Abstract
Myxoid liposarcoma is a mesenchymal malignancy that most commonly presents in young adults, with peak incidence between the ages of 30–50 years. The clinical behavior of myxoid liposarcoma has been well characterized in adults. However, little is known about the clinical features and treatment outcomes of myxoid liposarcoma in child, owing to its rarity. This case report describes an 11-year-old previously healthy female who presented with a painless mass in her right thigh. Ultrasonography, computed tomography, and magnetic resonance imaging demonstrated a soft tissue mass with clear margins in the subfascial plane superficial to the gracilis and sartorius muscles. She was diagnosed with myxoid liposarcoma based on histological and molecular cytogenetic examinations of the core-needle biopsy specimen. The patient subsequently underwent wide resection without any adjuvant treatment. The patient has not experienced any symptoms of local recurrence and metastases as of 2.5 years after surgery.
Keywords: Myxoid liposarcoma, Childhood tumors, Sarcoma
Introduction
Liposarcoma is the second most common type of soft tissue sarcoma. In the latest World Health Organization (WHO) Blue Books, there are five subtypes of liposarcoma [1]. Myxoid liposarcoma (MLS) accounts for approximately 20–30% of liposarcomas [2]. The peak incidence of MLS occurs in the fourth and fifth decades. Its clinical behavior has been well studied. On the other hand, MLS in children and adolescents is extremely rare; the clinicopathological and imaging features, optimal treatment strategy, and optimal surveillance after treatment remain unclear [3]. In this article, we report a case of MLS in an 11-year-old girl in detail.
Case report
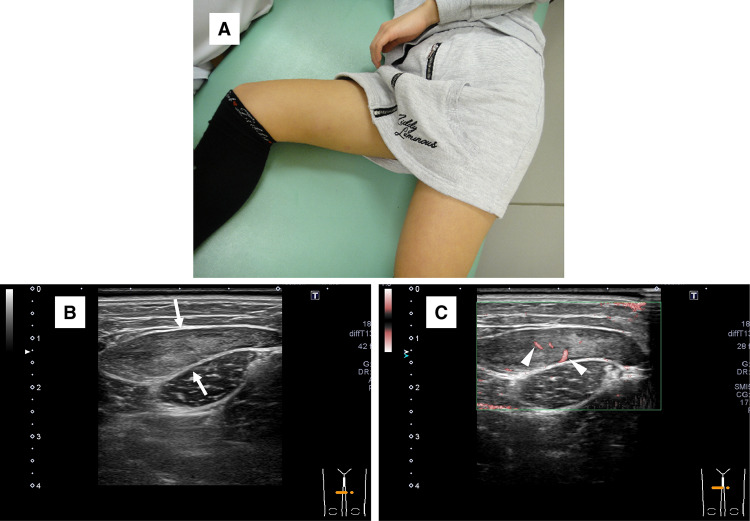
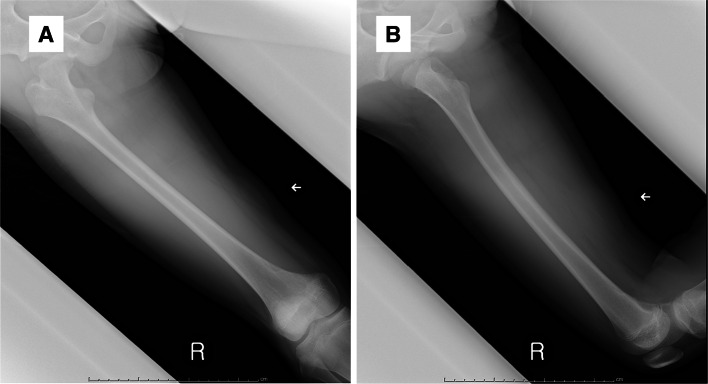
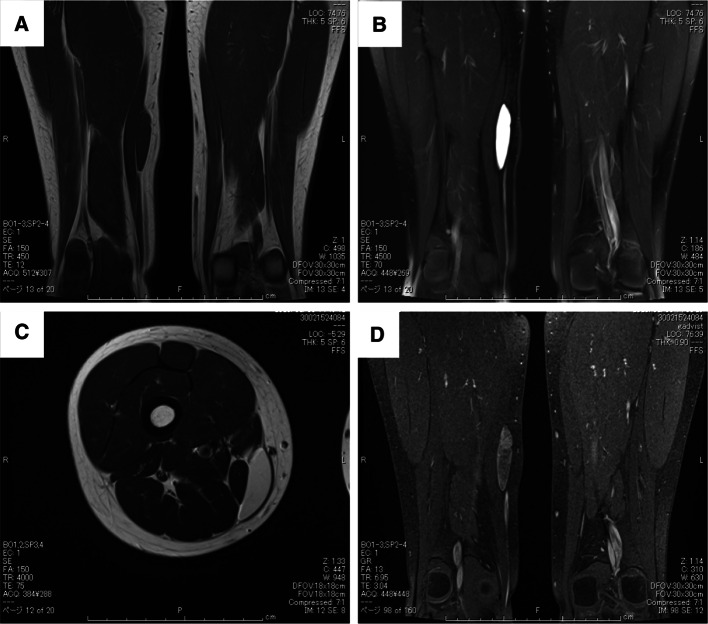
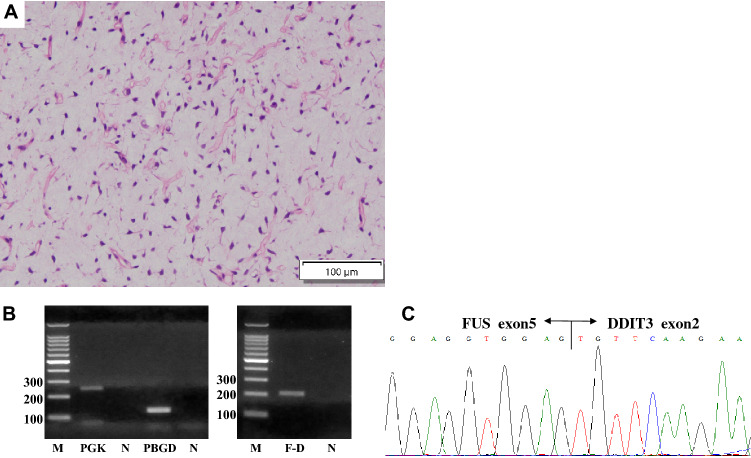
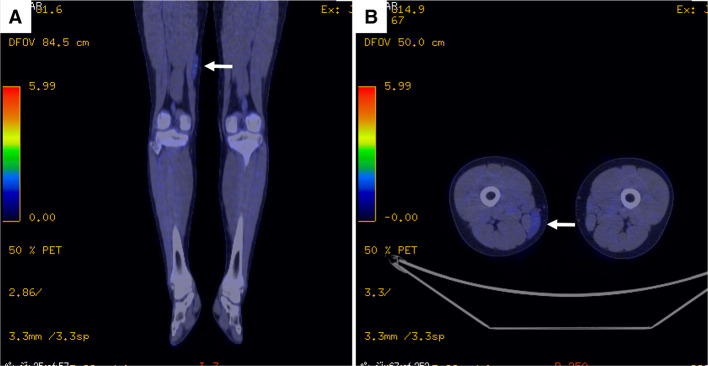
