Abstract
Earlier studies have shown that (i) the coding domain of the α22 gene encodes two proteins, the 420-amino-acid infected-cell protein 22 (ICP22) and a protein, US1.5, which is initiated from methionine 147 of ICP22 and which is colinear with the remaining portion of that protein; (ii) posttranslational processing of ICP22 mediated largely by the viral protein kinase UL13 yields several isoforms differing in electrophoretic mobility; and (iii) mutants lacking the carboxyl-terminal half of the ICP22 and therefore ΔUS1.5 are avirulent and fail to express normal levels of subsets of both α (e.g., ICP0) or γ2 (e.g., US11 and UL38) proteins. We have generated and analyzed two sets of recombinant viruses. The first lacked portions of or all of the sequences expressed solely by ICP22. The second set lacked 10 to 40 3′-terminal codons of ICP22 and US1.5. The results were as follows. (i) In cells infected with mutants lacking amino-terminal sequences, translation initiation begins at methionine 147. The resulting protein cannot be differentiated in mobility from authentic US1.5, and its posttranslational processing is mediated by the UL13 protein kinase. (ii) Expression of US11 and UL38 genes by mutants carrying only the US1.5 gene is similar to that of wild-type parent virus. (iii) Mutants which express only US1.5 protein are avirulent in mice. (iv) The coding sequences Met147 to Met171 are essential for posttranslational processing of the US1.5 protein. (v) ICP22 made by mutants lacking 15 or fewer of the 3′-terminal codons are posttranslationally processed whereas those lacking 18 or more codons are not processed. (vi) Wild-type and mutant ICP22 proteins localized in both nucleus and cytoplasm irrespective of posttranslational processing. We conclude that ICP22 encodes two sets of functions, one in the amino terminus unique to ICP22 and one shared by ICP22 and US1.5. These functions are required for viral replication in experimental animals. US1.5 protein must be posttranslationally modified by the UL13 protein kinase to enable expression of a subset of late genes exemplified by UL38 and US11. Posttranslational processing is determined by two sets of sequences, at the amino terminus and at the carboxyl terminus of US1.5, respectively, a finding consistent with the hypothesis that both domains interact with protein partners for specific functions.
The herpes simplex virus (HSV) genome encodes >80 genes whose expression is coordinately regulated and sequentially ordered during productive infection (9, 10, 29). The first set of genes expressed immediately after productive infection are the α genes, followed by β and γ genes. Of the five α genes initially described, four have regulatory functions, and of these three, the α genes 0, 4, and 27, have attracted considerable attention because they are essential for viral replication under all conditions tested. Thus, α0 encodes the infected-cell protein (ICP) 0, a promiscuous transactivator important in early stages of infection. ICP4, the product of the α4 gene, regulates gene expression both positively and negatively, whereas ICP27, the product of the α27 gene, regulates posttranslational processing and transport of RNA (30). ICP22, the product of the α22 gene, attracted less attention, possibly because its functions were less apparent, obscured as it were by the observation that it was dispensable for viral replication in cells in culture (21). Although the functions of the α22 gene are the least well understood, the evidence suggests that it plays an important role in viral replication. Specifically, and not in the order of discovery, we note the following.
(i) The domain of the α22 gene yields two mRNAs each expressed by its own promoter. The α22 mRNA initiates upstream from the open reading frame and is spliced; the first exon is in its 5′-noncoding domain (15, 28, 35). ICP22, its product, is a protein of 420 amino acids with alternating acidic and basic domains. The second mRNA initiates in the coding domain of the α22 gene and is driven by an independent promoter (5). It directs the synthesis of a protein of 274 amino acids beginning with Met147 of ICP22 and is colinear with the remainder of the protein. This protein, designated US1.5, is also expressed with α gene kinetics. The possibility that the sequences unique to ICP22 perform functions different from those of sequences shared by ICP22 and US1.5 emerged from the observation that insertion of a 20-codon linker at codon 200 or 240 had no apparent effect on the functions associated with ICP22 and described below.
(ii) ICP22 is extensively posttranslationally processed (1), as evidenced by phosphorylation and changes in electrophoretic mobility. ICP22 was shown to be phosphorylated largely by the protein kinase encoded by UL13 and to a lesser extent by protein kinase encoded by US3 (23, 24). ICP22 is also nucleotidylylated by casein kinase II (17, 18).
(iii) The deletion mutant R325 generated by Post and Roizman (21) lacked the carboxyl-terminal 220 amino acids. The mutant was highly attenuated in experimental animal systems (16, 33). It replicated to wild-type virus levels in Vero and HEp-2 cells, but its ability to replicate in rodent or rabbit cells or in primary human fibroblasts was diminished. In these restricted cell lines, a subset of γ2 proteins exemplified by the product of US11 was significantly reduced (24). In addition, the levels of mRNA and protein products of the α0 gene were also reduced (24). More detailed analyses showed that in rabbit skin cells infected with R325, ICP0 mRNA was unstable, and the alternate splice acceptor C of ICP0 intron 1 was not used (6). The studies by Purves et al. cited above showed that the phenotype of the R325 deletion mutant was similar to that of the mutant lacking a functional UL13 gene (24).
(iv) ICP22 localizes in both the nucleus and cytoplasm (11). Nuclear ICP22 localizes early in infection in small dense nuclear structures. At the onset of DNA synthesis, ICP22 colocalizes with ICP4, nascent DNA, RNA polymerase II, and other cellular proteins (14). The displacement of ICP22 from the small dense nuclear structures requires the expression of the protein kinase encoded by UL13. These results suggest that the products of the α22 gene are involved in transcription of late genes, a conclusion consistent with the report that α22 mediates an intermediate level of phosphorylation of the RNA polymerase II (26, 27).
(v) Studies in yeast two-hybrid systems with the entire α22 gene as bait yielded evidence of the interaction of ICP22 with at least two host proteins. The first, designated p78, was recently discovered to be identical in sequence to a protein reported to localize in nucleoli and designated MSP58 (25). Studies in our laboratory showed that p78 is made early in the S phase, has a short half-life, and binds the amino-terminal domain of ICP22. In synchronized cells, during the expression of p78, the ICP22 exhibits novel posttranslationally processed forms. These forms are replaced by the standard series of ICP22 isoforms with time after infection (3).
The second protein, designated p60, bound fast-migrating, underprocessed wild-type ICP22 and ICP22 lacking the carboxyl-terminal 24 amino acids but not ICP22 lacking the terminal 40 amino acids. p60 also bound ICP0, and this binding was independent of that of ICP22. In uninfected HEp-2 cells, p60 was distributed throughout the cell. In wild-type-virus-infected HEp-2 cells, p60 was translocated into the nucleus and formed dense bodies that colocalized with ICP0. The posttranslational processing of p60 present in HEp-2 cells infected with wild-type or ICP22 mutant viruses could not be differentiated from that of uninfected cells, whereas the p60 accumulating in rabbit skin cells infected with wild-type virus differed in electrophoretic mobility from that made in uninfected cells. The posttranslational processing of p60 was absent in rabbit skin cells infected with the virus lacking the sequences encoding the carboxyl-terminal half of ICP22. p60 appears to be a linker protein capable of binding to and mediating the interaction of ICP0 with the underprocessed form of ICP22 (4).
This report focuses on the functional anatomy of ICP22. In essence, we identified three functional domains. One domain maps in sequences unique to ICP22. The other two domains map in the domain of sequences shared by ICP22 and US1.5. The functions encoded by these domains are expressed by US1.5 in the absence of domain 1.
MATERIALS AND METHODS
Cells and Viruses.
Vero and HEp-2 cells were obtained from the American Type Culture Collection (Manassas, Va.). Rabbit skin cells were originally obtained from John McClaren. HSV-1(F) is the prototype HSV-1 strain used in this laboratory (8). The constructions of HSV-1 recombinant viruses R325, R7356 (ΔUL13), and R7905 were reported elsewhere (12, 21, 22).
Plasmids.
To construct pRB5210, the plasmid pRB138 containing BamHI N (bp 131,399 to 136,289 of the HSV-1 consensus sequence) was digested with EcoRI and BamHI, releasing a BamHI N fragment that was truncated by 121 bp. This fragment was cloned into the EcoRI-BamHI sites of the puc19 vector.
To construct pRB5212, a 110-bp PCR product was generated from pRB5210 with the aid of the Pfu polymerase (Stratagene, La Jolla, Calif.) and the primers P1 (GGA ACG TCC TCG TCG AGG CGA CCG) and P2 (GCC TGG GGA AAT GTC GGC CGT CCA GAA AAC GTC). P1 included in the final product the EcoNI site 100 bp upstream of the ICP22 open reading frame whereas P2 replaced the initiator methionine codon of ICP22 with an EagI site. The PCR product was then digested with EcoNI and EagI and subcloned into pRB5210 which had also been digested with EcoNI and EagI to remove the ICP22 open reading frame. In the resulting plasmid, pRB5212, the sequences encoding ICP22 and US1.5 from the initiation methionine to the carboxyl-terminal stop codon had been deleted, leaving only a unique EagI restriction endonuclease cleavage site.
To construct pRB5214, a 1.2-kb PCR product was generated from pRB5210 with the Pfu polymerase (Stratagene) and the primers P3 (GAC GTT TTC TGG CGG CCG ATG GCC GAC) and P4 (GAC GCT GGG ACA AAC GCT TTG ATT TTG GTC). P3 inserted an EagI site adjacent to the initiator methionine codon of ICP22, and the primer P4 represents a sequence located 50 bp downstream from the carboxyl-terminal EagI site of the carboxyl-terminal stop codon of the ICP22 open reading frame. The PCR product was then digested with EagI and subcloned into the EagI site of pRB5212. The resulting plasmid, pRB5214, contained ICP22 and US1.5 with an EagI site just preceding the initiation methionine.
