Abstract
The pseudorabies virus (PRV) gE gene encodes a multifunctional membrane protein found in infected cell membranes and in the virion envelope. Deletion of the gE gene results in marked attenuation of the virus in almost every animal species tested that is permissive for PRV. A common inference is that gE mutants are less virulent because they have reduced ability to spread from cell to cell; e.g., gE mutants infect fewer cells and, accordingly, animals live longer. In this report, we demonstrate that this inference does not hold in a rat experimental model for virus invasion of the brain. We find that animals infected with gE mutants live longer despite extensive retrograde, transneuronal spread of virus in the rat brain. In this model of brain infection, virus is injected into the stomach musculature and virions spread to the brain in long axons of brain stem neurons that give rise to the tenth cranial nerve (the vagus). The infection then spreads from neuron to neuron in well-defined, and physically separated, areas of the brain involved in autonomic regulation of the viscera. We examined the progression of infection of five PRV strains in this circuitry: the wild-type PRV-Becker strain, the attenuated PRV-Bartha vaccine strain, and three gE mutants isogenic with the PRV-Becker strain. By 60 to 67 h after infection, all PRV-Becker-infected animals were dead. Analysis of Becker-infected rats killed prior to virus-induced death demonstrated that the virus had established an infection only in the primary vagal neurons connected directly to the stomach and synaptically linked neurons in the immediate vicinity of the caudal brain stem. There was little spread to other neurons in the vagus circuitry. In contrast, rats infected with PRV-Bartha or PRV-Becker gE mutants survived to at least 96 h and exhibited few overt signs of disease. Despite this long survival and the lack of symptoms, brains of animals sacrificed at this time revealed extensive transsynaptic infection not only of the brain stem but also of areas of the forebrain synaptically linked to neurons in the brain stem. This finding provides evidence that the gE protein plays a role in promoting symptoms of infection and death in animals that is independent of neuron-to-neuron spread during brain infection. When this early virulence function is not active, animals live longer, resulting in more extensive spread of virus in the brain.
Alphaherpesviruses can infect the central nervous system (CNS) by invading neurons in the periphery and then replicating and spreading to the CNS through synaptically linked neurons. This ability to pass transsynaptically has led to the increasing use of these viruses for analysis of neuronal circuitry (8, 18, 29, 35, 44, 47). The attenuated pseudorabies virus (PRV) vaccine strain called Bartha is widely used for this purpose (5). Although the reduced virulence of this strain contributes to its usefulness for circuit analysis, we do not have a clear understanding of the genetic basis that underlies its efficient neuroinvasiveness (ability to infect the CNS). Several mutations in the PRV-Bartha genome have been characterized. For example, a deletion in the unique short region of the genome removes sequences coding for gI, gE, Us9, and Us2 (30, 31). Additionally, the gC gene in the unique long (UL) region harbors several mutations, including one in the signal sequence that reduces the concentration of gC in the viral envelope and in host cell membranes (42). There are eight nucleotide point mutations in the BglII-B and BamHI-4 segments of PRV-Bartha (23, 24, 32). Three of these mutations result in amino acid substitutions in the UL21 protein which is involved in capsid formation (14). Recently, Mettenleiter and colleagues found that PRV-Bartha carries two mutations in the gM gene, which result in a threonine-to-alanine change at amino acid position 59, eliminating an N-linked glycosylation signal, and a serine-to-proline substitution at position 60, which could potentially affect the secondary structure of the protein (15). The PRV-Bartha strain grows exceedingly well in most tissue culture cells, is more stable to temperature extremes than wild-type strains, and also is much less pathogenic than field isolates. Nevertheless, PRV-Bartha is still capable of efficient and extensive transneuronal, retrograde spread through the nervous systems of a wide variety of animals (18).
Of all the defects in the Bartha strain, the loss of membrane proteins gE and gI has demonstrable effects on virulence and spread in the nervous system (20). Homologous proteins of PRV gE and gI are found in all other members of the alphaherpesvirus subfamily (e.g., herpes simplex virus type 1, varicella-zoster virus, bovine herpesvirus 1, and equine herpesvirus 1), suggesting conserved biological function (34). While the PRV gE and gI gene products are not required for virus replication in tissue culture, null mutants display modest defects in viral release from certain cells and reduced spread from cell to cell as evidenced by smaller plaque size compared to those produced by wild-type virus (6, 22, 36, 37, 49, 51). Deletions or mutations of either gE or gI reduce virulence in chicken embryos, 1-day-old chicks, mice, and pigs (1, 4, 21, 22, 25, 38). In addition, analysis of PRV invasiveness of visual centers after retina infection in the rat has demonstrated that gE and gI mutants exhibit a restricted neurotropism relative to that of wild-type virus (48). Other studies of PRV have shown that gE may be involved in viral tropism for the thymus (25). Interestingly, PRV gE null mutants appeared to acquire tropism for the liver, an organ not infected by wild-type virus. Herpes simplex virus type 1 deletion mutants lacking gE or gI have also been shown to have restricted cell-to-cell spread in vitro, restricted rate of spread within the retina and to retinorecipient areas in the rat brain, and reduced pathogenesis in a mouse eye infection model (16, 17), as well as attenuated neurovirulence and neuroinvasiveness in mice (3, 40). Varicella-zoster virus mutants lacking gE show significant restriction in cell-to-cell spread and reduced yield of infectious virus (33). Collectively, these findings provide strong evidence that the gE and gI proteins play important roles in determining the extents of cell-to-cell spread, invasiveness, and virulence of alphaherpesviruses.
Given that PRV-Bartha is an attenuated strain, it may appear counterintuitive that in most animals, this strain is far more neuroinvasive than virulent field strains of the virus (11). Indeed, PRV-Bartha infects the CNS of a wide variety of animals after peripheral inoculation and consistently spreads by transsynaptic infection to more second- and third-order neurons than do virulent strains of PRV (18). This observation challenges the idea that virulence is directly related to the magnitude of neuroinvasiveness (e.g., the number of CNS neurons that are infected) and suggests that viral gene products other than those responsible for virus invasion and spread are responsible for the early demise of infected animals. In this report we provide direct evidence that PRV mutants with point mutations and deletions of the gE gene do not express an early virulence function, with the result that animals infected with these mutants survive longer than those infected with their isogenic parent. This longer survival facilitates widespread, retrograde, transsynaptic infections of the rat CNS that are far more extensive than those produced by the virulent parental virus.
MATERIALS AND METHODS
Model system: the neuronal circuitry innervating stomach muscles.
