Abstract
Analyses from administrative databases have suggested an increased cancer incidence among individuals who experienced a myocardial infarction, especially within the first 6 months. It remains unclear to what extent this represents an underlying biological link, or can be explained by detection of pre-symptomatic cancers and shared risk factors. Cancer incidence among 1809 consecutive patients surviving hospitalization for thrombotic ST-segment-elevation myocardial infarction (STEMI; mean age 62.6 years; 26% women; 115 incident cancers) was compared to the cancer incidence among 10,052 individuals of the general population (Rotterdam Study; mean age 63.1 years; 57% women; 677 incident cancers). Pathology-confirmed cancer diagnoses were obtained through identical linkage of both cohorts with the Netherlands Cancer Registry. Cox models were used to obtain hazards ratios (HRs) adjusted for factors associated with both atherosclerosis and cancer. Over 5-year follow-up, there was no significant difference in the incidence of cancer between STEMI patients and the general population (HR 0.96, 95% CI 0.78–1.19). In the first 3 months after STEMI, cancer incidence was markedly higher among STEMI patients compared to the general population (HR 2.45, 95% CI 1.13–5.30), which gradually dissolved during follow-up (P-for-trend 0.004). Among STEMI patients, higher C-reactive protein, higher platelet counts, and lower hemoglobin were associated with cancer incidence during the first year after STEMI (HRs 2.93 for C-reactive protein > 10 mg/dL, 2.10 for platelet count > 300*109, and 3.92 for hemoglobin < 7.5 mmol/L). Although rare, thrombotic STEMI might be a paraneoplastic manifestation of yet to be diagnosed cancer, and is hallmarked by a pro-inflammatory status and anemia.
Trial registration Registered into the Netherlands National Trial Register and WHO International Clinical Trials Registry Platform under shared catalogue number NTR6831.
Keywords: ST-segment-elevation myocardial infarction (STEMI), Cancer, Paraneoplastic, Prognosis, Population-based, Epidemiology
Introduction
Individuals diagnosed with cancer face increased short-term risks of cardiovascular events [1] which is often attributed to invasive procedures and chemotherapy. Conversely, analyses from large administrative databases have suggested an increased cancer incidence among individuals who experienced a myocardial infarction, especially during the first 6 months after hospital admission [2–4]. From these studies it remains unclear, however, to what extent this finding represents an underlying biological link, or can be explained by increased detection of pre-symptomatic cancers in a population receiving comprehensive clinical work-up (i.e. asymmetrical follow-up) and/or shared risk factors for both atherosclerosis and cancer (i.e. confounding) [2–6].
We aimed to study cancer incidence and its temporality among patients with thrombotic ST-segment-elevation myocardial infarction (STEMI). As a secondary objective, we sought to identify indicators of short-term cancer incidence among STEMI patients.
Methods
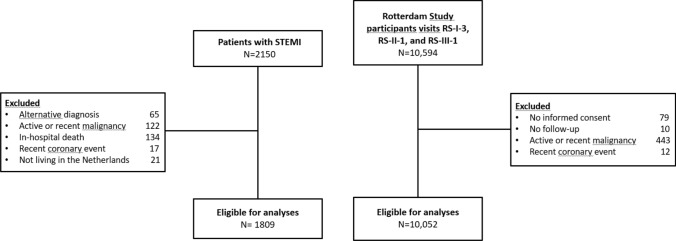
We reviewed all consecutive STEMI admissions between 2010 and 2017 at a single regional primary percutaneous coronary intervention center. Patients with rare non-thrombotic causes of STEMI and patients who died during the index hospitalization were excluded, resulting in 1809 patients for analysis. Cancer incidence in these patients was compared with that of an unselected prospective population-based cohort in a neighboring city (the Rotterdam Study; visits RS-I-3, RS-II-1, and RS-III-1; n = 10,052). The objectives and design of the Rotterdam Study have been described in detail previously [7]. For both the STEMI cohort and the Rotterdam Study cohort, individuals with active malignancy, malignancy diagnosed ≤ 5 years before baseline, or coronary intervention ≤ 90 days before baseline were excluded (Fig. 1) [8, 9].
