Abstract
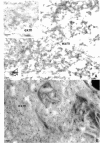
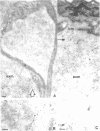
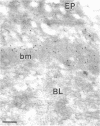
Immunoelectron microscopic studies of exfoliative iris tissue (seven specimens) revealed the presence of laminin in the fibrillar component of exfoliation material. The immunogold label was uniformly distributed on the exfoliation fibres. Deposition of laminin labelled exfoliation material in the dilator muscle was a noteworthy feature, as was an apparent depletion of laminin in the basement membranes of ostensibly unaffected vessels. In control iris tissue (five enucleated eyes) laminin was identified in the basement membrane round vascular contractile cells, but not beneath the endothelium.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott N. J. Developmental neurobiology. The milieu is the message. Nature. 1988 Apr 7;332(6164):490–491. doi: 10.1038/332490a0. [DOI] [PubMed] [Google Scholar]
- Abrahamson D. R. Recent studies on the structure and pathology of basement membranes. J Pathol. 1986 Aug;149(4):257–278. doi: 10.1002/path.1711490402. [DOI] [PubMed] [Google Scholar]
- Anastasi G., Puzzolo D., Romeo G., Santoro A., Scullica L. Iris ultrastructural changes in senile pseudo-exfoliation. So-called fibrillopathia epithelio-capsularis. Ophthalmologica. 1974;168(2):109–121. doi: 10.1159/000307028. [DOI] [PubMed] [Google Scholar]
- Ben-Zvi A., Rodrigues M. M., Krachmer J. H., Fujikawa L. S. Immunohistochemical characterization of extracellular matrix in the developing human cornea. Curr Eye Res. 1986 Feb;5(2):105–117. doi: 10.3109/02713688609015099. [DOI] [PubMed] [Google Scholar]
- Caldwell R. B. Extracellular matrix alterations precede vascularization of the retinal pigment epithelium in dystrophic rats. Curr Eye Res. 1989 Sep;8(9):907–921. [PubMed] [Google Scholar]
- Carpel E. F. Pupillary dilation in eyes with pseudoexfoliation syndrome. Am J Ophthalmol. 1988 Jun 15;105(6):692–694. doi: 10.1016/0002-9394(88)90073-6. [DOI] [PubMed] [Google Scholar]
- Davanger M. Pseudo-exfoliation material. Electron microscopy after the application of lanthanum as tracer particles and ionic stain. Acta Ophthalmol (Copenh) 1980 Aug;58(4):512–519. doi: 10.1111/j.1755-3768.1980.tb08292.x. [DOI] [PubMed] [Google Scholar]
- Dickson D. H., Ramsey M. S. Symposium on pseudocapsular exfoliation and glaucoma. Fibrillopathia epitheliocapsularis: review of the nature and origin of pseudoexfoliative deposits. Trans Ophthalmol Soc U K. 1979 Jul;99(2):284–292. [PubMed] [Google Scholar]
- Eagle R. C., Jr, Font R. L., Fine B. S. The basement membrane exfoliation syndrome. Arch Ophthalmol. 1979 Mar;97(3):510–515. doi: 10.1001/archopht.1979.01020010254014. [DOI] [PubMed] [Google Scholar]
- Engel J. EGF-like domains in extracellular matrix proteins: localized signals for growth and differentiation? FEBS Lett. 1989 Jul 17;251(1-2):1–7. doi: 10.1016/0014-5793(89)81417-6. [DOI] [PubMed] [Google Scholar]
- Essner E., Lin W. L. Immunocytochemical localization of laminin, type IV collagen and fibronectin in rat retinal vessels. Exp Eye Res. 1988 Aug;47(2):317–327. doi: 10.1016/0014-4835(88)90014-0. [DOI] [PubMed] [Google Scholar]
- Forsius H. Exfoliation syndrome in various ethnic populations. Acta Ophthalmol Suppl. 1988;184:71–85. doi: 10.1111/j.1755-3768.1988.tb02633.x. [DOI] [PubMed] [Google Scholar]
- Garner A., Alexander R. A. Pseudoexfoliative disease: histochemical evidence of an affinity with zonular fibres. Br J Ophthalmol. 1984 Aug;68(8):574–580. doi: 10.1136/bjo.68.8.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M., Speakman J. S. The iris in senile exfoliation of the lens. Can J Ophthalmol. 1974 Jul;9(3):289–297. [PubMed] [Google Scholar]
- Glaser B. M. Extracellular modulating factors and the control of intraocular neovascularization. An overview. Arch Ophthalmol. 1988 May;106(5):603–607. doi: 10.1001/archopht.1988.01060130657020. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Leblond C. P. Immunogold quantitation of laminin, type IV collagen, and heparan sulfate proteoglycan in a variety of basement membranes. J Histochem Cytochem. 1988 Mar;36(3):271–283. doi: 10.1177/36.3.2963856. [DOI] [PubMed] [Google Scholar]
- Harnisch J. P., Barrach H. J., Hassell J. R., Sinha P. K. Identification of a basement membrane proteoglycan in exfoliation material. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981;215(4):273–278. doi: 10.1007/BF00407666. [DOI] [PubMed] [Google Scholar]
