Abstract
The Tax protein of human T-cell leukemia virus type 1 (HTLV-1) is a transcriptional transactivator and viral oncogene. Since cellular transformation has been frequently linked to alterations in genome stability, we investigated the effect of Tax on nucleotide excision repair (NER), a prominent cellular DNA repair pathway. Cells expressing Tax exhibited a reduced capacity for NER as measured by unscheduled DNA synthesis and host cell reactivation assays. The cellular proliferating cell nuclear antigen (PCNA) gene product regulates DNA replication and repair pathways, including NER. Since Tax activates transcription of the PCNA promoter, we investigated whether this activity contributes to the reduction of NER. Tax increased endogenous PCNA protein expression, and analysis of Tax mutant proteins demonstrated that the reduction in NER correlated with Tax transactivation of PCNA gene expression. Direct overexpression of PCNA also reduced NER. We propose that overexpression of PCNA, and disruption of NER induced by Tax, predisposes cells to accumulate DNA damage and contributes to HTLV-1 transformation.
Human T-cell leukemia virus type 1 (HTLV-1) infects and transforms CD4+ T lymphocytes and is the etiologic agent of adult T-cell leukemia (ATL) (25). ATL develops in less than 5% of HTLV-1-infected individuals after a relatively long period of clinical latency lasting 2 or more decades (13). Cytogenetic studies of leukemic cells from ATL patients and of cells transformed with HTLV-1 in vitro have revealed clonal chromosomal abnormalities, most commonly involving deletions and translocations (6, 10, 16, 20, 22, 29). Although chromosomal changes are common in transformed ATL cells, no consistent abnormalities have been found in all ATL patients. These features suggest that accumulation of DNA damage induced by generalized deregulation of host DNA replication or repair contributes to the development of ATL.
The HTLV-1 Tax protein independently immortalizes T lymphocytes (11) and transforms rat fibroblasts (12, 26, 33). Cellular transformation by Tax is thought to involve a multistep pathway including induction of cell proliferation and accumulation of genetic damage. Effects of Tax on DNA repair have not been previously reported but are suggested by its ability to suppress expression of human DNA polymerase β, an enzyme involved in base excision repair (15), and the presence of micronuclei in Tax-expressing cells (21, 30).
Recently, we have shown that Tax can transactivate the proliferating cell nuclear antigen (PCNA) promoter (28). PCNA increases the processivity of DNA polymerase (Pol) δ, an enzyme involved in both DNA replication and repair (17). In the presence of DNA damage, elevated levels of the cyclin-dependent kinase inhibitor p21 interact directly with PCNA and block PCNA-dependent DNA replication without interfering with PCNA-dependent DNA repair (30, 34). Excess PCNA can overcome the p21 block of DNA replication (18), allowing DNA Pol δ synthesis past template lesions and resulting in increased nucleotide misincorporation rates (23).
The goal of this study was to determine the effects of Tax on cellular DNA repair synthesis. The results demonstrate that Tax inhibits nucleotide excision repair (NER), a prominent cellular DNA repair pathway, and that this effect correlates with Tax activation of PCNA gene expression. We further demonstrate that direct overexpression of PCNA also reduces NER. These activities may provide a mechanism for accumulation of chromosomal damage in HTLV-1-infected cells.
MATERIALS AND METHODS
Plasmids.
The PCNA cDNA from pGex-2T-PCNA (24) was cloned into pcDNA3.1Zeo(−) (Invitrogen) to create the PCNA expression plasmid. Antisense PCNA (ANCP) was created by cloning PCNA cDNA in the reverse orientation into pcDNA3.1Zeo(−). Tax expression plasmid pCMV-Tax and the mutant Tax expression plasmids used have been previously described (31). Plasmids −397PCNA-CAT, pSV-Tax, pMSV-Luc, and pSV-TaxΔHC have been previously described (28).
Transfections.
REF52 cells were grown in 60-mm-diameter dishes and transfected by calcium phosphate precipitation with a total of 14 μg of DNA, which included 1 μg of the pMSV-Luc plasmid, 4 μg of the −397PCNA-CAT reporter plasmid, and 6 μg of Tax expression vectors. Cells were harvested 72 h posttransfection and resuspended in 400 μl of reporter lysis buffer (Promega). The cell pellet was disrupted by a single freeze-thaw cycle.
CAT and luciferase assays.