An 11-year-old girl with no significant medical history presented with a painless mass over her medial thigh (Fig. 1a). She was referred to our hospital for surgical treatment after several examinations and a core-needle biopsy performed at a nearby hospital. The mass had grown in size over the preceding 2 months. Her height was 146.6 cm and her weight was 39.7 kg. Her body mass index was 18.5 kg/m2. Physical examination revealed a soft, palpable 5 × 4 cm mass with moderate mobility under the right distal thigh without restriction of the right knee’s range of motion. Ultrasonography visualized a homogeneous, slightly hyperechoic, and well-delineated mass with regular contours. Low vascular flow was observed (Fig. 1b and c, respectively). Plain radiographs of the right thigh showed a soft tissue tumor with discoidal morphology in the distal third of the right thigh without calcifications (Fig. 2). Computed tomography (CT) showed a soft tissue mass with slightly lower density than adjacent muscles (Fig. 3) and no apparent lung metastases (data not shown). On magnetic resonance imaging (MRI), the lesion measured 47 × 36 × 10 mm and appeared as a tumor with clear margins adjacent to the gracilis and sartorius muscles. The lesion had low signal intensity on T1-weighted images (Fig. 4a) and very high signal intensity T2-weighted images with and without fat suppression (Fig. 4b and c). Diffuse homogeneous internal enhancement was observed on gadolinium-enhanced T1-weighted images with fat suppression (Fig. 4d). Core needle biopsy was performed. Histopathologically, the specimens of the biopsy showed a proliferation of atypical short-spindle or oval cells having hyperchromatic nuclei and eosinophilic cytoplasm admixed with mono- or multi-vacuolated lipoblasts arranged in a haphazard fashion embedded in an abundant myxoid stroma containing delicate arborizing vascular channels (Fig. 5a). Mitotic figures were few. Reverse transcription-polymerase chain reaction (RT-PCR) was performed using total RNA extracted from the formalin-fixed