Abstract
Objective:
Weight loss of ≥10% improves glucose control and may remit type 2 diabetes (T2D). High protein (HP) diets are commonly used for weight loss, but whether protein sources, especially red meat, impact weight loss-induced T2D management is unknown. This trial compared a HP diet including red meat and normal protein (NP) without red meat for weight loss, body composition changes, and glucose control in individuals with T2D.
Methods:
106 adults (80 female) with T2D consumed a HP (40% protein) diet with ≥4 weekly servings of lean beef or a NP (21% protein) diet excluding red meat during a 52-week weight loss intervention. Body weight, body composition, and cardiometabolic parameters were measured before and after intervention.
Results:
Weight loss was not different between HP (−10.2±1.6 kg) and NP (−12.7±4.8 kg, p=0.336). Both groups reduced fat mass and increased fat free mass percent. Hemoglobin A1c, glucose, insulin, insulin resistance, blood pressure, and triglycerides improved with no differences between groups.
Conclusions:
The lack of observed effects of dietary protein and red meat consumption on weight loss and improved cardiometabolic health suggest that achieved weight loss – rather than diet composition – should be the principal target of dietary interventions for T2D management.
Keywords: obesity, weight loss, body composition, type 2 diabetes
Introduction
Type 2 diabetes (T2D) affects over 30 million adults in America and presents numerous public health challenges [1]. T2D is a major risk factor for cardiovascular disease [2], kidney disease [3, 4], amputation [5–7], certain cancers [8], and blindness [9, 10], which results in a major cost burden to the healthcare system [1]. The primary risk factor for T2D is obesity, with the majority of those with T2D having overweight or obesity [11, 12]. Obesity also increases the risk of several other co-morbid conditions including heart disease and stroke [11, 13, 14]. It has been demonstrated that both T2D and obesity can be treated with lifestyle modification. For example, in DiRECT (Diabetes Remission Clinical Trial) weight loss of 10 to 15 kg resulted in the remission of T2D in a majority of individuals who had been who had been diagnosed with T2D within the past 6 years. Nearly 9 in 10 of individuals achieving more than 15 kg of weight loss remitted their T2D [15]. While it is clear that weight loss is associated with improvements in T2D, the role of diet composition in the reversal of T2D presents a gap in knowledge.
Higher protein diets are an attractive target for lifestyle-based interventions for the treatment of T2D. High protein diets, especially when combined with exercise, produce greater weight loss and prevent losses of fat free mass (FFM) compared to lower protein diets [16–18]. In premenopausal women with obesity without diabetes, a high protein diet improved insulin sensitivity more than a high carbohydrate diet even though achieved weight loss was not different between diets (high protein: 9.8 ± 1.4%, high carbohydrate: 9.3 ± 1.6%, p = 0.9323) [19]. Conversely, a study in post-menopausal women found that consuming a high protein diet during weight loss eliminated the beneficial effects of 10% weight loss on insulin action and sensitivity [20]. These conflicting results suggest that additional studies on the amount and sources of dietary protein during weight loss are needed to determine its influence on weight loss induced improvements in T2D.
Red meats –and beef in particular –are important contributors to dietary protein intakes in the United States [21]. However, some observational studies have associated red meat consumption with higher risk of T2D, leading to recommendations to limit its consumption [22, 23]. Recommendations to limit red meat consumption are based mostly on observational data, while findings from randomized clinical trials usually find a neutral effect of red meat consumption on health outcomes [24–28]. A recent meta-analysis found no differences in most glycemic and insulinemic risk factors associated with T2D when comparing reduced or no red meat diets to diets that contained red meat [29]. However, the impact on red meat consumption during weight loss among people with T2D remains ambiguous. Thus, it would be important to know whether beef can be part of a HP dietary plan to reverse T2D by contributing to weight and fat loss and improving weight loss maintenance.
The purpose of this randomized clinical trial was to compare a high protein diet (HP) versus a normal protein diet (NP) for weight loss, body composition changes, and indicators of type 2 diabetes status during a 52-week behavioral weight loss intervention. Both intervention diets were energy-restricted, and the HP diet included recommendations to include lean beef in the diet whereas the NP was instructed to refrain from eating any red meats for the duration of the stud. The hypotheses were the HP diet would lead to greater weight loss, preferential loss of fat mass compared to fat free mass, and greater improvements glucose control and cardiometabolic health.
