Abstract
Background
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. Oral steroids are the standard treatment. We have updated this review, which was first published in 2002, because several new treatments have since been tried.
Objectives
To assess the effects of treatments for bullous pemphigoid.
Search methods
We updated searches of the following databases to November 2021: Cochrane Skin Specialised Register, CENTRAL, MEDLINE, and Embase. We searched five trial databases to January 2022, and checked the reference lists of included studies for further references to relevant randomised controlled trials (RCTs).
Selection criteria
RCTs of treatments for immunofluorescence‐confirmed bullous pemphigoid.
Data collection and analysis
At least two review authors, working independently, evaluated the studies against the review's inclusion criteria and extracted data from included studies. Using GRADE methodology, we assessed the certainty of the evidence for each outcome in each comparison. Our primary outcomes were healing of skin lesions and mortality.
Main results
We identified 14 RCTs (1442 participants). The main treatment modalities assessed were oral steroids, topical steroids, and the oral anti‐inflammatory antibiotic doxycycline. Most studies reported mortality but adverse events and quality of life were not well reported. We decided to look at the primary outcomes 'disease control' and 'mortality'.
Almost all studies investigated different comparisons; two studies were placebo‐controlled. The results are therefore based on a single study for each comparison except azathioprine. Most studies involved only small numbers of participants. We assessed the risk of bias for all key outcomes as having 'some concerns' or high risk, due to missing data, inappropriate analysis, or insufficient information.
Clobetasol propionate cream versus oral prednisone
Compared to oral prednisone, clobetasol propionate cream applied over the whole body probably increases skin healing at day 21 (risk ratio (RR 1.08, 95% confidence interval (CI) 1.03 to 1.13; 1 study, 341 participants; moderate‐certainty evidence). Skin healing at 21 days was seen in 99.8% of participants assigned to clobetasol and 92.4% of participants assigned to prednisone. Clobetasol propionate cream applied over the whole body compared to oral prednisone may reduce mortality at one year (RR 0.73, 95% CI 0.53 to 1.01; 1 study, 341 participants; low‐certainty evidence). Death occurred in 26.5% (45/170) of participants assigned to clobetasol and 36.3% (62/171) of participants assigned to oral prednisone. This study did not measure quality of life. Clobetasol propionate cream may reduce risk of severe complications by day 21 compared with oral prednisone (RR 0.65, 95% CI 0.50 to 0.86; 1 study, 341 participants; low‐certainty evidence).
Mild clobetasol propionate cream regimen (10 to 30 g/day) versus standard clobetasol propionate cream regimen (40 g/day)
A mild regimen of topical clobetasol propionate applied over the whole body compared to the standard regimen probably does not change skin healing at day 21 (RR 1.00, 95% CI 0.97 to 1.03; 1 study, 312 participants; moderate‐certainty evidence). Both groups showed complete healing of lesions at day 21 in 98% participants. A mild regimen of topical clobetasol propionate applied over the whole body compared to the standard regimen may not change mortality at one year (RR 1.00, 95% CI 0.75 to 1.32; 1 study, 312 participants; low‐certainty evidence), which occurred in 118/312 (37.9%) participants. This study did not measure quality of life. A mild regimen of topical clobetasol propionate applied over the whole body compared to the standard regimen may not change adverse events at one year (RR 0.94, 95% CI 0.78 to 1.14; 1 study, 309 participants; low‐certainty evidence).
Doxycycline versus prednisolone
Compared to prednisolone (0.5 mg/kg/day), doxycycline (200 mg/day) induces less skin healing at six weeks (RR 0.81, 95% CI 0.72 to 0.92; 1 study, 213 participants; high‐certainty evidence). Complete skin healing was reported in 73.8% of participants assigned to doxycycline and 91.1% assigned to prednisolone. Doxycycline compared to prednisolone probably decreases mortality at one year (RR 0.25, 95% CI 0.07 to 0.89; number needed to treat for an additional beneficial outcome (NNTB) = 14; 1 study, 234 participants; moderate‐certainty evidence). Mortality occurred in 2.3% (3/132) of participants with doxycycline and 9.1% (11/121) with prednisolone. Compared to prednisolone, doxycycline improved quality of life at one year (mean difference 1.8 points lower, which is more favourable on the Dermatology Life Quality Index, 95% CI 1.02 to 2.58 lower; 1 study, 234 participants; high‐certainty evidence). Doxycycline compared to prednisolone probably reduces severe or life‐threatening treatment‐related adverse events at one year (RR 0.59, 95% CI 0.35 to 0.99; 1 study, 234 participants; moderate‐certainty evidence).
Prednisone plus azathioprine versus prednisone
It is unclear whether azathioprine plus prednisone compared to prednisone alone affects skin healing or mortality because there was only very low‐certainty evidence from two trials (98 participants). These studies did not measure quality of life. Adverse events were reported in a total of 20/48 (42%) participants assigned to azathioprine plus prednisone and 15/44 (34%) participants assigned to prednisone.
Nicotinamide plus tetracycline versus prednisone
It is unclear whether nicotinamide plus tetracycline compared to prednisone affects skin healing or mortality because there was only very low‐certainty evidence from one trial (18 participants). This study did not measure quality of life. Fewer adverse events were reported in the nicotinamide group.
Methylprednisolone plus azathioprine versus methylprednisolone plus dapsone
It is unclear whether azathioprine plus methylprednisolone compared to dapsone plus methylprednisolone affects skin healing or mortality because there was only very low‐certainty evidence from one trial (54 participants). This study did not measure quality of life. A total of 18 adverse events were reported in the azathioprine group and 13 in the dapsone group.
Authors' conclusions
Clobetasol propionate cream applied over the whole body is probably similarly effective as, and may cause less mortality than, oral prednisone for treating bullous pemphigoid. Lower‐dose clobetasol propionate cream applied over the whole body is probably similarly effective as standard‐dose clobetasol propionate cream and has similar mortality. Doxycycline is less effective but causes less mortality than prednisolone for treating bullous pemphigoid. Other treatments need further investigation.
Keywords: Humans; Azathioprine; Azathioprine/therapeutic use; Clobetasol; Clobetasol/therapeutic use; Dapsone; Dapsone/therapeutic use; Doxycycline; Doxycycline/therapeutic use; Methylprednisolone; Methylprednisolone/therapeutic use; Niacinamide; Niacinamide/therapeutic use; Pemphigoid, Bullous; Pemphigoid, Bullous/drug therapy; Prednisone; Prednisone/therapeutic use
Plain language summary
Treatments for bullous pemphigoid
Which treatments work best for bullous pemphigoid (a rare, itchy skin disease that causes blisters)?
Key messages
• A cream, containing topical steroid clobetasol propionate, applied on the entire skin surface is as effective as oral steroids (prednisone), causes less severe unwanted or harmful effects, and may decrease deaths.
• Initiating treatment with doxycycline (200 mg/day), an antibiotic with anti‐inflammatory effect, leads to an acceptable short‐term blister control compared to the oral steroid prednisolone (0.5 mg/kg/day), and is superior in long‐term safety aspects, including deaths.
What is bullous pemphigoid?
Bullous pemphigoid is the most common autoimmune blistering disease. In autoimmune diseases, the body's immune system mistakes its own tissues as foreign and attacks them. In bullous pemphigoid, this causes blisters on the skin. Bullous pemphigoid usually occurs in the elderly, but may also affect younger people.
How is bullous pemphigoid treated?
Until recently, the leading treatment for bullous pemphigoid was oral steroids which suppress inflammation and the body's own immune system. However, given over a long period of time, oral steroids will cause severe adverse (i.e. harmful) effects.
This review assessed studies which investigated the effectiveness of other treatment options for bullous pemphigoid; for example, a steroid cream applied on the skin and the anti‐inflammatory antibiotic medicine, doxycycline.
What did we want to find out?
We wanted to find out which treatments work best for bullous pemphigoid with respect to the healing of blisters (efficacy) and reduction in adverse effects, such as death.
What did we do?
We searched for studies that looked at treatments for bullous pemphigoid. We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 14 studies including 1442 people with bullous pemphigoid. The main treatments assessed were oral steroids, topical steroids, and the oral anti‐inflammatory antibiotic doxycycline. Other treatments tested were oral (i.e. taken by mouth) immunosuppressives (medicines that keep your immune system in check) and immunoglobulins (also called antibodies. Antibodies are proteins that your immune system makes to fight germs, for example).
‐ Topical steroid cream, clobetasol propionate, applied over the whole body (40 grams of cream applied per day, with the amount decreased over 12 months) is an effective and safe treatment for bullous pemphigoid.
‐ Treatment with a lower amount of clobetasol propionate cream (10 to 30 grams per day, decreased over 4 months) is equally effective and safe.
‐ Prednisolone, an oral corticosteroid, in the dose of 0.5 mg/kg/day, may be adequate to control disease in most people and reduces adverse effects compared to higher doses of oral corticosteroid.
‐ Initiating treatment with 200 mg/day of doxycycline leads to acceptable blister control compared to oral prednisolone (0.5 mg/kg/day) and is safer.
‐ A study with 20 participants suggests that nicotinamide (a form of vitamin B3)and tetracycline (an antibiotic used to treat a wide variety of infections) may be an effective alternative to prednisone and may decrease treatment‐associated death.
‐ Adding azathioprine, a drug which suppresses the immune system, to an oral corticosteroid does not improve disease control; it may lead to a reduced need for oral corticosteroid.
‐ Further research is needed to fully understand the effectiveness of alternatives to oral steroids (such as dapsone or immunoglobulins), as well as the effectiveness of giving other medicines alongside an oral steroid.
What are the limitations of the evidence?
Except for the studies on topical clobetasol cream and doxycycline, the studies included relatively few participants. The methodological quality of these studies was further limited because of unclear methods of allocating people to different treatment groups; lack of masking (participants and researchers knew which treatments were given to which people, which are not good conditions for fair assessment); and the exclusion of people who dropped out of the studies from treatment analysis.
We are confident about the efficacy of initiating treatment with doxycycline and moderately confident about the efficacy of topical clobetasol cream for the treatment of bullous pemphigoid.
How up to date is this evidence?
The evidence is current to 11 November 2021.
Summary of findings
Summary of findings 1. Clobetasol propionate cream compared to oral prednisone for bullous pemphigoid.
| Clobetasol propionate cream compared to oral prednisone for bullous pemphigoid (Joly 2002) | ||||||
| Patient or population: bullous pemphigoid Setting: in‐patient, multicenter (20 dermatologic centres in France) Intervention: clobetasol propionate cream (40 grams daily, subsequently tapered) Comparison: oral prednisone (0.5 mg/kg for moderate disease and 1 mg/kg for extensive disease) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
Comments NNTB/H (95% CI) |
|
| Risk with oral prednisone | Risk with clobetasol propionate cream | |||||
| Disease control (healing of skin lesions) at day 21 | Study population | RR 1.08 (1.03 to 1.13) | 341 (1 RCT) | ⊕⊕⊕⊝ Moderatea | NNTB = 14 (11.0 to 17.4) for complete healing of skin lesions over 21 days | |
| 924 per 1000 | 998 per 1000 (952 to 1000) |
|||||
| Mortality at 1 year | Study population | RR 0.73 (0.53 to 1.01) | 341 (1 RCT) | ⊕⊕⊝⊝ Lowb | NNTB = 11 (7.2 to 17.4) for mortality at 1 year Mortality at 1 year ‐ prednisone 1 mg/kg for extensive disease: RR 0.58 (0.37 to 0.89); moderatec‐certainty evidence |
|
| 363 per 1000 | 265 per 1000 (192 to 366) |
|||||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events: severe complications at day 21 |
Study population | RR 0.65 (0.50 to 0.86) |
341 (1 RCT) |
⊕⊕⊝⊝ Lowb | NNTB = 7 (4.9 to 8.2) for severe complications at day 21d | |
| 468 per 1000 | 304 per 1000 (234 to 402) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for risk of bias (detection/performance). bDowngraded by two levels for imprecision (CI includes null effect and wide CI) and risk of bias (detection/performance). cDowngraded by one level for imprecision (low number of events). dAdverse events were very heterogeneously reported in the various studies. The number of deaths was the only reliable and comparable figure; we therefore decided to report adverse events only descriptively in most studies.
Summary of findings 2. Mild clobetasol propionate cream regimen (10 to 30 g/day) compared to standard clobetasol propionate cream regimen (40 g/day).
| Mild clobetasol propionate cream regimen (10 to 30 g/day) compared to standard clobetasol propionate cream regimen (40 g/day) for bullous pemphigoid (Joly 2009) | ||||||
| Patient or population: bullous pemphigoid Setting: in‐patient, multicentre (23 dermatologic centres in France) Intervention: mild clobetasol propionate cream regimen (10 to 30 grams per day, tapered over 4 months) Comparison: standard clobetasol propionate cream regimen (40 grams per day, tapered over 12 months) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
Comments NNTB/H (95% CI) |
|
| Risk with standard clobetasol propionate cream regimen | Risk with mild clobetasol propionate cream regimen | |||||
| Disease control (healing of skin lesions) at day 21. Intention‐to‐treat analysis, all participants |
Study population | RR 1.00 (0.97 to 1.03) | 312 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Since intervention and comparison had identical results, it is not possible to compute the NNTB. | |
| 980 per 1000 | 980 per 1000 (951 to 1000) | |||||
| Mortality at 1 year | Study population | RR 1.00 (0.75 to 1.32) | 312 (1 RCT) | ⊕⊕⊝⊝ Lowb | Since intervention and comparison had identical results, it is not possible to compute the NNTB. | |
| 379 per 1000 | 379 per 1000 (284 to 500) |
|||||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events at 1 year | 593 per 1000 | 558 per 1000 (463 to 676) |
RR 0.94 (0.78 to 1.14) |
309 (1 RCT) |
⊕⊕⊝⊝ Lowb | NNTB = 29 for adverse events at 1 year.c There were 194 grade 3 and 4 adverse eventsd,e (reported together) in 89 of 159 participants in the mild regimen compared to 227 in 89 of 150 participants in the standard regimen. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for risk of bias (detection/performance). bDowngraded by two levels for imprecision (CI includes null effect and wide CI) and risk of bias (detection/performance). cBecause the 95% CI for the absolute risk reduction extends from a negative number to a positive number, 95% CI for the NNT could not be calculated. dGrade 3 or 4 side effects were adverse events requiring hospitalisation or prolongation of hospitalisation or life‐threatening events. eAdverse events were very heterogeneously reported in the various studies. The number of deaths was the only reliable and comparable figure; we therefore decided to report adverse events only descriptively in most studies.
Summary of findings 3. Doxycycline compared to prednisolone for bullous pemphigoid.
| Doxycycline compared to prednisolone for bullous pemphigoid (Williams 2017) | ||||||
| Patient or population: bullous pemphigoid Setting: out‐patient, multicentre (54 UK and seven German dermatology centres) Intervention: doxycycline (200 mg/day) Comparison: prednisolone (0.5 mg/Kg of body weight/day) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
Comments NNTB/H (95% CI) |
|
| Risk with prednisolone | Risk with doxycycline | |||||
| Disease control at 6 weeks (unadjusted raw data) | Study population | RR 0.81 (0.72 to 0.92) | 213 (1 RCT) | ⊕⊕⊕⊕ High | NNTH = 6 (4.9 to 7.1) for complete healing of skin lesions at 6 weeks | |
| 911 per 1,000 | 738 per 1,000 (656 to 838) | |||||
| Treatment‐related mortality at 1 year (mITT) | Study population | RR 0.25 (0.07 to 0.89) | 234 (1 RCT) | ⊕⊕⊕⊝ Moderatea | NNTB = 14 (10.7 to 19.1) for mortality at 1 year | |
| 97 per 1000 | 24 per 1000 (7 to 87) | |||||
| Quality of life (DLQI) adjusted for baseline DLQI, disease severity, age, Karnovsky score, baseline versus week 52. A lower score is more favourable. | The mean quality of life (DLQI) score was not stated. | MD 1.8 lower (2.58 to 1.02 lower) | ‐ | 234 (1 RCT) | ⊕⊕⊕⊕ High | Only median and interquartile range (IQR) were provided: both treatments had a median (IQR) of 1 (0 to 3) at week 52. Both groups experienced similar improvement in DLQI scores with median improvement of 9 and 10 points from baseline in the doxycycline and prednisolone groups, respectively. |
| Number of participants with grade 3‐4 (severe or life‐threatening) treatment‐related adverse events at 1 year (raw data) | Study population | RR 0.59 (0.35 to 0.99) | 234 (1 RCT) |
⊕⊕⊕⊝ Moderatea |
NNTB = 10 (7.0 to 13.8) for severe or life‐threatening treatment‐related adverse events at 1 yearb | |
| 265 per 1000 |
157 per 1000 (93 to 263) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DLQI: Dermatology Life Quality Index; MD: mean difference; mITT: modified intention‐to‐treat; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for imprecision (wide CI). bAdverse events were very heterogeneously reported in the various studies. The number of deaths was the only reliable and comparable figure; we therefore decided to report adverse events only descriptively in most studies.
Summary of findings 4. Prednisone plus azathioprine compared to prednisone for bullous pemphigoid.
| Prednisone plus azathioprine compared to prednisone (Burton 1978, Guillaume 1993) | ||||||
| Patient or population: bullous pemphigoid Setting: in‐patient, single centre (UK) (Burton 1978); inpatient, multicentre (11 centres in France) (Guillaume 1993) Intervention: prednisone plus azathioprine (variable doses in the two studies) Comparison: prednisone (variable doses in the two studies) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
Comments NNTB/H (95% CI) |
|
| Risk with prednisone | Risk with Prednisone + azathioprine | |||||
| Disease control (at 6 months) | Study population | RR 0.93 (0.52 to 1.66) | 67 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTH = 35c for disease control at 6 months (Guillaume 1993) Burton 1978: n = 25, disease control at 3 years RR 1.08 (0.67 to 1.76); very low‐certainty evidenceb,d NNTB = 19 (10.6 to 63.7) |
|
| 419 per 1000 | 390 per 1000 (218 to 696) | |||||
| Mortality (at 6 months) | Study population | RR 1.03 (0.35 to 3.06) | 67 (1 RCT) | ⊕⊝⊝⊝ Very lowb,e | NNTH = 200c for mortality at 6 months (Guillaume 1993) Burton 1978: n=25, mortality at 3 years RR 0.81 (0.23 to 2.91); very low‐certainty evidenceb,c NNTB = 17 (10.2 to 50.5) |
|
| 161 per 1000 | 166 per 1000 (56 to 494) | |||||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events | See comments | ‐ | ‐ | ‐ | ⊕⊝⊝⊝ Very lowb,e | Total number of adverse events was reported in 5/13 (at 3 years) and 10/31 (at 6 months) participants in prednisone group; and 5/12 (at 3 years) and 15/36 (at 6 months) participants in azathioprine plus prednisone group.f |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for risk of bias (Burton 1978: method of sequence generation not mentioned, no blinding, no intention‐to‐treat analysis, results state that 25 participants completed a 3‐year follow‐up, but it is unclear how many were randomised to each group at the start, outcome measures were not clearly stated. Guillaume 1993: no blinding, no intention‐to‐treat analysis, reasons for dropouts not clear, only the composite measure of controlled disease reported, trial stopped early). bDowngraded by two levels for imprecision (low number of events, CI includes null effect and wide CI). cBecause the 95% CI for the absolute risk reduction extends from a negative number to a positive number, 95% CI for the NNT could not be calculated. dDowngraded by two levels for risk of bias (method of sequence generation not mentioned, no blinding, no intention‐to‐treat analysis, results state that 25 participants completed a 3‐year follow‐up, but it is unclear how many were randomised to each group at the start, outcome measures were not clearly stated). eDowngraded by one level for risk of bias (no blinding, no intention to treat analysis, reasons for dropouts not clear, only the composite measure of controlled disease reported, trial stopped early). fAdverse events were very heterogeneously reported in the various studies. The number of deaths was the only reliable and comparable figure; we therefore decided to report adverse events only descriptively in most studies.
Summary of findings 5. Nicotinamide plus tetracycline compared to prednisone for bullous pemphigoid.
| Nicotinamide plus tetracycline compared to prednisone (Fivenson 1994) | ||||||
| Patient or population: bullous pemphigoid Setting: outpatient, two centres (USA) Intervention: nicotinamide plus tetracycline 500 mg 4x/day Comparison: prednisone 40 to 80 mg/day | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
Comments NNTB/H (95% CI) |
|
| Risk with prednisone | Risk with nicotinamide plus tetracycline | |||||
| Disease control: complete response at 8 weeks: excluding dropouts | Study population | RR 2.50 (0.37 to 16.89) | 18 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTB = 4 (3.5 to 4.7) for complete response at 8 weeks | |
| 167 per 1000 | 417 per 1000 (62 to 1000) | |||||
| Mortality at 6 months | Study population | RR 0.18 (0.01 to 3.85) | 18 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTB = 8 (6.2 to 9.0) for mortality at 6 months | |
| 167 per 1000 | 30 per 1000 (2 to 642) | |||||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events at 1 year | See comments | See comments | ‐ | ‐ | ⊕⊝⊝⊝ Very lowa,b | A total of 8 adverse events were reported in 6 participants in the prednisone group and 4 in 14 participants in the tetracycline group.c |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for risk of bias (method of sequence generation not mentioned, allocation concealment not mentioned, no blinding, reasons for unavailability of two participants for follow‐up not mentioned, intention‐to‐treat analysis not performed, unclear if the participant groups were equivalent with respect to disease severity or demographics at the start of the therapy; "The study was originally designed to randomize a total of 96 patients. The study was terminated after the 20 patients presented were enrolled." bDowngraded by two levels for imprecision (low number of events, CI includes null effect, wide CI). cAdverse events were very heterogeneously reported in the various studies. The number of deaths was the only reliable and comparable figure; we therefore decided to report adverse events only descriptively in most studies.
Summary of findings 6. Methylprednisolone plus azathioprine compared to methylprednisolone plus dapsone for bullous pemphigoid.
| Methylprednisolone plus azathioprine compared to methylprednisolone plus dapsone for bullous pemphigoid (Sticherling 2017) | ||||||
| Patient or population: bullous pemphigoid Setting: outpatient, multicentre (nine university hospitals in Austria and Germany) Intervention: methylprednisolone 0.5 mg/kg/day plus dapsone 1.5 mg/kg/day Comparison: methylprednisolone 0.5 mg/kg/day plus azathioprine 1.5 to 2.5 mg/kg/day | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) |
Comments NNTB/H (95% CI) |
|
| Risk with methylprednisolone plus azathioprine | Risk with methylprednisolone plus dapsone | |||||
| Disease control (time when steroid could be discontinued) | Study population | 54 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | In the azathioprine group, 5 of 27 participants discontinued after a median of 251 days. In the dapsone group, 3 of 27 discontinued after a median of 81 days. | ||
| See comments | See comments | |||||
| Mortality at 1 year | Study population | RR 0.33 (0.04 to 3.01) | 54 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTB = 14 (10.3 to 19.5) for mortality at 1 year | |
| 111 per 1000 | 37 per 1000 (4 to 334) |
|||||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events at 1 year | See comments | ⊕⊝⊝⊝ Very lowa,b | A total of 18 adverse events (greater than grade 1)c were reported in the azathioprine group and 13 in the dapsone group.d | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for risk of bias (no blinding; outcome data are not fully reported; authors state recruitment of 88 was aimed for, however, only 54 participants were finally recruited; outcomes are still not reported for all, no reasons given; no intention‐to‐treat analysis; selective outcome reporting; trial not registered; “It cannot be excluded that healthier patients had been included resulting in a preselection bias”). bDowngraded by two levels for imprecision (low number of events, CI includes null effect, wide CI). cAdverse events were assessed and their severity graded; 1 for mild effects, 2 for moderate effects, 3 for severe effects, and 4 for life‐threatening effects, according to the standard criteria of the World Health Organization (WHO). dAdverse events were very heterogeneously reported in the various studies. The number of deaths was the only reliable and comparable figure; we therefore decided to report adverse events only descriptively in most studies.
Background
Description of the condition
Definition and epidemiology
Bullous pemphigoid is an acquired autoimmune disorder in which disease‐specific autoantibodies are directed against components of the basement membrane zone of the skin (Morrison 1990; Wojnarowska 1998). It is the most common autoimmune blistering disease in many countries. The incidence in England is 7.63 (95% confidence interval (CI) 7.35 to 7.93) per 100,000 person‐years and rises with increasing age, particularly for elderly men. The annual increase in incidence is 0.9% (95% CI 0.2 to 1.7). The prevalence in England almost doubled over the observation period from 1998 to 2017, reaching 47.99 (95% CI 43.09 to 53.46) per 100,000 people and 141.24 (95% CI 125.55 to 158.87) per 100,000 people over the age of 60 years (Langan 2008; Persson 2021). The risk of all‐cause mortality is highest in the two years after diagnosis (hazard ratio (HR) 2.96, 95% CI 2.68 to 3.26) and remains raised thereafter (HR 1.54, 95% CI 1.36 to 1.74). In central Europe, there are 42 new people with bullous pemphigoid per million inhabitants each year (Bernard 1995; Joly 2009; Zillikens 1995). Incidence figures are not available for most parts of the world, but bullous pemphigoid appears to be relatively rarer in the Far East (Adam 1992; Jin 1993; Tham 1998). Bullous pemphigoid is usually a disease of the elderly, but it can also affect younger people and children (Kirtschig 1994; Nemeth 1991; Orange 1989). Both sexes are similarly affected.
Clinical picture
The characteristic clinical picture is the development of tense blisters, which may arise on inflamed skin or skin of normal appearance (Miyamoto 2019). This may be heralded by an urticarial or eczematous rash. The degree of itch varies from none to intense and may precede the appearance of blisters, which contain either clear or bloodstained fluid. The blisters are usually generalised on the body with a tendency to appear on the creases of the limbs. Localised forms also occur. Bullous pemphigoid may affect mucosal surfaces such as the mouth; scarring is usually not observed.
Associated disease
Bullous pemphigoid is associated with increased morbidity and mortality (Joly 2012; Langan 2008), neurological diseases including dementia (Brick 2014; Taghipour 2010), Parkinson's disease, motor neurone disease, and stroke (Bastuji‐Garin 2011; Brick 2014; Taghipour 2010), haematological malignancies (Schulze 2015), and exposure to some medications (Schulze 2015), such as loop diuretics (Lloyd‐Lavery 2013).
Investigation and diagnosis
The most reliable test to achieve a diagnosis is a skin biopsy for immunopathological investigation. A direct immunofluorescence technique (on an individual's skin) demonstrates deposits of immunoglobulin G (IgG) autoantibodies and complement component 3 (C3) at the dermo‐epidermal junction binding to BP230 and BP180 autoantigens. Enzyme‐linked immunosorbent assay (ELISA) and indirect immunofluorescence (using serum) demonstrate circulating autoantibodies directed against basement membrane proteins (Giudice 1994; Morrison 1990; Wojnarowska 1998). When skin tissue is incubated in one molar sodium chloride, separation of the dermis from the epidermis occurs within the lamina lucida level of the basement membrane (visualised on electron microscopic examination). Immunofluorescence techniques performed on such split skin was first shown in the late 1980s to result in a more precise localisation of the antigen‐antibody‐binding site. This helps to separate other autoimmune bullous diseases, such as epidermolysis bullosa acquisita and bullous systemic lupus erythematosus (in which fluorescence is at the floor of the blister: dermal binding) from bullous pemphigoid (in which fluorescence is usually at the roof: epidermal binding) (Logan 1987). Immunoelectron microscopy and immunoblotting are more specific investigations, and in some cases, can lead to a change in the diagnosis (Kirtschig 1994). The latter investigations are not available for routine clinical use, being largely limited to research centres. However, ELISAs to detect circulating IgG autoantibodies to BP180 and BP230 antigens are now widely available. One limitation of ELISAs is that only autoantibody binding to BP180 and BP230 autoantigens will be demonstrated; other autoantigens remain undetected by this method.
Scoring of disease
A minimum set of outcomes, called a core outcome set (COS), for all clinical trials of a particular disease enables trials to be compared and included in meta‐analyses (Chalmers 2009; Prinsen 2016). Usually they consist of measures of effectiveness or harm, are relevant to patients and care providers, and all other stakeholders – for example, those making decisions about healthcare cost‐effectiveness. They need to be valid, repeatable, sensitive to change, and easy to use. Core outcomes may be different for clinical trials and routine care. A selection of outcomes for clinical trials in autoimmune bullous diseases was published in 2007 (Pfütze 2007), and in 2012 (Murrell 2012). These two sets were compared and validated in 2017 (Wijayanti 2017). However, a formal process to agree on a COS for bullous pemphigoid involving all stakeholders was not performed. Trials included in this review only partly used the existing selection of recommended outcomes and, thus, trials are not easy to compare.
Natural history
The natural history of both treated and untreated bullous pemphigoid is of a persistent disease with eventual remission occurring in the majority of cases. Remission is likely to occur within five years, although relapses and exacerbations may occur (Ahmed 1977; Hadi 1988; Nemeth 1991; Person 1977). The mortality rate in the initial 30 cases reported by Lever was 24% at one year; this was prior to the use of oral corticosteroids (Lever 1953). The mortality rate in other studies ranges from about 10% to 40% at one year (Colbert 2004; Gudi 2005; Roujeau 1998; Savin 1979; Savin 1987; Venning 1992), despite the use of topical and systemic treatments. This might suggest that treatment is at best suppressive (without really altering the prognosis of the disease) or at worst contributes to mortality (e.g. from sepsis secondary to immunosuppression) whilst relieving itch and preventing blisters. Savin suggested that death seemed to be more commonly related to underlying illness in the elderly, debilitation associated with severe illness, or the adverse effects of treatment (Savin 1979; Savin 1987). The study by Parker and colleagues supports this view: they evaluated 223 participants with pemphigoid and compared mortality data with the general population in the USA (Parker 2008). There was no difference between pemphigoid participants and age‐matched controls in expected mortality. They concluded that mortality of participants with bullous pemphigoid is more likely related to advanced age and associated medical conditions than disease‐specific factors, and that treatment will not alter the natural disease history but will alter the quality of life.
Description of the intervention
Bullous pemphigoid is a chronic disease and requires long‐term treatment. Current treatments include topical and oral steroids (e.g. prednisone or prednisolone); immunosuppressants such as azathioprine, mycophenolate mofetil, methotrexate, ciclosporin, and cyclophosphamide; plasma exchange; anti‐inflammatory acting antibiotics (e.g. tetracyclines including doxycycline, erythromycin, and dapsone); nicotinamide; biologics such as rituximab (anti‐CD20 antibody), and intravenous immunoglobulins in more severe cases. Some of these drugs or interventions have the potential for severe adverse effects, such as increased susceptibility to serious infections, liver and kidney damage, and bone marrow suppression. Some are very expensive.
For many years, oral corticosteroids were the standard of care. However, high‐potency topical steroids (clobetasol propionate cream) have been demonstrated to improve survival in people with bullous pemphigoid (Joly 2002). These topical steroids may be safer and more effective than high‐dose oral corticosteroids for controlling bullous pemphigoid and, therefore, may be suitable for treating those patients, often the elderly, who have a poor prognosis because they are at high risk of developing adverse effects with systemic steroids. Topical steroids are not without risk of adverse effects, both locally (increased susceptibility of the skin to damage, such as skin atrophy, bruising, and infections of the skin) and systemically, if enough steroid is absorbed through the skin. The latter can lead, for example, to Cushing syndrome with fluid retention, increased blood pressure and diabetes mellitus, adrenal gland suppression, and possibly osteoporosis.
More recently, antibiotics with anti‐inflammatory properties were introduced in the care of bullous pemphigoid. They are shown to be effective and safer in this usually aged population (Fivenson 1994; Williams 2017).
There are emerging reports of some bullous pemphigoid cases being treated with biological therapies; in particular, anti‐CD20 monoclonal antibodies (rituximab), omalizumab, and dupilumab (Cao 2022; Hall 2013; Hertl 2008; Kremer 2019).
Finally, plasma exchange and intravenously applied pooled immunoglobulins are used in selected cases.
New treatment options to improve healing and minimise adverse effects are continuously considered, but their effect in a clinical setting needs to be established.
How the intervention might work
Topical corticosteroids
Topical corticosteroids have been used for the treatment of many dermatological conditions for decades. Their mechanism of action for bullous pemphigoid is broad and non‐specific. They function chiefly as anti‐inflammatory, anti‐mitotic, and immunosuppressive agents (Ahluwalia 1998).
Topical corticosteroids induce vasoconstriction which in turn reduces the delivery of inflammatory mediators by blood flow to the dermis. In addition, topical corticosteroids inhibit phospholipase A2 and increase the expression of anti‐inflammatory genes to inhibit inflammatory transcription factors.
The anti‐mitotic effect of topical corticosteroids in basal cell layer and dermal fibroblasts leads to reduction in cell proliferation and collagen synthesis. This is a desired effect in disorders such as psoriasis, but may be an unwanted side effect in other conditions, as it may lead to skin atrophy with long‐term use. Topical corticosteroids can also affect proliferation, differentiation, and maturation of immune cells. They can also block the humoral factors that are important in an inflammatory response.
If used in sufficient quantities in generalised bullous pemphigoid, the costs of the creams for the health system may be high, limiting their utility in certain health systems.
Oral glucocorticosteroids
Regulation of the immune system and inflammatory cells is the main target of glucocorticosteroid actions. Glucocorticosteroids act through genomic and non‐genomic mechanisms. The human glucocorticoid receptor mediates most of the biologic effects of glucocorticosteroids: cytosolic glucocorticoid receptor binds glucocorticosteroids and is capable of binding to glucocorticoid response elements in DNA and either transactivate or transrepress genes, depending on the tissue and cell type. In addition, the glucocorticoid receptor exerts rapid, non‐genomic effects possibly mediated by membrane‐localised receptors or by translocation to mitochondria. Glucocorticosteroids can also interact directly with several enzymes and cytokines (Kubin 2017).
Prednisone is an inactive drug precursor that is metabolised by the liver and converted to biologically active prednisolone. The two forms are virtually identical therapeutically and can be used interchangeably in many situations. As prednisone is rapidly converted to prednisolone, prednisolone may be preferred in some patients who have liver disease or some other metabolic disorder. There are some differences in the appearance and taste of the two formulations: prednisolone sodium phosphate is very soluble with a not unpleasant taste, whereas prednisone is bitter and poorly soluble. Some reports have suggested that the use of prednisone is preferable to prednisolone in the treatment of bullous pemphigoid (Lebrun‐Vignes 1999), and this may account for differences in use of the drug, for example, in France. For the purposes of this review, prednisone and prednisolone are regarded as bio‐equivalent. However, for each of our included studies, we have used the drug name quoted in the study's report.
Immunosuppressants
Azathioprine
Azathioprine is a purine analogue which inhibits purine synthesis. It is converted to active metabolites, mercaptopurine and thioguanine, by the action of two main enzymes; namely, hypoxanthine‐guanine phosphoribosyltransferase (HPRT) and thiopurine methyltransferase (TPMT). Measuring TPMT in blood prior to commencing azathioprine is recommended, as individuals with low or no TPMT will be at risk of myelotoxicity due to accumulation of unmetabolised azathioprine. The metabolites prevent cell division by disturbing DNA replication (Mohammadi 2022).
Mycophenolate mofetil
Mycophenolate mofetil is a prodrug of mycophenolic acid (MPA) which inhibits inosine‐5'‐monophosphate dehydrogenase. This action in turn depletes guanosine nucleotide synthesis in T and B lymphocytes, thus inhibiting their proliferation. MPA has therefore a cytostatic effect on lymphocytes which leads to suppression of cell‐mediated immune response and antibody formation. MPA can also suppress glycosylation and expression of some adhesion molecules, resulting in reduced migration of lymphocytes and monocytes to the sites of inflammation. In addition, MPA suppresses production of inducible nitric oxide synthase and subsequently of nitric oxide, which in turn reduces tissue damage (Allison 2000).
Methotrexate
Originally developed as a chemotherapy drug, at lower doses, methotrexate is used to treat some inflammatory and autoimmune disorders. Methotrexate is a folic acid analogue and inhibits dihydrofolate reductase, which subsequently interferes with thymidylate synthesis. This in turn leads to suppression of nucleotide synthesis as well as DNA repair and replication.
The anti‐inflammatory effect of methotrexate is thought to be through inhibition of enzymes that are responsible for purine metabolism. This leads to accumulation of adenosine, which is a potent anti‐inflammatory mediator, which also accounts for suppression of T cells, T cell adhesion molecule expression, and down regulation of B cells.
Ciclosporin
Ciclosporin inhibits the action of calcineurin and is a substrate for cytochrome P450, and P‐glycoprotein. Ciclosporin binds to a cytosolic protein, namely cyclophilin, to make a complex which subsequently inhibits calcineurin phosphatase. This stops the activation and dephosphorylation of nuclear factor of activated T cells (NF‐AT), which normally cause inflammatory reactions. NF‐AT promotes the production of cytokines such as interleukin‐2 (IL‐2), which is required for the self‐activation and differentiation of T lymphocytes. This mechanism of inhibition of IL‐2 accounts for the cell‐mediated immunosuppressive effect of ciclosporin (Tapia 2021).
Cyclophosphamide
Cyclophosphamide is a nitrogen mustard DNA alkylating agent that is used to treat malignancy. In lower doses, it has immunomodulating and immunosuppressive effects, although the exact mechanism of these effects is not fully understood. Cyclophosphamide can selectively suppress regulatory T cells, induce T cell growth factor, increase Th1 cytokine production, and promote differentiation of Th17 (Ogino 2022).
Anti‐inflammatory acting antibiotics
Tetracyclines have an immunomodulatory effect via a number of mechanisms, including inhibition of matrix metalloproteinase (MMP), inducible nitric oxide synthase (iNOS) and cyclo‐oxygenase‐2 (COX‐2) (Chen 2000; Yrjanheikki 1999). In addition, they have been shown to be capable of inducing eosinophil apoptosis and down regulating eosinophil activating markers; a finding which may be of significance in the treatment of diseases associated with eosinophilia (Gehring 2021).
Macrolides, including erythromycin, reduce neutrophilic inflammation by inhibiting neutrophil function as well as reducing pro‐inflammatory cytokines, such as IL‐8 and IL‐1beta, which ultimately leads to lower tissue inflammation (Zimmermann 2018).
Dapsone has an anti‐inflammatory effect through inhibiting neutrophil migration and chemotaxis as well as reducing the cytotoxic activity which is mediated by myeloperoxidase in neutrophils and monocytes (Stendahl 1977; Wozel 1997).
Nicotinamide
Nicotinamide may exert its therapeutic function via electron scavenging, inhibition of phosphodiesterase, and/or increased tryptophan conversion to serotonin. Nicotinamide has direct antihistamine receptor effects and effects that inhibit histamine release. It also inhibits neutrophil and eosinophil chemotaxis and secretion (Fivenson 1994).
Biologics
New therapeutic pharmacologic biologic agents (such as rituximab, mepolizumab, omalizumab, and dupilumab) can selectively inhibit autoantibody formation and the inflammatory cascade. They may be an option to treat bullous pemphigoid (Cao 2022).
Anti‐CD20 antibody (rituximab): CD20 is a molecule which is expressed on the surface of B lymphocytes, immune cells which produce antibodies. Rituximab, a monoclonal antibody, binds specifically to this transmembranous CD20 antigen and the resulting lysis of the B lymphocyte is induced via a number of immune pathways. This limits the immune system's attack by depleting the number of B lymphocytes available to produce antibodies, including those directed at the skin in bullous pemphigoid. It has been proposed that rituximab may be used either as an alternative to standard treatments for bullous pemphigoid in patients that are refractory to standard treatment (Reguiaï 2009), or in patients unable to tolerate other treatments.
Anti‐IL‐5 antibody (mepolizumab) therapy and a recombinant DNA‐derived humanised IgG1k monoclonal antibody that specifically binds to free human IgE, shown to be effective in eosinophilic bronchial asthma and hypereosinophilic syndrome, was tried in the treatment of bullous pemphigoid (Kremer 2019; Simon 2020). Eosinophils are characteristically found in the skin at early stages of bullous pemphigoid before blisters occur; targeting eosinophils by reducing their number and activation promises an alternative therapeutic approach (Simon 2020).
Dupilumab is a fully human IgG4 monoclonal antibody directed against the IL‐4 receptor alpha subunit. It has been demonstrated to modulate chemokine‐ligand 18, IL‐4 and IL‐13. These are Th2‐related cytokines that show higher levels in patients with bullous pemphigoid (both in sera and in blister fluid) and play a role in the maintenance of Th2‐type responses, which are thought to be involved in the loss of tolerance against BP180 (Russo 2020). Dupilumab is licensed for the treatment of severe atopic eczema, asthma, and prurigo nodularis. It was tried off‐label for the treatment of recalcitrant bullous pemphigoid, and was shown in a few case series to potentially have a corticosteroid‐sparing effect without significant side effects in moderate‐to‐severe bullous pemphigoid (Liang 2023; Zhang 2021).
Omalizumab, a monoclonal antibody directed to IgE, is licensed for the treatment of severe allergic asthma and chronic urticaria. IgE autoantibodies are reported to play a role in the pathomechanism in bullous pemphigoid (Ishiura 2008; Van Beek 2017). Omalizumab has been used off‐label in a few case series of bullous pemphigoid, which suggest that it may be an effective add‐on therapy in treatment‐resistant patients (Alexandre 2022).
The combination of dupilumab and omalizumab in recalcitrant bullous pemphigoid is under investigation (Seyed Jafari 2021).
Good‐quality RCTs are needed to provide evidence for the efficacy and safety of biologics for the treatment of bullous pemphigoid.
Intravenous immunoglobulins
Treatment with high doses of pooled immunoglobulins is licensed for primary immunodeficiency, idiopathic thrombocytopenic purpura, chronic inflammatory demyelinating polyneuropathy, Kawasaki disease, certain cases of HIV/AIDS, measles, Guillain‐Barré syndrome, and other infections when a more specific immunoglobulin is not available. Immunoglobulins may be applied intravenously (IVIG) or be injected into the skin or muscle. Their effect lasts for a few weeks. They are generally well tolerated; severe adverse reactions include allergic reactions, kidney problems, haemolysis, and blood clots. IVIG are also used to treat a number of dermatological conditions, including toxic epidermal necrolysis and many autoimmune disorders (e.g. autoimmune pemphigus) (Amagai 2009; Jolles 1998), if resistant to conventional treatment or if patients are at risk of adverse effects. Immunoglobulins are expensive and their value for many of the conditions for which they are used is not quantified. They are also used in bullous pemphigoid but good quality studies to support their additional benefit are lacking.
Plasma exchange
Plasma exchange may act by mechanisms other than the removal of antibodies and immune complexes. It has been shown to favour the clearance of immune complexes by the reticuloendothelial system in vivo and to modulate function of monocytes. The depletion of complement components and of inflammatory mediators may also be beneficial (Roujeau 1984).
Why it is important to do this review
Mortality figures, based on uncontrolled studies, have not improved much since the introduction of systemic treatments. This may suggest that bullous pemphigoid is a self‐limiting condition – occurring in older people with a higher mortality than the general population – and that the prognosis is not altered by treatment. It is also possible that the improved skin care and medical support currently available, compared with Lever's time (Lever 1953), significantly lower the mortality rate, and that this benefit is masked by the adverse effects of systemic treatments. However, this does not tell us about morbidity and the quality of life of affected people and whether treatment alters the duration of the lesions. In fact, only Williams 2017 performed an accepted quality of life assessment. There is also variation in the long‐term toxicity of systemic agents, ranging from very little (e.g. antibiotics) to a lot (e.g. prednisolone or cyclophosphamide). Very potent topical steroid treatment may be adequate in localised disease and has minimal side effects. There is wide variation in practice amongst clinicians as to which drugs or interventions are used and in what order or combinations.
This review aims to establish:
which are the most effective drugs or interventions, with the fewest adverse effects;
whether combination therapy (e.g. azathioprine plus steroids) offers any advantages over single drugs (e.g. oral steroids alone);
whether antibiotics such as tetracyclines, erythromycin, dapsone, or sulphonamides are useful; and
whether systemic treatment is better than topical or no treatment.
Objectives
To assess the effects of treatments for bullous pemphigoid.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised controlled trials, including cluster‐RCTs, cross‐over RCTs, and multiple‐arm trials.
Types of participants
People of any age who have received treatment for a diagnosis of bullous pemphigoid confirmed by immunofluorescence studies.
We excluded studies involving participants with various dermatoses, including some with bullous pemphigoid, if we could not extract or calculate separate data for those with bullous pemphigoid.
Types of interventions
Any therapeutic intervention used to treat bullous pemphigoid compared to placebo.
Types of outcome measures
We did not use the following outcome measures as an eligibility criterion for studies' inclusion in the review.
Primary outcomes
-
Disease control, defined as:
initial regression or healing of lesions within six weeks; and
long‐term regression or healing of lesions at six months, one year, and beyond one year, including duration of remission after stopping treatment.
The included studies defined 'disease control' differently. In general, this outcome included regression or healing of skin lesions at time periods specified by individual trials.
Mortality (at any time during the trial and follow‐up period)
Secondary outcomes
Effect on quality of life; for example, relief of soreness or itching within six weeks (short‐term) and after six weeks (long‐term; at six months, one year and beyond one year)
Adverse effects of treatment (at any time); for example, systemic infection, organ failure, allergic reactions, or complications of the primary disease (bullous pemphigoid), such as localised skin infection (at any time during the trial)
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised and updated our search strategies in line with current Cochrane Skin practices (see Differences between protocol and review). Details of the previous search strategies are available in Kirtschig 2010.