and paraffin-embedded tumor tissue specimens, and FUS-DDIT3 fusion gene transcript was detected (Fig. 5b). Histopathological features and molecular analysis were compatible with MLS. A 18F-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) scan was performed. Faint FDG uptake was detected in the standard field of imaging (SUVmax 1.76) (Fig. 6). The patient underwent wide resection with sacrifice of the sartorius and gracilis muscles. The postoperative clinical course was uneventful.
Fig. 1.
a Photograph of the right thigh lump at the first visit. Ultrasonography: b A homogeneous mass (arrows), slightly hyperechoic relative to the adjacent muscle, is seen in the right medial thigh, mainly in the subfascial plane superficial to the gracilis muscle. Margins are well-defined. c A low level of internal vascularity is observed (arrowheads)
Fig. 2.
a Anteroposterior and b lateral views on plain radiographs show a soft tissue tumor with discoidal morphology in the distal third of the right thigh (arrow)
Fig. 3.
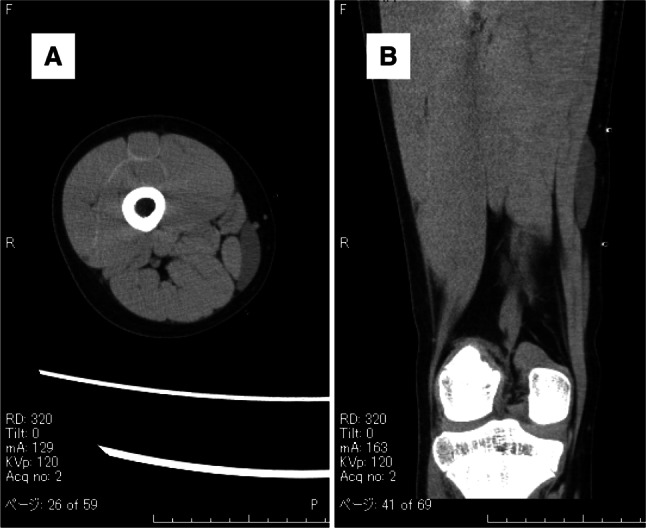
Computed tomography shows a soft tissue mass with slightly lower density than adjacent muscles (arrow)
Fig. 4.
Magnetic resonance imaging shows a tumor with clear margins adjacent to the gracilis and sartorius muscles. a T1-weighted coronal image. b T2-weighted coronal image with fat suppression. c T2-weighted axial image. d Gadolinium-enhanced T1-weighted coronal image with fat suppression
Fig. 5.
a Microscopic image of the biopsy specimen with hematoxylin–eosin staining shows a proliferation of atypical short-spindle or oval cells admixed with mono- or multi-vacuolated lipoblasts arranged in a haphazard fashion embedded in an abundant myxoid stroma containing delicate arborizing vascular channels. RT-PCR (b) and subsequent sequencing analysis (c) detected a transcript of the FUS-DDIT3 fusion gene. M size marker, F-D FUS-DDIT3, N negative control, PGK/PBGD housekeeping genes as internal positive controls
Fig. 6.