Fig. 1.
Flowchart of the study population included
Data on pathology-confirmed cancer diagnoses (not including non-melanoma skin cancers) were obtained through identical linkage of both cohorts with the Netherlands Cancer Registry of the of the Netherlands Comprehensive Cancer Organization [9]. Follow-up started at the date of STEMI hospitalization or Rotterdam Study research center visit, and was truncated at date of pathology confirmed-cancer diagnosis, death (14.6 per 1000 person-years among the STEMI patients and 12.1 per 1000 person-years among the Rotterdam Study population), loss to follow-up (0.9% of the STEMI patients and 0.3% of the Rotterdam Study population), linkage with the cancer registry, or 5 years of follow-up (to account for longer follow-up in the Rotterdam Study), whichever came first.
Following a pre-specified analysis plan, Cox models were used to obtain hazards ratios (HRs) for incident cancer among STEMI patients compared to the Rotterdam Study population. All analyses were adjusted for factors associated with both atherosclerotic cardiovascular disease and cancer: age, sex, smoking, diabetes, body mass index, and C-reactive protein [5, 6]. Based on prior data, [2–4] we stratified the 5-year follow-up period into pre-specified time strata of 0–3, 3–6, 6–12, 12–24, and 24–60 months.
Continuous candidate risk indicators for cancer diagnosis in the first year after STEMI hospitalization were standardized for comparison of corresponding age- and sex-adjusted HRs. However, if proven significant after Bonferroni correction, adjusted HRs for clinically meaningful thresholds were additionally presented in order to enhance clinical interpretation.
IBM SPSS Statistics version 25 was used for all statistical analyses.
Results
Baseline characteristics of the STEMI patients and Rotterdam Study general population sample are displayed in Table 1. A total of 26.0% of the STEMI patients were women, and the median age was 62.6 years. Among the Rotterdam Study participants 57.3% were women and median age was 63.1 years. Of the included STEMI patients, 1.7% presented with a resuscitated cardiac arrest. Nearly all STEMI patients (99.4%) underwent coronary angiography, of whom 95.2% underwent primary percutaneous coronary intervention and 2.5% underwent urgent coronary artery bypass grafting. Significant obstructive coronary disease in non-culprit coronary arteries was present in 38.5% of these patients.
Table 1.
Baseline characteristics
| STEMI patients | Rotterdam study | |
|---|---|---|
| n = 1809 | n = 10,052 | |
| Age, years * | 62.6 (54.0–71.8) | 63.1 (57.9–72.0) |
| Women | 471 (26.0) | 5762 (57.3) |
| Smoking status | ||
| Current | 357 (19.9) | 2253 (22.7) |
| Former | 817 (45.6) | 4596 (46.3) |
| Never | 618 (34.5) | 3072 (31.0) |
| Body mass index, kg/m2 * | 26.3 (24.4–29.1) | 26.7 (24.3–29.5) |
| Total cholesterol, mmol/L | 5.1 (1.2) | 5.7 (1.0) |
| High-density lipoprotein cholesterol, mmol/L | 1.2 (0.3) | 1.4 (0.4) |
| Hypertension | 901 (49.8) | 3876 (39.5) |
| Diabetes mellitus | 279 (15.4) | 755 (7.5) |