Chloramphenicol acetyltransferase (CAT) assays were performed by a single-phase extraction assay (28). Briefly, 25 μl of the total cell extract was added to a mixture of 50 μl of water, 10 μl of 1 M Tris (pH 7.4), 10 μl of 2.5 mM n-butyryl-coenzyme A, and 5 μl of xylene-extracted [3H]chloramphenicol (NEN) at 0.2 μCi/reaction. The CAT assay mixture was incubated overnight at 37°C and extracted with 200 μl of 2,6,10,14-tetramethylpentadecane and xylene (2:1). The organic phase was then scintillation counted. For luciferase assays, 25 μl of the total cell extract was added to 50 μl of luciferase substrate (Promega). Luciferase activity was quantitated in a Turner TD-20e luminometer.
Unscheduled DNA synthesis (UDS) assay.
Duplicate 60-mm-diameter dishes of confluent REF52 cells were transfected with a control plasmid (pSV2-neo or pCMV-1), a Tax expression plasmid (pSV-Tax or pCMV-Tax), or mutant Tax (M3, M21, M32, or M47) together with 1 μg of a luciferase reporter plasmid (pMSV-Luc) to control for transfection efficiency. To eliminate background semiconservative DNA replication, 48 h after transfection the cells were cultured in 0.5% serum for 3 h and 10 mM hydroxyurea was added 1 h before irradiation. One of the duplicate plates was then irradiated with UVC light (254 nm, 30 J/m2) by using a Stratalinker (Stratagene). The other plate was mock irradiated, and both plates were labeled with medium containing [3H]thymidine (5 μCi/ml; ICN Radiochemicals) for 2 h in the continued presence of 10 mM hydroxyurea. The cells were lysed by freeze-thawing in 400 μl of reporter lysis buffer (Promega) and transferred to 1.5-ml microcentrifuge tubes. A 50-μl aliquot was removed for analysis of luciferase activity, and the remaining DNA was precipitated by adding cold 100% trichloroacetic acid to a final concentration of 5%, and incubation at 4°C overnight. [3H]thymidine incorporation was measured by spotting the precipitated DNA onto glass fiber filters (GF/C; Whatman), sequential washing with 10 and 5% trichloroacetic acid, and counting in a Beckman scintillation counter. [3H]thymidine values for each plate were divided by luciferase activity to correct for transfection efficiency. The corrected [3H]thymidine value for each nonirradiated plate was set to 100%, and its irradiated partner was adjusted correspondingly so that different plasmid transfections could be compared. The adjusted [3H]thymidine value from irradiated cells that received the control plasmid was set to 100% repair activity, and the relative [3H]thymidine values from each test plasmid were reported as percent repair activity.
Host cell reactivation (HCR) assay.
The pMSV-Luc reporter plasmid was damaged in vitro by exposure to UVC light (254 nm) at 1,000 J/m2. REF52 cells or cloned rat embryo fibroblasts (CREF) were transfected with 4 μg of a UV-damaged or a control nonirradiated pMSV-Luc plasmid together with 4 μg of pSV2-CAT (to control for transfection efficiency). Certain plates also received plasmids encoding wild-type Tax, mutant Tax, or PCNA to test the effects of these gene products on DNA repair. Forty-eight hours after transfection, cells were lysed and luciferase activity was measured. Luciferase activity was normalized to the CAT activity of the same plate. Since UV damage reduces luciferase expression from pMSV-Luc, the level of normalized luciferase activity represents the degree of DNA repair. Normalized luciferase activity from each nonirradiated plate was set to 100%, and its irradiated partner was adjusted correspondingly so that different plasmid transfections could be compared. The adjusted luciferase value from irradiated cells that received the control plasmid was set to 100% repair activity, and the corrected luciferase values from each test plasmid were reported as percent repair activity.
RESULTS
Disruption of nucleotide excision repair by Tax.