The Cochrane Skin Information Specialist (Liz Doney) searched the following databases up to 11 November 2021:
the Cochrane Skin Specialised Register 2022 using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2021, Issue 10) using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3; and
Embase via Ovid (from 1974) using the strategy in Appendix 4.
We (GK, SS) searched the following trials registers up to 14 January 2022 using the term 'bullous pemphigoid':
the ISRCTN register (www.isrctn.com);
ClinicalTrials.gov (www.clinicaltrials.gov);
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/); and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References from published studies
We checked the bibliographies of included studies for further references to relevant trials.
Adverse effects
We did not perform a separate search for adverse effects of the target intervention. However, we did examine data on adverse effects from the included studies we identified.
Data collection and analysis
We imposed no restriction regarding the type of RCTs, including cross‐over, cluster‐RCTs, or within‐participant trials.
Selection of studies
We screened the abstracts of potentially relevant studies and obtained full articles if necessary. Working independently, three review authors (GK, KT, SS) assessed articles that were possible RCTs for eligibility using inclusion criteria outlined in the protocol. We discussed any disagreements with a fourth review author (CCC).
Data extraction and management
We extracted details of eligible studies (study identity, interventions, outcomes (e.g. disease control, mortality, quality of life, adverse events)) and summarised them using a data extraction sheet. Working independently, two authors (GK, SS) extracted data and subsequently checked for discrepancies (except the BLISTER study (Williams 2017), where data were extracted by SS, VA). We discussed any discrepancies with a third review author (primarily CCC) to reach consensus. One review author (CCC) kindly extracted data from Liu 2006 (published in Chinese). We planned to resolve any disagreements through discussion with the other review authors (PS, NK, DM), but this was not necessary.
Assessment of risk of bias in included studies
Working independently, three review authors (GK, SS, KT) assessed the risk of bias of the new studies identified by the updated search. We resolved any differences by consensus.
The risk of bias assessment entails an evaluation of the following components for each included study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008):
the method of generation of the randomisation sequence (selection bias);
the method of allocation concealment – it was considered 'adequate' if the assignment could not be foreseen (selection bias);
who was blinded or not blinded (participants, clinicians, outcome assessors) (performance bias and detection bias);
how many participants dropped out of the study overall, and whether participants were analysed in the groups to which they were originally randomised (attrition bias and intention‐to‐treat analysis);
selective reporting (reporting bias); and
other biases.
The original protocol of this review stated that we would use the Jadad quality assessment scale, which also similarly assesses randomisation, blinding, withdrawals, and dropouts (Jadad 1996). We assessed all these aspects but reported them individually (see Characteristics of included studies for details) rather than as a summary score.
Measures of treatment effect
We presented dichotomous measures as risk ratios (RR) with 95% confidence intervals (CI), and continuous measures as mean differences with 95% CI.
Unit of analysis issues
The unit of analysis is the randomised participants of the studies. We imposed no restriction on the type of RCT eligible for inclusion, but we did not find any cluster‐RCTs, cross‐over RCTs, or within‐participant trials. If we include these study designs in future review updates, we will use appropriate techniques, as described in Chapter 23 of the Cochrane Handbook (Higgins 2022), to analyse studies with these types of design.
Dealing with missing data
We contacted trial investigators to obtain missing data and clarify the specifics of the trial conditions when these were not clear to us from the published report of the trial.
Assessment of heterogeneity
For an assessment of heterogeneity, we used the I² statistic. If we found moderate to high levels of heterogeneity (I² > 50%) for the primary outcomes, we explored the possible sources of heterogeneity.
Assessment of reporting biases
We will use a funnel plot to detect publication bias when there are at least 10 studies for a primary outcome.
Data synthesis
We had planned to divide data analysis into two groups: (1) trials where the diagnosis of bullous pemphigoid was confirmed by immunofluorescence using intact skin; and (2) trials using split skin for immunofluorescence (this procedure helps, although not completely, to distinguish true bullous pemphigoid participants from those with other subepidermal immunobullous diseases) or enzyme‐linked immunosorbent assay (ELISA) detecting BP180 or BP230 antigens. However, this division was unnecessary as only four small studies (n = 213) used immunofluorescence on split skin or ELISAs (Amagai 2017; Beissert 2007; Simon 2020; Sticherling 2017).
We conducted a narrative synthesis of included trials, and present the characteristics of the trials and results in tables and figures. We were unable to pool data in a meta‐analysis as the studies were heterogeneous, especially in terms of the treatments used. We did, however, present some of the data in Review Manager 5.3 (RevMan 5) in the form of risk ratios and 95% confidence intervals for the results of single trials (Review Manager 2014).
We would use a random‐effects model for meta‐analysis if required.
We have summarised adverse events in Table 7. We have left some columns empty: it would have been misleading to enter "zero" when a paper was silent about a particular adverse event, because we are not sure that all adverse events were reported.
1. Adverse events in the included studies.
| Study ID | Drug and dose | Infection /Low white cell count | Organ impairment | Cardiovascular | Other | Total adverse events (AEs) | Death |
| Amagai 2017 | Intravenous immunoglobulin drip infusion 400 mg/kg/day for 5 consecutive days (n = 29) | 2 | 8 | 2 | 7 | 11 participants had 19 adverse drug reactions |
Assumed none (follow‐up 57 days) |
| Physiological saline intravenous drip infusion for 5 consecutive days (n = 27) |
0 | 3 | 2 | 1 | 5 participants had 6 adverse drug reactions |
Assumed none (follow‐up 57 days) |
|
| Beissert 2007 | Oral methylprednisolone 0.5 mg/kg/day plus azathioprine 2 mg/kg/day (n = 38) | 1 | 7 (1 hyperglycaemia 6 liver) |
0 | 3 | 11 (grade 3/4) |
2 |
| Oral methylprednisolone 0.5 mg/kg/day plus mycophenolate mofetil 2000 mg twice/day (n = 35) | 4 | 6 (5 hyperglycaemia 1 liver) |
0 | 3 | 13 (grade 3/4) |
0 | |
| Burton 1978 | Prednisone 30 to 80 mg/kg/day (n = 13) | 1 | 1 | 3 | ‐ | 5 | 4 |
| Azathioprine 2.5 mg/kg plus prednisone 30 to 80 mg/day (n = 12) | 2 | ‐ | 3 | ‐ | 5 | 3 | |
| Dreno 1993 | Prednisolone (average) 1.16 mg/kg/day (n = 29) | ‐ | 1 | 1 | 1 | 3 | 0 |
| Methylprednisolone (average) 1.17 mg/kg/day (n = 28) | 1 | 1 | 2 | 1 | 5 | 0 | |
| Fivenson 1994 | Prednisone 40 to 80 mg/day (n = 6) | 2 | 3 | 2 | 1 | 8 | 1 |
| Tetracycline 500mg 4x/day + nicotinamide (n = 14) | 1 | 1 | ‐ | 2 | 4 | 0 | |
| Guillaume 1993 | Prednisolone 1 mg/kg/day (n = 31) | ‐ | ‐ | ‐ | ‐ | 10 | 5 |
| Prednisolone 1 mg/kg/day plus azathioprine 100 to 150 mg/day (n = 36) | ‐ | ‐ | ‐ | ‐ | 15 | 6 | |
| Prednisolone 1 mg/kg/day plus plasma exchange (n = 31) | ‐ | ‐ | ‐ | ‐ | 6 | 3 | |
| Joly 2002 | Moderate disease:
topical steroids (n = 77) Prednisone 0.5 mg/kg/day (n = 76) |
11 16 | 5 14 | 15 16 | ‐ | 31 (severe AEs in 25 participants) 46 (severe AEs in 29) | 23 23 |
| Extensive disease: topical steroids (n = 93) Prednisone 1 mg/kg (n = 95) | 8 22 | 6 23 | 16 20 | ‐ | 30 (severe AEs in 27) 65 (severe AEs in 51) | 22 39 P = 0.02 | |
| Joly 2009 | Mild regimen topical steroids (n = 159) | 27 | 18 (DM) | 21 | 41% (skin) |
194 in 89 participants (grade 3/4) |
60 |
| Standard regimen topical steroids (n = 150) | 32 | 34 (DM) | 35 | 52% (skin) |
227 in 89 participants (grade 3/4) |
58 | |
| Liu 2006 | Jingui Shenqi Pill (JSP) 1# bid plus prednisone 0.5 to 1.0 mg/kg/day (n = 15) | ‐ | ‐ | ‐ | ‐ | Not mentioned | Not mentioned |
| Prednisone alone 0.5 to 1.0 mg/kg/day (n = 15) | ‐ | ‐ | ‐ | ‐ | Not mentioned | Not mentioned | |
| Morel 1984 | Prednisolone 0.75 mg/kg/day (n = 26) | 1 | 2 | ‐ | ‐ | 3 | 2 |
| Prednisolone 1.25 mg/kg/day (n = 26) | 1 | 1 | 1 | 2 | 5 | 3 | |
| Roujeau 1984 | Prednisolone 0.3 mg/kg/day (n = 17) |
‐ | 7 | ‐ | ‐ | 7 | 0 |
| Plasma exchange plus prednisolone 0.3 mg/kg/day (n = 24) | 10 | 7 | 7 | ‐ | 7 | 0 | |
| Sticherling 2017 | Oral methylprednisolone 0.5 mg/kg/day plus azathioprine 1.5 to 2.5 mg/kg/day (n = 27) | 0 | 9 | 1 | 4 | 18 > grade 1 |
3 |
| Oral methylprednisolone 0.5 mg/kg/day plus dapsone 1.5 mg/kg/day (n = 27) | 1 | 7 | 3 | 1 | 13 > grade 1 |
1 | |
| Williams 2017 | Doxycycline (200 mg/day) (modified intention‐to‐treat analysis (mITT) n = 121) |
31* | 183* | 21* | 95* | 22/121 (18%) grade 3‐5 *Total AEs for all categories = 330 |
3/121 (mITT) Total deaths: 13/132 randomised (9.9%) |
| (Per‐protocol (PP) analysis n = 94) | 18* | 127* | 15* | 63* | *Total AEs for all categories = 223 | Total death = 10 (10.6%) | |
| Prednisolone (0.5 mg/kg/day) (mITT n = 113) |
38* | 179* | 22* | 84* | 41/113 (36%) grade 3‐5 *Total AEs for all categories = 323 |
11/113 (mITT) Total deaths: 20/121 randomised (16.5%) |
|
| (PP n = 108) | 35* | 157* | 21* | 80* | *Total AEs for all categories = 293 | Total death = 16 (14.8%) | |
| Simon 2020 | Mepolizumab 750 mg plus prednisolone (0.5 mg/kg/day) (n = 20) |
‐ | ‐ | ‐ | ‐ | Total AEs for all categories = not mentioned | Total death = 0 |
| Placebo plus prednisolone (0.5 mg/kg/day) (n = 10) |
‐ | ‐ | ‐ | ‐ | Total AEs for all categories = not mentioned | Total death = 0 |
Not all adverse events (AEs) were classified/assigned to predefined groups in the different trials. An attempt of assignment to the predefined groups of events was done by the authors of this Cochrane Review.
Grade 1 = mild, 2 = moderate, 3 = severe, 4 = life‐threatening, 5 = fatal adverse events (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf)
*For each AE category, the number represents the number of participants who had an AE of that category at a visit (post baseline) at whatever severity, for each treatment. For each AE category (and population), each participant would only appear once; thus, for example, if they had the same AE on more than one occasion, they would appear only once for that AE.
DM: diabetes mellitus
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analysis because no subgroups were analysed in the available trials.
Sensitivity analysis
We did not plan to conduct sensitivity analysis by removing studies at high or unclear risk of bias. Instead, we assessed and discussed how risk of bias might influence our conclusions. We sought to obtain any missing data by requesting them from study authors, extracting the data from figures, or calculating missing values from data available in the articles.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence for each outcome in each comparison using the GRADE methodology. We (SS, VA) used the GRADE handbook and GRADEpro website (https://www.gradepro.org/) for this purpose (Schünemann 2013). We exported the summary of findings tables from this website to RevMan 5. We graded the certainty of the evidence as very low, low, moderate, or high. We included the following outcomes in the summary of findings tables:
disease control: for example, regression or healing of the skin lesions at time periods specified by individual trials;
mortality (at any time);
effect on the quality of life: for example, relief of soreness or itching within six weeks (short‐term), after six weeks (long‐term), at six months, one year, and beyond one year;
adverse effects of treatment (at any time).
We calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) for important (primary and key secondary) outcomes from the absolute risk reduction (ARR) values and added these to the summary of findings tables.
Of the 14 comparisons presented in the review, we considered the following six comparisons to be important for key decision‐makers: (1) clobetasol propionate cream versus oral prednisone; (2) mild clobetasol propionate cream regimen (10 to 30 g/day) versus standard clobetasol propionate cream regimen (40 g/day); (3) doxycycline versus prednisolone; (4) prednisone plus azathioprine versus prednisone; (5) nicotinamide plus tetracycline versus prednisone; and (6) methylprednisolone plus azathioprine versus methylprednisolone plus dapsone.
Results
Description of studies
Results of the search
Our update searches of electronic sources identified 189 records (see Electronic searches). We identified 319 potentially eligible records from other sources, giving a total of 508 records. We discarded 480 records as irrelevant, based on titles and abstracts. We examined the remaining 28 records in full text, and excluded eight studies (nine records) (see Characteristics of excluded studies). We included four new studies (reported in eight articles). Together with the 10 studies included in the previous version of this review, we now have a total of 14 included studies (see Characteristics of included studies). We identified seven ongoing studies (seven records) and listed five studies (five records) as 'awaiting classification'. Figure 1 shows a flow diagram summarising the study selection process.
1.
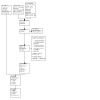
Included studies
We included four additional randomised controlled trials in this review update; there are now 14 published RCTs with 1442 participants available for analysis.
Design
All studies were parallel RCTs; there were two arms in all but one study, which had three arms (Guillaume 1993). We identified no cluster‐RCTs, within‐participant, or cross‐over trials. Six of the studies in this review were multicentre French studies (Dreno 1993; Guillaume 1993; Joly 2002; Joly 2009; Morel 1984; Roujeau 1984), and four multicentre studies in Japan, Germany/Austria and the UK/Germany (Amagai 2017; Beissert 2007; Sticherling 2017; Williams 2017). Williams 2017 was a pragmatic, non‐inferiority, randomised controlled trial. Morel was a co‐author in two studies (Morel 1984; Roujeau 1984), Guillaume in three (Guillaume 1993; Joly 2009; Roujeau 1984). Three trial authors (Crickx, Labeille, and Guillot) contributed to the same two trials (Guillaume 1993; Roujeau 1984), Dreno to three (Dreno 1993; Joly 2002; Joly 2009), and Roujeau to four studies (Guillaume 1993; Joly 2002; Joly 2009; Roujeau 1984). It is not clear if any of the studies included the same groups of participants. Burton 1978 was a single‐centre study in the UK; Fivenson 1994 was a two‐centre study in the USA; Liu 2006 was a single‐centre study in China; and Simon 2020 was a single‐centre study in Switzerland.
Sample size
There were 1442 participants in total. There were 11 small studies (between 20 and 100 participants in each) and three larger RCTs including more than half of the participants (909) in this review (Joly 2002; Joly 2009; Williams 2017).
Setting
Eight of the studies were conducted in centres outside France. Burton 1978 was conducted in the UK, Fivenson 1994 in the USA, Liu 2006 in China, Beissert 2007 in Germany, Sticherling 2017 in Germany and Austria, Amagai 2017 in Japan, Williams 2017 in the UK and Germany, and Simon 2020 in Switzerland. Although it is unclear what was the setting in the Liu 2006 study, the remaining 13 studies were carried out in hospital settings.
Participants
All participants had confirmed bullous pemphigoid (confirmed by immunofluorescence, except Liu 2006, in which this is unclear). The participants were older men and women (range of mean ages at baseline quoted in the included studies was 65.4 to 84.8 years of age).
Interventions
The interventions tested in the included studies included oral steroids, with or without other interventions (mycophenolate mofetil, azathioprine, or dapsone), topical steroids, tetracyclines with and without nicotinamide, and intravenous immunoglobulins versus placebo (Amagai 2017). Another study compared prednisolone plus mepolizumab to prednisolone plus placebo (Simon 2020). Amagai 2017 and Simon 2020 were the only studies including a placebo. All studies used different interventions, with only five studies overlapping. Thus, classification by intervention is intended to assist the reader, rather than to attempt to fit different interventions into broad classification groups. A brief summary of the type of interventions used is presented below. Full details of each trial are given in the Characteristics of included studies.
Oral steroid with or without other interventions, including plasma exchange
Beissert 2007 used oral methylprednisolone plus azathioprine versus oral methylprednisolone plus mycophenolate mofetil (Table 8); and Dreno 1993 administered prednisolone versus methylprednisolone (Table 9). Morel 1984 looked at prednisolone at two doses (0.75 mg/kg versus 1.25 mg/kg) (Table 10). Liu 2006 compared a traditional Chinese medicine, 'Jingui Shenqi Pill' (JSP) plus prednisone (0.5 to 1.0 mg/kg/day) to prednisone alone (0.5 to 1.0 mg/kg/day) (Table 11). In Guillaume 1993, participants received prednisolone versus prednisolone and azathioprine, versus plasma exchange and prednisolone (Table 12; Table 13), and Roujeau 1984 also investigated plasma exchange and prednisolone (Table 13). Burton 1978 compared azathioprine plus prednisone versus prednisone alone. We have used the drug names as reported in the included studies (i.e. prednisone or prednisolone); for the purposes of this review, prednisone and prednisolone are regarded as bio‐equivalent. Sticherling 2017 used oral methylprednisolone (0.5 mg/kg/day) plus azathioprine (1.5 to 2.5 mg/kg/day) versus oral methylprednisolone plus dapsone (1.5 mg/kg/day) (Table 14).
2. Azathioprine plus methylprednisolone compared to mycophenolate mofetil plus methylprednisolone for bullous pemphigoid.
| Azathioprine plus methylprednisolone compared to mycophenolate mofetil plus methylprednisolone for bullous pemphigoid (Beissert 2007) | ||||||
| Patient or population: bullous pemphigoid Setting: multicenter Intervention: azathioprine plus methylprednisolone Comparison: mycophenolate mofetil plus methylprednisolone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with mycophenolate mofetil plus methylprednisolone | Risk with azathioprine plus methylprednisolone | |||||
| Disease control: complete healing | Study population | RR 0.92 (0.83 to 1.03) | 73 (1 RCT) | ⊕⊕⊝⊝ Lowa | NNTH = 13 | |
| 1000 per 1000 | 920 per 1000 (830 to 1000) | |||||
| Mortality | ‐ | ‐ | ‐ | ‐ | ‐ | There were two deaths in the azathioprine group, described as not treatment‐related. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by two levels for imprecision (low number of events, CI includes null effect, wide CI).
3. Methylprednisolone compared to prednisolone for bullous pemphigoid.
| Methylprednisolone compared to prednisolone for bullous pemphigoid (Dreno 1993) | ||||||
| Patient or population: bullous pemphigoid Setting: multicentre Intervention: methylprednisolone Comparison: prednisolone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with prednisolone | Risk with methylprednisolone | |||||
| Disease control ‐ number of blisters at day 10 | Mean number of blisters: 13 | MD 7 lower (21.55 lower to 7.55 higher) | ‐ | 57 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Disease control ‐ overall improvement | Study population | RR 1.27 (0.90 to 1.79) | 57 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTB = 6 | |
| 621 per 1000 | 788 per 1000 (559 to 1000) | |||||
| Mortality | ‐ | ‐ | ‐ | ‐ | ‐ | There was no mortality, but the follow‐up period was only 10 days. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for unclear risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, and other bias, and no intention‐to‐treat analysis, short study duration, non‐validated assessment scales. bDowngraded by two levels for imprecision (low number of events, CI includes null effect, and wide CI).
4. Higher dose prednisolone (1.25 mg/kg) compared to lower dose prednisolone (0.75 mg/kg) for bullous pemphigoid.
| Higher dose prednisolone (1.25 mg/kg) compared to lower dose prednisolone (0.75 mg/kg) for bullous pemphigoid (Morel 1984) | ||||||
| Patient or population: bullous pemphigoid Setting: multicentre Intervention: higher dose prednisolone (1.25 mg/kg) Comparison: lower dose prednisolone (0.75 mg/kg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with lower dose prednisolone (0.75 mg/kg p/solone) | Risk with higher dose prednisolone (1.25 mg/kg p/solone) | |||||
| Disease control ‐ healing of skin lesions at day 21: ITT (assuming unknown = not healed) | Study population | RR 1.08 (0.66 to 1.77) |
50 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b | NNTB = 23 | |
| 538 per 1000 | 582 per 1000 (355 to 953) |
|||||
| Disease control ‐ healing of skin lesions at day 51: ITT (assuming unknown = not healed) | Study population | 1.63 (0.81 to 3.28) | 50 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTB = 8 NNTB = 6 |
|
| 308 per 1000 | 502 per 1000 (249 to 1000) |
|||||
| Mortality at day 51 | Study population | RR 1.64 (0.30 to 8.90) | 46 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTH = 19 | |
| 83 per 1000 | 137 per 1000 (25 to 742) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for risk of bias (allocation concealment not mentioned, no blinding, 2 dropouts in each group, no reasons given, and not included in analysis). bDowngraded by two levels for imprecision (low number of events, CI includes null effect and wide CI).
5. Jingui shenqi pill 1# bid plus prednisone compared to prednisone alone for bullous pemphigoid.
| Jingui shenqi pill1# bid plus prednisone compared to prednisone alone for bullous pemphigoid (Liu 2006) | ||||||
| Patient or population: bullous pemphigoid Setting: university hospital Intervention: jingui shenqi pill 1# bid plus prednisone Comparison: prednisone alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with prednisone alone | Risk with jingui shenqi pill 1# bid plus prednisone | |||||
| Healing at 4 weeks ‐ complete healing | Study population | RR 3.00 (0.13 to 68.26) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Healing at 4 weeks ‐ partial healing | Study population | RR 1.18 (0.82 to 1.70) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 733 per 1000 | 865 per 1000 (601 to 1000) | |||||
| Healing at 4 weeks ‐ overall healing ‐ participants experiencing any degree of healing | Study population | RR 1.27 (0.91 to 1.78) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 733 per 1000 | 931 per 1000 (667 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for risk of bias (method of sequence generation not mentioned, allocation concealment not mentioned, no blinding, exact method of diagnosis not mentioned). bDowngraded by two levels for imprecision (low number of events, CI includes null effect, wide CI).
6. Prednisolone plus azathioprine compared to prednisolone plus plasma exchange for bullous pemphigoid.
| Prednisolone plus azathioprine compared to prednisolone plus plasma exchange for bullous pemphigoid (Guillaume 1993) | ||||||
| Patient or population: bullous pemphigoid Setting: multicentre Intervention: prednisolone plus azathioprine Comparison: prednisolone plus plasma exchange | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with prednisolone plus plasma exchange | Risk with prednisolone plus azathioprine | |||||
| Disease control at 6 months | Study population | RR 1.34 (0.67 to 2.66) | 67 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTB = 11 | |
| 290 per 1000 | 389 per 1000 (195 to 772) | |||||
| Mortality at 6 months | Study population | RR 1.72 (0.47 to 6.32) | 67 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTH = 15 | |
| 97 per 1000 | 166 per 1000 (45 to 612) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for risk of bias (no blinding, reasons for dropout not clear, intention‐to‐treat analysis not performed, only the composite measure of controlled disease was reported, 120 patients were planned to be recruited but trial stopped after 100 patients). bDowngraded by two levels for imprecision (low number of events, CI includes null effect, wide CI)
7. Prednisolone plus plasma exchange compared to prednisolone for bullous pemphigoid.
| Prednisolone plus plasma exchange compared to prednisolone for bullous pemphigoid (Guillaume 1993, Roujeau 1984) | ||||||
| Patient or population: bullous pemphigoid Setting: multicentre Intervention: prednisolone plus plasma exchange Comparison: prednisolone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with prednisolone | Risk with prednisolone plus plasma exchange | |||||
| Disease control at 1 month (controlled with 0.3 mg/kg prednisolone) | Study population | RR 18.78 (1.20 to 293.70) | 37 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Disease control at 1 month (controlled with 1.0 mg/kg prednisolone or less) | Study population | RR 1.79 (1.11 to 2.90) | 37 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | NNTB = 3 | |
| 533 per 1000 | 955 per 1000 (592 to 1000) | |||||
| Disease control at 6 months | Study population | RR 0.69 (0.35 to 1.38) | 62 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | NNTB = 8 | |
| 419 per 1000 | 289 per 1000 (147 to 579) | |||||
| Mortality at 1 month: excluding dropouts | Study population | Not estimable | 37 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Mortality at 1 month: ITT worst case | Study population | RR 0.71 (0.11 to 4.55) | 41 (1 RCT) | ⊕⊝⊝⊝ Very lowa,d | NNTH = 30 | |
| 118 per 1000 | 84 per 1000 (13 to 535) | |||||
| Mortality at 6 months | Study population | RR 0.60 (0.16 to 2.30) | 62 (1 RCT) | ⊕⊝⊝⊝ Very lowc,d | NNTH = 16 | |
| 161 per 1000 | 97 per 1000 (26 to 371) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for risk of bias (Roujeau 1984: allocation concealment not mentioned, not blinded, intention‐to‐treat analysis not performed). bDowngraded by one level for imprecision (low number of events). cDowngraded by one level for risk of bias (Guillaume 1993: no blinding, reasons for 2 dropouts not clear, intention‐to‐treat analysis not performed, only the composite measure of controlled disease was reported, 120 patients were planned to be recruited but trial stopped after 100 patients). dDowngraded by two levels for imprecision (low number of events, CI includes null effect and wide CI).
Data from these studies were not analysed in a meta‐analysis because the doses of prednisone differed and the outcomes were measured at different time points.
8. Methylprednisolone plus azathioprine compared to methylprednisolone plus dapsone for bullous pemphigoid.
| Methylprednisolone plus azathioprine compared to methylprednisolone plus dapsone for bullous pemphigoid (Sticherling 2017) | ||||||
| Patient or population: bullous pemphigoid Setting: multicentre Intervention: methylprednisolone plus azathioprine Comparison: methylprednisolone plus dapsone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with methylprednisolone plus dapsone | Risk with methylprednisolone plus azathioprine | |||||
| Mortality at 1 year | Study population | RR 3.00 (0.33 to 27.06) | 54 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTH = 14 | |
| 37 per 1000 | 111 per 1000 (12 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by two levels for risk of bias (no blinding; outcome data are not fully reported; authors state recruitment of 88 was the aim, however, only 54 patients were finally recruited; outcomes are still not reported for all, no reasons given; no intention‐to‐treat analysis, selective outcome reporting, trial not registered, possible pharma bias, "Investigators may have been biased in favour of dapsone to confirm earlier results and therefore started tapering of corticosteroids earlier", “It cannot be excluded that healthier patients had been included resulting in a preselection bias”. bDowngraded by two levels for imprecision (low number of events, CI includes null effect, wide CI).
Topical steroid treatment
Joly 2002 used a very potent topical corticosteroid, clobetasol propionate, versus oral prednisolone. Joly 2009 investigated two different regimens of topical clobetasol propionate cream: 40 g clobetasol propionate cream/day versus a mild regimen of 10 to 30 g/day, depending on the body weight, were compared in a large, randomised study. The regimen was chosen according to disease severity.
Tetracyclines with or without nicotinamide
Fivenson 1994 used prednisolone versus nicotinamide and tetracycline. Williams 2017 used doxycycline (200 mg/day) versus prednisolone (0.5 mg/kg/day) for the initial treatment (six weeks) and permitted adjuvant potent topical steroid application on lesions (< 30 g/week) for the first three weeks. In this pragmatic trial design, treatment was allowed to be altered after week six according to participants' needs.
Intravenous immunoglobulins
Amagai 2017 used high‐dose intravenous immunoglobulins (400 mg/kg/day) for five days versus placebo in participants who showed no symptomatic improvement on ≥ 0.4 mg prednisolone/kg/day.
Mepolizumab
Simon 2020 (Table 15) used mepolizumab (750 mg) every four weeks over 12 weeks versus placebo as an add‐on therapy to oral corticosteroids in participants with an acute flare‐up of bullous pemphigoid. The oral corticosteroid dose was 0.5 mg prednisolone per kilogram of body weight until no further blisters and/or bullous pemphigoid lesions appeared, and it was then tapered by 20% every two weeks. Participants were followed over a period of up to six months after treatment. Nine participants (six in the mepolizumab group and three in the placebo group) discontinued the treatment phase prematurely, of which one participant withdrew from the trial. The sample size of this study was determined with a power of study of 60% (β = 0.6).
9. Prednisolone plus mepolizumab compared to prednisolone for bullous pemphigoid.
| Prednisolone plus mepolizumab compared to prednisolone for bullous pemphigoid (Simon 2020) | ||||||
| Patient or population: bullous pemphigoid Setting: single centre Intervention: prednisolone plus mepolizumab Comparison: prednisolone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with prednisolone | Risk with prednisolone plus mepolizumab | |||||
| Cumulative rate of relapse‐free patients at 16 weeks | Study population | RR 0.75 (0.27 to 2.06) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTH = 10 (favours prednisolone) | |
| 400 per 1000 | 300 per 1000 (108 to 824) | |||||
| Cumulative rate of relapse‐free patients at 36 weeks | Study population | RR 1.17 (0.65 to 2.09) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | NNTB = 10 (favours combination) | |
| 600 per 1000 | 702 per 1000 (390 to 1000) | |||||
| Number of patients with moderate to severe adverse events | Study population | RR 1.00 (0.47 to 2.14) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 500 per 1000 | 500 per 1000 (235 to 1000) | |||||
| Number of patients with serious adverse events | Study population | RR 1.50 (0.37 to 6.14) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 200 per 1000 | 300 per 1000 (74 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB/H: number needed to treat for an additional beneficial/harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by 1 level for risk of bias (unclear risk for random sequence generation, allocation concealment, incomplete outcome data, and other bias; and high risk for selective reporting). bDowngraded by 2 levels for imprecision (low number of events and wide confidence interval).
Outcomes
We specified a number of outcomes of interest for this review in Types of outcome measures. Our primary outcomes of regression or healing of skin lesions and mortality was reported in some form in all included studies except Liu 2006. However, these outcomes were not primary endpoints in all studies, and in some, they were only indirectly reported. Effects of the interventions on quality of life using a validated questionnaire were reported in Williams 2017. The duration of remission after stopping treatment was reported in Beissert 2007 and Joly 2009.
Adverse effects were recorded in Amagai 2017, Beissert 2007, Joly 2002, Sticherling 2017, and Williams 2017, and vaguely in Simon 2020. Mortality was reported in all but Liu 2006 and Simon 2020 (having contacted the authors, we learned that no deaths occurred during the study period in the Simon study), and only indirectly in Amagai 2017. None of the included studies reported all of our predefined outcomes.
The reports of the included studies focused on a variety of outcomes, including disease control, survival, and cumulative steroid doses, summarised as follows.
Amagai 2017 compared the disease activity score (DAS) and Japanese bullous pemphigoid activity score (jBPAS) on day 15. Other outcomes were the time to treatment reduction (defined as the length of time until symptoms improved leading to a reduction of treatment determined by the evaluator), oral steroid dosage, anti‐BP180 antibody titre, and the incidence of adverse events and adverse drug reactions (ADRs) up to day 57.
The outcomes reported in Beissert 2007 were complete healing (complete re‐epithelialisation of all lesions), and cumulative steroid dose. Secondary outcomes were duration of remission (disease‐free interval) and safety.
Only Burton 1978 did not have clearly stated outcome measures. We obtained the following outcome measures from the published report: cumulative dose of prednisone in both groups necessary for disease control, mortality, and adverse effects, including whether azathioprine and prednisolone (synergistic immunosuppression) was associated with increased risk of malignancy.
Dreno 1993 reported the number of blisters, intensity of erythema, and the intensity of pruritus (itch) at days five and 10.
Fivenson 1994 reported the number of bullous, crusted, urticarial lesions as the total highest score possible on each visit per participant.
Guillaume 1993 reported disease control in terms of blister formation, resolution of erythema, and no more than minimal pruritus at four weeks and six months after starting treatment.
Joly 2002 and Joly 2009 both reported survival after one year, disease control at three weeks, and occurrence of severe adverse events during the follow‐up year; Joly 2009 also reported occurrence of relapses during follow‐up and cumulative doses of steroid cream.
Liu 2006 reported compete healing at four weeks.
Morel 1984 assessed new blister formation at days 21 and 51.
Roujeau 1984 assessed the cumulative and daily corticosteroid dose to achieve disease control in terms of blister formation. Other parameters of disease control were intensity of pruritus and extent of erythema and urticarial lesions.
In the Simon 2020 study, the primary endpoint was the cumulative rate of relapse‐free participants after initiating therapy. Relapse was defined as manifestation of new bullous pemphigoid lesions and/or more than three blisters during or within four weeks after the treatment period. Secondary endpoints included the cumulative rate of participants attaining disease control and the rate of participants maintaining disease control, the absolute reduction of severity and pruritus as assessed by the autoimmune bullous skin disorder intensity score (ABSIS) and a pruritus numerical rating scale. Safety was evaluated by physical examination, monitoring white blood cell counts, liver and renal tests, concomitant therapies, adverse events, and serious adverse events.
Sticherling 2017 aimed for ceasing of (all) blister formation and re‐epithelialisation of lesions. The corticosteroid dose was then tapered. The primary outcome was time until complete tapering of methylprednisolone. An additional primary outcome of "time until the methylprednisolone dose could be reduced to ≤ 10 mg/d" was later determined. The cumulative amount of and number of days on methylprednisolone, and relapses were noted. Adverse events were assessed and their severity graded.
Williams 2017 determined the regression or healing of skin lesions at six weeks in a non‐inferiority approach (short‐term effectiveness) and long‐term safety (mortality at week 52) in a superiority approach. Secondary outcomes included long‐term effectiveness at 52 weeks, adverse events, and participants' quality of life.
Funding
Four of 14 included studies were supported by industry, either providing the study medication or financial support. Nihon Pharmaceutical Company Ltd. manufactured the investigational drugs used in the Amagai 2017 study. Hoffmann‐La Roche AG, producers and providers of mycophenolate mofetil ('CellCept') supported the Beissert 2007 study with an unrestricted grant. GlaxoSmithKline provided the study drug mepolizumab and a research grant to the Simon 2020 study. Riemser Inc. (Greifswald, Germany) supported the Sticherling 2017 study with unrestricted funding of EUR 10,000.
One study was funded by a government grant, the National Institute for Health and Care Research (NIHR) Health Technology Assessment Programme, UK (Williams 2017).
Excluded studies
We excluded eight trials for the following reasons (see Characteristics of excluded studies table):
four trials were terminated early (EUCTR2011‐004361‐32‐DE; NCT00472030; NCT01688882; NCT03286582);
bullous pemphigoid was not confirmed by immunofluorescence in one RCT testing cyclophosphamide (Kannan 2018);
one study looked at rituximab in pemphigus not bullous pemphigoid (EudraCT2008‐005266‐31);
NCT05061771 (investigating nomacopan) was withdrawn for strategical reasons;
Derhaschnig 2016 tested a mixed group of diseases; the number of participants with bullous pemphigoid was unknown.
Studies awaiting classification
We listed five completed trials (testing omalizumab, methotrexate, topical steroids, AKST4290, or tetracyclines) as awaiting classification (ChiCTR‐IOR‐15007146; ChiCTR‐TRC‐12003592; ChiCTR‐TRC‐12003593; EudraCT 2019‐001059‐37‐DE (AKST4290); NCT02313870).
Ongoing studies
Six ongoing randomised trials (seven references) are registered for the treatment of bullous pemphigoid: ChiCTR‐2000028707 (interleukin‐2); ChiCTR‐TRC‐12003538 (methotrexate); EudraCT 2020‐002912‐34 (avdoralimab); NCT02365675 (wound dressings); NCT04206553 (dupilumab); and NCT04612790 (benralizumab).
Risk of bias in included studies
Please see Figure 2 and Figure 3 for summaries of review authors' judgements about each methodological quality item for each included study and presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
We assessed selection bias by taking into account both random sequence generation (we considered nine studies at low risk, five unclear) and allocation concealment (five studies at low risk and nine unclear). Of the 14 studies, there was low risk of bias in four studies, unclear risk of bias in 10 studies, and there were no studies with high risk of bias.
Some attempt at randomisation was made in all included studies. Burton 1978, Dreno 1993, Fivenson 1994, Liu 2006, and Simon 2020 did not describe randomisation in detail. We assessed only Amagai 2017, Guillaume 1993, Joly 2009, and Williams 2017 as having adequate randomisation as both sequence generation and allocation concealment were adequate.
Burton 1978 did not explicitly state that the 25 participants were initially randomised, but this was implied in other sections of the article in that each participant was described as being assigned to treatment by the ward sister who drew a marked paper from an envelope. Since there were no details about how the envelopes were marked, we rated sequence generation as unclear risk.
Morel 1984 randomised 50 participants using a table of numbers, but allocation concealment was unclear. Dreno 1993 was randomised, but did not describe the method. Similarly, Fivenson 1994 mentioned randomisation but did not explain the method used. A full translation of the Liu 2006 study provided no details about the randomisation method used.
The prednisolone plus plasma exchange versus prednisolone‐only study had an adequate method of sequence generation (computer‐generated), but allocation was unclear (Roujeau 1984). The Joly 2002 study on topical versus oral corticosteroids had an adequate sequence generation method, but seemed marginal for allocation concealment; therefore we coded it as unclear.
A three‐arm study comparing the efficacy of azathioprine or plasma exchange when added to prednisolone used an adequate method of sequence generation (pre‐established lists) (Guillaume 1993). In this study, we rated allocation concealment as having a low risk of bias.
Beissert 2007 had adequate sequence generation by centrally‐generated random numbers to receive oral methylprednisolone plus azathioprine or mycophenolate mofetil. Sticherling 2017 performed computerised randomisation centrally; this was a non‐blinded study and allocation concealment was unclear.
Simon 2020 did not provide details about the randomisation method.
Blinding
We considered only two studies to be at low risk of performance bias (Amagai 2017; Simon 2020), two at unclear risk (Dreno 1993; Williams 2017), and the remaining 10 studies at high risk in this domain. For detection bias, we assessed only three studies as low risk (Amagai 2017; Dreno 1993; Simon 2020), one study as unclear risk (Williams 2017), and the remaining 10 studies as high risk.
Most of the studies had no masking of either participants or outcome assessors (see Figure 2). In Dreno 1993, the two products used as interventions differed in appearance, and were supplied to participants by someone other than the investigator. Clinical follow‐up after the end of the study was done by a masked outcome assessor. Amagai 2017 described adequate masking of participants and outcome assessors; the two products used were of similar appearance. Williams 2017 had outcome assessors masked for the primary effectiveness outcome, but not for the primary safety outcome. Participants in this study were not masked.
Two regimens of very potent topical corticosteroids – a standard regimen of 40 g clobetasol propionate cream/day versus a mild regimen of 10 to 30 g/day depending on the body weight – were compared in a large, randomised study (Joly 2009). Blinding was not deemed necessary by the authors as the primary outcome was event‐free survival.
The studies by Burton 1978, Fivenson 1994, Guillaume 1993, Liu 2006, Roujeau 1984, and Sticherling 2017 were also not masked. In Guillaume 1993, masking could be considered by some as unethical (this was given as a reason for not masking), because it would mean an invasive procedure (intravenous line) in the control group as well. Joly 2002 was not masked; however, the primary outcome was survival at one year which was considered unlikely to be biased by lack of masking (this was given as the reason for not "blinding"). However, assessments for disease control and complications were also made, which might potentially have been biased by the lack of masking. In Beissert 2007, complete healing (defined as complete re‐epithelialisation of the lesions) and cumulative steroid dose at complete healing were primary end points; judgement may have been biased because of the lack of masking. Simon 2020 was a double‐blind trial.
Incomplete outcome data
We assessed five studies as having a low risk of attrition bias, six as unclear risk, and three as high risk (Amagai 2017; Morel 1984; Sticherling 2017).
Burton 1978 and Liu 2006 seemed to have no dropouts, but the reports were short and no details were given. In Roujeau 1984, the number and reasons for dropouts – two from each arm of the study – were listed.
There was one dropout in Dreno 1993: treatment was stopped after eight days, as the participant was in a coma unrelated to treatment. Joly 2002 stated reasons for dropouts. This study was the largest, including 341 participants. In Beissert 2007, one participant was lost to follow‐up, and two participants died of causes not related to the treatment and were included in the intention‐to‐treat analysis. Williams 2017 aimed for 256 participants for their primary safety analysis. However, after randomisation of 278 participants, 25 were withdrawn because of ineligibility, resulting in 253 randomised eligible patients. There was a similar dropout rate in both groups, and reasons for withdrawals were given. The Health Technology Assessment report of this study provides additional data of the Williams 2017 trial, which is presented in the analysis of this review (Chalmers et al. 2017, see Williams 2017).
In the prednisone (six participants) versus tetracycline and nicotinamide (14 participants) trial (Fivenson 1994), the trial report stated that 18 of 20 participants enroled in the study were treated, and that two participants who were unavailable for follow‐up at eight weeks were both in the tetracycline/nicotinamide group. Fivenson 1994 did not give the reasons for dropout. Guillaume 1993 had three arms: prednisolone‐only (32 participants with one dropout), prednisolone plus azathioprine (36 participants, no dropouts), and prednisolone plus plasma exchange (32 participants with one dropout). The reasons for the two dropouts were not given. Joly 2009 did not fulfil an intention‐to‐treat analysis, as only 150 of 153 participants randomised to the standard regimen were analysed. However, this is only a small deviation. In Simon 2020, all participants were included in the intention‐to‐treat analysis; however, 9 of 30 participants left the trial prematurely.
Amagai 2017 had 15 dropouts at day 57 when final data were collected. As 56 participants were randomised and eligible, this entails a dropout rate greater than 20% (9/29 in the treatment group, 6/27 in the placebo group), and the reasons for dropout were not given for all. In Morel 1984, four participants were excluded from the analysis: two because they did not fit the inclusion criteria and two due to protocol deviation. The Sticherling 2017 study's recruitment target of 88 participants aimed for a 10% dropout rate, as 80 participants were needed for calculation. However, this study ultimately recruited only 54 participants, and did not report outcomes for all, with no reasons given.
Selective reporting
We assessed 10 studies as having a low risk of selective reporting bias, two as unclear risk (Burton 1978; Guillaume 1993), and two as high risk (Simon 2020; Sticherling 2017).
All included studies reported all of their prospectively‐stated outcomes except for Burton 1978 and Guillaume 1993. In Burton 1978, the prospectively‐stated outcome measures were unclear. In Guillaume 1993, outcome measurements of controlled disease were stated to be no more than one new blister occurring four weeks after starting treatment, resolution of erythema, and no more than minimal pruritus. However, only the composite measure of controlled disease was reported. Sticherling 2017 aimed for an intention‐to‐treat analysis, but did not report all outcome data for all randomised participants and gave no reasons. This study added another primary outcome for effectiveness analysis because of low numbers at the primary end point, resulting in no meaningful numbers for analysis. The Simon 2020 study provided no details regarding which groups showed which adverse events.
Other potential sources of bias
We rated three studies as having a low risk of other biases, eight as unclear risk, and three as high risk (Fivenson 1994; Liu 2006; Sticherling 2017).
Dreno 1993, Joly 2002, Liu 2006, Morel 1984, and Amagai 2017 had very short follow‐up periods (10, 21, 28, 51, and 57 days, respectively), which limits the applicability of their results to initial treatment effectiveness; long‐term effectiveness and adverse events relevant in clinical practice in a chronic disease such as bullous pemphigoid cannot be judged. Furthermore, Amagai 2017 did not want to capture initial treatment success but rather the effect of additional treatment in difficult‐to‐treat patients.
All but one study confirmed initial diagnosis by immunofluorescence (a pathological test was mentioned but not described further in Liu 2006).
In Beissert 2007, more participants in the azathioprine group had severe disease: 53% had 20% or more of body surface area involvement compared to only 27% of participants in the mycophenolate mofetil group, and more participants in the azathioprine group had raised liver enzyme tests. However, a test to check for thiopurine methyltransferase activity was not performed. Additionally, only those participants who were likely to attend for follow‐up were recruited. Eligibility was also determined by the consultant doctor after baseline testing.
The Fivenson 1994 study was originally designed to randomise 96 participants, but enrolment was terminated when 20 participants were enroled. No reasons were given for this.
The Guillaume 1993 study was stopped after the interim analysis became available, which showed no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone.
Sticherling 2017 had a very low mortality rate. Authors stated that it “cannot be excluded, however, that in the present trial, healthier patients had been included resulting in a preselection bias”. This may be a relevant factor for the lower‐than‐expected mortality rate.
The pharmaceutical industry supported the Amagai 2017, Beissert 2007, Simon 2020, and Sticherling 2017 studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
The assessment and reporting of disease control, symptoms, and adverse effects of medication were recorded in varying detail in the included studies. Mortality was the only outcome measure documented in all the studies. However, mortality was not a stated outcome of interest in most studies, and it was not always clear whether the deaths were related to treatment.
We have organised this section by comparison, describing the primary and secondary outcomes prespecified in Types of outcome measures, where available (primary outcomes were disease control and mortality; secondary outcomes were effect on quality of life and adverse effects).
Higher versus lower doses of prednisolone
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
The Morel 1984 study compared a starting dose of prednisolone of 0.75 mg/kg (26 participants) to a prednisolone dose of 1.25 mg/kg (24 participants). On the basis of intention‐to‐treat analysis (assuming unknown participants were not healed), healing of skin lesions at day 51 occurred in 8/26 (31%) participants with the 0.75 mg/kg dose and in 12/24 (50%) participants with the 1.25 mg/kg dose (risk ratio (RR) 1.63, 95% confidence interval (CI) 0.81 to 3.28; number needed to treat for an additional beneficial outcome (NNTB) = 6; 50 participants; very low‐certainty evidence; Analysis 1.1). Healing of skin lesions at day 21 occurred in 14/26 (54%) participants with the 0.75 mg/kg dose and in 14/24 (58%) participants with the 1.25 mg/kg dose (RR 1.08, 95% CI 0.66 to 1.77; NNTB = 23; 50 participants; very low‐certainty evidence; Analysis 1.1). Thus, the two doses were similarly effective.
1.1. Analysis.