The experiments were conducted using the stomach muscle infection model developed by Card and colleagues (11) and illustrated in Fig. 1. Injection of PRV into stomach muscles produces a retrograde infection of neurons in autonomic cell groups of the spinal cord and brain stem, followed by continued retrograde infection of synaptically linked neurons in other regions of the brain involved in regulating activity of the stomach muscles and other visceral organs. A retrograde infection is defined as spread of virus from the axon terminals to the parent neurons; the direction of retrograde spread of virus is opposite to that of the nerve impulse. We have previously used this well-defined circuitry to verify the specificity and direction of transsynaptic passage of PRV, as well as the brain’s response to infection that contributes to that specificity (10, 11, 41). Several areas of the brain were used to gauge the progression of infection produced by the different strains of virus. These included the dorsal motor vagal complex (DVC) and adjacent medulla in the caudal brain stem, as well as selected regions in the forebrain (paraventricular hypothalamic nucleus, central nucleus of the amygdala [CeAm], bed nucleus of the stria terminalis [BNST], subfornical organ [SFO], and insular cortex). The location of each of these regions in the brain is illustrated in Fig. 1. Other regions of the stomach circuitry are well known and were infected by PRV in these studies, but are not illustrated in this figure for simplicity of presentation and analysis.
FIG. 1.
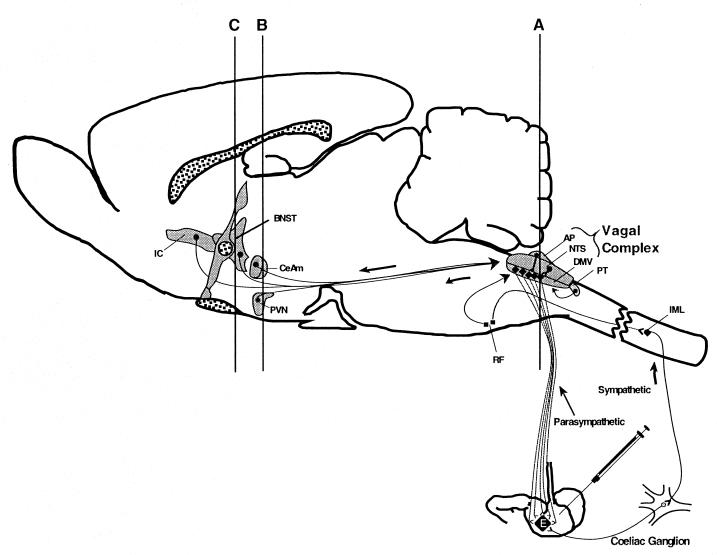
The circuitry analyzed in the present study is diagrammed schematically in a sagittal view of the rodent brain. Retrograde, transsynaptic infection of the CNS by PRV can take place via two pathways following injection of virus into stomach muscles. Neurons from two subdivisions of the autonomic nervous system innervate the stomach. Axon terminals provide sites of entry, and the axons provide conduits for viral infection of the cell bodies of these neurons in the brain stem. The most direct infection is achieved by retrograde infection of caudal brain stem neurons in the DMV. However, retrograde transsynaptic passage of virus through a disynaptic circuit involving pseudounipolar neurons in the celiac ganglion also leads to infection of sympathetic preganglionic neurons in the intermediolateral cell group (IML) in the spinal cord. Retrograde transsynaptic passage of virus from the DMV and IML produces an infection of other cell groups in the brain stem and forebrain. The organization of this circuitry is described in greater detail in the Materials and Methods section. The lines labeled A, B, and C define the planes of section used in subsequent illustrations. Abbreviations: AP, area postrema; BNST, bed nucleus of stria terminalis; CeAm, central nucleus of the amygdala; NTS, nucleus of the solitary tract; PT, paratrigeminal nucleus; PVN, paraventricular hypothalmic nucleus; IC, insular cortex; RF, reticular formation. The subfornical organ (SFO) lies in the C-plane, just above the BNST.
The dorsal motor vagal complex (DVC) and adjacent medulla provide an accurate index of early infection of the CNS. The DVC consists of a complex of three cell groups involved in the regulation of visceral function and, as such, has direct connections with the stomach. Neurons in the dorsal motor vagal nucleus (DMV) give rise to axons that project through the vagus nerve (cranial nerve X) to the stomach, where they synapse upon neurons that control the contractility of stomach musculature and gastric secretions. These DMV neurons are the first neurons in the brain to be infected. Injection of virus into the stomach wall leads to invasion of the axons of DMV neurons and ultimately, spread to and viral replication in the cell bodies of these neurons in the brain stem. The nucleus of the solitary tract (NTS) lies immediately above and adjacent to the DMV and contains neurons that synapse upon DMV neurons. NTS neurons can be infected by retrograde transsynaptic passage of virus through DMV neurons. In addition, there is some evidence that certain strains of virus can infect NTS neurons by transganglionic infection through sensory fibers of the nodose ganglion at long postinoculation intervals (10). The area postrema (AP) is a midline region lacking a blood-brain barrier that lies immediately adjacent to the NTS. AP neurons project axons into the NTS and can become infected via retrograde transsynaptic passage of virus from infected NTS neurons. Neurons in the DVC generally exhibit a progression of infection in which virus is seen first in the DMV, followed sequentially by infection of the NTS and the AP. Presence of viral antigen in these areas provides a precise and reliable indication of early infection.
Transsynaptic progression of infection can be monitored by the subsequent appearance of viral antigen in two additional regions of the medulla that are in synaptic contact with the DVC. These regions are the paratrigeminal cell groups (PT) in the dorsolateral portion of the caudal brain stem and neurons in the reticular formation at the ventral and lateral extent of the medulla. The locations of both regions are shown in Fig. 2. Neurons in each area project axons into the DVC and they become infected after viral antigen appears in neurons of the DMV, NTS, and AP. Thus, they provide added temporal precision to determining the progression of transsynaptic passage of PRV through the neuronal circuitry that regulates stomach muscle activities.
FIG. 2.
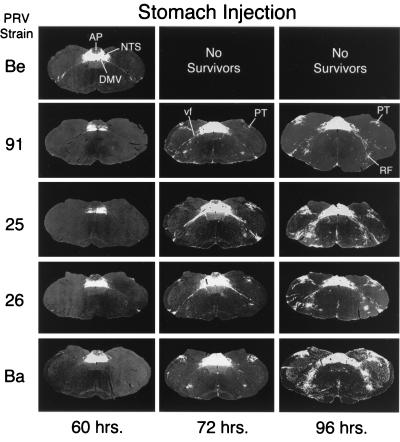
Low-power photomicrographs illustrate the progression of infection in the caudal brainstem 60, 72, and 96 h following injection of strains of PRV. Negative images, where viral immunoreactivity is revealed as a white signal on a dark background, are shown. PRV-Becker (PRV-Be), a wild-type strain of PRV, infects a large number of neurons in the DVC (DMV, NTS, and AP) 60 h postinoculation, but only scattered neurons are infected in the ventrolateral portion of the medulla. Animals infected by this virus did not survive beyond 67 h in this study. Photomicrographs in other rows illustrate the patterns of infection produced by PRV-91, PRV-25, PRV-26, and PRV-Bartha (PRV-Ba). PRV-Ba, an attenuated vaccine strain harboring a number of deletions and mutations, produces the most-extensive infection at all postinoculation intervals. PRV-25, PRV-26, and PRV-91 infect a subset of the circuitry infected by PRV-Ba, but they still produce extensive retrograde infections. RF, reticular formation.