Methods
Participants
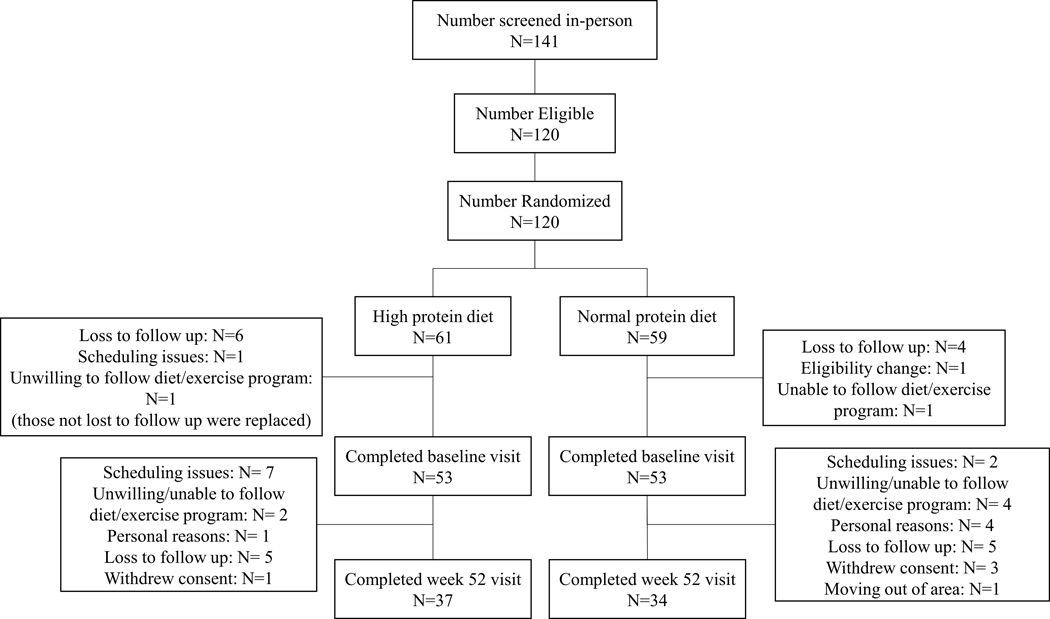
One hundred and six individuals (80 female) began the intervention and were recruited from the Denver, CO (n=39) and Birmingham, AL (n=67) metropolitan areas using letters, internet advertisements, and news advertisements to participate in the trial. A diagram depicting participant flow is presented in Figure 1. The study was conducted in three cohorts, with approximately 35 participants in each cohort. Cohort 1 was from the Denver, CO area and began the intervention in January 2020. Cohorts 2 and 3 were from the Birmingham, AL area and began in February 2020 and April 2021, respectively. Participants were required to be at least 18 years old, BMI ≥27 kg/m2, T2D diagnosis within the past 6 years (documented physician diagnosis, fasting glucose ≥126 mg/dl, or HbA1c ≥6.5%), be weight stable (±3 kg in the past 3 months), and be stable on all medications for the past 3 months. Regarding eligibility criteria for T2D diagnosis, participants were enrolled who had a recent diagnosis as described above without meeting the threshold for fasting glucose or HbA1c if that participant was on medication to manage their T2D, which would lower these values. Exclusion criteria were: HbA1c ≥ 12%, current eating disorder (anorexia or bulimia), dependence on illicit drugs or alcohol, untreated hypothyroidism, currently using insulin or other drugs known to cause weight loss or gain (including GLP-1 of SGLT-2 medications, steroids, tricyclic antidepressants, chemotherapy, antipsychotics, prescribed or OTC weight loss agent), following a vegetarian or vegan diet, any illness or injury that would make it unsafe to follow a diet and/or exercise up to 70 minutes at a moderate intensity regularly, and women who were pregnant, lactating, trying to become pregnant, or who had been pregnant or lactating in the last six months. Criteria for diabetes diagnoses were confirmed through medical records or doctor reports, blood biomarkers were confirmed via a blood test at the screening visit, and all other criteria were confirmed by self-report. The study protocol was reviewed and approved the Institutional Review Boards at the University of Alabama at Birmingham and University of Colorado Anschutz Medical Campus. The study was registered on clinicaltrials.gov as NCT03832933.
Figure 1. CONSORT Diagram.
Diagram of participant flow.
Experimental Design
All participants followed the State of Slim (SOS) weight management program for the first 16 weeks of the program, which consisted of weekly group classes led by a trained coach. Participants received copies of the SOS book, copies of the course materials, and access to the online community. After the first 16 weeks, participants participated in the SOS Next Steps program which consists of 18 bi-weekly group classes for the remainder of the intervention. Participants were randomly assigned to one of two diet groups: the high protein group (HP) with instructions to consume ≥4 weekly servings of lean beef as the only source of red meat or a normal protein group (NP) with instructions to not eat red meat for the duration of the study and followed a modified SOS diet that reduced protein intake.
Diet Intervention
The SOS plan is broken up into three distinct phases, each of which having food lists for participants to choose from as well as defined portion sizes for each food. Typically, the SOS plan is a high-protein, low fat diet plan that emphasizes non starchy and whole-grain carbohydrates. The SOS plan also has five diet rules that are to be followed throughout each phase: (1) Eat five to six times per day. (2) Eat breakfast within 1 hour of waking. (3) Do not count calories; instead, measure portions. (4) Have the right protein mix at each meal (one carbohydrate and one protein at each meal). (5) Eat a healthy fat twice a day. Food lists for the HP and NP groups were similar, with the exception of the HP group being asked to consume lean beef ≥4 times per week and the portion sizes for the protein being reduced for the NP group. Approximate carbohydrate and protein compositions were 32% and 40% of total energy for the HP diet, respectively and 53% and 21% for the NP diet, respectively. Recommended fat intakes were similar for HP (28% of total energy) and NP (26%). Food lists for each phase given to participants with adjusted portion sizes are presented in supplemental tables 1, 2, and 3. In addition, participants worked up to exercising up to 70 minutes per day, 6 days per week as a part of the program.
Self-reported energy intake and macronutrient distribution were not tracked during the study because a principal component of the SOS program is to focus on portion sizes as opposed to counting calories (Diet Rule #3). Participants did complete food logs throughout the intervention, however these were used as a self-monitoring tool to enhance weight loss [30], and not intended to measure energy intake or macronutrient distribution. Per the diet rules, a detailed food log designed to capture these data would be inconsistent with the program goals and structure. Further, self-reported measured of food intake are unreliable, and their suitability for clinical research has been questioned [31].
Participants were instructed that clinical decision making as regards T2D management was to be made with their primary care provider, but participants were asked to report any medication changes to research staff as soon as feasible. Study staff also queried participants on any medication changes on a monthly basis throughout the study period.
