Abstract
Objectives:
To describe the timing of tuberculosis (TB) presentation in relation to diagnosis of HIV infection and ART initiation and to evaluate whether the established impact from late presentation to care (LP) and late initiation (LI) of ART on the risk of TB is retained beyond the observation period of clinical trials.
Design:
We used marginal structural models to emulate a clinical trial with up to 5 years of follow-up to evaluate the impact of LI on TB risk.
Methods:
PLWH were enrolled from 2007–2016 in observational cohorts from Uganda, Peru, Mexico and Italy. The risk of TB was compared in LP (accessing care with CD4≤350 cells/μL) vs non-LP using survival curves and a weighted Cox regression. We emulated two strategies: initiating ART with CD4 count <350 cells/μL vs. CD4 count ≥350 cells/μL (LI). We estimated TB attributable risk and population attributable fraction up to 5 years from the emulated date of randomization.
Results:
20,112 patients and 1,936 TB cases were recorded. Over 50% of TB cases were diagnosed at presentation for HIV care. More than 50% of the incident cases of TB after ART initiation were attributable to LP; nearly 70% of TB cases during the first year of follow-up could be attributed to LP and more than 50%, five years after first attending HIV care.
Conclusions:
LP accounted for a large share of TB cases. Delaying ART initiation was detrimental for incident TB rates, and the impact of LP persisted up to 5 years from HIV care entry.
Keywords: Delayed ART initiation, HIV, Tuberculosis, co-infection, low- and middle-income countries
Introduction
HIV infection, is a major determinant of the risk for developing active tuberculosis (TB). It has been estimated that incidence of TB among persons living with HIV (PLWH), is 20 to 37 times higher than among HIV uninfected, depending on the local characteristics of the HIV epidemic [1].
Addressing TB-HIV coinfection is a tenet of the World Health Organization (WHO) strategy, which aims to end the global TB epidemic [2]. Scaling up and accelerating the initiation of antiretroviral therapy (ART) for PLWH is a central intervention in this context. ART reduces the risk of developing TB by 65–84%, both in low and high TB burden countries and less advanced HIV disease at time of ART initiation correlates with a greater protective effect of treatment [3,4]. A mathematical model has predicted that up to 98% of cases of TB attributable to HIV infection could be averted in high-burden countries by providing ART to all PLWH within one year of seroconversion [5].
In the past decade, we have witnessed an impressive scale-up of ART and an improvement in the timeliness of ART initiation. According to the UNAIDS estimate, by the end of 2020, 84% of all PLWH knew their status, 73% were on ART, and 66% had undetectable viral load [6]. In parallel, age-standardized incidence of TB decreased annually by 4% from 2006 to 2016 among PLWH, while the reduction recorded among HIV-negative individuals occurred at a slower rate (–1.3% per year) [7].
However, late presentation to care represents a 50% or more of those entering to care, contributing to AIDS-defining events and AIDS-related mortality [8, 9]. Therefore, TB-HIV coinfection remains a public health priority. It is estimated that in 2018, of the 10 million cases of TB which occurred globally, 860,000 were in persons with HIV. TB caused 250,000 deaths among PLWH, nearly one-third of all HIV-related mortality [10]. This may reflect both insufficient ART coverage and its late initiation. In addition, several studies suggest that PLWH successfully treated with ART may remain at increased risk of TB as compared to HIV-negative individuals [11].
Although we know that lower CD4 count is associated with a higher risk of TB and early ART reduces the risk of TB, the proportion of TB cases attributable to late presentation and late ART initiation have not been clearly quantified [12,13]. In this work, we aimed to use real-world data, collected in the observational setting in large HIV cohorts with long follow-up from four different countries, to describe the timing of TB presentation in relation to diagnosis of HIV infection and ART initiation, and to estimate the long-term impact (up to 5 years) of late presentation for HIV care and of delayed ART initiation on the risk of TB using a counterfactual prediction framework.
Methods
Our study population included PLWH enrolled in observational cohorts in four countries: Uganda (IDI: Infectious Disease Institute), Peru (IMTAvH: Instituto de Medicina Tropical von Humboldt) Mexico (INCMNSZ: Instituto Nacional de Ciencias Médicas y Nutrición, Salvador-Zubirán); and Italy (Icona: Italian Cohort Naive Antiretroviral) were included. The Italian site is a multi-center cohort while the other sites are mono-center institutions [14–16]. Institutional ethics review boards from each participating site reviewed and approved the project. Informed consent process was made at enrollment for Peru, Italy and Ugandás cohorts; and waived at Mexico site, because ethical regulations allow analysis of de-identified clinical data.