Representative a coronal and b axial images of 18F-fluorodeoxyglucose (FDG)-positron emission tomography/computed tomography show faint uptake of FDG in the lesion (arrow)
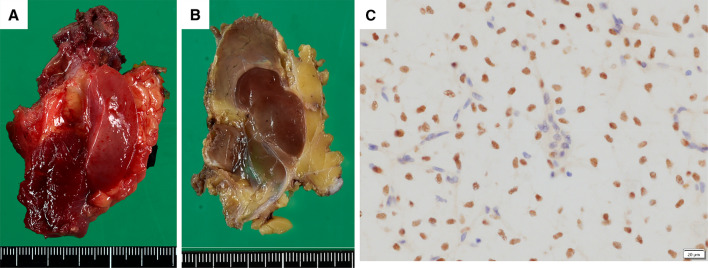
The resected tumor was well-circumscribed and lobulated gelatinous tumor and located in the subfascial plane superficial to the sartorius and gracilis muscles (Fig. 7a and b). Histological findings of the resected specimen were similar to those of the needle biopsy. Mature-appearing larger fat cells and mucous pools were also seen. Mitotic figures were few. Areas with round cells or necrosis were absent. Immunohistochemically, the tumor cells are positive for DDIT3 (Fig. 7c). The tumor was diagnosed as MLS. Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) was Grade 1 (score 2 + 1 + 0 = 3) and the surgical margins were negative.
Fig. 7.
Macroscopic appearance of the tumor with a gelatinous cut surface a before and b after formalin fixation. The tumor is well-circumscribed and lobulated tumor and located in the subfascial plane superficial to the sartorius and gracilis muscles. c Immunohistochemically, the tumor cells are positive for DDIT3, but vascular endothelial cells and inflammatory cells are negative
No adjuvant treatment such as radiation therapy or chemotherapy was administered. The patient did not experience any symptoms of local recurrence or metastasis as of 2.5 years after surgery.
Discussion
Pediatric soft tissue sarcomas are a heterogeneous group of malignant tumors that originate from primitive mesenchymal tissue. Rhabdomyosarcoma is the most common soft tissue sarcoma in children aged 0–14 years, accounting for 50% of tumors in this age group [4]. Liposarcomas are extremely rare in childhood, representing about 2% of all childhood soft tissue sarcomas. MLS is the most common liposarcoma subtype in children and adolescents [5]. In doubtful cases, detection of the typical chromosomal translocation is the best diagnostic tool. The detection of FUS-DDIT3 fusion leads to a definite diagnosis in the majority of patients with MLS [6].
The clinicopathological and imaging features, optimal treatment strategy, and prognosis of MLS have been well studied in adults. For example, age, presence of tumor necrosis, size (> 10 cm), and presence of a round cell component comprising more than 5% of the tumor have been reported as prognostic factors [2]. Surgical resection with adjuvant radiotherapy and possible chemotherapy is the primary management option for MLS in the extremities [7]. However, data on pediatric MLS are limited. A couple of case series have shown that pediatric liposarcoma has a different spectrum of presentation than in adults and MLS has excellent prognosis [3, 5, 8]. Baday et al. performed a literature review investigating the treatment and prognosis of pediatric MLS. They revealed that none of the patients had distant metastasis at presentation, 72.8% of patients underwent resection only, and 6.5% of patients died of disease, which generally occurred in the abdominal and pelvic regions and primary lesions were greater than 5 cm in size [3].
To further investigate the clinicopathological features, treatment strategies, and oncological prognosis of pediatric MLS, we conducted a literature search of articles about MLS in patients aged 12 years or younger published after 1990 [8–18] (Table 1). We identified 2 case series and 9 case reports in which 30 cases of MLS originated in an extremity (n = 22, 73.3%), trunk (n = 3, 10%), omentum (n = 1, 3.3%), or head and neck (n = 4, 13.3%). All patients had low-grade MLS, except for 6 patients for whom histological grade was not described. All but 1 underwent surgery as the primary treatment.