Transneuronal spread can be documented further by analysis of several areas in the forebrain that contain neurons projecting axons to cell groups in the DVC and spinal cord. These regions also are infected by injection of PRV into the stomach (Fig. 1). Two of these areas, the paraventricular nucleus (PVN) and bed nucleus of the stria terminalis (BNST), are among the areas of the forebrain that are infected earliest by retrograde transsynaptic transport of PRV from the stomach. Three other regions, the central nucleus of the amygdala (CeAM), the subfornical organ (SFO), and insular cortex, become infected after viral antigen is detected in the PVN and BNST. The locations of all of these areas in the brain are shown in Fig. 1 and 3. The PVN, BNST, and CeAm have direct connections to the DVC, but the temporal differences in the infection of these two areas suggests that they have different synaptic targets. For example, the temporal separation in the infection of these regions suggests that axons of PVN and BNST neurons synapse directly upon the DMV neurons while those of the CeAm synapse upon neurons of the NTS. Such a synaptic arrangement would require an extra round of viral replication in NTS neurons before retrograde transsynaptic infection of CeAm neurons and would thereby explain the delay of infection of this cell group relative to that of neurons in the PVN. The delayed infection of the SFO may occur because SFO neurons project to the PVN but do not have axons that extend to the caudal brain stem or spinal cord (2, 28, 39, 43). Thus, virus must first replicate in the PVN before passing transsynaptically to infect neurons in the SFO.
FIG. 3.
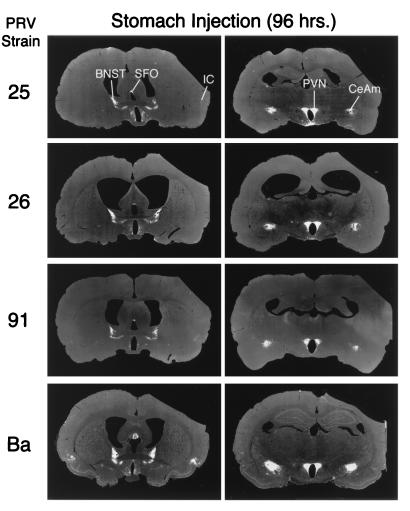
The extents of retrograde transsynaptic infection of the forebrain produced by injection of PRV mutants into the wall of the stomach are illustrated. Low-power, negative images, where circumscribed areas of viral immunoreactivity are revealed as a white signal on a dark background, are shown. Vertical columns illustrate selected coronal-plane sections obtained at the levels of B (right column) and C (left column) illustrated in Fig. 1. Horizontal rows illustrate the pattern of infection produced by PRV-25, PRV-26, PRV-91, and PRV-Bartha (PRV-Ba) 96 h following stomach inoculation. Animals injected with PRV-Becker did not survive that long and could not be analyzed. The most-extensive infection is produced by PRV-Ba. The other mutants infect a subset of the neurons infected by PRV-Ba. IC, insular cortex.
PRV strains.
Five strains of virus were used in this analysis. PRV-Becker is a virulent laboratory strain that has been used in prior characterizations of the invasiveness in this circuitry. It was also the parent virus for three of the other strains: PRV-25, PRV-26, and PRV-91. Construction of each of these mutants has been described in reports about prior investigations (45, 46, 48). Briefly, PRV-91 is a null mutant that is isogenic with PRV-Becker and lacks the gE gene. PRV-25 and PRV-26 are also isogenic with PRV-Becker. PRV 25 contains an insertion of one base in the sequences encoding the transmembrane domain of the protein (45). Translation of this gene produces a protein containing the wild-type N-terminal ectodomain of the protein fused to a novel cytoplasmic tail. gE is anchored in the membranes of infected cells. Functionally, this protein is equivalent to a gE protein lacking the cytoplasmic tail. PRV-26 has a stop codon at amino acid 428 and produces a form of the gE protein that lacks a transmembrane domain and cytoplasmic tail. The N-terminal ectodomain of gE produced in cells infected with this virus is secreted. PRV-Bartha is an attenuated vaccine strain containing the mutations and deletions described in the introduction (5). It has also been used in prior studies of the invasiveness of PRV in this circuitry (41). The 50% lethal doses (LD50) for wild-type PRV (Becker strain or Kaplan strain) and PRV gE null mutants in mice and rats, about 50 to 100 PFU, are virtually identical (references 9 and 51 and our unpublished observations). The LD50 in rats and mice for the attenuated Bartha strain is about 100 times greater than that for the Becker strain (9). In this study, we infected animals with the same numbers of PFU of all viruses because our model system involves uptake of the inoculum at axon terminals in the stomach followed by retrograde transport to the cell bodies of neurons in the brain stem, where primary replication occurs. Thus, we strove to infect the same number of primary neurons with the same number of virions. The amount of virus we used was at least 100-fold the LD50 to ensure that every animal became infected and that there was an amount of virus in the primary neurons sufficient to initiate a productive infection. As a result, every animal should die and the mean time to death can be quantitated. This parameter is highly characteristic of the genotype of the virus (9, 46, 50).
Experimental paradigm.
Adult male Sprague-Dawley albino rats were used in this study. Animals were housed in a biosafety level 2 facility with controlled temperature and photoperiod (12 h of light; light on at 0700). Five groups of animals that differed only in the strain of virus with which they were injected were included in the analysis. Table 1 provides information on the viruses, titers, times at which animals were euthanized after infection, and numbers of animals included in each experimental group. For the initial infections, each animal was anesthetized with ketamine (60 mg/kg of body weight) and xylazine (7 mg/kg) and the stomach was exposed after laparotomy. A total of 6 μl of virus was injected into the ventral and dorsal muscular wall of the stomach with a 10-μl Hamilton syringe equipped with a 26-gauge needle. Three injections, e.g., one each into the antrum, fundus, and ruminal segments, were made on each surface of the stomach wall to ensure efficient retrograde infection of the DMV as described previously (10, 41). Injection sites were swabbed with sterile saline, and the abdominal wall was closed with suture and wound clips. Animals recovered under a heat lamp and then were returned to their home cages for the balance of the experiment. Symptoms of infection were monitored throughout the experiment. These included nasal discharge, hardarian gland discharge, labored breathing, hunched posture, sensory irritation, and lethargy.