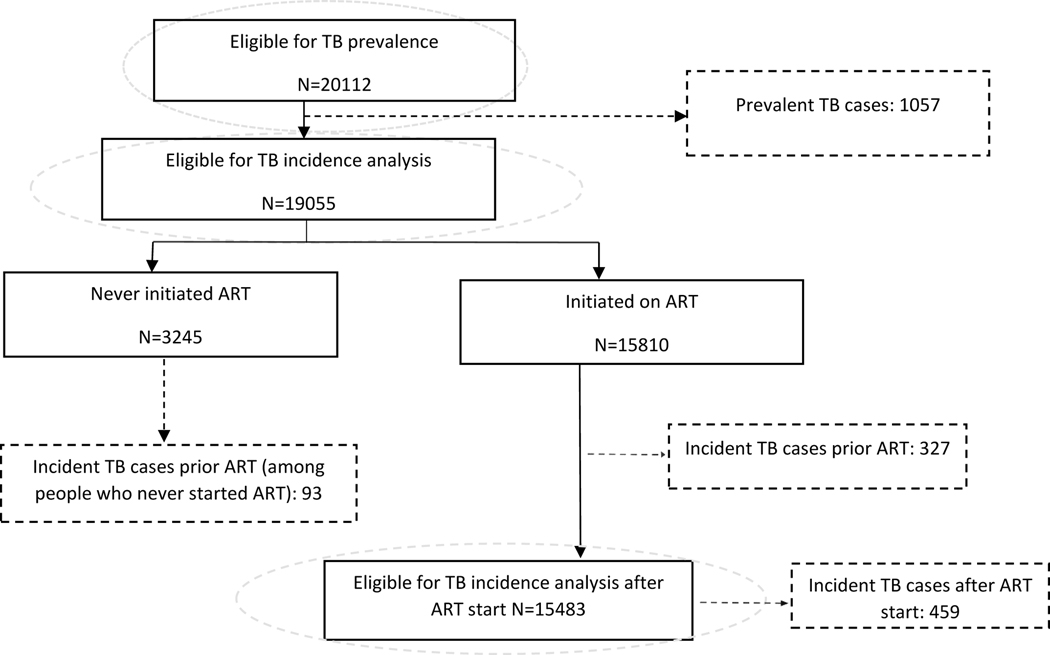
We included patients over the period from 2007 to 2016 who had an HIV diagnosis/initiation of HIV care within 3 months prior to the date of enrolment (baseline) in the cohorts, and had an available measure of CD4 count at baseline. We excluded patients who reported a TB episode or were on ART for longer than 3 months prior to enrollment. CD4 count was defined as the closest measurement to baseline in the time window −90; +180 days. The window −90; +90 days of the date of starting ART was used to define CD4 count at ART. A prevalent TB case was defined if a participant was diagnosed over the time window −90; +30 days of baseline. We also estimated the incidence of new TB cases after enrolment and the incidence after ART initiation, among patients who had ≥1follow-up clinical visit after baseline and did not have prevalent TB. An incident TB case before ART was defined as a newly diagnosed TB case after 1 month of enrolment but before the date of ART initiation. All TB cases newly diagnosed after the date of ART initiation were included as incident cases after ART. Distribution of eligible patients for each analysis: TB prevalence, TB incidence prior ART and TB incidence after ART; is shown in the flow diagram (Figure 1). TB incidence rates before and after ART were calculated overall and by cohort.
Figure 1.
Flow diagram with the number of patients included in each of TB analyses.
Estimation of attributable risk and population attributable fraction
We used attributable risk (AR) and population attributable fraction (PAF) to measure the impact of late presentation on the risk of TB incidence, among late presenters (LP, those with CD4 count <350 cells/μL at baseline) and among the whole study population. The AR was measured to account for the difference in the probability of developing TB between LP and non-LP [17,18] (Supplementary_Material_1). In addition, the PAF was calculated after accounting for the prevalence of LP (pLP) in the study population. These two risk estimates were used to measure the impact of late ART initiation (defined as starting ART with a CD4 count<350 cells/μL) on the risk of incident TB overall and in LP over time from enrollment. The probabilities p1(t) and p0(t), included in the ratios were estimated using dynamic marginal structural models. We aimed to provide 1, 3 and up to 5 years estimates for AR and PAF.
Estimation of models
Impact of late presentation on incident TB before ART
For the survival analysis of the causal effect of being a LP on the risk of TB incidence before ART, inverse probability weighting (IPW) of being LP were calculated using the following time-fixed patients’ characteristics: gender, age, cohort, educational level and calendar year. We estimated the AR and the PAF using the probabilities of TB estimated in LP and non-LP groups.
Impact of late presentation on incident TB after ART
We also estimated the impact of late presentation on TB after ART using a marginal structural model. We compared the risk of TB after ART initiation in a pooled logistic model among LP versus non-LP, using IPW from three models. The first model accounts for censoring from the study due to death or last visit recorded, the second for the ART initiation, and the last one for being a LP.