Table 1.
Summary of previous cases of patients with myxoid liposarcoma aged 12 years or younger published after 1990
| Reference | Year | Age (y) | Sex | Site | Size (cm) | Grade | Surgery | Radiotherapy | CT | Local relapse | Status | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quaglia [15] | 1993 | 0.5 | M | Buttock | 3 | Low grade | Wide local ex | – | – | – | ANED | 29.1y |
| 12 | M | Axilla | 9 | Low grade | Wide local ex | + | for metastases | – | DOD | 2y | ||
| Miller [16] | 1998 | 9 | M | Thigh | 10 | NA | Complete ex | NA | NA | NA | NA | NA |
| Ferrari [13] | 1999 | 9 | M | Groin | 6 | Low grade | Radical S | 54 Gy | adjuvant | – | CDF | 20y |
| 4 | F | Thigh | 12 | Low grade | Biopsy, non-radical S after CT | – | adjuvant | – | CDF | 15y | ||
| 5 | F | Groin | 3 | Low grade | Radical S | – | – | – | CDF | 12y | ||
| 6 | F | Thigh | 6 | Low grade | Radical S | – | adjuvant | – | CDF | 11y | ||
| 7 | M | Thigh | 6 | Low grade | Radical S | 60 Gy | – | Yes, at 43 m | ANED | 8y (from 1st S) | ||
| 1 | M | Groin | 3 | Low grade | Radical S | – | – | – | CDF | 2y | ||
| Brookenthal [10] | 2003 | 11 | F | Calf | 8 | Low grade | Wide local ex | – | – | – | CDF | 36 m |
| Alaggio [9] | 2009 | 11 | F | Thigh | NA | Low grade | + | NA | NA | – | ANED | 24 m |
| 9 | F | Thigh | NA | Low grade | + | NA | NA | – | ANED | 120 m | ||
| 10 | F | Calf | NA | Low grade | + | NA | NA | – | ANED | 36 m | ||
| 12 | F | Inguinal | NA | Low grade | + | NA | NA | NA | Lost | |||
| 7 | F | Thigh | NA | Low grade | + | NA | NA | – | ANED | 3y | ||
| 11 | F | Abdomen | NA | Low grade | + | NA | NA | NA | Lost | |||
| 9 | F | Tongue | NA | Low grade | + | NA | NA | – | ANED | 6y | ||
| 11 | F | Ankle | NA | Low grade | + | NA | NA | – | ANED | 3y | ||
| 12 | M | Oral cavity | NA | Low grade | + | + | NA | Yes, twice (at 2 and 3 y) | ANED | 5y | ||
| 12 | F | Arm | NA | Low grade | + | NA | NA | – | ANED | 1y | ||
| 12 | M | Leg | NA | Low grade | + | NA | NA | – | ANED | 2y | ||
| 11 | F | Inguinal | NA | Low grade | + | NA | NA | – | ANED | 5y | ||
| Nichols [17] | 2011 | 9 | M | Cheek | 3 | Low grade | Resection, 5 times | 59.4 Gy after 5th surgery | – | multiple | ANED | 55 m |
| De Corti [12] | 2012 | 8 | F | Cervicothoracic juncton | NA | NA | Resection | NA | neoadjuvant | – | CR | > 1.5y |
| Hightower [14] | 2014 | 11 | M | Omental | 21 | NA | + | – | – | NA | Multiple pulmonary nodules, at 6 m | NA |
| Dall’Igna [11] | 2014 | 12 | M | Knee | < 5 | NA | + | NA | NA | NA | NA | NA |
| 12 | F | Thigh | > 5, < 10 | NA | + | NA | NA | NA | NA | NA | ||
| Özşen [18] | 2019 | 12 | F | Popliteal fossa | 4 | NA | + | NA | NA | Yes, at 2y | ANED | NA |
| Peng [8] | 2021 | 11 | F | Waist | NA | Low grade | Complete ex | – | – | – | ANED | 32 m |
| 12 | M | Thigh | 10 | Low grade | Wide ex | – | – | – | ANED | 25 m |
F Female, M Male, NA not available, S surgery, ex excision, CT chemotherapy, y year, m month, ANED alive, no evidence of disease, CDF continuous disease free, DOD dead of disease, CR complete remission
Regarding (neo) adjuvant radiotherapy, recent studies have shown that MLS is more sensitive to radiotherapy than other histological soft tissue sarcoma subtypes [19]. On the other hand, Nishida et al. reported that good local control could be achieved with wide surgical margins without radiotherapy in MLS of the extremity and superficial trunk [20]. Our literature review showed that surgical resection with wide negative margins achieved good local control in children aged 12 years or younger. In addition, a case series reported by Stanelle et al. showed that among 25 pediatric and adolescent patients with MLS, only 8 patients received radiation therapy [21]. A multi-institutional retrospective analysis by Huh et al. showed that among 24 children and young adults with MLS, only 10 received radiation therapy and only 2 out of 24 patients developed recurrence that originated in the abdomen [5]. In another case series by Baday et al., none of the 8 pediatric patients with MLS received radiation therapy [3]. In order to avoid serious late radiation complications, especially in young patients, we recommend that (neo)adjuvant radiotherapy should be used only in patients with unresectable disease, positive margins, or incomplete resection.
