Abstract
Bdallophytum americanum (Cytinaceae) is an endoparasitic plant species, meaning only the flowers emerge from the host during the reproductive season. Reports on the pollination biology of this species state that its primary pollinators are carrion flies attracted by the smell of the flowers and nectar as a reward. However, the functional role of one of the most outstanding attributes of B. americanum has been neglected. These are the staminal appendages formed by the apical overgrowth of connective tissue during anther development. To determine whether these staminal appendages play a role in pollination, we monitored a nectarless population of B. americanum. We described the inflorescence emergence, floral movements, and pollination and performed field experiments to test whether the absence of the staminal connective appendages affected the visitation frequency. Male inflorescences emerge early, and both male and female flowers open during the day and do not close. Hoverflies are the most frequent visitors to both floral sexes and carry the most pollen. Moreover, the movement of staminal appendages matching the pollen viability changes is reported for the first time. The staminal appendages are the structures where pollinators land before foraging. The field experiments showed that the visitation frequency decreased sharply without staminal appendages. As a landing platform, the staminal connective appendages in B. americanum are crucial for pollinator positioning and collecting viable pollen.
Keywords: Copestylum, Floral movements, Hoverfly pollination, Landing platforms, Sapromyophily, Staminal appendages
Introduction
Parasitic plants obtain their resources from other plants through a specialized structure called the haustorium and have evolved 12 times during angiosperm evolution (Nickrent 2020; Twyford 2018). Parasitic plants can be classified as hemiparasites or holoparasites depending on their photosynthetic capacity. The former retains photosynthetic activity while the latter has lost all photosynthetic functions (Heide-Jørgensen 2008). A subset of holoparasites is classified as endoparasites because their vegetative body grows inside the host, and only the flowers emerge from the host (Teixeira-Costa et al. 2021; Thorogood et al. 2021). The endophytic holoparasites comprise four families from different orders: Apodanthaceae (Cucurbitales), Cytinaceae (Malvales), Mitrastemonaceae (Ericales), and Rafflesiaceae (Malpighiales), the latter being the most notable species within the parasitic angiosperms (Nickrent 2020; Thorogood et al. 2021). Since the species of these families externally comprise only flowers or inflorescences, floral biology studies are essential to understand part of the life cycle of these peculiar species.
Floral biology ranges from the flowers’ emergence, form, and function to advertisements and rewards concerning pollination (Gottsberger 1989; Willmer 2011). Pollination has been studied in some endoparasites, and due to their peculiar morphologies, the study of the form and function of the flowers has become important to understand aspects of the reproductive biology of the species (Bänziger 1991; Beaman et al. 1988; De Vega et al. 2009; García-Franco and Rico-Gray 1997; Hobbhahn and Johnson 2015; Johnson et al. 2011; Sipes et al. 2014; Suetsugu 2019). Within these endoparasites, the Rafflesiaceae species are the most studied, and their flowers have unique traits whose function has been related to carrion fly pollination (Faegri and van der Pijl 1979; Nikolov et al. 2014). An example is the presence of a barrier comprising floral acicular hairs allowing the entry of pollinators (Bänziger 1995). This prevents pollen robbery by non-pollinators, and the stigma form facilitates pollen deposition in Rafflesiaceae flowers (Beaman et al. 1988). Filiform appendages have been related to fly-pollination serving as landing platforms or scent-emitting structures, which influence the attraction of pollinators and their behaviour (Katsuhara et al. 2017; Suetsugu et al. 2021, 2022). In addition to filiform appendages, the strong foul smell, large or small flowers clustered in inflorescences, in some cases the presence of nectar, colours resembling decaying meat or dung, and the presence of hairy pads are some of the features that characterize the sapromyophilous pollination syndrome (Faegri and van der Pijl 1979; Moir et al. 2022; Shuttleworth et al. 2017; Suetsugu et al. 2022; Willmer 2011).
Sapromyophily has been highly reported for different non-parasitic angiosperm species such as Araceae, Apocynaceae, Aristolochiaceae, Orchidaceae, and others (Johnson 2016; Jürgens and Shuttleworth 2015), but as we mentioned before, the sapromyophilous endoparasites have received less attention. In that sense, in addition to Rafflesiaceae, other endoparasitic sapromyophilous species exist, such as those of the genus Bdallophytum of the Cytinaceae family (García-Franco and Rico-Gray 1997; Rios-Carrasco et al. 2022a, 2022b). Pollination studies confirmed that B. americanum (formerly B. bambusarum) is pollinated by carrion flies (García-Franco and Rico-Gray 1997), while B. andrieuxii and B. oxylepis are pollinated by butterflies and stingless bees respectively, indicating that carrion behaviour is not exclusive to carrion flies (Rios-Carrasco et al. 2022a, 2022b).
Cytinaceae is the second species-rich endoparasite family, following Rafflesiaceae, with 12 species in two genera (Nickrent 2020). The genus Cytinus encompasses eight species from the Mediterranean, South Africa, and Madagascar (Sanjust and Rinaldi 2021), and Bdallophytum includes four species from Mexico to Colombia (Nickrent 2020). The first pollination study in Cytinaceae was conducted in B. americanum in a population from a tropical semi-humid area (García-Franco and Rico-Gray 1997). However, this species is widely distributed in seasonal vegetation types characterized by marked dry and wet seasons (Alvarado-Cárdenas 2009). So far, the variation in pollination is unknown for other populations of B. americanum in different environments. Regarding floral traits, B. americanum is a dioecious species whose male flowers are noticeable because they have conspicuous staminal connective appendages resembling a big multilobed stigma. The growth of the apical connective tissue forms these appendages during flower development, creating long extensions, one per anther, that are not seen in other species of the genus (Rios-Carrasco and Vázquez-Santana 2021). The form-function relationship of the connective appendages on male flowers of B. americanum remains unknown. In order to know if staminal appendages of B. americanum male flowers play a role in pollination as reported for other flowers with appendages, this work aimed to study the floral biology of B. americanum, addressing the floral biology and pollination. We especially focus on the functionality of the staminal connective appendages during pollination.
Materials and methods
Species and study site
Bdallophytum americanum Harms is a dioecious endophytic holoparasite species belonging to Cytinaceae. This dioecious species is widely distributed, with populations from Mexico to Costa Rica (Alvarado-Cárdenas 2009). The species has the fewest flowers per inflorescence (12–18) and the largest flowers within the genus (Rios-Carrasco and Vázquez-Santana 2021). Based on the floral morph, the flowers have a dark purple perigone contrasting with the anthers or stigma. Male flowers have conspicuous bright yellow staminal connective appendages, and the female ones have a bright yellow stigma (Fig. 1a, b). Since each inflorescence emerges from a different site of the host root, here we consider that an inflorescence corresponds to an exophyte, which in turn corresponds to an individual.
Fig. 1.