Comparison 1: Higher‐dose prednisolone (1.25 mg/kg) versus lower‐dose prednisolone (0.75 mg/kg), Outcome 1: Disease control
When dropouts were excluded, healing of skin lesions at day 21 occurred in 14/24 (58%) cases with the 0.75 mg/kg dose and in 14/22 (64%) cases with the 1.25 mg/kg dose (RR 1.09, 95% CI 0.69 to 1.73; 44 participants; very low‐certainty evidence). When dropouts were excluded, healing of skin lesions at day 51 occurred in 8/24 (33%) participants with the 0.75 mg/kg dose and in 12/22 (55%) participants with the 1.25 mg/kg dose (RR 1.64, 95% CI 0.83 to 3.24; 43 participants; very low‐certainty evidence).
Mortality
At day 51, there were three deaths out of 22 participants in the higher‐dose group compared to two deaths out of 24 participants in the lower‐dose group (RR 1.64, 95% CI 0.30 to 8.90; number needed to treat for an additional harmful outcome (NNTH) = 19; 46 participants; very low‐certainty evidence; Analysis 1.2) (Morel 1984).
1.2. Analysis.

Comparison 1: Higher‐dose prednisolone (1.25 mg/kg) versus lower‐dose prednisolone (0.75 mg/kg), Outcome 2: Mortality at day 51
Secondary outcomes
Morel 1984 did not report effect on quality of life and adverse effects.
Methylprednisolone versus prednisolone
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
The Dreno 1993 study, comparing different formulations of the steroids methylprednisolone (28 participants) with prednisolone (29 participants), found a large reduction in the number of blisters in both groups. At day 10, the mean number of blisters was 6.0 (standard deviation (SD) 19) for methylprednisolone and 13.0 (SD 35) for prednisolone (mean difference (MD) ‐7.00, 95% CI ‐21.55 to 7.55; 57 participants; very low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Methylprednisolone versus prednisolone, Outcome 1: Disease control
Collective figures of overall improvement (22 of 28 participants in the methylprednisolone group, 78.6%, versus 18 of 29 participants in the prednisolone group, 62.1%) were reported (RR 1.27, 95% CI 0.90 to 1.79; NNTB = 6; 57 participants; very low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Methylprednisolone versus prednisolone, Outcome 3: Overall improvement (number of participants with good results)
Mortality
There were no deaths recorded in this study, but the follow‐up period was only 10 days (Analysis 2.2).
2.2. Analysis.