| Aspirin use | 287 (15.9) | 1359 (13.5) |
| Anticogulant use | 60 (3.3) | 314 (3.1) |
| History of coronary heart disease: | 250 (13.8) | 690 (6.9) |
| Myocardial infarction | 208 (11.5) | 502 (5.0) |
| Percutaneous coronary intervention | 174 (9.6) | 190 (1.9) |
| Coronary artery bypass grafting | 42 (2.3) | 239 (2.4) |
| History of cerebroascular disease | 117 (6.5) | 675 (6.7) |
| History of venous thrombo-embolism | 34 (1.9) | 324 (3.2) |
| Chronic obstructive pulmonary disease | 163 (9.0) | 646 (6.4) |
| Glomerular filtration rate, mL/kg/1.73 m2 * | 86 (72–97) | 81 (71–91) |
| Renal dialysis | 3 (0.2) | 1 (0.0) |
| Sodium, mmol/L | 138 (3) | 142 (2) |
| History of cancer ** | 100 (5.5) | 363 (3.6) |
| Hemoglobin, mmol/L | 8.9 (1.0) | 8.8 (0.8) |
| Platelet count, 109/L | 251 (70) | 266 (62) |
| Leukocyte count, 109/L * | 10.6 (8.4–13.2) | 6.6 (5.6–7.8) |
| C-reactive protein, mg/L * | 2.0 (2.0–7.0) | 1.6 (0.6–3.5) |
Unimputed data. Values are counts (percentages) or means (standard deviations)
*Median (25th–75th percentile) because of skewed distribution
**Not including non-melanoma skin cancer
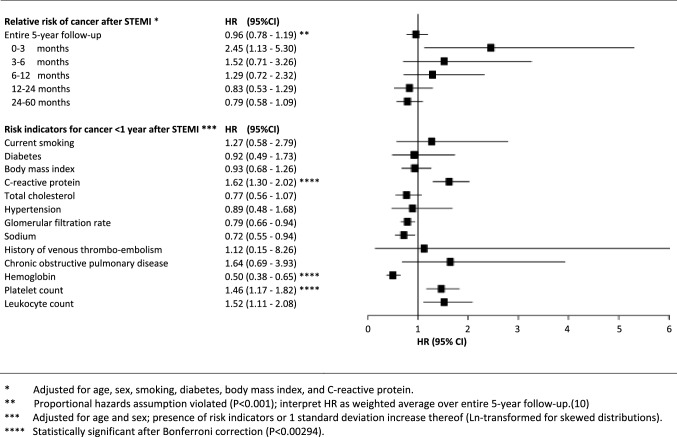
Over the entire 5-year period, 115 STEMI patients and 677 general population individuals were diagnosed with cancer (crude incidence rates 16.5 and 14.3 per 1000 person-years, respectively; Table 2). Over the full 5-year follow-up, there was no significant difference in the incidence of cancer between STEMI patients and the general population (Fig. 2; HR 0.96, 95% CI 0.78–1.19). However, the proportional hazards assumption was violated [P < 0.001 for Ln(follow-up time) interaction term], indicating that hazards were not stable over the 5-year follow-up period [10]. In the first 3 months after STEMI, cancer incidence was markedly higher among STEMI patients compared to the general population (HR 2.45, 95% CI 1.13–5.30). Differences in cancer incidence between STEMI patients and the general population gradually dissolved during follow-up (P-for-trend 0.004).
Table 2.
Incident cancer diagnoses
| STEMI patients | Rotterdam study | |
|---|---|---|
| n = 1809 | n = 10,052 | |
| All incident cancers | 115 | 677 |
| Cancer subtypes | ||
| Upper gastrointestinal | 10 (8.7) | 30 (4.4) |
| Hepatobiliary and panreatic | 2 (1.7) | 27 (4.0) |
| Colorectal | 15 (13.0) | 118 (17.4) |
| Breast | 7 (6.1) | 107 (15.8) |
| Prostate | 18 (15.7) | 111 (16.4) |
| Genital | 1 (0.9) | 24 (3.5) |
| Urologic | 11 (9.6) | 63 (9.3) |
| Otolaryngol | 3 (2.6) | 20 (3.0) |
| Lung | 23 (20.0) | 81 (12.0) |
| Melanoma | 9 (7.8) | 26 (3.8) |
| Hematologic | 10 (8.7) | 37 (5.5) |
| Other/unknown primary origin | 6 (5.2) | 33 (4.9) |
| Metastases at cancer diagnosis | 21 (18.3) | 97 (14.3) |
| Incident cancers by follow-up time strata | ||
| 0–3 Months | 15 (13.0) | 22 (3.2) |
| 3–6 Months | 11 (9.6) | 32 (4.7) |
| 6–12 Months | 16 (13.9) | 66 (9.7) |
| 12–24 Months | 26 (22.6) | 158 (23.3) |
| 24–60 Months | 47 (40.9) | 399 (58.9) |
Values are counts (percentages among total number of cancer cases)
Fig. 2.