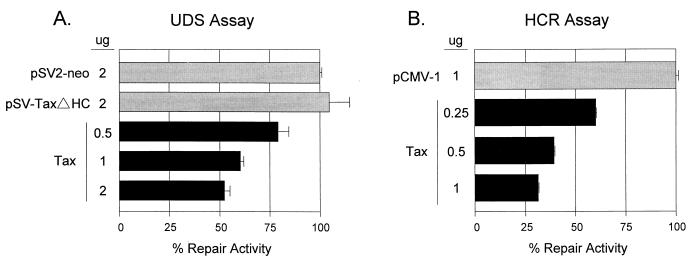
The effect of Tax on cellular DNA repair was investigated by using a UDS assay (Fig. 1A) which measures NER, the primary pathway for repair of UV-induced DNA lesions (7, 9). Vectors encoding Tax or a Tax frameshift mutant was transfected into REF52 cells. Rat cells were selected for this study because they can be transformed by Tax (12, 26, 33). Transfected cells were blocked in the cell cycle by serum starvation and addition of hydroxyurea and then UV irradiated to induce bulky pyrimidine-pyrimidine dimers and photoproducts. Control cells were similarly transfected but not UV damaged. After UV treatment, cells were labeled with [3H]thymidine. Cellular DNA repair activity was determined by comparing the incorporation of [3H]thymidine into irradiated and nonirradiated cells that had been similarly transfected. Since hydroxyurea was included to block DNA replication, [3H]thymidine incorporation served as a measure of DNA repair. Irradiated cells that received control plasmid pSV2-neo or Tax frameshift mutant plasmid pSV-TaxΔHC showed high levels of [3H]thymidine incorporation due to DNA repair synthesis. In contrast, irradiated cells that received Tax expression plasmid pSV-Tax showed reduced [3H]thymidine incorporation, indicating inhibition of DNA repair (Fig. 1A). At the highest concentration of Tax tested, [3H]thymidine incorporation was reduced to approximately 50% of that of irradiated, non-Tax-expressing cells. The Tax-dependent reduction of DNA repair was similar to that observed in cells from xeroderma pigmentosum patients, which have well-characterized defects in NER (2, 14).
FIG. 1.
Dose-dependent inhibition of cellular DNA repair by Tax. (A) UDS assay. REF52 fibroblasts were transfected with increasing concentrations of Tax expression plasmid pSV-Tax (0.5 to 2 μg) or 2 μg of negative control plasmid pSV-TaxΔHC (a Tax frameshift mutant plasmid) or pSV2-neo. The cells were serum starved, one dish of each duplicate was UV irradiated, and all cells were labeled with [3H]thymidine for 3 h. After labeling, the cells were lysed and the precipitated DNA was counted. The percent repair activity was calculated as described in Materials and Methods. The error bars indicate the standard deviations of three replicates. (B) HCR assay. The pMSV-Luc plasmid was UV irradiated and cotransfected into REF52 fibroblasts with increasing concentrations of Tax expression plasmid pCMV-Tax (0.25 to 1 μg) or 1 μg of negative control backbone plasmid pCMV-1. Duplicate dishes received the same transfection mixture except that the pMSV-Luc plasmid was not irradiated in one dish. Forty hours following transfection, the cells were harvested and luciferase and CAT assays were performed. Percent repair activity was calculated as described in Materials and Methods. The error bars indicate the standard deviations of three replicates.
An HCR assay (1) was also used to measure the effect of Tax on NER (Fig. 1B). In this assay, a reporter plasmid was UV treated to induce DNA damage prior to transfection. HCR is a particularly good measure of NER since only the reporter, and not the cells, is damaged by UV exposure, thus eliminating effects of DNA damage directly on DNA repair or cell cycle regulatory genes. A reporter plasmid (pMSV-Luc) was UV irradiated or mock treated and then transfected into REF52 cells together with Tax expression vector pCMV-Tax or vector backbone pCMV-1. We have previously demonstrated that Tax does not affect luciferase expression regulated by the murine sarcoma virus promoter (data not shown). Since UV-induced lesions provide a strong block to transcription, luciferase expression is reduced and only rescued if the damaged plasmid is repaired. If NER activity is inhibited, the damaged plasmid will not be repaired and luciferase expression will not be rescued. Thus, luciferase expression represents the ability of the cell to carry out NER. Cotransfection with backbone vector pCMV-1 allowed repair of the damaged luciferase reporter and rescue of luciferase activity by host repair machinery. The luciferase activity in cells cotransfected with pCMV-Tax and the damaged reporter plasmid was approximately 30% of that of cells in the absence of Tax, indicating that Tax reduced host NER activity.
Transactivation of the PCNA promoter by Tax mutants.