Comparison 2: Methylprednisolone versus prednisolone, Outcome 2: Mortality at day 10
Secondary outcomes
Effect on quality of life
Participants measured both erythema and pruritus (itch) on a scale from zero (absent) to three (severe). No difference was seen between the groups for either score: erythema 0.59 (SD 0.69) versus 0.93 (SD 0.72), mean difference ‐0.34 (95% CI ‐0.71 to 0.03; 57 participants; very low‐certainty evidence), and pruritus 0.59 (SD 0.8) versus 0.86 (SD 0.8), mean difference ‐0.27 (95% CI ‐0.69 to 0.15; 57 participants; very low‐certainty evidence; Analysis 2.4). The study investigators reported that the only statistically significant result was a reduction in pruritus, but it was unclear which statistical test they used.
2.4. Analysis.

Comparison 2: Methylprednisolone versus prednisolone, Outcome 4: Quality of life ‐ extent of itching (score out of 3)
Prednisone plus azathioprine versus prednisone
Two small studies evaluated this comparison: Burton 1978 with a three‐year follow‐up, and Guillaume 1993 with a six‐month follow‐up (see Table 4).
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
The Guillaume 1993 study failed to show improvements in disease control (14/36 versus 13/31; RR 0.93, 95% CI 0.52 to 1.66; NNTH = 35; 67 participants; very low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Prednisone plus azathioprine versus prednisone, Outcome 1: Disease control
In Burton 1978, 9/12 participants in the prednisone plus azathioprine group had their disease controlled at three years (seven participants off treatment and two still on treatment), and 9/13 participants in the prednisone‐only group had their disease controlled (four participants off treatment and five still on treatment) (RR 1.08, 95% CI 0.67 to 1.76; NNTB = 19; 25 participants; very low‐certainty evidence; Analysis 3.1). This study found a 45% reduction in the amount of prednisone required for disease control by the azathioprine group over a 3‐year period (mean total dose 3688 mg in the azathioprine group versus 6732 mg in the prednisone‐only group) (P < 0.01). The statistical test used was not reported.
Mortality
Mortality at six months was similar between the prednisone and the prednisone plus azathioprine group in Guillaume 1993 (RR 1.03, 95% CI 0.35 to 3.06; NNTH = 200; 67 participants; very low‐certainty evidence; Analysis 3.2). The Burton 1978 study had the longest follow‐up (three years) and an overall mortality of 7/25 participants (28%). There were three deaths in the prednisone plus azathioprine group (12 participants) and four in the prednisone‐only group (13 participants) (RR 0.81, 95% CI 0.23 to 2.91; NNTB = 18; P = 0.75; 25 participants; very low‐certainty evidence; Analysis 3.2) after three years of treatment.
3.2. Analysis.

Comparison 3: Prednisone plus azathioprine versus prednisone, Outcome 2: Mortality
Secondary outcomes
Adverse effects
In Burton 1978, one of the off‐treatment participants who was originally assigned to the prednisone‐only group withdrew from the prednisone group due to adverse effects and was subsequently successfully treated with azathioprine. In Guillaume 1993, severe complications were more often noted in the azathioprine group (RR 1.29, 95% CI 0.68 to 2.45; 67 participants; very low‐certainty evidence; Analysis 3.3). Unfortunately, the adverse effects were not given in detail for each group (see Adverse events; Table 7). The study investigators stated that "most of the adverse events could be attributed to corticosteroids". The main adverse effect associated with azathioprine was a reduction in the white cell count (two of 12 participants in the Burton 1978 trial and four of 36 participants in the Guillaume 1993 trial).
3.3. Analysis.

Comparison 3: Prednisone plus azathioprine versus prednisone, Outcome 3: Mortality and severe adverse events at 6 months
The Burton 1978 study found a beneficial effect of adding azathioprine without serious side effects; Guillaume 1993 found no benefit of adding azathioprine and serious side effects.
Prednisolone plus plasma exchange versus prednisolone
Primary outcome
Disease control (e.g. regression or healing of skin lesions)
Two small studies evaluated this comparison: Roujeau 1984 with a one‐month follow‐up, and Guillaume 1993 with a six‐month follow‐up.
In the study comparing prednisolone versus prednisolone and plasma exchange (Roujeau 1984), all participants were started on a low dose of prednisolone (0.3 mg/kg/day), which was increased (maximum 2 mg/kg/day methylprednisolone intramuscular plus 2 mg/kg/day oral cyclophosphamide) until disease control was achieved. The addition of plasma exchange appeared to reduce the amount of prednisolone required to achieve disease control. Disease control was achieved with a dose of 0.3 mg/kg/day in 13/22 participants in the prednisolone plus plasma exchange group but in none of the 15 participants in the prednisolone‐only group (risk ratio in favour of prednisolone plus plasma exchange: RR 18.78, 95% CI 1.20 to 293.70; P = 0.04; 37 participants; low‐certainty evidence; Analysis 4.1).
4.1. Analysis.

Comparison 4: Prednisolone plus plasma exchange versus prednisolone, Outcome 1: Disease control
More participants achieved disease control with prednisolone doses less than or equal to 1 mg/kg: 21/22 for prednisolone plus plasma exchange and 8/15 for prednisolone alone (RR 1.79, 95% CI 1.11 to 2.90; NNTB = 3; P = 0.02; 37 participants; low‐certainty evidence; Analysis 4.1). Disease control was achieved with less than half the total prednisolone dose in the plasma exchange group. Significantly lower doses of prednisolone were required to achieve disease control, both in terms of the cumulative dose (mean difference ‐1.53 g, 95% CI ‐2.40 to ‐0.66; 37 participants; low‐certainty evidence; Analysis 4.2) and the average daily dose: 0.52 (SD 0.28) mg/kg in the plasma exchange group versus 0.97 (SD 0.33) mg/kg in the prednisolone‐only group. Roujeau 1984 found a similar side‐effect profile in both groups and the disease was controlled within about four weeks in both groups.
4.2. Analysis.

Comparison 4: Prednisolone plus plasma exchange versus prednisolone, Outcome 2: Disease control at 1 month ‐ cumulative steroid dose (g)
However, this favourable effect of adding plasma exchange was not seen in the Guillaume 1993 study for disease control at six months: 9/31 prednisolone plus plasma exchange versus 13/31 prednisolone alone (RR 0.69, 95% CI 0.35 to 1.38; NNTH = 8; 62 participants; very low‐certainty evidence; Analysis 4.1, see Analysis 4.1.3). The study report indicates that the trial was "interrupted after the interim analysis showed no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone" at four weeks or at six weeks of follow‐up.
Mortality
Guillaume 1993 assessed mortality at six months as mortality alone (3/31 versus 5/31; RR 0.60, 95% CI 0.16 to 2.30; NNTB = 16; 62 participants; very low‐certainty evidence; Analysis 4.3), or total adverse events including mortality (10 major adverse events, including five deaths, in the prednisolone group versus six major adverse events, including three deaths, in the plasma exchange group) (RR 0.60, 95% CI 0.25 to 1.45; 62 participants; very low‐certainty evidence; Analysis 4.4).
4.3. Analysis.

Comparison 4: Prednisolone plus plasma exchange versus prednisolone, Outcome 3: Mortality
4.4. Analysis.

Comparison 4: Prednisolone plus plasma exchange versus prednisolone, Outcome 4: Mortality and severe adverse events at 6 months
In Roujeau 1984, no deaths occurred during the treatment period (Analysis 4.3). However, the study originally enroled 41 participants, of whom four were excluded for various reasons. Trial authors thus analysed only 37 participants, and of these, only 25 participants were available for follow‐up. Of these 25 participants, two participants in the prednisolone group died, and one in the prednisolone plus plasma exchange group died (the calculation for the worst‐case scenario includes the four lost participants, two in each group).
Secondary outcomes
Adverse effects
Guillaume 1993 reported major adverse effects including death, but provided few details. The study authors stated that most adverse effects were attributed to corticosteroids (6/31 in the plasma exchange group, including one myocardial infarction; 10/31 in the prednisolone group). Roujeau 1984 described glycosuria in five participants, myopathy in one, and mental disturbance in one of 10 participants in the prednisolone group, and three cases of glycosuria, three of mental disturbance, and one of gastritis in the 12 participants in the plasma exchange group. Seven episodes of hypotension and 10 chills/fever amongst 174 plasma exchange episodes occurred. No event required a modification of the treatment regimen. In eight of 22 participants, there was difficulty in venous access, restricting the volume of plasma exchange (Table 7).
Prednisolone plus azathioprine versus prednisolone plus plasma exchange
Primary outcome
Disease control (e.g. regression or healing of skin lesions)
Comparing the prednisolone plus azathioprine group with the prednisolone plus plasma exchange group (Guillaume 1993), no differences were found for disease control at six months: 14/36 and 9/31, respectively (RR 1.34, 95% CI 0.67 to 2.66; NNTB = 11; 67 participants; very low‐certainty evidence; Analysis 5.1). See Table 12.
5.1. Analysis.

Comparison 5: Prednisolone plus azathioprine versus prednisolone plus plasma exchange, Outcome 1: Disease control at 6 months
Mortality
Mortality at six months in Guillaume 1993 was 6/36 (azathioprine) versus 3/31 (plasma exchange) (RR 1.72, 95% CI 0.47 to 6.32; NNTH =15; 67 participants; very low‐certainty evidence).
Secondary outcomes
Adverse effects
Total adverse effects, including deaths, were more often noted in the azathioprine group (15/36 total adverse effects (including six deaths) versus 6/31 (including three deaths) in the plasma exchange group) (Guillaume 1993). The results at six months were in favour of the plasma exchange plus prednisolone group (RR 2.15, 95% CI 0.95 to 4.87; 67 participants; very low‐certainty evidence; Analysis 5.3).
5.3. Analysis.

Comparison 5: Prednisolone plus azathioprine versus prednisolone plus plasma exchange, Outcome 3: Mortality and adverse events at 6 months
Prednisone versus tetracycline and nicotinamide
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
Comparing prednisone with tetracycline and nicotinamide (Fivenson 1994), one complete and five partial responders were reported in the steroid group (6 participants), compared with five complete and five partial responders, one non‐responder, and one disease progression in the tetracycline group (12 participants). Two participants in the tetracycline group (n = 14) were unavailable for follow‐up at eight weeks. The results are similar for either complete response (RR 2.50, 95% CI 0.37 to 16.89; NNTB = 4; 18 participants; very low‐certainty evidence; Analysis 6.1) or complete and/or partial response (RR 0.87, 95% CI 0.62 to 1.22; 18 participants; very low‐certainty evidence; Analysis 6.1). The data for the one non‐responder, the participant whose disease progressed, and the two participants lost to follow‐up in the tetracycline group are not shown in the table. See Table 5.
6.1. Analysis.

Comparison 6: Nicotinamide plus tetracycline versus prednisone, Outcome 1: Disease control
Mortality
There was one death due to sepsis in the prednisone group, and no deaths in the tetracycline and nicotinamide group (Analysis 6.2).
6.2. Analysis.

Comparison 6: Nicotinamide plus tetracycline versus prednisone, Outcome 2: Mortality at 6 months
Secondary outcomes
Adverse effects
The Fivenson 1994 study report states that "fewer short‐term and long‐term adverse effects occurred in the participants treated with the nicotinamide/tetracycline combination compared with prednisone therapy" (there was one death due to sepsis in the prednisone group (Analysis 6.2)). Most of the side effects in the tetracycline/nicotinamide group in Fivenson 1994 were mild: two participants developed gastrointestinal symptoms which resolved after substitution of tetracycline with minocycline; one of them developed tinnitus on minocycline which resolved despite continuing treatment. One participant developed severe tubular necrosis. He had been enroled in the study with elevated serum creatinine (159 which peaked at 654 micromol/L: normal 60 to 120 micromol/L) and was also taking nonsteroidal anti‐inflammatory drugs (ibuprofen and aspirin). This participant's renal function returned to normal within two weeks of stopping treatment.
Other study outcomes
Duration of remission
Of the participants available for long‐term follow‐up in Fivenson 1994, all five in the tetracycline group remained disease‐free (mean 17.5 weeks) while two of the three participants in the steroid group had repeated flare‐up with tapered‐off treatment (mean 21.3 weeks). Unfortunately, this trial included very few participants, two‐thirds of whom were in the tetracycline group (14 of 20 participants). The randomisation in this study was unclear and there was a high dropout rate (2/20 at eight weeks and a further 10 participants at the end of study). At 10 months, only three participants remained in the steroid group (two of whom had multiple recurrences with tapering of medication), and only five participants remained in the nicotinamide plus tetracycline group, all of whom remained disease‐free during medication tapering.
Very potent topical steroid (clobetasol propionate) versus prednisone
The largest study had two study groups, with the study stratified by severity of disease (Joly 2002):
moderate disease (fewer than 10 new blisters a day): topical steroids (initial dose of 40 g of 0.05% clobetasol propionate twice daily applied to entire body surface) (77 participants), and oral prednisone 0.5 mg/kg (76 participants);
extensive disease (more than 10 new blisters a day): topical steroids (93 participants), and oral prednisone 1 mg/kg (95 participants).
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
In moderate disease, the rate of disease control at three weeks was similar in the topical steroid and 0.5 mg/kg oral steroid groups: 100% versus 95%, respectively (RR 1.06, 95% CI 1.00 to 1.12; NNTB = 18; P = 0.07; 153 participants; low‐certainty evidence; Analysis 7.1, see Analysis 7.1.1) See Table 1.
7.1. Analysis.

Comparison 7: Clobetasol propionate cream versus oral prednisone, Outcome 1: Disease control at day 21
Of those with extensive disease, both interventions resulted in nearly 100% of participants experiencing disease control. The disease was controlled in 99% of participants with extensive disease using topical steroids versus 91% of those on oral steroids at three weeks (RR 1.09, 95% CI 1.02 to 1.17; P = 0.01; NNTB = 13; 188 participants; moderate‐certainty evidence; Analysis 7.1, see Analysis 7.1.2), although this outcome was not assessed blindly, and therefore the possibility of bias exists.
Mortality (survival)
The major outcome in this study was survival, the study being designed to have 80% power to detect a reduction in the one‐year mortality rate for both moderate and extensive bullous pemphigoid (Joly 2002). To achieve this power, 75 participants were needed in each treatment group, which was accomplished.
In the extensive disease group, those using topical steroids had a better survival rate at one year compared to those on oral steroids (76% versus 58%, RR 0.58, 95% CI 0.37 to 0.89; NNTB = 6; P = 0.01; 188 participants; moderate‐certainty evidence; Analysis 7.2, see Analysis 7.3.2). This was consistent with the incidence of severe complications in the people with extensive disease. In the moderate disease group, similar results were seen between the topical steroid and 0.5 mg/kg oral steroid groups in terms of overall survival (30% versus 30%, RR 0.99, 95% CI 0.61 to 1.60; NNTB = 334; 153 participants; low‐certainty evidence; Analysis 7.2).
7.2. Analysis.

Comparison 7: Clobetasol propionate cream versus oral prednisone, Outcome 2: Mortality at 1 year
Secondary outcomes
Adverse effects
The incidence of severe complications was reported for people with extensive disease: 29% for topical steroids versus 54% for oral steroids (RR 0.54, 95% CI 0.37 to 0.78; P = 0.001; 188 participants; moderate‐certainty evidence); that is, there were fewer adverse events due to clobetasol (Analysis 7.3, see Analysis 7.2.2). The incidence of severe complications was similar in the moderate disease group (32% versus 38%, RR 0.85, 95% CI 0.55 to 1.31; P = 0.46; 153 participants; low‐certainty evidence; Analysis 7.3) (Joly 2002). Severe complications occurred in 47% of participants with oral prednisone versus 40% with topical clobetasol (RR 0.85, 95% CI 0.55 to 1.31; 341 participants; low‐certainty evidence).
7.3. Analysis.

Comparison 7: Clobetasol propionate cream versus oral prednisone, Outcome 3: Severe complications
Standard dose (40 g/day) of very potent topical steroid versus mild dose (10 to 30 g/day)
In a second large French study, Joly and colleagues compared two different topical steroid regimens (Joly 2009). In the mild regimen, participants received different amounts of clobetasol propionate cream applied to the whole body, depending on their body weight and severity of the disease. For moderate disease severity, defined as 10 or fewer new blisters/day, 69 participants received 20 g/day if their body weight was greater than 45 kg and 10 g/day if under 45 kg. For severe disease, defined as more than 10 new blisters/day, 90 participants received 30 g/day if their body weight was greater than 45 kg and 20 g/day if under 45 kg. In the standard regimen, all participants received 40 g of the cream/day (moderate disease: 65 participants; severe disease: 88 participants). See Table 2.
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
The Joly 2009 study report has a discrepancy in the number of participants evaluated at 21 days. It states that 150/153 participants were evaluable, as three participants were lost to follow‐up early after the initiation of treatment and were not available for evaluation of efficacy at day 21. However, disease control rates at 21 days are given for 153/153 participants. We wrote to the study investigator for clarification (Joly 2010 [pers comm]), who confirmed that the published report contains a typographical error. The correct figures are: 153 participants randomised, 150 analysed. We carried out an analysis of the results using the randomised number of participants (n = 153). In the mild regimen, 156/159 participants were controlled by day 21 and in the standard regimen, 150/153 were (RR 1.00, 95% CI 0.97 to 1.03; P = 0.96; 312 participants; high‐certainty evidence; Analysis 8.1, see Analysis 8.1.1).
8.1. Analysis.

Comparison 8: Mild clobetasol propionate cream (10 to 30 g/day) regimen versus standard clobetasol propionate cream (40 g/day) regimen, Outcome 1: Healing of skin lesions: complete (at day 21)
The study report stratified participants and their responses to the treatment regimens by disease severity. Using the correct figures supplied by the study investigator (Joly 2010 [pers comm]), of those with moderate disease, disease control was achieved in 68/69 using the mild regimen, and with the standard regimen, 63/65 were controlled (RR 1.02, 95% CI 0.97 to 1.07; 134 participants; low‐certainty evidence; Analysis 8.1, see Analysis 8.1.2). Of those with extensive disease, 88/90 achieved disease control in the mild regimen and 87/88 were controlled with the standard regimen (RR 0.99, 95% CI 0.95 to 1.03; 178 participants; low‐certainty evidence; Analysis 8.1, see Analysis 8.1.3).
The median cumulative doses of cream used during the study period were 5760 g in the standard regimen versus 1314 g in the mild regimen, which is a 70% reduction in cumulative doses of corticosteroid.
Mortality
In the mild regimen, 60/159 participants had died after one year (moderate disease 19/69, severe disease 41/90), and in the standard regimen, 58/153 had died (moderate disease 21/65, severe disease 37/88). Mortality was similar between the two groups for those participants with moderate (RR 0.85, 95% CI 0.51 to 1.43; 134 participants; low‐certainty evidence) or severe disease (RR 1.08, 95% CI 0.78 to 1.51; 178 participants; low‐certainty evidence; Analysis 8.2).
8.2. Analysis.

Comparison 8: Mild clobetasol propionate cream (10 to 30 g/day) regimen versus standard clobetasol propionate cream (40 g/day) regimen, Outcome 2: Mortality
The study report gives an adjusted analysis (Cox model adjusted for age and Karnofsky score), after which a beneficial effect of the mild regimen was observed in participants with moderate bullous pemphigoid, with an almost twofold decrease in the risk of death or life‐threatening adverse events relative to the standard regimen (hazard ratio = 0.54, 95% CI 0.30 to 0.97; P = 0.039).
Secondary outcomes
Adverse effects
Eighty‐nine participants in each group had severe adverse effects (RR 0.94, 95% CI 0.78 to 1.14; low‐certainty evidence; Analysis 8.4). There were 194 events in 89 participants in the mild regimen group and 227 in the standard regimen group. There were 42 life‐threatening adverse effects in 33 participants. The main severe side effects in both groups were diabetes mellitus (34 participants in the standard group; 18 participants in the mild group), cardiovascular and neurovascular disorders in 35 standard regimen participants and 21 mild regimen participants, and severe infections in 32 and 27 participants in the standard and mild regimen groups, respectively. There were also cutaneous side effects, including purpura, severe skin atrophy, and striae.
8.4. Analysis.

Comparison 8: Mild clobetasol propionate cream (10 to 30 g/day) regimen versus standard clobetasol propionate cream (40 g/day) regimen, Outcome 4: Adverse events (grade 3+4)
Other study outcomes
Duration of remissions
There were 67 relapses in 159 participants in the mild regimen and 52 in 153 participants in the standard regimen (RR 1.24, 95% CI 0.93 to 1.65; 312 participants, low‐certainty evidence; Analysis 8.3). This was similar between the two groups. There is insufficient evidence to conclude that the treatment regimens differ in effectiveness.
8.3. Analysis.

Comparison 8: Mild clobetasol propionate cream (10 to 30 g/day) regimen versus standard clobetasol propionate cream (40 g/day) regimen, Outcome 3: Number of relapses
Jingui Shenqi Pill (JSP) 1# bid plus prednisone versus prednisone
The Jingui Shenqi Pill (JSP) 1# bid plus prednisone (0.5 to 1.0 mg/kg/day) was compared to prednisone alone (0.5 to 1.0 mg/kg/day) in a small trial (Liu 2006). Thirty participants with bullous pemphigoid were included; the primary clinical outcome was healing of the skin lesions after four weeks of treatment. Authors defined a cure as more than 90% of the total number of lesions being healed; moderate healing as 60% to 89% of the affected area being healed; improved if 30% to 59% of the lesions had healed; and not effective if less than 30% of the lesions had healed.
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
Complete healing of the lesions at four weeks was achieved in one participant receiving the Jingui Shenqi Pill (1/15) and no participants in the prednisone group (0/15) (RR 3.00, 95% CI 0.13 to 68.26; 30 participants; very low‐certainty evidence; Analysis 9.1). Partial healing was achieved in 13 of 15 with JSP treatment compared to 11 of 15 participants with prednisone‐only treatment (RR 1.18, 95% CI 0.82 to 1.70; 30 participants; very low‐certainty evidence; Analysis 9.1).
9.1. Analysis.

Comparison 9: Jingui Shenqi Pill 1# bid plus prednisone versus prednisone alone, Outcome 1: Healing at 4 weeks
Overall, the treatment was effective (some degree of healing) in 14/15 participants (93.33%) in the treatment group compared to 11/15 (73.33%) in the prednisone group (RR 1.27, 95% CI 0.91 to 1.78; 30 participants; very low‐certainty evidence; Analysis 9.1).
Mortality
No deaths were reported during the four‐week follow‐up (Liu 2006).
Secondary outcomes
The Liu 2006 study reported no other outcomes and no adverse effects.
Azathioprine plus corticosteroid versus mycophenolate mofetil plus corticosteroid
Primary outcome
Disease control (e.g. regression or healing of skin lesions)
Comparing azathioprine (2 mg/kg/day) and mycophenolate mofetil (2000 mg twice/day), both in addition to oral methylprednisolone (0.5 mg/kg/day), all participants achieved some degree of healing (either partial or complete) (Beissert 2007).
The Beissert 2007 study defined complete healing and disease remission as complete re‐epithelialisation of all lesions. In the azathioprine group, 35/38 participants showed complete healing, and 35/35 of the mycophenolate mofetil group did (92% versus 100%) (RR 0.92, 95% CI 0.83 to 1.03; NNTH = 13; 73 participants; low‐certainty evidence; Analysis 10.1). Participants showed complete healing after 23.8 ± 18.9 days and 42.0 ± 55.3 days for the azathioprine and mycophenolate mofetil groups, respectively (P = 0.09).
10.1. Analysis.

Comparison 10: Azathioprine plus methylprednisolone versus mycophenolate mofetil plus methylprednisolone, Outcome 1: Healing of lesions
Mortality
There were two deaths in the azathioprine group, described as not treatment‐related.
Secondary outcomes
Adverse effects
Nine participants (24%) had grade 3/4 adverse effects in the azathioprine group, and six participants (17%) in the mycophenolate mofetil group (RR 1.38, 95% CI 0.55 to 3.49; NNTH = 16; 73 participants; low‐certainty evidence; Analysis 10.3). There were more elevated liver function tests in the azathioprine group (6/37 versus 1/35); however, participants were not checked for thiopurine methyltransferase activity prior to treatment. The number of grade 3 and 4 adverse events were 11 (azathioprine) and 13 (mycophenolate mofetil) (RR 0.78, 95% CI 0.40 to 1.51; 73 participants; low‐certainty evidence; Analysis 10.2) (Beissert 2007).
10.3. Analysis.

Comparison 10: Azathioprine plus methylprednisolone versus mycophenolate mofetil plus methylprednisolone, Outcome 3: Adverse events in patients (grade 3+4)
10.2. Analysis.

Comparison 10: Azathioprine plus methylprednisolone versus mycophenolate mofetil plus methylprednisolone, Outcome 2: Number of adverse events (grade 3+4)
Other study outcomes
Duration of remissions (weeks)
The disease‐free interval between complete remission and recurrence of lesions (new blister formation) was 23.5 weeks ± 19.4 weeks for the azathioprine group and 18 weeks ± 12.8 weeks for mycophenolate mofetil group; that is, 5.50 more weeks of remission for those participants treated with azathioprine (P = 0.74).
Azathioprine plus methylprednisolone versus dapsone plus methylprednisolone
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
Methylprednisolone (0.5 mg/kg body weight/day) plus azathioprine at a dose according to the thiopurine methyltransferase activity (2.8 to 10.0 nmol/mL erythrocytes/hour, azathioprine 1.5 mg/kg body weight; > 10.0 nmol/mL erythrocytes/hour, azathioprine 2.5 mg/kg body weight) was compared to methylprednisolone plus dapsone at a fixed dose of 1.5 mg/kg body weight/day. The primary outcome as determined by Sticherling 2017 was “time until complete tapering of methylprednisolone”. The initial methylprednisolone dose was maintained until blister formation had ceased and re‐epithelialisation of lesions started. The primary outcome was achieved in 5/19 after a median time of 251 (25% and 75% range 220 and 345) days in the azathioprine group and in 3/20 after a median time of 81 (25% and 75% range mentioned as 6‐∞) days in the dapsone group. See Table 6.
Sticherling 2017 later determined an additional primary outcome of "time until the methylprednisolone dose could be reduced to ≤10mg/d" because the small numbers for the prespecified primary outcome were not suitable for analysis. Twelve participants in the azathioprine and eight participants in the dapsone group (no denominator given) reached this endpoint after a median of 95 (25% and 75% range 85 and 112) days and 107 (55; 162) days, respectively.
The authors only present medians; means and medians can be very different from each other if the data are skewed. Furthermore, an estimate of the mean and variance cannot be calculated because the smallest and largest values are not given (Pudar Hozo 2005). We are therefore not able to present these data in the analysis of outcomes.
Mortality
There were three (of 27 = 11.1%) deaths in the azathioprine group and one (of 27 = 3.7%) in the dapsone group at the end of the observation period of 12 months (RR 3.00, 95% CI 0.33 to 27.06; NNTH = 14; 54 participants; very low‐certainty evidence; Analysis 12.1). The one death was probably related to dapsone (hepatic toxicity, renal failure, and arrhythmia). Pneumonia, bronchial carcinoma, and unknown cause were the causes of death in the azathioprine group; pneumonia was possibly related to treatment, as judged by the review authors. The study authors stated an overall death rate of 7% at one year; this calculation includes all randomised participants (not stated in the article); for treatment response at 12 months, they analysed only 44 participants. We calculated the study groups’ death rates separately: the death rate in the dapsone group was 3.7% and in the azathioprine group, it was 11.1% (P = 0.61, Fisher’s exact test).
12.1. Analysis.

Comparison 12: Methylprednisolone plus dapsone versus methylprednisolone plus azathioprine, Outcome 1: Mortality at 1 year
Secondary outcomes
Adverse effects
The Sticherling 2017 study recorded 31 adverse events above grade 1 (including deaths): 18 in the azathioprine group and 13 in the dapsone group. However, we do not know in how many participants these adverse events occurred (Table 7).
Other study outcomes
Daily and cumulative dose and number of days on methylprednisolone
In a post hoc analysis, the Sticherling 2017 study authors calculated the daily methylprednisolone dose at two and four weeks to address whether the longer disease duration in the azathioprine group required more intense initial treatment. After two weeks, the mean ± standard deviation methylprednisolone dose was 36.5 mg/day ± 8.3 mg/day (median 34.0 mg/day), and after four weeks, it was 30.0 mg/day ± 15.8 mg/day (median 28.8 mg/day) in the azathioprine group, compared to 39.6 mg/day ± 20.5 mg/day (median 36.0 mg/day) and 30.1 mg/day ± 15.9 mg/day (median 31.8 mg/day) in the dapsone group, respectively. There was no further interpretation of this calculation.
The median time until complete cessation of new blisters was 89 (25% and 75% range 59; 331) days in the azathioprine (22 participants) and 42 (19; 277) days in the dapsone group (17 participants) (P = 0.26).
The median (25%; 75% range) cumulative corticosteroid dose was 2654 (2120; 4052) mg in the azathioprine group (19 participants) and 1917 (1052; 3334) mg in the dapsone group (20 participants) (P = 0.06).
The median number of days of administered corticosteroids was 148 (64; 245) in the azathioprine group (19 participants) and 51 (51; 169) in the dapsone group (20 participants) (P = 0.24).
Duration of remission
At 12 months, complete remission on therapy was achieved in 70% of 24 participants in the azathioprine group and in 65% of 20 participants in the dapsone group (P = 0.75). At that time point, complete remission off therapy was achieved in one participant from the azathioprine group and in four participants from the dapsone group. Two participants in each of the treatment groups (two of 24 in the azathioprine group; two of 20 in the dapsone group) relapsed during the observation period.
Intravenous immunoglobulins versus placebo
The Amagai 2017 study investigated the therapeutic effect of high‐dose intravenous immunoglobulin (IVIG; 400 mg/kg/day for five days) versus placebo in 56 people with bullous pemphigoid who showed no symptomatic improvement with prednisolone (≥ 0.4 mg/kg/day). The study's primary endpoint was efficacy using the disease activity score (DAS) on day 15 (DAS15). The secondary endpoints were changes in the DAS over time, time to treatment reduction (defined as the length of time until the symptoms were improved and the evaluator determined that a reduction in treatment was required), oral steroid dosage, the anti‐BP180 antibody titre, and safety for a period of 57 days. The bullous pemphigoid disease area index had not been established at the start of the study.
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
The DAS15 was 12.5 points lower in the IVIG group (26 participants completed day 15; 29 participants randomised and included in analysis; DAS baseline = 45.3 +/‐ 23.6, DAS15 = 19.8 +/‐ 22.2) than in the placebo group (26 participants completed day 15; 27 participants randomised and included in analysis; DAS baseline = 45.0 +/‐24.5, DAS15 = 32.3 +/‐ 31.5) (P = 0.089; Analysis 11.1). The outcomes were similar between the groups.
11.1. Analysis.