The role of chemotherapy as (neo)adjuvant treatment in the management of resectable pediatric MLS has not been clearly defined. Very recently, results of the prospective ARTS0332 study have been reported. The 551 patients with non-rhabdomyosarcoma soft tissue sarcoma younger than 30 years (which included 25 patients with liposarcoma) had undergone risk-based treatment [22]. They defined non-metastatic R0 tumors, low-grade R1 tumors, or high-grade R1 tumors ≤ 5 cm as low risk. It is recommended that most low-risk patients can be cured without adjuvant therapy, thereby avoiding known long-term treatment complications. We considered our patient to be at low risk because she had no metastatic lesions, a small tumor (< 5 cm), and no round cell components. Our patient did not undergo adjuvant therapy. Taking into consideration our patient and those described in other case series, surgical resection without any adjuvant treatment would be recommended, especially in pediatric MLS defined as low risk. Further studies are required to develop the optimal treatment strategy for MLS in children.
Regarding patient surveillance after treatment for MLS, we should take consideration that MLS has a higher propensity for extrapulmonary metastasis [23]. As MLS in our patient had faint FDG accumulation, it has been reported that MLS might present with low FDG uptake [24]. In addition, Sakamoto et al. reported a case of a MLS patient with multiple vertebral metastases not detected with FDG-PET/CT [25]. Taken together, FDG-PET/CT surveillance would be inappropriate for the detection of pediatric MLS, while FDG-PET/CT was ordered and performed by the previous hospital in the current case. We should be concerned about radiation risks to children from medical imaging. Kleinerman reported that we should pay close attention to the possible risk of secondary cancer following diagnostic radiation exposure in children [26]. Stevenson et al. reported whole-body MRI in MLS to detect extrapulmonary metastatic disease [23]. Currently, we have used a surveillance protocol consisting of whole-body MRI and chest x-ray every 6 months.
In conclusion, the present article reports a rare case of MLS in an 11-year old female. MLS typically presents in young adults. However, this tumor can affect younger individuals. Molecular cytogenetic studies to detect FUS-DDIT3 gene rearrangement are helpful for the diagnosis of MLS. Surgical resection with negative margins is the mainstay of treatment for pediatric patients with MLS. We should be concerned about radiation risks to children from medical imaging and consider a posttreatment surveillance strategy based on a risk-based approach, such as whole-body MRI.
Author contributions
All listed authors contributed to the original manuscript. TM: is the main orthopaedic oncologist of this case and wrote the manuscript draft. YI: coordinated and completed the manuscript. AM: supported orthopaedic surgical management. YI, KM and MH: supported pathohistological and molecular analyses. All authors have read and approved the manuscript of this case report.
Funding
This work was supported by in part by the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (20ck0106614h0001).