Impact of late initiation of ART on incident TB
A dynamic marginal structural model was used to emulate a clinical trial designed to answer the question ‘when best to start ART according to current CD4 count’. Two strategies were compared: starting ART immediately at any CD4 >350 (non-LI strategy) versus starting ART only after CD4 had dropped ≤350 (LI strategy). We made a copy of every patient to account for the time each one contributed to both strategies, using the so-called method of ‘cloning and censoring’ or the ‘doppelganger method’ [19]. We used a grace period of 3 months after the CD4 count declined below 350 to allow variation in CD4 count monitoring practices across studies [20]. An example of the artificial censoring created by the procedure is shown in Supplementary Figure 1 (Supplementary_Material_1). Inverse probability of censoring weights (using the same set of covariates previously mentioned and splines for continuous variables) was used to maintain the conditional exchangeability. We estimated the risk of developing TB after following each of the treatment strategies using Cox regression model. Variables in this model were selected following the Dagitty Acyclic Graph (Supplementary_Figure 2). The estimates from this model were used to calculate the AR and PAF. Hazard ratios for TB in LI vs. non-LI were estimated by introducing an interaction term between time and the strategy of ART initiation in the pooled logistic regression model. Bootstrap with 200 replications was used to calculate the 95% confidence intervals for AR and PAF.
Results
General characteristics of study population.
A total of 20,112 PLWH were included in the analysis; 10,822 (54%) from Uganda, 5,827 (29%) from Italy, 2,898 (14%) from Peru and 565 (3%) from Mexico. Overall, the majority of patients (56%) were male, and (53%) aged between 19 and 35 years, 14% had primary schooling, and 41% a CD4 count < 200cells/μL. Median follow-up was 2.91 years (IQR: 0.69 − 5.62). Characteristics of the study population by cohort are shown in Table 1.
Table 1.
Main characteristics of the study population
| Italy | Mexico | Peru | Uganda | Total | *p-value | |
|---|---|---|---|---|---|---|
| N= 5827 | N= 565 | N= 2898 | N= 10822 | N= 20112 | ||
|
| ||||||
| Gender, n(%) | <·001 | |||||
|
| ||||||
| Male | 4656 (79·9%) | 512 (90·6%) | 2197 (75·8%) | 3928 (36·3%) | 11293 (56·2%) | |
|
| ||||||
| Age, years n(%) | <·001 | |||||
|
| ||||||
| 0–18 | 6 (0·1%) | 1 (0·2%) | 58 (2·1%) | 115 (1·1%) | 180 (0·9%) | |
| 19–35 | 2246 (40·0%) | 324 (59·6%) | 1649 (59·5%) | 5895 (57·6%) | 10114 (52·7%) | |
| 35–55 | 2736 (48·7%) | 189 (34·7%) | 906 (32·7%) | 3878 (37·9%) | 7709 (40·2%) | |
| 56+ | 631 (11·2%) | 30 (5·5%) | 158 (5·7%) | 353 (3·4%) | 1172 (6·1%) | |
|
| ||||||
| Level of education, n(%) | <·001 | |||||
|
| ||||||
| Primary | 323 (5·5%) | 17 (3·0%) | 227 (7·8%) | 2207 (20·4%) | 2774 (13·8%) | |
| Secondary | 2724 (46·7%) | 277 (49·0%) | 1447 (49·9%) | 1845 (17·0%) | 6293 (31·3%) | |
| Tertiary | 730 (12·5%) | 264 (46·7%) | 1175 (40·5%) | 649 (6·0%) | 2818 (14·0%) | |
| Unknown | 2050 (35·2%) | 7 (1·2%) | 49 (1·7%) | 6121 (56·6%) | 8227 (40·9%) | |
|
| ||||||
| Mode of HIV transmission, n(%) | <·001 | |||||
|
| ||||||
| MSM | 2602 (44·7%) | 392 (69·4%) | 1180 (40·7%) | 0 (0·0%) | 4174 (20·8%) | |
| Heterosexual contacts | 0 (0·0%) | 138 (24·4%) | 1656 (57·1%) | 0 (0·0%) | 1794 (8·9%) | |
| Other | 3225 (55·3%) | 35 (6·2%) | 62 (2·1%) | 0 (0·0%) | 3322 (16·5%) | |
| Unknown | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) | 10822 (100·0%) | 10822 (53·8%) | |
|
| ||||||
| CD4 count, cells/μL n(%) | <·001 | |||||
|
| ||||||
| 0–200 | 2012 (34·5%) | 329 (58·2%) | 1484 (51·2%) | 4376 (40·4%) | 8201 (40·8%) | |
| 201–350 | 1205 (20·7%) | 111 (19·6%) | 623 (21·5%) | 2203 (20·4%) | 4142 (20·6%) | |
| > 350 | 2610 (44·8%) | 125 (22·1%) | 791 (27·3%) | 4243 (39·2%) | 7769 (38·6%) | |
|
| ||||||
| Calendar year of enrolment, n(%) | <·001 | |||||
|
| ||||||
| 2007–2009 | 633 (10·9%) | 122 (21·6%) | 694 (23·9%) | 4248 (39·3%) | 5697 (28·3%) | |
| 2010–2012 | 1750 (30·0%) | 165 (29·2%) | 810 (28·0%) | 4450 (41·1%) | 7175 (35·7%) | |
| 2013–2016 | 3444 (59·1%) | 278 (49·2%) | 1394 (48·1%) | 2124 (19·6%) | 7240 (36·0%) | |
|
| ||||||
| Follow-up time, years | <·001 | |||||
|
| ||||||
| Median (IQR) | 3·83 (2·17, 6·22) | 4·50 (1·86, 6·86) | 2·66 (0·58, 5·76) | 2·07 (0·38, 5·17) | 2·91 (0·69, 5·62) | |
Chi-square test. MSM: Men who have sex with men, Other includes perinatal transmission, transfusion and injecting drug user. n: Number of patients, IQR: interquartile range.