Protocol Modifications due to COVID-19
The original plan for this intervention was for SOS classes to be held in-person. The onset of lockdown orders in the spring of 2020 due to the COVID-19 pandemic required that classes be moved to an online platform (Zoom). The online intervention format was used for the remainder of the trial. The group classes were switched to the online format for cohort 1 (Colorado) at week 7 of the intervention and at week 4 for cohort 2 (Alabama). The intervention for cohort 3 (Alabama) was conducted entirely online. Weekly self-weighing was completed at home with pictures of scale weight sent to the health coaches in lieu of weighing in-person before each class. Additionally, the Week 16 study visits became at-home study visits for cohorts 1 and 2 with limited data collection due to university-wide restrictions on in-person clinical research at the University of Colorado Anschutz Medical Campus and the University of Alabama at Birmingham. Participants were sent a link to a video call with research staff, who conducted the visit. For this manuscript, baseline and week 52 data were used to assess study outcomes due to the limited data collection techniques used during the week 16 visits.
Anthropometric Measurements
Body weight was measured at baseline and week 52 using a digital platform scale (Colorado: Tanita BWB-800 Digital scale, Tanita Cooperation of America, Inc., Arlington Heights, Illinois; Alabama: DETECTO BRW1000, DETECTO, Webb City, MO) in a fasted state in clinic with participants wearing light clothing after voiding. Height was measured using a stadiometer in clinic at the screening visit. Body Mass Index (BMI, kg/m2) was calculated using these measurements. Body composition (fat and lean mass) was measured using dual x‐ray absorptiometry at baseline and week 52 (Colorado: Horizon W, APEX software version 5.6.05 Hologic, Inc., Marlborough, MA, USA; Alabama GE Lunar Prodigy Primo, enCORE software version 15.10.046, GE Healthcare, Chicago, IL, USA). Waist circumference (WC) was measured at the border of the iliac crest in duplicate in accordance with the National Institutes of Health recommendations [32], measured at baseline and week 52.
Cardiometabolic Health
Blood samples were obtained at baseline and week 52 from an antecubital vein by a trained phlebotomist. Samples were processed and analyzed for glucose, total cholesterol, low‐density lipoprotein cholesterol (LDL; calculated), high‐density lipoprotein cholesterol (HDL), triglycerides, HbA1c, and blood urea nitrogen (BUN). Blood samples were processed at the University of Alabama Outreach Lab for Alabama samples and at University of Colorado Hospital Clinical Lab for screening and at the Adult CTRC Core lab for baseline and week 52 visits for Colorado samples. Blood pressure was measured at each in-person study visit using at the left upper arm using an automatic sphygmomanometer (Colorado: Datascope Trio, (Serial# MC07547-A5) Digital Patient Monitor; Alabama, Omron 3-Series Upper Arm Blood Pressure Monitor, OMRON Healthcare Inc., Kyoto, Japan). Blood pressure was measured after the participant rested quietly in a seated position for ≥5 min with participants’ legs uncrossed and back and arms supported. The measurement was taken two times and if the reading differed by more than 5 mmHg, a third measurement was obtained.
Statistical Analyses
All study data were collected and managed using REDCap electronic data capture tools hosted at the University of Alabama at Birmingham and University of Colorado Denver [33, 34]. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for data integration and interoperability with external sources. All analyses were completed using SAS (version 9.4, 2002–2012 by SAS Institute Inc.).
A sample size of 120 participants was targeted based on findings from the Beef WISE Study [25] to detect a 2.75 kg difference in weight loss between the HP and NP. Weight loss achieved by the ‘Beef’ group in the Beef WISE Study was 8.9±6.0 kg, and this group received the same dietary plan and counseling as the HP group in the current study. Assuming a similar amount and variability in achieved weight loss, statistical power calculations indicated that sample size of 112 (56 per group) would provide >80% power (α = 0.05) to detect a 2.75 kg difference in weight loss.
Baseline characteristics were assessed by diet group (HP and NP) as well as the total of the whole sample. Randomization was performed by the statistician and was stratified by age, sex, BMI, and years since diagnosis of T2D. Differences in baseline characteristic were assessed using paired t-tests or Chi-squared tests. Linear mixed models (LMM) with unstructured covariance were used to test the effect of diet group, time, and their interaction term for changes in body weight and composition, and cardiometabolic health using and intention to treat approach (ITT), meaning that all participants that were randomized and have one or more measures were included in LMM analyses regardless of completion of protocol or adherence. Differences in frequency of reducing or discontinuing medication for T2D was assessed using Chi-squared tests. SAS 9.4 was used for all the analyses. P<0.05 is deemed statistically significant.
Results
Baseline Characteristics of Participants
Baseline characteristic of participants are presented in Table 1. No differences in any baseline characteristics were detected between HP and NP. Participant retention at 52 weeks did not differ between HP and NP (completed: HP n=37 (69.8%); NP n=34 (64.2%), p= 0.51).
Table 1. Baseline Characteristics of Participants.