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee. For this type of study, formal consent is not required.
Consent for publication
Written informed consent was obtained from the patient and her mother. No identifiable information was included in this report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO classification of Tumours of soft tissue and bone . WHO classification of Tumours of soft tissue and bone. Lyon: IARC Press; 2020. [Google Scholar]
- 2.Orvieto E, Furlanetto A, Laurino L, Dei Tos AP. Myxoid and round cell liposarcoma: a spectrum of myxoid adipocytic neoplasia. Semin Diagn Pathol. 2001;18(4):267–273. [PubMed] [Google Scholar]
- 3.Baday YI, Navai SA, Hicks MJ, Venkatramani R, Whittle SB. Pediatric liposarcoma: a case series and literature review. Pediatr Blood Cancer. 2021;68(12):e29327. doi: 10.1002/pbc.29327. [DOI] [PubMed] [Google Scholar]
- 4.Nakata K, Ito Y, Magadi W, Bonaventure A, Stiller CA, Katanoda K, Matsuda T, Miyashiro I, Pritchard-Jones K, Rachet B. Childhood cancer incidence and survival in Japan and England: a population-based study (1993–2010) Cancer Sci. 2018;109(2):422–434. doi: 10.1111/cas.13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh WW, Yuen C, Munsell M, Hayes-Jordan A, Lazar AJ, Patel S, Wang WL, Barahmani N, Okcu MF, Hicks J, Debelenko L, Spunt SL. Liposarcoma in children and young adults: a multi-institutional experience. Pediatr Blood Cancer. 2011;57(7):1142–1146. doi: 10.1002/pbc.23095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonescu CR, Elahi A, Humphrey M, Lui MY, Healey JH, Brennan MF, Woodruff JM, Jhanwar SC, Ladanyi M. Specificity of TLS-CHOP rearrangement for classic myxoid/round cell liposarcoma: absence in predominantly myxoid well-differentiated liposarcomas. J Mol Diagn. 2000;2(3):132–138. doi: 10.1016/S1525-1578(10)60628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng K, Yu XC, Xu M, Yang Y. Surgical outcomes and prognostic factors of myxoid liposarcoma in extremities: a retrospective study. Orthop Surg. 2019;11(6):1020–1028. doi: 10.1111/os.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng R, Li N, Lan T, Chen H, Du T, He X, Chen M, Xie Y, Zhang Z, Zhao W, Zhang H. Liposarcoma in children and young adults: a clinicopathologic and molecular study of 23 cases in one of the largest institutions of China. Virchows Arch. 2021;479(3):537–549. doi: 10.1007/s00428-021-03076-8. [DOI] [PubMed] [Google Scholar]
- 9.Alaggio R, Coffin CM, Weiss SW, Bridge JA, Issakov J, Oliveira AM, Folpe AL. Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol. 2009;33(5):645–658. doi: 10.1097/PAS.0b013e3181963c9c. [DOI] [PubMed] [Google Scholar]
- 10.Brookenthal KR, Meyer JS, Pill SG, Matthews MR, Huff DS, Dormans JP. Calf mass in an 11-year-old girl. Clin Orthop Relat Res. 2003;407:268–277. doi: 10.1097/00003086-200302000-00036. [DOI] [PubMed] [Google Scholar]
- 11.Dall'Igna P, De Corti F, Alaggio R, Cecchetto G. Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg. 2014;24(6):482–487. doi: 10.1055/s-0034-1396422. [DOI] [PubMed] [Google Scholar]
- 12.De Corti F, Avanzini S, Cecchetto G, Buffa P, Guida E, Zanon GF, Jasonni V. The surgical approach for cervicothoracic masses in children. J Pediatr Surg. 2012;47(9):1662–1668. doi: 10.1016/j.jpedsurg.2012.03.087. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari A, Casanova M, Spreafico F, Luksch R, Terenziani M, Cefalo G, Massimino M, Gandola L, Navarria P, Fossati-Bellani F. Childhood liposarcoma: a single-institutional twenty-year experience. Pediatr Hematol Oncol. 1999;16(5):415–421. doi: 10.1080/088800199276967. [DOI] [PubMed] [Google Scholar]
