Abstract
Heparin-induced thrombocytopenia (HIT) and vaccine-induced immune thrombotic thrombocytopenia (VITT) are highly prothrombotic (thrombosis frequency ≥50%). Both are caused by platelet-activating anti-platelet factor 4 (PF4) antibodies, forming PF4/IgG-containing immune complexes that engage platelet FcγIIa receptors, producing strong platelet activation. In HIT, heparin crosslinks several PF4 molecules, whereas in VITT, anti-PF4 antibodies alone crosslink PF4. Sufficient levels of circulating anti-PF4 antibodies are needed to create the pathogenic immune complexes on platelet surfaces; this explains why certain serum (plasma)-based assays are highly sensitive for detecting HIT/VITT antibodies. Accordingly, HIT and VITT are “clinical-pathological” disorders, that is, positive testing for such antibodies—together with a compatible clinical picture—is integral for diagnosis. Heparin (low concentrations) enhances HIT antibody-induced platelet activation, but platelet activation by VITT sera is usually inhibited by heparin. For both HIT and VITT, high sensitivity (>99% and >95%, respectively) characterizes PF4-dependent enzyme immunoassays (EIAs) and PF4-enhanced platelet activation assays; in contrast, certain rapid immunoassays have high sensitivity for HIT (>90-97%) but poor sensitivity (<25%) for VITT. HIT and VITT antibodies are directed at distinct sites on PF4: solid-phase EIAs and platelet activation assays are indifferent to these distinct antigen targets, but rapid immunoassays are not. We discuss a conceptual model where PF4 is viewed as a “globe,” with the heparin-binding site the “equator”; in this model, HIT antibodies are primarily directed at antigen site(s) at the north and south “poles” of PF4 (formed when PF4 binds to heparin), whereas VITT antibodies recognize sites on the equator.
Keywords: enzyme immunoassay, heparin-induced thrombocytopenia, platelet factor 4, platelet-activating antibodies, vaccine-induced immune thrombotic thrombocytopenia
Heparin-induced thrombocytopenia (HIT) is an antibody-mediated, prothrombotic drug reaction characterized in 1973 as a distinct disorder, featuring thrombocytopenia beginning approximately 1 week after the start of heparin, frequent association with thrombosis, and detectability of heparin-dependent, platelet-activating antibodies. 1 The platelet-activating nature of HIT became established over the next decade. 2 Two groups subsequently showed that using “washed” platelets enhanced antibody detectability. 3 4 In 1992, Amiral and colleagues 5 identified the platelet-associated protein, platelet factor 4 (PF4), as the target protein of HIT: in essence, (cationic) PF4 bound to (anionic) heparin, resulting in formation of heparin-dependent antigens on PF4.
In the past half century, the concept of platelet-activating anti-PF4 disorders has expanded beyond that of classic heparin-dependent HIT. 6 As we will discuss, anti-PF4 disorders induced by heparin sometimes feature platelet-activating antibodies that do not require heparin for pathogenicity. Furthermore, platelet-activating anti-PF4 antibodies rarely are triggered by environmental factors distinct from heparin itself, an entity called “spontaneous HIT” 7 (some authors prefer alternate designations—such as “spontaneous HIT-like syndrome” 8 —to avoid inferring involvement of heparin).
Furthermore, in March 2021, a novel prothrombotic disorder 9 was recognized that was precipitated by vaccination with an adenoviral vector vaccine, ChAdOx1 nCoV-19, used for preventing symptomatic infection with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), the cause of coronavirus disease 2019 (COVID-19). This striking disorder, subsequently named “vaccine-induced immune thrombotic thrombocytopenia (VITT),” was promptly identified as a HIT-mimicking anti-PF4 disorder, based on the following: (1) its clinical picture (thrombocytopenia, venous/arterial thromboses, hypercoagulability) resembled severe HIT; (2) the temporal onset approximately in the second week post-vaccination suggested a “point immunization” event akin to the heparin trigger of HIT; and (3) screening patients with putative VITT showed generally strong reactivity in PF4-dependent enzyme immunoassays (EIAs). Finally, (4) tests for platelet-activating antibodies were also generally positive, although supplementation with PF4 was frequently required, and heparin often appeared to inhibit—rather than augment—platelet activation.
Anti-PF4 disorders should be viewed as “clinical-pathological” disorders; that is, diagnosis requires a compatible clinical picture (thrombocytopenia, thrombosis, or both) and detectability of platelet-activating anti-PF4 antibodies. Pathogenic antibodies against PF4 can be directed against different regions on PF4, some corresponding to wholly or predominantly heparin-dependent antibodies but also against other antigen sites that do not require heparin for pathogenicity. Our review will address laboratory testing for HIT and VITT antibodies, in historical order of assay development 10 : (1) platelet activation assays, (2) PF4-dependent EIAs, and (3) rapid PF4-dependent immunoassays. Reference laboratories should be able to perform more than one type of EIA, and also possess capacity for PF4 supplementation for platelet activation assays.
Dimeric Antigen Structure of PF4
PF4 is a cationic homotetrameric protein to which anionic heparin binds. 11 The PF4 tetramer bears a circumferential “ring of positive charge,” which comprises the heparin-binding sites. A key concept is that the tetrameric protein, PF4, is composed of two identical homodimers; thus, the HIT antigen sites are duplicated, which allows for binding of two HIT (or VITT) antibodies to one PF4 molecule. PF4's dimeric antigenic structure is the prerequisite to form large multimeric PF4–IgG immune complexes (discussed subsequently). Thus, the anti-PF4 immune response is rather unique in comparison with other drug-dependent antibodies or autoantibodies.
Heparin produces close approximation of several PF4 molecules, which results in conformational changes on PF4, exposing heparin-dependent antigens. Resulting binding of HIT IgG, if present, creates multimolecular PF4/IgG/heparin immune complexes, which bind to (low affinity) FcγIIa receptors on platelet surfaces; cross-linking of FcγIIa receptors causes strong platelet activation. 12 13 14 Experimental data show that HIT antibodies also activate leukocytes, 15 16 a feature irrelevant for diagnostic assays.
A highly schematized depiction of PF4 emphasizes its resemblance to a “globe,” with the “equator” representing the cationic ring of positive charge to which heparin binds ( Fig. 1 ). 6 In the presence of heparin, antigens are formed at the north and south “poles.” In contrast, heparin-independent antigens are formed at two sites on the “equator,” on opposite sides of the globe (which for illustrative purposes can be considered as the two sites where the “prime meridian” intersects the equator, at 0 and 180 degrees of longitude). VITT antibodies are oligoclonal/monoclonal, 17 and so an individual patient's VITT antibodies will all react to the same (or closely situated) target region on PF4; however, the target antigens likely differ to a minor extent among different VITT patients.
Fig. 1.
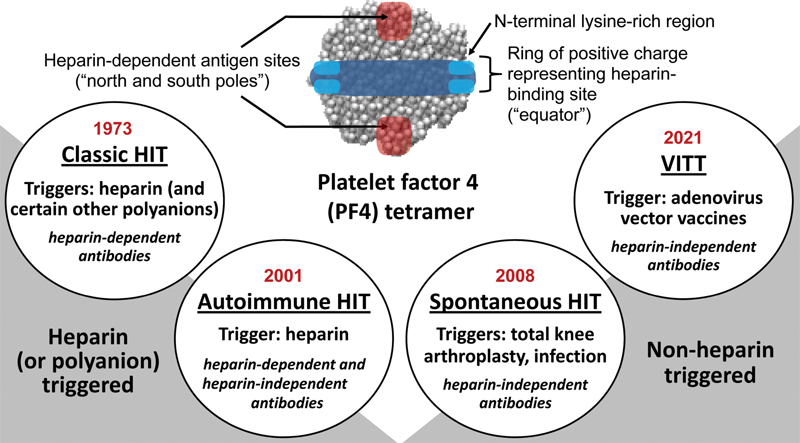
Four anti–platelet factor 4 (PF4) disorders. A highly schematized depiction of the PF4 tetramer shows the “ring of positive charge” composed of four N-terminal lysine-rich regions (light blue) and a circumferential region rich in arginine and other lysine residues (dark blue). (Arginine and lysine are cationic amino acids.) The ring of positive charge represents the heparin-binding region; when heparin binds to the ring of positive charge, two (duplicate) heparin-dependent antigen sites form at opposite sites on the PF4 tetramer. In this model, PF4 can be conceptualized as the “globe,” with an “equator” indicating the ring of positive charge, and the “north and south poles” the duplicated heparin-dependent antigen sites. Each circle represents one of the four anti-PF4 disorders, listed in historical order (from left to right), indicating year of recognition, trigger(s), and nature of platelet-activating antibodies (heparin-dependent and/or heparin-independent antibodies). Note that very high (suprapharmacologic) heparin concentrations will inhibit reactivity of all anti-PF4 antibodies. Thus, the designation, heparin-dependent and heparin-independent, refers to whether—at pharmacological heparin concentrations (0.1–0.5 U/mL heparin)—heparin enhances platelet activation by anti-PF4 antibodies (heparin-dependent), or whether heparin has no enhancing platelet-activating effect (it may have platelet-inhibiting effects) and/or there is substantial antibody-induced platelet activation even in the absence of any heparin (heparin-independent). The figure also illustrates that classic HIT and autoimmune HIT (aHIT) are heparin-triggered, whereas nonheparin triggers are implicated in spontaneous HIT and VITT. HIT, heparin-induced thrombocytopenia; VITT, vaccine-induced immune thrombotic thrombocytopenia. (Reprinted with permission from Warkentin TE. Platelet-activating anti-PF4 disorders: an overview. Semin Hematol 2022;59(02):59-71.)