To construct pRB5215, a 870-bp PCR product was generated from pRB5212 with Pfu polymerase (Stratagene) and the primers P5 (ACG CAG CCC CGG GCC CCC CGG CCG TCG GCC) and P4. P5 created an EagI site 25 bp upstream of the US1.5 initiation Met171 and the primer P4. The PCR product was then digested with EagI and subcloned into pRB5212. The resulting plasmid, pRB5215, contained a US1.5 open reading frame driven by the α22 promoter.
To construct pRB458, the EcoRI site of plasmid puc19 was destroyed with the T4 polymerase (Stratagene).
To construct pRB5211, the 3.9-kb SacI (bp 129,088 to 133,046) fragment from the HindIII M (bp 126,526 to 133,466) fragment of HSV-1(F) was subcloned from pRB201 and cloned into pRB458.
To construct pRB5213, the 3.2-kb SacI-XbaI fragment (nucleotides 133,049 to 136,289 of BamHI N) from pRB5212 was cloned into pRB5211. The resulting plasmid, pRB5213, extended BamHI N by 2 kb to nucleotide 129,088.
To construct pRB5216, a 740-bp PCR product was generated from pRB5210 with the Pfu polymerase and the primers P5 and P7 (GGC CCG GGC CGT TCC ACG GAG CTG GTA TC). P7 inserted an EagI site 120 bp upstream from the stop codon of α22/US1.5. The PCR product was digested with EagI and DraIII and subcloned into pRB5210 digested with DraIII and EagI. In the resulting plasmid, pRB5216, both ICP22 and US1.5 open reading frames were truncated by 40 3′ codons.
To construct pRB5217, a 1.2-kb PCR product was generated from pRB5210 with the Pfu polymerase and the primers P3 and P8 (ATA GGG CGG CCG GGT GGA GAA GCG CAT TTT). P8 inserted an EagI site 30 bp upstream from the carboxyl-terminal stop codon of ICP22 and US1.5. The PCR product was digested with EagI and subcloned into pRB5212 digested with EagI. In the resulting plasmid, pRB5217, both ICP22 and US1.5 open reading frames were truncated by 10 3′ codons.
To construct pRB5218, a 1.2-kb PCR product was generated from pRB5210 with the Pfu polymerase and the primers P3 and P9 (GCA GCC CGG CCG ACA CTT GCG GTC TTC TGC). P9 inserted an EagI site 66 bp upstream from the stop codon of ICP22 and US1.5. The PCR product was digested with EagI and subcloned into EagI-digested pRB5212. In the resulting plasmid, pRB5218, both ICP22 and US1.5 open reading frames are truncated by 22 3′ codons.
To construct pRB5219, a 1.3-kb PCR product was generated from pRB5210 with the Pfu polymerase and the primers P10 (CCG GTA CCT TTT CTG GAT GGC CGA CAT TTC CCC AGG) and P11 (GCC GGT ACC ACG CTG GGA CAA ACG CTT TGA TTT TGG). P10 inserted a KpnI site adjacent to the initiator methionine codon of ICP22, whereas P11 placed a KpnI site downstream and adjacent to the stop codon of ICP22 and US1.5. The PCR product was digested with KpnI and subcloned into the KpnI site of pRB4297 downstream of the αβγ promoter. The αβγ promoter was constructed by cloning the −12 to −520 bp upstream promoter region of α4 in front of a polylinker. Next, 200 bp of the 240-bp γ1 promoter of UL19 (VP5) was cloned in at the −12 position. This created a promoter which allows the expression of an inserted gene throughout the herpesvirus infection cycle (1a). The resulting plasmid, pRB5219, contained the open reading frames of the α22 gene driven by the αβγ promoter.
To construct pRB5243, a 1.2-kb PCR product was generated from pRB5210 with the Pfu polymerase and the primers P3 and P12 (GGT GGA CGG CCG CAT TTT CCG GCA GCC GTC). P12 inserted an EagI site 45 bp upstream from the stop codon of ICP22 and US1.5. The PCR product was digested with EagI and subcloned into EagI-digested pRB5212. In the resulting plasmid, pRB5243, both ICP22 and US1.5 open reading frames are truncated by 15 3′ codons.
To construct pRB5244, a 1.2-kb PCR product was generated from pRB5210 with the Pfu polymerase and the primers P3 and P13 (GCG CAT CGG CCG GCA GCC GTC CAG ACA CTT GC). P13 inserted an EagI site 54 bp upstream from the stop codon of ICP22 and US1.5. The PCR product was digested with EagI and subcloned into EagI-digested pRB5212. In the resulting plasmid, pRB5244, both ICP22 and US1.5 open reading frames are truncated by 18 3′ codons.
To construct pRB5251, a 1.1-kb PCR product was generated from pRB5210 with the Pfu polymerase and the primers P3 and P14 (TCT GAG CGG CCG TCC GAT ACA GCC TTG GAG TCT). P14 inserted an EagI site 115 bp downstream from the initiation methionine (Met1) of ICP22 and US1.5. The PCR product was digested with EagI and subcloned into EagI-digested pRB5212. In the resulting plasmid, pRB5251, the ICP22 open reading frame is truncated by 47 5′ codons, and the first available codon for translational initiation is Met90.
Cosmids.
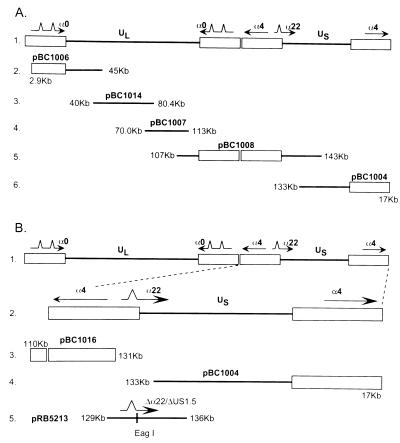
The set of cosmids used in this study was derived from HSV-1(F) DNA as described elsewhere (7, 12) and is illustrated in Fig. 1A, lines 2 through 6. The sequences contained in the cosmid set were as follows: pBC1004, nucleotides 133,052 to 17,029; pBC1006, nucleotides 2,945 to 45,035; pBC1007, nucleotides 77,933 to 116,016; pBC1008, nucleotides 106,750 to 142,759; and pBC1014, nucleotides 40,617 to 80,454. Cosmid pBC1016, containing the nucleotides 110,095 to 131,534, was constructed by digesting pBC1008 with EcoRI, followed by gel purification. The desired DNA fragment was then ligated into the multicloning site of the SuperCos1 cosmid vector (Stratagene) and packaged into lambda phage with the Gigapack XLII (Stratagene) packaging extract according to the manufacturer’s instructions.
FIG. 1.
Schematic representation of the construction of recombinant viruses. (A) Line 1, sequence arrangement of the HSV-1 genome showing the unique long (UL) and unique short (US) sequences and the location of the α genes 0, 4, and 22. Lines 2 to 6, domains of the HSV-1 cosmid set used for the construction of recombinants. (B) Construction of the Δα22/ΔUS1.5 virus. Line 2, expansion of the S component of HSV-1 DNA. Lines 3, 4, and 5, domains of cosmids pBC1016 and pBC1004 and plasmid pRB5213. Plasmid pBR5213 was constructed to bridge the gap in the nonoverlapping cosmids pBC1016 and pBC1004. In this plasmid, the α22/US1.5 open reading frame was replaced with a unique EagI restriction site.
Construction of recombinant viruses.
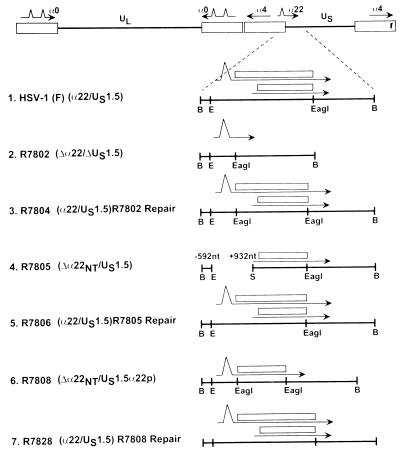
The construction of recombinant virus R7802 (Δα22/ΔUS1.5) involved the following steps. (i) The cosmid pBC1008 was digested with the restriction enzyme EcoRI to create a gap in the cosmid within the α22/US1.5 region (Fig. 1B, lines 3 and 4), creating cosmid pBC1016. (ii) The cosmids (pBC1006, pBC1014, pBC1007, pBC1016, and pBC1004) were digested with the restriction enzyme PacI to release the HSV-1 sequences from the cosmid vector. (iii) A bridging plasmid (pBR5213) from which the entire α22/US1.5 open reading frame had been deleted was constructed. This plasmid has a 2.5-kb overlap with pBC1016 and a 1-kb overlap with the cosmid pBC1004 (Fig. 1B, lines 3, 4, and 5). (iv) A second plasmid (pBR5219), which contained the open reading frame for α22/US1.5 driven by the recombinant herpesvirus αβγ promoter but lacking sequences overlapping within the cosmid set, was constructed. (v) The modified cosmid set in amounts of 1 μg each (pBC1004, pBC1006, pBC1007, pBC1014, and pBC1016), the linearized bridging plasmid pBR5213 in amounts ranging from 0 to 0.8 μg lacking the α22/US1.5 gene, and the α22/US1.5 expression plasmid (pBR5219; 0.1 μg) were transfected into Vero cells with Lipofectamine (Gibco BRL, Gaithersburg, Md.) according to the manufacturer’s instructions. The progeny virus was designated R7802 (Δα22/ΔUS1.5). In addition, the cells were transfected with the cosmid set (pBC1006, pBC1014, pBC1007, pBC1004, and pBC1016) without the bridging plasmid (pBR5213) to yield R7805 (Δα22NT/US1.5) (Fig. 2, line 4).
