helpful discussion. Parts of Figs. 1 (A and C), 2D, 4A, and 5A were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Funding: This work was supported by National Natural Science Foundation of China 52125201 (Yi. Zhang), National Natural Science Foundation of China 21975141 (Yi. Zhang), Beijing Municipal Commission of Science and Technology Z22110000272 (Yi. Zhang), and National Key Basic Research and Development Program 2020YFA0210702 (Yi. Zhang).
Author contributions: Conceptualization: Yi. Zhang and S.L. Methodology: S.L., H.W., Yi. Zhang, K.X., and Yo. Zhang. Investigation: S.L., H.W., Yi. Zhang, W.M., L.Q., Yo. Zhang, H.L., M.Z., X.L., X.-E.W., and H.L. Visualization: S.L. and H.W. Funding acquisition: Yi. Zhang. Project administration: Yi. Zhang. Supervision: Yi. Zhang. Writing—original draft: S.L. Writing—review and editing: Yi. Zhang, S.L., W.M., and L.Q.
Competing interests: Yi. Zhang and S.L. are inventors on patent application (application number: CN 202310563126.9, application date: 19 May 2023, current state: under review) submitted by Tsinghua University that covers the blood pressure and cardiac function monitoring system. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The source codes for pulse signal preprocess and deep learning, and codes for the blood pressure and cardiac function monitoring system used in this study are available at Zenodo database: https://doi.org/10.5281/zenodo.8000626.
Supplementary Materials
This PDF file includes:
Figs. S1 to S18
Table S1
Legend for movie S1
Other Supplementary Material for this manuscript includes the following:
Movie S1
REFERENCES AND NOTES
- 1.E. J. Benjamin, P. Muntner, A. Alonso, M. S. Bittencourt, C. W. Callaway, A. P. Carson, A. M. Chamberlain, A. R. Chang, S. Cheng, S. R. Das, F. N. Delling, L. Djousse, M. S. V. Elkind, J. F. Ferguson, M. Fornage, L. C. Jordan, S. S. Khan, B. M. Kissela, K. L. Knutson, T. W. Kwan, D. T. Lackland, T. T. Lewis, J. H. Lichtman, C. T. Longenecker, M. S. Loop, P. L. Lutsey, S. S. Martin, K. Matsushita, A. E. Moran, M. E. Mussolino, M. O’Flaherty, A. Pandey, A. M. Perak, W. D. Rosamond, G. A. Roth, U. K. A. Sampson, G. M. Satou, E. B. Schroeder, S. H. Shah, N. L. Spartano, A. Stokes, D. L. Tirschwell, C. W. Tsao, M. P. Turakhia, L. B. VanWagner, J. T. Wilkins, S. S. Wong, S. S. Virani; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee , Heart disease and stroke statistics—2019 Update: A report from the american heart association. Circulation 139, e56–e528 (2019). [DOI] [PubMed] [Google Scholar]
- 2.K. Whelton Paul, M. Carey Robert, S. Aronow Wilbert, E. Casey Donald, J. Collins Karen, C. Dennison Himmelfarb, M. DePalma Sondra, S. Gidding, A. Jamerson Kenneth, W. Jones Daniel, J. MacLaughlin Eric, P. Muntner, B. Ovbiagele, C. Smith Sidney, C. Spencer Crystal, S. Stafford Randall, J. Taler Sandra, J. Thomas Randal, A. Williams Kim Sr., D. Williamson Jeff, T. Wright Jackson Jr., 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J. Am. Coll. Cardiol. 71, e127–e248 (2018). [DOI] [PubMed] [Google Scholar]
- 3.A. V. Chobanian, G. L. Bakris, H. R. Black, W. C. Cushman, L. A. Green, J. J. L. Izzo Jr., D. W. Jones, B. J. Materson, S. Oparil, J. J. T. Wright, E. J. Roccella; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee , The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 289, 2560–2572 (2003). [DOI] [PubMed] [Google Scholar]