- 14.Hightower JL, Jr, Dire DJ. Omental liposarcoma presenting as chronic constipation. Pediatr Emerg Care. 2014;30(7):483–484. doi: 10.1097/PEC.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 15.La Quaglia MP, Spiro SA, Ghavimi F, Hajdu SI, Meyers P, Exelby PR. Liposarcoma in patients younger than or equal to 22 years of age. Cancer. 1993;72(10):3114–3119. doi: 10.1002/1097-0142(19931115)72:10<3114::aid-cncr2820721037>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Miller GG, Yanchar NL, Magee JF, Blair GK. Lipoblastoma and liposarcoma in children: an analysis of 9 cases and a review of the literature. Can J Surg. 1998;41(6):455–458. [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols EM, Mirmiran A, Garofalo MC, Sun CC, Hatten K, Wolf J. Recurrent myxoid liposarcoma of the buccal mucosa in a young boy: a case report and review of the literature. Ear Nose Throat J. 2011;90(12):E27–31. doi: 10.1177/014556131109001215. [DOI] [PubMed] [Google Scholar]
- 18.Ozsen M, Yalcinkaya U, Yazici Z, Sarisozen MB. Lipomatous tumors in pediatric patients: a retrospective analysis of 50 cases. Turk Patoloji Derg. 2020;36(1):1–10. doi: 10.5146/tjpath.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansu J, Bovee J, Braam P, van Boven H, Flucke U, Bonenkamp JJ, Miah AB, Zaidi SH, Thway K, Bruland OS, Baldini EH, Jebsen NL, Scholten AN, van den Ende PLA, Krol ADG, Ubbels JF, van der Hage JA, van Werkhoven E, Klomp HM, van der Graaf WTA, van Coevorden F, Schrage Y, van Houdt WJ, Haas RL. Dose reduction of preoperative radiotherapy in myxoid liposarcoma: a nonrandomized controlled trial. JAMA Oncol. 2021;7(1):e205865. doi: 10.1001/jamaoncol.2020.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida Y, Tsukushi S, Nakashima H, Ishiguro N. Clinicopathologic prognostic factors of pure myxoid liposarcoma of the extremities and trunk wall. Clin Orthop Relat Res. 2010;468(11):3041–3046. doi: 10.1007/s11999-010-1396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanelle EJ, Christison-Lagay ER, Sidebotham EL, Singer S, Antonescu CR, Meyers PA, La Quaglia MP. Prognostic factors and survival in pediatric and adolescent liposarcoma. Sarcoma. 2012;2012:870910. doi: 10.1155/2012/870910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spunt SL, Million L, Chi YY, Anderson J, Tian J, Hibbitts E, Coffin C, McCarville MB, Randall RL, Parham DM, Black JO, Kao SC, Hayes-Jordan A, Wolden S, Laurie F, Speights R, Kawashima E, Skapek SX, Meyer W, Pappo AS, Hawkins DS. A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): a Children's Oncology Group prospective study. Lancet Oncol. 2020;21(1):145–161. doi: 10.1016/S1470-2045(19)30672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson JD, Watson JJ, Cool P, Cribb GL, Jenkins JP, Leahy M, Gregory JJ. Whole-body magnetic resonance imaging in myxoid liposarcoma: a useful adjunct for the detection of extra-pulmonary metastatic disease. Eur J Surg Oncol. 2016;42(4):574–580. doi: 10.1016/j.ejso.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Baffour FI, Wenger DE, Broski SM. (18)F-FDG PET/CT imaging features of lipomatous tumors. Am J Nucl Med Mol Imaging. 2020;10(1):74–82. [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto A, Fukutoku Y, Matsumoto Y, Harimaya K, Oda Y, Iwamoto Y. Myxoid liposarcoma with negative features on bone scan and [18F]-2-fluoro-2-deoxy-D-glucose-positron emission tomography. World J Surg Oncol. 2012;10:214. doi: 10.1186/1477-7819-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36(Suppl 2):121–125. doi: 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.