Historical Overview of Anti-PF4 Disorders
Fig. 1 also provides a historical overview of anti-PF4 disorders. 6 Four anti-PF4 disorders are listed: classic HIT (cHIT), autoimmune HIT (aHIT), spontaneous HIT (SpHIT), and VITT. The first two entities are triggered by heparin, whereas the latter two entities have non-heparin triggers.
Classic HIT
Classic HIT indicates HIT featuring wholly or predominantly heparin-dependent antibodies. In general, the HIT-related platelet count fall begins in a narrow time range following the immunizing heparin exposure, generally between 5 and 10 days following the start of heparin (i.e., typical-onset HIT). 18 19 If heparin is administered to a sensitized patient whose blood already contains HIT antibodies, the platelet count fall can begin abruptly (i.e., rapid-onset HIT). 18 cHIT is strongly associated with thrombosis (relative risk, 12–15; absolute risk of thrombosis, 50–70%). 20
Autoimmune HIT
Autoimmune HIT refers to a subset of HIT characterized by atypical clinical presentations, 21 for example, onset or worsening of thrombocytopenia after stopping heparin (“delayed-onset HIT”) 22 ; thrombocytopenia lasting more than 1 week after stopping heparin (“persisting” or “refractory” HIT) 23 24 ; and HIT associated only with exposure to heparin flushes (heparin “flush” HIT). 25 26 A striking laboratory feature underlying these disorders is strong serum-induced platelet activation in the absence of heparin (heparin-“independent” platelet-activating properties). aHIT antibodies either require no polyanion for pathogenicity or are able to utilize endogenous platelet-associated polyanions (chondroitin sulfate, polyphosphates) 27 28 as a heparin substitute.
Spontaneous HIT
Spontaneous HIT (SpHIT) denotes patients who have thrombocytopenia, thrombosis, and platelet-activating anti-PF4 antibodies despite no proximate heparin exposure. Three subtypes are recognized: post–orthopaedic surgery (almost always, knee replacement), post-infectious, and monoclonal paraprotein-associated. 7 29 30 31 Sometimes no trigger is recognized. Most cases are self-limited, analogous with the immune transience typical for HIT. 18 32 However, paraprotein-associated SpHIT can resemble a chronic autoimmune disorder, as the anti-PF4 activity resides within the monoclonal protein. The medical (post-infectious) subtype of SpHIT has a high risk of stroke, 7 33 resembling the clinical picture of VITT.
Vaccine-Induced Immune Thrombotic Thrombocytopenia
VITT is a highly prothrombotic disorder that begins 5 to 30 days following vaccination with an adenoviral vector vaccine, ChAdOx1 nCoV-19 (Oxford/AstraZeneca), 34 35 36 37 Ad26.COV2.S (Janssen/Johnson & Johnson), 38 and likely also Sputnik V. 39 Patients with onset after 30 days typically present with deep-vein thrombosis or pulmonary embolism, which likely indicates progression of earlier asymptomatic thrombosis. VITT is relatively rare: the frequency ranges from approximately 1/25,000 to approximately 1/250,000 for preceding vaccination with ChAdOx1 nCoV-19 and Ad26.COV2.S, respectively. 35 38 The severity of thrombocytopenia is variable, with some patients developing severe thrombocytopenia and overt DIC.
Reported thrombosis frequency is high (>95%), and often unusual, for example, cerebral venous (sinus) thrombosis and splanchnic vein thrombosis. 37 The much higher rate of thrombosis in VITT (vs. cHIT) could reflect recognition bias: given absence of platelet count monitoring after vaccination, and resulting lack of data regarding frequency of VITT-associated isolated thrombocytopenia post-vaccination, patients are recognized because of symptomatic thrombosis. However, it is also possible that the frequency of VITT-associated thrombosis is truly high, by analogy with other anti-PF4 disorders with heparin-independent platelet-activating properties. 22 25
The term “thrombosis with thrombocytopenia syndrome” (TTS) is used by the World Health Organization, the Centers for Disease Control and Prevention (the United States), and the Therapeutic Goods Administration (Australia) to classify patients who develop the duad of thrombocytopenia and thrombosis post-vaccination. 40 Not all such patients will have detectable platelet-activating anti-PF4 antibodies, however; so, the term “VITT” should be reserved for the subset of TTS patients in whom VITT antibodies are demonstrable. 41 Nevertheless, VITT comprises the vast majority of patients otherwise recognized with TTS. 42 Rarely, mRNA-based COVID-19 vaccines, as well as non-COVID-19 vaccines (e.g., Gardasil 9 vaccination for human papillomavirus [HPV]), have been implicated in causing TTS. 43 44 Although these cases are also associated with platelet-activating anti-PF4 antibodies, their ultralow frequency suggests they could represent a “background” rate of SpHIT. 38 41
Spectrum of Anti-PF4 Reactivity: cHIT versus VITT
As noted, PF4 is a highly cationic homotetrameric protein bearing a circumferential ring of positive charge 11 to which anionic heparin binds, forming antigens elsewhere on PF4 recognized by cHIT antibodies. 45 46 47 48 In contrast, for VITT antibodies and aHIT antibodies (i.e., HIT sera with heparin-independent platelet-activating properties), the heparin-binding site within PF4 itself comprises the antigen target site. 49 Thus, whether the “polar” (cHIT) or “equatorial” (aHIT, VITT) antigen sites are recognized, large multimolecular complexes composed of several PF4 molecules and IgG antibodies can be formed in situ on platelet surfaces, which engage with (low-affinity) platelet FcγIIa receptors, leading to strong platelet activation response ( Fig. 2 ). Even though each PF4 molecule has two antigen sites, these cannot be recognized by a single HIT/VITT antibody due to spatial considerations (namely, the diameter of a PF4 tetramer is only ∼5 nm, and the two Fab arms of a single IgG cannot bind so closely to one another). Rather, single antibodies bind to two different PF4 molecules, resulting in large complexes. Fundamentally, cHIT antibody reactivity against PF4 is enhanced in the presence of heparin, whereas VITT antibody reactivity is inhibited by heparin (heparin competes with the antibodies for binding to PF4). 34 aHIT and VITT antibodies appear able to crosslink PF4 directly, without requirement for heparin or other polyanions. 49 50
Fig. 2.
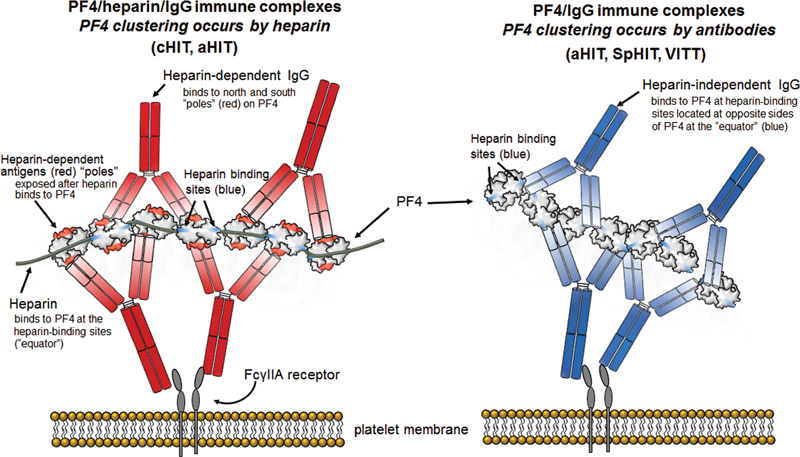
Heparin-dependent versus heparin-independent antibodies. Left : Platelet activation by heparin-dependent antibodies. Shown is a small immune complex composed of 7 PF4 molecules bound together (“clustered”) by a lengthy heparin molecule, and 4 IgG antibodies. PF4 is a highly charged (cationic) molecule; thus, PF4 clustering requires (polyanionic) heparin. Binding of the globular tetrameric protein, PF4, to heparin results in formation of antigen sites at the north and south “poles” (indicated in red). The IgG Fc “tails” bind to the platelet FcγIIa receptors; crosslinking of the FcγIIa receptors results in strong platelet activation. Right : Platelet activation by heparin-independent antibodies. Shown is a small immune complex composed of 7 PF4 molecules and 4 IgG antibodies. The heparin-independent antibodies bind to the heparin-binding site on PF4 (“equator”). In this case, PF4 clustering is caused by the IgG antibodies themselves. This is the general mechanism for VITT antibodies as well as those heparin-independent antibodies within aHIT sera. HIT, heparin-induced thrombocytopenia; VITT, vaccine-induced immune thrombotic thrombocytopenia.