Exophytes of Bdallophytum americanum. a Female inflorescences (exophytes). b Male inflorescences (exophytes). c Percentage of emerged exophytes of B. americanum per sex through time in the population of Calvillo, Aguascalientes, Mexico. The withered male inflorescences were not counted
The fieldwork was conducted in the municipality of Calvillo in the state of Aguascalientes, Mexico. The weather is predominantly semi-warm, with an average annual temperature of 16.6–20.3 °C and a total annual precipitation of 612.2 mm (Instituto Nacional de Estadística y Geografía 2017). The locality is in a remnant patch of a seasonally dry tropical forest where some species such as Albizia plurijuga, Conzattia multiflora, Lysiloma spp., Leucaena spp. (Fabaceae), Bursera fagaroides (Burseraceae), Myrtillocactus geometrizans, Stenocereus queretaroensis, and S. drummondii (Cactaceae) are dominant in the area (Siqueiros-Delgado et al. 2016). Within the municipality, the original vegetation is scarce since fields of guava cultivation have replaced it. This population of B. americanum is parasitizing B. fagaroides, and we considered each parasitized host as a cluster. The specimen vouchers were deposited at the Herbarium Luz María Villarreal de Puga of the Universidad de Guadalajara as follows: Sánchez 681 IBUG (male inflorescences of B. americanum), Sánchez 682 IBUG (female inflorescences of B. americanum), and Sánchez 679 IBUG (host Bursera fagaroides).
Bdallophytum americanum population
During three flowering seasons from 2018 to 2020, we counted the number of B. americanum hosts within 1 km2 in a remnant patch of seasonally dry tropical forest between cultivated areas. We counted the number of exophytes on roots for each host (equal to one cluster) and classified them according to sex. It is noteworthy that neither sexual morph showed any secretions or nectar during the fieldwork at any moment or year of study. Thus, this is a nectarless population.
Floral movements, stigmatic receptivity, and pollen viability
We observed at least 50 pre-anthetic flowers per sex (belonging to 10 inflorescences per sex) to detect any change or movement during opening, as well as the moment of anther dehiscence and movement in the connective appendages throughout the flower lifespan in male flowers. Additionally, we used hydrogen peroxide to determine the moment when stigmatic receptivity begins and its duration (Galen and Plowright 1987). We tagged and bagged 10 female inflorescences to avoid pollen interference on stigmas. At least 15 flowers per age were used to measure stigmatic receptivity in newly opened flowers in 1-, 2-, 3-, and 4-day-old flowers; 30 µL syringes were used to place a few drops of hydrogen peroxide on the stigmas.
Regarding male flowers, we collected pollen samples from different anthers from each flower with a dissection needle. We placed them on glass slides to stain the pollen grains with Alexander’s reagent (Alexander 1980) to determine the duration of pollen viability over time. We classified flowers according to their age, and we calculated the percentage of viable pollen in flowers of different ages (1- to 6-day-old flowers). We used at least 15 flowers per age.
Floral visitors
We recorded the floral visitors in two flowering seasons during August and September of 2019 and 2020, respectively, for 4 days each year in six clusters (three clusters per sex). Each cluster had at least five flowering inflorescences of B. americanum. The records of diurnal floral visitors were produced through direct observations in five inflorescences per cluster from 9:00 to 16:00 h in 15 min observations followed by 15 min of rest. We also used trap cameras programmed in two video modes, (1) to record a one-minute video every 15 min (to detect small visitors) and (2) we activated the motion sensor (to detect larger visitors); both modes were active from 16:00 to 9:00 h. We obtained the number of visitors per inflorescence (male or female), the visited flowers, the foraging time, and the number of visited inflorescences per visitor. We used lethal chambers to capture samples of floral visitors. Mounted specimens were identified at least at the family level and to the finest level possible, using the following keys: de Carvalho et al. (2003), Elberg et al. (2009), Engel (2000), Goulet and Huber (1993), Hernández et al. (2013), Knutson and Orth (2001), Thompson (1999) and Triplehorn and Johnson (2005). When the species level was not reached, we used the morphospecies criterion.
We classified the floral visitors into functional groups according to their foraging behaviour (Fenster et al. 2004). Particularly, we split the fly functional group into two according to the foraging behaviour we observed in the field. The first group was treated as “flies” and the second as “hoverflies”, given their pollinivorous and landing behaviour described in the results section. To determine differences in the visitation frequency among functional groups, we performed a generalized linear model (GLM) with a Poisson distribution and log-linkage function using the package stat in the software R (R Development Core Team 2019). We used the visitation frequency as the response variable and the functional groups, years of observation, and sexes as the explanatory variables. Then, a Chi-square test was performed to examine if differences existed between each explanatory variable. Additionally, we performed a multiple comparison analysis of the visitation frequency between functional groups. We considered pollinators those that visited female and male inflorescences, consistently touching the sexual organs and carrying pollen from donors on their bodies. Visitors who approached only one sex or did not touch sexual organs were discarded as pollinators.
To identify the functional group that contributes the most to pollination, we evaluated the pollination important index (PII) following Lindsey (1984) and Mochizuki and Kawakita (2018). We calculated the PII considering (1) the relative abundance expressed as the proportion of the visitors collected per functional group (A) of each functional group; (2) the pollen carrying capacity (PCC) expressed as the average of pollen load; (3) relative host fidelity (F) expressed as the proportion of carried host pollen, and (4) pollinator effectiveness (PE) which indicates the probability of a visitor to pollinate. Following Mochizuki and Kawakita (2018), we assigned a PE value of 1 if the visitors (belonging to the same species or morphospecies) always touched both sexual organs (indicating that they visited both male and female inflorescences), and 0 if the same visitor species or morphospecies touched only one sexual organ (only visited one inflorescence sex) or none. As some visitors occasionally touched both sexual organs, we assigned them a PE value of 0.5. We estimate the pollination importance value (PIV) for each functional group through the following equation: PIV = A × PCC × F × PE. Then, to obtain the PII per functional group, including both years of study, we used: PII = PIV∕ƩPIV (Lindsey 1984; Mochizuki and Kawakita 2018). We excluded functional groups whose visit frequency was so low that it was not possible to capture specimens.
Role of connective appendages in pollination
We performed a field experiment in 2020 to explore the role of the staminal connective appendages by evaluating whether their absence affected the visitation frequency of pollinators to male inflorescences. The experiment comprised two floral conditions, the mutilated and the intact flowers. In the first, the apical connective appendages were removed from all flowers of an inflorescence. In the intact, the flowers were not handled, serving as a control. Both treatments were applied to all inflorescences from two clusters namely pure clusters since all the flowers presented the same condition, one cluster with only mutilated flowers (n = 10 inflorescences), and in the second only intact flowers (n = 10 inflorescences). To discard the cluster effect and evaluate the decision-making by floral visitors, we randomly applied both treatments (mutilated and intact) into a single cluster, namely the mixed cluster (n = 10 inflorescences, 5 per treatment). The visitation frequency was recorded following the same method described above for floral visitors. We compared the total visitation frequency (response variable) between treatments (mutilated vs intact) and between cluster types (pure vs mixed). The comparisons were performed through a GLM with a Poisson distribution, log-linkage function, and chi-square to determine differences between treatments and clusters using the software R Development Core Team (2019). The analysis was conducted considering a daytime observation time from 9:00 to 19:00 h to avoid data overdispersion since the pollinators are diurnal.