FIG. 2.
Schematic representation of the recombinants with amino-terminal deletion in the α22 gene. Line 1, BamHI N fragment of HSV-1(F) showing the open reading frames of ICP22 (open rectangles) and US1.5 along with their mRNA transcripts (thin lines). Line 2, R7802 (Δα22/ΔUS1.5) and a schematic representation of the BamHI N fragment (pRB5212). The open reading frames of ICP22 and US1.5 were replaced with a unique EagI restriction site. Line 3, R7804 (R7802 repair). The BamHI N fragment (pRB5214) was restored. Line 4, schematic diagram of the BamHI N fragment in R7805 (Δα22/US1.5) recombinant virus. Line 5, representation of the BamHI N fragment of R7806 (R7805 repair). The BamHI N fragment (pRB5210) was restored. Line 6, representation of the BamHI N fragment in R7808 (Δα22/US1.5). The open reading frame of US1.5 was cloned into the unique EagI site of pBR5212 to yield pRB5215. Line 7, representation of the BamHI N fragment of R7828 (R7808 repair). The BamHI N fragment (pRB5210) from wild-type virus HSV-1(F) was restored. Abbreviations: B, BamHI; E, EcoRI; S, Sau3AI.
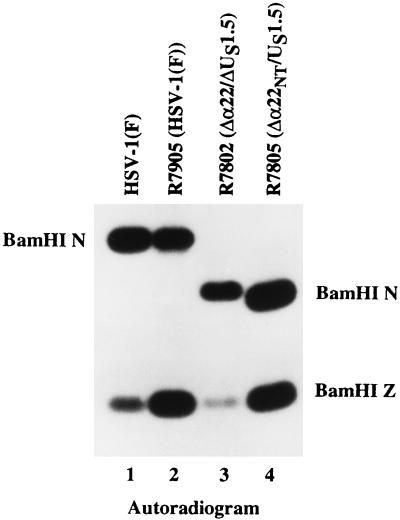
The genotypes of the wild-type parent HSV-1(F) and of the recombinant viruses R7905 [HSV-1(F), derived from the original cosmid set], R7802 (Δα22/ΔUS1.5), and R7805 (Δα22/US1.5) were analyzed as follows. Viral DNAs were isolated and digested with BamHI, electrophoretically separated in agarose gels, transferred to a nylon membrane, and hybridized to 32P-labeled BamHI N (pBR5210) as described above. The probe pBR5210 (BamHI N) hybridized to the 4.9-kb BamHI N fragment of wild-type HSV-1(F) and R7905 [HSV-1(F)] (Fig. 3, lanes 1 and 2). The probe hybridized to a 3.6-kb BamHI fragment corresponding to the predicted size of BamHI N in R7802 (Δα22/US1.5) (Fig. 3, lane 3). The probe also hybridized to a 3.4-kb fragment corresponding to the predicted size of BamHI N in R7805 (Δα22NT/US1.5) (Fig. 3, lane 4). The probe hybridizes to the BamHI Z fragment since this fragment contains a portion of the inverted repeat common with BamHI N. These results are consistent with the predicted size of the BamHI N region with the open reading frame of α22/US1.5 (R7802) deleted and with the deletion of the promoter elements and amino-terminal region of α22 (R7805).
FIG. 3.
Autoradiographic images of electrophoretically separated BamHI digests of recombinant viral DNA hybridized with 32P-labeled pRB5210. The digests were electrophoretically separated in a 0.8% agarose gel, transferred to Zeta-probe membrane, and hybridized with 32P-labeled pRB5210 carrying the HSV-1(F) BamHI N fragment. Lanes: 1, the 4.9-kb BamHI N fragment from HSV-1(F); 2, the 4.9-kb BamHI N fragment from the R7905 [HSV-1(F)] cosmid virus; 3, the 3.6-kb BamHI N fragment from R7802 recombinant virus; 4, the 3.4-kb BamHI N fragment from the R7805 recombinant virus.
To construct R7808 (Δα22/US1.5αp), the predicted open reading frame of US1.5, that is, the sequence encoding codons 171 to 420 of ICP22, was PCR amplified and cloned into plasmid pRB5212. The new plasmid, pRB5215, contained an additional EagI restriction site at the beginning of the US1.5 open reading frame (Fig. 2, line 6). This plasmid was cotransfected with the R7802 (Δα22/ΔUS1.5) recombinant viral DNA into rabbit skin cells. Several plaques were isolated, and the structure of the recombinant virus R7808 (Δα22/US1.5αp) was verified by hybridization of electrophoretically separated BamHI-digested viral DNA with nick-translated pRB5210 (BamHI N fragment; data not shown).
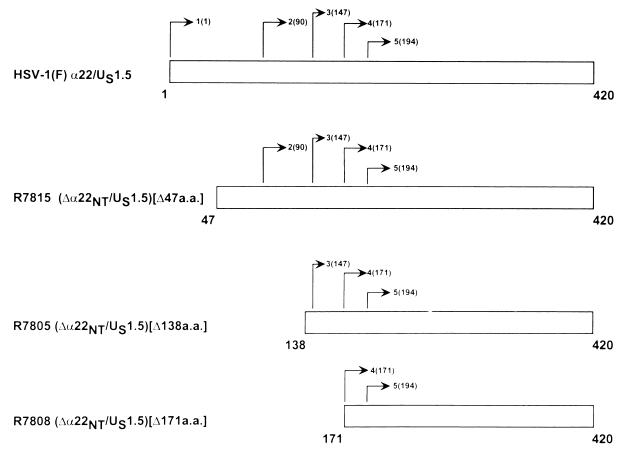
To construct R7815 (Δα22NT/US1.5)[Δ47a.a.], the predicted open reading frame of ICP22/US1.5, that is, the sequence encoding codons 47 to 420 of ICP22, was PCR amplified and cloned into plasmid pRB5251. This plasmid was cotransfected with the R7802 (Δα22/ΔUS1.5) recombinant viral DNA into rabbit skin cells. Several plaques were isolated, and the structure of the recombinant virus R7815 (Δα22NT/US1.5)[Δ47a.a.] (see Fig. 7) was verified by hybridization of electrophoretically separated BamHI-digested viral DNA with nick-translated pRB5210 (BamHI N fragment; data not shown).
FIG. 7.
Schematic representations of the ICP22 open reading frame of wild-type and mutant HSV-1 showing the location of methionine codons. Line 1, positions of the four methionine codons in the first 200 amino acids of HSV-1. Line 2, R7815 (Δα22NT/US1.5)[Δ47a.a.] lacking 47 amino acids deleted from the amino terminus. The first methionine available for translational initiation is at codon 90. Line 3, R7805 (Δα22NT/US1.5)[Δ138a.a.], lacking the amino-terminal 138 amino acids. The first methionine available for translational initiation is at codon 147. Line 4, R7808 (Δα22NT/US1.5)[Δ171a.a.] lacking amino-terminal 170 amino acids. The first methionine available for translational initiation is at codon 171.
Construction of ICP22/US1.5 carboxyl-terminal truncation viruses.
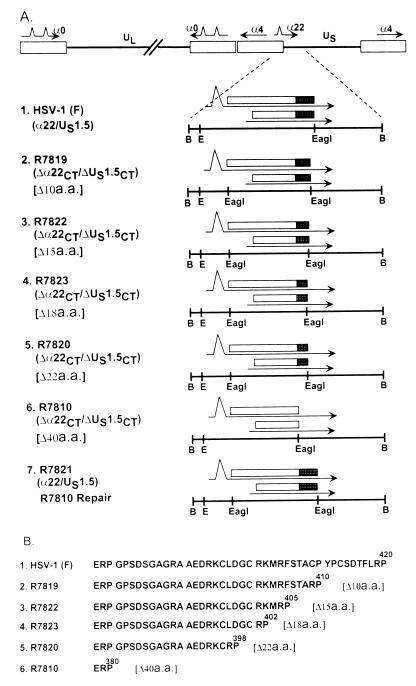
To investigate the function of the carboxyl-terminal domain of ICP22/US1.5, a series of recombinant viruses lacking the terminal 10, 15, 18, 22, or 40 codons of the genes were constructed. To construct the recombinant virus R7819, which lacks the 3′-terminal 10 codons of the α22/US1.5 open reading frames (Fig. 4A, line 2, and 4B, line 2), the ICP22/US1.5 gene was amplified using PCR. This amplified product extended from the initiation methionine codon to codon 410 of α22 and contained a diagnostic EagI restriction endonuclease site at the amino terminus of the α22/US1.5 genes. The PCR product was cloned into plasmid pRB5212 to create pRB5217. This plasmid (pRB5217) was cotransfected with R7802 (Δα22/ΔUS1.5) viral DNA into rabbit skin cells. Several plaques were isolated, and the structure of the mutant virus R7819 was verified by hybridization of electrophoretically separated BamHI-digested viral DNA with nick-translated pRB5210.
FIG. 4.