TABLE 1.
Animals used in this study
| Virus | Stock titer | No. of animalsa examined at:
|
||||
|---|---|---|---|---|---|---|
| 60 h | 72 h | 84 h | 93 h | 96 h | ||
| PRV-Be | 3.5 × 108 | 4 | Noneb | None | None | None |
| PRV-Be | 4.0 × 108 | 2 | 3 | 1 | 2 | 1 |
| PRV-25 | 3.5 × 108 | 2 | 3c | 4 | 2 | 1 |
| PRV-26 | 2.3 × 108 | 2 | 3d | 3 | 2 | 3 |
| PRV-91 | 3.0 × 108 | 1 | 3 | 1 | 1 | 1 |
Numbers of animals that were euthanized and brains that were analyzed at the indicated times postinfection.
No animals infected with PRV-Becker survived more than 67 h postinfection.
Four animals infected with PRV-25 died unexpectedly at approximately 72 h postinfection, and these tissues were not suitable for processing. They are not included in the numbers shown in the table.
One animal infected with PRV-26 died unexpectedly at approximately 72 h postinfection, and this tissue was not suitable for processing. This animal is not included in the number shown in the table.
At approximately 60, 72, 84, 93 and 96 h after infection, surviving animals were anesthetized and sacrificed by transcardiac perfusion fixation with buffered aldehyde solutions by using published procedures (8, 44). The brains were removed, postfixed, cryoprotected, and sectioned serially in the coronal plane at 30-μm section thickness with a rotary microtome equipped with a freezing stage. Sections separated by distances of 180 μm (every sixth section) were processed for immunohistochemical localization of viral antigens by using rabbit polyclonal antiserum prepared against acetone-inactivated PRV and the avidin-biotin modification of the immunoperoxidase procedure (8). This antibody detects the viral capsid, tegument, and membrane proteins in fixed, infected tissue. Thus, any neuron that reacts with the antiserum is producing PRV structural proteins, most of which require DNA replication for synthesis. Processed sections were mounted on gelatin-coated slides, dehydrated, cleared, and coverslipped. The aforementioned regions were examined with a light microscope, and the magnitude of infection (number of infected neurons) in serial sections made through each region was documented by photomicroscopy. In all cases, sections covering the entire brain and brain stem were examined. In Fig. 2 and 3 low-power, negative images of selected single sections, where viral immunoreactivity is revealed as a white signal on a dark background, are shown. The patterns of infected neurons extend rostrally and caudally for several sections, such that we can outline the entire caudal brain stem with precision. In Fig. 4 to 7, selected high-power, positive images, where immunoreactivity representing virus replication in individual neurons is detected as a black signal on a light background, are shown. Experimental protocols were approved by the Animal Welfare Committees of the University of Pennsylvania and Princeton University and were consistent with the regulations of the American Association for Accreditation of Laboratory Animal Care and those in the Animal Welfare Act (public law 99-198).
FIG. 4.
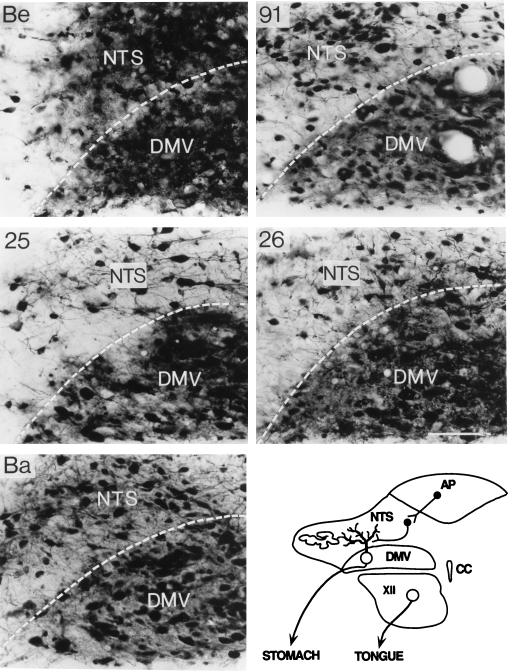
High-power photomicrographs illustrate the extents of infection in primary neurons of the DMV and synaptically connected second-order neurons of the NTS 60 h after inoculation of the stomach with different strains of PRV. Positive images, where viral immunoreactivity in individual neurons is detected as a black signal on a light background, are shown. The most-extensive infection is observed in animals infected with PRV-Becker (Be), which also produces the most-pronounced neuropathology, indicated by extensive cytopathic effect. PRV-Bartha (Ba) also infects a large number of DMV and NTS neurons at this time after inoculation, but both the extent of infection and the degree of cytopathology are reduced relative to those produced by PRV-Becker. PRV-25, PRV-26, and PRV-91 infect a subset of the neurons infected by PRV-Ba. The schematic diagram in the lower right corner illustrates the organization of synaptic connections through which this circuitry is infected. CC, corpus callosum; XII, 12th cranial nerve. Calibration bar = 100 mm.
FIG. 7.
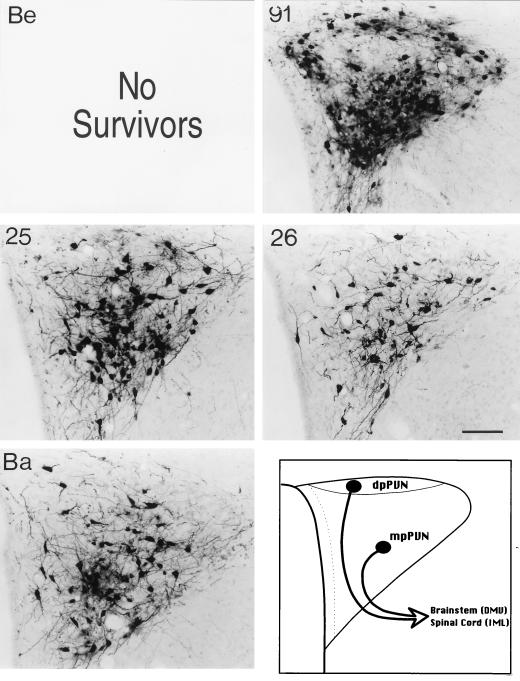
The distributions of infected neurons in the PVN observed 96 h following inoculation of the stomach with PRV-91, PRV-25, PRV-26, and PRV-Bartha (Ba) are illustrated. Positive images, where viral immunoreactivity in individual neurons is detected as a black signal on a light background, are shown. Animals infected with PRV-Becker (Be) did not survive beyond 67 h in this study and could not be analyzed at this late time after infection. All of the mutants infect large numbers of neurons throughout the medial and dorsal parvicellular subdivisions of the nucleus (mpPVN and dpPVN, respectively) at this postinoculation interval. The schematic diagram illustrates the subdivisions of this hypothalamic nucleus. Calibration bar = 200 mm.