Results
Bdallophytum americanum population
The studied population of Bdallophytum americanum comprised nine clusters within approximately 1 km2 in a seasonally dry tropical forest patch. During the first year of monitoring (2018), we found six clusters, of which three had exophytes from that season, while the other three had remains of exophytes apparently from the previous season. Moreover, the same clusters were found in the next two years, and three clusters more during the last year. Most exophytes had the same sex within a cluster, and this was maintained through the years (Table 1), indicating re-emergence.
Table 1.
Number and sex of exophytes per cluster (host) of B. americanum indicating reemergence through years in the population of Calvillo, Aguascalientes
| Cluster | Number of exophytes and their sex | ||
|---|---|---|---|
| 2018 | 2019 | 2020 | |
| 1 | 47 ♂ | 47 ♂ | 40 ♂ |
| 2 | 4 ♀, 13♂ | 38 ♀, 8 ♂ | 41 ♀, 10 ♂ |
| 3 | 10 ♀ | 17 ♀ | 11 ♀ |
| 4 | 14 ♀ | 13 ♀ | 14 ♀ |
| 5 | 24 ♀ | 30 ♀ | 25 ♀ |
| 6 | 41 ♂ | 33 ♂ | 40 ♂ |
| 7 | – | – | 10 ♂ |
| 8 | – | – | 2 ♀ |
| 9 | – | – | 4 ♀ |
The emergence of the inflorescences begins after the first rains in the area. The exophytes or young inflorescences can be observed from July, and as time passes, the number of exophytes increases. The first exophytes to emerge in the studied population corresponded to the male inflorescences, which predominated during the first month, mainly flower buds. The female inflorescences predominated from August to September, while only infructescences were present in October (Fig. 1). Despite the asynchrony at emergence, female and male inflorescences emit a strong unpleasant smell when the flowers are open. This smell is easily detectable by the human nose when close to a patch.
Floral movements, stigmatic receptivity, and pollen viability
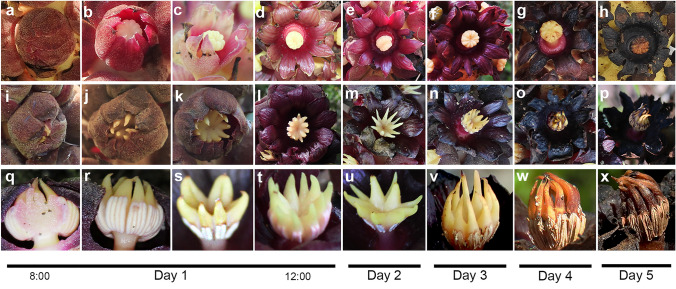
During the morning, the flowers are closed (Fig. 2a, i). The flower opening occurs around 8:00 to 9:00 h in the morning (Fig. 2b–c), reaching a full aperture at approximately noon (Fig. 2d, l). As the days pass, female flowers exhibit colour changes; they are initially pale red (Fig. 2b–d) and turn darker (Fig. 2e–g). The perigone remains open and withers around day 5 (Fig. 2h), indicating the start of fruit development if fertilization has occurred. In male flowers, the perigone is dark red from the beginning (Fig. 2i–k). Female flowers reach the full aperture at noon (Fig. 2l); the flowers remain open until they wither on day 4 or 5 (Fig. 2m–o), later appearing whiter (Fig. 2p). In the male flowers, the anthers and appendages of the connective tissue also showed movements and changes along the flower lifespan (Fig. 2q–x). The apical connective appendages are towards the centre when the flowers open, so the tips touch each other and protrude above the perigone (Fig. 2q, r). The appendages then extend out as the perigone unfolds to achieve a complete opening (Fig. 2s–u). The anther dehiscence is extrorse. This occurs when the flowers are completely open, and the connective appendages are fully extended (Fig. 2t, u). The appendages remain extended during the first 2 days of anthesis (Fig. 2t, u). The appendages begin to darken and retract towards their initial position on the third day (Fig. 2v–x).
Fig. 2.
Changes and movements through time in B. americanum flowers. a–h Female flowers showing their colour change since their opening until their withering. i–p Male flowers opening. q–x Movements and changes of connective appendages of stamens
Regarding sexual functionality, the pollen grains are viable in male flowers during the first 4 or 5 days. Nevertheless, from the third day, viability decreases abruptly. The pollen grains are no longer viable on the sixth day (Fig. 3). The stigmas in female flowers start to be receptive as soon as the flowers start to open. The receptivity lasts 3 days. The stigmas are no longer receptive on day 4 (Fig. 3).
Fig. 3.
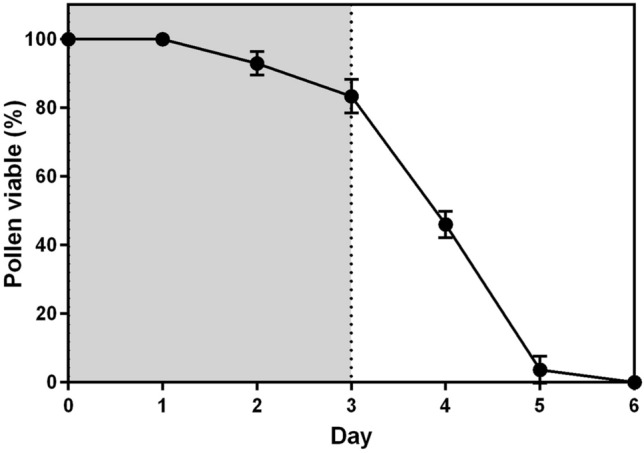
Decreasing in viability of pollen grains in male flowers of B. americanum over the days. The gray area indicates the duration of stigmatic receptivity in female flowers
Floral visitors
We recorded the visits of two species and 12 different morphospecies belonging to eight functional groups (Table 2) in a total of 2052 min of observation during both years of study. Most visits were recorded in a diurnal schedule and only rodents and moths were rarely seen at night-time (Fig. 4). Male inflorescences had more frequent visits than female inflorescences (χ2 = 6.166, df = 1, p < 0.0001). Additionally, we registered more visits in 2019 from more functional groups than in 2020 (χ2 = 14.156, df = 1, p < 0.0001; see Fig. 4). Regarding the visitation frequency, differences occurred between the functional groups (χ2 = 294.431, df = 7, p < 0.0001; Table 3). Hoverflies were the most important pollinators in both years of study. Their PII was the highest value compared to other important potential pollinators such as bees, beetles, and flies (see Table 4).
Table 2.