- 4.G. Mancia, R. Fagard, K. Narkiewicz, J. Redón, A. Zanchetti, M. Böhm, T. Christiaens, R. Cifkova, G. De Backer, A. Dominiczak, M. Galderisi, D. E. Grobbee, T. Jaarsma, P. Kirchhof, S. E. Kjeldsen, S. Laurent, A. J. Manolis, P. M. Nilsson, L. M. Ruilope, R. E. Schmieder, P. A. Sirnes, P. Sleight, M. Viigimaa, B. Waeber, F. Zannad; Task force members , 2013 ESH/ESC Guidelines for the management of arterial hypertension. J. Hypertens. 31, 1281–1357 (2013). [DOI] [PubMed] [Google Scholar]
- 5.A. Pelliccia, S. Sharma, S. Gati, M. Back, M. Borjesson, S. Caselli, J. P. Collet, D. Corrado, J. A. Drezner, M. Halle, D. Hansen, H. Heidbuchel, J. Myers, J. Niebauer, M. Papadakis, M. F. Piepoli, E. Prescott, J. W. Roos-Hesselink, A. G. Stuart, R. Taylor, P. D. Thompson, M. Tiberi, L. Vanhees, M. Wilhelm; ESC Scientific Document Group , 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 42, 17–96 (2021). [DOI] [PubMed] [Google Scholar]
- 6.F. Cosentino, P. J. Grant, V. Aboyans, C. J. Bailey, A. Ceriello, V. Delgado, M. Federici, G. Filippatos, D. E. Grobbee, T. B. Hansen, H. V. Huikuri, I. Johansson, P. Jüni, M. Lettino, N. Marx, L. G. Mellbin, C. J. Östgren, B. Rocca, M. Roffi, N. Sattar, P. M. Seferović, M. Sousa-Uva, P. Valensi, D. C. Wheeler; ESC Scientific Document Group , 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 41, 255–323 (2020). [DOI] [PubMed] [Google Scholar]
- 7.A. P. Avolio, M. Butlin, A. Walsh, Arterial blood pressure measurement and pulse wave analysis—Their role in enhancing cardiovascular assessment. Physiol. Meas. 31, R1–R47 (2010). [DOI] [PubMed] [Google Scholar]
- 8.M. Carey Robert, P. Muntner, B. Bosworth Hayden, K. Whelton Paul, Prevention and control of hypertension. J. Am. Coll. Cardiol. 72, 1278–1293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.K. Kario, Management of hypertension in the digital era. Hypertension 76, 640–650 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.K. Kario, I. Saito, T. Kushiro, S. Teramukai, Y. Tomono, Y. Okuda, K. Shimada, Morning home blood pressure is a strong predictor of coronary artery disease. J. Am. Coll. Cardiol. 67, 1519–1527 (2016). [DOI] [PubMed] [Google Scholar]
- 11.G. J. Langewouters, J. J. Settels, R. Roelandt, K. H. Wesseling, Why use Finapres or Portapres rather than intraarterial or intermittent non-invasive techniques of blood pressure measurement? J. Med. Eng. Technol. 22, 37–43 (1998). [DOI] [PubMed] [Google Scholar]
- 12.A. L. Siu, Screening for high blood pressure in adults: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 163, 778–786 (2015). [DOI] [PubMed] [Google Scholar]
- 13.A. C. Flint, C. Conell, X. Ren, N. M. Banki, S. L. Chan, V. A. Rao, R. B. Melles, D. L. Bhatt, Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N. Engl. J. Med. 381, 243–251 (2019). [DOI] [PubMed] [Google Scholar]
- 14.M. Jiang, C. Lu, C. Zhang, J. Yang, Y. Tan, A. P. Lu, K. Chan, Syndrome differentiation in modern research of traditional Chinese medicine. J. Ethnopharmacol. 140, 634–642 (2012). [DOI] [PubMed] [Google Scholar]
- 15.J. C. Dong, The relationship between traditional chinese medicine and modern medicine. Evid. Based Complement. Alternat. Med. 2013, 153148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.S. Magder, The meaning of blood pressure. Crit. Care 22, 257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.E. Agabiti-Rosei, G. Mancia, M. F. O’Rourke, M. J. Roman, M. E. Safar, H. Smulyan, J.-G. Wang, I. B. Wilkinson, B. Williams, C. Vlachopoulos, Central blood pressure measurements and antihypertensive therapy. Hypertension 50, 154–160 (2007). [DOI] [PubMed] [Google Scholar]