RESULTS
Our experiments produced two primary results. First, deletions or mutations of gE substantially reduced the symptoms of viral infection in the stomach model and resulted in extended survival of infected animals relative to that of animals infected with wild-type virus. Second, because of this longer survival, virus continued to spread in the vagus circuitry, with the result that infected neurons were found throughout most of the brain centers in this autonomic control system. These centers were not infected by wild-type virus because of early animal death.
gE mutants exhibit reduced virulence.
Rats infected with PRV mutants harboring defined gE mutations exhibited a delay in the onset of symptoms, a reduction in symptom severity, and extended survival compared to rats infected by wild-type virus. In this study, no animals infected with the virulent PRV-Becker strain survived longer than 67 h, and rats infected with this strain exhibited pronounced and severe symptoms of infection as early as 48 h postinoculation. Typically, these symptoms included a hunched posture, piloerection, and lethargy. Infected rats were hyperresponsive to touch and characteristically exhibited signs of sensory irritation. Secretions from the mouth, nares, and eyes were evident by 50 h and were pronounced by 60 h after infection. In addition, PRV-Becker-infected animals lost approximately 20% of their initial body weight during the experiment.
The effects of gE mutants upon the health of infected animals were markedly different from those produced by PRV-Becker. Animals infected with PRV-Bartha routinely survived to 96 h postinoculation and exhibited only modest signs of infection that became apparent in the longest surviving animals. These animals showed no signs of weight loss, continued to groom, and remained quite active before 72 h postinoculation. The only overt sign of infection, even at late times after infection, was the appearance of secretions at the corners of the eyes as well as from the nares and mouth. Extended survival and similar mild symptoms were exhibited by animals infected with PRV-91 (gE null mutant). Similarly, animals infected with PRV-25 and PRV-26 (both are mutants that express gE proteins lacking the cytoplasmic domain) exhibited noticeable reductions in virulence, but symptoms were more varied from animal to animal, more so than those observed after infection with PRV-91 or PRV-Bartha (46). We noted that occasionally animals infected with either PRV-25 or PRV-26 died abruptly and unexpectedly at approximately 72 h after infection (e.g., they showed no signs indicating death was imminent). However, these animals were the exception rather than the rule: 4 of a total of 12 PRV-25-infected rats that developed infection died early, and 1 of a total of 11 PRV-26 infected rats that developed infection died early. Even these early deaths occurred well beyond the time at which all PRV-Becker-infected animals showed severe symptoms and had succumbed to infection.
Reduction in virulence of gE mutants did not correlate with neuroinvasiveness.
The increased survival of animals infected with the gE mutants resulted in extensive retrograde infection of neurons synaptically linked with the autonomic neurons that project axons to the stomach. This extensive spread is readily apparent in Fig. 2, which illustrates the magnitudes of infection in one typical section through the caudal brain stem produced by injection of equivalent concentrations of the different strains of PRV. The longest surviving PRV-Becker-infected animals (60 h) exhibited extensive infections of the primary neurons in the DMV and second-order neurons in the NTS, with only scattered infected neurons in the AP, the PT, and ventrolateral medulla. Equivalent sections through the caudal brain stem of animals infected with gE mutants sacrificed at 60 h demonstrated that the magnitude of infection occasionally was reduced relative to that produced by PRV-Becker, but the infection was still extensive (see Fig. 4 for a close-up view). In all of the infections by mutant viruses at this time point, the infection was largely confined to the DVC, with only occasionally infected neurons in either the PT or ventrolateral medulla being noted. Furthermore, the magnitude of infection within the DVC was often equal to that observed in the same area of PRV-Becker-infected animals (compare Fig. 2 and Fig. 4). The magnitude of infection in the caudal brain stem by gE mutants increased progressively. Retrograde transsynaptic passage of the gE mutants led to infection of neurons throughout the DVC, including the AP, as well as infection of large numbers of neurons in adjacent regions of the medulla (compare Fig. 2 and Fig. 4). By 72 h postinfection, the entire vagus complex in the brain stem was infected, and this infection was more extensive than that produced by PRV-Becker at 60 h, a time at which most PRV-Becker-infected animals were dead or exhibited terminal symptoms. PRV-Bartha-infected rats exhibited the most-extensive infections, with the least-overt symptoms. In addition to the robust infection of the DVC, these animals had large numbers of infected neurons in the paratrigeminal complex and in the portion of the ventrolateral medulla that contains brain stem catecholamine neurons. Infected neurons were also present along the midline in the raphe nuclei. All of these areas have documented synaptic connections with neurons in the DVC, and they became infected subsequent to the appearance of detectable viral antigen in that region.