Relative risk of cancer after STEMI and risk indicators for cancer among STEMI patients
Sensitivity analyses, including competing risk regression to account for non-cancer mortality, age- and sex-matched analysis, and complete-case analysis yielded similar estimates and patterns (data available upon request). No significant associations were observed with common cancer locations or the presence of disseminated disease at time of cancer diagnosis (Table 2).
Among STEMI patients, higher C-reactive protein, higher platelet counts, and lower hemoglobin were significantly associated with cancer incidence during the first year after STEMI (Fig. 2; age- and sex-adjusted HRs 2.93 for C-reactive protein > 10 mg/dL, 2.10 for platelet count > 300*109, and 3.92 for hemoglobin < 7.5 mmol/L). History of coronary heart disease and presence of non-culprit coronary stenosis were not significantly associated with cancer incidence in any of the follow-up time strata.
Discussion
During the initial period after hospitalization, STEMI patients appeared at increased risk of being diagnosed with (likely pre-existing) cancer. This might be explained by some STEMIs being of 'paraneoplastic' thrombotic origin, for instance due to a pro-thrombotic state induced by an asymptomatic undiagnosed cancer, as we postulated previously [11].
Our results are generally in line with findings from several administrative databases [2–4]. These previous studies were hampered by asymmetrical data collection among individuals with and without myocardial infarction, detection bias through clinical work-up of patients with myocardial infarction, or lack of available data on confounding shared risk factors.
In order to study the existence of paraneoplastic STEMI, we chose our reference population carefully to address several issues. First, we ensured identical follow-up using linkage to a nationwide cancer registry, hence ruling out asymmetrical data collection. Next, we aimed to reduce the likelihood of spurious associations by detecting asymptomatic cancers through routine clinical work-up in STEMI patients, since Rotterdam Study participants underwent comparable blood work and examinations. The only marked difference was the prevalence of thoracic imaging: > 90% of STEMI patients underwent chest radiography, while only 35.3% of the Rotterdam Study participants underwent non-enhanced cardiac CT as part of the study protocol. However, lung cancers accounted for only 13.5% of all diagnoses during the initial 3 months of follow-up. Next, in order to address shared etiology, we adjusted for cardiovascular risk factors that have been implicated in cancer development [5]. Nonetheless, residual confounding cannot be ruled out.
The lack of ethnic diversity in our study populations (95.8% of the Rotterdam Study is white) warrants replication in other populations. Also, absolute cancer risks are likely somewhat underestimated, since we only had data available on pathology-confirmed cases [9]. However, this was the case for both populations and hence is unlikely to have affected the presented relative risk estimates.
Conclusion
In conclusion, although rare, thrombotic STEMI might be a paraneoplastic manifestation of yet to be diagnosed cancer, and is hallmarked by a pro-inflammatory status and anemia.
Acknowledgements
The authors thank the registration team of the Netherlands Comprehensive Cancer Organization (IKNL, https://iknl.nl/en/) for the collection of data for the Netherlands Cancer Registry. The dedication, commitment, and contribution of inhabitants, general practitioners, and pharmacists to the Rotterdam Study are gratefully acknowledged.
Author contributions
Dr Leening was responsible for the study conception and design, and obtaining funding. Material preparation and data collection were performed by Drs Leening, Bouwer, and Deckers. STEMI case verification was supervised by Drs van den Bos and Weevers. Statistical analysis and drafting the manuscript was performed by Dr Leening. All authors revised the draft critically for important intellectual content. All authors read and approved the manuscript.