Comparison 11: Intravenous human IgG 400 mg/kg/day for 5 days versus placebo, Outcome 1: Disease control
Mortality
Amagai 2017 reported no deaths (Analysis 11.2).
11.2. Analysis.

Comparison 11: Intravenous human IgG 400 mg/kg/day for 5 days versus placebo, Outcome 2: Mortality at day 57
Secondary outcomes
Adverse effects
The authors reported 19 adverse events in 11 of 29 treated participants in the IVIG group and six adverse events in five of 27 participants in the placebo group. Comparing events in number of participants at day 57 results in 11/29 (37.9%) participant events in the IVIG group and 5/27 (18.5%) in the placebo group (RR 2.05, 95% CI 0.82 to 3.65; NNTH = 6; P = 0.143; 56 participants; low‐certainty evidence; Analysis 11.3). The authors stated that there was no serious event; however, there was one pulmonary embolism in the placebo group and one participant had chest pain, not further specified, in the IVIG group (Table 7).
11.3. Analysis.

Comparison 11: Intravenous human IgG 400 mg/kg/day for 5 days versus placebo, Outcome 3: Adverse events at day 57
Other study outcomes
Changes in DAS over time (disease control)
A post hoc analysis of covariance comparing changes between DAS1 and DAS15 revealed the following differences: IVIG group (DAS1 = 46.6, DAS15 = 19.7) and the placebo group (DAS1 = 46.3, DAS15 = 32.4) (P = 0.041); standard deviations were not provided.
DAS57 were 19.3 and 27.1 for the IVIG group and placebo group, respectively; these were lower than DAS on day 1 (DAS1) for each group (P < 0.05). Furthermore, in the severe patient subgroup (DAS1 ≥ 40), the IVIG group provided lower values than the placebo group on days 8, 15, and 22 (P < 0.05).
Throughout the course of the observation, the mean DAS of the IVIG group was lower than that of the placebo group. Time to treatment reduction was reduced in the IVIG group (P = 0.01).
Oral steroid dosage/day
The oral steroid dose per day was not increased from day 1 by day 15 in the IVIG group, but increased in the placebo group (P = 0.031). On day 15, the placebo group had a higher dose of steroids (P = 0.042).
Changes in antibody titre over time
The anti‐BP180 antibody titres showed no difference between groups.
Withdrawals and safety
Twenty participants from the IVIG group and 21 from the placebo group completed the study. There were nine dropouts in the IVIG group and six in the placebo group. Three participants from the IVIG group and one from the placebo group were withdrawn before day 15. Study authors provided the reasons for these withdrawals on request: two protocol deviations, one adverse event (IVIG group; no details given), and one due to investigator's judgement (placebo group). The reasons for the other dropouts were not provided.
Doxycycline versus prednisolone
The Williams 2017 study tested whether a strategy of starting oral doxycycline 200 mg per day produces an acceptable degree of short‐term blister control (three or fewer blisters) compared with oral prednisolone 0.5 mg/kg per day (a non‐inferiority comparison measured at week 6), while conferring a long‐term safety advantage over oral corticosteroids (a superiority comparison of severe, life‐threatening, or fatal adverse events measured at week 52) in a pragmatic way that could inform everyday clinical practice. Participants were allowed to apply up to 30 g of a potent topical steroid (mometasone furoate) per week to lesions during weeks 1 to 3 and again after week 6. See Table 3.
For estimating the number of participants required for the efficacy analysis, it was assumed, based on published data and expert opinion, that prednisolone would produce three or fewer blisters at six weeks in 95% of participants and doxycycline in 70% of participants (absolute difference of 25%). As a 25% difference gave rise to an unrealistically large sample size, a 37% non‐inferiority margin (upper limit of 90% CI for 25% difference) was used for sample size calculation.
For initial treatment with doxycycline to be considered an acceptable alternative strategy to prednisolone, both non‐inferiority for efficacy and superiority for safety had to be shown.
The study randomised 132 eligible participants to doxycycline and 121 to prednisolone. The mean participant age was 77.7 years and baseline disease severity was mild in 31.6% (three to nine blisters), moderate in 39.1% (10 to 30 blisters) and severe in 29.3% (> 30 blisters). Baseline characteristics (age, sex, ethnicity, bullous pemphigoid severity, and Karnofsky score of functional impairment) were well‐matched between the two groups. The proportion who withdrew or died was 13.0% at six weeks and 36.4% (Health Technology Assessment report page xxvi (28/122)) at week 52 (Williams 2017).
Modified intention‐to‐treat analysis
The intention‐to‐treat principle requires that all participants randomised must be included in the final analysis and analysed according to the treatment group to which they were originally assigned, regardless of the treatment received, withdrawals, losses to follow‐up, or cross‐overs. There is no universally accepted definition for a modified intention‐to‐treat (mITT) analysis. However, the mITT population for this trial consisted of those participants who fulfilled the eligibility criteria, who were randomised to receive either study drug, and who had data on the outcome of interest. For the primary efficacy outcome at week 6 and the primary safety outcome at week 52, 112 and 121 of 132 participants who were started on doxycycline and 101 and 113 of 121 participants who were started on prednisolone, respectively, were included.
Per‐protocol analysis
Seventy‐eight of the 132 participants who started on doxycycline and 91 of the 121 participants who started on prednisolone and who did not switch treatment were included in the efficacy analysis. Participants were excluded from the per‐protocol (PP) analysis for deviations from the protocol that could affect the outcome. The PP population for mortality included all participants who started on a medication and did not switch to another treatment, even if they had missed all visits. Ninety‐four participants received doxycycline and 108 received prednisolone. For the safety analysis, the Williams 2017 study specified that a participant needed to have at least one visit's worth of data in order to be included. Of those treated with doxycycline, 87 participants fulfilled these requirements, as did 103 participants treated with prednisolone.
All superiority analyses were conducted on a mITT basis and all non‐inferiority analyses were performed on both the mITT and PP population according to recommended practice (D'Agostino 2003).
Primary outcomes
Disease control (e.g. regression or healing of skin lesions)
For the primary efficacy outcome of three or fewer blisters at six weeks, 92 of 101 (91.1%) of those randomised to prednisolone achieved success compared with 83 of 112 (74.1%) of those randomised to doxycycline using a mITT analysis, an adjusted difference of 18.6% (90% CI 11.1% to 26.1%, P < 0.001) in favour of prednisolone (difference adjusted for baseline severity of bullous pemphigoid and Karnofsky score) (RR 1.23, 95% CI 1.08 to 1.39; NNTB = 6; 213 participants; high‐certainty evidence; Analysis 13.1). The result for doxycycline falls within the predefined inferiority margin of 37%. A PP analysis showed a similar result, with 58 of 78 (74.4%) and 84 of 91 (92.3%) achieving success in the doxycycline and prednisolone groups, respectively, an adjusted difference of 18.68% (95% CI 8.83% to 29.27%; SE = 5.40, P = 0.001, and 90% CI 9.8% to 27.6%). There was no evidence to support a difference in efficacy according to baseline disease severity (P values for an interaction test on the mITT population for severe and moderate disease compared with mild disease at baseline of 0.863 and 0.417, respectively).
Of all randomised participants, death occurred in 13 of 132 (9.9%) randomised to doxycycline and 20 of 121 (16.5%) randomised to prednisolone (P = 0.173); treatment‐related death was three of 132 (2.3%) in the doxycycline group and 11 of 121 (9.1%) in the prednisolone group (Williams 2017). Using a mITT analysis, treatment‐related death occurred in three of 121 (2.48%) randomised to doxycycline and 11 of 113 (9.73%) randomised to prednisolone (RR 3.93, 95% CI 1.12 to 13.71; NNTH = 14; 234 participants; high‐certainty evidence; Analysis 13.2) (Williams 2017). In the PP population (all participant included, even if all visits were missed), death occurred in 10 of 94 (10.64%) and 16 of 108 (14.81%) participants in the doxycycline and prednisolone groups, respectively (P = 0.565).
Of note, there were only 13 deaths in total in the doxycycline group at week 52. However, the Williams 2017 study report stated there were 14 deaths. We needed additional calculations for this review, and in reviewing numbers, we noticed that one previously included participant died one week after week 52. The study report included this one borderline participant, bringing total deaths to 14. However, strictly speaking, this participant should not have been included. It may have been that the site and the study team made the decision to include this participant. We corrected the numbers in this review. The participant in question was not a treatment success at week 6, so was included correctly as having not achieved the primary endpoint “treatment success at week 6 AND alive at week 52”, regardless of their subsequent death.
Secondary outcomes
Adverse effects
Treatment‐related severe, life‐threatening, and fatal events at 52 weeks occurred in 22 of 121 (18.2%) participants started on doxycycline and in 41 of 113 (36.3%) participants started on prednisolone (mITT analysis), an unadjusted difference of 18.1% in favour of doxycycline (95% CI 6.9 to 29.3; P = 0.002) and 19.0% (95% CI 7.9% to 30.1%; SE = 5.7, P = 0.001) after adjusting for baseline disease severity (RR 2.00, 95% CI 1.27 to 3.13; 234 participants; high‐certainty evidence; Analysis 13.3), Williams 2017, Table 7. The PP analysis showed an adjusted difference of 23.12% in favour of doxycycline (95% CI 11.69% to 34.57%; SE = 5.8, P < 0.001), affecting 11 of 87 (12.6%) participants randomised to the doxycycline group and 31 of 103 (35%) randomised to the prednisolone group. Estimates for treatment‐related severe, life‐threatening, and fatal events at week 52 were taken from a model on the raw data; that is, not on imputed data. The analyses include an adjusted estimate for baseline severity of blisters (age and Karnofsky score were not adjusted for, due to the model not converging with them in).
Participants in the prednisolone group were more likely to experience treatment‐related adverse events of any grade during the study than were those in the doxycycline group (96% versus 86%, difference 9.5% (95% CI 1.8 to 17.2; P = 0.016), unadjusted because of non‐convergence in the model; numbers shown in the appendix page 5, Table 7, Williams 2017 are the basis for this calculation).
Quality of life
For quality of life, assessed by the European Quality of Life–5 Dimension, three‐level version (EuroQoL EQ‐5D‐3L), the difference in score was similar when adjusted for baseline score, baseline disease severity, age, or Karnofsky score (adjusted difference 0.045, 95% CI ‐0.015 to 0.106; P = 0.143). Both groups experienced similar improvement in Dermatology Life Quality Index (DLQI) scores, with median improvement of 9 and 10 points from baseline in the doxycycline and prednisolone groups, respectively. When adjusted for baseline DLQI, disease severity, age, and Karnofsky score, there was a difference of ‐1.8 (95% CI ‐2.58 to ‐1.01; P < 0.001) in favour of doxycycline.
Mepolizumab versus placebo as an add‐on therapy to oral corticosteroids
The Simon 2020 study tested the efficacy and safety of mepolizumab at a dose of 750 mg, versus matching placebo, every four weeks over 12 weeks as an add‐on therapy to oral corticosteroids in participants with an acute flare‐up of bullous pemphigoid.
Primary outcomes
There was no difference in the primary endpoint – the cumulative rate of relapse‐free participants after initiating therapy – between the mepolizumab group, reached by six of 20 participants (30%) and the placebo group, reached by four of 10 participants (40%) at week 16 (RR 0.75, 95% CI 0.27 to 2.06; NNTH = 10; 30 participants; low‐certainty evidence; Analysis 14.1). Relapse was defined as manifestation of new bullous pemphigoid lesions, more than three blisters during or within four weeks after the treatment period, or both. At week 36, 14 of 20 (70%) participants in the mepolizumab group were relapse‐free and six of 10 (60%) in the placebo group (RR 1.17, 95% CI 0.65 to 2.09; NNTB = 10; 30 participants; very low‐certainty evidence; Analysis 14.2).
14.1. Analysis.

Comparison 14: Prednisolone plus mepolizumab versus prednisolone, Outcome 1: Cumulative rate of relapse‐free participants at 16 weeks
14.2. Analysis.

Comparison 14: Prednisolone plus mepolizumab versus prednisolone, Outcome 2: Cumulative rate of relapse‐free participants at 36 weeks
Mortality
Having contacted the authors, we learned that no deaths occurred during the study period.
Secondary outcomes
Adverse effects
All participants had adverse events; 27.5% in the mepolizumab group and 16.7% in the placebo group had serious adverse events (RR 1.50, 95% CI 0.37 to 6.14; 30 participants; very low‐certainty evidence; Analysis 14.4). According to Simon 2020, 13 were possibly, one was likely, and one was certainly associated with the investigational product (not specified in which group). The study report stated, "Despite the relatively high number of serious adverse events, none of them was related to mepolizumab. The uncontrolled diabetes observed in one patient was most likely due to the OCS [oral corticosteroid] therapy."
14.4. Analysis.

Comparison 14: Prednisolone plus mepolizumab versus prednisolone, Outcome 4: Number of participants with serious adverse events
Nine participants (six in the mepolizumab group (five because of inefficacy) and three in the placebo group) discontinued the treatment phase prematurely. Of these, one participant withdrew from the trial.
Other study outcomes
The Simon 2020 study's secondary endpoints were the cumulative rate of participants attaining disease control and the rate of participants maintaining disease control; the absolute reduction of severity and pruritus, as assessed by the autoimmune bullous skin disorder intensity score (ABSIS) and a pruritus numerical rating scale (NRS) from 1 to 10, respectively; the absolute reduction of serum levels of BP180 and BP230; the peripheral blood eosinophil count; and the cumulative dose of systemic corticosteroids administered until clinical remission was achieved. Furthermore, biopsies were taken before and after therapy in order to evaluate subepidermal blister formation and the presence of eosinophils, antibody or complement C3 deposition at the dermal‐epidermal junction, as well as the number and pattern of inflammatory cells and cytokine expression. Safety was evaluated by physical examination, monitoring white blood cell counts, liver and renal tests, concomitant therapies, adverse events, and serious adverse events. Mepolizumab had no impact on eosinophil numbers in the skin, but lowered blood eosinophil numbers compared with placebo (P = 0.007).
The authors concluded that mepolizumab therapy failed to be superior to placebo in bullous pemphigoid patients treated with systemic corticosteroids with respect to clinical outcome. However, participants treated with mepolizumab had lower peripheral blood eosinophil levels compared to those receiving placebo.
Discussion
Summary of main results
We included 14 studies in this update review (the previous version included 10 studies, and we found and included four new studies for this version: Amagai 2017; Simon 2020; Sticherling 2017; Williams 2017). Included studies used mainly oral prednisolone or prednisone in the control group; two studies were placebo‐controlled (Amagai 2017; Simon 2020). All were small trials, apart from three comparing different amounts of topical corticosteroids or doxycycline and oral steroids (Joly 2002; Joly 2009; Williams 2017). For the purposes of this review, prednisone and prednisolone are regarded as bio‐equivalent.
No meta‐analysis was possible because of the clinical heterogeneity of the studies in terms of interventions, measures of disease control, and follow‐up. The five studies that had overlapping treatments compared prednisone versus prednisone plus azathioprine (Beissert 2007; Burton 1978; Sticherling 2017), prednisolone versus prednisolone plus plasma exchange (Roujeau 1984), and a three‐armed study comparing prednisolone alone to prednisolone plus azathioprine and prednisolone plus plasma exchange (Guillaume 1993). However, these studies were heterogeneous, especially in terms of treatment doses used.
In total, there were 14 comparisons. The main results of six comparisons are summarised below. Statements about the comparative efficacy of treatments take into account the entirety of evidence (risk ratio (RR), width of confidence interval of RR, number needed to treat for an additional beneficial/harmful outcome (NNTB/H) and certainty of the evidence).
Clobetasol propionate cream compared to oral prednisone
Low‐certainty evidence showed that both treatments had similar efficacy at day 21 in moderate disease. However, in extensive disease, topical clobetasol was more effective than oral prednisone (moderate‐certainty evidence). Low‐certainty evidence showed that both treatments had similar mortality at one year in moderate disease. In extensive disease, topical clobetasol caused less mortality than oral prednisone (moderate‐certainty evidence). This study provided evidence that the whole‐body application of clobetasol propionate cream is an effective treatment for bullous pemphigoid.
Mild regimen of clobetasol propionate cream regimen compared to standard regimen of clobetasol propionate cream
High‐certainty evidence showed that both regimens had similar efficacy at day 21. Low‐certainty evidence showed that mortality rates with the two regimens were similar at one year in both moderate and extensive disease.
Doxycycline compared to prednisolone
High‐certainty evidence showed that doxycycline was non‐inferior to prednisolone with regard to disease control at six weeks. Moderate‐certainty evidence showed that treatment‐related mortality at one year was lower with doxycycline. This study provided evidence that initiating treatment with doxycycline is an effective strategy for bullous pemphigoid, and one which also helps avoid the adverse effects associated with oral corticosteroid treatment.
Prednisone plus azathioprine compared to prednisone
Very low‐certainty evidence showed that the two treatments were similar for disease control at six months (Guillaume 1993) and at three years (Burton 1978), and for mortality rates at six months and three years. The results suggest that adding azathioprine to prednisolone may not be beneficial.
Nicotinamide plus tetracycline compared to prednisone
Very low‐certainty evidence showed that complete response at eight weeks was similar with the two treatments. There was one death due to sepsis in the prednisone group, whilst no deaths occurred in the tetracycline and nicotinamide group. The result suggests that a treatment regimen consisting of two non‐immunosuppressive drugs (nicotinamide and tetracycline) may be as effective as oral prednisone; however, the trial included only 20 participants.
Methylprednisolone plus azathioprine compared to methylprednisolone plus dapsone
Comparing dapsone with azathioprine, both in addition to methylprednisolone, did not show conclusive results, with evidence certainty being very low. This study suffered from several shortcomings, including slow recruitment and early closure due to the low recruitment rate. Moreover, the primary endpoint of ‘time until the oral corticosteroid could be omitted’ was achieved by only eight of 54 participants.
Overall completeness and applicability of evidence
The outcome measures in these studies are very varied, as can be seen when looking at the definition of disease control and the interventions used (see Characteristics of included studies). Our primary outcome was regression or healing of skin lesions. We did not prespecify follow‐up times in our protocol as there is no established optimum treatment for bullous pemphigoid (Wojnarowska 2002), and we did not want to exclude potentially effective therapies from our analyses because they did not meet strict inclusion criteria in relation to follow‐up times. We found that there were relatively few reports of trials for this rare disease and that there is variation in the time points reported.
Some of the included studies reported short follow‐up periods (e.g. only 10 days in Dreno 1993), which makes judgements about the practical significance of the results difficult, especially in view of the chronic nature of this disease. The Morel 1984 study compared the starting dose of prednisolone, and reported results at 21 and 51 days, so perhaps the follow‐up of 51 days may be more reasonable. We reported the results of that study at both time points in this review, although there were no differences in healing when the length of time or the dose given were compared. The Burton 1978 trial had the longest follow‐up period, but unfortunately, it gave no details about how disease control was evaluated, and few clinical data were available. Participants with contraindications to oral steroids or azathioprine and those "unlikely to attend follow‐up" were excluded from the trial.
The Fivenson 1994 study had an unclear method of randomisation, a high dropout rate, and small numbers, but may suggest some merit in the use of tetracycline and nicotinamide. Williams 2017 investigated this option further and compared doxycycline (no nicotinamide) with prednisolone in one of the biggest of the included studies of this review; the study was methodologically well‐conducted. An initial treatment with low‐dose prednisolone (0.5 mg/kg of body weight/day) was compared with doxycycline (200 mg/day). Prednisolone led to suppression of disease in 91.1% and doxycycline in 74.1% of the participants within six weeks. The difference favoured prednisolone but fell within the predefined 37% non‐inferiority margin. There was an adjusted difference of 19.0% favouring doxycycline of treatment‐related severe, life‐threatening, and fatal events at 52 weeks between participants (95% CI 7.9 to 30.1; P = 0.001, modified intention‐to‐treat (mITT) analysis). This pragmatic, non‐inferiority trial showed that starting patients on doxycycline is non‐inferior, within the predefined non‐inferiority margins, to standard treatment with oral prednisolone for short‐term blister control in bullous pemphigoid and is safer in the long term.
Probably the most interesting feature of the Roujeau 1984 study was the lower doses of prednisolone used in both treatment groups. Strict measures of disease control were used (complete disappearance of blisters, pruritus, and erythema) and, in both groups, the disease was controlled within about four weeks in all participants. However, higher doses than the initial low dose of 0.3 mg/kg prednisone were needed in all participants of the prednisolone‐only group and in two‐thirds of the participants in the plasma exchange group to achieve disease control. There were no deaths during the study, but this may be partly because of the exclusion of participants older than 80 years of age. This study found that the plasma exchange group required much less prednisolone than the prednisolone‐only group. This benefit was, however, not confirmed by Guillaume 1993. This latter study also failed to confirm the benefit of the addition of azathioprine to prednisolone.
Beissert 2007 added either azathioprine or mycophenolate mofetil to an initial dose of 0.5 mg methylprednisolone/kg/day; there was no difference in effectiveness. The cumulative steroid dose until the end of the documentation (> 720 days) was 4967 ± 12,191 mg for the azathioprine group and 5754 ± 9693 mg for the mycophenolate mofetil group. The similarity between the two groups possibly reflects a comparable immunosuppressive effect of the two drugs. Interestingly, there were more participants with severe disease in the azathioprine group: 53% had 20% or higher of body surface area involvement compared to only 27% in the mycophenolate mofetil group. Sticherling 2017 aimed for a sample size of 88 participants for analyses, but only 54 could be enroled. Authors claim a similar corticosteroid‐sparing effect and safety profile of dapsone and azathioprine in the treatment of bullous pemphigoid. There were fewer deaths in the dapsone group, but this was not significant. All results were either not significant or numbers were too small for any meaningful calculation (time until oral corticosteroid could be discontinued, days on steroids, time until blistering ceased were in favour of dapsone, but there appears to be a high risk of bias; time until methylprednisolone dose ≤ 10 mg/day and cumulative corticosteroid dose received by the participants did not differ significantly between treatments).
In a small, methodologically unclear trial, Liu 2006 added the Jingui Shenqi Pill to oral steroids and described a beneficial effect after four weeks of treatment compared to the control group. They found an increased expression of glucocorticosteroid receptor (GCr) α and a decreased expression of GCr β in skin lesions of the treatment group, which may improve the sensitivity of the skin to glucocorticosteroids. However, the effectiveness of this intervention was not proven in our analyses.
As it is unlikely that future studies on interventions for bullous pemphigoid including a placebo group would be ethically justifiable, a comparison of low‐dose prednisolone with tetracyclines and nicotinamide (or potent topical corticosteroids, for mild and/or localised disease) may prove a worthy alternative. Uncontrolled studies have suggested the successful use of topical steroids as first‐line for the treatment of both localised and mild disease (Garg 1994; Rollin 1993; Zimmermann 1999); two randomised controlled trials which used steroid applications to the entire body surface confirm this view (Joly 2002; Joly 2009). The use of potent topical steroids is favoured because they have minimal side effects and few contraindications. Joly 2002 showed benefit of 40 g 0.05% clobetasol propionate cream/day over 1 mg/kg/day of prednisone in extensive disease for disease control, adverse events, and mortality. No differences between clobetasol propionate cream and 0.5 mg/kg/day prednisone were found in the moderate disease group for disease control, adverse events, and mortality. However, even though there were less severe adverse effects (pneumonia, diabetes requiring insulin, myocardial infarction, psychiatric symptoms, stroke, thrombosis, bone fracture) noted in the group of participants treated with topical clobetasol compared to prednisolone 1 mg/kg/day, the study did not mention if participants in the different groups had similar adverse effects regarding, for example, blood pressure and bone mineral density (Joly 2002). Also, the effort needed in applying the creams twice daily to the whole body is a major limitation in people with bullous pemphigoid, who are mostly elderly and may have other coexisting disease. Even in physically competent patients, application of a topical steroid to difficult‐to‐access areas of the body, such as the back, will require help from a caregiver. Furthermore, application of high potency topical steroid over the face may be avoided in view of the possibility of local side effects. Another aspect is that topical preparations and nursing care may be more costly than oral steroid preparations.
It is likely that very potent topical corticosteroids applied in such large quantities may have systemic effects (perhaps comparable to 0.5 mg/kg/day prednisone). However, we do expect that there is also a local immunosuppressive and anti‐inflammatory effect of the topically applied corticosteroids, because there are reports of participants with localised bullous pemphigoid effectively treated with potent topical steroids only. In fact, a later trial by the same group showed that smaller amounts of topical steroids (≤ 30 g 0.05% clobetasol propionate cream/day) are as effective in disease control after 21 days, and that the mean cumulative dose was 71% lower in a mild treatment regimen than in a standard treatment regimen. The mild regimen was associated with less severe adverse effects (Joly 2009).
Amagai 2017 explored the effect of intravenous immunoglobulins (IVIG) for five days in treatment‐resistant patients, compared to placebo. The primary outcome was measured at 15 days. This study had a high dropout rate and reasons for dropouts were only partly given. Authors state that IVIG provide a beneficial therapeutic outcome for people with bullous pemphigoid; however, according to their predefined analysis of the results, this could not be shown.
In our last update, we emphasised that an important research question for the future is to evaluate whether a lower dose of steroid (0.5 mg/kg/day) would be adequate for disease control in extensive disease (Kirtschig 2010). This question is answered by the Williams 2017 study: 0.5 mg prednisolone per kg of body weight led to three or fewer blisters after six weeks of treatment in 97% of participants with mild (three to nine blisters), 98% with moderate (10 to 30 blisters), and 75% with severe (> 30 blisters) disease, and in 76%, 78%, and 66%, respectively, in the doxycycline group. There was no evidence of an interaction between disease severity and treatment effect in either the mITT or the per‐protocol (PP) population.
A small trial showed that the addition of mepolizumab to oral prednisolone did not increase its efficacy; however, the power of the study was 60% (Simon 2020). Definitive conclusions about the efficacy of mepolizumab may not be drawn.
Future studies should include primary effectiveness (e.g. reduction of blistering and pruritus) and safety outcomes (adverse effects, including mortality), evaluating short‐term (e.g. two, four, and six weeks) and long‐term (e.g. at one year) effects. A uniform measure for treatment effect should be used. Attempts have been made to introduce the bullous pemphigoid index (Murrell 2012; Pfütze 2007; Wijayanti 2017). However, a formal procedure developing core outcome sets has not been performed for bullous pemphigoid or other blistering diseases (Chalmers 2009; Prinsen 2016), which may need to be done before future trials are conducted. Furthermore, recruitment of participants for trials in rare diseases is a challenge. Some of the included studies, as well as studies that await publication, have not reached the needed number to treat to show meaningful results. Attempts should be made to ensure sufficient recruitment, including conducting multicentre trials.
People with dementia are usually excluded from clinical trials because written informed consent cannot be obtained. However, a considerable proportion of people with bullous pemphigoid have dementia; there is a recognised association of disease (Brick 2014; Taghipour 2010). Future trials should address this aspect and include people with dementia, who may respond to treatment differently and may need different treatment options.
There may also be a need for a Priority Setting Partnership to prioritise research questions into these rare autoimmune bullous diseases (Thomas 2017).
Certainty of the evidence
Most of the studies were very small and of poor methodological quality because of:
an unclear method of randomisation;
lack of masking in the majority of studies; and
exclusion of dropouts from the analysis in most studies.
Figure 2 summarises these limitations and risks of bias. The 14 comparisons in this review looked at 47 different primary outcomes (regression or healing of lesions, and mortality) and a key secondary outcome (adverse effects of treatment). GRADE assessments showed that the certainty of the evidence was high for four outcomes (8.5%), moderate for five outcomes (10.6%), low for 15 outcomes (31.9%), and very low for 23 outcomes (48.9%).
Some studies which did not describe the method of randomisation were published some time ago (e.g. Burton 1978; Dreno 1993; Morel 1984). We did not attempt to gain further information as it was unlikely that further details of the studies would be available.
Our main concern with Liu 2006 is that the published report was not absolutely clear that the diagnosis of bullous pemphigoid was confirmed by immunofluorescence. The trial report was translated from Chinese and refers to the confirmation of the diagnosis, listing the usual clinical features, histology, direct immunofluorescence, indirect immunofluorescence on salt split skin, and immuno‐electron microscopy, using a method given in a reference. We had the reference source of the methods translated, but it remained unclear which of the methods listed were used. We contacted the trial investigators on two occasions, regarding the method used to confirm diagnosis, but did not receive a reply. Given that the description of the diagnosis of bullous pemphigoid in the published report is not precise, it is possible that inclusion of the trial into a meta‐analysis could introduce bias. However, Chinese traditional medicine plus prednisone was not shown to be effective in this single trial.
In Burton 1978, there may have been selection bias, as participants were "started on oral prednisone 30 to 80 mg/day, to suppress new blisters" and only then "did the consultant decide whether to include the participant in the trial". In addition, Joly 2002 switched three participants from one intervention group to another because of treatment side effects, although this was done in accordance with the study protocol.
The Guillaume 1993 study was stopped after the interim analysis became available, showing no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone. The trial investigators calculated that "the inclusion of 120 participants as initially scheduled, could not change these negative results".
The Fivenson 1994 study was terminated after enroling 20 participants (the study was originally designed to randomise 96 participants in four centres). No reasons were given for this early ending, but the published report of the trial mentioned a further randomised, double‐blind, multicenter trial as being 'underway'. We attempted to contact the trial investigators but have been unsuccessful in obtaining any further details or data from the later study.
Amagai 2017 was a small trial with a high and unbalanced dropout rate; the reasons for dropout were only partially given. There were 10.3% and 3.7% dropout rates for the primary outcome at day 15 for the IVIG and placebo groups, respectively. For secondary outcomes at day 56, the dropout rate was 31.0% and 22.2% for IVIG and placebo, respectively. Significant results for the primary outcome could only be shown in a post hoc analysis of covariance using disease activity score (DAS) on day 1 as covariate.
The Sticherling 2017 study had difficulties recruiting participants. The trial was terminated, resulting in insufficient numbers of participants for analyses. Neither investigators nor participants were masked, and the bias following these limitations makes it difficult to accept the authors' conclusion favouring dapsone.
Williams 2017 was the only pragmatic trial with a non‐inferiority approach. Pragmatic clinical trials seek to determine the effectiveness of an intervention in a real‐world setting to inform clinical decision‐making, and try to ensure that the study population is as similar as possible to the population on which the intervention is meant to be used, thus ensuring a high external validity. This design will, for example, allow adjustment or even change of trial medication at a defined point, according to participants' requirements (Williams 2015). Williams 2017 was one of the bigger included trials with good methodology. The number of participants required for analyses was reached. Data were analysed using a modified ITT analysis (mITT) that was clearly defined and a per‐protocol (PP) analysis; results of these analyses did not differ to an extent that conclusions needed to be changed. However, participants were not masked for their treatment and investigators were only masked for the primary efficacy outcome.
Simon 2020 was a phase 2 pilot, double‐blind, placebo‐controlled study comparing efficacy of mepolizumab plus oral prednisolone versus prednisolone alone. Sample size was calculated with power of study of 60%. There was an unclear risk of bias for random sequence generation, allocation concealment, incomplete outcome data, and other bias, and high risk of selective reporting bias.
Overall, there were relatively few included studies and these were of variable methodological quality. Therefore, caution should be exercised in interpreting the results. Additionally, statistical pooling of the data was not possible because of the clinical heterogeneity of the studies in terms of interventions, measures of disease control, and follow‐up.
Potential biases in the review process
There may be a potential bias in the review process because one review author (GK) was involved in the development and conduction of the BLISTER study (Williams 2017). However, another review author (SS), who also screened the literature and evaluated the trials, was not involved in any included or excluded studies. Two review authors (SS, VA), working independently, extracted data from the BLISTER study and checked for discrepancies.
Agreements and disagreements with other studies or reviews
The last published version of this review included ten randomised controlled trials (Kirtschig 2010). The authors concluded at that time that large amounts of very potent topical steroid applied over the entire body surface are effective and safe treatments for bullous pemphigoid, but their use in extensive disease may be limited by side effects and practical factors (Joly 2002). Milder regimens (using lower doses of topical steroids) are safe and effective in mild and moderate bullous pemphigoid (Joly 2009). Starting doses of prednisolone greater than 0.75 mg/kg/day do not give additional benefit; lower doses may be adequate to control disease and reduce the incidence and severity of adverse reactions. The effectiveness of adding plasma exchange, azathioprine, mycophenolate mofetil, or Chinese herbal medicine (Jingui Shenqi Pill) to corticosteroids, and combination treatment with tetracycline and nicotinamide, needed further investigation.
Since the 2010 review update, we have found and included four new RCTs and our conclusions have changed. A starting dose of 0.5 mg prednisolone/kg/day will control disease in almost 100% of people with mild and moderate disease and in 75% of people with severe disease (Williams 2017). Initial treatment with 200 mg doxycycline/day proved to be effective in two‐thirds of patients with bullous pemphigoid, but was less effective than prednisolone 0.5 mg/kg/day. There were fewer treatment‐related severe, life‐threatening, and fatal events when participants received initial treatment with doxycycline (Williams 2017). The benefit of doxycycline and low‐dose oral steroids (0.5 mg/day) diminished in severe disease. However, even though there were no clear differences in this study, much larger studies may show differences. Therefore, a strategy to initiate treatment with doxycycline is an effective and safe alternative to corticosteroids for all patients with bullous pemphigoid; however, this strategy may be of less value in severe disease and needs further investigation. The effectiveness of adding dapsone to corticosteroids (Sticherling 2017), and the benefit of high‐dose IVIG in treatment‐resistant bullous pemphigoid (Amagai 2017), could not be demonstrated in two small trials; these need further investigation. There are no new data regarding the effectiveness of adding plasma exchange, mycophenolate mofetil, or azathioprine to corticosteroids; their effectiveness in the treatment of bullous pemphigoid is not shown. Unchanged is the conclusion that very potent topical steroids applied to the whole body are effective and safe treatment for bullous pemphigoid. A small trial with 60% power of study showed that addition of mepolizumab to oral prednisolone did not increase efficacy (Simon 2020); further investigations may be needed.
We identified 13 registered RCTs involving bullous pemphigoid that were not completed when updating this review in 2010. Of these 13 trials, two were completed and published (Amagai 2017 (NCT01408550); Williams 2017 (ISRCTN13704604)); we included them in this update review. One other newly‐included RCT was not pre‐registered (Sticherling 2017). We contacted investigators of the remaining 11 registered RCTs to obtain unpublished data on completed trials. Responses revealed that one trial using methotrexate (NCT02313870, publication expected in 2018) is completed, but data are not available. Of the remaining nine trials, one trial using simvastatin was terminated because of poor recruitment (EUCTR2011‐004361‐32‐DE); one using QGE031 (i.e. ligelizumab, a monoclonal antibody) was terminated because of inefficacy (NCT01688882, data available on the register); one using TNT009 (a humanised monoclonal antibody) is completed, but involved an unknown number of participants with bullous pemphigoid and has been listed as an excluded study in this update (Derhaschnig 2016); and there is no information on six trials (ChiCTR‐IOR‐15007146 (topical steroids); NCT02365675 (wound dressings); ChiCTR‐TRC‐12003593 (methotrexate); ChiCTR‐TRC‐12003592 (tetracyclines); ChiCTR‐TRC‐12003538 (methotrexate); NCT00472030 (omalizumab)). We identified contact persons for all trials and attempted to contact them, but received no response for the six registered studies mentioned last.
Monoclonal antibody therapies could offer alternatives to long‐term steroid use, or may permit the dose of steroids or immune suppressive drugs to be reduced. The efficacy of anti‐IL‐5 antibody (mepolizumab) treatment is tested against placebo in a small randomised trial (Simon 2020); data are published and included in this update. Three ongoing studies are investigating the use of such biological agents. The use of rituximab as an adjuvant treatment in bullous pemphigoid is being studied in ACTRN12607000104459, a prospective open‐label pilot study in three participants, studying remission of disease with rituximab. The efficacy and tolerance of a single cycle of rituximab in the control of bullous pemphigoid is being examined in NCT00525616 (non‐randomised). The safety of rituximab plus systemic corticosteroids in people resistant to therapy with systemic corticosteroids is being tested in NCT00286325 (non‐randomised). While monoclonal antibody therapy may have a role to play in treatment of bullous pemphigoid, it is not without adverse reactions, including infusion reactions, fever, neutropenia, chills, increased risk of infection, weakness, and fatigue. Participants would potentially also require re‐treatment with monoclonal antibodies, and there is a risk of neutralising antibodies that would interfere with therapeutic efficacy. Furthermore, it is a very costly treatment and, if found effective and safe in randomised controlled trials, will probably be reserved for treatment‐resistant cases. The efficacy and safety of newer biology therapies (monoclonal antibodies) should be further investigated in the context of randomised controlled trials. Methotrexate is a registered trial medication in three studies; results are awaited for one (NCT02313870).
Finally, the Williams 2017 study was the only study to assess cost‐effectiveness. There was no robust difference in costs or quality‐adjusted life‐years (QALYs) per participant at one year between doxycycline‐initiated therapy with prednisolone‐initiated therapy (net cost: GBP (pounds sterling) 959, 95% confidence interval GBP 24 to GBP 1941; net QALYs: ‐0.024, 95% CI ‐0.088 to 0.041). However, findings varied by baseline blister severity. For participants with mild or moderate blistering (≤ 30 blisters) net costs and outcomes were similar. For participants with severe blistering (> 30 blisters), net costs were higher (GBP 2558, 95% CI 82 to 5198) and quality of life poorer (‐0.090 QALYs, 95% CI ‐0.222 to 0.042) for those starting on doxycycline. The probability that doxycycline would be cost‐effective for those with severe pemphigoid was 1.5% at a willingness to pay of GBP 20,000/QALY. Neither strategy is clearly a preferred use of UK National Health Service (NHS) resources. However, prednisolone‐initiated treatment may be more cost‐effective for people with severe blistering (Mason 2017).
Authors' conclusions
Implications for practice.
Starting doses of prednisolone greater than 0.75 mg/kg/day may not give additional benefit. Starting doses of prednisolone of 0.5 mg/kg/day may be adequate for disease control in most people with bullous pemphigoid. This is expected to reduce the incidence and severity of adverse reactions (especially death) associated with treatment.
Very potent topical steroid (clobetasol propionate) applied over the entire body is an effective treatment for bullous pemphigoid. It seems to have less serious adverse effects compared to high‐dose systemic steroids; however, its use in extensive disease may be limited by practical factors (ability of participant or availability of carer to apply the treatment). When feasible, it should be considered for first‐line treatment, especially in localised disease. However, if large quantities are needed to be applied topically, it may be associated with systemic absorption and adverse events. It is advisable to carefully monitor adverse effects when using topical steroid therapies, including changes in serum cortisol levels, especially when applied to large areas. Milder regimens (lower doses of clobetasol propionate cream applied over the whole body) are safe and effective in moderate and extensive bullous pemphigoid.
The effectiveness of the addition of plasma exchange, azathioprine, mycophenolate mofetil, dapsone, or mepolizumab to prednisolone or prednisone has not been established. The effect of adding nicotinamide to tetracycline has not been established. The addition of a Chinese traditional herbal medicine to prednisone was not beneficial.
The effectiveness of high‐dose intravenous immunoglobulins in addition to conventional treatment in treatment‐resistant patients is of unknown benefit and may be associated with severe adverse events.
A strategy of starting treatment with doxycycline (200 mg/day) is an effective approach for most people with bullous pemphigoid, and results in fewer adverse effects compared to initial treatment with prednisolone. Future studies may show that people with severe disease may benefit less from this approach.
Implications for research.
The optimum dose for treatment with doxycycline is not known; this needs further investigation.
The approach for people with severe disease may be different from that for people with mild and moderate disease. Combined treatment of oral prednisolone and doxycycline may be beneficial; this needs further investigation.
People with dementia or with other neurological disorders suffering from bullous pemphigoid are under‐investigated. Future trials should pay special attention to these populations.
The efficacy of doxycycline therapy may be compared with topical steroid therapy.
Masked randomised controlled trials comparing topical steroids with low doses of prednisolone/prednisone are needed.
The effect of agents such as azathioprine, mycophenolate mofetil, dapsone, methotrexate, or mepolizumab in addition to steroids is still not known and may need further investigation. However, the publications of ongoing trials on methotrexate are awaited.
The effect of nicotinamide alone or with tetracycline needs further investigation.
The efficacy and safety of newer biologic therapies (monoclonal antibodies) should be investigated in randomised controlled trials.
Future trial should include uniform effectiveness and safety measures to make trials comparable. A set of core outcomes for autoimmune blistering diseases needs to be established.
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2023 | Amended | Fixed minor typographical error in the abstract of review |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 10 August 2023 | New citation required and conclusions have changed | Initiating treatment with 200 mg/day doxycycline is non‐inferior to oral prednisolone (0.5 mg/kg/day) and is safe. |
| 10 August 2023 | New search has been performed | Four new included studies were found in updated literature searches; change in authorship. |
| 11 September 2013 | Amended | Contact author's out‐of‐date email address removed and current email address and second affiliation added. Another author's affiliation also updated |
| 7 November 2011 | Amended | Correction made to the data relating to the Beissert 2007 study ('1000' mg MMF amended to '2000' mg MMF) |
| 6 September 2010 | New citation required but conclusions have not changed | Change in authorship |
| 6 September 2010 | New search has been performed | Review updated with 3 new studies |
| 5 February 2010 | New search has been performed | Updated |
| 5 February 2010 | New citation required but conclusions have not changed | New studies found and included or excluded, authors changed |
| 8 August 2008 | Amended | Converted to new review format |
| 5 June 2008 | New citation required but conclusions have not changed | New studies found and included or excluded |
| 16 May 2005 | New citation required and conclusions have changed | Substantive amendment |
| 5 June 2003 | New search has been performed | Minor update |
Acknowledgements
Acknowledgements from the authors
Penelope Standen was the consumer co‐author on the review but died in July 2022. She checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
Editorial and peer‐reviewer contributions
Cochrane Skin supported the authors in the development of this review update.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Martin Burton, Robert Dellavalle
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Anne‐Marie Stephani
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynsk
Peer‐reviewers (provided comments and recommended an editorial decision): Dr Antonia Lloyd‐Lavery, Oxford University Hospitals NHS Foundation Trust, UK (clinical/content review), Roses Parker, Research Fellow, The Cochrane Collaboration (methods review), Yuan Chi, Cochrane Campbell Global Ageing Partnership (search review). One additional peer reviewer provided clinical peer review, but chose not to be publicly acknowledged.
Robert Boyle and Ching‐Chi Chi are Editors for Cochrane Skin but were not involved in the editorial process.
Appendices
Appendix 1. Cochrane Skin Specialised Register/CRSW search strategy
(Bullous pemphigoid*) AND INREGISTER
Appendix 2. CENTRAL (Cochrane Library) strategy
#1 MeSH descriptor: [Pemphigoid, Bullous] explode all trees #2 bullous pemphigoid*:ti,ab,kw #3 #1 or #2
Appendix 3. MEDLINE (Ovid) strategy
1. Pemphigoid, Bullous/ 2. bullous pemphigoid$.mp. 3. 1 or 2 4. randomized controlled trial.pt. 5. controlled clinical trial.pt. 6. randomized.ab. 7. placebo.ab. 8. clinical trials as topic.sh. 9. randomly.ab. 10. trial.ti. 11. 4 or 5 or 6 or 7 or 8 or 9 or 10 12. exp animals/ not humans.sh. 13. 11 not 12 14. 3 and 13
[Lines 4‐13: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 4. Embase (Ovid) strategy
1. bullous pemphigoid/ 2. bullous pemphigoid$.mp. 3. 1 or 2 4. crossover procedure.sh. 5. double‐blind procedure.sh. 6. single‐blind procedure.sh. 7. (crossover$ or cross over$).tw. 8. placebo$.tw. 9. (doubl$ adj blind$).tw. 10. allocat$.tw. 11. trial.ti. 12. randomized controlled trial.sh. 13. random$.tw. 14. or/4‐13 15. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 16. human/ or normal human/ 17. 15 and 16 18. 15 not 17 19. 14 not 18 20. 3 and 19
[Lines 4‐19: Based on terms suggested for identifying RCTs in Embase (section 3.6.2) in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Data and analyses
Comparison 1. Higher‐dose prednisolone (1.25 mg/kg) versus lower‐dose prednisolone (0.75 mg/kg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Disease control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Healing of skin lesions at day 51: ITT (assuming unknown = not healed) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 Healing of skin lesions at day 21: ITT (assuming unknown = not healed) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Mortality at day 51 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Methylprednisolone versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Disease control | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 Disease control ‐ number of blisters at day 10 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 Disease control ‐ extent of erythema at day 10 (score out of 3) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Mortality at day 10 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Overall improvement (number of participants with good results) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 Quality of life ‐ extent of itching (score out of 3) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Prednisone plus azathioprine versus prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Disease control | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Disease control at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 Disease control: well at 3 years, either needing or not needing further treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 Mortality at 3 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Mortality and severe adverse events at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Prednisolone plus plasma exchange versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Disease control | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 Disease control at 1 month (controlled with 0.3 mg/kg prednisolone) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1.2 Disease control at 1 month (controlled with 1.0 mg/kg prednisolone or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1.3 Disease control at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Disease control at 1 month ‐ cumulative steroid dose (g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3.1 Mortality at 1 month: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3.2 Mortality at 1 month: ITT worst case | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3.3 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4 Mortality and severe adverse events at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Prednisolone plus azathioprine versus prednisolone plus plasma exchange.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Disease control at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.3 Mortality and adverse events at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.2. Analysis.