A characteristic feature of HIT is the high sensitivity (>99%) for PF4-dependent EIAs in detecting the pathogenic antibodies. Indeed, availability of serial plasma samples from clinical trials of heparin therapy has shown that HIT antibodies are readily detected at the onset of the HIT-related platelet count decline, including at such early time points that a diagnosis of HIT would not be considered. 51 A simple explanation is that high quantities of free antibodies in plasma are required to form dynamically the in situ immune complexes that underlie the pathogenesis of HIT. 52 53
Table 1 summarizes the generally high sensitivity of assays for HIT and VITT antibodies; however, one remarkable feature is the poor sensitivity of rapid immunoassays (RIAs) for VITT antibodies. Also, PF4 supplementation 34 and, sometimes, serum dilution 54 are required for optimal sensitivity of platelet activation assays for detecting VITT antibodies (discussed subsequently). Diagnostic specificity depends on the clinical context, but in general is highest for platelet activation assays and lowest for EIAs.
Table 1. Diagnostic sensitivity of various laboratory tests for anti-PF4 disorders.
| cHIT | aHIT | SpHIT | VITT | |
|---|---|---|---|---|
| PF4/polyanion-EIA | >99% | >99% | >99% | >99% |
| Rapid immunoassay a | >95% | >95% | >80% b | <25% |
| PAA (washed): heparin | >95% | >95% c | >80% b | ∼50% d |
| PAA (washed): PF4 | >95% | >95% | >95% b | >95% |
Abbreviations: aHIT, autoimmune heparin-induced thrombocytopenia; cHIT, classic heparin-induced thrombocytopenia; EIA, enzyme immunoassay; PAA, platelet activation assay (e.g., serotonin-release assay [SRA], heparin-induced platelet activation [HIPA] test); PF4, platelet factor 4; RIA, rapid immunoassay; SpHIT, spontaneous HIT; VITT, vaccine-induced immune thrombotic thrombocytopenia.
Note: Diagnostic specificity depends on the clinical context, but, in general, specificity is highest for the PAAs and lowest for the EIAs.
Rapid immunoassays include chemiluminescence immunoassay, latex immunoturbidimetric assay, lateral flow immunoassay, and particle gel assay.
Very rough estimate (given the paucity of cases of SpHIT reported in the literature).
Platelet activation can be seen even in the absence of adding heparin.
Estimated frequency based on any positive reaction at 0, 0.1, or 0.3 U/mL heparin (without regard to whether there is enhanced or inhibited reactivity in the presence of heparin).
Iceberg Model
A key principle of HIT is the “iceberg model,” which depicts the spectrum of the anti-PF4 immune response among heparin-exposed patients, including numerous non-HIT patients forming nonpathogenic antibodies of IgM, IgA, and/or (non–platelet-activating) IgG classes. In this model, the “tip of the iceberg” represents those patients with clinically overt HIT, whose sera contain platelet-activating antibodies of IgG class. 53 Indeed, antibody pathogenicity and risk for HIT relate most closely to in vitro platelet-activating properties. 55 56 However, to what extent the iceberg model applies to VITT is uncertain: although prospective studies of heparin-exposed individuals show frequent formation of nonpathogenic anti-PF4 antibodies, it is less clear whether VITT analogously occurs in a small subset of those patients who form platelet-activating anti-PF4 antibodies among a larger post-vaccination group who form non–platelet-activating anti-PF4 antibodies. 57 58 This uncertainty reflects the reality that COVID-19 vaccination occurs without post-vaccination platelet count monitoring (unlike in-hospital platelet count monitoring in heparin-treated patients); furthermore, there are relatively few studies evaluating frequency of anti-PF4 antibody formation post-vaccination.
Platelet Activation Assays for HIT and VITT
Historically, platelet activation assays have been central for the diagnosis of HIT. 1 59 60 61 However, conventional platelet aggregometry, using citrated platelet-rich plasma (PRP), has unacceptably low sensitivity (∼65–70%). 2 Fortunately, technical developments enhanced detectability of HIT antibodies. Table 2 lists various platelet activation tests used to detect HIT and VITT antibodies.
Table 2. Platelet activation assays for detecting HIT and VITT antibodies.
| Assay | Comment |
|---|---|
| Washed platelets | |
| SRA, a PF4-SRA a | PF4-SRA more sensitive than SRA for detecting HIT and VITT antibodies |
| PF4/H-SRA a | PF4/H-SRA more sensitive than SRA for detecting HIT antibodies |
| HIPA, b PIPA b | PIPA more sensitive than HIPA for detecting VITT antibodies |
| PEA c | PEA more sensitive than SRA for detecting VITT antibodies |
| Whole blood | |
| PIFPA c | PIFPA has high sensitivity and specificity for VITT |
| Multiplate d | Minimal experience reported to date for diagnosis of VITT |
| PPA e | Exploits synergistic platelet activation by PAR-1 agonist and HIT/VITT antibodies |
| Platelet-rich plasma (citrated) | |
| HitAlert c | Minimal experience reported to date for diagnosis of VITT 35 |
Abbreviations: HIPA, heparin-induced platelet activation (assay); HIT, heparin-induced thrombocytopenia; PEA, platelet factor 4–enhanced P-selectin expression assay; PF4/H-SRA, platelet factor 4/heparin-serotonin release assay; PF4-SRA, PF4-enhanced serotonin-release assay; PIFPA, PF4-induced flow cytometry-based platelet activation (assay); PIPA, PF4-induced platelet activation (assay); SRA, serotonin-release assay; VITT, vaccine-induced immune thrombotic thrombocytopenia.
Source: Reprinted with permission from Warkentin TE, Greinacher A. Laboratory testing for VITT antibodies. Semin Hematol 2022;59(02):80–88.
Measurement of 14 C-radiolabeled serotonin (or other methods of serotonin measurement) released from platelets.
Platelet aggregation, assessed visually.
Flow cytometry (detection of P-selectin as platelet activation marker).
Impedance aggregometry performing using Multiplate instrument.
Washed Platelet Assays: Serotonin-Release Assay, HIPA
Sheridan and colleagues (McMaster University), 3 in 1986, found that “washing” platelets in the presence of apyrase (which retains platelet reactivity to the potentiator, adenosine diphosphate 62 ), and resuspending the platelets in divalent cation-containing buffer, renders the platelets highly sensitive to activation by HIT antibodies. Indeed, test sensitivity is estimated to be approximately 95% for the detection of HIT antibodies. 63 64 Although Sheridan et al utilized release of radiolabeled serotonin as the platelet activation endpoint marker (serotonin-release assay [SRA]), German investigators (Giessen, Greifswald) showed that simple platelet aggregation—also using washed platelets—was a suitable platelet activation endpoint; this assay is called the heparin-induced platelet activation (HIPA) test. 4 65
A key additional observation was that high heparin concentrations reliably inhibit platelet activation by HIT sera, yielding high diagnostic specificity. 3 Although the explanation was not known at the time, high heparin concentrations—by binding individual PF4 molecules—prevent the close approximation of PF4 tetramers required to form platelet-activating PF4/IgG immune complexes. 66
In both the SRA and HIPA, optimal platelet activation occurs with heparin concentrations between 0.1 and 0.5 U/mL (peak reactivity, ∼0.2 U/mL). Both these assays yield measurable endpoints, either percent SRA or lag time (in minutes) to platelet aggregation (HIPA). Use of pedigree donors (i.e., platelets from donors known to react well to HIT sera 67 ) or testing three to four randomly selected donors (e.g., at a blood donation center), 4 65 and employing weak-positive HIT controls (to evaluate assay performance), 67 help attain high diagnostic sensitivity (∼95%) and specificity (∼95%) for HIT.
PF4-Enhanced Washed Platelet Activation Assays: PF4-SRA, PF4/H-SRA, PEA, PIPA
Two groups independently showed in 2015 that addition of PF4 enhances detectability of HIT antibodies in platelet activation assays. For the McMaster group (originators of the standard SRA), the additional sensitivity of the “PF4-SRA” for HIT was judged to be minor. 68 Nonetheless, on occasion HIT sera for which the conventional SRA yields a negative result test positive by PF4-SRA. 69 70 71
Also in 2015, a group in Milwaukee, WI, part of a reference laboratory for HIT antibody testing (Versiti), developed an assay called the PF4-dependent P-selectin expression assay (PEA). 72 The PEA also uses PF4 supplementation to enhance HIT antibody detection. In this assay, washed platelets are incubated with PF4 (3.75 μg/mL) prior to adding patient serum; after 1 hour, labeled anti-P-selectin and anti-glycoprotein IIb/IIIA antibodies are added, with P-selectin expression (per flow cytometry) used to quantitate platelet activation. The Milwaukee investigators concluded that the PEA was much more sensitive than their SRA 73 ; however, the sensitivity of their SRA was estimated to be only approximately 50% (vs. McMaster group reported SRA sensitivity of ∼95%), making the issue of avoiding “SRA-negative HIT” more relevant to the U.S. laboratory.