Schematic representation of terminal sequences of the recombinants carrying 3′-terminal deletions in the α22/US1.5 genes of HSV-1(F). (A) BamHI N sequence arrangements in recombinants. The rectangles represent the open reading frames. The filled boxes represent the 40 carboxyl-terminal codons. The numbers in brackets indicate the number of codons deleted from the termini of the open reading frames. Abbreviations: B, BamHI; E, EcoRI. (B) Schematic diagram of the 43 carboxyl-terminal amino acids of α22/US1.5 protein from amino acid 377 to the end of the α22/US1.5 protein.
In a similar fashion, cotransfection of R7802 DNA with plasmid pRB5216 yielded R7810 (Δα22CT/US1.5CT)[Δ40a.a.]. Cotransfection of R7802 DNA with plasmid pRB5243 yielded R7822 (Δα22CT/US1.5CT)[Δ15a.a.], cotransfection with plasmid pRB5244 yielded R7823 (Δα22CT/US1.5CT)[Δ18a.a.], and cotransfection with plasmid pRB5218 yielded R7820 (Δα22CT/US1.5CT)[Δ22a.a.] (Fig. 4).
Repair of sequences deleted from the viral genomes.
The deletion of R7802 (Δα22/ΔUS1.5) was repaired to yield the repair virus R7804 (Fig. 2, line 3). The open reading frame of α22/US1.5 was PCR amplified and cloned into plasmid (pRB5212) to create pRB5214, which was identical to BamHI N except for the presence of an additional EagI restriction site at the beginning of the α22/US1.5 open reading frame (Fig. 2; compare lines 2 and 3). This plasmid (pRB5214) was cotransfected with viral DNA of R7802 (Δα22/ΔUS1.5) into rabbit skin cells. The selection for the recombinant virus took advantage of the observation that R7802 viral DNA did not form plaques in transfected rabbit skin cells. Therefore, the presence of plaques on this cell line would signal the presence of a recombinant virus. Several plaques were isolated, and the structure of the recombinant R7804 virus was verified by hybridization of electrophoretically separated BamHI-digested viral DNA with nick-translated pRB5210 (BamHI N fragment; data not shown).
The recombinant viruses R7805 (Δα22NT/US1.5) and R7808 (Δα22NT/US1.5) were repaired by blind selection on rabbit skin cells to yield R7806 (α22/US1.5) and R7828 (α22/US1.5), respectively (Fig. 2, lines 6 and 7). In a similar fashion, R7810 (Δα22CT/US1.5CT)[Δ40a.a.] was repaired with plasmid pRB5212 to yield R7821 (α22/US1.5) (Fig. 4, line 7).
Antibodies.
The US11, ICP0 (H1083) mouse monoclonal antibodies, the rabbit polyclonal antibody R77 amino-terminal ICP22, W2 against the carboxyl terminal of ICP22, and the rabbit polyclonal W1 against UL38 were described previously (1, 13, 31, 34). Goat anti-rabbit or anti-mouse alkaline phosphatase-conjugated secondary antibody was purchased from Bio-Rad (Hercules, Calif.).
Electrophoretic separation and immunoblotting of viral proteins.
Replicate cultures of Vero or rabbit skin cells in 25-cm2 flasks were exposed to 10 PFU of the appropriate virus per cell. The cells were maintained in medium 199V, consisting of a mixture of 199 supplemented with 1% calf serum. At 18 h after infection, the cells were rinsed and scraped into 1 ml of ice-cold phosphate-buffered saline lacking Ca2+ and Mg2+ (PBS-A), centrifuged for 5 min in a microcentrifuge at 4°C, and resuspended in 350 μl of PBS-A* (PBS-A with 0.1 mM TPCK [tolylsulfonyl phenylalanyl chloromethyl ketone], 0.1 mM TLCK [tosyl-l-phenylalanine chloromethyl ketone], 0.1 mM PMSF [phenylmethylsulfonyl fluoride], 1.0% [vol/vol] Nonidet P-40 [NP-40], 40 mM b-glycerophosphate, and 1.0% [wt/vol] sodium deoxycholate). The lysates were sonicated briefly and frozen in aliquots at −70°C. Aliquots were thawed on wet ice, and 20 μl of disruption buffer (12.5 mM Tris-HCl [pH 6.8], 0.5% sodium dodecyl sulfate (SDS), 2.5% glycerol, 5% β-mercaptoethanol) was added to 40 μl of infected cell lysate and boiled for 5 min. The solubilized proteins were subjected to electrophoresis in denaturing polyacrylamide gels (60 μl per lane), transferred to a nitrocellulose membrane (Schleicher & Schuell), and reacted with the appropriate antibody. The bound antibody was visualized with antibody conjugated to alkaline phosphatase (Bio-Rad) and visualized according to the manufacturer’s instructions.
Analyses of viral DNA by hybridization.
Cytoplasmic DNAs from infected cells were harvested by resuspending two roller bottle cultures (approximately 4 × 108 cells) in 20 ml of 150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 1.5 mM MgCl2 with NP-40 added to a final concentration of 0.1% (vol/vol). The nuclei were pelleted by centrifugation at 2,500 rpm for 5 min in a Beckman (model TJ-6) tabletop centrifuge. The supernatant fluid containing cytoplasmic virions was collected, and SDS, EDTA, and β-mercaptoethanol were added at final concentrations of 0.2%, 5 mM, and 50 mM, respectively. Phenol-chloroform extraction was performed twice followed by a chloroform extraction. Viral DNA was then precipitated with 2 volumes of 100% ethanol and centrifuged at 10,000 rpm in an SS-34 rotor. Viral DNA pellet was resuspended in 1 ml of sterile H2O and RNase A was added at a final concentration of 20 μg/ml. The mixture was incubated for 15 min at 37°C and centrifuged through a linear 5 to 20% potassium acetate gradient in 10 mM Tris-HCl (pH 8.0)–5 mM EDTA in an SW41 rotor (Beckman) at 40,000 rpm for 3.5 h at 20°C. The pellet was gently rinsed once with H2O, resuspended in 0.4 ml of H2O, precipitated by the addition of 2 volumes of 100% ethanol, solubilized, digested with BamHI, electrophoretically separated on an 0.8% agarose gel, and transferred to a nylon membrane (Bio-Rad). The hybridization and membrane-stripping procedures were performed as recommended by the manufacturer. The plasmid pRB5210 was used to make [32P]dCTP-labeled probe using a Nick Translation Kit (Promega, Madison, Wis.).
Cell fractionation.
HEp-2 cells grown in 25-cm2 flask cultures were infected with 10 PFU of HSV-1(F), R7805(Δα22NT/US1.5), R7808 (Δα22NT/US1.5α22), and R7810 (Δα22CT/ΔUS1.5CT) per cell. At 18 h after infection, the cells were washed with 5 ml of PBS(A) and then scraped into 1 ml of PBS(A) and resuspended into 100 μl of buffer A (50 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 1 mM PMSF, 0.1 mM TLCK, 0.1 mM TPCK). The cells were lysed by the addition of 4 μl of 10% NP-40 and stored at 25°C for 5 min. The nuclei were separated from the cytoplasm by centrifugation in a microcentrifuge. The supernatant (cytoplasmic fraction) was removed, and 50 μl of disruption buffer was added. The nuclei were resuspended in 75 μl of PBS(A) and 50 μl of disruption buffer.
RESULTS
Construction of the Δα22/ΔUS1.5 and US1.5 recombinant viruses.
To conduct a comprehensive analysis of ICP22/US1.5, it was necessary to construct a virus from which the entire open reading frame of α22/US1.5 had been deleted. To delete the entire α22/US1.5 domain, we used a modified cosmid system as described previously (7, 12) and in Materials and Methods. This cosmid set is illustrated in Fig. 1A, lines 2 to 6. Construction of the Δα22/ΔUS1.5 cosmid virus involved the following steps. (i) The cosmid pBC1008 was digested with the restriction enzyme EcoRI to create a gap within the α22/US1.5 region (Fig. 1B, lines 3 and 4) to create the cosmid pBC1016 (see Materials and Methods). (ii) A bridging plasmid (pBR5213) from which the entire α22/US1.5 open reading frame had been deleted was constructed (Fig. 1B, line 5). (iii) A second plasmid (pBR5219) which contained the open reading frame for α22/US1.5 driven by the recombinant herpesvirus αβγ promoter but without any overlapping sequences within the cosmid set was constructed. (iv) The modified cosmid set (pBC1004, pBC1006, pBC1007, pBC1014, and pBC1016), the bridging plasmid pBR5213, lacking the α22/US1.5 genes, and the α22/US1.5 expression plasmid (pBR5219) were transfected into Vero cells to yield R7802 (Fig. 2, line 2). In addition, the cells were transfected with the cosmid set (pBC1006, pBC1014, pBC1007, pBC1004, and pBC1016) without the bridging plasmid (pBR5213) to yield R7805 (Fig. 2, line 4). The subsequent plaques were isolated, and the genotypes were analyzed.
The genotypes of the wild-type parent HSV-1(F) and of the recombinant viruses R7905 [HSV-1(F) derived from the original cosmid set], R7802 (Δα22/ΔUS1.5), and R7805 (Δα22/US1.5) were analyzed as follows. Viral DNAs were isolated and digested with BamHI, electrophoretically separated in agarose gels, transferred to a nylon membrane, and hybridized to 32P-labeled BamHI N (pBR5210) as described in Materials and Methods. The probe hybridized to the 4.9-kb BamHI N fragment of wild-type HSV-1(F) and R7905 [HSV-1(F)] (Fig. 3, lanes 1 and 2). The probe hybridized to a 3.6-kb BamHI fragment corresponding to the predicted size of BamHI N in R7802 (Δα22/ΔUS1.5) (Fig. 3, lane 3) and to a 3.4-kb fragment corresponding to the predicted size of BamHI N in R7805 (Δα22/US1.5) (Fig. 3, lane 4). These results are consistent with the predicted size of the BamHI N region deleted for the open reading frame of α22/US1.5 (R7802) and the deletion of the promoter elements and amino terminus of α22 (R7805).