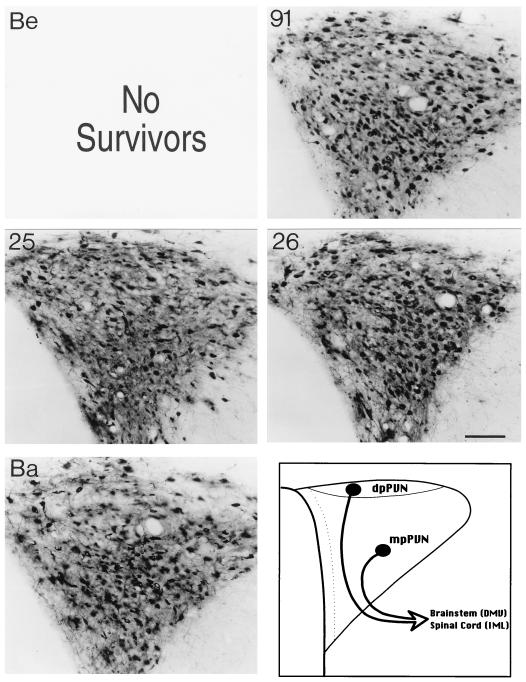
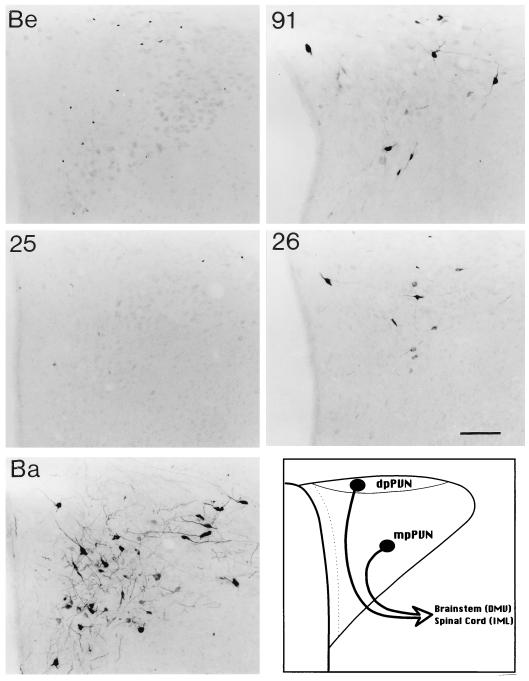
Infected forebrain neurons located some distance from the brain stem became apparent as early as 66 h after inoculation, and their numbers increased thereafter. Again, the most-robust infection was observed in PRV-Bartha-infected rats that were sacrificed 96 h after inoculation. These animals exhibited exceptionally large numbers of infected neurons in the PVN, CeAm, BNST, SFO, and insular cortex (Fig. 3). The same cell groups were infected by gE mutants, but often subsets of cells were infected (see the following paragraph). An example of the temporal progression of infection is shown in Fig. 5 through 7, which illustrate infected neurons in the PVN 60, 72, and 96 h postinoculation. This cell group contains large numbers of parvicellular neurons that participate in the regulation of autonomic function and are sequestered in distinct subdivisions that project to the brain stem and spinal cord. The earliest infection of PVN neurons was observed in the medial parvicellular subdivision of the nucleus (Fig. 5). With advancing survival time, the number of infected cells in the medial parvicellular subdivision increased and infected neurons also became apparent in the dorsal parvicellular subdivisions. Similar patterns of progression of infection were noted in other forebrain nuclei. The observation of the progression of infection in the CeAm was particularly informative from the standpoint of defining the temporal sequence of infection. This cell group has four distinct subdivisions that are synaptically connected, but only the medial subdivision projects to the DVC. Thus, early infections are restricted to the medial subdivision whereas infections at longer postinoculation intervals characteristically exhibit infected neurons throughout all four subfields. This is evident in comparing the CeAm infections produced by PRV-26 and PRV-Bartha illustrated in Fig. 3.
FIG. 5.
The distributions of infected neurons in the PVN observed 60 h following inoculation of the stomach with PRV-Becker (Be), PRV-91, PRV-25, PRV-26, and PRV-Bartha (Ba) are illustrated. Positive images, where viral immunoreactivity in individual neurons is detected as a black signal on a light background, are shown. PRV-Ba produces the most-extensive infection of neurons in the medial parvicellular subdivision of the PVN (mpPVN) that give rise to descending projections to autonomic cell groups in the brain stem (DMV) and spinal cord (IML). The schematic diagram in the lower right panel illustrates the subdivisions of this hypothalamic nucleus that is synaptically connected to the DMC in the brain stem. Other strains of virus produce a more restricted pattern of infection. Calibration bar = 200 mm. dpPVN, dorsal parvicellular subdivision of the PVN (dpPVN).
The extents of progression of retrograde transsynaptic infection differ among gE mutants.
Distinct differences in the extents of retrograde transsynaptic infection were observed in animals infected with the gE mutants. As noted previously, the most-extensive infection was observed in animals infected with PRV-Bartha, while the other gE mutants infected a significant subset of that circuitry. Examination of the entire caudal brain stem and forebrain suggested that deletion of gE (as in PRV-91) produced a more restricted pattern of infection than those produced by mutants that expressed only the ectodomain of gE (PRV-25 and PRV-26). This is particularly evident in comparing the extents of infection in the PT and ventrolateral medulla. In these areas, animals infected with PRV-25 or PRV-26 showed a robust retrograde transsynaptic infection of neurons that approached that produced by PRV-Bartha, whereas PRV-91 infected a smaller subset of these neurons at the same postinoculation intervals (Fig. 2). Similarly, fewer forebrain neurons were infected by PRV-91 than by either of the mutants with carboxy-terminal deletions. Thus, the extent of retrograde, transsynaptic infection by mutants that express the N-terminal ectodomain of gE appears to be greater than that produced by mutants in which the entire gE glycoprotein gene has been deleted.
DISCUSSION
Many groups have shown that PRV encodes a variety of gene products that promote virulence, i.e., the ability to cause disease (35). Operationally, such genes can be essential or nonessential for growth in tissue culture cells and are usually defined by mutations that reduce or abrogate the ability of a virus to cause disease. These mutations affect genes that fall into the following four general classes: (i) genes whose products affect virus replication (e.g., thymidine kinase mutations or any mutation that reduces yield of infectious virions), (ii) genes whose products are involved in movement of virus in the host away from the site of initial infection (e.g., genes promoting cell-to-cell spread of virus or neuroinvasion genes), (iii) genes that defeat host defenses (by producing complement binding proteins, like gC, Fc receptors, like gE/gI, or proteins that reduce major histocompatibility class I expression), and (iv) genes whose products cause pathogenic effects on their own (toxins or intrinsic virulence factors). The multifunctional gE gene product is a virulence factor that, under certain circumstances, may affect at least three of these four functions in promoting virulence, e.g., classes ii, iii, and iv. In this study, we extend our previous work indicating that gE expression promotes early virulence (rapid appearance of symptoms and death) independently of its role in cell-to-cell spread (46). Moreover, we demonstrate that the expression of early virulence requires the cytoplasmic domain of the gE protein.
The multifunctional actions of gE in promoting virulence and spread have been deduced from several lines of experimentation. First, it is well known that gE is required for efficient cell-to-cell spread after primary infection of epithelial cells (16, 20). Without this function of gE, it is likely that a primary infection will be poorly established because virus will not spread well among epithelial cells at the primary site. Consequently, infections by gE mutants are expected to be cleared rapidly by host innate defenses (e.g., interferons, NK cells, and complement) (20). It is likely that lack of the gE-mediated, cell spread function in epithelial cells is a primary reason for the attenuation of gE-deleted viruses in the wide variety of animals susceptible to infection by PRV. Second, gE is required for directional spread of virus infection in peripheral and central neurons (18). This is a neuroinvasive function of gE required for anterograde transneuronal transfer (passage from presynaptic neurons to postsynaptic neurons) of virus in many neurons. In pigs, gE and gI are required for PRV to spread to the olfactory bulb after infection of the nasal olfactory mucosa, and in pigs and rodents, it is required for transganglionic infection of trigeminal sensory nuclei in the brain stem through the fifth (trigeminal) cranial nerve (1, 26, 27). In rodents, a heterooligomer of gE and/or gI is thought to be required for anterograde transsynaptic infection of the optic tectum and lateral geniculate but not for infection of neurons in CNS nuclei involved in the control of circadian function (12, 13, 19, 48). Similarly, in the rodent CNS, gE and gI are required, at least in part, for anterograde transneuronal infection of the striatum after injection into the prefrontal cortex (7). This directional spread-and-release function of gE also may be required for spread of newly produced virus to the mucosal surface after reactivation from latency in sensory and autonomic neurons, rather than spread to the CNS. A critical point is that gE is not required for retrograde transsynaptic infection (passage from postsynaptic neurons to presynaptic neurons) (18). Finally, gE expression results in rapid appearance of symptoms and early death of infected animals (18, 46). In this report, we demonstrate that this last function is independent of the cell-to-cell spread and neuroinvasive functions of the virus.