A group in Tours, France, which also frequently uses the SRA for diagnostic purposes, confirmed the improved sensitivity of the PF4-SRA (vs. the SRA) for HIT diagnosis. 74 These workers suggested another modification of the SRA, namely, to add a fixed amount of heparin (0.5 IU/mL) and PF4 (10 μg/mL), 74 which we refer to here as the “PF4/H-SRA.”
As discussed in the next section, another PF4-enhanced platelet activation assay—the “PIPA”—was developed specifically to enhance detection of VITT antibodies.
PF4-Enhanced Assays for Detection of VITT Antibodies
In parallel with the development of various PF4-enhanced platelet activation assays (PF4-SRA, PF4/H-SRA, PEA), with the goal of optimizing test sensitivity for HIT antibodies, there occurred in March 2021 the initial recognition of the HIT-mimicking disorder, VITT. When the initial putative VITT sera were studied by the Greifswald (Germany) laboratory, the sera yielded negative results in the classic HIPA test, prompting development of PF4-enhanced HIPA, called the “PIPA.” 34 By including PF4 (10 µg/mL, final) in the reaction mixture, VITT sera typically produce greater platelet activation (i.e., shorter lag time to platelet aggregation) in the presence of PF4 versus buffer control (low-dose heparin or low-molecular-weight heparin generally inhibits VITT antibody-induced platelet activation), with relatively few (∼5%) of VITT sera showing enhancement of platelet activation with heparin. 75 VITT antibodies—as is characteristic of HIT antibodies 3 —also show inhibition of platelet activation at high (suprapharmacologic) concentrations of heparin (100 U/mL). This phenomenon is caused by the disruption of PF4 complexes as well as detachment of PF4 from the platelet surface by high heparin concentrations. 76 Historical information regarding the development of the PIPA test—including the first iteration of this assay on March 17, 2022—has been reported elsewhere. 77 Details regarding assay performance is provided by Handtke et al (supplementary material 78 ; see also video tutorial available online at https://www.youtube.com/watch?v=hFs-_85YJX4 ).
The recognition that PF4-enhanced platelet activation assays provided a sensitive approach to the detection of VITT antibodies was confirmed a few weeks later, when laboratories in North America and elsewhere in Europe began to receive referred VITT sera (the ChAdOx1 nCoV-19 vaccine was introduced a few weeks earlier in Europe than in North America). Both the McMaster 79 and French (Tours) 80 laboratories observed greater diagnostic sensitivity for VITT antibodies with the PF4-SRA versus the (conventional) SRA. Both groups also found that increasing (pharmacologic) concentrations of heparin usually inhibited VITT serum-induced platelet activation in the PF4-SRA. The French group also showed that the PF4/H-SRA—while providing a more sensitive test for HIT antibodies 74 —was not as sensitive as the PF4-SRA (without addition of heparin) for detecting VITT antibodies. 80 A caveat that emerged in the studies of VITT was that a false-negative platelet activation assay could result if the patient was treated with high-dose intravenous immunoglobulin (IVIG), 81 due to inhibition of FcγIIa receptor-mediated VITT antibody-induced platelet activation. 79 82
Application of the various PF4-enhanced platelet activation assays altered the cHIT paradigm. Although previously the concept that EIA +/SRA+ (or EIA +/HIPA + ) status was reasonably correlated with the clinically significant anti-PF4 disorder, HIT, the new paradigm was that EIA +/PF4-SRA+ (or EIA +/PIPA + ) status better reflected a greater spectrum of anti-PF4 reactivities, including patients with VITT. One implication is that certain atypical patients—e.g., ones with thrombocytopenia and headaches (but no cerebral thrombosis by imaging) were classified as having likely VITT (with subthreshold cerebral thrombosis) when their sera had the striking serological picture of EIA +/PIPA+ status. 83
Although to date few data are reported using the PEA for VITT diagnosis, it appears likely that the PEA has greater sensitivity for the diagnosis of VITT versus the SRA. 84 Thus, the four PF4-enhanced washed platelet assays—PF4-SRA, PF4/H-SRA, PIPA, and PEA—appear to have high sensitivity for detecting VITT antibodies. Thus, reference laboratories should be able to perform conventional platelet activation assays for HIT, as well as PF4-enhanced assays.
Serum Dilution and Detection of VITT Antibodies
Recently, Schönborn and colleagues observed that false-negative platelet activation testing results (by PIPA) could be reduced by diluting the serum by 1/5 to 1/10. 54 Such a phenomenon has never been observed in HIT. Fig. 3 shows that the explanation for this unique phenomenon could be high levels of VITT antibodies; by diluting patient serum, the stoichiometrically “correct” quantities of VITT antibodies and PF4 molecules are more likely to be attained.
Fig. 3.
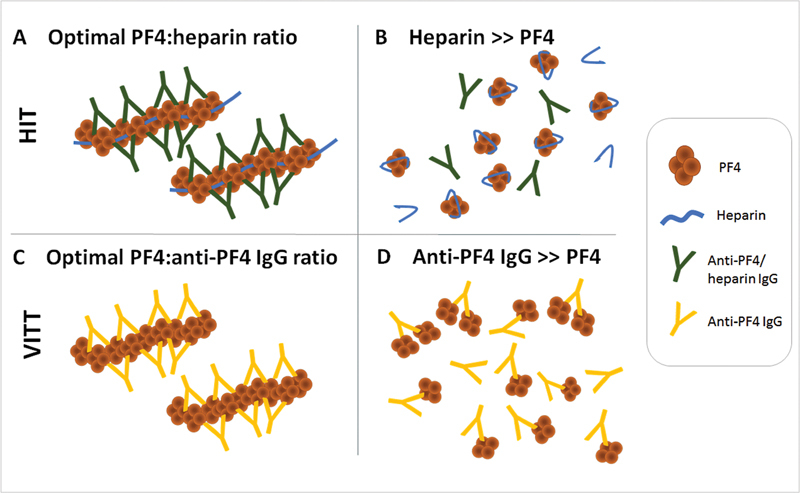
HIT versus VITT: dependency of immune complex formation on stoichiometric ratios. ( A ) HIT: optimal ratio of PF4 (cationic) and heparin (anionic) results in formation of multimolecular complexes. Anti-PF4/heparin IgG binds to these complexes. The resulting immune complexes activate platelets in functional assays. Even if anti-PF4/heparin antibodies are present in excess, interaction of PF4 and heparin is so strong that the state of lowest energy is always formation of multimolecular PF4/heparin complexes, to which subsequently HIT antibodies bind. ( B ) HIT: in case of heparin excess, the long heparin molecules wrap around the PF4 tetramer (or several shorter heparin chains bind along the rim of positive charge) and no complexes are formed. Accordingly, anti-PF4/heparin IgG antibodies do not bind to PF4 and no platelet activation is observed in the functional assay. ( C ) VITT: anti-PF4 antibodies bind to PF4 alone and form multimolecular complexes without addition of a polyanion. ( D ) In case of very high concentrations of anti-PF4 VITT antibodies, there are not enough PF4 tetramers for all IgG Fab. Overall, the state of lowest energy is reached if each antibody can bind to one PF4. A situation in which few antibodies form complexes with PF4 while others have no binding partner would be thermodynamically unfavorable. Dilution of these sera lowers anti-PF4 IgG concentration and subsequent formation of immune complexes causing platelet activation in the PIPA. HIT, heparin-induced thrombocytopenia; VITT, vaccine-induced immune thrombotic thrombocytopenia. (Reprinted with permission from Schönborn L, et al. Quantitative interpretation of PF4/heparin-EIA optical densities in predicting for platelet-activating VITT antibodies. J Thromb Haemost 2022;20(11):2579-2586.)
Whole Blood Assays: Multiplate, PIFPA, Procoagulant Platelet Assay
A drawback of washed platelet activation assays is the handling of platelets, including wash steps. Accordingly, there is interest in using whole blood assays, in which patient serum is incubated with donor whole blood. The “Multiplate” (Dynabyte Medical), also known as “heparin-induced multiple-electrode aggregometry” (HIMEA), has been used for the diagnosis of HIT. 85 86 In one small study, 5/5 VITT sera triggered platelet aggregation with saline buffer, with a low concentration of heparin (∼1 U/mL) inhibiting ( n = 3), enhancing ( n = 1), or having minimal effect ( n = 1) on platelet aggregation.
A novel whole blood assay, “PF4-induced flow cytometry-based platelet activation” assay, or PIFPA test, was developed using heat-inactivated patient serum incubated with hirudin-anticoagulated (or PPACK-anticoagulated) whole blood obtained from normal healthy donors. 78 This assay is performed with different concentrations of PF4 (0, 5, or 20 μg/mL PF4), as well as different heparin concentrations (1 and 100 U/mL of heparin). Like the PEA, flow cytometry is used to assess platelet activation, using fluorescent markers against platelet GPIIb/IIIa (CD61-PE) and P-selectin (CD62P-PE-Cy5). All 16 VITT sera tested positive when PF4 (5 μg/mL) was added (in contrast, 13/16 sera were positive also at buffer). The assay appeared to have comparable specificity for VITT as the PIPA.