- Herbst T. J., McCarthy J. B., Tsilibary E. C., Furcht L. T. Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. J Cell Biol. 1988 Apr;106(4):1365–1373. doi: 10.1083/jcb.106.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay J. L., Murray S. B. Early trabeculectomy versus conventional management in primary open angle glaucoma. Br J Ophthalmol. 1988 Dec;72(12):881–889. doi: 10.1136/bjo.72.12.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstas A. G., Marshall G. E., Lee W. R. Immunocytochemical localisation of collagens (I-V) in the human iris. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):180–186. doi: 10.1007/BF00935730. [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Streeten B. W., Wallace R. N. Association of elastin with pseudoexfoliative material: an immunoelectron microscopic study. Curr Eye Res. 1988 Dec;7(12):1163–1172. doi: 10.3109/02713688809033220. [DOI] [PubMed] [Google Scholar]
- Li Z. Y., Streeten B. W., Yohai N. Amyloid P protein in pseudoexfoliative fibrillopathy. Curr Eye Res. 1989 Feb;8(2):217–227. doi: 10.3109/02713688908995194. [DOI] [PubMed] [Google Scholar]
- Marshall G. E., Konstas A. G., Lee W. R. Ultrastructural distribution of collagen types I-VI in aging human retinal vessels. Br J Ophthalmol. 1990 Apr;74(4):228–232. doi: 10.1136/bjo.74.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Morrison J. C., Green W. R. Light microscopy of the exfoliation syndrome. Acta Ophthalmol Suppl. 1988;184:5–27. doi: 10.1111/j.1755-3768.1988.tb02624.x. [DOI] [PubMed] [Google Scholar]
- Panayotou G., End P., Aumailley M., Timpl R., Engel J. Domains of laminin with growth-factor activity. Cell. 1989 Jan 13;56(1):93–101. doi: 10.1016/0092-8674(89)90987-2. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Streeten B. W., Ritch R., Dark A. J., Sperling M. Preclinical diagnosis of pseudoexfoliation syndrome. Arch Ophthalmol. 1987 Aug;105(8):1076–1082. doi: 10.1001/archopht.1987.01060080078032. [DOI] [PubMed] [Google Scholar]
- Ringvold A. Exfoliation syndrome immunological aspects. Acta Ophthalmol Suppl. 1988;184:35–43. doi: 10.1111/j.1755-3768.1988.tb02626.x. [DOI] [PubMed] [Google Scholar]
- Ringvold A. Light and electron microscopy of the wall of iris vessels in eyes with and without exfoliation syndrome (pseudoexfoliation of the lens capsule). Virchows Arch A Pathol Pathol Anat. 1970;349(1):1–9. doi: 10.1007/BF00548518. [DOI] [PubMed] [Google Scholar]
- Ringvold A. The distribution of the exfoliation material in the iris from eyes with exfoliation syndrome (pseudoexfoliation of the lens capsule). Virchows Arch A Pathol Pathol Anat. 1970;351(2):168–178. doi: 10.1007/BF00542941. [DOI] [PubMed] [Google Scholar]
- SHAKIB M., ASHTON N., BLACH R. ELECTRON MICROSCOPIC STUDY OF PSEUDO-EXFOLIATION OF THE LENS CAPSULE. II. IRIS AND CILIARY BODY. Invest Ophthalmol. 1965 Apr;4:154–161. [PubMed] [Google Scholar]
- Schittny J. C., Timpl R., Engel J. High resolution immunoelectron microscopic localization of functional domains of laminin, nidogen, and heparan sulfate proteoglycan in epithelial basement membrane of mouse cornea reveals different topological orientations. J Cell Biol. 1988 Oct;107(4):1599–1610. doi: 10.1083/jcb.107.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T. Changes of iris vessels in capsular glaucoma: three-dimensional and electron microscopic studies. Jpn J Ophthalmol. 1985;29(4):434–452. [PubMed] [Google Scholar]
- Streeten B. W., Dark A. J., Barnes C. W. Pseudoexfoliative material and oxytalan fibers. Exp Eye Res. 1984 May;38(5):523–531. doi: 10.1016/0014-4835(84)90130-1. [DOI] [PubMed] [Google Scholar]
- Streeten B. W., Gibson S. A., Dark A. J. Pseudoexfoliative material contains an elastic microfibrillar-associated glycoprotein. Trans Am Ophthalmol Soc. 1986;84:304–320. [PMC free article] [PubMed] [Google Scholar]
- Streeten B. W., Gibson S. A., Li Z. Y. Lectin binding to pseudoexfoliative material and the ocular zonules. Invest Ophthalmol Vis Sci. 1986 Oct;27(10):1516–1521. [PubMed] [Google Scholar]
- Sugita A., Ishibashi R., Shiotani N., Yoshioka H. Morphological features of iris fibroblasts in dilator muscle region. Jpn J Ophthalmol. 1988;32(2):151–158. [PubMed] [Google Scholar]
- Vannas A. Fluorescein angiography of the vessels of the iris in pseudoexfoliation of the lens capsule, capsular glaucoma and some other forms of glaucoma. Acta Ophthalmol Suppl. 1969;105:1–75. [PubMed] [Google Scholar]