Species or morphospecies of floral visitors recorded for male and female inflorescences of Bdallophytum americanum in Calvillo, Aguascalientes, Mexico during 2019 and 2020
| Visitors to female inflorescences | Visitors to male inflorescences | ||||
|---|---|---|---|---|---|
| Functional group | Taxonomical family | Visitor | Functional group | Taxonomical family | Visitor |
| Bees | Hallictidae | Augochlorini sp. 1 | Bees | Hallictidae | Augochlorini sp. 1 |
| Beetles | Curculionidae | Epimechus adspersus | Augochlorini sp. 2 | ||
| Butterflies | – | Butterfly 1 | Beetles | Curculionidae | Epimechus adspersus |
| Flies | Heleomyzidae | Heleomyzidae sp. 1 | Nitidulidae | Nitidulidae sp. 1 | |
| Sciomyzidae | Sepedon sp. 1 | Nitidulidae sp. 2 | |||
| Hoverflies | Syrphidae | Copestylum sp. 1 | Nitidulidaae sp. 3 | ||
| Moths | – | Moth 1 | Flies | Fannidae | Fannia canicularis |
| Orthopterans | Acrididae | Acrididae sp. 1 | Heleomyzidae | Heleomyzidae sp. 1 | |
| Sciomyzidae | Sepedon sp. 1 | ||||
| Hoverflies | Syrphidae | Copestylum sp.1 | |||
| Moths | – | Moth 1 | |||
| Rodents | – | Rodent 1 | |||
The visitors were classified into functional groups based on their behaviour. Visitors recorded on both inflorescences are in bold
Fig. 4.
Floral visitors to B. americanum by functional groups to see the frequency of visits to male and female inflorescences along the day in both years of study. Potential pollinators can be distinguished by overlapping different coloured bubbles
Table 3.
Characteristics of the visits from different functional groups to the male and female inflorescences of B. americanum in Calvillo, Aguascalientes, Mexico during both years of study
| Female inflorescences | Male inflorescences | |||||||
|---|---|---|---|---|---|---|---|---|
| Functional group | Visited inflorescences per cluster Mean ± SEM | Visited flowers per inflorescence Mean ± SEM | Handling time per visit s | Total records | Visited inflorescences per cluster Mean ± SEM | Visited flowers per inflorescence Mean ± SEM | Handling time per visit s | Total records |
| Beesac | 1.5 ± 0.28 | 1 | 7.5 ± 2.5 | 2 | 2.12 ± 0.35 | 2.9 ± 0.64 | 19 ± 5.56 | 13 |
| Beetlesbc | 1.4 ± 0.4 | 2.14 ± 0.4 | 17.44 ± 4.6 | 11 | 1.22 ± 0.22 | 1.42 ± 0.29 | 41 ± 23.61 | 7 |
| Butterfliesac | 1.33 ± 0.33 | 1.66 ± 0.67 | 6.55 ± 1.3 | 8 | – | – | – | 0 |
| Fliesc | 1.2 ± 0.13 | 2.27 ± 0.33 | 34.8 ± 9.6 | 36 | 1 | 1.57 ± 0.29 | 132.5 ± 25.87 | 13 |
| Hoverfliesd | 2.12 ± 0.24 | 3.7 ± 0.48 | 25.7 ± 4.49 | 75 | 1.72 ± 0.23 | 3.91 ± 0.57 | 80.86 ± 16.4 | 87 |
| Mothsa | 1 | 1 | 2 | 1 | 1 | 1 | 4 | 1 |
| Orthopteransac | 1 | 1.5 ± 0.5 | 212 ± 66 | 5 | – | – | – | 0 |
| Rodentsab | – | – | – | 0 | 2 | 1.5 ± 0.5 | 4.33 ± 0.3 | 1 |
Superscripts indicate differences in the visitation frequency between functional groups resulting from comparison multiple analysis at p < 0.001
Table 4.
Importance of functional groups as pollinators of B. americanum in Calvillo, Aguascalientes, Mexico during the two years of study
| Functional group | Visitors captured | A | PCC | F | PE | PIV | PII |
|---|---|---|---|---|---|---|---|
| Bee | 4 | 0.15 | 17,500 | 0.7 | 0.5 | 907.407 | 0.3 |
| Beetles | 5 | 0.19 | 370 | 1 | 1 | 68.518 | 0.02 |
| Flies | 3 | 0.11 | 375 | 1 | 0.5 | 20.833 | 0.01 |
| Hoverflies | 15 | 0.56 | 3680 | 1 | 1 | 2044.444 | 0.67 |
PII values are in bold
A relative visitor abundance, PCC pollen carrying capacity, F relative plant fidelity, PE pollinator effectiveness, PIV pollination importance value, PII pollination importance index
Regarding the behaviour of visitors, butterflies, moths, orthopterans, and rodents were discarded as pollinators. Most of them only visited one type of inflorescence, or their visits were rare, and they spent a few seconds foraging (Table 3). Additionally, the orthopterans spent a long time eating floral parts. Thus, they were florivorous rather than pollinators. The halictid bees of the tribe Augochlorini (Augochlorini sp. 1 and sp. 2) were the visitors carrying the largest number of pollen grains of B. americanum. However, only Augochlorini sp. 1 visited both sexes, meaning that only this bee species could contribute to pollination. On their visits, the bees spent more time collecting pollen than on their visits to female flowers. Besides, the pollen found on the bees’ bodies was not exclusive to B. americanum (Table 4). Despite the low visitation frequency to female flowers, the PII of bees was above that of beetles and flies; thus, bees may contribute to pollination due to their high pollen loads. Beetles of the species Epimechus adspersus (Curculionidae) were registered to visit both floral sexes. Although beetles spend more time resting in the perigone than touching the sexual organs, they can pollinate as they arrive at stigmas with B. americanum pollen. Flies were more frequent than beetles (Fig. 4). They occasionally touched the sexual organs and spent more time resting on the perigone (Table 3). We found that Sepedon sp. 1 flies reached the female flowers with B. americanum pollen, occasionally touching the sexual organs of both inflorescences. Thus, they also contributed to pollination but are not as important as hoverflies (Table 4).
Finally, Copestylum sp. 1 hoverflies were the most frequent visitors in both male and female inflorescences (Fig. 4) during both years of study. They carried large amounts of pollen, exclusive to B. americanum, which was transported from male to female flowers. Also, Copestylum sp. 1 were the insects with the most records and visited the most flowers and inflorescences per cluster (Tables 3 and, 4). These floral visitors consistently touched the sexual organs. In male flowers, hoverflies landed on staminal connective appendages and used them as perches to move underneath the anthers and the rest of the flower (Fig. 5a). When they reached the female flowers, they landed on the stigma and began to move along the perigone of the flower (Fig. 5b). Once the hoverflies landed on stigmas or connective appendages according to sex, they moved to visit different flowers along the same inflorescence and spent less time on female flowers than on male ones. During most of their visits, we noticed that hoverflies could remain for more than 30 min resting on the perigone of the male flowers after foraging on the anthers. Therefore, given the amount of pollen on the body, the visitation frequency on both types of flowers, the constant contact with sexual organs, and the higher PII, hoverflies were the most important pollinators of B. americanum in the study area.
Fig. 5.