The circuit described in the present analysis has been used to define the invasiveness of PRV and the specificity of transsynaptic passage of virus (10, 11, 41). Its value lies in providing the knowledge of the precise anatomical locations of multiple neuronal groups involved in the circuit that enables one to discern the time course and direction of infection through a complicated chain of neurons. Another critical point is that virions injected into the stomach wall are taken up at axon terminals by neurons and transported to cell bodies that lie in the caudal brain stem. Thus, virus enters the brain without any requirement for replication in the periphery. As gE mutants have defects that reduce spread in epithelial cells and affect anterograde spread in neurons, this model allows us to bypass such potential complications and enables us to study gE functions directly in CNS neurons. Early studies that compared the invasiveness of PRV-Becker and PRV-Bartha in this circuitry demonstrated that the primary neurons were first infected by PRV-Becker approximately 20 h earlier than by PRV-Bartha (10, 11, 41). The present data confirm and extend those observations to encompass the role of the gE protein, and more specifically the ectodomain of the protein, in this transsynaptic spread. These data demonstrated that differences in the extents of retrograde transneuronal spread of virus infection produced by the gE mutants correlated with the type of mutation. Total elimination of the gE gene (as in PRV-91) resulted in extensive retrograde spread with a more restricted pattern of infection in second- and third-order neurons than observed for PRV mutants that encoded to varying degrees the N-terminal portion of the gE glycoprotein. Therefore, the ectodomain of the gE protein permitted more extensive retrograde spread of virus through neural circuits. However, the most-extensive retrograde infection of the vagal autonomic circuitry was produced by PRV-Bartha, which harbors a number of additional mutations.
All of the PRV gE mutants exhibited a marked decrease in virulence that did not correlate with the magnitude of neuroinvasiveness. The reduction in virulence occurred in spite of extensive retrograde transsynaptic passage of virus through the vagus autonomic circuitry, involving multiple areas in the brain stem and forebrain. We had noted previously in our studies of the neuroinvasiveness of these mutants in the rodent visual system a similar reduction in virulence and also demonstrated that this reduction was directly related to the elimination of the cytoplasmic domain of gE (46). It must be noted that in the visual circuitry, the primary route of CNS invasion is by anterograde spread. Importantly, those studies also revealed that mutation of the gE gene did not compromise the neuroinvasiveness of PRV in visual projections to regions in the brain involved in circadian function (46). Taken together, these observations strongly support the conclusion that virulence and neuroinvasiveness are separate functions subserved by different portions of the gE glycoprotein. While the N-terminal ectodomain of the protein is sufficient to mediate anterograde transneuronal spread of the virus, the C-terminal cytoplasmic domain of gE is required for full expression of early virulence. This observation is the basis for our assertion that the gene that encodes gE is a multifunctional virulence gene: it is involved both in direct cell-to-cell spread among epithelial cells and in directional spread between synaptically linked neurons, and it also promotes symptoms and rapid death of infected animals. The mechanism of gE-induced virulence subserved by the cytoplasmic domain currently is under investigation.
FIG. 6.
The distributions of infected neurons in the PVN observed 72 h following inoculation of the stomach with PRV-91, PRV-25, PRV-26, and PRV-Bartha (Ba) are illustrated. Positive images, where viral immunoreactivity in individual neurons is detected as a black signal on a light background, are shown. Animals infected with PRV-Becker (Be) did not survive beyond 67 h in this study and could not be analyzed at this time after infection. All of the mutants infect a substantial number of neurons in the medial parvicellular subdivision of the PVN (mpPVN) as well as scattered neurons in the dorsal parvicellular subdivision (dpPVN). Both of these cell groups give rise to descending projections to autonomic nuclei in the brain stem and spinal cord. The schematic diagram illustrates the subdivisions of this hypothalamic nucleus. Calibration bar = 200 mm.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of Marlies Eldridge. Members of the Enquist lab provided support and constructive criticism.
This work was supported by NIH RO1s MH53574 (J.P.C.), NINDS33506 (L.W.E.), NIH grant 5T32GM07388 (R.S.T.), and GM27739 (R.R.M.).
REFERENCES
- 1.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 2.Bains J S, Ferguson A V. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 3.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 4.Banfield B W, Yap G S, Knapp A C, Enquist L W. A chicken embryo eye model for the analysis of alphaherpesvirus neuronal spread and virulence. J Virol. 1998;72:4580–4588. doi: 10.1128/jvi.72.6.4580-4588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartha A. Experimental reduction of virulence of Aujeszky’s disease virus. Magy Allatorv Lapja. 1961;16:42–45. [Google Scholar]
- 6.Ben-Porat T, DeMarchi J, Pendrys J, Veach R A, Kaplan A S. Proteins specified by the short unique region of the genome of pseudorabies virus play a role in the release of virions from certain cells. J Virol. 1986;57:191–196. doi: 10.1128/jvi.57.1.191-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card J P, Levitt P, Enquist L W. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J Virol. 1998;72:4434–4441. doi: 10.1128/jvi.72.5.4434-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Card J P, Enquist L W. Use of pseudorabies virus for definition of synaptically linked populations of neurons. Methods Mol Genet. 1994;4:363–382. [Google Scholar]
- 9.Card J P, Dubin J R, Whealy M E, Enquist L W. Influence of infectious dose upon productive replication and transsynaptic passage of pseudorabies virus in rat central nervous system. J Neurovirol. 1995;1:349–358. doi: 10.3109/13550289509111024. [DOI] [PubMed] [Google Scholar]
- 10.Card J P, Rinaman L, Lynn R B, Lee B H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Card J P, Whealy M E, Robbins A K, Moore R Y, Enquist L W. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 14.de Wind N, Wagenaar F, Pol J, Kimman T, Berns A. The pseudorabies virus homolog of the herpes simplex virus UL21 gene product is a capsid protein which is involved in capsid maturation. J Virol. 1992;66:7096–7103. doi: 10.1128/jvi.66.12.7096-7103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dijkstra J M, Mettenleiter T C, Klupp B G. Intracellular processing of pseudorabies virus glycoprotein M (gM): gM of strain Bartha lacks N-glycosylation. Virology. 1997;237:113–122. doi: 10.1006/viro.1997.8766. [DOI] [PubMed] [Google Scholar]