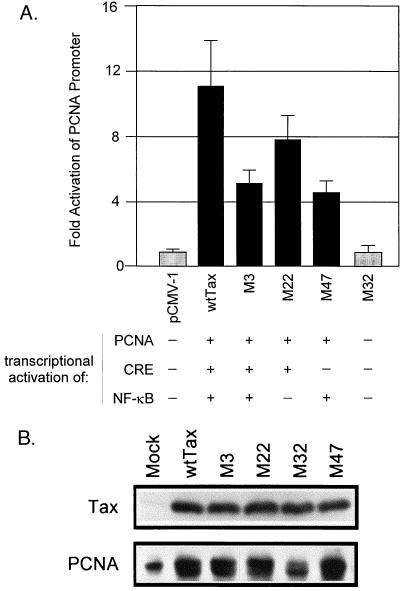
We have previously demonstrated that Tax can activate PCNA expression through a novel Tax-responsive motif in the PCNA promoter which does not require either cyclic AMP response element (CRE) binding protein or NF-κB (28), and elevated levels of PCNA have been shown to allow synthesis in the presence of DNA damage (23). To determine whether Tax inhibition of cellular DNA repair synthesis requires activation of PCNA gene expression, a set of 38 mutant Tax expression vectors (31) were characterized. The abilities of these mutant proteins to transactivate the PCNA promoter (data not shown) were compared with their previously reported abilities to activate CRE- and NF-κB-dependent promoters. The phenotypes segregated into four groups represented by the mutants shown in Fig. 2A. A representative Tax mutant (M3, M22, M47, or M32) from each phenotypic group, as well as wild-type Tax, was cotransfected into REF52 cells with a CAT reporter containing the Tax-responsive element of the human PCNA promoter. Each of these mutants has previously been shown to localize predominantly in the nucleus (31). CAT assays were performed to determine the abilities of the mutants to activate the PCNA promoter (Fig. 2A). The M32 Tax mutant protein did not transactivate the PCNA promoter while mutants M3, M22, and M47 did activate the PCNA promoter, albeit to levels slightly lower than that of wild-type Tax (Fig. 2A and Table 1). We had previously demonstrated that Tax activation of the PCNA promoter does not require the CRE pathway (28). These results confirm that finding and further demonstrate that the NF-κB pathway is not required for Tax activation of the PCNA promoter. This screen did not identify a mutant that was specifically defective for PCNA activation while retaining activation of the CRE and NF-κB pathways. However, the mutant phenotypes can be used to deduce the relationship between Tax activation of the PCNA promoter and the role of this activation in cellular DNA repair. Before initiating this effort, it was necessary to test the abilities of the mutants to activate endogenous PCNA protein expression.
FIG. 2.
(A) Activation of the PCNA promoter by Tax mutants. REF52 fibroblasts were transfected with 4 μg of the −397 PCNA CAT reporter, 6 μg of the indicated mutant Tax protein or the pCMV-1 negative control plasmid, and 1 μg of pMSV-Luc. Fold activation was determined by dividing the CAT activity in the presence of Tax mutant proteins by the basal CAT activity in the presence of pCMV-1. CAT units were normalized to luciferase values to control for transfection efficiency. The ability of each mutant protein to activate the NF-κB, CRE, and PCNA pathways is shown. Activation of the NF-κB and CRE pathways is based on previously published data (16) and confirmed by us (data not shown). wt, wild type. (B) Activation of endogenous PCNA expression by Tax mutants. Twenty micrograms of the indicated Tax mutant was transfected into 100-mm-diameter dishes of CREF. Seventy-two hours after transfection, cells were lysed in 500 μl of reporter lysis buffer (Promega) and 20 μl of the extracts was loaded onto duplicate sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis gels. After transfer onto Immobilon membranes (Millipore), the membranes were probed with either a rabbit anti-Tax polyclonal antibody or a mouse anti-human PCNA monoclonal antibody (Santa Cruz Biotech).
TABLE 1.
Functional activities of wild-type and mutant Tax proteins
| Protein | % Inhibition of NER (HCR) | % Activation of:
|
||
|---|---|---|---|---|
| PCNAa | CREb | NF-κBb | ||
| Wild-type Tax | 100 | 100 | 100 | 100 |
| M3 | 67 | 48 | 21 | 33 |
| M22 | 81 | 72 | 56 | <5 |
| M47 | 69 | 41 | <5 | >120 |
| M32 | 13 | 12 | <5 | <5 |
Transactivation of the −397PCNA reporter.
As reported by Smith and Greene (31).