- 18.J. R. Jennings, M. F. Muldoon, B. Allen, A. T. Ginty, P. J. Gianaros, Cerebrovascular function in hypertension: Does high blood pressure make you old? Psychophysiology 58, e13654 (2021). [DOI] [PubMed] [Google Scholar]
- 19.B. H. McGhee, E. J. Bridges, Monitoring arterial blood pressure: What you may not know. Crit. Care Nurse 22, 60–79 (2002). [PubMed] [Google Scholar]
- 20.J. Allen, Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 28, R1–R39 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Reference Values for Arterial Stiffness' Collaboration , Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J. 31, 2338–2350 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.M. F. O'Rourke, A. Pauca, X.-J. Jiang, Pulse wave analysis. Br. J. Clin. Pharmacol. 51, 507–522 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.K. Bartels, S. A. Esper, R. H. Thiele, Blood pressure monitoring for the Anesthesiologist. Anesth. Analg. 122, 1866–1879 (2016). [DOI] [PubMed] [Google Scholar]
- 24.A. E. Ulloa Cerna, L. Jing, C. W. Good, D. P. vanMaanen, S. Raghunath, J. D. Suever, C. D. Nevius, G. J. Wehner, D. N. Hartzel, J. B. Leader, A. Alsaid, A. A. Patel, H. L. Kirchner, J. M. Pfeifer, B. J. Carry, M. S. Pattichis, C. M. Haggerty, B. K. Fornwalt, Deep-learning-assisted analysis of echocardiographic videos improves predictions of all-cause mortality. Nat. Biomed. Eng. 5, 546–554 (2021). [DOI] [PubMed] [Google Scholar]
- 25.S. R. Ommen, S. Mital, M. A. Burke, S. M. Day, A. Deswal, P. Elliott, L. L. Evanovich, J. Hung, J. A. Joglar, P. Kantor, C. Kimmelstiel, M. Kittleson, M. S. Link, M. S. Maron, M. W. Martinez, C. Y. Miyake, H. V. Schaff, C. Semsarian, P. Sorajja, 2020 AHA/ACC Guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 142, e558–e631 (2020). [DOI] [PubMed] [Google Scholar]
- 26.J. Knuuti, W. Wijns, A. Saraste, D. Capodanno, E. Barbato, C. Funck-Brentano, E. Prescott, R. F. Storey, C. Deaton, T. Cuisset, S. Agewall, K. Dickstein, T. Edvardsen, J. Escaned, B. J. Gersh, P. Svitil, M. Gilard, D. Hasdai, R. Hatala, F. Mahfoud, J. Masip, C. Muneretto, M. Valgimigli, S. Achenbach, J. J. Bax; ESC Scientific Document Group , 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2020). [DOI] [PubMed] [Google Scholar]
- 27.H. Hu, H. Huang, M. Li, X. Gao, L. Yin, R. Qi, R. S. Wu, X. Chen, Y. Ma, K. Shi, C. Li, T. M. Maus, B. Huang, C. Lu, M. Lin, S. Zhou, Z. Lou, Y. Gu, Y. Chen, Y. Lei, X. Wang, R. Wang, W. Yue, X. Yang, Y. Bian, J. Mu, G. Park, S. Xiang, S. Cai, P. W. Corey, J. Wang, S. Xu, A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Y. Kwon, P. L. Stafford, D. C. Lim, S. Park, S.-H. Kim, R. B. Berry, D. A. Calhoun, Blood pressure monitoring in sleep: Time to wake up. Blood Press. Monit. 25, 61–68 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.K. Meng, X. Xiao, W. Wei, G. Chen, A. Nashalian, S. Shen, X. Xiao, J. Chen, Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 34, 2109357 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Z. Xiang, M. Han, H. Zhang, Nanomaterials based flexible devices for monitoring and treatment of cardiovascular diseases (CVDs). Nano Res. 16, 3939–3955 (2023). [Google Scholar]
- 31.K. Sim, F. Ershad, Y. Zhang, P. Yang, H. Shim, Z. Rao, Y. Lu, A. Thukral, A. Elgalad, Y. Xi, B. Tian, D. A. Taylor, C. Yu, An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity. Nat. Electron. 3, 775–784 (2020). [Google Scholar]