Present baseline characteristics of participants by diet group assignment. Percent of sample (i.e. % female, % using medications for T2D), waist circumference, and blood biomarkers rounded to the nearest whole number (except HOMA-IR, HbA1c %, and insulin)
| Parameter | HP | NP |
|---|---|---|
| Age, y (M±SD) | 54.1 ± 12.0 | 55.4 ± 9.6 |
| Duration of T2D, y (M±SD) | 3.2 ± 1.8 | 3.2 ± 1.6 |
| Female, n (%) | 38 (72) | 42 (79) |
| Using Medications for T2D, n (%) | 48 (91) | 46 (87) |
| Anthropometrics (M±SD) | ||
| Weight, kg | 108.0 ± 22.8 | 107.8 ± 26.6 |
| BMI, kg/m2 | 38.7 ± 6.8 | 38.8 ± 7.3 |
| Waist Circumference, cm | 118 ± 14 | 117 ± 15 |
| Fat Mass, % | 46.2 ± 6.2 | 46.6 ± 5.9 |
| Fat-Free Mass, % | 52.9 ± 6.1 | 52.4 ± 5.8 |
| Biomarkers (M±SD) | ||
| HOMA-IR | 7.6 ± 7.0 | 5.9 ± 3.4 |
| Glucose, mg/dL | 134 ± 38 | 130 ± 40 |
| HbA1c, % | 7.2 ± 1.0 | 7.0 ± 1.3 |
| Insulin, μIU/mL | 22.3 ± 16.5 | 18.1 ± 11.1 |
| BP Systolic, mmHg | 135 ± 15 | 135 ± 15 |
| BP Diastolic, mmHg | 86 ± 9 | 86 ± 9 |
| Triglycerides, mg/dL | 138 ± 71 | 139 ± 58 |
| HDL Cholesterol, mg/dL | 47 ± 11 | 45 ± 9 |
| LDL Cholesterol, mg/dL | 94 ± 28 | 95 ± 36 |
| Race, n (%) | ||
| White | 37 (70) | 30 (57) |
| Black | 11 (21) | 19 (39) |
| Asian | 3 (6) | 1 (2) |
| Native Hawaiian or Pacific Islander | 1 (2) | 0 (0) |
| Other | 1 (2) | 3 (6) |
| Ethnicity, n (%) | ||
| Hispanic | 6 (11) | 9 (17) |
| Non-Hispanic | 46 (87) | 43 (81) |
| Did not report | 1 (2) | 1 (2) |
Abbreviations: BMI, body mass index; BP, blood pressure; cm, centimeter; dL, deciliter; HDL, high‐density lipoprotein cholesterol; HbA1c, hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; HP, high protein; kg, kilogram; LDL, low‐density lipoprotein cholesterol; M, mean; m, meter; mg, milligrams; ml, milliliter; mmHg, millimeters of mercury; NP, normal protein; SD, standard deviation; T2D, type 2 diabetes; y, years; μIU, micro international unit.
Adverse Events
No adverse events likely to be related to the study were reported by participants in either group.
Weight Loss and Body Composition
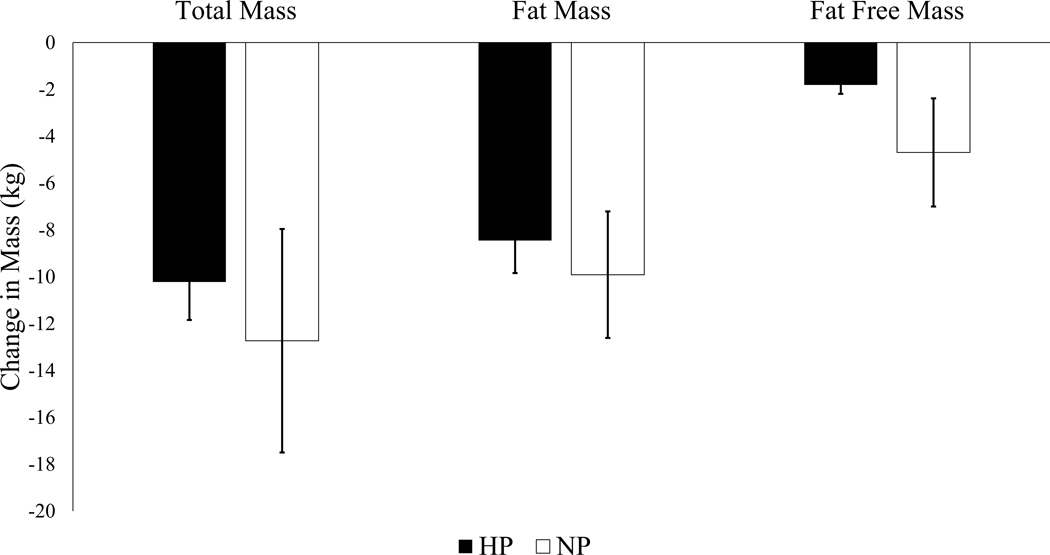
Changes in body weight and composition are shown in Figure 2. Total mass was reduced by 10.2±1.6 kg (9.4%) in the HP group and 12.7±4.8 kg (11.8%) in the NP group (Figure 2), with no difference between groups (p=0.336). Fat mass percent decreased (HP, 46.2±0.8% vs. 41.9±1.1%, p<0.001; NP, 46.6±0.8% vs. 42.8±1.6%, p<0.001) and fat free mass percent increased (HP, 52.9±0.8% vs. 57.1±1.1%, p<0.001; NP, 51.8±1.6%, vs. 55.4±0.8% p<0.001) in both groups during the intervention period with no significant difference between HP and NP (fat mass percent, p = 0.665; fat free mass percent, p = 0.689). Both the HP and NP groups reduced waist circumference (HP, 118±2.0 cm vs. 111±2.1 cm, p=<0.001; NP, 117±2 cm vs. 109±2 cm, p=<0.001) and BMI (HP, 38.7±1.0 kg/m2 vs. 35.0±1.0 kg/m2, p=<0.001; NP, 38.8±1.0 kg/m2 cm vs. 34.4±1.0 kg/m2, p=<0.001) from baseline to week 52, with no differences between groups (waist circumference, p=0.934; BMI, p=0.421). Supplemental figure 1 includes additional plots related to body composition changes by group.