The effects of the wild-type and mutant Tax proteins on endogenous PCNA protein expression were examined by Western blot analysis of transfected cells. Wild-type and mutant Tax proteins were expressed at similar levels (Fig. 2B). Cells transfected with wild-type Tax or mutants that reduced DNA repair (M3, M22, and M47) displayed an approximately fourfold increase in total endogenous PCNA expression (Fig. 2B). Expression of endogenous PCNA protein in the absence of Tax (mock transfection) was similar to that of cells in the presence of the M32 Tax mutant that failed to reduce DNA repair (1.6-fold). The PCNA doublet observed in cells expressing wild-type and mutant Tax is presumed to represent the phosphorylated and unphosphorylated forms of PCNA (27). These results are consistent with a previous report that endogenous PCNA is overexpressed in the HTLV-1-transformed Molt-4 T-cell line (5). It has been shown that PCNA expression is increased in transformed cells from 1.7-fold to 9.3-fold (4), and in normal cells, the fluctuation of PCNA expression throughout the cell cycle is about 2.7-fold (3). Therefore, the fourfold increase in endogenous PCNA expression induced by Tax is likely to be biologically significant.
The ability of Tax to inhibit NER correlates with its ability to transactivate the PCNA promoter.
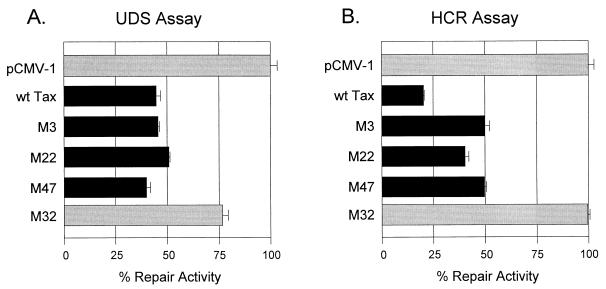
Tax mutants were transfected into REF52 cells to determine their effect on cellular DNA repair activity by using both UDS and HCR assays. In the UDS assay, the M32 Tax mutant that failed to transactivate the PCNA promoter showed minimal effects on NER (Fig. 3A) with repair activity greater than 75% of that of irradiated cells transfected with the control plasmid. In contrast, those Tax mutants that transactivated the PCNA promoter (M3, M22, and M47) retained the ability to reduce NER, with repair activity equal to or less than 50% of that of irradiated cells transfected with the control plasmid. These results showed that the ability of Tax to reduce NER correlated with its ability to transactivate PCNA but did not require functional NF-κB and CRE pathways.
FIG. 3.
Effects of Tax mutant proteins on cellular DNA repair synthesis. The abilities of the M3, M22, M47, and M32 Tax mutants to impair cellular DNA synthesis were tested. (A) UDS assay. (B) HCR assay. The experiments were performed as described in the legend to Fig. 1. wt, wild type.
The ability of Tax mutants to inhibit NER activity was also examined by using the HCR assay (Fig. 3B). Cells expressing the M32 Tax mutant that did not transactivate the PCNA promoter showed NER activity similar to that of non-Tax-expressing cells. In contrast, mutants M3, M22, and M47, which retained transactivation of the PCNA promoter, also retained the ability to reduce NER, although they were somewhat less efficient in this activity than wild-type Tax (Table 1). In Table 1, the ability of Tax mutants to activate the PCNA promoter, as well as CRE- and NF-κB-dependent promoters, is compared with the reduction in NER induced by the mutants in the HCR assay. In this comparison, it is clear that inhibition of NER correlates most closely with the ability to activate PCNA gene expression. Thus, overexpression of PCNA protein induced by Tax may play a central role in its ability to reduce cellular DNA repair.
Direct overexpression of PCNA reduces NER.