The recombinant virus R7808 (Δα22/US1.5) and the repair viruses of R7802 (Δα22/ΔUS1.5), R7805 (Δα22/US1.5), and R7808 (Δα22/US1.5) were constructed by blind selection on rabbit skin cells to yield R7804 (α22/US1.5), R7806 (α22/US1.5), and R7828 (α22/US1.5) (Fig. 2, lines 3, 5, 6, and 7) as described in Materials and Methods.
Biologic properties of R7802 (Δα22/ΔUS1.5) and R7805 (Δα22/US1.5).
Of the various recombinants produced in these studies, two are of key importance. These are R7802 and R7805. R7802 lacked the coding domains of both α22 and US1.5 (Fig. 2, line 2), whereas R7805 contained the coding sequences of US1.5 but not the 5′ sequence that codes the amino terminus of ICP22 (Fig. 2, line 4). In our studies, R7802 could not be differentiated from R325 with respect to biologic properties. Thus, it replicated in primate cell lines (Vero and HEp-2) and to a lesser extent in rabbit skin cells in a manner consistent with the findings of previous studies of R325 (24, 33) and the report on a homologue of R7802 described by Poffenberger et al. (19, 20). One remarkable observation with significant consequences was that R7802 did not yield plaques on transfection of rabbit skin cells. Plaques were formed, however, if a plasmid expressing ICP22 was cotransfected with the cosmid set, but under conditions in which ICP22 could not recombine with the cosmids to form an infectious virus. The failure of transfected R7802 DNA to yield plaques was of special significance, since virtually any plasmid containing coding sequences of ICP22 or US1.5 genes rescued the capacity to make plaques, and recombinant viruses could easily be selected on the basis of that property.
R7805 was tested for its ability to cause morbidity or mortality upon intracerebral inoculation in mice. The 50% lethal doses (PFU) were 5.6 × 104 for R7805, 3.4 × 101 for R7806 in which the α22/US1.5 lesions were repaired, and 1.1 × 102 for the wild-type parent, HSV-1(F).
The posttranslational modification of US1.5 is determined by the UL13 protein kinase.
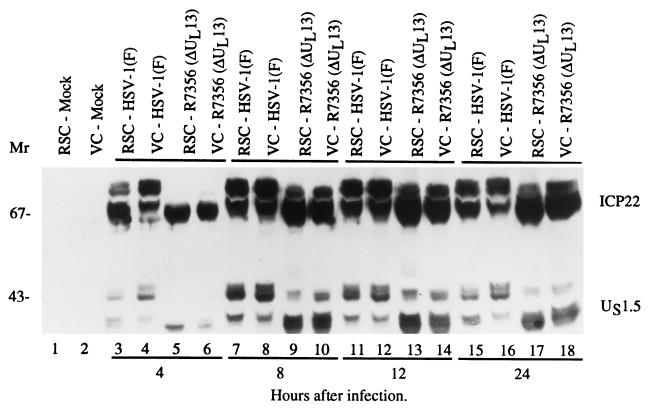
An earlier study (23) has shown that UL13 mediates the phosphorylation and posttranslational processing of ICP22. A central question was whether the UL13 protein kinase also mediates the posttranslational processing of US1.5 protein. To investigate this question, replicate cultures of Vero or rabbit skin cells were exposed to 10 PFU of wild-type [HSV-1(F)] or ΔUL13 (R7356) viruses per cell. At 4, 8, 12, and 24 h after infection, the cells were harvested, solubilized, electrophoretically separated on a denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with a polyclonal antibody to α22/US1.5 (Fig. 5).
FIG. 5.
Photograph of an immunoblot of electrophoretically separated lysates of cells mock infected or infected with HSV-1(F) or R7356 (ΔUL13) and reacted with antibody to ICP22/US1.5. Vero cells (VC) and rabbit skin cells (RSC) harvested at various times (4 to 24 h) after infection were solubilized, subjected to electrophoresis in a denaturing 10% polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the rabbit polyclonal antibody (W2) made against the 138 carboxyl-terminal amino acids of ICP22/US1.5.
The antibody to the carboxyl-terminal 138 amino acids of ICP22/US1.5 reacted with several bands of ICP22 (Mr, 67,000 to 72,000) and US1.5 (Mr, 35,000 to 48,000) corresponding to the isoforms resulting from posttranslational processing of the two proteins (Fig. 5, lanes 7 and 8). ICP22 and US1.5 proteins were not processed at 4 h after infection and exhibited a reduced number of isoforms in lysates of cells infected with the ΔUL13 (R7356) virus. The decrease in the number of isoforms of US1.5 paralleled the decrease in the number of isoforms of ICP22 (Fig. 5; compare lane 8 with lane 9). These results indicate that the UL13 protein kinase mediated some of the posttranslational processing of the US1.5 protein. The results also indicate that (i) at least one domain targeted by the UL13 protein kinase is located in the amino-terminal 250 amino acids shared by ICP22 and US1.5 protein, and (ii) the same domain is responsible for the differentiation of several isoforms of these proteins.
Processing of US1.5 protein expressed by mutant viruses.
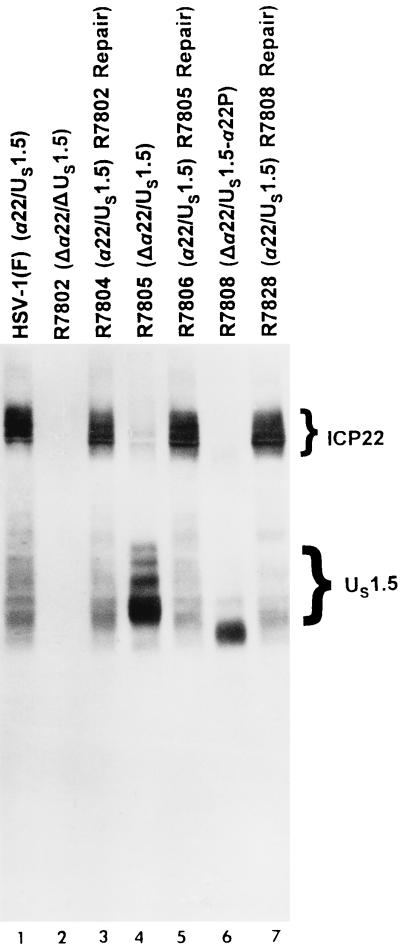
The purpose of the next series of experiments was to verify that R7802 did not express α22/US1.5 proteins and to examine the expression of US1.5 in R7805 and R7808. Replicate cultures of Vero cells were exposed to 10 PFU of HSV-1(F), R7802 (Δα22/ΔUS1.5), R7804 (R7802 repair), R7805 (Δα22/US1.5), R7806 (R7805 repair), R7808 (Δα22/US1.5), or R7828 (R7808 repair) virus per cell. At 18 h after infection, the cells were harvested, solubilized, electrophoretically separated on a denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with the polyclonal antibody to the carboxyl terminal of ICP22/US1.5 proteins as described in Materials and Methods. The results (Fig. 6) were as follows. (i) Both ICP22 and US1.5 were present in lysates of HSV-1(F) (Fig. 6, lane 1). (ii) Both ICP22 and US1.5 were absent in lysates of R7802 (Δα22/ΔUS1.5)-infected cell lysates (Fig. 6, lane 2). (iii) The US1.5 protein was detected in lysates of cells infected with R7805 (Δα22/US1.5) and R7808 (Δα22/US1.5) (Fig. 6, lanes 4 and 6). In R7805, US1.5 was overexpressed compared to US1.5 in HSV-1(F)-infected cell extracts (Fig. 6; compare lanes 1 and 4). The US1.5 protein in R7805 was posttranslationally processed and exhibited a range of proteins having an Mr of 33,000 to 48,000, similar to US1.5 of HSV-1(F). In R7808, US1.5 was also overexpressed compared to HSV-1(F) (Fig. 6; compare lanes 1 and 6). However, in this recombinant, US1.5 was not posttranslationally processed and formed two predominant bands, a major band with an apparent Mr of 33,000 and a minor band with an apparent Mr of 36,000.
FIG. 6.
Photograph of an immunoblot of electrophoretically separated lysates of cells mock-infected or infected with HSV-1(F), R7802, R7804, R7805, R7806, R7808, or R7828 and reacted with antibody to ICP22/US1.5. Vero cells harvested 18 h after infection were solubilized, subjected to electrophoresis in a denaturing 8% polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the polyclonal antibody W2. Lanes: 1, HSV-1(F); 2, R7802 (Δα22/ΔUS1.5); 3, R7804 (R7802 repair); 4, R7805 (Δα22/US1.5); 5, R7806 (R7805 repair); 6, R7808 (Δα22/US1.5-α22P); 7, R7828 (R7808 repair).
(iv) The repair viruses R7804 (R7802 repair), R7806 (R7805 repair), and R7828 (R7808 repair) exhibited wild-type levels of expression and posttranslational processing of ICP22/US1.5 proteins (Fig. 6; compare lane 1 with lanes 3, 5, and 7). ICP4, measured by its reactivity with a corresponding monoclonal antibody (data not shown), served as a loading control.