Figure 2. Change in Body Weight and Composition.
Depicts changes in weight and composition (fat and fat free mass) in kilograms from baseline to week 52 by diet group. Mixed effects models used to test the effect of diet group, time, and their interaction term on body weight and composition changes. Presented as LSMEANS±SE. Both groups had a significantly reduced weight (HP −10.2±1.6 kg; NP −12.5±1.6 kg), fat mass (HP −8.4±1.4 kg; NP −9.1±1.5 kg), and fat-free mass (HP −1.8±0.4 kg; NP −2.9±0.4 kg), but the majority of weight loss was due to fat mass loss. There were no differences in outcomes by diet group in changes in total mass (p=0.333), fat mass (p=0.735), or fat free mass p=0.056).
Indicators of Type 2 Diabetes
Changes in indicators of type 2 diabetes are presented in Table 2. In general, participants reduced HbA1c, fasting glucose and HOMA-IR with no differences in changes between HP and NP. Data describing participants with HbA1c ≥6.5% or fasting glucose ≥126 mg/dL (clinically diagnostic of T2D) at baseline and week 52 by diet group are presented in Table 3. At baseline, 73.6% of participants in the HP group and 62.3% of participants in the NP group had biomarkers in the range for T2D. Using the ITT approach, 24.5% and 22.6% of participants in the HP and NP groups respectively reduced these values to no longer meet diagnostic criteria for T2D. Using the completer analyses, these numbers are 38.2% and 38.5% for the HP and NP groups respectively. Four participants in the HP group and 1 participant in the NP group were classified as having a negative change, with values at baseline not meeting criteria for T2D diagnosis, but meeting these criteria at week 52. There were no differences between diet groups for any changes in T2D indicators. Also during the trial, n=14 participants (HP, n=5; NP, n=9) discontinued all T2D medications and n=16 (HP, n=7; NP, n=9) reduced at least one T2D medication. The frequency of discontinuing or reducing T2D medications was not statistically different between HP and NP (p=0.4358).
Table 2.
Changes in Indicators of Type 2 Diabetes by Diet Group
| Parameter | Group | Baseline | Week 52 | Mean Change | 95% CI for Change | P-value |
|---|---|---|---|---|---|---|
| Glucose, mg/dL | HP | 134 (5) | 115 (6) | −19 (6) | (−31.0, −6.8) | |
| NP | 130 (5) | 111 (6) | −19 (6) | (−31.4, −6.4) | 0.999 | |
| HbA1c, % | HP | 7.2 (0.2) | 6.4 (0.2) | −0.8 (0.2) | (−1.1, −0.4) | |
| NP | 7.0 (0.2) | 6.5 (0.2) | −0.5 (0.2) | (−0.9, −0.1) | 0.329 | |
| Insulin, μIU/mL | HP | 22.3 (1.9) | 14.5 (1.7) | −7.8 (2.0) | (−11.8, −3.7) | |
| NP | 18.8 (1.9) | 11.0 (1.8) | −7.8 (2.0) | (−12.0, −3.7) | 0.979 | |
| HOMA-IR | HP | 7.6 (0.8) | 4.5 (0.7) | −3.1 (0.8) | (−4.7, −1.6) | |
| NP | 5.9 (0.8) | 3.2 (0.7) | −2.7 (.8) | (−4.2, −1.1) | 0.657 |
Values presented as LS MEANS (SE) with glucose values rounded to nearest whole number. Values are model based from the ITT analysis performed, including LS MEANS, change, and 95% CI.
P-value represents differences in change between diet groups (HP vs. NP)
Mixed effects model used to test the effect of time, group, and their interaction term on changes in indicators of type 2 diabetes. Both diet groups reduced, glucose, HbA1c, insulin, and HOMA-IR from baseline to week 52. There were no differences in change in any parameters by diet group (HP vs NP)
Abbreviations: dL, deciliter; HbA1c, hemoglobin A1c; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; HP, high protein; mg, milligrams; mL, milliliter; NP, normal protein; μIU, micro international unit.
Table 3. Changes in Type 2 Diabetes Status.
Presents changes in status of Type 2 Diabetes as indicated by HbA1c ≥6.5% or fasting glucose ≥126 mg/dL (clinically diagnostic of T2D) from baseline to week 52 of the trial.
| ITT Approach | HP n (%) | NP n (%) |
|---|---|---|
| Remained in abnormal range | 7 (13) | 12 (23) |
| Remained in normal range | 12 (19) | 9 (17) |
| Positive change (abnormal to normal value) | 13 (25) | 12 (23) |
| Negative change (normal to abnormal) | 4 (8) | 1 (2) |
| Unknown (no paired value) | 19 (36) | 16 (30) |
|
| ||
| Completers Analysis | ||
|
| ||
| Remained in abnormal range | 7 (21) | 12 (33) |
| Remained in normal range | 10 (2) | 9 (25) |
| Positive change (abnormal to normal value) | 13 (38) | 14 (39) |
| Negative change (normal to abnormal) | 4 (12) | 1 (3) |
Intention to treat (ITT) and completers analyses are presented. Unknown in the ITT approach refers to participants without either a baseline or week 52 value, therefore no conclusions could be made regarding change in status. Abnormal value refers to lab values consistent with T2D diagnosis where normal range refers to values below the criteria for T2D diagnosis. Percentages rounded to nearest whole number.