Pollinators of B. americanum in the studied population. a Copestylum sp. 1 landing on the stigma of female flowers. b Copestylum sp. 1 landing on connective appendages of male flowers, using them as perch to move around the flower
Role of staminal appendages in pollination
The frequency of visits differed between mutilated and intact (control) male flowers (χ2 = 114.56, df = 1, p < 0.0001). In the absence of connective appendages, pollinators approach clusters but do not land on flowers. The visitation frequency was lower in mutilated flowers, which was observed in both clusters (pure and mixed). However, the mixed cluster was less visited than the pure intact cluster (χ2 = 46.79, df = 1, p < 0.0001) indicating that mutilated flowers affected the visits to the rest of the inflorescences within the cluster (Fig. 6). Also, visitors seem to discern between mutilated and intact flowers because the visits were more frequent in the control than in the mutilated inflorescences within the same cluster. Visiting hours were mainly daytime, and the visits were consistent with the pollination observations where hoverflies were noticeable as the most frequent visitors in both treatments and clusters (Fig. 6).
Fig. 6.

Visits to intact (control) and mutilated male flowers from mixed and pure clusters resulted in the connective appendages experiments. Pure clusters: patch with all inflorescences with the same condition (either intact or mutilated). Mixed cluster: patch with both mutilated and intact inflorescences randomly distributed. Asterisks show differences in the visitation frequency between treatments and clusters at p < 0.001
Discussion
Bdallophytum americanum population
Bdallophytum americanum growth showed re-emergence of same-sex inflorescences over the years. The inflorescence emergence occurs gradually during blooming, so the variation between the number of exophytes between years and between clusters depends on the moment they are observed. The same pattern of re-emergence according to sex was reported for other species of Cytinaceae, such as Cytinus hypocistis (De Vega et al. 2007) and C. sanguineus (Hobbhahn and Johnson 2015). This feature has also been observed in Pilostyles thurberi, another endoparasite of the family Apodanthaceae (Ortega-González et al. 2023). In C. hypocistis, the re-emergence of exophytes has been followed over 5 years, indicating that this species has perennial plants (De Vega et al. 2007).
Bdallophytum americanum is a dioecious species whose male and female plants differ during emergence. The male inflorescences emerge before the female ones. This asynchronous emergence could affect reproduction. Female inflorescences take more time to appear as they need more resources to reproduce (Conn and Blum 1981). While male inflorescences can be advantageous since they can compete for pollinators with other species growing on the same site and keep floral visitors close to the area, taking advantage of their learning skills and awareness of the resource offered by B. americanum flowers (Purrington and Schmitt 1998; Weiss 2001).
Floral movements and their implications in pollination
Here, we described for the first time the floral movements in a species of Cytinaceae. In B. americanum, the perigone movements occur during the morning and stop at noon, when the flowers are fully open, exposing the sexual organs. Stigmas from female flowers of B. americanum remain receptive for three days, while pollen grains from male flowers are viable for up to five days. Thus, all-day exposure to sexual organs allows a wide range of pollinators to visit flowers at different schedules favouring the reception and collection of pollen (Ganie et al. 2021; van Doorn and van Meeteren 2003). On the other hand, the lack of movements that cause the flowers to close can be disadvantageous since sexual organs are exposed to florivores, robbers, or damage (van Doorn and van Meeteren 2003). In this sense, floral movements are essential to flower and pollination ecology (Henning et al. 2018; Sibaoka 1969).
Additionally, a novel floral movement is described here for the family. The apical staminal connective appendages move synchronously in all stamens and parallel with the direction of the perigone when it opens. This parallel movement of stamens and perianth is described for other angiosperms where anthers and filaments are the moving structures (Henning et al. 2018; Zhang et al. 2019). In B. americanum, the staminal structures that move are the connective appendages that have an important role during pollen collection. In the first two days of anthesis, when the staminal appendages are completely extended or “open”, the pollen is at its maximum viability, favouring viable pollen presentation. When the connective appendages begin to “close” or return to their initial position on the third day of anthesis, the pollen viability decreases abruptly. This match between changes in pollen viability, the extended staminal appendages, and pollination is discussed in depth in the following sections.
Floral visitors and pollination
The pollination patterns can vary depending on the habitat composition, availability of resources, and surrounding communities of organisms (Evans et al. 2017). A previous study on the pollination biology of B. americanum (formerly B. bambusarum) described how both male and female flowers produce nectar; however, it is more concentrated in female flowers (García-Franco and Rico-Gray 1997). The presence of floral rewards in both morphs explains the floral visits to male and female flowers, but in the studied population of B. americanum, all flowers lacked nectar. Still, hoverflies carried pollen exclusively from B. americanum and consistently touched the stigma. Thus, pollination can occur despite the lack of nectar. Despite the differences in the floral rewards offered by B. americanum to pollinators in different populations, dipterans with carrion behaviour are maintained as pollinators (García-Franco and Rico-Gray 1997). This pattern is also observed in other endoparasites such as Sapria ram (Rafflesiaceae), where the pollinators in different populations are consistently carrion flies, indicating a specialized interaction (Bänziger and Pape 2004; Pape and Bänziger 2000).
Studies of the floral ecology of other sapromyophilous endoparasites, such as the Rafflesiaceae species, have described the floral scent as one of the main attractants to pollinators (Zain et al. 2020). The foetid smell of the sapromyophilous species is strongly related to attracting carrion flies (Chakraborty and Adhikary 2018). Although syrphids are known to feed on pollen and nectar, members of the subfamily Eristalinae (where Copestylum belongs) have been reported as helpful in forensic entomology due to their consumption of carrion material (Martins et al. 2010). Specifically, hoverflies of the genus Copestylum were found in rodent carcasses, indicating that these organisms exhibit carrion behaviour (Moretti et al. 2008). Thus, although the potential pollinators are hoverflies of the genus Copestylum rather than the expected carrion flies, the carrion pollination is maintained given the carrion foraging behaviour of Copestylum sp. 1 in B. americanum flowers. Visits of Copestylum to female flowers are shorter and less frequent than to male ones. This might be because the female flowers do not produce rewards such as pollen or nectar, nor do they display large appendages like the male ones. Other attractants, such as scents may be conspicuous enough to attract pollinators, mostly in cases of sapromyophily, in which foetid smells are crucial for pollination to occur (Jürgens and Shuttleworth 2015). As hoverflies of the genus Copestylum can be attracted to carrion, pollination in this population appears to occur by deception. The female flowers do not offer a reward but expel a strong unpleasant smell. To confirm deceit pollination in this nectarless population it is necessary to study the emission of volatile organic compounds (VOCs) and the morphoanatomy of flowers to assess the functionality of nectaries.