Distribution of TB cases relative to enrollment and ART treatment time
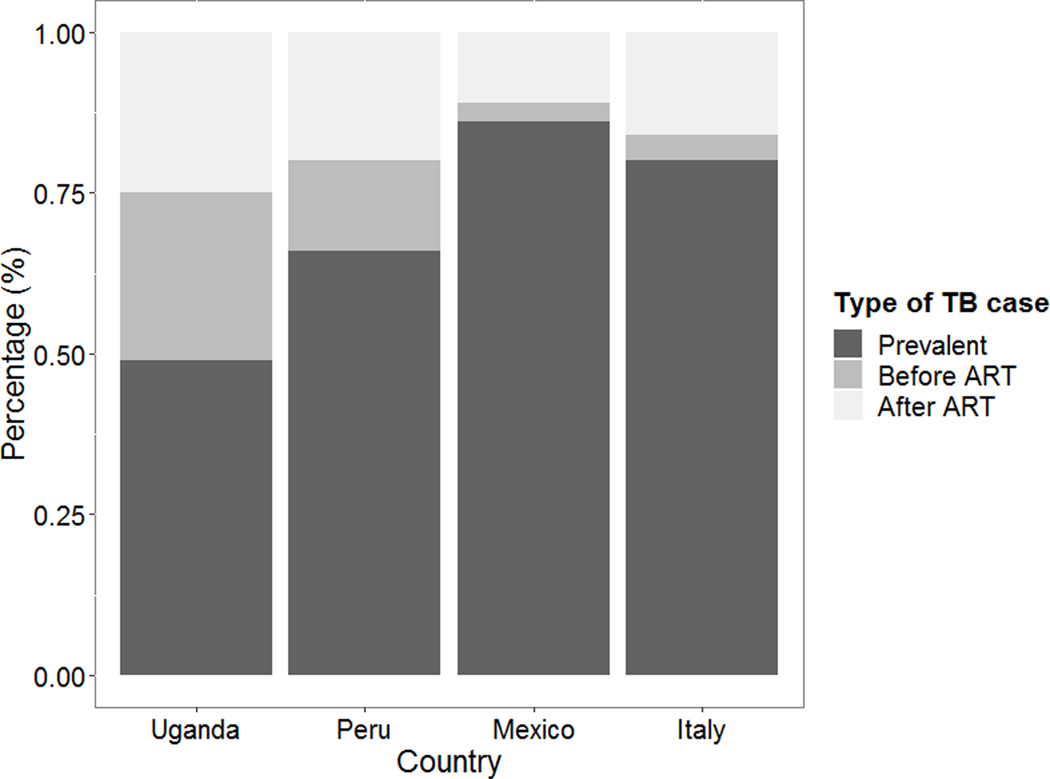
A total of 1,936 TB cases were reported: 1,412 (73%) from Uganda, 364 (19%) from Peru, 102 (5%) from Italy; and 58 (3%) from Mexico. Most of these TB cases were prevalent cases (1057, 55%), while the remaining were incident cases: 420 (21%) occurred before ART initiation, and 459 (24%) after ART initiation. In Italy and Mexico, more than 80% of the cases were prevalent cases, while lower proportions were seen in Peru and Uganda. The distribution of TB cases by time of presentation and by country is shown in Figure 2.
Figure 2.
Percentage of TB cases by time of presentation and country.
Estimated Incident TB before ART and after ART
Four-hundred and twenty newly diagnosed TB cases were observed in participants who were still ART-naïve. Overall, estimated incidence of TB before ART was 23.0 cases per 1,000-PYFU (95%CI: 20.9 −25.3); 327 (78%) of these diagnoses, occurred before ART initiation and 93 (22%) among patients who never started ART. The highest incidence was seen in Uganda (29.5 cases per 1000-PYFU) and the lowest in Italy (0.06 cases per 1000-PYFU).