- 32.Y.-S. Guan, F. Ershad, Z. Rao, Z. Ke, E. C. da Costa, Q. Xiang, Y. Lu, X. Wang, J. Mei, P. Vanderslice, C. Hochman-Mendez, C. Yu, Elastic electronics based on micromesh-structured rubbery semiconductor films. Nat. Electron. 5, 881–892 (2022). [Google Scholar]
- 33.W. Yan, G. Noel, G. Loke, E. Meiklejohn, T. Khudiyev, J. Marion, G. Rui, J. Lin, J. Cherston, A. Sahasrabudhe, J. Wilbert, I. Wicaksono, R. W. Hoyt, A. Missakian, L. Zhu, C. Ma, J. Joannopoulos, Y. Fink, Single fibre enables acoustic fabrics via nanometre-scale vibrations. Nature 603, 616–623 (2022). [DOI] [PubMed] [Google Scholar]
- 34.H. U. Chung, B. H. Kim, J. Y. Lee, J. Lee, Z. Xie, E. M. Ibler, K. Lee, A. Banks, J. Y. Jeong, J. Kim, C. Ogle, D. Grande, Y. Yu, H. Jang, P. Assem, D. Ryu, J. W. Kwak, M. Namkoong, J. B. Park, Y. Lee, D. H. Kim, A. Ryu, J. Jeong, K. You, B. Ji, Z. Liu, Q. Huo, X. Feng, Y. Deng, Y. Xu, K.-I. Jang, J. Kim, Y. Zhang, R. Ghaffari, C. M. Rand, M. Schau, A. Hamvas, D. E. Weese-Mayer, Y. Huang, S. M. Lee, C. H. Lee, N. R. Shanbhag, A. S. Paller, S. Xu, J. A. Rogers, Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363, eaau0780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.X. Shi, Y. Zuo, P. Zhai, J. Shen, Y. Yang, Z. Gao, M. Liao, J. Wu, J. Wang, X. Xu, Q. Tong, B. Zhang, B. Wang, X. Sun, L. Zhang, Q. Pei, D. Jin, P. Chen, H. Peng, Large-area display textiles integrated with functional systems. Nature 591, 240–245 (2021). [DOI] [PubMed] [Google Scholar]
- 36.S. Lee, S. Franklin, F. A. Hassani, T. Yokota, M. O. G. Nayeem, Y. Wang, R. Leib, G. Cheng, D. W. Franklin, T. Someya, Nanomesh pressure sensor for monitoring finger manipulation without sensory interference. Science 370, 966–970 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Y. Jiang, Z. Zhang, Y.-X. Wang, D. Li, C.-T. Coen, E. Hwaun, G. Chen, H.-C. Wu, D. Zhong, S. Niu, W. Wang, A. Saberi, J.-C. Lai, Y. Wu, Y. Wang, A. A. Trotsyuk, K. Y. Loh, C.-C. Shih, W. Xu, K. Liang, K. Zhang, Y. Bai, G. Gurusankar, W. Hu, W. Jia, Z. Cheng, R. H. Dauskardt, G. C. Gurtner, J. B. H. Tok, K. Deisseroth, I. Soltesz, Z. Bao, Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics. Science 375, 1411–1417 (2022). [DOI] [PubMed] [Google Scholar]
- 38.S. Li, Y. Zhang, X. Liang, H. Wang, H. Lu, M. Zhu, H. Wang, M. Zhang, X. Qiu, Y. Song, Y. Zhang, Humidity-sensitive chemoelectric flexible sensors based on metal-air redox reaction for health management. Nat. Commun. 13, 5416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.H. Wang, Y. Zhang, X. Liang, Y. Zhang, Smart fibers and textiles for personal health management. ACS Nano 15, 12497–12508 (2021). [DOI] [PubMed] [Google Scholar]
- 40.X. Liang, H. Li, J. Dou, Q. Wang, W. He, C. Wang, D. Li, J.-M. Lin, Y. Zhang, Stable and biocompatible carbon nanotube ink mediated by silk protein for printed electronics. Adv. Mater. 32, 2000165 (2020). [DOI] [PubMed] [Google Scholar]
- 41.H. Wang, S. Li, Y. Wang, H. Wang, X. Shen, M. Zhang, H. Lu, M. He, Y. Zhang, Bioinspired fluffy fabric with in situ grown carbon nanotubes for ultrasensitive wearable airflow sensor. Adv. Mater. 32, e1908214 (2020). [DOI] [PubMed] [Google Scholar]
- 42.W. He, C. Wang, H. Wang, M. Jian, W. Lu, X. Liang, X. Zhang, F. Yang, Y. Zhang, Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 5, eaax0649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.W. Wang, A. Yu, J. Zhai, Z. L. Wang, Recent progress of functional fiber and textile triboelectric nanogenerators: Towards electricity power generation and intelligent sensing. Adv. Fiber Mater. 3, 394–412 (2021). [Google Scholar]