Recently, Lee and colleagues 87 reported a novel flow cytometry-based procoagulant platelet assay, which uses two platelet activation markers (cell death marker, GSAO; P-selectin), in which putative VITT plasma is incubated with a protease-activated receptor-1 (PAR-1) agonist. This assay exploits the synergistic procoagulant effects 88 of a G protein-coupled receptor (e.g., PAR-1) and an FcγIIa receptor-mediated agonist (e.g., VITT antibodies) in producing a platelet procoagulant response.
PF4-Dependent Enzyme Immunoassays
The key discovery by Jean Amiral and colleagues, 5 in 1992, that PF4 (bound to heparin) represented the target protein of HIT, heralded the ensuing 30-year era of HIT diagnostic testing using the EIA (or ELISA, i.e., enzyme-linked immunosorbent assay). In this solid-phase assay, PF4—together with stoichiometric concentrations of heparin or polyvinyl sulfonate (PVS)—is coated onto microtiter plates. Patient (or control) serum (or plasma), usually diluted 1:50, is added to the microtiter plate wells. Anti-PF4/heparin antibodies, if present, bind to the complexed surface-bound PF4, and after washing, the residual bound antibodies are detected by enzyme-conjugated antihuman immunoglobulin. 10 Although both IgG-specific and poly-specific EIAs (detecting IgG, IgA, and/or IgM class antibodies) are available, IgG-specific assays have higher diagnostic specificity. 55 56 89 90 The sensitivity of PF4-dependent EIAs for HIT antibodies is regarded as being high (>99%).
All four anti-PF4 disorders—cHIT, aHIT, SpHIT, as well as the novel disorder VITT—are likely to test positive in a PF4-dependent EIA. Indeed, the first studies 34 35 36 reporting on VITT noted generally strong-positive EIA reactivity (median optical density [OD] values, ∼3.0), together with a positive platelet activation test.
Different commercial EIAs utilize different antigen targets. EIAs utilizing PF4/heparin complexes are available both commercially (Diagnostica Stago) and as “in-house” assays developed by HIT research laboratories, 91 92 although there are interassay technical differences. Heparin is not the only polyanion that can create antigens on PF4, 93 a concept exploited by Visentin and coworkers 94 when they developed a PF4/PVS EIA recognized by HIT antibodies; the resulting commercial IgG-specific and poly-specific EIAs (Immucor GTI Diagnostics) are widely used. A third commercially available EIA (Zymutest), from Hyphen Biomed, permits PF4 (and other heparin-binding proteins within platelet/leukocyte lysate) to form antigen complexes with heparin that has been covalently linked to the microtiter plate. 95
PF4-dependent EIAs, both IgG-specific and polyspecific, appear to have high sensitivity (∼99%) for detecting VITT antibodies: this high estimate assumes that an occasional negative assay in one type of EIA will yield a positive result with another EIA. 96 97 Accordingly, if VITT is strongly suspected on clinical grounds, a sample yielding an unexpected negative EIA should be tested in another EIA (or a functional assay). Interestingly, a VITT study 98 evaluating serial sample EIA reactivity showed that from initial EIA-positive reactivity (at the time of initial diagnosis), different EIAs differed with respect to seroreversion, namely, the IgG-specific Zymutest became negative in all patients prior to another IgG-specific PF4/PVS-EIA becoming negative. This observation suggests there may well be inherent differences in diagnostic sensitivity between these two EIAs. An important advantage of the EIA (vs. platelet activation assay) is that high-dose IVIG treatment (a recommended treatment for acute VITT 99 ) does not result in false-negative EIA testing. 79
In parallel with HIT, diagnostic specificity of any positive EIA in a case of putative VITT is lower than for the same patient who has a positive functional (platelet activation) test result. Also as seen in HIT, greater magnitude of a positive OD will predict for a greater likelihood for a true diagnosis of VITT. 54 Since vaccinated patients who do not develop any clinical evidence of VITT have an approximately 3 to 5% chance of testing EIA positive, 57 58 it is advisable to confirm a putative diagnosis of VITT with a platelet activation test, whenever possible.
Immunoassays to Distinguish between HIT and VITT Antibodies
The key report by Huynh and colleagues 49 indicating that there are distinct epitopes on PF4 recognized by cHIT versus VITT antibodies implies that it ought to be possible to develop immunoassays that distinguish reliably between VITT and HIT antibodies. VITT sera can yield positive results when tested in EIAs in which uncomplexed PF4 alone (i.e., without added heparin or other polyanions) is the target. 34 84 100 However, since all sera that test positive with uncomplexed PF4 also test positive with complexed PF4, this approach does not adequately differentiate between those sera that contain heparin-dependent antibodies versus those that are purely reactive against PF4 alone. Given our view that there are four anti-PF4 disorders—cHIT HIT, aHIT, SpHIT, and VITT—simple laboratory approaches to distinguish between different categories of pathogenic anti-PF4 antibodies—such as heparin-enhanced versus heparin-inhibited—could be invaluable, such as for clinical decision-making regarding safety of heparin treatment.
Rapid Immunoassays
Unlike EIAs, RIAs for HIT are designed to provide a result within an hour of sample acquisition and processing (preparation of serum or plasma). Unlike standard EIAs, where binding of PF4/heparin (or PF4/PVS) complexes to the solid-phase results in antigens on PF4 recognized by both heparin-dependent and heparin-independent (or heparin-inhibited) antigens, the RIAs do not usually yield positive testing with VITT sera/plasmas. This simple observation underscores key differences between antibodies implicated in HIT versus the other anti-PF4 disorders, as underscored by Huynh and colleagues, 49 who found that the target antigens on PF4 recognized by VITT antibodies (heparin-binding site) differ from sites targeted by HIT antibodies (heparin-dependent antigens distinct from the heparin-binding site).
Four RIAs are in clinical use for the diagnosis of HIT, with sensitivities >95% (estimated sensitivity shown in parentheses): (1) particle gel immunoassay (PaGIA; ∼98% 101 ); (2) lateral-flow assay (LFA; ∼97% 102 ); (3) latex-enhanced immunoturbidimetric assay (LIA; ∼97% 103 ); and (4) chemiluminescence immunoassay (CLIA; ∼96–98% 104 105 ). In contrast, sensitivity of these rapid assays for the detection of VITT antibodies is poor, with the highest sensitivity (only ∼45%) observed with the PaGIA (sensitivity <10% for the LIA, CLIA, and LFA). 97 Sachs and coworkers 96 also noted PaGIA sensitivity (25%) to be higher than the LFA (8%) and CLIA (0%). Indeed, these authors propose that the serological profile, EIA +/CLIA − , could indicate a presumptive diagnosis of VITT in the appropriate clinical context.
That the LIA usually yields negative results for VITT antibodies is not surprising, given that this assay is designed to be positive only if the patient's anti-PF4 antibodies inhibit binding of a HIT-like monoclonal antibody (KKO) to PF4/PVS complexes, 103 a target site distinct from the epitopes recognized by VITT. Another monoclonal antibody, 1E12, recognizes the same/similar binding site on PF4 recognized by VITT antibodies. 31 106 Given that the CLIA almost invariably yields negative results for VITT antibodies, this indicates that the binding of PF4/heparin complexes to the beads does not allow for expression of the VITT epitopes.
Conclusions and Future Directions
Solid-phase EIAs are excellent for detecting anti-PF4 antibodies from all four anti-PF4 disorders, irrespective of the specific target epitopes. This is in striking distinction from the RIAs, which are designed to detect antibodies directed against heparin-dependent antigens, where HIT antibodies—but not VITT antibodies—are detectable. In our view, it seems likely that the (solid-phase) microtiter plates bind not only PF4/heparin complexes but also uncomplexed PF4. This allows expression of PF4 with multiple conformational changes on the plastic surface, such that antigens recognized by VITT antibodies are exposed (uncomplexed PF4) as well as those epitopes (on PF4/polyanion complexes) recognized by (heparin-dependent) HIT antibodies. The concept that proteins undergo significant conformational changes when binding to solid (plastic) surfaces is well-known, such as for antiphospholipid antibody assays (e.g., detection of anti-β 2 glycoprotein 1 antibodies). 107 These concepts suggest that fluid-phase EIAs (which avoid conformational alterations that result when PF4 binds to plastic surfaces) might be helpful in distinguishing between HIT and VITT antibodies, which is supported by preliminary findings from the McMaster laboratory.
Acknowledgements
The authors thank Jo-Ann I. Sheppard for assistance in the preparation of the figures.