In total, 15,180 patients initiated ART; of those, 93 (1%) initiated ART before they were enrolled, and 1,355 (9%) the same day of enrollment. Among the remaining 90% (n=13,732) the median time to initiation was 60 days (IQR: 22−280) and the median CD4 count at ART initiation was 215 cells/μL (IQR: 85−374). Characteristics of the population starting ART by cohort are included in the Supplementary Table 1; and survival analysis of the causal effect of being a LP on the risk of TB incidence before ART, in Supplementary Figure 3 (Supplementary_Material_1). There were 459 TB cases that occurred after the date of ART initiation, and overall incidence was estimated to be 8.77 per 1000-PYFU (95%CI: 8.0 − 9.61) . TB incidence rates after stratifying by cohort, current CD4 count categories (0–200 cells/μL, 201–350 cells/μL and ≥350 cells/μL) and by length of time since baseline (0–4; 4–12 and >12 months) are also shown as Supplementary material.
Impact of late presentation on incident TB before ART
19,055 patients (95% of total) where included in this analysis who didńt have a TB diagnosis at the time of enrollment; the main characteristics of these patients by cohort are shown in Supplementary Table 2 (Supplementary_Material_1). Among these, 11,371 (59%) had a CD4 count <350 cells/μL at baseline and were classified as LP. There was a total of 420 TB cases diagnosed prior to ART initiation; 284 (68%) of these, were in the LP group. The cumulative risk for developing TB was 27.4% (95%CI: 17−36) vs. 8.0% (95%CI: 7−10) in the LP vs. non-LP group. From fitting a weighted Cox regression model, the adjusted hazard ratio of TB incidence before ART was 4.94 (95%CI: 4.27−5.71) in LP compared to the non-LP participants. Probability of incident TB cases after ART among LP and non-LP is shown in Supplementary Figure 3 (Supplementary_Material_1). Among LP, the AR estimated for late presentation at 1 year after enrolment was 81% (95%CI: 75–87). The PAF among the whole population for LP was 72% (95%CI: 64–80). These figures decreased slightly with longer time from enrolment but the difference persisted up to 5 years from HIV care entry (Table 2).
Table 2.
Attributable risk and population attributable fraction of tuberculosis due to late presentation for care, before and after initiation of antiretroviral therapy.
| Attributable Risk of TB for late presenters | Population Attributable fraction of TB | |||
|---|---|---|---|---|
| Percentage (95%CI) | Percentage (95%CI) | |||
|
| ||||
| Time after enrollment | before ART initiation | after ART initiation | before ART initiation | after ART initiation |
| One year | 81 (75 ─ 87) | 68 (61 – 79) | 72 (64 – 80) | 57 (49 – 70) |
| Three years | 76 (70 ─ 82) | 68 (61 −79) | 66 (58 – 73) | 56 (48 – 70) |
| Five years | 69 (60 ─ 79) | 67 (60 – 78) | 58 (46 – 69) | 56 (48 – 69) |
ART= antiretroviral therapy; CI =confidence interval
Impact of late ART initiation on incident TB
In total, 7,684 non-LP individuals at baseline were included in this final analysis aiming to estimate the causal effect of initiating ART immediately vs. initiating when the CD4 count fell <350 cells/μL. The characteristics of the individuals in this analysis are shown in Supplementary Table 3 (Supplementary_Material_1). Overall, 2,322 (30%) individuals remained ART-naïve, 4,607 (60%) initiated ART while having a CD4 count above 350 cells/μL (non-LI) and 755 (9.8%) initiated ART after CD4 count dropped below 350 cells/μL (LI). A total of 195 incident TB diagnosed cases were observed. Of these cases, 34 (17.4%) were recorded among participants who never initiated ART; in 14 of these (38%) current CD4 count was ≤350 cells/μl. The remaining 161 (82.6 %) TB cases occurred among people initiating ART, 87 of them among non-LI and 74 among LI. The adjusted hazard ratio of having TB from fitting a weighted Cox regression model comparing non-LI with LI was 0.54 (95%CI: 0.23−1.26). Among LI, the AR for LI by 1, 3 and 5 years were −3% (95%CI: −18−14), 21% (95%CI: 1−39) and 31% (95%CI: 5−48) respectively. PAF for late initiation of ART among non-LP were −2% (95%CI: −9 − 8); 15% (95%CI: 1−30) and 26% (95%CI: 9−59), by 1, 3 and 5 years respectively. Adjusted survival probability of TB incidence after ART initiation for LI and non-LI is shown in Supplementary Figure 4 (Supplementary_Material_1).
Discussion
Our analyses of TB in PLWH enrolled in four countries with different burden of TB and HIV, showed that over 50% of TB cases were diagnosed at presentation for HIV care. Prevalent cases were particularly frequent in Mexico and Italy. Our data confirms that more than 50% of the incident cases of TB occurring either before or after ART initiation are attributable to late presentation for HIV care. Indeed, our data replicated the results of randomized studies, but also extended the observation up to 5 years from HIV care entry. Our data are also useful to inform stochastic models of the HIV-TB epidemic in resource-limited countries. In contrast, there was little evidence that late initiation of ART among non-late presenters was a major determinant of TB risk in this population.