Comparison 5: Prednisolone plus azathioprine versus prednisolone plus plasma exchange, Outcome 2: Mortality at 6 months
Comparison 6. Nicotinamide plus tetracycline versus prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Disease control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1.1 Complete response at 8 weeks: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1.2 Complete or partial response at 8 weeks: excluding dropouts | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 Mortality at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Clobetasol propionate cream versus oral prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Disease control at day 21 | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.03, 1.13] |
| 7.1.1 Prednisone 0.5 mg/kg for moderate disease | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| 7.1.2 Prednisone 1 mg/kg for extensive disease | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.02, 1.17] |
| 7.2 Mortality at 1 year | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.44, 1.26] |
| 7.2.1 Prednisone 0.5 mg/kg for moderate disease | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.61, 1.60] |
| 7.2.2 Prednisone 1 mg/kg for extensive disease | 1 | 188 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.89] |
| 7.3 Severe complications | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.86] |
| 7.3.1 Prednisone 0.5 mg/kg for moderate disease | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.55, 1.31] |
| 7.3.2 Prednisone 1 mg/kg for severe disease | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.78] |
Comparison 8. Mild clobetasol propionate cream (10 to 30 g/day) regimen versus standard clobetasol propionate cream (40 g/day) regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Healing of skin lesions: complete (at day 21) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1.1 Intention‐to‐treat analysis, all participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1.2 Moderate disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1.3 Extensive disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.2 Mortality | 1 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.75, 1.32] |
| 8.2.1 Moderate disease | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.43] |
| 8.2.2 Extensive disease | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.78, 1.51] |
| 8.3 Number of relapses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.4 Adverse events (grade 3+4) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Jingui Shenqi Pill 1# bid plus prednisone versus prednisone alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Healing at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1.1 Complete healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1.2 Partial healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1.3 Overall healing ‐ participants experiencing any degree of healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Azathioprine plus methylprednisolone versus mycophenolate mofetil plus methylprednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Healing of lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1.1 Complete healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2 Number of adverse events (grade 3+4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.3 Adverse events in patients (grade 3+4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. Intravenous human IgG 400 mg/kg/day for 5 days versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Disease control | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1.1 Disease activity score (DAS) on day 15 using DAS at baseline for calculation (day before treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.2 Mortality at day 57 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3 Adverse events at day 57 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Methylprednisolone plus dapsone versus methylprednisolone plus azathioprine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Mortality at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 13. Doxycycline versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Disease control at 6 weeks (primary efficacy outcome) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.2 Mortality at week 52 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.2.1 Modified intention‐to‐treat analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.2.2 Total deaths of all randomized patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.2.3 Per protocol analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.3 Adverse events at week 52 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.3.1 Number of patients with adverse event of any grade | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.3.2 Number of patients with grade 1 (mild) and 2 (moderate) adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.3.3 Number of patients with grade 3 (severe) and 4 (life‐threatening) adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.3.4 Grade 3 and 4 adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.3.5 Treatment‐related severe, life treatening or fatal adverse events at week 52 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.4 Quality of life (DLQI) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.4.1 Mean difference of DLQI adjusted for baseline DLQI, disease severity, age & Karnovsky score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
13.1. Analysis.

Comparison 13: Doxycycline versus prednisolone, Outcome 1: Disease control at 6 weeks (primary efficacy outcome)
13.2. Analysis.

Comparison 13: Doxycycline versus prednisolone, Outcome 2: Mortality at week 52
13.3. Analysis.

Comparison 13: Doxycycline versus prednisolone, Outcome 3: Adverse events at week 52
13.4. Analysis.

Comparison 13: Doxycycline versus prednisolone, Outcome 4: Quality of life (DLQI)
Comparison 14. Prednisolone plus mepolizumab versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Cumulative rate of relapse‐free participants at 16 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.2 Cumulative rate of relapse‐free participants at 36 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.3 Number of participants with moderate to severe adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.4 Number of participants with serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
14.3. Analysis.