Funding
The Rotterdam Study is supported by the Erasmus MC and Erasmus University Rotterdam; the Netherlands Organisation for Scientific Research (NWO); the Netherlands Organisation for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Genomics Initiative (NGI); the Ministry of Education, Culture, and Science; the Ministry of Health, Welfare, and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. Dr Leening is supported by grants from the Albert Schweitzer Hospital Research Fund (stipend 2018-05); Foundation for Oncologic Research Albert Schweitzer (ORAS); and Foundation for Promoting Advanced Cardiology through Education (PACE). Dr Kavousi is supported by a grant from the Netherlands Organisation for Health Research and Development (ZonMw, VENI 91616079). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declarations
Conflict of interest
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Leening reports receiving speaker fees from Sanofi, and Novartis; and served on an advisory board for Boehringer Ingelheim; all unrelated to the submitted work. No other disclosures were reported.
Ethics approval
This study was approved by the Institutional Review Board of the Albert Schweitzer Hospital, Dordrecht (WOAC 2018.93), and the requirement for informed consent for the retrospective clinical cohort of STEMI patients was waived. The Rotterdam Study was approved by the Institutional Review Board of the Erasmus MC (MEC 02.1015) and by the Ministry of Health, Welfare, and Sport of the Netherlands, implementing the Population Screening Act: Rotterdam Study (license number 1071272-159521-PG). The Rotterdam Study has been entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the WHO International Clinical Trials Registry Platform (www.who.int/ictrp/network/primary/en/) under shared catalogue number NTR6831. All Rotterdam Study participants provided written informed consent to participate and to obtain information from their treating physicians.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maarten J. G. Leening and Nathalie I. Bouwer contributed equally.
References
- 1.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70(8):926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rinde LB, Smabrekke B, Hald EM, Brodin EE, Njolstad I, Mathiesen EB, et al. Myocardial infarction and future risk of cancer in the general population: the Tromso Study. Eur J Epidemiol. 2017;32(3):193–201. doi: 10.1007/s10654-017-0231-5. [DOI] [PubMed] [Google Scholar]
- 3.Malmborg M, Christiansen CB, Schmiegelow MD, Torp-Pedersen C, Gislason G, Schou M. Incidence of new onset cancer in patients with a myocardial infarction: a nationwide cohort study. BMC Cardiovasc Disord. 2018;18(1):198. doi: 10.1186/s12872-018-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Tagawa ST, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2019;133(8):781–789. doi: 10.1182/blood-2018-06-860874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van 't Klooster CC, Ridker PM, Cook NR, Aerts JGJV, Westerink J, Asselbergs FW, et al. Prediction of lifetime and 10-year risk of cancer in individual patients with established cardiovascular disease. JACC: CardioOncol. 2020;2(3):400–410. doi: 10.1016/j.jaccao.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikram MA, Brusselle G, Ghanbari M, Goedegebure A, Ikram MK, Kavousi M, et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol. 2020;35(5):483–517. doi: 10.1007/s10654-020-00640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leening MJG, Kavousi M, Heeringa J, van Rooij FJA, Verkroost-van Heemst J, Deckers JW, et al. Methods of data collection and definitions of cardiac outcomes in the Rotterdam Study. Eur J Epidemiol. 2012;27(3):173–185. doi: 10.1007/s10654-012-9668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Willik KD, Rojas-Saunero LP, Labrecque JA, Ikram MA, Schagen SB, Stricker BHC, et al. Pathology-confirmed versus non pathology-confirmed cancer diagnoses: incidence, participant characteristics, and survival. Eur J Epidemiol. 2020;35(6):557–565. doi: 10.1007/s10654-019-00592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leening MJG, Weevers APJD, van Geuns RM, Deckers JW, Levin MD. Recurrent late bioresorbable scaffold thrombosis as a presenting symptom of underlying cancer. J Am Coll Cardiol. 2018;71(2):259–260. doi: 10.1016/j.jacc.2017.10.086. [DOI] [PubMed] [Google Scholar]