Sapromyophilous traits are exclusive of the genus Bdallophytum within the Cytinaceae (Rios-Carrasco et al. 2022a, 2022b). Despite those floral traits such as dark floral colour and foetid smell resembling decaying material, pollination by carrion flies has only been reported in one population of B. americanum (García-Franco and Rico-Gray 1997). The other Bdallophytum species B. andrieuxii and B. oxylepis are pollinated by butterflies, and stingless bees, respectively (Rios-Carrasco et al. 2022a, 2022b), in addition to the present study in which B. americanum flowers are pollinated by hoverflies. Although butterflies, stingless bees, and hoverflies are not typically classified as carrion pollinators like certain groups of flies or beetles (Jürgens and Shuttleworth 2015), their carrion behaviour includes them as carrion pollinators. Thus, it is necessary to discuss based on the background information on visitor behaviour to better understand their role as pollinators.
Staminal connective appendages as landing platforms
Flowers of B. americanum display a set of signals to attract pollinators, including the unpleasant smell, the dark colour of the flowers, and the long and apical connective appendages. Previous studies on B. americanum pollination did not address the role of apical connective appendages (García-Franco and Rico-Gray 1997). Thus, here, we describe for the first time their role in pollination. Our results indicated that the visitation frequency decreases sharply without the connective appendages in all flowers within a cluster. It is necessary to consider that the mutilation of staminal connective appendages could cause the releasing of VOCs associated with plant defence against herbivory affecting the visitation frequency (Suetsugu et al. 2022). To confirm this, studies are needed to assess the VOCs emission under herbivory conditions.
In a second scenario where both treatments were on the same patch, the visitation frequency was less in mutilated than in the intact flowers. Moreover, almost all visits were performed by hoverflies. The preference for intact flowers within the same cluster indicates a pollinator’s decision-making. Thus, there is a possibility that staminal appendages function as a visual attractant. According to the behaviour recorded for hoverflies (the most important pollinators in this population), they landed and perched on the staminal appendages. In Mitella pauciflora (Saxifragaceae), a fly-pollinated species, the petals have long filiform structures serving as landing platforms. In their absence, the visitation rate decreases (Katsuhara et al. 2017) as in B. americanum. As we mentioned previously, the “opening” of connective appendages matches the maximum pollen viability and is the structure where the hoverflies land. Studies on stamen movements mentioned that motion is an adaptation that facilitates pollen removal (Abdusalam et al. 2021). In B. americanum, apical connective appendages enhance the removal of viable pollen, given the match between the highest pollen viability and the fully extended connective appendages (that form the landing platform). Thus, the connective appendages in B. americanum can serve as a landing platform that improves pollen presentation, collection, and transfer favouring reproduction (Henning et al. 2018; Lawson and Rands 2018; Tan and Tan 2018).
Additional floral appendages have been related to fly pollination (Faegri and van der Pijl 1979). Some fly-pollinated species have flowers with filiform appendages with different roles in pollination. For instance, in Rafflesiaceae, filiform structures resembling a filter to stamens allow the flies to traverse to the anthers but avoid the entry of other non-pollinators (Bänziger 1995). In a recent study on Arisaema urashima (Araceae), authors demonstrate that the long filiform appendage on inflorescences plays an important role in pollination carried out mainly by fungus gnats; in the absence of the appendage, visitation frequency decreases affecting the fruit and seed set (Suetsugu et al. 2022). In this study, we explore the role of staminal connective appendages during pollination, but their importance related to fruit and seed formation remains to be explored. Suetsugu et al. (2021) hypothesize that the long appendage plays a role in the emission of VOCs as pollinator attractant thus, in their absence, the pollinators did not approach inflorescences (Suetsugu et al. 2022). We cannot discard that staminal appendages of B. americanum male flowers could participate in the release of volatile attractants. It is necessary to confirm if staminal appendages emit attractive VOCs.
The pollination of a nectarless population of Bdallophytum americanum was studied, emphasizing the functional role of connective appendages. Our results show that pollination by dipterans is maintained in B. americanum despite lacking nectar. We described a novel type of movement in the staminal connective appendages of the androecium that coincides with the changes in pollen viability. Potential pollinators were bees, beetles, flies, and hoverflies as they visited male and female flowers carrying pollen grains on their bodies. Hoverflies of the genus Copestylum stood out as the most important pollinators in the studied population of B. americanum. The connective staminal appendages served as a landing platform for hoverflies. This landing platform appears crucial for pollinator positioning and the collection of viable pollen, but it cannot be discarded as a visual attractant. The form-function relationship of specialized structures must be studied in detail to better understand the animal-plant interactions, particularly in the misunderstood endoparasites.
Acknowledgements
SRC acknowledges scholarship and financial support provided by the Consejo Nacional de Ciencia y Tecnología (CONACyT). We thank Rocío Hernández for their help in the fieldwork. We also thank Santiago R. Montes de Oca for his help with technical work for pollen counting. We thank the two anonymous reviewers who kindly helped us to improve the manuscript. This study was funded by the DGAPA-PAPIIT (IN-223118, IN-222021) for SVS.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/13/2023
A Correction to this paper has been published: 10.1007/s10265-023-01481-5
References
- Abdusalam A, Maimaitituerxun R, Hashan H, Abdukirim G. Pollination adaptations of group-by-group stamen movement in a meadow plant with temporal floral closure. Plant Divers. 2021;43:308–316. doi: 10.1016/j.pld.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP. A versatile stain for pollen fungi, yeast and bacteria. Stain Technol. 1980;55:13–18. doi: 10.3109/10520298009067890. [DOI] [PubMed] [Google Scholar]