We conclude from these studies that the ICP22 amino acids 147 to 171 are required for posttranslational processing of a truncated product of ICP22 that corresponds to US1.5. Forced translation initiation at Met171 yielded a product that did not appear to be posttranslationally processed.
Translational initiation within the domain containing US1.5 preferentially occurs at amino acid 147 of the ICP22 sequence.
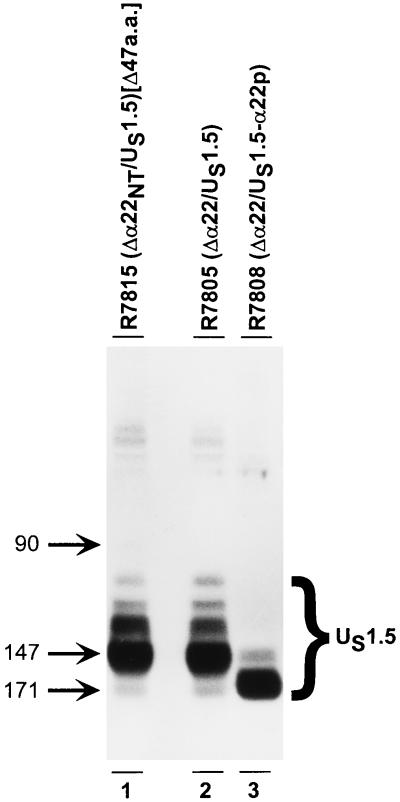
The purpose of this series of experiments was twofold. The first objective was to attempt to produce amino-terminal truncations of ICP22 other than those which correspond to the sequence encoding the US1.5 protein. The second objective was to characterize further the product of the ICP22/US1.5 genes encoded by R7808 and R7805. Preliminary experiments designed to meet the first objective indicated that all 5′-terminal truncations of the α22 gene 5′ of the Met147 codon yielded proteins that resembled the US1.5 protein. The hypothesis that emerged from the studies described above was that the preferred translation initiation methionine within the US1.5 transcript was at codon 147. To test this hypothesis, we constructed a mutant in which the amino-terminal 47 codons of the α22 coding sequence were deleted. The truncated α22 gene in the resulting virus, R7815, contained three possible initiator methionine codons, Met90, Met147, and Met171 (Fig. 7, line 2). Vero cells were exposed to 10 PFU of R7805 (Δα22/US1.5), R7808 (Δα22/US1.5), or R7815 (Δα22NT/US1.5) virus per cell. At 18 h after infection, the cells were harvested, solubilized, electrophoretically separated in a denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with the polyclonal antibody to the carboxy terminus of ICP22. The expression of US1.5 was examined, and the results (Fig. 8) were as follows.
FIG. 8.
Photograph of an immunoblot of electrophoretically separated lysates of cells infected with R7815, R7805, or R7808 and reacted with the polyclonal antibody W2 to ICP22/US1.5. Vero cells harvested at 18 h after infection were solubilized, subjected to electrophoresis in a denaturing 10% polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the polyclonal antibody prepared against the carboxyl-terminal amino acids of ICP22/US1.5. Lanes: 1, R7815 (Δα22NT/US1.5)[Δ47a.a.]; 2, R7805 (Δα22/US1.5); 3, R7808 (Δα22/US1.5). The numbers next to the arrows indicate the initiator methionine for the unprocessed protein product in each lane.
(i) In cell extracts infected with R7815, the predominant species of protein initiated at amino acid 147, even though the first methionine in this construct is at amino acid 90 (Fig. 7A, lane 1). Because of the variability seen between strains of HSV-1, the presence of this methionine in HSV-1(F) was verified by sequencing.
(ii) In cell extracts infected with R7805, the predominant species of protein initiated at amino acid 147, with weak initiation at amino acid 171 (Fig. 8, lane 2). The initiation at amino acid 171 can be seen in extracts infected with R7808 (Fig. 8, lane 3).
(iii) In cell extracts infected with R7808, the predominant species of protein initiates at amino acid 171, with no apparent initiation at amino acid 194 (Fig. 8, lane 3). We conclude from these studies that in the absence of the initiator methionine, the preferred initiator methionine is the codon Met147.
The ICP22 function that enhances the expression of a subset of γ2 genes is located at the carboxyl-terminal domain shared with the US1.5 protein and can be expressed by the latter protein.
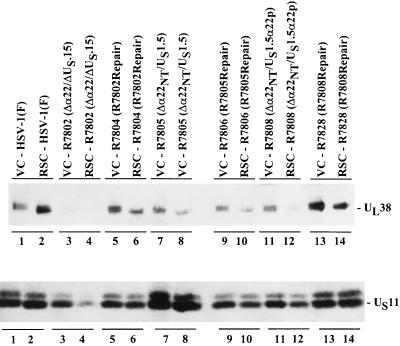
In this series of experiments replicate cultures of Vero or rabbit skin cells were exposed to 10 PFU of HSV-1(F), R7802 (Δα22/ΔUS1.5), R7804 (R7802 repair), R7805 (Δα22/US1.5), R7806 (R7805 repair), R7808 (Δα22/US1.5), or R7828 (R7808 repair) virus per cell. At 18 h after infection, the cells were harvested, solubilized, electrophoretically separated in a denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted sequentially with the monoclonal antibody to US11 and the polyclonal antibody (W1) to UL38. The expression levels of US11 and UL38 were examined. The results (Fig. 9) were as follows.
FIG. 9.
Photograph of an immunoblot of electrophoretically separated lysates of cells mock-infected or infected with HSV-1(F), R7802, R7804, R7805, R7806, R7808, or R7828 and reacted with antibodies to UL38 and US11. Replicate cultures of Vero cells (VC) (odd-numbered lanes) or rabbit skin cells (RSC) (even-numbered lanes) harvested at 18 h after infection were solubilized, subjected to electrophoresis in a denaturing polyacrylamide gel, transferred to a nitrocellulose sheet, then sequentially reacted with the monoclonal antibody to US11, and the polyclonal antibody to UL38. Lanes: 1 and 2, HSV-1(F); 3 and 4, R7802 (Δα22/ΔUS1.5); 5 and 6, R7804 (R7802 repair); 7 and 8, R7805 (Δα22NT/US1.5); 9 and 10, R7806 (R7805 repair); 11 and 12, R7808 (Δα22NT/US1.5α22p); 13 and 14, R7828 (R7808 repair).
(i) Vero and rabbit skin cells infected with HSV-1(F) expressed equivalent levels of US11 protein whereas the level of UL38 protein in rabbit skin cells was greater than that detected in Vero cells (Fig. 9; compare lanes 1 and 2).
(ii) Rabbit skin cells infected with R7802 (Δα22/ΔUS1.5) expressed less US11 and UL38 proteins than infected Vero cells (Fig. 9; compare lanes 3 and 4). This phenotype is comparable to the phenotype seen with infection of these cell lines with R7356, a recombinant virus deleted in the UL13 protein kinase, and R325, a recombinant virus with US1.5 deleted (24).
(iii) Vero and rabbit skin cells infected with a US1.5-expressing virus (R7805) expressed equivalent levels of US11 and UL38 (Fig. 9; compare lanes 7 and 8).
(iv) Vero and rabbit skin cells infected with R7808 did not express equivalent levels of US11 and UL38, which is consistent with the observation that US1.5 in this construct is not processed (Fig. 9; compare lanes 11 and 12). The decrease of US11 protein in cells infected with R7808 was not as great as that seen in cells infected with R7802.
(v) Vero and rabbit skin cells infected with R7804 (R7802 repair), R7806 (R7805 repair), and R7828 (R7808 repair) expressed equivalent levels of US11 and UL38 proteins in each cell line (Fig. 9; compare lanes 5, 6, 9, and 10 with lanes 13 and 14).
We conclude that the genetic information required for optimal expression of a subset of late γ2 genes exemplified by US11 and UL38 resides in the domain shared by US1.5 and ICP22. Earlier studies have shown that optimal expression of this subset of γ2 genes requires a functional UL13 protein kinase that, coincidentally, also mediates the posttranslational processing and phosphorylation of ICP22 and US1.5.
All isoforms of US1.5 protein are translocated into the nucleus.
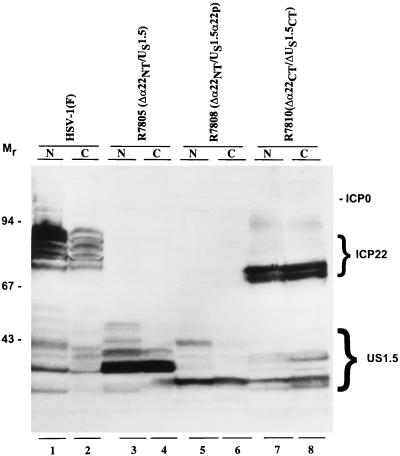
The purpose of the next series of experiments was to determine whether posttranslational processing of the isoforms of US1.5 proteins was dependent on nuclear localization. HEp-2 cells were exposed to 10 PFU of HSV-1(F), R7805 (Δα22/US1.5), R7808 (Δα22/US1.5), or R7810 (Δα22/ΔUS1.5ΔCT) per cell and harvested at 18 h after infection. The nuclei and cytoplasm were separated as described in Materials and Methods. Each fraction was subjected to electrophoresis in a denaturing polyacrylamide gel and was then reacted with the polyclonal antibody made against the carboxyl terminus of ICP22. The fractionation was validated by reacting the nitrocellulose sheet a second time but with antibody against ICP4. As expected, ICP4 localized in the nuclear fractions (data not shown). The results (Fig. 10) were as follows.