Comparison 14: Prednisolone plus mepolizumab versus prednisolone, Outcome 3: Number of participants with moderate to severe adverse events
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amagai 2017.
| Study characteristics | ||
| Methods | Randomised, masked, placebo‐controlled Multicentre study; Japan Disease control = DAS (pemphigus disease activity score) on day 15 Follow‐up: 57 days (= treatment and follow‐up) Collection of data: August 2011 until September 2013 |
|
| Participants | 56 participants with clinical bullous pemphigoid, confirmed by histology, direct, indirect immunofluorescence and/or ELISA. Mean age in the placebo group was 66 years (9 males, 18 females), and in the intervention group 64 years (10 males, 19 females). Disease severity was variable, mild to severe. Inclusion criteria
Exclusion criteria
|
|
| Interventions | A) 20 of 29 completed intravenous drip infusion of human IgG at 400 mg/kg/day for 5 consecutive days. B) 21 of 27 completed intravenous drip infusion of physiological saline for 5 consecutive days. | |
| Outcomes | Primary
Secondary
|
|
| Notes | Registered 27 July 2011: NCT01408550, confirmatory phase 3 study Investigational drugs manufactured by Nihon Pharmaceutical Co. Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code was computer‐generated by independent staff members and was not revealed until completion of the study. |
| Allocation concealment (selection bias) | Low risk | Random allocation to the treatment groups by a central enrolment system controlled by a dynamic allocation scheme in order to ensure that there were no between‐group differences in the dose of prior steroids or the DAS (further details given on page 79 of study report). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Adequate masking of participants was performed, masking of personnel not clear. Quote: "Investigational drugs were distinguishable in terms of appearance and viscosity after reconstitution, independent staff at each study institution separately prepared and administered the dosing solution, and evaluated the efficacy and safety in each patient to maintain blinding" (page 79). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adequate masking of outcome assessors was performed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four participants (placebo, 1; IVIG, 3) withdrawn before day 15 and 11 participants (placebo, 5; IVIG, 6) after day 15. Some details given on enquiry: Reasons for withdrawals before day 15:
High risk because of unbalanced number of dropouts, different and unknown reasons for dropout, and high proportion of missing outcomes (> 20% dropout rate at day 57) |
| Selective reporting (reporting bias) | Low risk | Nine participants withdrawn from group A and 6 from group B but analysed in an ITT analysis. |
| Other bias | Unclear risk | ‐ |
10. Intravenous immunoglobulin 400 mg/kg/day for 5 days compared to placebo for bullous pemphigoid.
| Intravenous immunoglobulin 400 mg/kg/day for 5 days compared to placebo for bullous pemphigoid(Amagai 2017) | ||||||
| Patient or population: bullous pemphigoid Setting: multicentre Intervention: intravenous human IgG 400 mg/kg/day for 5 days Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with intravenous human IgG 400 mg/kg/day for 5 days | |||||
| Disease control: DAS on day 15 from baseline: last day pre‐treatment | The mean disease control ‐ DAS on day 15 from baseline: last day pre‐treatment was 0 | MD 12.5 lower (26.87 lower to 1.87 higher) | ‐ | 56 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| Mortality at day 57 | Study population | Not estimable | 56 (1 RCT) | ⊕⊕⊕⊝ Moderateb | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DAS: disease activity score; OR: odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by two levels for imprecision (low number of events, CI includes null effect, wide CI). bDowngraded by one level for imprecision (low number of events).
Beissert 2007.
| Study characteristics | ||
| Methods | Multicentre study in Germany; central randomisation; not masked Two parallel groups; initial dose was maintained until blister formation ceased and re‐epithelialisation started. Corticosteroid dose was then reduced every 2 weeks. After discontinuation of corticosteroid, azathioprine, or mycophenolate mofetil (MMF) dose was maintained for 4 more weeks, then reduced (see taper regimen on page 1537 of study report). Follow‐up of 720 days |
|
| Participants | 73 participants with bullous pemphigoid, confirmed by direct and indirect immunofluorescence on salt split skin Mean age in the azathioprine group was 76 years (12 males, 26 females), and in the MMF group 75 years (15 males, 20 females). Disease severity was variable, mild to severe. Inclusion criteria
Exclusion criteria
|
|
| Interventions | A) 38/38 oral methylprednisolone 0.5 mg/kg/day plus azathioprine sodium 2 mg/kg/day B) 35/35 oral methylprednisolone 0.5 mg/kg/day plus mycophenolate mofetil 2000 mg/day (tapering described on page 1537 of study report). |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Registered trial: NCT00431119 Cumulative corticosteroid dose: described as primary outcome until complete healing was achieved (page 1537); on page 1539, the cumulative corticosteroid dose was defined as corticosteroid dose until the end of the documentation period (> 720 days) (Table 11, Table 14). We presumed that calculated cumulative corticosteroid dose was calculated until the end of the documentation period. This trial was supported by an unrestricted grant from Hoffmann‐La Roche AG, producers and providers of the mycophenolate mofetil (CellCept) used in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was stratified according to the clinical centre and performed centrally with the use of random number of three for each stratum" (Page 1537). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not well described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study design: "non blinded clinical trial" (Page 1448). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not masked. Quote: "Since complete healing was a primary outcome measure, blinding was not considered necessary" (Page 1537). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes: 1 participant was lost, 2 died of non‐treatment‐related causes ‐ they were included in the intention‐to‐treat analysis. The same number of participants who started the trial were analysed at the end of the trial. (Figure 1, page 1538) |
| Selective reporting (reporting bias) | Low risk | Outcomes reported for both outcome measures. Primary: complete healing Secondary: cumulative corticosteroid doses used |
| Other bias | Unclear risk | ‐ |
Burton 1978.
| Study characteristics | ||
| Methods | Randomised but not masked Intervention took place over 3 years. Treatment maintained at starting doses until 'rash suppressed', at which point, prednisone doses were gradually reduced in both groups. If rash did not recur then azathioprine was also gradually withdrawn in the azathioprine group. Follow‐up: at the end of the 3‐year treatment period |
|
| Participants | 25 participants with bullous pemphigoid confirmed by immunofluorescence studies. Mean age in the azathioprine plus prednisone group was 76 years (6 males, 6 females) and in the prednisone group 74 years (3 males, 10 females). Disease severity was not mentioned. Inclusion criteria: people were included only if the diagnosis was supported by clinical, histological (a subepidermal blister), and immunological evidence (IgG directed against the basement membrane zone on direct immunofluorescence). Exclusion criteria: people who satisfied the diagnostic criteria were excluded from the trial only if they were unlikely to attend for regular follow‐up or if there was some definite reason (such as known malignancy or hypertension) for not receiving azathioprine or prednisone. |
|
| Interventions | A) 12/12 participants prednisone (30 to 80 mg/day) plus azathioprine (2.5 mg/kg/day) B) 13/13 participants prednisone (30 to 80 mg/kg/day) |
|
| Outcomes | Unclear outcome measures:
|
|
| Notes | After 1 week of prednisolone to suppress lesions, consultant decided whether to include participants in trial. Not clear how prednisolone dose was decided or numbers of participants on lower or higher doses in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Low risk | Quote: "Once included, each participant was randomly assigned ......by the ward sister who drew a marked paper from an envelope" (Page 1190). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The results section states that 25 participants completed a 3‐year follow‐up, but it is unclear how many were randomised to each group at the start. Table 7 page 1190 reports outcomes over 3 years for all 25 participants. |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures were not clearly stated. |
| Other bias | Unclear risk | Only those participants who were likely to attend for follow‐up were recruited. Eligibility was also determined by the consultant doctor after baseline testing. |
Dreno 1993.
| Study characteristics | ||
| Methods | Randomised, investigator masked. Disease control = reduction of blisters, redness, and itch > 50% Follow‐up: 10 days (= treatment period) |
|
| Participants | 57 participants with bullous pemphigoid confirmed by immunofluorescence studies Mean age in the methylprednisolone group was 77 years (11 males, 17 females) and in the prednisolone group 77 years (14 males, 15 females). Disease severity was not stated. The numbers of blisters when people were included in the study were 67 +/‐ 34 in the methylprednisolone group and 56 +/‐ 85 in the prednisolone group on average. Inclusion criteria: people with immunologically‐confirmed pemphigoid Exclusion criteria
|
|
| Interventions | A) 29/29 participants prednisolone (1.16 mg/kg/day) B) 28/28 participants methylprednisolone (1.17 mg/kg/day) |
|
| Outcomes |
|
|
| Notes | Problem: 'scale' for measuring symptoms & signs. Very short study duration | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Taking into account the difference in presentation between the 2 products, the supply of the products to the participants was made by a person other than the investigator; additionally clinical follow‐up after the end of the study was done by a masked (blinded) investigator" (Translation, page 518). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Taking into account the difference in presentation between the 2 products, the supply of the products to the participants was made by a person other than the investigator; additionally clinical follow‐up after the end of the study was done by a masked (blinded) investigator" (Translation, page 518). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29/29 evaluated at both time points for prednisolone group. 27/28 evaluated at both times points for the methylprednisolone group. One participant dropped out (ceased treatment) after 8 days of treatment, due to a coma unrelated to the treatment (page 519). |
| Selective reporting (reporting bias) | Low risk | All outcomes (number of blisters, itching, and redness) reported on at 5 and 10 days of treatment. |
| Other bias | Unclear risk | Short study duration. Non‐validated assessment scales. |
Fivenson 1994.
| Study characteristics | ||
| Methods | Randomised open‐label, but randomisation method not stated; not masked. Disease control after 8 weeks of treatment: complete response = 100% clearing of all lesions, partial response ≥ 50%, no response < 25%. Pruritus and physician's global assessment were also recorded. Disease recurrence in the follow‐up period was recorded if new blisters, urticarial lesions, and/or crusts were noted. Follow‐up: 10 months (= treatment period) |
|
| Participants | 20 participants with bullous pemphigoid confirmed by immunofluorescence studies Mean age in the nicotinamide and tetracycline group was 78 years (5 males, 9 females) and in the prednisone group 77 years (1 male, 5 females). Disease severity was variable, mild to severe. Inclusion criteria
Exclusion criteria
|
|
| Interventions | A) 6/6 participants prednisone 40 to 80 mg/day. B) 14/14 participants nicotinamide 1500 mg/day in 3 divided doses plus tetracycline 2 g/day in 4 divided doses |
|
| Outcomes | Number of bullous, crusted, urticarial lesions as follows: none = 0, 1 to 5 = 1+, 6 to 10 = 2+, 11 to 20 = 3+, 20 to 40 = 4+, more than 40 lesions = 5+. All three of these recorded as less than or more than 1 cm in size. Total highest score possible on each visit per participant. Mean scores for each group used to calculate P values | |
| Notes | Not clear how the prednisone dose was decided or number of participants on higher or lower dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | Unclear; no details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding; open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 were randomised, 18 were treated. 2 were unavailable for follow‐up within the initial 8 weeks, both from the tetracycline/nicotinamide group. There was 1 death in the prednisone group due to sepsis complicated by aspiration pneumonia. The time point was not given, but the participant was available for follow‐up at week 8 as the results for all 8 participants who received prednisolone are given in Table 7 (page 755). At longer‐term follow‐up, only 3 participants in the prednisolone group and 5 in the tetracycline/nicotinamide group are reported. No detail of the reasons for loss to follow‐up at this later follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported at each time point |
| Other bias | High risk | Unclear if the participant groups were equivalent with respect to disease severity or demographics at the start of the therapy. Quote: "The study was originally designed to randomise a total of 96 patients... The study was terminated after the 20 patients presented were enrolled" (page 754). |
Guillaume 1993.
| Study characteristics | ||
| Methods | Randomised, not masked. Disease control = no new blisters for 4 weeks, prednisolone dose decreased gradually to 0.5 mg/kg at 3 months and 0.2 mg/kg at 6 months Follow‐up: 6 months (= treatment period) |
|
| Participants | 100 participants with bullous pemphigoid confirmed by immunofluorescence studies Mean age in the prednisolone group was 75 years (17 males, 14 females), in the azathioprine plus prednisolone group 77 years (19 males, 17 females), and in the plasma exchange plus prednisolone group 75 years (14 males, 17 females). Disease severity was variable, mild to severe. Inclusion criteria: the diagnosis of BP required a skin biopsy demonstrating a subepidermal blister and deposits of immune reactants (IgG and/or C3) in a linear pattern along the basement‐membrane zone of the epidermis. Exclusion criteria
|
|
| Interventions | A) 31/32 participants prednisolone 1 mg/kg/day B) 36/36 participants azathioprine plus prednisolone 1 mg/kg/day C) 31/32 participants plasma exchange plus prednisolone 1 mg/kg/day |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial stopped at interim period due to no appreciable benefit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. Quote: "According to pre‐established randomisation lists equilibrated in blocks of 3 for each centre" (Page 50). |
| Allocation concealment (selection bias) | Low risk | Quote: "After determining a patient's eligibility, the attending physician telephoned the co‐ordinator of the study, who assigned the patient to one of three treatment groups according to pre‐established randomisation lists, equilibrated in blocks of three for each centre." Thus, allocation concealment was performed.(Page 50) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for 2 dropouts not clear. Quote: "unavailable for follow‐up after having withdrawn their consent" (Page 51) (not clear if this was before or after starting treatment). Dropouts not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Controlled disease was stated to be no more than 1 new blister occurring at 4 weeks after starting treatment, resolution of erythema, and no more than minimal pruritis. Only the composite measure of controlled disease was reported (Table 11, Page 52). |
| Other bias | Unclear risk | Trial stopped early. Quote: "Our trial was interrupted after the interim analysis showed no appreciable benefit resulting from the addition of azathioprine or plasma exchange to prednisolone in the initial (at 4 weeks) and maintenance (at 6 months) treatments of BP" (Page 52). |
Joly 2002.
| Study characteristics | ||
| Methods | Randomised, not masked. Disease control = number of new blisters after 3 weeks (21 days) of treatment. (Not clear if participant kept record of new blisters daily or if all new blisters since previous visit were averaged out to get a daily rate) |
|
| Participants | 341 participants with bullous pemphigoid confirmed by immunofluorescence studies. Mean age in the oral prednisone group moderate disease was 81 years (28 males, 48 females), extensive disease 81 years (32 males, 68 females). Mean age in the topical steroid group moderate disease was 82 years (29 males, 48 females), extensive disease 80 years (40 males, 53 females). Disease severity was variable, mild to severe. Inclusion criteria: consecutive patients with newly diagnosed bullous pemphigoid were eligible for entry if the following criteria were met:
Exclusion criteria: predominant or exclusive mucosal involvement and treatment with oral or topical corticosteroids, dapsone, or immunosuppressive drugs during the previous six months. |
|
| Interventions | Moderate disease: A) 77/77 participants 40 g topical clobetasol propionate cream twice daily to entire body B) 76/76 participants 0.5 mg/kg/day oral prednisone Extensive disease: A) 93/93 participants topical clobetasol B) 95/95 participants prednisone 1 mg/kg/day |
|
| Outcomes |
|
|
| Notes | Originally there were 364 participants recruited: 14 did not meet the inclusion criteria, 8 did not give consent, 1 withdrew his consent in the beginning of the study: left with 341 study participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed centrally with the use of random numbers in permuted blocks of four within each stratum" (Page 322). |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not well described. Probably adequate as it was done centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded. A nurse not otherwise associated with the study assessed the number of new bullae, daily. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded. A nurse not otherwise associated with the study assessed the number of new bullae, daily. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants and outcomes adequately reported |
| Selective reporting (reporting bias) | Low risk | Adequately reported |
| Other bias | Unclear risk | Quote: "In accordance with the study protocol, the investigators switched three patients with moderate bullous pemphigoid and four with extensive bullous pemphigoid from the oral‐prednisone group to the topical‐corticosteroid group because of side‐effects of treatment." (Page 324) 'Compliance with treatment and adverse effects'. |
Joly 2009.
| Study characteristics | ||
| Methods | Multicentre study in France, centrally randomised; not masked Two different regimens were applied. In the mild regimen, participants received doses depending on their body weight; follow‐up of 360 days |
|
| Participants | 312 participants with bullous pemphigoid; confirmed by direct immunofluorescence test. Mean age in the mild regimen group (10 to 30 g of clobetasol propionate cream per day) in moderate disease was 85 years (25 males, 44 females), in extensive disease 82 years (39 males, 51 females). Mean age in the standard regimen (40 g) group in moderate disease was 82 years (28 males, 37 females), in extensive disease 80 years (37 males, 51 females). Disease severity was variable, mild to severe. Inclusion criteria: consecutive patients with newly diagnosed BP were eligible for entry with the following criteria:
Exclusion criteria
|
|
| Interventions | Mild regimen: clobetasol propionate cream 10 to 30 g/day, until 15 days after disease control; thereafter corticosteroid tapering over 4 months (159 participants).
Standard regimen: clobetasol propionate cream 40 g/day initially, until 15 days after disease control; corticosteroid tapering over 12 months (study report page 1686) (153 participants). |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Page 1686 typing error: should be 0.05% clobetasol propionate cream, not 0.005% Discrepancy between the numbers of participants as follows: the numbers in figure 1 (153 participants randomised, 150 participants analysed), text (153 participants randomised, 150 participants analysed), and table 2 (153 randomised and 153 analysed) for the standard regimen do not match. Clarification from the study investigator (Joly 2010 [pers comm]): numbers in table 2 on page 1684, are wrong, should be 150 in the standard regimen Only 150 were analysed, therefore intention‐to‐treat (ITT) analysis was not fulfilled Worst case scenario calculation (none of the 3 missing cases in the standard group had complete healing): ‐ 156/159 v 150/153 (ITT analysis), Analysis 8.1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed centrally with the use of random numbers in permuted blocks of four within each stratum" (Page 1686). |
| Allocation concealment (selection bias) | Low risk | Allocation concealment is not well described. Probably adequate as it was done centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intentionally not masked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Intentionally not masked |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not all participants were accounted for at each stage of the trial. See Figure 1, page 1683, and Table 11, page 1686: typing error in table 2, only 150 participants were analysed. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported adequately. |
| Other bias | Low risk | No other bias. |
Liu 2006.
| Study characteristics | ||
| Methods | No details provided | |
| Participants | 30 participants with bullous pemphigoid; diagnosis confirmed by pathological tests Mean age in the Jingui Shenqi Pill (JSP) 1# bid plus prednisone group was 65 years (11 males, 4 females) and in the prednisone alone group 68 years (10 males, 5 females). Disease severity not stated, but the authors stated there were no significant differences in the distribution of disease severity. Inclusion criteria: people with both clinically and pathologically explicitly diagnosed BP with yang‐deficient constitution The "yang‐deficient constitution" is a type of constitution defined by traditional Chinese medicine. Exclusion criteria: not stated |
|
| Interventions | A) Jingui Shenqi Pill (JSP) 1# bid plus prednisone 0.5 to 1.0 mg/kg/day (15 participants) B) prednisone alone 0.5 to 1.0 mg/kg/day (15 participants) |
|
| Outcomes |
|
|
| Notes | Article in Chinese. Translation obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomisation details in English abstract, nor in paper |
| Allocation concealment (selection bias) | Unclear risk | No details in English abstract, nor in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No masking described: no details in English abstract, nor in paper |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masking described: no details in English abstract, nor in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Adequately reported |
| Selective reporting (reporting bias) | Low risk | Outcome is adequately reported |
| Other bias | High risk | Few details about any methods in translated paper. Precise method of diagnosis not specified (referred to as 'clinical' and 'pathological' tests). No further details available from the trial investigator. |
Morel 1984.
| Study characteristics | ||
| Methods | Randomised but not masked. Disease control = number of new blisters between days 21 to 51 Follow‐up: 51 days (= treatment period) |
|
| Participants | 50 participants with bullous pemphigoid confirmed by immunofluorescence studies. Mean age in the prednisolone 0.75 mg group was 81 years (8 males, 16 females) and in the prednisolone 1.25 mg group 77 years (13 males, 9 females). Disease severity was not stated. Inclusion criteria: participants should not have been on corticosteroid treatment before enrolment, or if they were, they should have stopped treatment 7 days before enrolment Exclusion criteria: people with BP who had a recurrence during treatment (GK: means treatment resistant cases?) |
|
| Interventions | A) 24/26 prednisolone 0.75 mg/kg/day B) 22/24 prednisolone 1.25 mg/kg/day |
|
| Outcomes | New blister formation: at day 21 and 51 | |
| Notes | Two dropouts in each group, no reasons given, and not included in analysis Erythromycin used for infection but its anti‐inflammatory effect not evaluated or commented upon |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a single table of pre‐established and balanced randomisation for all 8 patients." (Translation, page 926). |
| Allocation concealment (selection bias) | Unclear risk | No; no details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded; no details given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded; no details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two dropouts in each group, no reasons given, and not included in analysis. |
| Selective reporting (reporting bias) | Low risk | New blister formation at days 21 and 51 reported. |
| Other bias | Unclear risk | Some participants who could have been recruited were excluded on the grounds that they were able to take part in a parallel study involving plasma exchanges. |
Roujeau 1984.
| Study characteristics | ||
| Methods | Randomised but not masked. Disease control = complete disappearance of blisters, itch, and erythema Follow‐up: 6 months (= treatment period) |
|
| Participants | 41 participants with bullous pemphigoid confirmed by immunofluorescence studies; 4 participants were withdrawn. Mean age in the prednisolone group was 70 years (9 males, 6 females) and in the plasma exchange plus prednisolone group 67 years (12 males, 10 females). Disease severity was variable, mild to severe. Inclusion criteria
Exclusion criteria
|
|
| Interventions | A) 15/17 prednisolone 0.3 mg/kg/day B) 22/24 plasma exchange plus prednisolone 0.3 mg/kg/day Both groups were treated with an identical protocol based on weekly dose adjustments according to the therapeutic results observed. Therapy was started at a dose of 0.3 mg/kg oral prednisolone daily for 1 week. If the treatment was ineffective, the dose was increased weekly to 1.5 mg/kg/day oral prednisolone, 2 mg/kg/day intramuscular methylprednisolone, and 2 mg/kg/day intramuscular methylprednisolone with (maximum permissible) 3 mg/kg/day oral cyclophosphamide. |
|
| Outcomes |
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised. Quote: "Assigned to groups according to lists drawn by microcomputer for each participating centre and equilibrated after every 4 patients" (Page 487). |
| Allocation concealment (selection bias) | Unclear risk | "The patients were allocated randomly to two groups"; exact method not mentioned (Page 487, left column). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 41 randomised, 2 from each group were excluded from analysis, reasons accounted for in Results (Page 487). 2 participants from each group withdrawn from study, before treatment was initiated (1 had no BP, 2 accidentally received higher initial doses of prednisolone, 1 no plasma exchange). Dropouts not included in the analysis (they were randomised, but did not really start proper trial treatment and were excluded). Outcomes (disease control) were reported for the remaining 37 participants in the groups they were randomised to (Table 11, Page 488). Side effects and mortality are reported in the text (Tables II and III). |
| Selective reporting (reporting bias) | Low risk | The main objective testing whether plasma exchange leads to a significant reduction in corticosteroid administration, next to other outcomes, is reported. |
| Other bias | Low risk | ‐ |
Simon 2020.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled, double‐masked, parallel‐group, phase 2 pilot study Treatment period: 12 weeks Follow‐up: 6 months |
|
| Participants | 30 participants with bullous pemphigoid, defined by typical clinical signs and symptoms, typical histology in a lesional skin biopsy, proof of linear deposits of IgG or complement C3 along the dermal‐epidermal junction assessed by direct immunofluorescence analysis and/or detection of anti‐basement membrane autoantibodies by indirect immunofluorescence and/or BP180 and BP230 autoantibodies in serum assessed by ELISA. Mean age in the placebo group was 78 years (5 males, 5 females) and in the mepolizumab group 74 years (7 males, 13 females). Disease severity was variable; there were fewer mild cases than severe disease, mainly in the mepolizumab group. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intravenous mepolizumab at a dose of 750 mg or matching placebo every 4 weeks over 12 weeks in addition to 0.5 mg prednisolone per kg body weight. | |
| Outcomes | Primary
Secondary
|
|
| Notes | See appendix of the publication; no figures on mortality presented. However, we contacted the first author and confirmed that there were no deaths during the study period. GlaxoSmithKline provided the study drug mepolizumab and supported the study with a research grant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation (sequence generation, random allocation as well as preparation of mepolizumab and placebo infusions, identical in appearance) were done at the Institute of Hospital Pharmacy, Inselspital, by staff otherwise not involved in the trial. Exact method not stated. Method of randomisation not mentioned. |
| Allocation concealment (selection bias) | Unclear risk | Random allocation as well as preparation of mepolizumab and placebo infusions, identical in appearance, were done at the Institute of Hospital Pharmacy, Inselspital, by staff otherwise not involved in the trial. Exact method not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "intravenous mepolizumab or matching placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Preparation of mepolizumab and placebo infusions, identical in appearance, were done at the Institute of Hospital Pharmacy, Inselspital, by staff otherwise not involved in the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were included in the intention‐to‐treat analysis; however, 9 of 30 participants left the trial prematurely. |
| Selective reporting (reporting bias) | High risk | No details regarding which adverse events/serious adverse events happened in which group. Authors stated by email that there were no deaths during the study period. |
| Other bias | Unclear risk | ‐ |
Sticherling 2017.
| Study characteristics | ||
| Methods | Randomised but not masked. Multicentre study in Germany (12 centres) and Austria (1 centre) Disease control = time until complete tapering of methylprednisolone Follow‐up: 12 months (= treatment period) Collection of data: December 2001 until March 2005 |
|
| Participants | 54 participants with bullous pemphigoid confirmed by immunofluorescence studies or ELISA; demented patients were included Mean age in the azathioprine group was 74 years (6 males, 21 females) and in the dapsone group 79 years (9 males, 18 females). Disease severity was variable, mild to severe. Inclusion criteria
Exclusion criteria
|
|
| Interventions | A) Oral methylprednisolone 0.5 mg/kg/day plus azathioprine 1.5 to 2.5 mg/kg/day (27 participants). Dose according to the thiopurine methyltransferase activity (2.8 to 10.0 nmol/mL erythrocytes/hour, azathioprine 1.5 mg/kg body weight; > 10.0 nmol/mL erythrocytes/hour, azathioprine 2.5 mg/kg body weight). B) oral methylprednisolone 0.5 mg/kg/day plus dapsone 1.5 mg/kg/day (27 participants). |
|
| Outcomes | Primary
A second primary outcome was later determined because of small numbers due to low recruitment:
Secondary
|
|
| Notes | The study was terminated in March 2005 due to low recruitment. This study was not pre‐registered. 5 mg prednisolone = 4 mg methylprednisolone. Riemser Inc. (Greifswald, Germany) supported the study with unrestricted funding of EUR 10,000. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed centrally by facsimile using a unique computer‐generated master list of random numbers (allocation ration 1:1) without stratification for disease severity or any other patient characteristic. |
| Allocation concealment (selection bias) | Unclear risk | Probably done as randomisation was performed centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No masking (see study design) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masking. Quote: "Investigators may have been biased in favour of dapsone to confirm earlier results and therefore started tapering of corticosteroids earlier" (Page 1304). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data are not fully reported. Authors state recruitment of 88 was the target, allowing 10% dropout as 80 participants were needed for calculation. However, only 54 participants were finally recruited, but outcomes are still not reported for all, and no reasons given. |
| Selective reporting (reporting bias) | High risk | ITT analysis was intended; however, not all outcome data are reported for all randomised patients; no reasons given. Additional primary outcome was added for analysis. |
| Other bias | High risk | This study had extensive exclusion criteria regarding the health status of patients. “It cannot be excluded, that in the present trial, healthier patients had been included resulting in a preselection bias”. |
Williams 2017.
| Study characteristics | ||
| Methods | Parallel‐group, randomised controlled trial, pragmatic. Multicentre study in UK and Germany Disease control: non‐inferiority approach to compare short‐term effectiveness (week 6) Safety: superiority approach to compare long‐term safety (week 52) Follow‐up: 52 weeks (= treatment and follow‐up) Collection of data: 1 March 2009 until 31 October 2013 |
|
| Participants | Mean age in the prednisolone group was 77 years (64 males, 57 females) and in the doxycycline group 78 years (69 males, 63 females). Disease severity was variable, mild to severe. Inclusion criteria: adults (18 years and older) with clinical signs of bullous pemphigoid with blisters appearing on at least two body sites within the last week, confirmed by immunofluorescence, and who were able to provide written informed consent Exclusion criteria
|
|
| Interventions | A) Doxycycline (200 mg per day): 140 participants allocated, 132 included, 112 effectiveness analysis week 6 (modified (m) ITT), 121 primary safety analysis week 52 (mITT). B) Prednisolone (0.5 mg/kg per day): 138 participants allocated, 121 included, 101 effectiveness analysis week 6 (mITT), 113 primary safety analysis week 52 (mITT). |
|
| Outcomes | Primary
Secondary effectiveness outcomes
Secondary safety outcomes
Tertiary outcomes
|
|
| Notes | Trial registration: 11/11/2008 ISRCTN13704604; TR2007‐006658‐24‐GB Funding: NIHR Health Technology Assessment Programme, UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (1:1); randomisation was done by the Internet and occurred once recruited participants’ details had been entered by local physicians and research nurses onto a study database. |
| Allocation concealment (selection bias) | Low risk | Treatment was allocated using random permuted blocks of randomly varying size generated by the Nottingham Clinical Trials Unit and was stratified by baseline severity. Treatment allocation was sent directly to the local pharmacist who dispensed the appropriate medication directly in the UK or to an unmasked study nurse or physician in Germany, allowing the investigator to remain masked for the first 6 weeks. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Personnel was masked up to week 6 (primary end point); participants were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were masked for the first 6 weeks; they were subsequently unmasked to adjust or switch medication to reflect normal clinical practice (pragmatic study design). This means no masking for the primary safety outcome (including mortality) assessed at 52 weeks. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | According to Health Technology Assessment report, 256 participants were needed for the safety analysis. However, after randomisation of 278 participants, 25 were withdrawn because of ineligibility, resulting in 253 randomised eligible participants. The reason for withdrawals is given. 112/132 analysed for primary effectiveness outcome in group A and 101/121 in group B (modified ITT) 121/132 analysed for primary safety outcome in group A and 113/121 in group B (modified ITT) (compare HTA report that provides additional data) |
| Selective reporting (reporting bias) | Low risk | ITT analysis was aimed for and performed; all outcomes reported. |
| Other bias | Low risk | No other bias detected |
BMZ: basement‐membrane zone; BP: bullous pemphigoid; C3: complement component 3; DAS: disease activity score; ELISA: enzyme‐linked immunosorbent assay; IgA: immunoglobulin A; IgG: immunoglobulin G; ITT: intention‐to‐treat; IVIG: intravenous immunoglobulin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Derhaschnig 2016 | Tested a mixed group of diseases; the number of participants with bullous pemphigoid was unknown |
| EUCTR2011‐004361‐32‐DE | Study terminated because of poor enrolment |
| EudraCT2008‐005266‐31 | Ineligible condition: pemphigus patients (rituximab) |
| Kannan 2018 | Bullous pemphigoid was not confirmed by immunofluorescence |
| NCT00472030 | Study terminated because of poor enrolment (omalizumab) |
| NCT01688882 | Study terminated. This study was stopped after Part 1 completed and was terminated because the predefined efficacy criterion was not reached (> 50% better than placebo). |
| NCT03286582 | Study terminated with partial enrolment completed, no results available |
| NCT05061771 | This study was withdrawn by the investigator before recruitment began. |
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR‐IOR‐15007146.
| Methods | Randomised controlled trial |
| Participants | People with bullous pemphigoid |
| Interventions | Control group: 0.05% halometasone cream daily Treatment group: 0.05% halometasone cream daily plus low‐dose systemic steroids |
| Outcomes | Efficacy of low‐dose systemic steroids combined with topical steroids in the treatment of bullous pemphigoid and risk factors for treatment‐refractory patients |
| Notes | Finalised study; no data available. We contacted authors in July 2020, but received no response. panmeng@medmail.com.cn |
ChiCTR‐TRC‐12003592.
| Methods | Randomised parallel controlled trial |
| Participants |
Inclusion criteria
|
| Interventions | Tripterygium glycosides group: take tripterygium glycosides orally at 20 mg, 3 times a day Nicotinamide plus minocycline group: take nicotinamide at 500 mg, 3 times a day and minocycline 100 mg, twice a day |
| Outcomes | Primary
|
| Notes | Finalised trial; no results data available. We contacted authors in July 2020, but received no response. hangyezhuanxiang@163.com; wangbx@ncstdlc.org |
ChiCTR‐TRC‐12003593.
| Methods | Randomised parallel controlled trial |
| Participants |
Inclusion criteria
|
| Interventions | Glucocorticoids group: "this group receives systemic glucocorticoid, initially, with prednisone at 1mg/kg/day. Then we adjust the dose of glucocorticoid according to the patients' responses." Glucocorticoid plus Methotrexate (MTX) group: "This group receives systemic glucocorticoid plus MTX and starts with prednisone at 1mg/kg/day and MTX IV drip infusion at 15mg/week. Then we adjust the dose of glucocorticoid according to the patients' responses." |
| Outcomes | Primary