- Alvarado-Cárdenas LO. Sistemática del género Bdallophytum (Cytinaceae) Acta Bot Mex. 2009;87:1–21. doi: 10.21829/abm87.2009.1079. [DOI] [Google Scholar]
- Bänziger H. Stench and fragrance: unique pollination lure of Thailand's largest flower, Rafflesia kerrii Meijer. Nat Hist Bull Siam Soc. 1991;39:19–52. [Google Scholar]
- Bänziger H. Ecological, morphological and taxonomic studies on Thailand’s fifth species of Rafflesiaceae: Rhizanthes zippelii (Blume) Spach. Nat Hist Bull Siam Soc. 1995;43:337–365. [Google Scholar]
- Bänziger H, Pape T. Flowers, faeces and cadavers: natural feeding and laying habits of flesh flies in Thailand (Diptera: Sarcophagidae, Sarcophaga spp.) J Nat Hist. 2004;38:1677–1694. doi: 10.1080/0022293031000156303. [DOI] [Google Scholar]
- Beaman RS, Decker PJ, Beaman J. Pollination of Rafflesia (Rafflesiaceae) Am J Bot. 1988;75:1148–1162. doi: 10.2307/2444098. [DOI] [Google Scholar]
- Chakraborty P, Adhikary P. Sapromyophily: mimicry of the dead to get reproductive success. In: Chaudhuri PK, editor. Environment a multidisciplinary approach. Knowledge Based. Kolkata: Jogamaya Devi College; 2018. pp. 72–78. [Google Scholar]
- Conn JS, Blum U. Differentiation between the sexes of Rumex hastatulus in net energy allocation, flowering and height. Bull Torrey Bot Club. 1981;108:446–455. doi: 10.2307/2484445. [DOI] [Google Scholar]
- de Carvalho CJB, Pont A, Couri M, Pamplona D. A catalogue of the Fanniidae (Diptera) of the Neotropical region. Zootaxa. 2003;219:1–32. doi: 10.11646/zootaxa.219.1.1. [DOI] [Google Scholar]
- De Vega C, Ortiz PL, Arista M, Talavera S. The endophytic system of Mediterranean Cytinus (Cytinaceae) developing on five host Cistaceae species. Ann Bot. 2007;100:1209–1217. doi: 10.1093/aob/mcm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vega C, Arista M, Ortiz PL, Herrera CM, Talavera S. The ant-pollination system of Cytinus hypocistis (Cytinaceae), a Mediterranean root holoparasite. Ann Bot. 2009;103:1065–1075. doi: 10.1093/aob/mcp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberg K, Rozkošný R, Knutson L. A review of of the Holarctic Sepedon fuscipennis and S. spinipes groups with description of a new species (Diptera: Sciomyzidae) Zootaxa. 2009;2288:51–60. doi: 10.11646/zootaxa.2288.1.3. [DOI] [Google Scholar]
- Engel MS. Classification of the bee tribe Augochlorini (Hymenoptera: Halictidae) Bull Am Mus Nat Hist. 2000;250:1–89. doi: 10.1206/0003. [DOI] [Google Scholar]
- Evans TM, Cavers S, Ennos R, Vanbergen AJ, Heard MS. Florally rich habitats reduce insect pollination and the reproductive success of isolated plants. Ecol Evol. 2017;7:6507–6518. doi: 10.1002/ece3.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst. 2004;35:375–403. doi: 10.1146/annurev.ecolsys.34.011802.132347. [DOI] [Google Scholar]
- Galen C, Plowright RC. Testing the accuracy of using peroxidase activity to indicate stigma receptivity. Can J Bot. 1987;65:107–111. doi: 10.1139/b87-015. [DOI] [Google Scholar]
- Ganie AH, Tali BA, Reshi ZA, Nawchoo IA. Inflorescence architecture, floral part movements and pollinator attraction by androecia–contrivance for successful mating in Eremurus himalaicus Baker. J Plant Sci Conserv. 2021;10:29–34. doi: 10.17581/bp.2021.10206. [DOI] [Google Scholar]
- García-Franco JG, Rico-Gray V. Reproductive biology of the holoparasitic endophyte Bdallophyton bambusarum (Rafflesiaceae) Bot J Linn Soc. 1997;123:237–247. doi: 10.1111/j.1095-8339.1997.tb01416.x. [DOI] [Google Scholar]
- Gottsberger G. Floral ecology. In: Behnke HD, Esser K, Kubitzki K, Runge M, Ziegler H, editors. Progress in Botany/Fortschritte der Botanik. Springer; 1989. pp. 352–379. [Google Scholar]
- Goulet H, Huber JT. Centre for land and biological resources research. Ottawa: Agriculture Canada; 1993. Hymenoptera of the world: an identification guide to families. [Google Scholar]
- Heide-Jørgensen H. Parasitic flowering plants. Leiden: AAGE V. Jensens Fonde; 2008. [Google Scholar]
- Henning T, Mittelbach M, Ismail SA, Acuña-Castillo RH, Weigend M. A case of behavioural diversification in male floral function–the evolution of thigmonastic pollen presentation. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-32384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández MS, Jones RW, Castillo PR. A key to the Mexican and central America genera of Anthonomini (Curculionidae, Curculioninae) ZooKeys. 2013;260:31–47. doi: 10.3897/zookeys.260.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbhahn N, Johnson SD. Sunbird pollination of the dioecious root parasite Cytinus sanguineus (Cytinaceae) S Afr J Bot. 2015;99:138–143. doi: 10.1016/j.sajb.2015.04.003. [DOI] [Google Scholar]
- Instituto Nacional de Estadística y Geografía (INEGI) Anuario estadístico y geográfico de Aguascalientes 2017. Aguascalientes: INEGI; 2017. [Google Scholar]
- Johnson SD. Carrion flowers. Curr Biol. 2016;26:R556–R558. doi: 10.1016/j.cub.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Burgoyne PM, Harder LD, Dötterl S. Mammal pollinators lured by the scent of a parasitic plant. Proc R Soc B Biol Sci. 2011;278:2303–2310. doi: 10.1016/j.sajb.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens A, Shuttleworth A. Carrion and dung mimicry in plants. In: Benbow ME, Tomberlin JK, Tarone AM, editors. Carrion ecology, evolution, and their applications. 1. Boca Raton: CRC Press; 2015. pp. 361–386. [Google Scholar]
- Katsuhara KR, Kitamura S, Ushimaru A. Functional significance of petals as landing sites in fungus-gnat pollinated flowers of Mitella pauciflora (Saxifragaceae) Funct Ecol. 2017;31:1193–1200. doi: 10.1111/1365-2435.12842. [DOI] [Google Scholar]
- Knutson L, Orth RE. Sepedon mcphersoni, N. Sp., Key to North American Sepedon, groups in Sepedon S.S., and intra and intergeneric comparison (Diptera:Sciomyzidae) Proc Entomol Soc Wash. 2001;103:620–635. [Google Scholar]
- Lawson DA, Rands SA. The evolution of floral guides: using a genetic algorithm to investigate the evolution of floral cue arrangements. Biol J Linn Soc. 2018;123:739–753. doi: 10.1093/biolinnean/bly011. [DOI] [Google Scholar]
- Lindsey AH. Reproductive biology of Apiaceae. I. Floral visitors to Thaspium and Zizia and their importance in pollination. Am J Bot. 1984;71:375–387. doi: 10.1002/j.1537-2197.1984.tb12524.x. [DOI] [Google Scholar]