- 16.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1999;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 19.Enquist L W, Miselis R R, Card J P. Specific infection of rat neuronal circuits by pseudorabies virus. Gene Ther. 1994;1:S10. [PubMed] [Google Scholar]
- 20.Jacobs L. Glycoprotein E of pseudorabies virus and homologous proteins in other alphaherpesvirinae. Arch Virol. 1994;137:209–228. doi: 10.1007/BF01309470. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs L, Mulder W A, Van Oirschot J T, Gielkens A L, Kimman T G. Deleting two amino acids in glycoprotein gI of pseudorabies virus decreases virulence and neurotropism for pigs, but does not affect immunogenicity. J Gen Virol. 1993;74:2201–2206. doi: 10.1099/0022-1317-74-10-2201. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs L, Rziha H J, Kimman T G, Gielkens A L, Van Oirschot J T. Deleting valine-125 and cysteine-126 in glycoprotein gI of pseudorabies virus strain NIA-3 decreases plaque size and reduces virulence in mice. Arch Virol. 1993;131:251–264. doi: 10.1007/BF01378630. [DOI] [PubMed] [Google Scholar]
- 23.Klupp B G, Kern H, Mettenleiter T C. The virulence-determining genomic BamHI fragment 4 of pseudorabies virus contains genes corresponding to the UL15 (partial), UL18, UL19, UL20, and UL21 genes of herpes simplex virus and a putative origin of replication. Virology. 1992;191:900–908. doi: 10.1016/0042-6822(92)90265-q. [DOI] [PubMed] [Google Scholar]
- 24.Klupp B G, Lomniczi B, Visser N, Fuchs W, Mettenleiter T C. Mutations affecting the UL21 gene contribute to avirulence of pseudorabies virus vaccine strain Bartha. Virology. 1995;212:466–473. doi: 10.1006/viro.1995.1504. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs F, Mettenleiter T C. Firefly luciferase as a marker for herpesvirus (pseudorabies virus) replication in vitro and in vivo. J Gen Virol. 1991;72:2999–3008. doi: 10.1099/0022-1317-72-12-2999. [DOI] [PubMed] [Google Scholar]
- 26.Kritas S K, Nauwynck H J, Pensaert M B. Dissemination of wild-type and gC-, gE- and gI-deleted mutants of Aujeszky’s disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J Gen Virol. 1995;76:2063–2066. doi: 10.1099/0022-1317-76-8-2063. [DOI] [PubMed] [Google Scholar]
- 27.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky’s disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Gerguson A V. Subfornical organ efferents to paraventricular nucleus utilize angiotensin as a neurotransmitter. Am J Physiol. 1993;265:R302–R309. doi: 10.1152/ajpregu.1993.265.2.R302. [DOI] [PubMed] [Google Scholar]
- 29.Loewy A D. Pseudorabies virus: a transneuronal tracer for neuroanatomical studies. In: Knipe M G, Loewy A D, editors. Viral vectors. Gene therapy and neuroscience applications. San Diego, Calif: Academic Press, Inc.; 1995. pp. 349–366. [Google Scholar]
- 30.Lomniczi B, Blankenship M L, Ben-Porat T. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J Virol. 1984;49:970–979. doi: 10.1128/jvi.49.3.970-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984;52:198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomniczi B, Watanabe S, Ben-Porat T, Kaplan A S. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J Virol. 1987;61:796–801. doi: 10.1128/jvi.61.3.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallory S, Sommer M, Arvin A M. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGeoch D J. Evolutionary relationships of virion glycoprotein genes in the S regions of alphaherpesvirus genomes. J Gen Virol. 1990;71:2361–2367. doi: 10.1099/0022-1317-71-10-2361. [DOI] [PubMed] [Google Scholar]
- 35.Mettenleiter T C. Pseudorabies (Aujeszk’s disease) virus: state of the art. Acta Vet Hung. 1994;42:153–177. [PubMed] [Google Scholar]
- 36.Mettenleiter T C, Lomniczi B, Sugg N, Schreurs C, Ben-Porat T. Host cell-specific growth advantage of pseudorabies virus with a deletion in the genome sequences encoding a structural glycoprotein. J Virol. 1988;62:12–19. doi: 10.1128/jvi.62.1.12-19.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mettenleiter T C, Schreurs C, Zuckermann F, Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987;61:2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mettenleiter T C, Zsak L, Kaplan A S, Ben-Porat T, Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987;61:4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miselis R R. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural circuitry subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- 40.Rajcani J, Herget U, Kaerner H C. Spread of herpes simplex virus (HSV) strains SC16, ANG, ANGpath and its glyC minus and GlyE minus mutants in DBA-2 mice. Acta Virol. 1990;34:305–320. [PubMed] [Google Scholar]
- 41.Rinaman L, Card J P, Enquist L W. Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci. 1993;13:685–702. doi: 10.1523/JNEUROSCI.13-02-00685.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins A K, Ryan J P, Whealy M E, Enquist L W. The gene encoding the gIII envelope protein of pseudorabies virus vaccine strain Bartha contains a mutation affecting protein localization. J Virol. 1989;63:250–258. doi: 10.1128/jvi.63.1.250-258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shioya M, Tanaka J. Inputs from the nucleus of the solitary tract to subfornical organ neurons projecting to the paraventricular nucleus in the rat. Brain Res. 1989;483:192–195. doi: 10.1016/0006-8993(89)90054-1. [DOI] [PubMed] [Google Scholar]
- 44.Strick P L, Card J P. Transneuronal mapping of neural circuits with alphaherpesviruses. In: Bolam J P, editor. Experimental neuroanatomy. A practical approach. Oxford, United Kingdom: IRL Press; 1992. pp. 81–101. [Google Scholar]
- 45.Tirabassi R S, Enquist L W. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J Virol. 1999;73:2717–2728. doi: 10.1128/jvi.73.4.2717-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ugolini G. Transneuronal tracing with alpha-herpesviruses: a review of the methodology. In: Kaplitt M G, Loewy A D, editors. Viral vectors: gene therapy and neuroscience applications. San Diego, Calif: Academic Press, Inc.; 1995. pp. 293–317. [Google Scholar]
- 48.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zsak L, Mettenleiter T C, Sugg N, Ben-Porat T. Release of pseudorabies virus from infected cells is controlled by several viral functions and is modulated by cellular components. J Virol. 1989;63:5475–5477. doi: 10.1128/jvi.63.12.5475-5477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zsak L, Sugg N, Ben-Porat T. The different interactions of a gIII mutant of pseudorabies virus with several different cell types. J Gen Virol. 1992;73:821–827. doi: 10.1099/0022-1317-73-4-821. [DOI] [PubMed] [Google Scholar]
- 51.Zsak L, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992;66:2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]