- 44.J. R. Sempionatto, J. A. Lasalde-Ramírez, K. Mahato, J. Wang, W. Gao, Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.S. Sundaram, P. Kellnhofer, Y. Li, J.-Y. Zhu, A. Torralba, W. Matusik, Learning the signatures of the human grasp using a scalable tactile glove. Nature 569, 698–702 (2019). [DOI] [PubMed] [Google Scholar]
- 46.O. A. Araromi, M. A. Graule, K. L. Dorsey, S. Castellanos, J. R. Foster, W.-H. Hsu, A. E. Passy, J. J. Vlassak, J. C. Weaver, C. J. Walsh, R. J. Wood, Ultra-sensitive and resilient compliant strain gauges for soft machines. Nature 587, 219–224 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Z. Zhou, K. Chen, X. Li, S. Zhang, Y. Wu, Y. Zhou, K. Meng, C. Sun, Q. He, W. Fan, E. Fan, Z. Lin, X. Tan, W. Deng, J. Yang, J. Chen, Sign-to-speech translation using machine-learning-assisted stretchable sensor arrays. Nat. Electron. 3, 571–578 (2020). [Google Scholar]
- 48.A. Moin, A. Zhou, A. Rahimi, A. Menon, S. Benatti, G. Alexandrov, S. Tamakloe, J. Ting, N. Yamamoto, Y. Khan, F. Burghardt, L. Benini, A. C. Arias, J. M. Rabaey, A wearable biosensing system with in-sensor adaptive machine learning for hand gesture recognition. Nat. Electron. 4, 54–63 (2021). [Google Scholar]
- 49.Y. Luo, Y. Li, P. Sharma, W. Shou, K. Wu, M. Foshey, B. Li, T. Palacios, A. Torralba, W. Matusik, Learning human–environment interactions using conformal tactile textiles. Nat. Electron. 4, 193–201 (2021). [Google Scholar]
- 50.M. Wang, Z. Yan, T. Wang, P. Cai, S. Gao, Y. Zeng, C. Wan, H. Wang, L. Pan, J. Yu, S. Pan, K. He, J. Lu, X. Chen, Gesture recognition using a bioinspired learning architecture that integrates visual data with somatosensory data from stretchable sensors. Nat. Electron. 3, 563–570 (2020). [Google Scholar]
- 51.M. Zhu, Z. Sun, Z. Zhang, Q. Shi, T. He, H. Liu, T. Chen, C. Lee, Haptic-feedback smart glove as a creative human-machine interface (HMI) for virtual/augmented reality applications. Sci. Adv. 6, eaaz8693 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.W. Lin, D. Zhang, W. W. Lee, X. Li, Y. Hong, Q. Pan, R. Zhang, G. Peng, H. Z. Tan, Z. Zhang, L. Wei, Z. Yang, Super-resolution wearable electrotactile rendering system. Sci. Adv. 8, eabp8738 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.H. Li, Y. Ma, Z. Liang, Z. Wang, Y. Cao, Y. Xu, H. Zhou, B. Lu, Y. Chen, Z. Han, S. Cai, X. Feng, Wearable skin-like optoelectronic systems with suppression of motion artifacts for cuff-less continuous blood pressure monitor. Natl. Sci. Rev. 7, 849–862 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.G. H. Lee, H. Kang, J. W. Chung, Y. Lee, H. Yoo, S. Jeong, H. Cho, J.-Y. Kim, S.-G. Kang, J. Y. Jung, S. G. Hahm, J. Lee, I.-J. Jeong, M. Park, G. Park, I. H. Yun, J. Y. Kim, Y. Hong, Y. Yun, S.-H. Kim, B. K. Choi, Stretchable PPG sensor with light polarization for physical activity–permissible monitoring. Sci. Adv. 8, eabm3622 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.J. Chen, J. Zhang, J. Hu, N. Luo, F. Sun, H. Venkatesan, N. Zhao, Y. Zhang, Ultrafast-response/recovery flexible piezoresistive sensors with DNA-Like double helix yarns for epidermal pulse monitoring. Adv. Mater. 34, e2104313 (2022). [DOI] [PubMed] [Google Scholar]