Footnotes
Conflict of Interest T.E.W. has received lecture honoraria from Alexion and Werfen (Instrumentation Laboratory), and royalties from Informa (Taylor & Francis); has provided consulting services to Aspen Canada, Aspen Global, CSL Behring, Ergomed, Paradigm Pharmaceuticals, Octapharma, and Veralox Therapeutics; has received research funding from Werfen (Instrumentation Laboratory); and has provided expert witness testimony relating to heparin-induced thrombocytopenia (HIT) and non-HIT thrombocytopenic and coagulopathic disorders. A.D. reports personal fees from Aspen, grants from Ergomed, grants from Boehringer Ingelheim, personal fees from Bayer Vital, grants from Rovi, grants from Sagent, personal fees from Chromatec, personal fees from Instrumentation Laboratory, grants and personal fees from Macopharma, grants from Portola, grants from Biokit, personal fees from Sanofi-Aventis, grants from Blau Farmaceutics, grants from Prosensa/Biomarin, grants and other from DRK-BSD NSTOB, grants from DRK-BSD Baden-Württemberg/Hessen, personal fees from Roche, personal fees from GTH e.V., grants from Deutsche Forschungsgemeinschaft, grants from Robert-Koch-Institut, nonfinancial support from Veralox, grants from Dilaflor, nonfinancial support from Vakzine Projekt Management GmbH, grants from GIZ Else-Körner-Stiftung, grants from GIZ Else-Körner-Stiftung, nonfinancial support from AstraZeneca, nonfinancial support from Janssen Vaccines & Prevention B.V., personal fees from Takeda Pharma, personal fees from Falk Foundation e.V., grants from European Medicines Agency, personal fees from Mylan Germany, outside the submitted work. In addition, A.G. has a patent Screening Methods for transfusion-related acute lung injury (TRALI) with royalties paid to EP2321644, 18.05.2011.
References
- 1.Rhodes G R, Dixon R H, Silver D. Heparin induced thrombocytopenia with thrombotic and hemorrhagic manifestations. Surg Gynecol Obstet. 1973;136(03):409–416. [PubMed] [Google Scholar]
- 2.Chong B H, Pitney W R, Castaldi P A.Heparin-induced thrombocytopenia: association of thrombotic complications with heparin-dependent IgG antibody that induces thromboxane synthesis in platelet aggregation Lancet 19822(8310):1246–1249. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan D, Carter C, Kelton J G. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(01):27–30. [PubMed] [Google Scholar]
- 4.Greinacher A, Michels I, Kiefel V, Mueller-Eckhardt C. A rapid and sensitive test for diagnosing heparin-associated thrombocytopenia. Thromb Haemost. 1991;66(06):734–736. [PubMed] [Google Scholar]
- 5.Amiral J, Bridey F, Dreyfus M et al. Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost. 1992;68(01):95–96. [PubMed] [Google Scholar]
- 6.Warkentin T E. Platelet-activating anti-PF4 disorders: an overview. Semin Hematol. 2022;59(02):59–71. doi: 10.1053/j.seminhematol.2022.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Warkentin T E, Greinacher A. Spontaneous HIT syndrome: knee replacement, infection, and parallels with vaccine-induced immune thrombotic thrombocytopenia. Thromb Res. 2021;204:40–51. doi: 10.1016/j.thromres.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Favaloro E J, Pasalic L, Lippi G. Antibodies against platelet factor 4 and their associated pathologies: from HIT/HITT to spontaneous HIT-like syndrome, to COVID-19, to VITT/TTS. Antibodies (Basel) 2022;11(01):7. doi: 10.3390/antib11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greinacher A, Thiele T, Warkentin T E, Weisser K, Kyrle P, Eichinger S.A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination N Engl J Med 2021384222092–2101.. Preprint available at:https://assets.researchsquare.com/files/rs-362354/v2/c3d5df68-3cc0-40e4-a779-e19498b50d6f.pdf?c=1631880543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warkentin T E, Greinacher A. Boca Raton, FL: CRC Press; 2013. Laboratory testing for heparin-induced thrombocytopenia; pp. 272–314. [Google Scholar]
- 11.Mayo K H, Ilyina E, Roongta Vet al. Heparin binding to platelet factor-4. An NMR and site-directed mutagenesis study: arginine residues are crucial for binding Biochem J 1995312(Pt 2):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greinacher A, Pötzsch B, Amiral J, Dummel V, Eichner A, Mueller-Eckhardt C. Heparin-associated thrombocytopenia: isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen. Thromb Haemost. 1994;71(02):247–251. [PubMed] [Google Scholar]
- 13.Kelton J G, Smith J W, Warkentin T E, Hayward C PM, Denomme G A, Horsewood P. Immunoglobulin G from patients with heparin-induced thrombocytopenia binds to a complex of heparin and platelet factor 4. Blood. 1994;83(11):3232–3239. [PubMed] [Google Scholar]
- 14.Visentin G P, Ford S E, Scott J P, Aster R H. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J Clin Invest. 1994;93(01):81–88. doi: 10.1172/JCI116987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gollomp K, Kim M, Johnston I et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight. 2018;3(18):e99445. doi: 10.1172/jci.insight.99445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perdomo J, Leung H HL, Ahmadi Z et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun. 2019;10(01):1322. doi: 10.1038/s41467-019-09160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanack A J, Bayas A, George G et al. Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT. Blood. 2022;140(01):73–77. doi: 10.1182/blood.2021014588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warkentin T E, Kelton J G. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 19.Warkentin T E, Sheppard J I, Whitlock R P. Temporal presentations of heparin-induced thrombocytopenia following cardiac surgery: a single-center, retrospective cohort study. J Thromb Haemost. 2022;20(11):2601–2616. doi: 10.1111/jth.15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warkentin T E.HITlights: a career perspective on heparin-induced thrombocytopenia Am J Hematol 201287(Suppl 1):S92–S99. [DOI] [PubMed] [Google Scholar]
- 21.Greinacher A, Selleng K, Warkentin T E. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 22.Warkentin T E, Kelton J G. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135(07):502–506. doi: 10.7326/0003-4819-135-7-200110020-00009. [DOI] [PubMed] [Google Scholar]
- 23.Warkentin T E. Clinical picture of heparin-induced thrombocytopenia (HIT) and its differentiation from non-HIT thrombocytopenia. Thromb Haemost. 2016;116(05):813–822. doi: 10.1160/TH16-06-0435. [DOI] [PubMed] [Google Scholar]
- 24.Padmanabhan A, Jones C G, Pechauer S M et al. IVIg for treatment of severe refractory heparin-induced thrombocytopenia. Chest. 2017;152(03):478–485. doi: 10.1016/j.chest.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mian H, Warkentin T E, Sheppard J I et al. Autoimmune HIT due to apheresis catheter heparin flushes for stem cell harvesting before autotransplantation for myeloma. Blood. 2017;130(14):1679–1682. doi: 10.1182/blood-2017-06-788679. [DOI] [PubMed] [Google Scholar]
- 26.Refaai M A, Warkentin T E, Axelson M, Matevosyan K, Sarode R. Delayed-onset heparin-induced thrombocytopenia, venous thromboembolism, and cerebral venous thrombosis: a consequence of heparin “flushes”. Thromb Haemost. 2007;98(05):1139–1140. [PubMed] [Google Scholar]
- 27.Padmanabhan A, Jones C G, Bougie D W et al. Heparin-independent, PF4-dependent binding of HIT antibodies to platelets: implications for HIT pathogenesis. Blood. 2015;125(01):155–161. doi: 10.1182/blood-2014-06-580894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cines D B, Yarovoi S V, Zaitsev S V et al. Polyphosphate/platelet factor 4 complexes can mediate heparin-independent platelet activation in heparin-induced thrombocytopenia. Blood Adv. 2016;1(01):62–74. doi: 10.1182/bloodadvances.2016000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warkentin T E, Makris M, Jay R M, Kelton J G. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am J Med. 2008;121(07):632–636. doi: 10.1016/j.amjmed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Jay R M, Warkentin T E. Fatal heparin-induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: another example of ‘spontaneous’ HIT? J Thromb Haemost. 2008;6(09):1598–1600. doi: 10.1111/j.1538-7836.2008.03040.x. [DOI] [PubMed] [Google Scholar]
- 31.Greinacher A, Langer F, Schönborn L et al. Platelet-activating anti-PF4 antibodies mimic VITT antibodies in an unvaccinated patient with monoclonal gammopathy. Haematologica. 2022;107(05):1219–1221. doi: 10.3324/haematol.2021.280366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih A W, Sheppard J I, Warkentin T E. Platelet count recovery and seroreversion in immune HIT despite continuation of heparin: further observations and literature review. Thromb Haemost. 2017;117(10):1868–1874. doi: 10.1160/TH17-03-0212. [DOI] [PubMed] [Google Scholar]