Laboratory Markers
Changes in blood pressure, lipids, and BUN are summarized in Table 4. Participants reduced systolic and diastolic blood pressures, and triglycerides with no differences between groups. BUN was increased in both groups at week 52 compared to baseline with no difference between HP and NP. There were no changes in total cholesterol, HDL cholesterol, or LDL cholesterol in either group over the duration of the intervention.
Table 4. Laboratory Markers Changes by Diet Group.
Values presented as LS MEANS (SE) rounded to the nearest whole number. Values are model based from the ITT analysis performed, including LS MEANS, change, and 95% CI.
| Parameter | Group | Baseline | Week 52 | Mean Change | 95% CI for Change | P-value |
|---|---|---|---|---|---|---|
| BP Systolic, mmHg | HP | 135 (2) | 126 (3) | −9 (2) | (−13.0, −5.1) | |
| NP | 135 (2) | 127 (3) | −8 (2) | (−12.2, −3.9) | 0.719 | |
| BP Diastolic, mmHg | HP | 86 (1) | 78 (1) | −7 (2) | (−10.2, −4.2) | |
| NP | 86 (1) | 81 (2) | −6 (2) | (−8.6, −2.3) | 0.430 | |
| Triglycerides, mg/dL | HP | 138 (9) | 114 (10) | −25 (9) | (−42.8, −6.1) | |
| NP | 139 (9) | 110 (10) | −29 (10) | (−48.0, −10.0) | 0.732 | |
| HDL Cholesterol, mg/dL | HP | 47 (1) | 47 (2) | 2 (1) | (−2.1, 2.9) | |
| NP | 45 (1) | 47 (2) | 1 (2) | (−0.8, 4.4) | 0.448 | |
| LDL Cholesterol, mg/dL | HP | 94 (4) | 91 (5) | −3 (4) | (−11.1, 4.6) | |
| NP | 95 (4) | 94 (5) | −1 (4) | (−9.3, 6.9) | 0.718 | |
| BUN | HP | 15 (1) | 17 (1) | 3 (1) | (1.5, 4.2) | |
| NP | 15 (6) | 18 (1) | 3 (1) | (1.2, 4.0) | 0.770 |
P-value represents differences in change between diet groups (HP vs. NP)
Mixed effects model used to test the effect of time, group, and their interaction term on changes in laboratory markers. Both diet groups reduced systolic and diastolic blood pressures and triglycerides from baseline to week 52. Neither group had changes in HDL or LDL cholesterol from baseline to week 52. Both groups increased BUN from baseline to week 52. There were no differences in change in any parameters by diet group (HP vs NP)
Abbreviations: BUN, blood urea nitrogen; BP, blood pressure; dL, deciliter; HDL, high‐density lipoprotein cholesterol; HP, high protein; LDL, low‐density lipoprotein cholesterol; mg, milligrams; mmHg, millimeters of mercury; NP, normal protein.
Discussion
Both the HP and NP diet groups significantly reduced weight and improved in key indicators of T2D with no difference between groups. These findings support data suggesting that weight loss is the primary driver of improvements in glucose control. Results from the Look AHEAD (Action for Health in Diabetes) study found that those with a 5–10% reduction in weight had increased odds of achieving a 0.5% reduction in HbA1c (odds ratio 3.52) [35]. Evidence from review papers also suggests that modest weight loss can successfully result in remission of T2D in many individuals [36]. Findings from the current study further support the notion that weight loss can produce improvements in glucose control in many with T2D. Average weight loss in the current study was consistent with that found to cause improvements in T2D in the majority of participants in DiRECT [15]. Our results extend those of previous research by demonstrating that weight loss is an effective treatment for T2D.
Contrary to the hypothesis, the HP diet did not result in greater weight loss when compared to the NP diet. Instead, the groups had similar weight loss and body composition changes following the intervention. It was also hypothesized that the HP group would result in preferential loss of fat mass compared to fat free mass which was also not supported. A recent review suggested that a weight loss could increase risk of mortality for those recently diagnosed with T2D potentially as a result in a decline in appendicular lean mass [37]. While appendicular body composition was not analyzed for the present study, whole-body fat free mass was only slightly reduced with no significant differences between groups. The preferential loss of fat mass in both diet groups in the present study lessens concerns related to potential weight loss-related adverse events.
Results from this study also add to the literature regarding the impact of HP diets during weight loss for individuals with T2D. The HP and NP groups had similar reduction in indices of T2D, including HbA1c and fasting glucose. Some trials have demonstrated that a HP diet is more beneficial than a high carbohydrate diet in outcomes associated with T2D [19, 38]. However, One trial in post-menopausal women demonstrated that a HP diet during weight loss could negatively insulin action [20], however this did not seem to be the case in the present study, as marked by similar improvements in HOMA-IR across groups. In the present study, at baseline, 73.6% of participants in the HP group and 62.3% of participants in the NP group had biomarkers in the range for T2D. Notably, inclusion into the study was based on previously diagnosed T2D but did not require participants to present with a diagnostic value for A1c or fasting glucose at baseline. This resulted in some participants having A1c or fasting glucose below the diabetic range at study entry. This is likely because many participants were taking T2D medications at baseline and they were not required to discontinue medications at study entry as has been done in other studies such as DiRECT [15]. Participants exhibiting indices of T2D was reduced to 35.1% and 32.4% in the HP and NP groups, respectively. These findings strongly suggest that achieved weight loss – regardless of dietary pattern – is the primary factor driving improvements in glucose control. This finding has substantial public health implications as it provides a degree of individual-level flexibility in choosing a dietary pattern that is consistent with patient preferences.