- 56.X. Shi, X. Fan, Y. Zhu, Y. Liu, P. Wu, R. Jiang, B. Wu, H.-A. Wu, H. Zheng, J. Wang, X. Ji, Y. Chen, J. Liang, Pushing detectability and sensitivity for subtle force to new limits with shrinkable nanochannel structured aerogel. Nat. Commun. 13, 1119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.C. Dagdeviren, Y. Su, P. Joe, R. Yona, Y. Liu, Y.-S. Kim, Y. Huang, A. R. Damadoran, J. Xia, L. W. Martin, Y. Huang, J. A. Rogers, Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 5, 4496 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Z. Yi, Z. Liu, W. Li, T. Ruan, X. Chen, J. Liu, B. Yang, W. Zhang, Piezoelectric dynamics of arterial pulse for wearable continuous blood pressure monitoring. Adv. Mater. 34, 2110291 (2022). [DOI] [PubMed] [Google Scholar]
- 59.W. Fan, Q. He, K. Meng, X. Tan, Z. Zhou, G. Zhang, J. Yang, Z. L. Wang, Machine-knitted washable sensor array textile for precise epidermal physiological signal monitoring. Sci. Adv. 6, eaay2840 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Y. Fang, Y. Zou, J. Xu, G. Chen, Y. Zhou, W. Deng, X. Zhao, M. Roustaei, T. K. Hsiai, J. Chen, Ambulatory cardiovascular monitoring via a machine-learning-assisted textile triboelectric sensor. Adv. Mater. 33, 2104178 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.K. Meng, X. Xiao, Z. Liu, S. Shen, T. Tat, Z. Wang, C. Lu, W. Ding, X. He, J. Yang, J. Chen, Kirigami-inspired pressure sensors for wearable dynamic cardiovascular monitoring. Adv. Mater. 34, 2202478 (2022). [DOI] [PubMed] [Google Scholar]
- 62.C. Wang, X. Li, H. Hu, L. Zhang, Z. Huang, M. Lin, Z. Zhang, Z. Yin, B. Huang, H. Gong, S. Bhaskaran, Y. Gu, M. Makihata, Y. Guo, Y. Lei, Y. Chen, C. Wang, Y. Li, T. Zhang, Z. Chen, A. P. Pisano, L. Zhang, Q. Zhou, S. Xu, Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2, 687–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.M. Wang, Y. Yang, J. Min, Y. Song, J. Tu, D. Mukasa, C. Ye, C. Xu, N. Heflin, J. S. McCune, T. K. Hsiai, Z. Li, W. Gao, A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D. Kireev, K. Sel, B. Ibrahim, N. Kumar, A. Akbari, R. Jafari, D. Akinwande, Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat. Nanotechnol. 17, 864–870 (2022). [DOI] [PubMed] [Google Scholar]
- 65.S. Li, Y. Zhang, Y. Wang, K. Xia, Z. Yin, H. Wang, M. Zhang, X. Liang, H. Lu, M. Zhu, H. Wang, X. Shen, Y. Zhang, Physical sensors for skin-inspired electronics. InfoMat 2, 184–211 (2020). [Google Scholar]
- 66.C. Wang, K. Xia, H. Wang, X. Liang, Z. Yin, Y. Zhang, Advanced Carbon for flexible and wearable electronics. Adv. Mater. 31, 1801072 (2019). [DOI] [PubMed] [Google Scholar]
- 67.C. Wang, K. Xia, M. Jian, H. Wang, M. Zhang, Y. Zhang, Carbonized silk georgette as an ultrasensitive wearable strain sensor for full-range human activity monitoring. J. Mater. Chem. C 5, 7604–7611 (2017). [Google Scholar]
- 68.W. Wahood, S. Ghozy, A. Al-Abdulghani, D. F. Kallmes, Radial artery diameter: A comprehensive systematic review of anatomy. J. NeuroInterv. Surg. 14, 1274–1278 (2022). [DOI] [PubMed] [Google Scholar]
- 69.Z. Domagała, J. Grzelak, N. Pospiech, N. Hunter, J. Klekowski, A. Lach, K. Stój, B. Kurc-Darak, M. Trzaska, Ultrasound evaluation of the radial artery in young adults—A pilot study. Ann. Anat. 238, 151763 (2021). [DOI] [PubMed] [Google Scholar]
- 70.G. Thambiraj, U. Gandhi, U. Mangalanathan, V. J. M. Jose, M. Anand, Investigation on the effect of Womersley number, ECG and PPG features for cuff less blood pressure estimation using machine learning. Biomed. Signal Process. Control 60, 101942 (2020). [Google Scholar]
- 71.C. J. Porth, V. S. Bamrah, F. E. Tristani, J. J. Smith, The Valsalva maneuver: Mechanisms and clinical implications. Heart Lung 13, 507–518 (1984). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S18
Table S1
Legend for movie S1
Movie S1