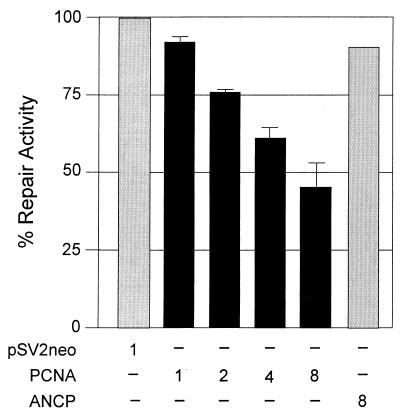
The M32 mutant Tax protein was defective in several biological activities, including activation of PCNA gene expression, as well as activation of CRE- and NF-κB-dependent promoters. An HCR assay was used to determine whether direct overexpression of PCNA would affect DNA repair (Fig. 4). UV-irradiated reporter plasmid pMSV-Luc was transfected into CREF together with increasing concentrations of a PCNA expression vector or antisense PCNA expression vector. Overexpression of PCNA in these cells resulted in a dose-dependent reduction in NER, suggesting that elevated levels of PCNA may inhibit DNA repair. These results are consistent with our hypothesis that the Tax-mediated increase in PCNA expression is responsible for the reduced NER observed in Tax-expressing cells. This activity may predispose cells to accumulate DNA damage and contribute to HTLV-1 transformation.
FIG. 4.
Effect of PCNA overexpression on NER. A host cell reactivation assay was used to measure NER. The pMSV-Luc plasmid was UV irradiated and cotransfected into CREF with pSV-neo (1 μg), various amounts of a PCNA expression plasmid (1, 2, or 4 μg), or 4 μg of ANCP. Duplicate dishes received the same transfection mixture, except that the pMSV-Luc plasmid was not irradiated in one dish. Forty hours following transfection, the cells were harvested and luciferase and CAT assays were performed. Luciferase activity was normalized to CAT expression to correct for the difference in transfection efficiency. Percent repair activity was calculated as described in Materials and Methods. The error bars indicate the standard deviations of three replicates.
DISCUSSION
This study investigated the possibility that HTLV-1 Tax may contribute to genome instability by negatively influencing NER. We demonstrated that Tax expression reduced NER and that this reduction correlated closely with Tax transactivation of PCNA gene expression. These results support a causal relationship between Tax activation of PCNA gene expression and reduced DNA repair capacity in Tax-expressing cells. Other viral transforming proteins, such as hepatitis B virus X (2) and human papillomavirus E6 (8), have also been shown to disrupt cellular DNA repair, suggesting that this may be a common feature by which viruses transform cells.
Cellular transformation is thought to proceed by sequential steps, and accumulation of DNA mutations is one feature common to many transformed cells. It has been proposed that induction of a mutator phenotype (19) that allows an increase in the mutation rate is likely to be an early step in tumor progression. An increased mutation rate can be achieved by replication of damaged DNA such that the damage becomes fixed in the genome. Further amplification of the mutation rate could result if the cell’s DNA repair capacity were reduced, in combination with the ability to replicate damaged DNA. This mutator phenotype model predicts that disruption of cellular mechanisms that coordinate DNA replication and repair may play an important role in transformation.
Despite accumulating knowledge regarding transcription regulation by Tax, it remains unclear how its diverse biological activities contribute to cell transformation. We hypothesize that the HTLV-1-transforming protein Tax can induce a mutator phenotype by altering the cellular environment such that DNA repair capacity is reduced and the ability to replicate DNA through damage is increased. The resulting phenotype would favor accumulation of DNA mutations and may lay the foundation for subsequent steps in HTLV-1 transformation. The studies presented here address the first aspect of this hypothesis, demonstrating that Tax can reduce the cell’s ability to repair DNA damage.
Although not directly addressed in this study, the ability of Tax to stimulate PCNA gene expression may also contribute to the second step of our mutator phenotype model of Tax transformation by promoting replication through DNA damage. This is suggested by previous observations that PCNA overexpression promotes DNA replication, even in the presence of template lesions, and increases nucleotide misincorporation rates (18, 23). Thus, activation of PCNA gene expression by Tax may contribute to genome instability by allowing replication through DNA damage. Additional studies are necessary to determine whether Tax can, indeed, stimulate replication of damaged DNA.
PCNA is a required cofactor of DNA Pol δ, an enzyme involved in both DNA replication and repair. Increasing the PCNA-to-Pol δ ratio can have a significant impact on the functions of Pol δ in DNA replication. The present study provides the first evidence that excess PCNA can inhibit DNA repair and implies that excess PCNA may deregulate Pol δ DNA repair activity.