Our findings are consistent with previous data describing TB occurrence in PLWH in sub-Saharan Africa and high-income countries [21–24]. However, the proportion of prevalent TB cases differed by cohort, and was inversely associated with TB incidence in the country (the lower the proportion of prevalent TB, the higher the incidence). Twenty percent of the TB cases in our study occurred in persons already in care who were not yet receiving ART. We estimated that 70% of cases occurring in this population during the first year of follow-up could be attributed to late presentation and, although this fraction diminished with time from enrolment, it was still above 50% five years after initiation of HIV care. We think that the following factors may explain the significant contribution of late ART initiation to TB occurrence: a) in our cohort, 40% of patients were enrolled prior 2011, and 47% of TB cases which occurred before ART initiation were enrolled before 2011. Before 2011, there was little evidence of the benefit of ART initiation while on TB treatment [25]. b) we have documented that time to ART initiation started to decrease up to 2013 in Latin America [26] and up to 2016 in Africa [27], while the proportion of late ART initiation is still high after those years, it may be related to a slow the introduction of the universal ART initiation criteria regardless of CD4 cell counts.
In our study, approximately 75% of patients started ART during follow up and, consistent with previous studies, estimated incidence of TB decreased dramatically during the course of ART in all cohorts. Nonetheless, after ART initiation the risk of TB remained approximately three times higher, for those who presented late to HIV care, compared to non-LP even after controlling for most recent CD4 [3]. Similar figures were recently reported in a meta-analysis for Ethiopia [12]. Additionally, we estimated that more than 50% of cases occurring after ART initiation could be attributed to LP suggesting that entering late to care increases TB risk to a level that cannot be fully compensated by ART [28]. Our data are in line with those of a recent clinical trial conducted in high TB-HIV burden countries, in which early HIV diagnosis and treatment by annual HIV screening and universal ART, resulted in a 59% reduction in the estimated incidence rate of TB at 3 years, when compared to performing a one-time TB screening and CD4-guided ART initiation [29]. Our estimates extend the observation to 5 years of follow-up.
When investigating the potential causal link between delaying ART initiation and risk of developing TB, among those who presented to care with a CD4 count >350/μL, we found that the risk of incident TB was reduced for non-LI of ART compared to LI, although this was not significant due to the small number of non-late ART initiators and the 30% of non-ART initiators. Additionally, in the short term, no excess incidence of TB could be attributed to delaying ART. Because some TB cases are clinically unmasked by ART, it is possible that latent TB revealed by ART initiation equaled those not prevented by delayed initiation amounting to no overall difference [30]. However, 16% and 25% of cases occurring after 3 and 5 years of starting care respectively, could be attributed to delayed treatment initiation.
Late HIV diagnosis is still common and drives TB incidence among PLWH, which remains the most common cause of AIDS-related deaths and our findings show that the impact of late presentation, can persist for years [10,31–32]. Efforts to increase access to HIV screening (self-testing, community testing, universal testing in health care systems, etc.) are needed, in resource-limited countries and resource-rich settings, with large migrant populations such as in Italy and other countries in Europe. The finding of the elevated risk of occurrence of TB despite ART initiation for a prolonged period of time, has important clinical implications, such as, the important role of preventive TB therapy, that has been recommended for PLWH by WHO since 2011. However, scale-up of this intervention has been inefficient in most contexts [33, 34]. Preventive therapy may provide protection against TB when administered to patients on ART and may have a significant impact. Such impact has been perceived particularly relevant in countries with high TB burden [33–35], but our findings may suggest that the benefit of the preventive therapy could be also important in countries with lower burden of TB.
A strength of this analysis is that we used techniques such as marginal structural models to appropriately adjust by the effect of time-varying confounders affected by prior treatment strategies on outcomes [36]. However, these models cannot control for unmeasured confounders and rely on very strict, mainly untestable, assumptions. Nevertheless, our short-term estimates are entirely consistent with those shown in randomized studies. We also used attributable risk and population attributable fraction to estimate the impact of late presentation and late initiation of ART on incident TB cases observed. These measures have been previously used to evaluate the potential impact of reducing or eliminating a specific exposure in a population but never in the context of HIV/TB [37].
Another limitation of our study is the lack of information regarding the prevalence of latent TB and the proportion of TB preventive therapy provided in the four settings included. Latent TB is estimated very prevalent in Mexico, Peru and Uganda, and the treatment with preventive therapy may be important, as it is early ART, for the clinical outcomes and may impact in our results. It is unlikely that a significant proportion of persons entering HIV care in our cohorts, received TB preventive therapy since, during this study period the uptake of this intervention was low [38]. Nonetheless, if some people actually initiating preventive therapy along with ART, this could have mitigated the risk of incident TB especially in those with low CD4 at treatment initiation. Furthermore, we didńt control for the types infrastructure and tools to diagnose TB, which are different in the four settings. Finally, the database was created by joining the data of four different cohorts, who dońt share a common platform for data collection; however, we included variables which were defined and collected in the same way across the cohorts in order to maximize standardization.