|
| Notes | Completed, no results data available. We contacted authors in July 2020, but received no response. hangyezhuanxiang@163.com; wangbx@ncstdlc.org |
EudraCT 2019‐001059‐37‐DE.
| Methods | Randomised, double‐blind, placebo‐controlled study |
| Participants | People with bullous pemphigoid |
| Interventions | Adjunctive AKST4290 versus placebo |
| Outcomes | "To investigate the proportion of subjects who achieve disease control (defined as ≤ 3 new blisters/day and healing of existing blisters) following topical steroid treatment with adjunctive AKST4290 without receiving rescue therapy. To assess treatment safety of the proposed dosing regimen. Additional secondary endpoints include assessment of time to disease control; time to rescue therapy; change in BP Disease Area Index (BPDAI) score by treatment week and at disease control; and change in pruritis as assessed by the BPDAI‐Visual Analog Scale (BPDAI‐VAS) by treatment week and at disease control. In addition, change in skin (biopsy) eosinophil counts and overall steroid use required to achieve disease control will be assessed." |
| Notes | Completed; no results data available in November 2021 |
NCT02313870.
| Methods | Randomised controlled trial |
| Participants | People with bullous pemphigoid |
| Interventions | Clobetasol propionate plus methotrexate versus clobetasol propionate alone |
| Outcomes | The primary endpoint is the actuarial survival rate with or without recurrence at 9 months in the topical steroid plus methotrexate group (Arm A) compared to exclusive topical steroid group (Arm B). Secondary outcomes:
|
| Notes | We contacted the investigator in July 2020: the data were about to be written up, but results were not available. https://clinicaltrials.gov/ct2/show/NCT02313870 UF 7850, Topical Steroids Alone or Associated With Methotrexate in Bullous Pemphigoid o‐dereure@chu‐montpellier.fr |
ELISA: enzyme‐linked immunosorbent assay
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐2000028707.
| Study name | Randomized, controlled clinical trial for low‐dose interleukin‐2 in the treatment of moderate to severe bullous pemphigoid |
| Methods | Randomised, controlled clinical trial |
| Participants | People with bullous pemphigoid |
| Interventions | Low‐dose interleukin‐2 versus "standard treatment plan" |
| Outcomes |
|
| Starting date | 1 January 2020 |
| Contact information | gdpfkjk@vip.163.com; xueruzeng@163.com |
| Notes | Recruiting |
ChiCTR‐TRC‐12003538.
| Study name | Use of intravenous methotrexate plus glucocorticoid for the treatment of bullous pemphigoid: a multicenter, randomised and controlled clinical trial |
| Methods | Randomised and controlled clinical trial |
| Participants | People with bullous pemphigoid |
| Interventions | Glucocorticoids group (90 participants) versus glucocorticoid plus methotrexate group (90 participants) |
| Outcomes |
|
| Starting date | Enrolment started: 01 August 2011 Registration: 27 December 2012 |
| Contact information | Yi Liu, hangyezhuanxiang@163.com Baoxi Wang, wangbx@ncstdlc.org |
| Notes |
http://www.chictr.org.cn/showproj.aspx?proj=6022 Trial was still recruiting in April 2017. We contacted the investigator in July 2020, but received no response. |
EudraCT 2020‐002912‐34.
| Study name | Treatment of bullous pemphigoid with avdoralimab (IPH5401), an anti‐C5aR1 monoclonal antibody |
| Methods | Randomised, controlled clinical trial |
| Participants | People with autoimmune bullous diseases |
| Interventions | Avdoralimab (IPH5401) plus super potent topical steroids versus super potent topical steroids |
| Outcomes | Complete clinical remission, at 3 months |
| Starting date | 11 August 2020 |
| Contact information | Centre Hospitalier Universitaire de Nice, Nice, France Email: caillon.c@chu‐nice.fr Tel. 0033492034589 |
| Notes | Sponsor protocol number: 20‐PP‐13 No further details found |
NCT02365675.
| Study name | Wound dressings for pemphigus and pemphigoid |
| Methods | Randomised, parallel assignment |
| Participants | People with pemphigus or pemphigoid |
| Interventions | Cotton gauze with petrolatum/cellulose acetate with petrolatum versus nanocrystalline silver dressing (Acticoat)/carboxymethylcellulose with ionic silver (Aquacel AG) |
| Outcomes | Healing and decreasing pain and itch |
| Starting date | January 2015 |
| Contact information | Jose Contreras‐Ruiz, Hospital General Dr. Manuel Gea González, Mexico. NCT02365675, 06‐106‐2014 Jose Contreras‐Ruiz: dermayheridas@gmail.com Karla Lopez‐Ortiz: karlitaav24@hotmail.com |
| Notes | We contacted investigator in July 2020, but received no response. Recruitment status unknown; ongoing trial? |
NCT04206553.
| Study name | A multicenter, randomized, double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy and safety of dupilumab in adult patients with bullous pemphigoid (LIBERTY‐BP) |
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled, parallel group study |
| Participants | People with bullous pemphigoid |
| Interventions | Dupilumab versus placebo versus oral corticosteroids |
| Outcomes | The primary objective of the study is to demonstrate that dupilumab is superior to placebo in achieving sustained remission off oral corticosteroids in patients with bullous pemphigoid (Time frame: Week 36) |
| Starting date | June 2020 |
| Contact information |
NCT04206553 Clinical Trials Administrator Tel. 844‐734‐6643 clinicaltrials@regeneron.com |
| Notes | Recruiting |
NCT04612790.
| Study name | A multinational, randomized, double‐blind, parallel‐group, placebo‐controlled study to investigate the use of benralizumab as a treatment option for patients with bullous pemphigoid (FJORD) |
| Methods | Multinational, randomised, double‐blind, parallel‐group, placebo‐controlled study |
| Participants | People with bullous pemphigoid |
| Interventions | Benralizumab versus placebo |
| Outcomes | Proportion of participants who are in complete remission while off oral corticosteroids for ≥ 2 months at week 36 |
| Starting date | 31 March 2021 |
| Contact information | information.center@astrazeneca.com |
| Notes | ‐ |
Differences between protocol and review
This is the third update of the review first published in 2002. The review question, eligibility criteria, and methods have not changed since the first review and the first protocol.
However, for this update, we revised and updated our search strategies in line with current Cochrane Skin practices. We also included for the first time a consumer author.
We changed the wording of the primary outcome "disease control" (e.g. regression or healing of skin lesions), which had referred to the "rate of" and "when/how soon?", as these are time‐to‐event measures which are complicated to measure and analyse (and not often reported in trials). We added "at time periods specified by individual trials".
We made minor changes to the secondary outcomes of systemic infection and mortality. We had originally intended to look at systemic infection and mortality as a result of the primary disease and as a result of treatment. At the time of the first published version of the review, we decided that these data were unlikely to be available and we no longer include them.
The original protocol of this review stated that we would use the Jadad quality assessment scale, which also similarly assesses randomisation, blinding, withdrawals, and dropouts (Jadad 1996). We assessed all these aspects but reported them individually (see risk of bias tables in the Characteristics of included studies) rather than as a summary score, as the use of scales for assessing quality or risk of bias is explicitly discouraged in Cochrane reviews (Higgins 2008; section 8.3.3).
We have reclassified the outcomes as primary and secondary outcomes.
We have changed the measures of treatment effect to risk ratio (RR) from odds ratio (OR) in accordance with Cochrane Skin Group policy.
In March 2015, we updated our search strategies slightly. This was to incorporate the latest randomised controlled trial (RCT) filters for MEDLINE and Embase. Additionally, we omitted the term 'pemphigoid gestationis' which was used with the NOT command in previous searches. Using the term with NOT only removed two or three references from each database, and in one instance the paper removed referred to both bullous and gestational pemphigoid patients, so may be relevant.
Contributions of authors
GK was the contact person with the editorial base, GK co‐ordinated contributions from the co‐authors, and wrote the final draft of the review with the help of SS.
GK and KT screened abstracts/papers against eligibility criteria. GK and SS screened trial registries against eligibility criteria.
GK obtained data on ongoing and unpublished studies.
GK, SS, and CCC appraised the quality of papers.
GK and SS extracted data for this review update (except BLISTER study, Williams 2017) with the help of CCC and sought additional information about papers from the authors.
SS and VA extracted data from the BLISTER study (Williams 2017), and checked for discrepancies.
SS and VA performed GRADE assessments and created summary of findings tables.
GK, with the help of SS, entered data into RevMan.
GK, SS, and CCC analysed and interpreted data.
GK, SS, CCC, and Emma Mead worked on the methods sections.
GK, with the help of SS and KT, drafted the clinical sections of the background and responded to the clinical comments of the referees.
SS and VA responded to the methodology and statistics comments of the referees.
SS and GK performed the final revision in 2023 supported by the Cochrane support team.
DM commented on quality issues of trials.
RJB reviewed summary of findings tables and data analyses and helped to revise and edit the manuscript.
Sources of support
Internal sources
-
New Source of support, Other
none
External sources
-
The National Institute for Health and Care Research (NIHR), UK
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Sanjay Singh: none to declare
Gudula Kirtschig was a co‐investigator of the BLISTER study but was not involved in data extraction or analysis for this study.
Vinayak N Anchan: none to declare
Ching‐Chi Chi: none to declare
Kathy Taghipour: none to declare
Robert Boyle: none to declare
Dedee Murrell declares the following interests: ArgenX ‐ Consultant for Efgartigimod trials in Pemphigus and Bullous Pemphigoid AstraZeneca ‐ Investigator PRINCIPIA BIOPHARMA INC. ‐ Chief consultant for trial designs from phase 1, 2 to 3 End; Principal investigator in clinical trials SANOFI PASTEUR BIOLOGICS LLC ‐ Chief Investigator and advisor for Phase 3 PEGASUS trial of rilzabrutinib to treat pemphigus SANOFI PASTEUR BIOLOGICS LLC ‐ Principle investigator and advisor for design of trials for bullous pemphigoid with dupilumab Premier Specialists ‐ Employment, private practice. In my private practice, I treat patients with blistering diseases. I was an advisor and trial site investigator for the design of the Roche trial of rituximab for pemphigus but did not recruit any patients for that trial.
Robert Boyle and Ching‐Chi Chi are Editors for Cochrane Skin but were not involved in the editorial process.
Edited (no change to conclusions)
References
References to studies included in this review
Amagai 2017 {published data only}01408550
- Amagai M, Ikeda S, Hashimoto T, Mizuashi M, Fujisawa A, Ihn H, et al. A randomized double-blind trial of intravenous immunoglobulin for bullous pemphigoid. Journal of Dermatological Science 2017;85(2):77-84. [CENTRAL: CN-01298892] [PMID: ] [DOI] [PubMed] [Google Scholar]
Beissert 2007 {published data only}
- Beissert S, Werfel T, Frieling U, Böhm M, Sticherling M, Stadler R, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Archives of Dermatology 2007;143(12):1536-42. [DOI] [PubMed] [Google Scholar]
Burton 1978 {published data only}
- Burton JL, Harman RR, Peachy RD, Warin RP. Azathioprine plus prednisone in treatment of pemphigoid. British Medical Journal 1978;2:1190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dreno 1993 {published data only}
- Dreno B, Sassolas B, Lacour P, Montpoint S, Lota I, Giordano F, et al. Methylprednisolone versus prednisolone methylsulfobenzoate in pemphigoid: A comparative multicenter study. Annales de Dermatologie et de Vénéréologie 1993;120:518-21. [PubMed] [Google Scholar]
Fivenson 1994 {published data only}
- Fivenson DP, Breneman DL, Rosen GB, Hersh CS, Cardone S, Mutasim D. Nicotinamide and tetracycline therapy of bullous pemphigoid. Archives of Dermatology 1994;130(6):753-8. [PubMed] [Google Scholar]
Guillaume 1993 {published data only}
- Guillaume JC, Vaillant L, Bernard P, Picard C, Prost C, Labeille B, et al. Controlled trial of azathioprine and plasma exchange in addition to prednisolone in the treatment of bullous pemphigoid. Archives of Dermatology 1993;129(1):49-53. [PubMed] [Google Scholar]
Joly 2002 {published data only}
- Joly P, Benichou J, Lok C, Hellot MF, Saiag P, Trancrede-Bohin E, et al. Prediction of survival for patients with bullous pemphigoid. A prospective study. Archives of Dermatology 2005;141:691-98. [DOI] [PubMed] [Google Scholar]
- Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. New England Journal of Medicine 2002;346(5):321-7. [DOI] [PubMed] [Google Scholar]
- Joly P. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. Annales de Dermatologie et de Vénéréologie 2002;129 Suppl 1 Pt 1:1S235. [Google Scholar]
Joly 2009 {published data only}
- Joly P, Roujeau JC, Benichou J, Delaporte E, D'Incan M, Dreno B, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: A multicenter randomized study. Journal of Investigative Dermatology 2009;129(7):1681-7. [DOI] [PubMed] [Google Scholar]
- Joly P. Personal communication. Email communication clarifying the published report. 30 January 2010.
Liu 2006 {published data only}
- Liu BG, Li ZY, Du M. Effects of Jingui Shenqi Pill combined prednisone on expression of glucocorticoid receptor and its clinical effect in treating bullous pemphigoid patients. Chinese Journal of Integrated Traditional and Western Medicine 2006;26(10):881-4. [PubMed] [Google Scholar]
Morel 1984 {published data only}
- Morel P, Guillaume JC. Treatment of bullous pemphigoid with prednisolone only: 0.75 mg/kg/day versus 1.25 mg/kg/day. A multicenter randomized study. Annales de Dermatologie et de Vénéréologie 1984;111(10):925-8. [PubMed] [Google Scholar]
Roujeau 1984 {published data only}
- Roujeau JC, Guillaume JC, Morel P, Crickx B, Dalle E, Doutre MS, et al. Plasma exchange in bullous pemphigoid. Lancet 1984;2(8401):486-8. [DOI] [PubMed] [Google Scholar]
Simon 2020 {published data only}01705795
- Simon D, Yousefi S, Cazzaniga S, Bürgler C, Radonjic S, Houriet C, et al. Mepolizumab failed to affect bullous pemphigoid: a randomized, placebo-controlled, double-blind phase 2 pilot study. Allergy 2020;75(3):669-672. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sticherling 2017 {published data only}
- Sticherling M, Franke A, Aberer E, Gläser R, Hertl M, Pfeiffer C, et al. An open, multicenter, randomized clinical study in patients with bullous pemphigoid comparing methylprednisolone and azathioprine with methylprednisolone and dapsone. British Journal of Dermatology 2017;177(5):1299-1305. [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 2017 {published data only}13704604
- Chalmers JR, Wojnarowska F, Kirtschig G, Mason J, Childs M, Whitham D, et al. A randomised controlled trial to compare the safety, effectiveness and cost-effectiveness of doxycycline (200 mg/day) with that of oral prednisolone (0.5 mg/kg/day) for initial treatment of bullous pemphigoid: the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) trial. Health Technology Assessment 2017;21(10):1-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JR, Wojnarowska F, Kirtschig G, Nunn AJ, Bratton DJ, Mason J, et al. A randomized controlled trial to compare the safety and effectiveness of doxycycline (200 mg daily) with oral prednisolone (0.5 mg kg(-1) daily) for initial treatment of bullous pemphigoid: a protocol for the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Trial. British Journal of Dermatology 2015;173(1):227-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec T, Schmidt E, et al. Doxycycline vs. prednisolone for initial treatment of bullous pemphigoid: A pragmatic noninferiority randomized controlled trial. British Journal of Dermatology 2016;175(S1):11. [Google Scholar]
- Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet 2017;389(10079):1630-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Derhaschnig 2016 {published data only}
- Bratko J, Schoergenhofer C, Schwameis M, Firbas C, Beliveau M, Chang C, et al. A randomized, first-in-human, healthy volunteer trial of sutimlimab, a humanized antibody for the specific inhibition of the classical complement pathway. Clinical Pharmacology & Therapeutics 2018;104(4):655-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derhaschnig U, Gilbert J, Jager U, Böhmig G, Stingl G, Jilma B. Combined integrated protocol/basket trial design for a first-in-human trial. Orphanet Journal of Rare Diseases 2016;11(134):1-6. [EUDRA CT: 2014-003881-26] [NCT: NCT02502903] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2011‐004361‐32‐DE {published and unpublished data}
- EUCTR2011-004361-32-DE. Effect of simvastatin in combination with a superpotent topical corticosteroid in bullous pemphigoid. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011-004361-32-DE (first received 22 May 2012).
EudraCT2008‐005266‐31 {published and unpublished data}
- Joly P. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet 2017;20(389 (10083)):2031-40. [DOI] [PubMed] [Google Scholar]
Kannan 2018 {published data only}
- Kannan R, Vijayananth J, Chandrasekar M. A comparative study on the efficacy of i.V. cyclophosphamide pulse versus oral cyclophosphamide in bullous pemphigoid. Journal of Clinical and Diagnostic Research 2018;12(10):WC01-WC03. [DOI: 10.7860/JCDR/2018/37164/12270] [DOI] [Google Scholar]
NCT00472030 {published and unpublished data}
- NCT00472030. Efficacy and safety of omalizumab in bullous pemphigoid. clinicaltrials.gov/ct2/show/NCT00472030 (first received 10 May 2007).
NCT01688882 {published and unpublished data}
- NCT01688882. Safety, efficacy and PK/PD of QGE031 vs. placebo in patients with active bullous pemphigoid despite oral steroid treatment. clinicaltrials.gov/ct2/show/NCT01688882 (first received 20 September 2012).
NCT03286582 {published and unpublished data}
- NCT03286582. A proof-of-concept study of topical AC-203 in patients with bullous pemphigoid. clinicaltrials.gov/ct2/show/NCT03286582 (first received 18 September 2017).
NCT05061771 {published and unpublished data}
- NCT05061771. Nomacopan Therapy in Adult Patients With Bullous Pemphigoid Receiving Adjunct Oral Corticosteroid Therapy (ARREST-BP). clinicaltrials.gov/ct2/show/NCT05061771 (first received 30 September 2021).
References to studies awaiting assessment
ChiCTR‐IOR‐15007146 {published and unpublished data}
- ChiCTR-IOR-15007146. Combined treatment with low dose systemic steroids and topical steroids in bullous pemphigoid and factors of treatment-refractory bullous pemphigoid. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IOR-15007146 (first received 17 September 2015).
ChiCTR‐TRC‐12003592 {published and unpublished data}
- ChiCTR-TRC-12003592. Use of tripterygium glycosides and nicotinamide plus minocycline for the treatment of bullous pemphigoid: A multicenter, randomized and controlled clinincal trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-12003592 (first received 30 December 2012).
ChiCTR‐TRC‐12003593 {published and unpublished data}
- ChiCTR-TRC-12003593. Use of oral methotrexate plus glucocorticoid for the treatment of bullous pemphigoid: a multicenter, randomized and controlled clinical trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-12003593 (first received 30 December 2012).
EudraCT 2019‐001059‐37‐DE {published and unpublished data}
- EudraCT 2019-001059-37-DE. Double-blind, randomized, placebo-controlled trial of AKST4290 for adjunctive treatment of mild to moderate bullous pemphigoid. www.clinicaltrialsregister.eu/ctr-search/search?query=2019-001059-37 (accessed 24 May 2022).
NCT02313870 {published and unpublished data}
- NCT02313870. Topical steroids alone or associated with methotrexate in bullous pemphigoid (BP/MTX). clinicaltrials.gov/ct2/show/NCT02313870 (first received 10 December 2014).
References to ongoing studies
ChiCTR‐2000028707 {published and unpublished data}
- ChiCTR2000028707. Randomized, controlled clinical trial for low-dose interleukin-2 in the treatment of moderate to severe bullous pemphigoid. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000028707 (first received 31 December 2019).
ChiCTR‐TRC‐12003538 {published and unpublished data}
- ChiCTR-TRC-12003538. Use of inravenous methotrexate plus glucocorticoid for the treatment of bullous pemphigoid: A multicenter, randomized and controlled clinincal trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-12003538 (first received 27 December 2012).
EudraCT 2020‐002912‐34 {published and unpublished data}
- EudraCT 2020-002912-34. Treatment of bullous pemphigoid with avdoralimab (IPH5401), an anti-C5aR1 monoclonal antibody. www.clinicaltrialsregister.eu/ctr-search/trial/2020-002912-34/FR (first received 22 June 2020).
- NCT04563923. Treatment of bullous pemphigoid with avdoralimab (IPH5401), an anti-C5aR1 monoclonal antibody. clinicaltrials.gov/ct2/show/NCT04563923 (first received 25 September 2020).
NCT02365675 {published and unpublished data}
- NCT02365675. Wound dressings for pemphigus and pemphigoid. clinicaltrials.gov/ct2/show/NCT02365675 (first received 19 February 2015).
NCT04206553 {published and unpublished data}
- NCT04206553. A study to evaluate the efficacy and safety of dupilumab in adult patients with bullous pemphigoid (LIBERTY-BP). clinicaltrials.gov/ct2/show/NCT04206553 (first received 20 December 2019).
NCT04612790 {published and unpublished data}
- NCT04612790. A multinational, randomized, double-blind, parallel-group, placebo-controlled study to investigate the use of benralizumab as a treatment option for patients with bullous pemphigoid (FJORD). clinicaltrials.gov/ct2/show/NCT04612790 (first received 3 November 2020).
Additional references
Adam 1992
- Adam BA. Bullous diseases in Malaysia: Epidemiological and natural history. International Journal of Dermatology 1992;31(1):42-5. [DOI] [PubMed] [Google Scholar]
Ahluwalia 1998
- Ahluwalia A. Topical glucocorticoids and the skin--mechanisms of action: an update. Mediators of Inflammation 1998;7(3):183-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ahmed 1977
- Ahmed AR, Maize JC, Provost TT. Bullous pemphigoid: Clinical and immunologic follow-up after successful treatment. Archives of Dermatology 1977;113(8):1043-6. [DOI] [PubMed] [Google Scholar]
Alexandre 2022
- Alexandre M, Bohelay G, Gille T, Le Roux-Villet C, Soued I, Morin F, et al. Rapid disease control in first-line therapy-resistant mucous membrane pemphigoid and bullous pemphigoid with omalizumab as add-on therapy: a case series of 13 patients. Frontiers of Immunology 2022;13:874108. doi: 10.3389/fimmu.2022.874108. PMID: 35514989; PMCID: PMC9065717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allison 2000
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 2000;47:85-118. [DOI] [PubMed] [Google Scholar]
Amagai 2009
- Amagai M, Ikeda S, Shimizu H, Iizuka H, Hanada K, Aiba S, et al. A randomized double-blind trial of intravenous immunoglobulin for pemphigus. Journal of the American Academy of Dermatology 2009;60:595–603. [DOI] [PubMed] [Google Scholar]
Bastuji‐Garin 2011
- Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. Journal of Investigative Dermatology 2011;131(3):637–43. [DOI] [PubMed] [Google Scholar]
Bernard 1995
- Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Archives of Dermatology 1995;131(1):48-52. [PubMed] [Google Scholar]
Brick 2014
- Brick KE, Weaver CH, Savica R, Lohse CM, Pittelkowet MR, Boeve BF, et al. A population-based study of the association between bullous pemphigoid and neurologic disorders. Journal of the American Academy of Dermatology 2014;71(6):1191–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2022
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Frontiers of Immunology 2022;13:928621. DOI: 10.3389/fimmu.2022.928621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalmers 2009
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374(9683):86–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chen 2000
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Medicine 2000;6(7):797-801. [DOI] [PubMed] [Google Scholar]
Cochrane Skin Specialised Register 2022
- Cochrane Skin. More about the Cochrane Skin Specialised Register. skin.cochrane.org/search-methods (accessed 24 May 2022).
Colbert 2004
- Colbert RL, Allen DM, Eastwood D, Fairley JA. Mortality rate of bullous pemphigoid in a US medical centre. Journal of Investigative Dermatology 2004;122(5):1091-5. [DOI] [PubMed] [Google Scholar]
D'Agostino 2003
- D'Agostino RB Sr, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues –the encounters of academic consultants in statistics. Statistics in Medicine 2003;22(2):169–86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garg 1994
- Garg V, Jusko WJ. Bioavailability and reversible metabolism of prednisone and prednisolone in man. Biopharmaceutics and Drug Disposition 1994;15(2):163-72. [DOI] [PubMed] [Google Scholar]
Gehring 2021
- Gehring M, Wieczorek D, Kapp A, Wedi B. Potent anti-inflammatory effects of tetracyclines on human eosinophils. Frontiers in Allergy 2021;2:754501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giudice 1994
- Giudice GJ, Wilske KC, Anhalt GJ, Fairley JA, Taylor AF, Emery DJ, et al. Development of an ELISA to detect anti-BP180 autoantibodies in bullous pemphigoid and herpes gestationis. Journal of Investigative Dermatology 1994;102:878-81. [DOI] [PubMed] [Google Scholar]
Gudi 2005
- Gudi VS, White MI, Cruickshank N, Herriot R, Edwards SL, Nimmo F, et al. Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North-east Scotland. British Journal of Dermatology 2005;153(2):424-7. [DOI] [PubMed] [Google Scholar]
Hadi 1988
- Hadi SM, Barnetson RS, Gawkrodger DJ, Saxena U, Bird P, Merrett TG. Clinical, histological and immunological studies in 50 patients with bullous pemphigoid. Dermatologica 1988;176(1):6-17. [DOI] [PubMed] [Google Scholar]
Hall 2013
- Hall RP, Streilein RD, Hannah DL, McNair PD, Fairley JA, Ronaghy A, et al. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. Journal of Investigative Dermatology 2013;133(12):2786-8. [NCT00286325] [DOI] [PubMed] [Google Scholar]
Hertl 2008
- Hertl M, Zillikens D, Borradori L, Bruckner-Tuderman L, Burckhard H, Eming R, et al. Recommendations for the use of rituximab (anti-CD20 antibody) in the treatment of autoimmune bullous skin diseases. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2008;6(5):366-73. [DOI: DOI: 10.1111/j.1610-0387.2007.06602.x] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. Available from www.cochrane-handbook.org.
Higgins 2022
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Ishiura 2008
- Ishiura N, Fujimoto M, Watanabe R, Nakashima H, Kuwano Y, Yazawa N, et al. Serum levels of IgE anti-BP180 and anti-BP230 autoantibodies in patients with bullous pemphigoid. Journal of Dermatological Science 2008;49(2):153-61. DOI: 10.1016/j.jdermsci.2007.08.008. Epub 2007 Oct 24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Jin 1993
- Jin P, Shao C, Ye G. Chronic bullous dermatoses in China. International Journal of Dermatology 1993;32(2):89-92. [DOI] [PubMed] [Google Scholar]
Jolles 1998
- Jolles S, Hughes J, Whittaker S. Dermatological uses of high-dose intravenous immunoglobulin. Archives of Dermatology 1998;134:80-6. [DOI] [PubMed] [Google Scholar]
Joly 2010 [pers comm]
- Personal communication. Email to P Joly clarifying the published report 30 January 2010.
Joly 2012
- Joly P, Baricault S, Sparsa A, Bernard P, Bédane C, Duvert-Lehembre S, et al. Incidence and mortality of bullous pemphigoid in France. Journal of Investigative Dermatology 2012;132:1998-2004. [DOI] [PubMed] [Google Scholar]
Kirtschig 1994
- Kirtschig G, Wojnarowska F, Marsden RA, Edwards S, Bhogal B, Black MM. Acquired bullous disease of childhood: Re-evaluation of diagnosis by indirect immunofluorescence examination on 1 M NaCl split skin and immunoblotting. British Journal of Dermatology 1994;130(5):610-6. [DOI] [PubMed] [Google Scholar]
Kremer 2019
- Kremer N, Snast I, Cohen ES, Hodak E, Mimouni D, Lapidoth M, et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. American Journal of Clinical Dermatology 2019;20(2):209-21. [DOI] [PubMed] [Google Scholar]
Kubin 2017
- Kubin ME, Hellberg L, Palatsi R. Glucocorticoids: The mode of action in bullous pemphigoid. Experimental Dermatology 2017;26(12):1253-60. [DOI] [PubMed] [Google Scholar]
Langan 2008
- Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: population based cohort study. BMJ 2008;337:a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lebrun‐Vignes 1999
- Lebrun-Vignes B, Roujeau JC, Bernard P, Delaporte E, Joly P, Prost C, et al. Prednisone is more effective than prednisolone metasulfobenzoate in the treatment of bullous pemphigoid. Archives of Dermatology 1999;135(1):89-90. [DOI] [PubMed] [Google Scholar]
Lever 1953
- Lever WF. Pemphigus. Medicine 1953;32(1):1-123. [DOI] [PubMed] [Google Scholar]
Liang 2023
- Liang J, Abulikemu K, Maolidan, Hu F, Zhao J, Qiu Y, et al. Nine cases of refractory bullous pemphigoid treated with dupilumab and literature review. International Immunopharmacology 2023;116:109788. DOI: 10.1016/j.intimp.2023.109788. Epub 2023 Feb 1. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lloyd‐Lavery 2013
- Lloyd-Lavery A, Chi CC, Wojnarowska F, Taghipour K. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatology 2013;149:58–62. [DOI] [PubMed] [Google Scholar]
Logan 1987
- Logan RA, Bhogal B, Das AK, McKee PM, Black MM. Localization of bullous pemphigoid antibody - An indirect immunofluorescence study of 228 cases using a split-skin technique. British Journal of Dermatology 1987;117(4):471-8. [DOI] [PubMed] [Google Scholar]
Mason 2017
- Mason JM, Chalmers JR, Godec T, Nunn AJ, Kirtschig G, Wojnarowska F, et al. Doxycycline compared to prednisolone therapy for patients with bullous pemphigoid: cost-effectiveness analysis of the BLISTER trial. British Journal of Dermatology 2017;178(2):415-23. [DOI: 10.1111/bjd.16006] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miyamoto 2019
- Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. Anais Brasileiros de Dermatologia 2019;94:133-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammadi 2022
- Mohammadi O, Kassim TA. Azathioprine. www.statpearls.com/ArticleLibrary/viewarticle/18065 (Updated 8 May 2022). [PMID: 31194347]31194347
Morrison 1990
- Morrison LH, Diaz L, Anhalt GJ. Bullous pemphigoid. In: Wojnarowska F, Briggaman RN (Editors), editors(s). Management of Blistering Diseases. London, UK: Chapman and Hall Medical, 1990:63-82. [Google Scholar]
Murrell 2012
- Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. Journal of the American Academy of Dermatology 2012;66(3):479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nemeth 1991
- Nemeth AJ, Klein AO, Gould EW, Schachner LA. Childhood bullous pemphigoid. Clinical and immunologic features, treatment, and prognosis. Archives of Dermatology 1991;127(3):378-86. [DOI] [PubMed] [Google Scholar]
Ogino 2022
- Ogino MH, Tadi P. Cyclophosphamide. www.statpearls.com/ArticleLibrary/viewarticle/20199 (Updated 30 April 2022). [PMID: 31971727]31971727
Orange 1989
- Orange AP, Joost T. Pemphigoid in children. Pediatric Dermatology 1989;6(4):267-74. [DOI] [PubMed] [Google Scholar]
Parker 2008
- Parker SR, Dyson S, Brisman S, Pennie M, Swerlick RA, Khan R, et al. Mortality of bullous pemphigoid: An evaluation of 223 patients and comparison with the mortality in the general population in the United States. Journal of the American Academy of Dermatology 2008;59(4):582-8. [DOI] [PubMed] [Google Scholar]
Person 1977
- Person JR, Rogers RS. Bullous and cicatricial pemphigoid: Clinical, pathological, and immunologic collerations. Mayo Clinic Proceedings 1977;52:54-66. [PubMed] [Google Scholar]
Persson 2021
- Persson MS, Harman KE, Vinogradova Y, Langan SM, Hippisley-Cox J, Thomas KS, et al. Incidence, prevalence and mortality of bullous pemphigoid in England 1998-2017: a population-based cohort study. British Journal of Dermatology 2021;184(1):68-77. [DOI] [PubMed] [Google Scholar]
Pfütze 2007
- Pfütze M, Niedermeier A, Hertl M, Eming R. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. European Journal of Dermatology 2007;17:4–11. [DOI] [PubMed] [Google Scholar]
Prinsen 2016
- Prinsen CAC, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials 2016;17(1):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pudar Hozo 2005
- Pudar Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reguiaï 2009
- Reguiaï Z, Tchen T, Perceau G, Bernard P. Efficacy of rituximab in a case of refractory bullous pemphigoid [Efficacite du rituximab dans une pemphigoide bulleuse en echec therapeutique.]. Annales de Dermatologie et de Venereologie 2009;136(5):431-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rollin 1993
- Rollin C, Chosidow O, Diquet B, Dutreuil C, Herson S, Revuz J, et al. Comparative study of availability of prednisolone after intestinal infusion of prednisolone metasulfobenzoate and prednisone. European Journal of Clinical Pharmacology 1993;44(4):395-9. [DOI] [PubMed] [Google Scholar]
Roujeau 1998
- Roujeau JC, Lok C, Bastuji-Garin S, Mhalla S, Enginger V, Bernard P. High risk of death in elderly patients with extensive bullous pemphigoid. Archives of Dermatology 1998;134(4):465-9. [DOI] [PubMed] [Google Scholar]
Russo 2020
- Russo R, Cozzani E, Gasparini G, Parodi A. Targeting interleukin 4 receptor α: A new approach to the treatment of cutaneous autoimmune bullous diseases? Dermatol Ther 2020;33:e13190; doi: 10.1111/dth.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savin 1979
- Savin JA. The events leading to the death of patients with pemphigus and pemphigoid. British Journal of Dermatology 1979;101(5):521-34. [DOI] [PubMed] [Google Scholar]
Savin 1987
- Savin JA. Death in bullous pemphigoid. Clinics in Dermatology 1987;5(1):52-9. [DOI] [PubMed] [Google Scholar]
Schulze 2015
- Schulze F, Neumann K, Recke A, Zillikens D, Linder R, Schmidt E. Malignancies in pemphigus and pemphigoid diseases. Journal of Investigative Dermatology 2015;135(5):1445-7. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.htm.
Seyed Jafari 2021
- Seyed Jafari SM, Feldmeyer L, Bossart S, Simon D, Schlapbach C, Borradori L. Case report: combination of omalizumab and dupilumab for recalcitrant bullous pemphigoid. Frontiers of Immunology 2021;11:611549. DOI: 10.3389/fimmu.2020.611549. PMCID: PMC7879677. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stendahl 1977
- Stendahl O, Dahlgren C. The inhibition of polymorphonuclear leukocyte cytotoxicity by dapsone; a possible mechanism in the treatment of dermatitis herpetiformis. Journal of Clinical Investigation 1977;62(1):214-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taghipour 2010
- Taghipour K, Chi CC, Vincent A, Groves RW, Venning V, Wojnarowska F. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case-control study. Archives of Dermatology 2010;146:1251–54. [DOI] [PubMed] [Google Scholar]
Tapia 2021
- Tapia C, Nessel TA, Zito PM. Cyclosporine. www.statpearls.com/ArticleLibrary/viewarticle/20202 (Updated 11 November 2021). [PMID: 29494057]29494057
Tham 1998
- Tham SN, Thirumoorthy T, Rajan VS. Bullous pemphigoid: Clinico-epidemiological study of patients seen at a Singapore skin hospital. Australasian Journal of Dermatology 1998;25(2):68-72. [DOI] [PubMed] [Google Scholar]
Thomas 2017
- Thomas KS, Brindle R, Chalmers JR, Gamble B, Francis NA, Hardy D, et al. Identifying priority areas for research into the diagnosis, treatment and prevention of cellulitis (erysipelas): results of a James Lind Alliance Priority Setting Partnership. British Journal of Dermatology 2017;177:541-3. [DOI] [PubMed] [Google Scholar]
Van Beek 2017
- Van Beek N, Lüttmann N, Huebner F, Recke A, Karl I, Schulze FS, et al. Correlation of serum levels of IgE autoantibodies against BP180 with bullous pemphigoid disease activity. JAMA Dermatology 2017;153(1):30-38. Erratum in: JAMA Dermatology 2017; 153(7):729. [DOI: 10.1001/jamadermatol.2016.3357] [PMID: ] [DOI] [PubMed] [Google Scholar]
Venning 1992
- Venning VA, Wojnarowska F. Lack of predictive factors for the clinical course of bullous pemphigoid. Journal of the American Academy of Dermatology 1992;26(4):585-9. [DOI] [PubMed] [Google Scholar]
Wijayanti 2017
- Wijayanti A, Zhao CY, Boettiger D, Chiang YZ, Ishii N, Hashimoto T, et al. The reliability, validity and responsiveness of two disease scores (BPDAI and ABSIS) for bullous pemphigoid: Which one to use? Acta Dermato-venereologica 2017;97:24-31. [DOI] [PubMed] [Google Scholar]
Williams 2015
- Williams HC, Burden-Teh E, Nunn AJ. What Is a pragmatic clinical trial? Journal of Investigative Dermatology 2015;135:e33. [DOI] [PubMed] [Google Scholar]
Wojnarowska 1998
- Wojnarowska F, Eady RA, Burge SM. Bullous pemphigoid. In: Champion RH, Burton JL, Burns DA, Breathnach SM, editors(s). Textbook of Dermatology. 6th edition. Vol. 3. Oxford, UK: Blackwell Science Ltd, 1998:1866-73. [Google Scholar]
Wojnarowska 2002
- Wojnarowska F, Kirtschig G, Highet AS, Venning VA, Khumalo NP. Guidelines for the management of bullous pemphigoid. British Journal of Dermatology 2002;147(2):214-21. [DOI] [PubMed] [Google Scholar]
Wozel 1997
- Wozel G, Blasum C, Winter C, Gerlac B. Dapsone hydroxylamine inhibits the LTB4-induced chemotaxis of polymorphonuclear leukocytes into human skin: Results of a pilot study. Inflammation Research 1997;46:420. [DOI] [PubMed] [Google Scholar]
Yrjanheikki 1999
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proceedings of the National Academy of Sciences of the United States of America 1999;96(23):13496-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2021
- Zhang Y, Xu Q, Chen L, Chen J, Zhang J, Zou Y, et al. Efficacy and safety of dupilumab in moderate-to-severe bullous pemphigoid. Frontiers of Immunology 2021;12:738907. DOI: 10.3389/fimmu.2021.738907. PMID: 34721404; PMCID: PMC855203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zillikens 1995
- Zillikens D, Wever S, Roth A, Weidenthaler-Barth B, Hashimoto T, Bröcker EB. Incidence of autoimmune subepidermal blistering dermatoses in a region of central Germany (Comment). Archives of Dermatology 1995;131(1):48-52. [DOI] [PubMed] [Google Scholar]
Zimmermann 1999
- Zimmermann R, Faure M, Claudy A. Prospective study of treatment of bullous pemphigoid by a class I topical corticosteroid. Annales de Dermatologie et de Vénéréologie 1999;126(1):13-6. [PubMed] [Google Scholar]
Zimmermann 2018
- Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides—a systematic review of the underlying mechanisms. Frontiers in Immunology 2018;9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Khumalo 2002
- Khumalo NP, Murrell DF, Wojnarowska F, Kirtschig G. A systematic review of treatments for bullous pemphigoid. Archives of Dermatology 2002;138(3):385-9. [DOI] [PubMed] [Google Scholar]
Khumalo 2005
- Khumalo N, Kirtschig G, Middleton P, Hollis S, Wojnarowska F, Murrell DF. Interventions for bullous pemphigoid. Cochrane Database of Systematic Reviews 2005, Issue 3. Art. No: CD002292. [DOI: 10.1002/14651858.CD002292.pub3] [DOI] [PubMed] [Google Scholar]
Kirtschig 2010
- Kirtschig G, Middleton P, Bennett C, Murrell DF, Wojnarowska F, Khumalo NP. Interventions for bullous pemphigoid. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD002292. [DOI: 10.1002/14651858.CD002292.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