Three major DNA excision repair pathways have been described: base excision repair, NER, and mismatch repair. Of the five characterized nuclear DNA polymerases, DNA Pol β is responsible for base excision repair and DNA Pol δ and ɛ are responsible for both NER and mismatch repair. Tax has previously been shown to repress expression of the DNA Pol β promoter, and it was proposed that this activity may allow accumulation of DNA damage (15). In this report, we show that Tax reduces NER and that this effect correlates with its ability to activate PCNA gene expression. The presence of excess PCNA may reduce NER by disrupting Pol δ repair activity. This effect, in combination with the ability of excess PCNA to stimulate DNA replication by Pol δ (18, 23), could result in replication of damaged DNA. Since Pol δ is involved in NER, as well as mismatch repair, these results, together with earlier studies showing Tax repression of the Pol β promoter, predict that Tax has the ability to disrupt all three major DNA excision repair pathways.
Although we propose that inhibition of DNA repair synthesis may play an important role in Tax-mediated transformation, other steps are certainly required. Tax has been shown to activate a large number of cellular genes, many of which have been implicated in the transformation process. The panel of Tax mutants used in this study demonstrated that activation of CRE- or NF-κB-dependent genes is not required for Tax activation of the PCNA promoter or for inhibition of DNA repair synthesis (Table 1). This finding is supported by our previous report that Tax can activate the PCNA promoter through an element which does not contain CRE binding protein- or NF-κB-binding sites (28). However, CRE- and NF-κB-dependent genes are likely to play important roles at later stages of Tax transformation (32, 35).
A reduced ability to efficiently repair DNA damage, coupled with an ability to stimulate DNA replication and cell proliferation, would be expected to introduce random mutations into the genome. Thus, the ability of Tax to reduce NER provides a basis for the long and variable period of clinical latency before the onset of ATL and the development of ATL in only a low percentage of infected individuals. In addition, the ability of Tax to disrupt cellular DNA repair may explain why ATL cells have a high incidence of chromosomal instability and abnormalities.
ACKNOWLEDGMENTS
We thank Steve Ressler and Sherry Becker for helpful discussions and Larry Donehower, Betty Slagle, and John Brady for critical evaluation of the manuscript.
This work was supported by grants from the National Institutes of Health and the American Cancer Society to S.J.M.
REFERENCES
- 1.Athas W F, Hedayati M A, Matanoski G M, Farmer E R, Grossman L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991;51:5786–5793. [PubMed] [Google Scholar]
- 2.Becker S A, Lee T H, Butel J S, Slagle B L. Hepatitis B virus X protein interferes with cellular DNA repair. J Virol. 1998;72:266–272. doi: 10.1128/jvi.72.1.266-272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bravo R, Celis J E. Gene expression in normal and virally transformed mouse 3T3 and hamster BHK21 cells. Exp Cell Res. 1980;127:249–260. doi: 10.1016/0014-4827(80)90430-9. [DOI] [PubMed] [Google Scholar]
- 4.Bravo R, Celis J E. Human proteins sensitive to neoplpastic transformation in cultured epithelial and fibroblast cells. Clin Chem. 1982;28:949–954. [PubMed] [Google Scholar]
- 5.Celis J E, Bravo R, Larsen P M, Fey S J. Cyclin: a nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leuk Res. 1984;8:143–157. doi: 10.1016/0145-2126(84)90135-8. [DOI] [PubMed] [Google Scholar]
- 6.Chieco-Bianchi L, Saggioro D, DelMistro A, Montaldi A, Majone F, Levis A G. Chromosome damage induced in cord blood T-lymphocytes infected in vitro by HTLV-I. Leukemia. 1988;2:223s–232s. [PubMed] [Google Scholar]
- 7.Fautz R, Husein B, Efstathiou E, Hechenberger-Freudi C. Assessment of the relation between the initial viability and the attachment of freshly isolated rat hepatocytes used for the in vivo/in vitro DNA repair assay (UDS) Mutat Res. 1993;291:21–27. doi: 10.1016/0165-1161(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 8.Ford J M, Baron E L, Hanawalt P C. Human fibroblasts expressing the human papillomavirus E6 gene are deficient in global genomic nucleotide excision repair and sensitive to ultraviolet irradiation. Cancer Res. 1998;58:599–603. [PubMed] [Google Scholar]
- 9.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 10.Fujita K, Yamasaki Y, Sawada H, Izumi Y, Fukuhara S, Uchino H. Cytogenetic studies on the adult T cell leukemia in Japan. Leuk Res. 1989;13:535–543. doi: 10.1016/0145-2126(89)90120-3. [DOI] [PubMed] [Google Scholar]