- 33.Moores G, Warkentin T E, Farooqi M AM, Jevtic S D, Zeller M P, Perera K S. Spontaneous heparin-induced thrombocytopenia presenting as cerebral venous sinus thrombosis. Neurol Clin Pract. 2021;11(06):e929–e931. doi: 10.1212/CPJ.0000000000000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greinacher A, Thiele T, Warkentin T E, Weisser K, Kyrle P A, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz N H, Sørvoll I H, Michelsen A E et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scully M, Singh D, Lown R et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavord S, Scully M, Hunt B J et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.See I, Lale A, Marquez P et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination-United States, December 2020 to August 2021. Ann Intern Med. 2022;175(04):513–522. doi: 10.7326/M21-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrera-Comoglio R, Lane S. Vaccine-induced immune thrombocytopenia and thrombosis after the Sputnik V vaccine. N Engl J Med. 2022;387(15):1431–1432. doi: 10.1056/NEJMc2210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19)Accessed October 15, 2022 at:https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-TTS-2021.1
- 41.Warkentin T E, Pai M. The epidemiology of thrombosis with thrombocytopenia syndrome: analogies with heparin-induced thrombocytopenia. Ann Intern Med. 2022;175(04):604–605. doi: 10.7326/M22-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makris M, Pavord S. Most cases of thrombosis and thrombocytopenia syndrome (TTS) post ChAdOx1 nCov-19 are vaccine-induced immune thrombotic thrombocytopenia (VITT) Lancet Reg Health Eur. 2021;12:100274. doi: 10.1016/j.lanepe.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huynh A, Arnold D M, Michael J V et al. Characteristics of VITT antibodies in patients vaccinated with Ad26.COV2.S. Blood Adv. 2023;7(02):246–250. doi: 10.1182/bloodadvances.2022007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen S, Laegreid I J, Ernstsen S L et al. Thrombosis and thrombocytopenia after HPV vaccination. J Thromb Haemost. 2022;20(03):700–704. doi: 10.1111/jth.15604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh J S, Aster R H, Visentin G P. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis recognize different epitopes on heparin: platelet factor 4. Blood. 1998;91(03):916–922. [PubMed] [Google Scholar]
- 46.Ziporen L, Li Z Q, Park K S et al. Defining an antigenic epitope on platelet factor 4 associated with heparin-induced thrombocytopenia. Blood. 1998;92(09):3250–3259. [PubMed] [Google Scholar]
- 47.Li Z Q, Liu W, Park K S et al. Defining a second epitope for heparin-induced thrombocytopenia/thrombosis antibodies using KKO, a murine HIT-like monoclonal antibody. Blood. 2002;99(04):1230–1236. doi: 10.1182/blood.v99.4.1230. [DOI] [PubMed] [Google Scholar]
- 48.Cai Z, Yarovoi S V, Zhu Z et al. Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat Commun. 2015;6:8277. doi: 10.1038/ncomms9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huynh A, Kelton J G, Arnold D M, Daka M, Nazy I.Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia Nature 2021596(7873):565–569. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen T H, Medvedev N, Delcea M, Greinacher A. Anti-platelet factor 4/polyanion antibodies mediate a new mechanism of autoimmunity. Nat Commun. 2017;8:14945. doi: 10.1038/ncomms14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warkentin T E, Sheppard J A, Moore J C, Cook R J, Kelton J G. Studies of the immune response in heparin-induced thrombocytopenia. Blood. 2009;113(20):4963–4969. doi: 10.1182/blood-2008-10-186064. [DOI] [PubMed] [Google Scholar]
- 52.Newman P M, Chong B H. Heparin-induced thrombocytopenia: new evidence for the dynamic binding of purified anti-PF4-heparin antibodies to platelets and the resultant platelet activation. Blood. 2000;96(01):182–187. [PubMed] [Google Scholar]
- 53.Warkentin T E.Laboratory diagnosis of heparin-induced thrombocytopenia Int J Lab Hematol 201941(Suppl 1):15–25. [DOI] [PubMed] [Google Scholar]
- 54.Schönborn L, Thiele T, Esefeld M et al. Quantitative interpretation of PF4/heparin-EIA optical densities in predicting platelet-activating VITT antibodies. J Thromb Haemost. 2022;20(11):2579–2586. doi: 10.1111/jth.15862. [DOI] [PubMed] [Google Scholar]
- 55.Greinacher A, Juhl D, Strobel U et al. Heparin-induced thrombocytopenia: a prospective study on the incidence, platelet-activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J Thromb Haemost. 2007;5(08):1666–1673. doi: 10.1111/j.1538-7836.2007.02617.x. [DOI] [PubMed] [Google Scholar]
- 56.Warkentin T E, Sheppard J I, Moore J C, Sigouin C S, Kelton J G. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(08):1304–1312. doi: 10.1111/j.1538-7836.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 57.Thiele T, Ulm L, Holtfreter S et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138(04):299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barefah A S, Radhwi O O, Alamri S S et al. Low clinical utility of testing for anti-platelet factor 4 in asymptomatic individuals after ChAdOx1 nCoV-19 vaccine. Int J Lab Hematol. 2022;44(02):424–429. doi: 10.1111/ijlh.13774. [DOI] [PubMed] [Google Scholar]
- 59.Babcock R B, Dumper C W, Scharfman W B. Heparin-induced immune thrombocytopenia. N Engl J Med. 1976;295(05):237–241. doi: 10.1056/NEJM197607292950501. [DOI] [PubMed] [Google Scholar]
- 60.Green D, Harris K, Reynolds N, Roberts M, Patterson R. Heparin immune thrombocytopenia: evidence for a heparin-platelet complex as the antigenic determinant. J Lab Clin Med. 1978;91(01):167–175. [PubMed] [Google Scholar]
- 61.Nelson J C, Lerner R G, Goldstein R, Cagin N A. Heparin-induced thrombocytopenia. Arch Intern Med. 1978;138(04):548–552. [PubMed] [Google Scholar]
- 62.Polgár J, Eichler P, Greinacher A, Clemetson K J. Adenosine diphosphate (ADP) and ADP receptor play a major role in platelet activation/aggregation induced by sera from heparin-induced thrombocytopenia patients. Blood. 1998;91(02):549–554. [PubMed] [Google Scholar]
- 63.Warkentin T E. How I diagnose and manage HIT. Hematology (Am Soc Hematol Educ Program) 2011;2011:143–149. doi: 10.1182/asheducation-2011.1.143. [DOI] [PubMed] [Google Scholar]
- 64.Warkentin T E, Arnold D M, Nazi I, Kelton J G. The platelet serotonin-release assay. Am J Hematol. 2015;90(06):564–572. doi: 10.1002/ajh.24006. [DOI] [PubMed] [Google Scholar]
- 65.Eichler P, Budde U, Haas S et al. First workshop for detection of heparin-induced antibodies: validation of the heparin-induced platelet-activation test (HIPA) in comparison with a PF4/heparin ELISA. Thromb Haemost. 1999;81(04):625–629. [PubMed] [Google Scholar]
- 66.Greinacher A, Gopinadhan M, Günther J U et al. Close approximation of two platelet factor 4 tetramers by charge neutralization forms the antigens recognized by HIT antibodies. Arterioscler Thromb Vasc Biol. 2006;26(10):2386–2393. doi: 10.1161/01.ATV.0000238350.89477.88. [DOI] [PubMed] [Google Scholar]
- 67.Warkentin T E, Hayward C PM, Smith C A, Kelly P M, Kelton J G. Determinants of donor platelet variability when testing for heparin-induced thrombocytopenia. J Lab Clin Med. 1992;120(03):371–379. [PubMed] [Google Scholar]
- 68.Nazi I, Arnold D M, Warkentin T E, Smith J W, Staibano P, Kelton J G. Distinguishing between anti-platelet factor 4/heparin antibodies that can and cannot cause heparin-induced thrombocytopenia. J Thromb Haemost. 2015;13(10):1900–1907. doi: 10.1111/jth.13066. [DOI] [PubMed] [Google Scholar]
- 69.Warkentin T E, Sheppard J I, Smith J W, Arnold D M, Nazy I. Timeline of heparin-induced thrombocytopenia seroconversion in serial plasma samples tested using an automated latex immunoturbidimetric assay. Int J Lab Hematol. 2019;41(04):493–502. doi: 10.1111/ijlh.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Warkentin T E, Nazy I, Sheppard J I, Smith J W, Kelton J G, Arnold D M. Serotonin-release assay-negative heparin-induced thrombocytopenia. Am J Hematol. 2020;95(01):38–47. doi: 10.1002/ajh.25660. [DOI] [PubMed] [Google Scholar]
- 71.Koster A, Nazy I, Birschmann I E, Smith J W, Sheppard J I, Warkentin T E. High-dose IVIG plus cangrelor platelet “anesthesia” during urgent heparin-CPB in a patient with recent SRA-negative HIT-thrombosis with persisting platelet-activating antibodies. Res Pract Thromb Haemost. 2020;4(06):1060–1064. doi: 10.1002/rth2.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Padmanabhan A, Jones C G, Bougie D W et al. A modified PF4-dependent, CD62p expression assay selectively detects serotonin-releasing antibodies in patients suspected of HIT. Thromb Haemost. 2015;114(06):1322–1323. doi: 10.1160/TH15-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Padmanabhan A, Jones C G, Curtis B R et al. A novel PF4-dependent platelet activation assay identifies patients likely to have heparin-induced thrombocytopenia/thrombosis. Chest. 2016;150(03):506–515. doi: 10.1016/j.chest.2016.02.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vayne C, Guery E A, Kizlik-Masson C et al. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin-induced thrombocytopenia antibodies. Br J Haematol. 2017;179(05):811–819. doi: 10.1111/bjh.14955. [DOI] [PubMed] [Google Scholar]