Conclusions
Our results suggest that the persistently high burden of TB in an era of increasingly high uptake of ART may be largely due to late HIV diagnosis; the impact of late presentation is likely to persist up to 5 years from first attending HIV care. Our data extends those coming from randomized studies to a longer follow-up period and are useful to inform stochastic models of the HIV/TB epidemic. Interventions for promoting early diagnosis and treatment of HIV infection is needed to realize the full potential of ART in reducing the risk of TB for PLWH.
Supplementary Material
Acknowledgements
We acknowledge the participation of all cohortś team investigators, physicians and patients’ participants.
Funding
This work was supported by the National Institutes of Health–funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiology Databases to Evaluate AIDS [leDEA; U01AI069923]. This award is funded by the following institutes: Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Office of the Director (OD), National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID), National Cancer Institute (NCI), and the National Institute of Mental Health (NIMH). Enrico Girardi was supported by the Italian Ministry of Health- Grant “Ricerca Corrente Linea 4” to INMI Spallanzani. Icona Foundation received unrestricted grants from Gilead, ViiV, MSD and Janssen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Dr. Girardi reports grants from Gilead Sciences, grants from Mylan, personal fees from Gilead Sciences, personal fees from ViiV, outside the submitted work.
Footnotes
Additional files
Additional Material 1: Supplementary Material 1.
This material contains supporting information about the design of the study, and tables and figures of additional analyses.
Competing interests
All the other authors declare no conflicts of interest.
References
- 1.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50 Suppl 3:S201–S207. [DOI] [PubMed] [Google Scholar]
- 2.Uplekar M, Weil D, Lonnroth K, et al. WHO’s new End TB Strategy. Lancet 2015; 385: 1799–1801. [DOI] [PubMed] [Google Scholar]
- 3.Suthar AB, Lawn SD, del Amo J, et al. (2012) Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis. PLoS Med 2012. 9(7): e1001270. 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor J, Vjecha MJ, Phillips AN, et al. Effect of immediate initiation of antiretroviral therapy on risk of severe bacterial infections in HIV-positive people with CD4 cell counts of more than 500 cells per μL: secondary outcome results from a randomised controlled trial Lancet HIV 2017; 4: e105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams BG, Granich R, De Cock KM, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci U S A 2010; 107: 19485–19489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNAIDS. UNAIDS Data 2020. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- 7.GBD Tuberculosis Collaborators. Global, regional, and national burden of tuberculosis, 1990–2016: results from the Global Burden of Diseases, Injuries, and Risk Factors 2016 Study. Lancet Infect Dis 2018; 18: 1329–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Late Presentation Working Groups in Euro, S. and Cohere (2020). Estimating the burden of HIV late presentation and its attributable morbidity and mortality across Europe 2010–2016. BMC Infectious Diseases 20(1): 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belaunzarán-Zamudio P, Caro-Vega Y, Shepherd B, et al. The Population Impact of Late Presentation with Advanced HIV Disease and Delayed Antiretroviral Therapy in Adults Receiving HIV Care in Latin America. Am J Epidemiol 2020. Jun 1;189(6):564–572. doi: 10.1093/aje/kwz252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Global tuberculosis report. 2019. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1.
- 11.Lawn SD, Wood R. Antiretroviral therapy for control of the HIV-associated MDR and XDR tuberculosis epidemic in South Africa. Am J Respir Crit Care Med. 2010;182(12):1567–1569. doi: 10.1164/ajrccm.182.12.1567 [DOI] [PubMed] [Google Scholar]
- 12.Geremew D, Melku M, Endalamaw A, et al. Tuberculosis and its association with CD4+ T cell count among adult HIV positive patients in Ethiopian settings: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):325. Published 2020 May 7. doi: 10.1186/s12879-020-05040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis PK, Martin WJ, Dodd PJ. 2017. CD4 count and tuberculosis risk in HIV-positive adults not on ART: a systematic review and meta-analysis. PeerJ 5:e4165 10.7717/peerj.4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan CC, Cahn P, Gotuzzo E, et al. Cohort Profile: Caribbean, Central and South America Network for HIV research (CCASAnet) collaboration within the International Epidemiologic Databases to Evaluate AIDS (IeDEA) programme. Int J Epidemiol. 2007. Oct;36(5):969–76. Epub 2007 [DOI] [PubMed] [Google Scholar]
- 15.d’Arminio Monforte A, Cozzi Lepri A, Rezza G, Pezzotti P, et al. I.CO.N.A. Study Group. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naïve patients. AIDS. 2000. Mar 31;14(5):499–507. [DOI] [PubMed] [Google Scholar]