These results demonstrate that the inclusion of lean, minimally processed beef does not impact the effectiveness of an energy restricted diet to induce weight loss and improvements in cardiometabolic health. Previous work from our group found equivalent changes in body weight and composition between a HP diet including beef and a HP diet excluding all red meat [25]. The present study builds on this work, through the investigation of two diets with differing recommended macronutrient compositions. From these data, it is evident that minimally processed, lean beef can be safely included in diets when attempting to lose weight and control glucose.
A limitation of this trial was that despite giving explicit diet rules and lists to participants, both groups saw an equivalent increase in BUN from baseline to week 52. This finding could indicate that the protein composition of the diet groups likely did not reach the intended macronutrient distributions, with the groups consuming similar amounts of protein. The study might not have been able to detect the true impact on improvements in T2D markers because participants discontinued medication as recommended by their primary care providers. This could mean that the observed intervention could have had a greater effect if those participants had stayed on their medications. Additionally, the COVID-19 pandemic began during the first cohort of the AL and CO groups and lasted throughout the rest of the study. The onset of the pandemic resulted in several methodological changes of the study and impacted the lives of participants taking part in the intervention. Fortunately, the SOS program had been previously delivered in a virtual format, so changes to course content were minor. Classes were still able to be held at the usual time, but in a virtual setting. However, research study visits were completely halted for a period during the study, hence the completion of some of the week 16 visits virtually instead of in-person. This change limited the data that could be collected at this timepoint. In addition, some outcome measures tests that were originally collected in person could no longer be collected at all, even when restrictions were lifted, including resting energy expenditure and six-minute walk test. These two tests were removed because masks were still required in the research facility and since baseline measurements were collected without masks, it was unclear if the use of masks at future visits would impact results. Some participants who withdrew from the study cited COVID-19 as a primary reason for leaving, such as increased work demands. Total attrition overall was high at 33%, which is likely due in part to the challenges faced during the COVID-19 pandemic. Despite challenges, the study team was able to adapt swiftly to the nature of the pandemic and continue the intervention and data collection.
These result show that behavioral weight loss programs can produce significant weight loss in those with T2D and that this weight loss can improve glucose control and many other aspects of cardiometabolic health. Results also show that avoiding red meat does not provide an advantage either in weight loss or in disease management.
Supplementary Material
What is already known about this subject?
Weight loss of ≥10% improves can improve glucose control and even remit type 2 diabetes for some
High protein diets can produce greater weight loss and prevent loss of fat free mass compared to diets lower in protein
Some observational studies recommend limiting red meat consumption to reduce risk for type 2 diabetes, but data from randomized clinical trials generally find little to no independent effect of lean red meat consumption.
What are the new findings in your manuscript?
Both a normal protein diet excluding red meat and a high protein diet containing red meat are effective at producing weight loss and improvements in glucose control
How might your results change the direction of research or the focus of clinical practice?
Weight loss, not diet composition, is the primary driver of type 2 diabetes management
Avoiding red meat does not provide additional benefit for weight loss or improvements in glucose control during a weight loss intervention
Acknowledgements
The authors acknowledge Jeanne Anne Breen and Aaron Chestnut for their effort in the recruitment, retention, and completion of study visits. The authors would also like to acknowledge Marsha Miller for coaching the SOS classes at the CO site. Finally, the authors acknowledge the participants for their contribution to the study and time and effort put forth as a part of this trial. Funding for the parent trial was provided by the Beef Checkoff. This work was also supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 and by Award Number P30DK056336 from the National Institute Of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health. The corresponding author will cooperate with any requests for data sharing. Any data described in the manuscript will be made available upon request pending application and approval.
Funding:
Funding for this study was provided by The Beef Checkoff/National Cattlemen’s Beef Association and the University of Alabama at Birmingham, Nutrition Obesity Research Center. The financial supporters had no role in the design and conduct of the study or collection, analysis, and interpretation of the data.
JOH and HRW have received royalties from the book, State of Slim. They have ownership in Shakabuku LLC, which offers weight loss to the public. HRW has received grant support for unrelated studies from Gelesis, Novo Nordisk, Epitome, and General Mills, Inc., and has done consulting for Gelesis and reports speaking fees from Novo Nordisk, and the National Cattlemen’s Beef Association. JOH has received grant funding for unrelated studies from Gelesis and has done consulting for Gelesis, General Mills, Inc., and McCormick Science Institute. RDS has received grant support for an unrelated study from General Mills, Inc., and reports speaking fees from the Texas Beef Council.
Footnotes
Clinical Trial Registration: National Clinical Trial NCT03832933
Disclosure:
All other authors declare no conflicts of interest.