- 11.Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski J G, Haseltine W A, Ramstedt U. Role of human T-cell leukemia virus type I X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinrichs S H, Nerenberg M, Reynolds R K, Khoury G, Jay G. A transgenic mouse model for human neurofibromatosis. Science. 1987;237:1340–1343. doi: 10.1126/science.2888191. [DOI] [PubMed] [Google Scholar]
- 13.Hinuma Y, Nagata K, Misoka M, Nakai T, Matsumoto T, Kiroshita K, Shirakwa S, Miyoshi I. Adult T-cell leukemia: antigen in ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoejmakers J H J. Nucleotide excision repair II: from yeast to mammals. Trends Genet. 1993;9:211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- 15.Jeang K-T, Widen S G, Semmes O J, Wilson S H. HTLV-1 trans-activator protein, Tax, is a trans-repressor of the human β-polymerase gene. Science. 1990;247:1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- 16.Kamada N, Sakurai M, Miyamoto K, Sancar A, Sadamori N, Fukuhara S, Abe S, Shiraishi Y, Abe T, Kaneko Y, Shimoyama M. Chromosome abnormalities in adult T-cell leukemia/lymphoma: a karyotype review committee report. Cancer Res. 1992;52:1482–1493. [PubMed] [Google Scholar]
- 17.Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Waga S, Hannon G J, Beach D, Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994;371:534–537. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- 19.Loeb L A. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- 20.Macera M J, Hyde P, Peddanna N, Szabo P, Gogineni S K, Verma R S. T-cell receptor Jβ1/β2 locus rearrangements in an HTLV-1-positive T-cell lymphoma with complex chromosomal aberrations. Am J Hematol. 1996;52:53–57. doi: 10.1002/(SICI)1096-8652(199605)52:1<53::AID-AJH9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Majone F, Semmes O J, Jeang K-T. Induction of micronuclei by HTLV-I Tax: a cellular assay for function. Virology. 1993;193:456–459. doi: 10.1006/viro.1993.1145. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama K, Fukushima T, Kawamura K, Mochizuki S. Chromosome and gene rearrangements in immortalized human lymphocytes infected with human T-lymphotropic virus type I. Cancer Res. 1990;50:5697s–5702s. [PubMed] [Google Scholar]
- 23.Mozzherin D J, Shibutani S, Tan C K, Downey K M, Fisher P A. Proliferating cell nuclear antigen promotes DNA synthesis past template lesions by mammalian DNA polymerase δ. Proc Natl Acad Sci USA. 1997;94:6126–6131. doi: 10.1073/pnas.94.12.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi M, Robetorye R S, Pereira-Smith O M, Smith J R. The C-terminal region of p21SDI1/WAF1/CIP1 is involved in proliferating cell nuclear antigen binding but does not appear to be required for growth inhibition. J Biol Chem. 1995;270:17060–17063. doi: 10.1074/jbc.270.29.17060. [DOI] [PubMed] [Google Scholar]
- 25.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from freshly cultured lumphocytes of a patient with cutaneous T cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozzatti R, Vogel J, Jay G. The human T-lymphotropic virus I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prosperi E, Scovassi A I, Stivala L A, Bianchi L. Proliferating cell nuclear antigen bound to DNA synthesis sites: phosphorylation and association with cyclin D1 and cyclin A. Exp Cell Res. 1994;215:257–262. doi: 10.1006/excr.1994.1341. [DOI] [PubMed] [Google Scholar]
- 28.Ressler S, Morris G F, Marriott S J. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J Virol. 1997;71:1181–1190. doi: 10.1128/jvi.71.2.1181-1190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowley J D, Haren J M, Wong-Staal F, Franchini G, Gallo R C, Blattner W. Chromosome patterns in cells from patients positive for human T-cell leukemia/lymphoma virus. In: Gallo R C, Essex M E, Gross L, editors. Human T-cell leukemia/lymphoma virus. New York, N.Y: Cold Spring Harbor Laboratory; 1984. pp. 85–89. [Google Scholar]
- 30.Saggioro D, Majone F, Forino M, Turchetto L, Leszl A, Chieco-Bianchi L. Tax protein of human T-lymphotropic virus type I triggers DNA damage. Leuk Lymphoma. 1994;12:281. doi: 10.3109/10428199409059600. [DOI] [PubMed] [Google Scholar]
- 31.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 32.Smith M R, Greene W C. Type I human T cell leukemia virus Tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka A, Takahashi G, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waga S, Hannon G J, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- 35.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]