- 16.Castelnuovo B, Kiragga A, Musaazi J, et al. Outcomes in a Cohort of patients started on Antiretroviral treatment and followed up for a decade in an Urban Clinic in Uganda. PLoS One. 2015;10(12):e0142722. doi: 10.1371/journal.pone.0142722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter SD. The Estimation and Interpretation of Attributable Risk in Health Research. Biometrics. 1976;32(4):829–849 [PubMed] [Google Scholar]
- 18.Miettinen O Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99(5):325–332. [DOI] [PubMed] [Google Scholar]
- 19.Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ. 2018;360: k182. Published 2018 Feb 1. doi: 10.1136/bmj.k182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewings FM, Ford D, Walker AS, Carpenter J, Copas A. Optimal CD4 count for initiating HIV treatment: impact of CD4 observation frequency and grace periods, and performance of dynamic marginal structural models. Epidemiology. 2014;25(2):194–202. doi: 10.1097/EDE.0000000000000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang CA, Meloni ST, Eisen G, et al. Tuberculosis Incidence and Risk Factors Among Human Immunodeficiency Virus (HIV)-Infected Adults Receiving Antiretroviral Therapy in a Large HIV Program in Nigeria. Open Forum Infect Dis. 2015. Oct 17;2(4): ofv154. doi: 10.1093/ofid/ofv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Auld AF, Mbofana F, Shiraishi RW, et al. (2013) Incidence and Determinants of Tuberculosis among Adults Initiating Antiretroviral Therapy – Mozambique, 2004–2008. PLoS ONE 8(1): e54665. 10.1371/journal.pone.0054665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capocci S, Smith C, Morris S, et al. Decreasing cost effectiveness of testing for latent TB in HIV in a low TB incidence area. Eur Respir J. 2015. Jul;46(1):165–74. doi: 10.1183/09031936.00067114. [DOI] [PubMed] [Google Scholar]
- 24.Norrby M, Wannheden C, Ekström AM, Berggren I, Lindquist L. Incidence of tuberculosis and the need of prophylactic treatment in persons living with HIV in Stockholm during the era of anti-retroviral therapy 1996–2013. Infect Dis (Lond). 2018. Nov - Dec;50(11–12):807–816. doi: 10.1080/23744235.2018.1486511. [DOI] [PubMed] [Google Scholar]
- 25.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011. Oct 20;365(16):1492–501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crabtree-Ramírez BE, Caro-Vega Y, Belaunzarán-Zamudio PF, et al. ; Caribbean, Central, South America Network for HIV Epidemiology (CCASAnet). Temporal changes in ART initiation in adults with high CD4 counts in Latin America: a cohort study. J Int AIDS Soc. 2019. Dec;22(12):e25413. doi: 10.1002/jia2.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esber AL, Coakley P, Ake JA, et al. Decreasing time to antiretroviral therapy initiation after HIV diagnosis in a clinic-based observational cohort study in four African countries. J Int AIDS Soc. 2020;23(2):e25446. doi: 10.1002/jia2.25446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riou C, Jhilmeet N, Rangaka MX, Wilkinson RJ, Wilkinson KA. Tuberculosis Antigen-Specific T-Cell Responses During the First 6 Months of Antiretroviral Treatment. J Infect Dis. 2020;221(1):162–167. doi: 10.1093/infdis/jiz417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Havlir DV, Balzer LB, Charlebois ED, et al. HIV Testing and Treatment with the Use of a Community Health Approach in Rural Africa N Engl J Med 2019; 381:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manabe YC, Breen R, Perti T, Girardi E, Sterling TR. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis. 2009;199 :437–444. [DOI] [PubMed] [Google Scholar]
- 31.Koenig SP, Kim A, Shepherd BE, et al. Increased Mortality After Tuberculosis Treatment Completion in Persons Living With Human Immunodeficiency Virus in Latin America. Clin Infect Dis. 2020. Jun 24;71(1):215–217. doi: 10.1093/cid/ciz1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphrey JM, Mpofu P, Pettit AC, et al. Mortality Among People With HIV Treated for Tuberculosis Based on Positive, Negative, or No Bacteriologic Test Results for Tuberculosis: The IeDEA Consortium. Open Forum Infect Dis. 2020. Jan 10;7(1):ofaa006. doi: 10.1093/ofid/ofaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. 2011. [Google Scholar]
- 34.World Health Organization. Consolidated Guidelines on the use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization. 2013. [PubMed] [Google Scholar]
- 35.Group TAS, Danel C, Moh R, Gabillard D, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–22. [DOI] [PubMed] [Google Scholar]
- 36.Fewell Z, Hernan MA, Wolfe F, Tilling K, Choi H, Sterne JA. Controlling for time-dependent confounding using marginal structural models. Stata J. 2004; 4:402–20. [Google Scholar]
- 37.Biostatistics Benichou J. and epidemiology: measuring the risk attributable to an environmental or genetic factor. C R Biol. 2007. Apr;330(4):281–98. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed AA, Grammatico M, Moll AP, Malinga S, Makhunga P, Charalambous S, et al. Factors associated with low tuberculosis preventive therapy prescription rates among health care workers in rural South Africa. Glob Health Action. 2021;14(1):1979281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.