FIG. 10.
Photograph of an immunoblot of electrophoretically separated nuclear and cytoplasmic fractions of HEp-2 cells infected with HSV-1(F), R7805, R7808, or R7810. Infected HEp-2 cells were harvested and lysed by the addition of 0.4% NP-40. Nuclear and cytoplasmic fractions prepared as described in Materials and Methods were solubilized, subjected to electrophoresis on an SDS–10% polyacrylamide gel, transferred to nitrocellulose, and reacted with the polyclonal antibody to α22/US1.5 protein. ICP22 and US1.5 protein are indicated on the right, and molecular weights (in thousands) are shown on the left. Lanes: 1 and 2, HSV-1(F); 3 and 4, R7805 (Δα22NT/US1.5); 5 and 6, R7808 (Δα22NT/US1.5α22p); 7 and 8, R7810 (Δα22CT/ΔUS1.5CT). Abbreviations: N, nuclear fraction; C, cytoplasmic fraction.
(i) ICP22 was detected in both the nucleus and the cytoplasm of cells infected with HSV-1(F). The nuclear and cytoplasmic ICP22 were posttranslationally processed to the same extent, but the slowest migrating forms of ICP22 were more abundant in the nucleus than in the cytoplasm. US1.5 protein made in cells infected with HSV-1(F) was present in greater abundance in the nucleus. Moreover, the ratio of the various electrophoretically distinct isoforms of ICP22 in the cytoplasm differed from those in the nucleus (Fig. 10; compare lanes 1 and 2).
(ii) In cells infected with R7805 (Δα22NT/US1.5), the US1.5 protein was more abundant but also present in both nucleus and cytoplasm. Some of the slow-migrating forms of US1.5 were absent or present in smaller amounts in the cytoplasm (Fig. 10; compare lanes 3 and 4).
(iii) In cells infected with R7808 (Δα22NT/US1.5αp), US1.5 was present in both fractions. The nuclear form of US1.5 has an additional major band which is lacking in the cytoplasmic fraction (Fig. 10; compare lanes 5 and 6).
(iv) In cells infected with R7810 (Δα22CT/ΔUS1.5CT) (Fig. 4A, line 6), both ICP22 and the US1.5 protein are unprocessed and distributed in both fractions (Fig. 10; compare lanes 7 and 8).
We conclude the following. (i) All isoforms of ICP22 and US1.5 localized in both the nucleus and cytoplasm. Implicit in this observation is that either US1.5 contains an as-yet unidentified nuclear localization signal or it is transported to the nucleus in association with another protein. (ii) Posttranslational processing of US1.5 protein requires two domains, the amino-terminal domain between amino acids 147 and 171 and the carboxyl-terminal 40 amino acids, although partial processing was noted in US1.5 protein lacking the amino-terminal domain. (iii) Processing of the US1.5 protein does not require the presence of an intact ICP22.
The amino acids required for posttranslational modification of ICP22/US1.5 map to three amino acids within the carboxyl-terminal domain.
In the preceding section, we showed that the deletion of 40 carboxyl-terminal codons yielded a truncated protein that was not posttranslationally processed. To map the sequence required for processing, we constructed the series of carboxyl-terminal deletion mutants shown in Fig. 4A and B. Replicate cultures of Vero cells were exposed to 10 PFU of HSV-1(F), R7819 (Δ10 codons), R7822 (Δ15 codons), R7823 (Δ18 codons), R7820 (Δ22 codons), R7810 (Δ40 codons), or R7821 (repair of R7810) per cell. At 18 h after infection, the cells were harvested, solubilized, electrophoretically separated in a denaturing polyacrylamide gel, electrically transferred to a nitrocellulose sheet, and reacted with the polyclonal antibody (R77) to ICP22. The results (Figure 11) were as follows.
FIG. 11.
Photograph of an immunoblot of electrophoretically separated lysates of cells infected with HSV-1(F), R7819, R7822, R7823, R7820, R7810, or R7821 and reacted with polyclonal rabbit antibody R77 to ICP22. Vero cells harvested at 18 h after infection were solubilized and subjected to electrophoresis in a denaturing 10% polyacrylamide gel, transferred to a nitrocellulose sheet, and reacted with the polyclonal antibody R77 against ICP22. The cells were infected as follows. Lanes: 1, HSV-1(F); 2, R7819 (Δα22/US1.5ΔCT10a.a.); 3, R7822 (Δα22/US1.5ΔCT15a.a.); 4, R7823 (Δα22/US1.5ΔCT18a.a); 5, R7820 (Δα22/US1.5ΔCT22a.a.); 6, R7810 (Δα22/US1.5ΔCT40a.a.); 7, R7821 (R7810 repair).
(i) Processed forms of ICP22 were present in lysates of cells infected with all viruses except those infected with R7810, R7820, or R7823 (Fig. 11, lanes 4 to 6). We noted a slightly higher accumulation of the fastest migrating forms of ICP22 in cells infected with mutants carrying carboxyl-terminal deletions (e.g., R7822 and R7823 [Fig. 11, lanes 3 and 4]).
(ii) Only the fastest migrating forms of ICP22 accumulated in cells infected with R7823 or R7820 (Fig. 11, lanes 4 and 5).
The substitution of lysine for the arginine 404 had no effect on the processing of ICP22, although again, the fastest migrating form accumulated in the infected cells (data not shown).
We conclude from these studies the following. (i) ICP22 encoded by mutants lacking 15 or fewer carboxyl-terminal amino acids were posttranslationally processed by the UL13 protein kinase whereas ICP22 encoded by mutants lacking 18 or more carboxyl-terminal amino acids were not processed. (ii) The three carboxyl-terminal amino acids—Lys402, Met403, and Arg404—appear to be required for posttranslational processing of ICP22.
DISCUSSION
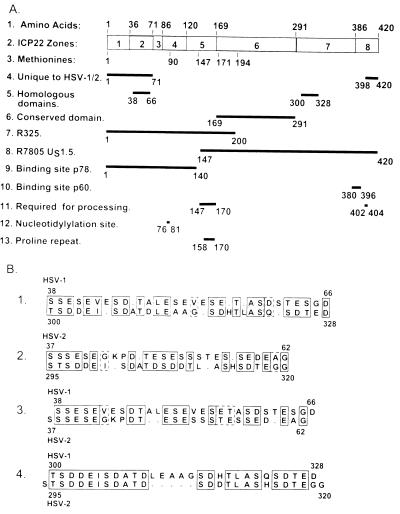
The overall objective of the studies described in this report was to initiate a functional dissection of the α22 gene. Schwyzer et al. (32) in examining the sequence of several homologs of ICP22 identified four distinct zones within the amino acid sequence of ICP22. A key conclusion, echoed in this report, is that ICP22 contains two sets of sequences (one at the amino terminus of ICP22 and one at the carboxyl terminus) that are unique to HSV-1 and HSV-2 and a sequence conserved among the various homologs located between amino acids 169 and 291 of HSV-1 ICP22. Our analyses, shown at the top of Fig. 12A, differentiate eight zones on the basis of amino acid composition or other criteria. We also noted that a sequence of approximately 28 amino acids at positions 38 to 66 is repeated at positions 300 to 328 and that both are conserved in HSV-2 (Fig. 12B). It is convenient to consider the salient features of our results in reference to the amino acid sequence arrangement of the α22 gene. To simplify the discussion, it is also convenient to define two domains in contradistinction to either zones or proteins per se. Thus, the ICP22 domain comprises the amino acid sequence 1 to 146 and is unique to ICP22. The US1.5 domain is shared by both ICP22 and US1.5 protein and extends from amino acid 147 to 420 of ICP22. The thesis we propose to defend on the basis of our results is as follows.
FIG. 12.
Schematic representation of the functional domains of the α22 gene and its products, ICP22 and US1.5 protein. (A) Functional maps. The zones are assigned on the basis of amino acid composition. Zones 1, 3, 5, and 8 are basic whereas zones 2, 4, and 7 are acidic. (B) The sequence of the internal homologous repeats. The sequence alignments are as follows. Lines: 1, HSV-1 amino terminal versus HSV-1 carboxyl terminal; 2, HSV-2 amino terminal versus HSV-2 carboxyl terminal; 3, HSV-1 amino terminal versus HSV-2 amino terminal; 4, HSV-1 carboxyl terminal versus HSV-2 carboxyl terminal. The numbers above and below refer to amino acid numbers of the corresponding ICP22.
(i) Optimal expression of the γ2 subset exemplified by US11 and UL38 genes maps entirely in the US1.5 domain and does not require the ICP22 domain. This conclusion is based on the observation that cells infected with a mutant, R7805, lacking the ICP22 domain, are not defective in the expression of US11 or UL38 proteins.
(ii) Optimal expression of US11 and UL38 requires posttranslational processing of US1.5 determined by signals located at both amino (zone 5) and carboxyl (zone 8) termini of the US1.5 protein. The site of phosphorylation of US1.5 has not been mapped, but the signals for posttranslational processing associated primarily with the UL13 protein kinase were mapped to amino acids 147 to 170 and to amino acids 402 to 404. The sequence around amino acids 402 to 404 is not reproduced at the second, amino-terminal site. These observations are consistent with the hypothesis that one or both sites involve the association of the US1.5 domain with other proteins as a requirement for posttranslational processing. Two observations are particularly noteworthy. First, the sequence between amino acids 158 and 170 is particularly rich in prolines, raising the possibility that it forms a site for protein-protein interactions. Second, p60 binds solely nonprocessed forms of ICP22. The site for binding of the p60 protein has been mapped to a position (amino acids 380 to 396) in zone 8 adjacent to that of the carboxyl-terminal domain processing signal, which suggests either that posttranslational modification at the carboxyl terminus alters the secondary structure of ICP22 or that a protein binding to the signal site displaces p60.