- 75.Greinacher A, Langer F, Makris M et al. Vaccine-induced immune thrombotic thrombocytopenia (VITT): update on diagnosis and management considering different resources. J Thromb Haemost. 2022;20(01):149–156. doi: 10.1111/jth.15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krauel K, Fürll B, Warkentin T E et al. Heparin-induced thrombocytopenia–therapeutic concentrations of danaparoid, unlike fondaparinux and direct thrombin inhibitors, inhibit formation of platelet factor 4-heparin complexes. J Thromb Haemost. 2008;6(12):2160–2167. doi: 10.1111/j.1538-7836.2008.03171.x. [DOI] [PubMed] [Google Scholar]
- 77.Warkentin T E, Greinacher A. Laboratory testing for VITT antibodies. Semin Hematol. 2022;59(02):80–88. doi: 10.1053/j.seminhematol.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Handtke S, Wolff M, Zaninetti C et al. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. 2021;137(26):3656–3659. doi: 10.1182/blood.2021012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bourguignon A, Arnold D M, Warkentin T E et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385(08):720–728. doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vayne C, Rollin J, Gruel Y et al. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med. 2021;385(04):376–378. doi: 10.1056/NEJMc2106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiele T, Weisser K, Schönborn L et al. Laboratory confirmed vaccine-induced immune thrombotic thrombocytopenia: retrospective analysis of reported cases after vaccination with ChAdOx-1 nCoV-19 in Germany. Lancet Reg Health Eur. 2022;12:100270. doi: 10.1016/j.lanepe.2021.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uzun G, Althaus K, Singh A et al. The use of IV immunoglobulin in the treatment of vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(11):992–996. doi: 10.1182/blood.2021012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salih F, Schönborn L, Kohler S et al. Vaccine-induced thrombocytopenia with severe headache. N Engl J Med. 2021;385(22):2103–2105. doi: 10.1056/NEJMc2112974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kanack A J, Singh B, George G et al. Persistence of Ad26.COV2.S-associated vaccine-induced immune thrombotic thrombocytopenia (VITT) and specific detection of VITT antibodies. Am J Hematol. 2022;97(05):519–526. doi: 10.1002/ajh.26488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galea V, Khaterchi A, Robert F, Gerotziafas G, Hatmi M, Elalamy I. Heparin-induced multiple electrode aggregometry is a promising and useful functional tool for heparin-induced thrombocytopenia diagnosis: confirmation in a prospective study. Platelets. 2013;24(06):441–447. doi: 10.3109/09537104.2012.724736. [DOI] [PubMed] [Google Scholar]
- 86.Subcommittee on Platelet Immunology . Morel-Kopp M C, Mullier F, Gkalea V et al. Heparin-induced multi-electrode aggregometry method for heparin-induced thrombocytopenia testing: communication from the SSC of the ISTH. J Thromb Haemost. 2016;14(12):2548–2552. doi: 10.1111/jth.13516. [DOI] [PubMed] [Google Scholar]
- 87.Lee C SM, Liang H PH, Connor D E et al. A novel flow cytometry procoagulant assay for diagnosis of vaccine-induced immune thrombotic thrombocytopenia. Blood Adv. 2022;6(11):3494–3506. doi: 10.1182/bloodadvances.2021006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee C SM, Selvadurai M V, Pasalic L et al. Measurement of procoagulant platelets provides mechanistic insight and diagnostic potential in heparin-induced thrombocytopenia. J Thromb Haemost. 2022;20(04):975–988. doi: 10.1111/jth.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lindhoff-Last E, Gerdsen F, Ackermann H, Bauersachs R. Determination of heparin-platelet factor 4-IgG antibodies improves diagnosis of heparin-induced thrombocytopenia. Br J Haematol. 2001;113(04):886–890. doi: 10.1046/j.1365-2141.2001.02869.x. [DOI] [PubMed] [Google Scholar]
- 90.Warkentin T E, Sheppard J I, Moore J C, Kelton J G. The use of well-characterized sera for the assessment of new diagnostic enzyme-immunoassays for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2010;8(01):216–218. doi: 10.1111/j.1538-7836.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 91.Greinacher A, Amiral J, Dummel V, Vissac A, Kiefel V, Mueller-Eckhardt C. Laboratory diagnosis of heparin-associated thrombocytopenia and comparison of platelet aggregation test, heparin-induced platelet activation test, and platelet factor 4/heparin enzyme-linked immunosorbent assay. Transfusion. 1994;34(05):381–385. doi: 10.1046/j.1537-2995.1994.34594249047.x. [DOI] [PubMed] [Google Scholar]
- 92.Horsewood P, Warkentin T E, Hayward C PM, Kelton J G. The epitope specificity of heparin-induced thrombocytopenia. Br J Haematol. 1996;95(01):161–167. doi: 10.1046/j.1365-2141.1996.d01-1876.x. [DOI] [PubMed] [Google Scholar]
- 93.Greinacher A, Michels I, Mueller-Eckhardt C. Heparin-associated thrombocytopenia: the antibody is not heparin specific. Thromb Haemost. 1992;67(05):545–549. [PubMed] [Google Scholar]
- 94.Visentin G P, Moghaddam M, Beery S E, McFarland J G, Aster R H. Heparin is not required for detection of antibodies associated with heparin-induced thrombocytopenia/thrombosis. J Lab Clin Med. 2001;138(01):22–31. doi: 10.1067/mlc.2001.115525. [DOI] [PubMed] [Google Scholar]
- 95.Pouplard C, Leroux D, Regina S, Rollin J, Gruel Y. Effectiveness of a new immunoassay for the diagnosis of heparin-induced thrombocytopenia and improved specificity when detecting IgG antibodies. Thromb Haemost. 2010;103(01):145–150. doi: 10.1160/TH09-04-0253. [DOI] [PubMed] [Google Scholar]
- 96.Sachs U J, Cooper N, Czwalinna A et al. PF4-dependent immunoassays in patients with vaccine-induced immune thrombotic thrombocytopenia: results of an interlaboratory comparison. Thromb Haemost. 2021;121(12):1622–1627. doi: 10.1055/a-1535-9002. [DOI] [PubMed] [Google Scholar]
- 97.Platton S, Bartlett A, MacCallum P et al. Evaluation of laboratory assays for anti-platelet factor 4 antibodies after ChAdOx1 nCOV-19 vaccination. J Thromb Haemost. 2021;19(08):2007–2013. doi: 10.1111/jth.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Platton S, Schönborn L, Charrot S et al. Vaccine-induced immune thrombocytopenia and thrombosis: the decline in anti-platelet factor 4 antibodies is assay-dependent. Br J Haematol. 2022;197(04):428–430. doi: 10.1111/bjh.18022. [DOI] [PubMed] [Google Scholar]
- 99.Gabarin N, Arnold D M, Nazy I, Warkentin T E. Treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT) Semin Hematol. 2022;59(02):89–96. doi: 10.1053/j.seminhematol.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gabarin N, Patterson S, Pai M et al. Venous thromboembolism and mild thrombocytopenia after ChAdOx1 nCoV-1 vaccination. Thromb Haemost. 2021;121(12):1677–1680. doi: 10.1055/a-1585-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nellen V, Sulzer I, Barizzi G, Lämmle B, Alberio L. Rapid exclusion or confirmation of heparin-induced thrombocytopenia: a single-center experience with 1,291 patients. Haematologica. 2012;97(01):89–97. doi: 10.3324/haematol.2011.048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leroux D, Hezard N, Lebreton A et al. Prospective evaluation of a rapid nanoparticle-based lateral flow immunoassay (STic Expert(®) HIT) for the diagnosis of heparin-induced thrombocytopenia. Br J Haematol. 2014;166(05):774–782. doi: 10.1111/bjh.12939. [DOI] [PubMed] [Google Scholar]
- 103.Warkentin T E, Sheppard J I, Linkins L A, Arnold D M, Nazy I. Performance characteristics of an automated latex immunoturbidimetric assay [HemosIL ® HIT-Ab (PF4-H) ] for the diagnosis of immune heparin-induced thrombocytopenia . Thromb Res. 2017;153:108–117. doi: 10.1016/j.thromres.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 104.Althaus K, Hron G, Strobel U et al. Evaluation of automated immunoassays in the diagnosis of heparin induced thrombocytopenia. Thromb Res. 2013;131(03):e85–e90. doi: 10.1016/j.thromres.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 105.Warkentin T E, Sheppard J I, Linkins L A, Arnold D M, Nazy I. High sensitivity and specificity of an automated IgG-specific chemiluminescence immunoassay for diagnosis of HIT. Blood. 2018;132(12):1345–1349. doi: 10.1182/blood-2018-04-847483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vayne C, Nguyen T H, Rollin J et al. Characterization of new monoclonal PF4-specific antibodies as useful tools for studies on typical and autoimmune heparin-induced thrombocytopenia. Thromb Haemost. 2021;121(03):322–331. doi: 10.1055/s-0040-1717078. [DOI] [PubMed] [Google Scholar]
- 107.Galli M, Barbui T. Antiprothrombin antibodies: detection and clinical significance in the antiphospholipid syndrome. Blood. 1999;93(07):2149–2157. [PubMed] [Google Scholar]