- Martins E, Neves JA, Moretti TC, Godoy WAC, Thyssen PJ. Breeding of Ornidia obesa (Diptera: Syrphidae: Eristalinae) on pig carcasses in Brazil. J Med Entomol. 2010;47:690–694. doi: 10.1603/ME09254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K, Kawakita A. Pollination by fungus gnats and associated floral characteristics in five families of the Japanese flora. Ann Bot. 2018;121:651–663. doi: 10.1093/aob/mcx196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir M, Johnson SD, Anderson B. Remarkable floral colour variation in the functionally specialized fly-pollinated iris, Moraea lurida. Bot J Linn Soc. 2022;200:218–232. doi: 10.1093/botlinnean/boac009. [DOI] [Google Scholar]
- Moretti TDC, Ribeiro OB, Thyssen PJ, Solis DR. Insects on decomposing carcasses of small rodents in a secondary forest in Southeastern Brazil. Eur J Entomol. 2008;105:691–696. doi: 10.14411/eje.2008.094. [DOI] [Google Scholar]
- Nickrent DL. Parasitic angiosperms: how often and how many? Taxon. 2020;69:5–27. doi: 10.1002/tax.12195. [DOI] [Google Scholar]
- Nikolov LA, Staedler YM, Manickam S, et al. Floral structure and development in Rafflesiaceae with emphasis on their exceptional gynoecia. Am J Bot. 2014;101:225–243. doi: 10.3732/ajb.1400009. [DOI] [PubMed] [Google Scholar]
- Ortega-González PF, Rios-Carrasco S, Mandujano MC, Sánchez D, Vázquez-Santana S. Reproductive aspects and pollination biology in endoparasitic Pilostyles thurberi (Apodanthaceae) Plant Species Biol. 2023;38:40–53. doi: 10.1111/1442-1984.12395. [DOI] [Google Scholar]
- Pape T, Banziger H. Two new species of Sarcophaga (Diptera: Sarcophagidae) among pollinators of newly discovered Sapria ram (Rafflesiaceae) Raffles Bull Zool. 2000;48:201–208. doi: 10.1603/033.046.0503. [DOI] [Google Scholar]
- Purrington CB, Schmitt J. Consequences of sexually dimorphic timing of emergence and flowering in Silene latifolia. J Ecol. 1998;86:397–404. doi: 10.1046/j.1365-2745.1998.00262.x. [DOI] [Google Scholar]
- R Development Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org
- Rios-Carrasco S, Vázquez-Santana S. Comparative morphology and ontogenetic patterns of Bdallophytum species (Cytinaceae, Malvales): insight into the biology of an endoparasitic genus. Botany. 2021;99:221–238. doi: 10.1139/cjb-2020-0025. [DOI] [Google Scholar]
- Rios-Carrasco S, de Jesús-Celestino L, Ortega-González PF, Mandujano MC, Hernández-Najarro F, Vázquez-Santana S. The pollination of the gynomonoecious Bdallophytum oxylepis (Cytinaceae, Malvales) Plant Species Biol. 2022;37:66–77. doi: 10.1111/1442-1984.12354. [DOI] [Google Scholar]
- Rios-Carrasco S, González-Martínez CA, Vázquez-Santana S. Floral visitors of the holoparasite Bdallophytum andrieuxii Eichler: a new report of brood-site pollination and thermogenesis for Cytinaceae (Malvales) Braz J Bot. 2022;45:1047–1055. doi: 10.1007/s40415-022-00816-1. [DOI] [Google Scholar]
- Sanjust E, Rinaldi AC. Cytinus under the microscope: disclosing the secrets of a parasitic plant. Plants. 2021;10:146. doi: 10.3390/plants10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth A, Johnson SD, Jürgens A. Entering through the narrow gate: a morphological filter explains specialized pollination of a carrion-scented stapeliad. Flora. 2017;232:92–103. doi: 10.1016/j.flora.2016.09.003. [DOI] [Google Scholar]
- Sibaoka T. Physiology of rapid movements in higher plants. Annu Rev Plant Physiol. 1969;20:165–184. doi: 10.1146/annurev.pp.20.060169.001121. [DOI] [Google Scholar]
- Sipes SD, Huff Hartz KE, Amin H, Anterola A, Nickrent DL. Floral scent and pollinators of the holoparasite Pilostyles thurberi (Apodanthaceae) J Pollinat Ecol. 2014;12:31–39. doi: 10.26786/1920-7603(2014)4. [DOI] [Google Scholar]
- Siqueiros-Delgado ME, Rodríguez-Avalos JA, Martínez-Ramírez J, Sierra-Muñoz JC. Situación actual de la vegetación del estado de Aguascalientes, México. Bot Sci. 2016;94:455–470. doi: 10.17129/botsci.466. [DOI] [Google Scholar]
- Suetsugu K. Social wasps, crickets and cockroaches contribute to pollination of the holoparasitic plant Mitrastemon yamamotoi (Mitrastemonaceae) in southern Japan. Plant Biol. 2019;21:176–182. doi: 10.1111/plb.12889. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Sato R, Kakishima S, Okuyama Y, Sueyoshi M. The sterile appendix of two sympatric Arisaema species lures each specific pollinator into deadly trap flowers. Ecology. 2021;102:e03242. doi: 10.1002/ecy.3242. [DOI] [PubMed] [Google Scholar]
- Suetsugu K, Nishigaki H, Fukushima S, Ishitani E, Kakishima S, Sueyoshi M. Thread-like appendix on Arisaema urashima (Araceae) attracts fungus gnat pollinators. Ecology. 2022;103:e3782. doi: 10.1002/ecy.3782. [DOI] [PubMed] [Google Scholar]
- Tan KH, Tan LT. Movements of floral parts and roles of the tooth on the column wall of Bulbophyllum praetervisum (Orchidaceae) flower in pollination by Dacini fruit flies (Diptera: Tephritidae) J Pollinat Ecol. 2018;24:157–163. doi: 10.26786/1920-7603(2018)19. [DOI] [Google Scholar]
- Teixeira-Costa L, Davis CC, Ceccantini G. Striking developmental convergence in angiosperm endoparasites. Am J Bot. 2021;108:756–768. doi: 10.1002/ajb2.1658. [DOI] [PubMed] [Google Scholar]
- Thompson C. A key to the genera of the flower flies (Diptera: Syrphidae) of the Neotropical Region including descriptions of new genera and species and a glossary of taxonomic terms. Contrib Ent Int. 1999;3:321–378. [Google Scholar]
- Thorogood CJ, Teixeira-Costa L, Ceccantini G, Davis C, Hiscock SJ. Endoparasitic plants and fungi show evolutionary convergence across phylogenetic divisions. New Phytol. 2021;232:1159–1167. doi: 10.1111/nph.17556. [DOI] [PubMed] [Google Scholar]
- Triplehorn C, Johnson N. Borror and DeLong’s introduction to the study of insects. 7. Thomson Learnings; 2005. [Google Scholar]
- Twyford AD. Parasitic plants. Curr Biol. 2018;28:R857–R859. doi: 10.1016/j.cub.2018.06.030. [DOI] [PubMed] [Google Scholar]
- van Doorn WG, van Meeteren U. Flower opening and closure: a review. J Exp Bot. 2003;54:1801–1812. doi: 10.1093/jxb/erg213. [DOI] [PubMed] [Google Scholar]
- Weiss MR. Vision and learning in some neglected pollinators: beetles, flies, moths, and butterflies. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination: animal behaviour and floral evolution. Cambridge: Cambridge University Press; 2001. pp. 171–190. [Google Scholar]
- Willmer P. Pollination and floral ecology. New Jersey: Princeton University Press; 2011. [Google Scholar]
- Zain NM, Jamil M, Markandan S, Ali NAM, Hamzah Z. Assessing the floral volatile constituents of male and female Rafflesia kerri Meijer from Lojing Highlands, Peninsular Malaysia. IOP Conf Ser Earth Environ Sci. 2020;549:012068. doi: 10.1088/1755-1315/549/1/012068. [DOI] [Google Scholar]
- Zhang Q, Fu W, Wang X, Huang L. Ingenious floral structure drives explosive pollination in Hydrilla verticillata (Hydrocharitaceae) Plant Biol. 2019;22:480–486. doi: 10.1111/plb.13085. [DOI] [PubMed] [Google Scholar]