(iii) The amino-terminal and carboxyl-terminal signals of the US1.5 domain may function independently. This hypothesis is based on the studies by Carter and Roizman (5), who inserted in-frame 20-amino-acid linkers at amino acid 200 or 240 of ICP22 without effective loss of the wild-type phenotype in cell culture. These data suggest that the US1.5 domain contains two independent functional sites, each of which must be present to bring about posttranslational modification of the protein. One example of a situation in which two functional sites operating independently would direct the same posttranslational modification would be the direction of ICP22 by one site to a site at which the modification was to take place.
Earlier in the text, we noted that the ICP22 and US1.5 domains share homologs of a 28-amino-acid sequence located in zones 2 and 7 and conserved in both HSV-1 and HSV-2 (Fig. 12B). It is conceivable that the repeat is involved in the binding of one or more identical proteins but with different results.
(iv) Earlier studies have shown that zones 6 to 8, lacking in the recombinant R325, are required for viral replication in experimental animal systems and for wild-type virus yields in restricted cell lines. In this study, we showed that the same phenotype is reproduced by a recombinant expressing US1.5. A noteworthy observation is that in restricted rabbit skin cells infected with recombinants lacking the carboxyl-terminal 40 amino acids of ICP22/US1.5 protein, the p60 protein is not posttranslationally processed to a slower electrophoretic mobility. The data suggest that in order for the wild-type phenotype to be fully expressed, p60 must be modified or sequestered in rabbit skin cells or sequestered in nuclear structures of permissive cells by the carboxyl-terminal amino acid sequences of the US1.5 domain. The amino acid sequences in zone 8 may have additional functions in experimental animal tissues that are not discernible in cells in culture.
(v) The function of the ICP22 domain contained in zones 1 to 5 is less clear. The attributes mapped to that domain are a putative nuclear localization signal in zone 1, a nucleotidylylation signal in zone 4, and one 28-amino-acid repeat in zone 2. The only clear phenotype attributed to that domain is replication in experimental animal systems. The function of the ICP22 domain appears to be distinct from that of the US1.5 and raises the question of whether the two can be physically separated onto different proteins.
The hypothesis we present does not limit the number of functional sites to the two mapped above. We have not, for example, accounted for the ICP22 zone 6 conserved in ICP22 homologs or for the 28-amino-acid repeats at amino acids 38 to 66 and 300 to 328. We should also note that while ICP22 contains a readily identifiable nuclear localization signal in zone 1 at amino acids 16 to 32, no such signal was identified in the US1.5 protein.
The key conclusion to be drawn from the studies presented in this report is that the domain of the α22 gene encodes several functions strung together and distributed on two proteins. At least two of the functions map to the US1.5 protein, whereas all of the functions are contained in ICP22. The physical separation of the two sets of proteins suggest the possibility that they either complement or are antithetical to each other. The evidence in favor of the latter activity is not compelling since it rests on transfection assays in which ICP22 homologs appeared to repress measured expression of other proteins (13, 22). Multifunctional proteins appear to be a hallmark of HSV regulatory proteins. While it could safely be predicted that the function of the proteins in their totality is the sum of their individual functions, the dissection to define the contribution of each protein presents a formidable challenge.
ACKNOWLEDGMENTS
These studies were aided by Public Health Service grants from the National Cancer Institute (CA47451, CA71933, and CA78766).
REFERENCES
- 1.Ackermann M, Sarmiento M, Roizman B. Application of antibody to synthetic peptides for characterization of the intact and truncated α22 protein specified by herpes simplex virus 1 and the R325 α22− deletion mutant. J Virol. 1985;56:207–215. doi: 10.1128/jvi.56.1.207-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Barker, D., R. King, and B. Roizman. Unpublished results.
- 2.Blaho J A, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 α regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. [PubMed] [Google Scholar]
- 3.Bruni R, Roizman B. Herpes simplex virus 1 regulatory protein ICP22 interacts with a new cell cycle-regulated factor and accumulates in a cell cycle-dependent fashion in infected cells. J Virol. 1998;72:8525–8531. doi: 10.1128/jvi.72.11.8525-8531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruni R, Fineshi B, Ogle W O, Roizman B. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J Virol. 1999;73:3810–3817. doi: 10.1128/jvi.73.5.3810-3817.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter K L, Roizman B. The promoter and transcriptional unit of a novel herpes simplex virus 1 α gene are contained in, and encode a protein in frame with, the open reading frame of the α22 gene. J Virol. 1996;70:172–178. doi: 10.1128/jvi.70.1.172-178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter K L, Roizman B. Alternatively spliced mRNAs predicted to yield frameshift proteins and stable intron 1 RNAs of the herpes simplex virus 1 regulatory gene α0 accumulate in the cytoplasm of infected cells. Proc Natl Acad Sci USA. 1996;93:12535–12540. doi: 10.1073/pnas.93.22.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Y E, Van Sant C, Krug P W, Sears A E, Roizman B. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol. 1997;71:8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejercito P, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 9.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacquemont B, Verrier B, Epstein A L, Machuca I. Expression of immediate-early genes in herpes simplex virus type 1 infected XC cells: lack of ICP22 (68K) polypeptide. J Gen Virol. 1984;65:1331–1340. doi: 10.1099/0022-1317-65-8-1331. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp L M, Latchman D S. Induction and repression of cellular gene transcription during herpes simplex virus infection are mediated by different viral immediate-early gene products. Eur J Biochem. 1988;174:443–449. doi: 10.1111/j.1432-1033.1988.tb14118.x. [DOI] [PubMed] [Google Scholar]
- 14.Leopardi R, Ward P L, Ogle W O, Roizman B. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the Ul13 protein kinase. J Virol. 1997;71:1133–1139. doi: 10.1128/jvi.71.2.1133-1139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackem S, Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of α genes. Proc Natl Acad Sci USA. 1980;77:7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meignier B, Longnecker R, Mavromara-Nazos P, Sears A E, Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus I. Virology. 1988;162:251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell C, Blaho J A, Roizman B. Casein kinase II specifically nucleotidylylates in vitro the amino acid sequence of the protein encoded by the α22 gene of herpes simplex virus 1. Proc Natl Acad Sci USA. 1994;91:11864–11868. doi: 10.1073/pnas.91.25.11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell C, Blaho J A, McCormick L, Roizman B. The nucleotidylylation of herpes simplex virus 1 regulatory protein α22 by human casein kinase II. J Biol Chem. 1997;272:25394–25400. doi: 10.1074/jbc.272.40.25394. [DOI] [PubMed] [Google Scholar]
- 19.Poffenberger K L, Raichlen P E, Herman R C. In vitro characterization of a herpes simplex virus type 1 ICP22 deletion mutant. Virus Genes. 1993;7:171–186. doi: 10.1007/BF01702397. [DOI] [PubMed] [Google Scholar]
- 20.Poffenberger K L, Idowu A D, Fraser-Smith E B, Raichlen P E, Herman R C. A herpes simplex virus type 1 ICP22 deletion mutant is altered for virulence and latency in vivo. Arch Virol. 1994;139:111–119. doi: 10.1007/BF01309458. [DOI] [PubMed] [Google Scholar]
- 21.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: α gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 22.Prod’hon C, Machuca I, Berthomme H, Epstein A L, Jacquemont B. Characterization of regulatory functions of the HSV-1 immediate-early protein ICP22. Virology. 1996;226:393–402. doi: 10.1006/viro.1996.0667. [DOI] [PubMed] [Google Scholar]
- 23.Purves F P, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purves F P, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren Y, Busch R, Busch H. The 58-kDa microspherule protein (MSP58), a nucleolar protein, interacts with nucleolar protein p120. Eur J Biochem. 1998;253:734–742. doi: 10.1046/j.1432-1327.1998.2530734.x. [DOI] [PubMed] [Google Scholar]
- 26.Rice S A, Long M C, Lam V, Spencer C A. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol. 1994;68:988–1001. doi: 10.1128/jvi.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice S A, Long M C, Lam V, Schaffer P A, Spencer C A. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J Virol. 1995;69:5550–5559. doi: 10.1128/jvi.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rixon F J, Clements J B. Detailed structural analysis of two spliced HSV-1 immediate-early mRNAs. Nucleic Acids Res. 1982;10:2241–2256. doi: 10.1093/nar/10.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, et al., editors. Fields virology. New York, N.Y: Raven Press; 1996. pp. 2231–2296. [Google Scholar]
- 31.Roller R J, Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J Virol. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwyzer M, Wirth U V, Vogt B, Fraefel C. BICP22 of bovine herpesvirus 1 is encoded by a spliced 1.7 kb RNA which exhibits immediate early and late transcription kinetics. J Gen Virol. 1994;75:1703–1711. doi: 10.1099/0022-1317-75-7-1703. [DOI] [PubMed] [Google Scholar]
- 33.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward P L, Ogle W O, Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson R J, Sullivan M, Vande Woude G F. Structures of two spliced herpes simplex virus type 1 immediate-early mRNA’s which map at the junctions of the unique and reiterated regions of the virus DNA S component. J Virol. 1981;37:431–444. doi: 10.1128/jvi.37.1.431-444.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]