References
- 1.Control, C.f.D. and Prevention, National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, 2014. 2014. [Google Scholar]
- 2.Einarson TR, et al. , Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovascular Diabetology, 2018. 17(1): p. 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkarian M, et al. , Kidney disease and increased mortality risk in type 2 diabetes. Journal of the American Society of Nephrology, 2013. 24(2): p. 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koye DN, et al. , The global epidemiology of diabetes and kidney disease. Advances in chronic kidney disease, 2018. 25(2): p. 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller IS, et al. , Foot ulceration and lower limb amputation in type 2 diabetic patients in Dutch primary health care. Diabetes care, 2002. 25(3): p. 570–574. [DOI] [PubMed] [Google Scholar]
- 6.Shatnawi NJ, et al. , Predictors of major lower limb amputation in type 2 diabetic patients referred for hospital care with diabetic foot syndrome. Diabetes, metabolic syndrome and obesity: targets and therapy, 2018. 11: p. 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh N, Armstrong DG, and Lipsky BA, Preventing foot ulcers in patients with diabetes. Jama, 2005. 293(2): p. 217–228. [DOI] [PubMed] [Google Scholar]
- 8.Berster JM and Göke B, Type 2 diabetes mellitus as risk factor for colorectal cancer. Archives of physiology and biochemistry, 2008. 114(1): p. 84–98. [DOI] [PubMed] [Google Scholar]
- 9.Cheung N. and Wong TY, Diabetic retinopathy and systemic vascular complications. Progress in retinal and eye research, 2008. 27(2): p. 161–176. [DOI] [PubMed] [Google Scholar]
- 10.Sasongko MB, et al. , Prevalence of diabetic retinopathy and blindness in Indonesian adults with type 2 diabetes. American journal of ophthalmology, 2017. 181: p. 79–87. [DOI] [PubMed] [Google Scholar]
- 11.Albu J. and Pi-Sunyer FX, Obesity and diabetes, in Handbook of obesity. 2003, CRC Press. p. 915–934. [Google Scholar]
- 12.Maggio CA and Pi-Sunyer FX, Obesity and type 2 diabetes. Endocrinology and Metabolism Clinics, 2003. 32(4): p. 805–822. [DOI] [PubMed] [Google Scholar]
- 13.Apovian CM, Obesity: definition, comorbidities, causes, and burden. Am J Manag Care, 2016. 22(7 Suppl): p. s176–85. [PubMed] [Google Scholar]
- 14.Schelbert KB, Comorbidities of obesity. Primary Care: Clinics in Office Practice, 2009. 36(2): p. 271–285. [DOI] [PubMed] [Google Scholar]
- 15.Lean ME, et al. , Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. The Lancet, 2018. 391(10120): p. 541–551. [DOI] [PubMed] [Google Scholar]
- 16.Wycherley TP, et al. , Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. The American journal of clinical nutrition, 2012. 96(6): p. 1281–1298. [DOI] [PubMed] [Google Scholar]
- 17.Wycherley TP, et al. , A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes care, 2010. 33(5): p. 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leidy HJ, et al. , The role of protein in weight loss and maintenance. The American journal of clinical nutrition, 2015. 101(6): p. 1320S–1329S. [DOI] [PubMed] [Google Scholar]
- 19.Kitabchi AE, et al. , Effects of high-protein versus high-carbohydrate diets on markers of β-cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes: a randomized controlled trial. Diabetes care, 2013. 36(7): p. 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith Gordon I., et al. , High-Protein Intake during Weight Loss Therapy Eliminates the Weight-Loss-Induced Improvement in Insulin Action in Obese Postmenopausal Women. Cell Reports, 2016. 17(3): p. 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniel CR, et al. , Trends in meat consumption in the USA. Public health nutrition, 2011. 14(4): p. 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnard N, Levin S, and Trapp C, Correction: Barnard N, et al. Meat Consumption as a Risk Factor for Type 2 Diabetes. Nutrients 2014, 6, 897–910. Nutrients, 2014. 6(10): p. 4317–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan A, et al. , Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA internal medicine, 2013. 173(14): p. 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayer RD, et al. , Dietary Approaches to Stop Hypertension diet retains effectiveness to reduce blood pressure when lean pork is substituted for chicken and fish as the predominant source of protein. The American journal of clinical nutrition, 2015. 102(2): p. 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayer R, et al. , Equivalent reductions in body weight during the Beef WISE Study: beef’s role in weight improvement, satisfaction and energy. Obesity science & practice, 2017. 3(3): p. 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roussell MA, et al. , Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. The American journal of clinical nutrition, 2012. 95(1): p. 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roussell M, et al. , Effects of a DASH-like diet containing lean beef on vascular health. Journal of human hypertension, 2014. 28(10): p. 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magkos F, et al. , Unprocessed red meat in the dietary treatment of obesity: a randomized controlled trial of beef supplementation during weight maintenance after successful weight loss. The American Journal of Clinical Nutrition, 2022. [DOI] [PMC free article] [PubMed]
- 29.Sanders LM, Wilcox ML, and Maki KC, Red meat consumption and risk factors for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. European Journal of Clinical Nutrition, 2022. [DOI] [PMC free article] [PubMed]
- 30.Butryn ML, et al. , Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring), 2007. 15(12): p. 3091–6. [DOI] [PubMed] [Google Scholar]
- 31.Dhurandhar NV, et al. , Energy balance measurement: when something is not better than nothing. International journal of obesity, 2015. 39(7): p. 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obesity, N.A.A.f.t.S.o., et al. , The practical guide: identification, evaluation, and treatment of overweight and obesity in adults. 2000: National Institutes of Health, National Heart, Lung, and Blood Institute; …. [Google Scholar]
- 33.Harris PA, et al. , The REDCap consortium: Building an international community of software platform partners. Journal of biomedical informatics, 2019. 95: p. 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, et al. , Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 2009. 42(2): p. 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing RR, et al. , Benefits of Modest Weight Loss in Improving Cardiovascular Risk Factors in Overweight and Obese Individuals With Type 2 Diabetes. Diabetes Care, 2011. 34(7): p. 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau DCW and Teoh H, Benefits of Modest Weight Loss on the Management of Type 2 Diabetes Mellitus. Canadian Journal of Diabetes, 2013. 37(2): p. 128–134. [DOI] [PubMed] [Google Scholar]
- 37.Huang S, et al. , Association of magnitude of weight loss and weight variability with mortality and major cardiovascular events among individuals with type 2 diabetes mellitus: a systematic review and meta-analysis. Cardiovascular Diabetology, 2022. 21(1): p. 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAuley K, et al. , Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia, 2005. 48: p. 8–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.